Introduction
Colorectal carcinoma (CRC) is the third most
prevalent cancer globally, the incidence rate of which increases
with advancing age (1). Despite
advances in chemotherapy, radiotherapy and novel drug development,
the prognosis in patients with CRC remains poor. Furthermore, the
occurrence of severe side effects and toxicities limit the existing
therapeutic regimens. Therefore, alternative medications with
pronounced effectiveness and low-toxicity are necessary (2,3).
Formononetin (Form) is an O-methylated isoflavone
phytoestrogen obtained from the root of Astragalus
membranaceus, an essential herb used in Chinese medicine for
>2,000 years which offers various pharmacological effects
including inhibition of tumor cell proliferation, migration and
invasion (4,5). Furthermore, a previous study
indicated that Form controlled CRC progression without causing
significant toxicity in drug-treated animals, with no mortality or
decline in body weight. In addition, it did not exert any
neutropenic effects on drug-treated animals (6). The discovery of low-toxicity Form,
which possesses potential for cancer chemoprevention or treatment
is essential for the development of colon cancer therapy.
MicroRNAs (miRs) are endogenous, small,
single-stranded and non-coding RNAs that function as gene
expression repressors by binding to target sites in the
3′-untranslated regions of messenger RNA (mRNA) (7). These miRs can be categorized as
oncogenes or tumor suppressors and by targeting various
transcripts, they participate in the proliferation, apoptosis,
differentiation, and invasion processes (8). Recent studies have suggested that
Form inhibits various human cancers via miR regulation. For
example, it inhibited human bladder cancer cell proliferation and
invasiveness (9) and induced
apoptosis in the human osteosarcoma cell line U2OS (10) and breast cancer cells (11) via miR-21 or miR-375 regulation.
Another recent study has demonstrated that EphB3-targeted miR-149
regulation serves a role in the migration and invasion of HCT116
and SW620 cells (12). However,
whether Form inhibits colon carcinoma cell proliferation and
invasion by EphB3-targeted miR-149 regulation has not yet been
investigated.
Previous studies have demonstrated that Form induces
growth inhibition and promotes caspase-dependent apoptosis, which
involves the inhibition of antiapoptotic proteins including B-cell
lymphoma-2 and B-cell lymphoma-extra large, and the activation of
the novel proapoptotic protein nonsteroidal anti-inflammatory drug
activated gene-1 in human colon cancer cells (13). In addition, Form inhibits
angiogenesis and tumor cell invasion involving matrix
metalloproteinase (MMP) inhibition in human colon cancer cells
(14). However, detailed
investigations on Form-induced proliferation and invasion of colon
carcinoma as well as its underlying molecular mechanisms still
remain limited.
Accumulating evidence indicates that Form may serve
as an agent that can deal with several cancer types including
breast (15), bladder (16) and prostate (15) cancer. Treatment of these cancer
cells with Form significantly decreased the cyclin D1 protein and
gene expression, which was demonstrated to be associated with
phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT),
extracellular signal-regulated kinase (ERK), or signal transducer
and activator of transcription 3 (STAT3) signaling pathways.
However, which signaling pathway is involved in the Form-mediated
antitumor effects on human CRC cell growth and invasion remains
unclear. The present study aimed to test the effects of Form on
colon cancer cell proliferation and invasion and further dissected
the molecular mechanisms underlying the regulation of cell
proliferation and invasion. The present study also demonstrated for
the first time to the best of the authors' knowledge that Form
suppresses colon carcinoma cell growth and invasion via the
miR-149-mediated EphB3 downregulation and the inhibition of the
PI3K/AKT and STAT3 signaling pathways.
Materials and methods
Reagents
Form (purity >98%), provided by Phytomarker,
Ltd., (Tianjing, China), was verified by high-performance liquid
chromatography and its chemical structure is illustrated in
Fig. 1A. Form was dissolved in
dimethyl sulfoxide (DMSO) as a 200 mM stock solution and stored at
4°C for further use.
Cell culture
The human colon carcinoma cell lines SW1116, HCT116
and normal colon epithelial NCM460 cells were obtained from the
Cancer Hospital, Chinese Academy of Medical sciences (Beijing,
China). The cells were cultured in Dulbecco's modified Eagle's
media (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
containing 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life
Sciences), 100 kU/l penicillin and 100 mg/l streptomycin at 37°C in
a humidified atmosphere of 5% CO2. These cells were
exposed to stimulation with 0, 20, 50, 100 and 200 µM of Form for 2
h.
Adenovirus infection
Adenovirus encoding Ephrin type-B receptor 3
(Ad-EphB3) and green fluorescent protein control (GFP-control) were
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). When HCT116 cells had grown to 70–80% confluence, the
cells were infected by Ad-EphB3 or GFP-control virus (6 MOI) for 24
h and treated with Form for 12 h. Then cells were collected for
assays as previously described (17).
Transfection of miRNA mimics or
siRNAs
The human miR-149 mimic and negative control
(mimic-NC) were designed and provided by Guangzhou RiboBio Co.,
Ltd., (Guangzhou, China). The siEphB3 and the negative control RNA
(si-NS) were synthesized and purified by Takara Biotechnology Co.,
Ltd. (Dalian, China). The sequences of miRNAs and siRNAs were
described in Table I. Cultured
HCT116 and SW620 cells were transfected with miRNAs or siRNAs with
the Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol (12).
 | Table I.Primers used for cell
transfection. |
Table I.
Primers used for cell
transfection.
| Name | Primers (5′-3′) |
|---|
| miR-149 | Forward:
UCUGGCUCCGUGUCUUCACUCCC |
|
| Reverse:
GGGAGUGAAGACACGGAGCCAGA |
| mimic-NC | Forward:
UUCUCCGAACGUGUCACGUTT |
|
| Reverse:
ACGUGACACGUUCGGAGAATT |
| siEphB3 | Forward:
GGACCCUAAUGAGGCUGUU |
|
| Reverse:
AACAGCCUCAUUAGGGUCC |
| si-NS | Forward:
GGAAAUCGAGUUCGCCGUU |
|
| Reverse:
AACGGCGAACUCGAUUUCC |
MTT assay
Following appropriate treatment, the viability of
SW1116 and HCT116 cells cultured in 96-well plates was measured
using the MTT assay, as previously described (13). Following 24 h, various
concentrations (0, 20, 50, 100 and 200 µM) of Form were added and
incubated for 24, 48, or 72 h. After the media and Form were
discarded, the cells were incubated with 0.5 mg/ml MTT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 4 h.
The medium was then removed and 150 µl DMSO was added to every
well. The mixtures were agitated for 15 min in the dark. The
optical density of each well was measured using a microculture
plate reader (Omega Bio-Tek, Inc., Norcross, GA, USA) at a
wavelength of 490 nm.
Cell cycle analysis
Cells were treated with the Form for 48 h and
harvested by trypsinization, and then cells were washed with
ice-cold PBS, fixed with 70% ethanol at 4°C for 24 h, incubated for
5 min with 0.5% Triton X-100 and stained with propidium iodide at
37°C for 30 min in PBS containing 25 mg/ml RNase. Stained cells
were analyzed by BD ACCURI C6 flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) and the mean intensity of the cells in
each sample was determined by BD Accuri C6 software (BD
Biosciences) as previously described (6).
Cell invasion assay
The cell invasion ability was examined using 24-well
matrigel-coated Transwell chambers (BD Biosciences) as previously
described (6). In brief, the
SW1116 and HCT116 cells were treated with Form for 48 h, washed
with PBS and resuspended at 1×105 cells/ml in serum-free
medium. Then 0.2 ml cell suspension was added to the upper chamber
and 0.5 ml medium containing 10% FBS was added to the bottom
chamber. Following 24 h incubation, all non-invaded cells were
removed from the upper face of the filters and the invaded cells
were fixed with 4.0% paraformaldehyde at room temperature for 15
min and stained by 0.1% crystal violet solution at room temperature
for 2 min. The experiments were repeated in triplicate wells and
the invaded cells were counted using a light microscope (×400) in
five different fields of view per filter.
Western blot analysis
The protein concentration of cell lysates of SW1116
and HCT116 by 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% Nonidet
P-40, 0.5% sodium deoxycholic acid, and phenylmethane sulfonyl
fluoride (78830-1G; Sigma-Aldrich; Merck KGaA) were determined by
Coomassie brilliant blue method, then 50 ng protein were separated
by 10% SDS-PAGE and transferred onto a polyvinlidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). Membranes were
blocked with 5% milk in 0.1% Tween-20 TBS for 2 h at 37°C and
incubated overnight at 4°C with the following primary antibodies:
Rabbit anti-cyclin D1 (1:1,000; cat. no. 12363-1-AP; Proteintech
Group, Inc., Chicago, IL, USA), rabbit anti-cyclin B1 (1:1,000;
cat. no. 21644-1-AP; Proteintech Group, Inc.), rabbit anti-MMP2
(1:1,000; cat. no. 10373-2-AP; Proteintech Group, Inc.) and rabbit
anti-MMP9 (1:1,000; cat. no. 10375-2-AP; Proteintech Group, Inc.),
mouse anti-phosphorylated (p)-AKT (Ser473; 1:1,000; cat. no.
66444-1-Ig; Proteintech Group, Inc.), mouse anti-AKT (1:1,000; cat.
no. 60203-2-Ig; Proteintech Group, Inc.), mouse anti-p-PI3K
(Tyr458; 1:1,000; cat. no. 4228T; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit anti-PI3K (1:1,000; cat. no. 20584-1-AP;
Proteintech Group, Inc.), rabbit anti-p-ERK (Thr202/Tyr204;
1:1,000; cat. no. 66192-1-lg; Proteintech Group, Inc.), rabbit
anti-ERK (1:1,000; cat. no. 51068-1-AP; Proteintech Group, Inc.),
rabbit anti-p-STAT3 (Ser 727; 1:1,000; cat. no. sc-7993; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit anti-STAT3 (1:1,000;
cat. no. 10253-2-AP; Proteintech Group, Inc.) and rabbit
anti-β-actin (1:5,000; cat. no. 12363-1-AP; Proteintech Group,
Inc.). Membranes were washed and incubated with the appropriate
horseradish peroxidase-conjugated rabbit anti-mouse secondary
antibody (1:1,000; cat. no. 21644-1-AP; Cell Signaling Technology,
Inc.). Protein bands were detected by enhanced chemiluminescent
plus (Thermo Fisher Scientific, Inc.) and β-actin was served as an
internal control for protein quantitation with cell imaging
densitometry (E-Gel GelQuant Express Analysis Software, version
1.7; Thermo Fisher Scientific, Inc.).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SW1116 and HCT116 cells
using the TRizol™ reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. A total 1 mg of RNA was
subjected to reverse transcription using first-strand cDNA
synthesis kit (Beijing Transgen Biotech Co., Ltd., Beijing, China)
according to the manufacturer's protocol. qPCR (SYBR Premix Ex
Taq™; Takara Biotechnology, Co., Ltd.) experiments were carried on
an ABI 7500 FAST system (Thermo Fisher Scientific, Inc.). Relative
amount of transcripts were normalized with U6 or GAPDH and
calculated using the 2−ΔΔCq formula as previously
described (12). All PCRs were
performed in triplicate. PCR amplification procedure: 94°C, 5 min;
(94°C, 30 sec; 55°C, 30 sec; 72°C, 1 min) ×30; 72°C, 5 min. The
primer sequences were as follows: Hsa-miR-149 forward (F),
5′-GGCTCTGGCTCCGTGTCTT-3′ and reverse (R),
5′-CAGTGCAGGGTCCGAGGTATT-3′; U6 F, 5′-CAAATTCGTGAAGCGTTCCATA-3′ and
R, 5′-AGTGCAGGGTCCGAGGTATTC-3′; EphB3 F, 5′-CCTGTACGCCAACACAGTGC-3′
and R, 5′-CTCGAGCCGGATTATATTG-3′; and GAPDH F,
5′-GAAAGCCTGCCGGTGACTAA-3′ and R,
5′-GCCCAATACGACCAAATCAGAGA-3′.
Xenograft experiments in vivo
A total of 30 Male BALB/c nude mice, 6–8 weeks,
weighting ~20–24 g, were purchased from the Experimental Animal
Center of Hebei Medical University. The present study was approved
by the Laboratory Animal Ethics Committee of Fourth Hospital Hebei
Medical University (Shijiazhuang, China). Experimental procedures
were implemented according to the guidelines and regulations of the
Institutional Animal Care and Use Committee of Hebei Medical
University.
The design HCT116 cell numbers (3×108
density) were subcutaneously injected into the back of the nude
mice. Following the solid tumor growing to 5 mm in diameter, nude
mice were randomly grouped as follows: Vehicle control (n=10), Form
treated groups (15 mg/kg, n=10) and Ad-EhpB3+Form groups (n=10)
intragastrically given 15 mg/kg daily for 14 days. Tumor size was
measured using a digital vernier caliper. The tumor volume was
calculated according to the following formula: mm3 =
d2 × L/2, where d and L represent the shortest and
longest diameters, respectively. The isolated solid tumor was then
weighed.
Statistical analysis
All the experiments were repeated three times. The
data are presented as the mean ± standard deviation. The SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA) was used for statistical
analyses including analysis of variance and Bonferroni post hoc
tests was used for cell viability, cell number, band density, gene
expression data and Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Form suppresses SW1116 and HCT116 cell
proliferation and invasion
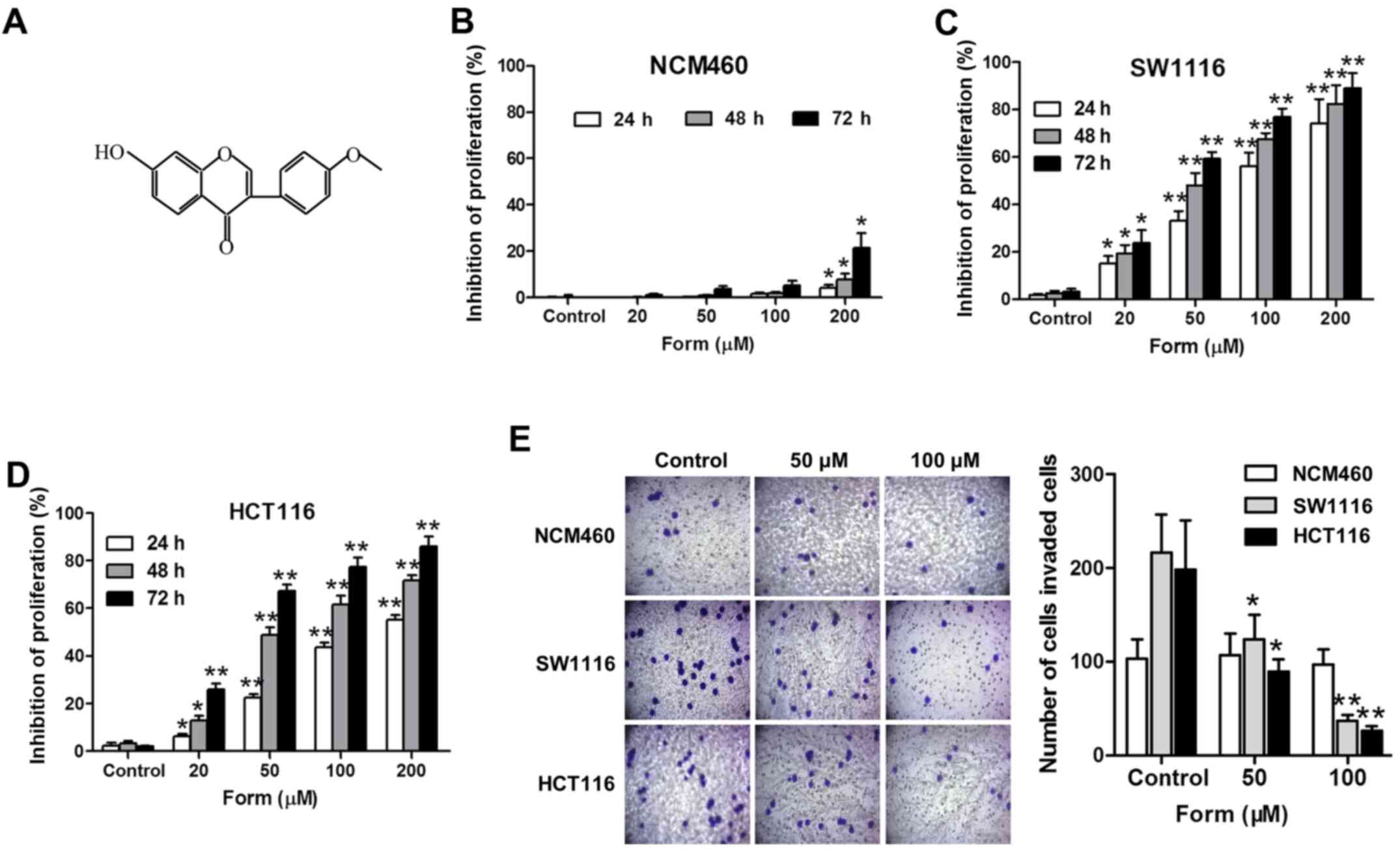
The inhibition rates of Form (0, 20, 50, 100 and 200
µM) in SW1116 and HCT116 cells and normal epithelial colon NCM460
cells were detected using an MTT assay, and the results suggested
that Form inhibited the HCT cell proliferation in a dose- and
time-dependent manner (Fig. 1).
Compared with the control, the inhibitory effect of Form on SW1116
and HCT116 cells was significantly increased at 24, 48, and 72 h
(P<0.05; Fig. 1C and D). The
Transwell invasion assay performed to examine the effects of Form
on SW1116 and HCT116 cell invasiveness demonstrated that Form
significantly inhibited the cell invasiveness in a
concentration-dependent manner compared with the negative control
(P<0.05; Fig. 1E). Overall,
these results indicated strong anti-proliferation and anti-invasion
effects of Form on SW1116 and HCT116 cells.
Form induces cell-cycle arrest and
suppresses cell invasion in SW1116 and HCT116 cells
Flow cytometry was performed to assess the cell
cycle in the 4 groups (control, 20, 50 and 100 µM Form) to further
elucidate how Form inhibits SW1116 and HCT116 cell proliferation.
Cells treated with different Form concentrations (20, 50 and 100
µM) for 48 h increased the proportion in the
G0-G1 phase compared with the control
(P<0.05); the proportion of cells undergoing this phase
increased to 79.7% following treatment with 100 µM Form (P<0.05;
Fig. 2A and B). Form was also
observed to suppress cyclin D1 but not cyclin B1 expression (data
not shown) in a dose-dependent manner (Fig. 2C). The expression of extracellular
matrix-degrading enzymes MMP2 and MMP9, which serve roles in cell
invasion regulation, were examined to delineate the mechanism by
which Form protects human colon carcinoma cell invasion (18). Fig. 2D
and E demonstrates that Form treatment significantly attenuated
MMP2 and MMP9 protein expression, as determined using western
blotting analysis. These results indicate that the suppressive
effects of Form on human colon carcinoma cell proliferation and
invasion are mediated by the inhibition of cyclin D1 and MMP2/MMP9
expressions.
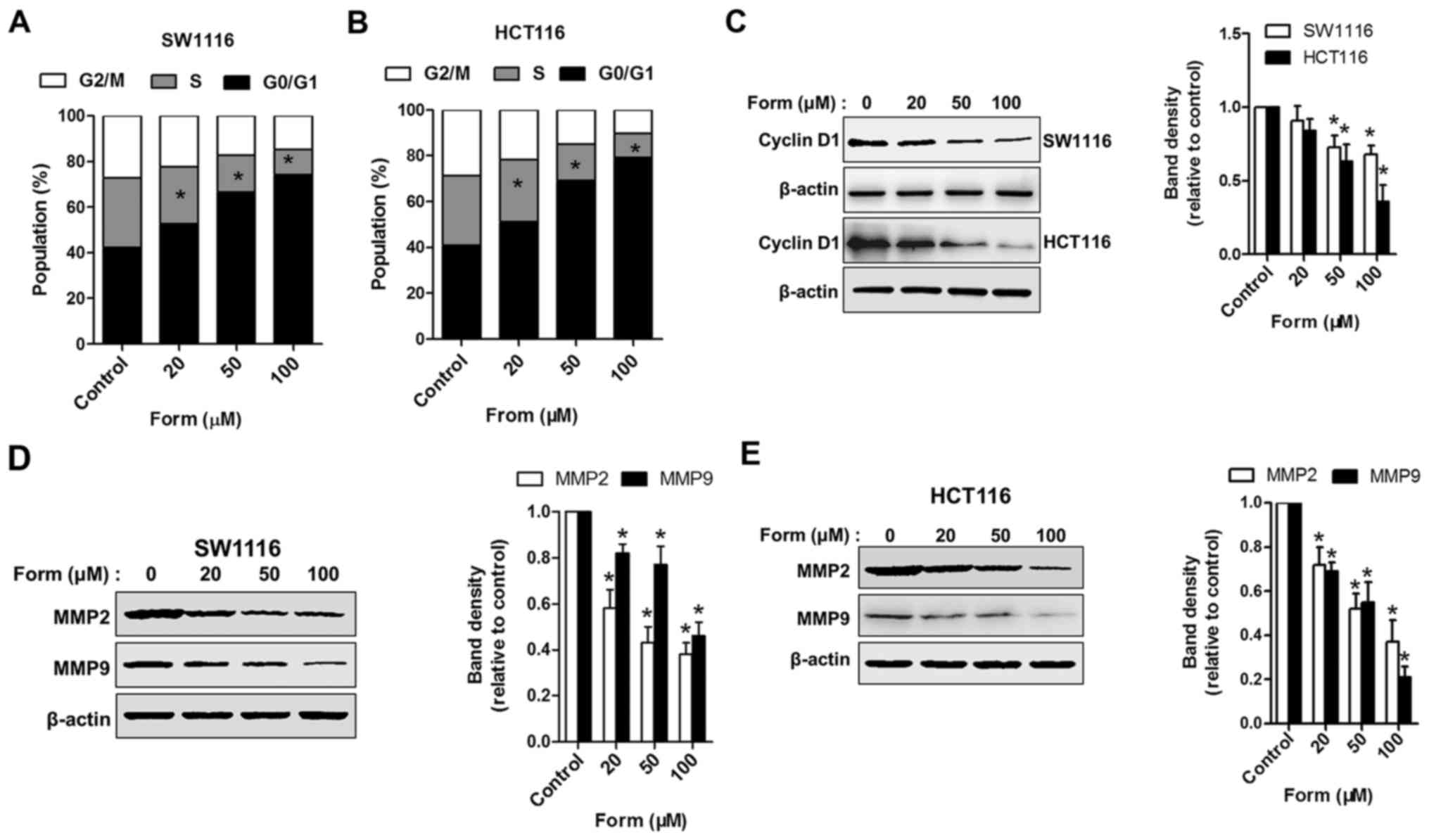 | Figure 2.Form induces cell-cycle arrest and
inhibits MMP2/9 expression in SW1116 and HCT116 cells. Both (A)
SW1116 and (B) HCT116 cells treated with 0, 20, 50, and 100 µM Form
for 48 h were stained using propidium iodide, and cell-cycle
distributions were analyzed using flow cytometry. Bar graph,
percentage cells in G1, S and G2 cell-cycle
phases. SW1116 and HCT116 cells treated with Form (0, 20, 50, and
100 µM) for 48 h and whole-cell extracts collected for the western
blot analysis using (C) cyclin D1, (D) MMP2, MMP9, and β-actin
antibodies in SW1116 cells and (E) HCT116 cells. The band
intensities relative to β-actin are presented as the mean ±
standard deviation; n=6; *P<0.05 vs. control. MMP, matrix
metalloproteinase; Form, Formononetin. |
Form regulates the expression of
miR-149 and EphB3 and the phosphorylation of Akt/PI3K and STAT3 in
SW1116 and HCT116 cells
A recent study has demonstrated the suppressive role
of the EphB3-targeted miR-149 regulation in the migration and
invasion of human colon carcinoma (12). Therefore, the present study aimed
to determine the inhibitory effects of Form on SW1116 and HCT116
cells and whether different Form concentrations altered miR-149 and
EphB3 (the direct target of miR-149) expression. Fig. 3A indicates that Form upregulated
miR-149 expression in SW1116 and HCT116 cells compared with
untreated cells in a dose-dependent manner (P<0.05 and
P<0.01). Although the EphB3 mRNA expression was significantly
downregulated (P<0.05 and P<0.01; Fig. 3B) when treated with Form, miR-149
and EphB3 expression demonstrated no apparent alterations in normal
epithelial colon NCM460 cells (Fig.
3C). In addition, the ERK, AKT, PI3K and STAT3 signaling
pathways have been implicated in the regulation of cell
proliferation, and invasion, in addition to cell cycle-associated
protein and MMP expression (19–21).
Therefore, the roles of p-ERK, p-AKT, p-PI3K and p-STAT3 signaling
in SW1116, and HCT116 cells were investigated following treatment
with Form. Fig. 3D indicated that
Form treatment significantly decreased p-AKT, p-PI3K and p-STAT3
protein expression compared with the control, suggesting that Form
alters p-AKT, p-PI3K, and p-STAT3 protein levels in SW1116 and
HCT116 cells (P<0.05). However, the total protein levels of AKT,
PI3K, STAT3 and ERK signaling remained unaffected. These data
suggested that Form suppresses cell proliferation and invasion by
inhibition of cyclin D1 and MMP2/9 expression via p-AKT, p-PI3K,
and p-STAT3 inactivation in colon carcinoma cells. These data
indicate that Form significantly upregulates miR-149 expression,
downregulates EphB3 and inhibits PI3K/AKT and STAT3 phosphorylation
in SW1116 and HCT116 cells.
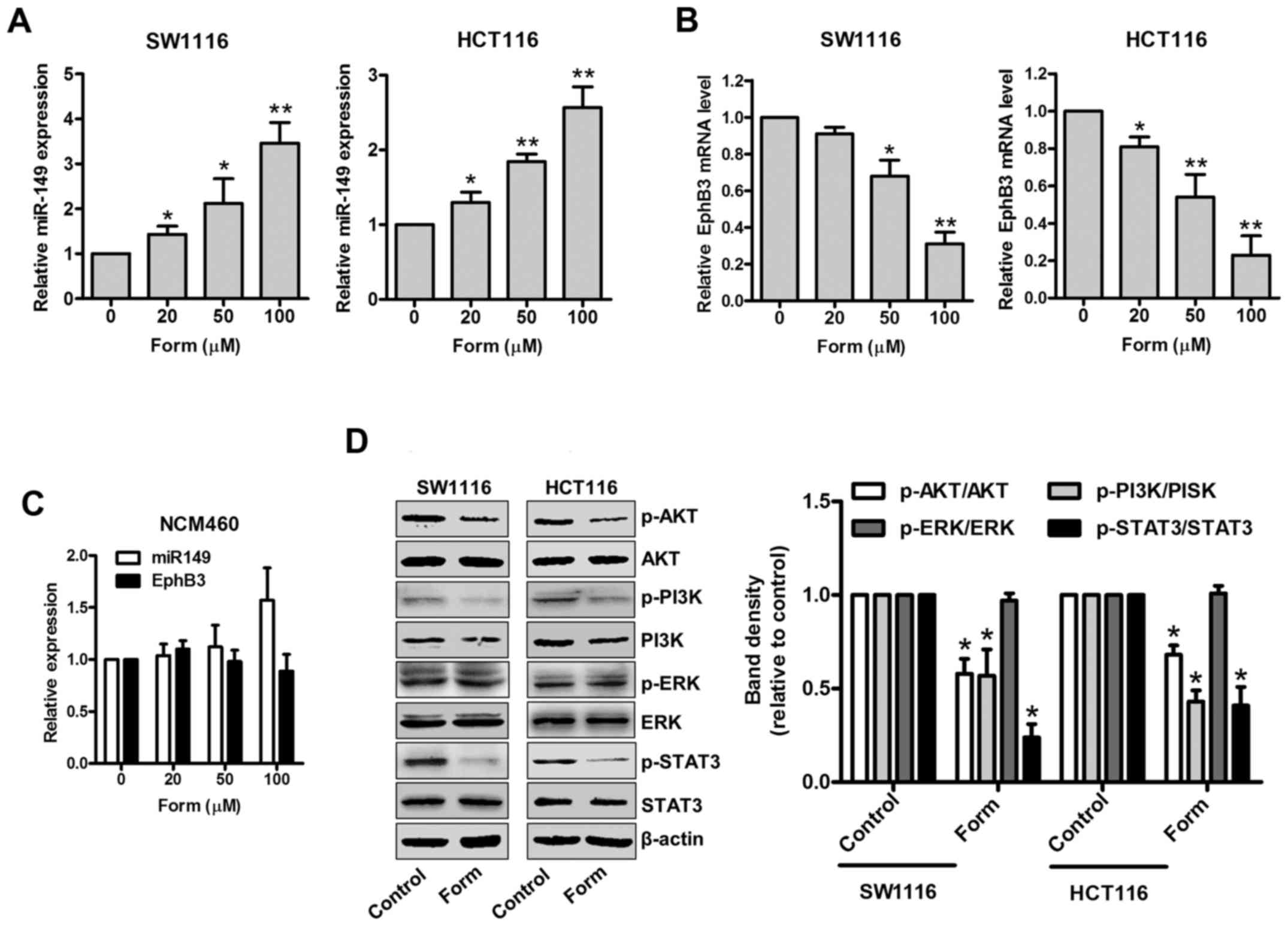 | Figure 3.Form regulates miR-149 and EphB3
expression and suppresses AKT/PI3K expression and STAT3 signaling
in SW1116 and HCT116 cells. (A) SW1116, (B) HCT116 and (C) NCM460
cells were treated with Form (20, 50, and 100 µM) for 48 h, then
miR-149 and EphB3 mRNA were determined using reverse
transcription-quantitative polymerase chain reaction. Data are
expressed as the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. control, n=5. (D) SW1116 and HCT116 cells were
incubated with Form (100 µM) for 12 h, and the protein levels of p-
and total PI3K, AKT, ERK, and STAT3 were determined using western
blotting. The band intensities relative to AKT, PI3K, ERK and STAT3
are presented as the mean ± standard deviation. *P<0.05 vs. the
control; n=6. Form, Formononetin; p-PI3K,
phosphorylated-phosphoinositide 3 kinase; AKT, protein kinase B;
ERK, extracellular regulated signal kinase; STAT3, signal
transducer and activator of transcription 3; EphB3, Ephrin type-B
receptor 3. |
Ectopic miR-149 expression and small
interfering (siRNA)-mediated EphB3 silencing promotes
Form-inhibition of cell growth and invasion in SW1116 and HCT116
cells
To elucidate the role of miR-149 and
EphB3 in Form-inhibited cell growth and invasion in colon
carcinoma cells, SW1116 and HCT116 cells were transiently
transfected with an miR-149 mimic, miR-149 mimic control
(mimic-NS), or EphB3 siRNA for EphB3 expression knockdown, and its
efficacy was confirmed using RT-qPCR and western blot analysis. The
results of RT-qPCR revealed that the miR-149 mimic significantly
upregulated the miR-149 expression in SW1116 and HCT116 cells
compared with the mimic-NS (P<0.01; Fig. 4A). SW1116 and HCT116 cells
transfected with miR-149 mimic demonstrated lower EphB3 expression
levels compared with the mimic control groups, as determined using
western blot analysis (Fig. 4B).
Different siRNA concentrations were used in both cell lines to
standardize the conditions and inhibit expression of EphB3
(Fig. 4C). Based on the obtained
results, SW1116 and HCT116 cells were transfected with the miR-149
mimic, mimic-NS, siNS, or EphB3 siRNA, and following 24 h, they
were treated with Form for 2 h. The results revealed that cell
viability was significantly decreased in the mimic miR-149+Form
group compared with the other 3 groups (P<0.01; Fig. 4D); Cell viability was also
significantly decreased in the siEphB3+Form group compared to the
other 3 groups (P<0.01; Fig.
4E), suggesting a role EphB3 in Form-inhibited colon carcinoma
cell growth. Similarly, Transwell assays indicated that Form
induced the inhibition of HCT116 cell invasion where miR-149
overexpression or EphB3 knockdown significantly increased compared
with the negative control (P<0.05; Fig. 4F and G). These results indicated
the role of miR-149 and EphB3 in the Form-inhibited cell growth and
invasion in colon carcinoma cells.
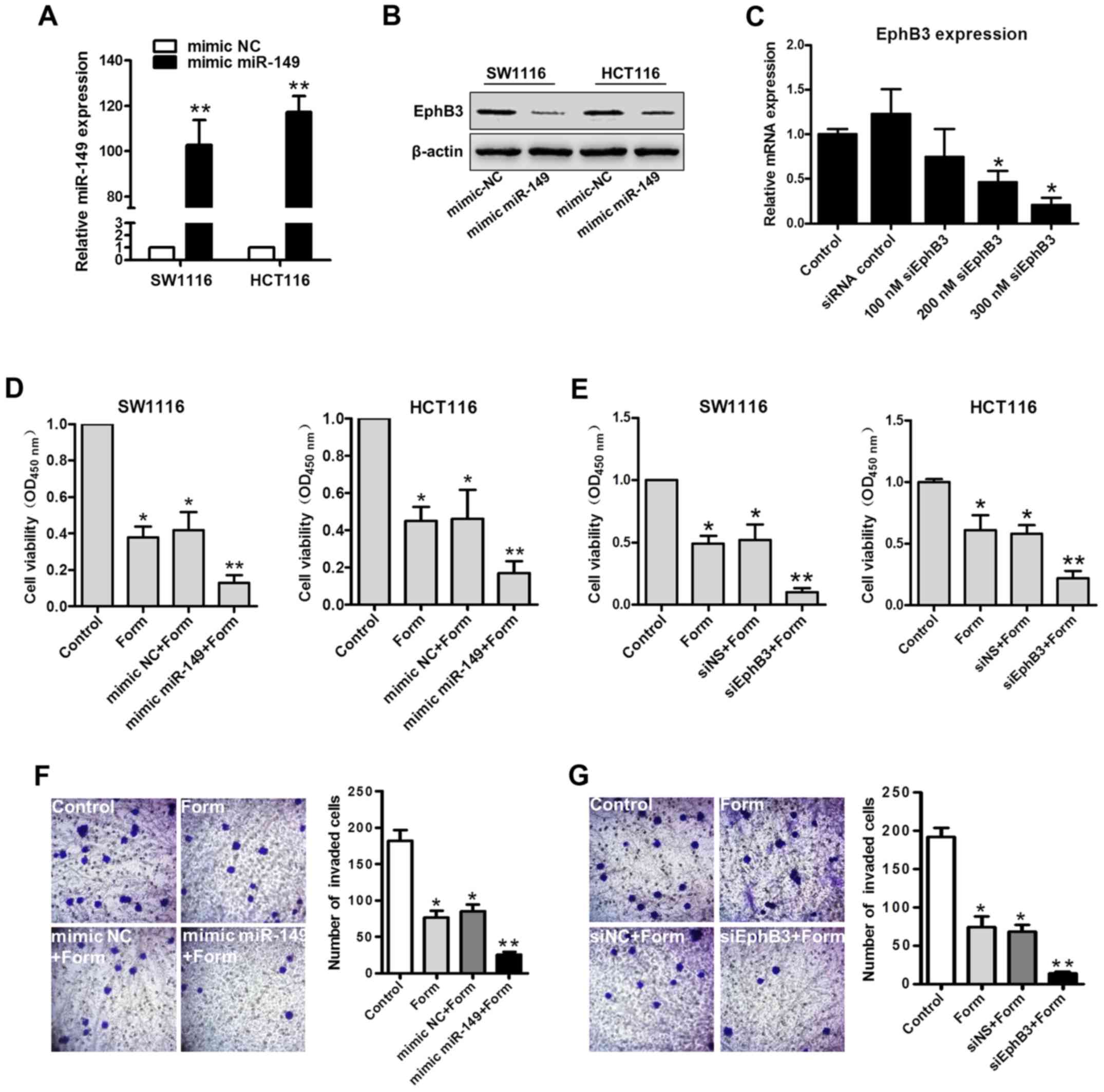 | Figure 4.Both the mimic miR-149 and siEphB3
enhance Form-induced inhibition of proliferation of colon cancer
cells. (A) RT-qPCR analysis of miR-149 in SW1116 and HCT116 cells
transfected with mimic miR-149 or negative control. Data are
depicted as the mean ± standard deviation. **P<0.01 vs. control,
n=5. (B) Western blot analysis for EphB3 expression detection in
SW1116 and HCT116 cells transfected with mimic miR-149. (C) RT-qPCR
for siRNA-mediated silencing verification of EphB3 mRNA in SW1116
and HCT116 cells transfected with siEphB3 or siRNA control.
*P<0.05 vs. control, n=5. SW1116 and HCT116 cells transfected
with (D) mimic-NC or mimic miR-149 for 24 h or transfected with (E)
siEphB3 or siNS for 24 h. Transfected cells were then treated with
100 µM Form for 24 h. Cell viability was determined using the MTT
assay. Data are illustrated as the mean ± standard deviation,
*P<0.05 and **P<0.01 vs. control, n=5. (F) Transwell assay
demonstrated that miR-149 overexpression and (G) EphB3
downregulation enhanced Form-inhibited cell invasion in HCT116
cells (magnification, ×400). Data are presented as the mean ±
standard deviation, *P<0.05 and **P<0.01 vs. the control,
n=5. RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; si, small interfering; miR, microRNA; NS, normal control;
EphB3, Ephrin type-B receptor 3; Form, Formononetin. |
EphB3 overexpression partially
decreases the Form-inhibited colon carcinoma cell growth
The EphB3 expression was enhanced using Ad-EphB3 in
HCT116 cells to elucidate the role of miR-149 and EphB3 in
Form-inhibited cell growth and invasion in colon carcinoma cells.
In Fig. 5A-C, the western blot
analysis demonstrated that Ad-EphB3 infection enhanced EphB3
expression in HCT116 cells and that its overexpression could rescue
Form-inhibited cell viability and invasion. The effects of Form on
colon carcinoma cell growth in xenograft nude mice were analyzed to
confirm the in vitro results. As illustrated in Fig. 5D-F, xenograft nude mice treated by
subcutaneous injection for 2 weeks demonstrated a significant
increase in tumor volume and weight, whereas Form significantly
reduced growth of tumor xenografts compared with the control
(P<0.05). Furthermore, the suppressive effects of Form on colon
cancer cell growth could be partially abolished by overexpressing
EphB3. These results indicated the role of EphB3 in the
Form-inhibited colon carcinoma cell growth.
 | Figure 5.EphB3 overexpression by Ad-EphB3
partially decreased Form-induced inhibition of cell viability and
invasion in colon cancer cells. HCT116 cells were infected with the
Ad-GFP control or Ad-EphB3, 24 h following infection cells were
treated with 100 µM Form for 24 h. (A) The expression of EphB3 was
analyzed by western blotting. (B) MTT assay and (C) Transwell assay
were performed to determine cell viability and invasion. Data are
presented as the mean ± standard deviation, *P<0.05 vs. the
Control, n=5. Ad-GFP, adenovirus-green fluorescent protein; EphB3,
Ephrin type-B receptor 3; Form, Formononetin. (D) HCT116 (Control),
Form treatment and Ad-EphB3 infection and Form treatment
(Ad-EphB3+Form) xenograft tumour masses were harvested on day 28.
Photographs of tumor removed from mice in each group. (E) Form
treatment significantly decreased and Ad-EphB3+Form rescued the
xenograft tumour volumes and (F) tumor weights, compared with
Control. *P<0.05, **P<0.01, ***P<0.001 vs. the
Control. |
Discussion
The present study aimed to elucidate the molecular
mechanisms of Form and its inhibitory effect exerted on the
proliferation and invasion of colon carcinoma cells in
vitro. It was demonstrated that Form inhibits colon carcinoma
cell proliferation and invasion by suppressing cyclin D1 and MMP2/9
expression via miR-149-induced EphB3 downregulation, and
downregulates PI3K/AKT activity and STAT3 signaling pathways.
The anticarcinogenic activities of different herbal
flavonoids may involve both common and novel mechanisms of action,
which could be developed into potential anticancer drugs. Form, a
traditional Chinese herbal medicine isolated from red clover, not
only possesses a number of properties, including antioxidant,
antiviral and cardioprotective effects, but is also gaining
prominence for its potential antitumor effect (22). Previous studies have demonstrated
that Form exerts anticarcinogenic effects on cancer cells by
miR-mediated target gene regulation and associated signaling
pathways (9). Furthermore, a
recent study has highlighted that EphB3 is a direct target gene for
miR-149 and regulates colon carcinoma cell development, including
cell proliferation, invasion, and cell cycle progression, via EphB3
expression regulation (12). The
present study demonstrated that Form dose-dependently upregulated
miR-149 expression and downregulated EphB3 mRNA expression in
SW1116 and HCT116 cells. These results were in agreement with those
of previous studies that demonstrated variable expression patterns
of miR-149 and its target gene in colorectal cancer cells (23,24).
A role of miR-149 and EphB3 in Form-inhibited cell growth and
invasion in colon carcinoma cells was demonstrated.
Additionally, previous studies have reported that
Form can attenuate the growth and invasion of various cancer cell
types and suppress cyclin and MMP expression by inhibiting a number
of mitogen-activated protein kinase pathways, including the ERK1/2,
p38, JNK (25), PI3K/AKT, and
STAT3 pathways (26). Furthermore,
cyclin D1 and MMP2/9 expression was demonstrated to decrease in
SW1116 and HCT116 cells treated with Form and that cyclin D1 and
MMP2/9 mediated Form-elicited SW1116 and HCT116 cell growth and
invasion through PI3K/AKT signaling pathways. These results
corroborate those of previous studies demonstrating that Form
promoted cell-cycle arrest and invasion via AKT/cyclin D1/STAT3
downregulation in breast (6) and
prostate cancer cells (5).
Particularly, Form treatment was demonstrated to inhibit CRC
proliferation and invasion by suppressing cyclin D1 and MMP2/9
expression via PI3K/AKT downregulation and STAT3 signaling
pathways.
Accumulating evidence suggests that cyclin D1 is
essential in cell-cycle control and that its expression is
regulated by PI3K/AKT (27). A
previous study highlighted the role of cyclin D1 in several human
cancers, including breast cancer (15). SW1116 and HCT116 cells both treated
with Form were demonstrated to accumulate at the
G0-G1 phase. Form significantly stimulated
p-PI3K/AKT signaling pathway inactivation and decreased cyclin D1
expression, which is consistent with the MTT assay results. These
results indicated that Form inhibited SW1116 and HCT116 cell growth
by suppressing cyclin D1 expression, which promotes cell-cycle
arrest through PI3K/AKT signaling pathway inactivation.
In colon cancer, tumor cells must invade the
muscularis mucosa and migrate into the submucosa prior to reaching
the lymphatic channels or blood vessels. This proteolytic
degradation of the extra cellular membrane by proteolytic enzymes
like MMP2/9 is a necessary step in cancer metastasis. MMP2/9
expression is mediated by the PI3K/AKT signaling pathway. STAT3 is
a well-known MMP inducer as well as a downstream component of the
PI3K/AKT pathway. p-STAT3 is translocated into the nucleus to
transmit extracellular signals that regulate tumor cell
proliferation and migration (28).
PI3K/AKT and STAT3 inactivation as well as a decrease in the
extracellular MMP2/9 expression in SW1116 and HCT116 cells, were
observed upon cell treatment with Form, which inhibited tumor
growth and invasion. In accordance with the results of the present
study, Huang et al (13)
reported the antiproliferative effects of Form on human CRC through
the suppression of cell growth and invasion both in vitro
and in vivo. In addition, Zhang et al (12) reported that the EphB3-targeted
regulation of miR-149 served a suppressive role in the migration
and invasion of human colonic carcinoma. Although it was confirmed
that Form affected the expression of miR-149 and the EphB3 gene in
SW1116 and HCT116 cells, there remains a question as to how miR149
regulates the expression of MMP and cyclin D in the present study,
which should be the focus of a future study. Overall, these results
suggest that Form inhibits tumor growth and cell invasion, thereby
highlighting its potential for use in advanced and metastatic colon
cancer treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ALW carried out the experimental work, and data
collection and interpretation. YL participated in the design and
coordination of experimental work, and the acquisition of data. QZ
participated in the study design, data collection, analysis of data
and preparation of the manuscript. LQF carried out the study
design, the analysis and interpretation of data, and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Labratory
Animal Ethics Committee of Fourh Hospital Hebei Medical University
(Shijiazhuang, China). Experimental procedures were implemented in
according to the guidelines and regulations of the Institutional
Animal Care and Use Committee of Hebei Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Bodalal Z and Bendardaf R: Coloectal
carcinoma in aSouthern Mediterranean country: The Libyan scenario.
World J Gastrointest Oncol. 6:98–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Touil Y, Igoudjil W, Corvaisier M, Dessein
AF, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G,
et al: Colon cancer cells escape 5FU chemotherapy-induced cell
death by entering stemness and quiescence associated with the
c-Yes/YAP axis. Clin Cancer Res. 20:837–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Misale S, Yaeger R, Hobor S, Scala E,
Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M,
Siravegna G, et al: Emergence of KRAS mutations and acquired
resistance to anti-EGFR therapy in colorectal cancer. Nature.
486:532–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin M, Zhao K, Huang Q and Shang P:
Structural features and biological activities of the
polysaccharides from Astragalus membranaceus. Int J Biol
Macromol. 64:257–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z
and Ye Y: Formononetin promotes cell cycle arrest via
downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer
cells. Cell Physiol Biochem. 34:1351–1358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barbato S, Solaini G and Fabbri M:
MicroRNAs in oncogenesis and tumor suppression. Int Rev Cell Mol
Biol. 333:229–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Zhang X, Li Z, Yan H, Qin J and Li
T: Formononetin inhibits human bladder cancer cell proliferation
and invasiveness via regulation of miR-21 and PTEN. Food Funct.
8:1061–1066. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu W and Xiao Z: Formononetin induces
apoptosis of human osteosarcoma cell line U2OS by regulating the
expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell
Physiol Biochem. 37:933–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Zhang X, Wang Y, Ye Y and Huang Z:
Formononetin promotes proliferation that involves a feedback loop
of microRNA-375 and estrogen receptor alpha in estrogen
receptor-positive cells. Mol Carcinog. 55:312–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Liu X, Li Y, Wang Y, Liang H, Li
K, Li L, Chen C, Sun W, Ren S, et al: EphB3-targeted regulation of
miR-149 in the migration and invasion of human colonic carcinoma
HCT116 and SW620 cells. Cancer Sci. 108:408–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Xie M, Gao P, Ye Y, Liu Y, Zhao
Y, Luo W, Ling Z, Cao Y, Zhang S, et al: Antiproliferative effects
of formononetin on human colorectal cancer via, suppressing cell
growth in vitro, and in vivo. Process Biochem. 50:912–917. 2015.
View Article : Google Scholar
|
|
14
|
Auyeung KK, Law PC and Ko JK: Novel
anti-angiogenic effects of formononetin in human colon cancer cells
and tumor xenograft. Oncol Rep. 28:2188–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Zeng J, Xin M, Huang W and Chen X:
Formononetin induces cell cycle arrest of human breast cancer cells
via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm Metab Res.
43:681–686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Begnini KR, de Leon Moura PM, Thurow H,
Schultze E, Campos VF, Rodrigues Martins F, Borsuk S, Dellagostin
OA, Savegnago L, Roesch-Ely M, et al: Brazilian red propolis
induces apoptosis-like cell death and decreases migration potential
in bladder cancer cells. Evid Based Complement Alternat Med.
2014:6398562014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li G, Ji XD, Gao H, Zhao JS, Xu JF, Sun
ZJ, Deng YZ, Shi S, Feng YX, Zhu YQ, et al: EphB3 suppresses
non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt
signalling complex. Nat Commun. 3:6672012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J and Sun L: Formononetin-induced
apoptosis by activation of Ras/p38 mitogen-activated protein kinase
in estrogen receptor-positive human breast cancer cells. Horm Metab
Res. 44:943–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galliher AJ and Schiemann WP: Src
phosphorylates Tyr284 in TGF-beta type II receptor and regulates
TGF-beta stimulation of p38 MAPK during breast cancer cell
proliferation and invasion. Cancer Res. 67:3752–3758. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun M, Liu C, Nadiminty N, Lou W, Zhu Y,
Yang J, Evans CP, Zhou Q and Gao AC: Inhibition of Stat3 activation
by sanguinarine suppresses prostate cancer cell growth and
invasion. Prostate. 72:82–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ko JK, Lam FY and Cheung AP: Amelioration
of experimental colitis by Astragalus membranaceus through
anti-oxidation and inhibition of adhesion molecule synthesis. World
J Gastroenterol. 11:5787–5794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Auyeung KK and Ko JK: Novel herbal
flavonoids promote apoptosis but differentially induce cell cycle
arrest in human colon cancer cell. Invest New Drugs. 28:1–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang F, Ma YL, Zhang P, Shen TY, Shi CZ,
Yang YZ, Moyer MP, Zhang HZ, Chen HQ, Liang Y and Qin HL: SP1
mediates the link between methylation of the tumour suppressor
miR-149 and outcome in colorectal cancer. J Pathol. 229:12–24.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Xie T, Mao X, Xue L, Chu X and Chen
L: MicroRNA-149 increases the sensitivity of colorectal cancer
cells to 5-fluorouracil by targeting forkhead box transcription
factor FOXM1. Cell Physiol Biochem. 39:617–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu XY, Xu H, Wu ZF, Chen C, Liu JY, Wu GN,
Yao XQ, Liu FK, Li G and Shen L: Formononetin, a novel FGFR2
inhibitor, potently inhibits angiogenesis and tumor growth in
preclinical models. Oncotarget. 6:44563–44578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signaling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liabakk NB, Talbot I, Smith RA, Wilkinson
K and Balkwill F: Matrix metalloprotease 2 (MMP-2) and matrix
metalloprotease 9 (MMP-9) type IV collagenases in colorectal
cancer. Cancer Res. 56:190–196. 1996.PubMed/NCBI
|
|
28
|
Timofeeva OA, Tarasova NI, Zhang X,
Chasovskikh S, Cheema AK, Wang H, Brown ML and Dritschilo A: STAT3
suppresses transcription of proapoptotic genes in cancer cells with
the involvement of its N-terminal domain. Proc Natl Acad Sci USA.
110:1267–1272. 2013. View Article : Google Scholar : PubMed/NCBI
|