Introduction
Mammary gland tumors are among the most common
malignant tumors with high morbidity among female canines (1,2). As
previously determined by histological examination, ~50% of cases
are malignant (2). Metastasis is a
primary cause of treatment failure and mortality in human and
veterinary patients (3). Because
canines and humans live in the same environment and have similar
genetic profiles, canine mammary gland neoplasia can serve as a
model to study human mammary gland tumors (3). Surgical resection and chemotherapy
are the most commonly used methods of clinical treatment of mammary
gland tumors (3–5). Paclitaxel belongs to the class of
diterpenoid compounds (mitotic inhibitors) derived from Taxus
brevifolia, exerting efficient, broad-spectrum chemotherapeutic
effects against various cancer types, including human ovarian
cancer (6,7), breast cancer (8,9),
gastric cancer (10) and other
malignancies (11,12). The molecular formula of paclitaxel
is C47H51NO14 and the relative
molecular mass is 853.890. As an antimicrotubule agent, paclitaxel
has been demonstrated to arrest the G2/M-phase
transition, interfere with several signal transduction pathways and
induce apoptosis through the stabilization of microtubules
(13,14). However, which signaling pathways
are altered by paclitaxel to induce the antitumor effects in canine
mammary gland tumors remains to be elucidated.
Previous studies have demonstrated that
chemotherapeutic drugs control growth of cancerous tissue through
induction of apoptosis (10,12,13,15).
Therefore, the assessment of apoptosis following treatment with a
novel chemotherapeutic drug is a marker of efficacy (16). Paclitaxel induces apoptosis in
multiple cell types through different signal transduction pathways,
including the phosphatidylinositol-4,5-bisphosphate 3-kinase
(PI3K)/RAC-α serine/threonine-protein kinase (AKT) pathway
(17), the epidermal growth factor
receptor pathway (12), and the
mitogen-activated protein kinase (MAPK) pathway (18). Targeted inhibition of
phosphorylated-phosphatidylinositol-3-kinase (p-PI3K) was
demonstrated to enhance the induction of apoptosis and increase the
sensitivity of paclitaxel-resistant ovarian cancer cells to
treatment (19). MAPK signaling is
a redox-sensitive signaling pathway (20). Oxidative stress can regulate cell
proliferation, differentiation and apoptosis through the activation
of the MAPK signaling pathway (21). It has been previously reported that
elevated levels of reactive oxygen species (ROS) can increase the
phosphorylation of JNK, P38 MAPK and extracellular signal-regulated
kinase 1/2, regulate the expression of Bcl-2 family proteins and
mitochondrial membrane depolarization, ultimately resulting in
apoptosis (19,22). Although these processes are
generally well understood, the mode of action of paclitaxel in the
context of canine mammary gland tumors remains to be
elucidated.
The present study aimed to determine the mechanism
underlying the antitumor effect of paclitaxel and the role of the
AKT/MAPK signal transduction pathway in CHMm cells in vitro,
in order to provide theoretical and experimental basis for clinical
applications and further research.
Materials and methods
Cell culture
Canine CHMm cell line was kindly provided by the
Department of Veterinary Medical Sciences, University of Tokyo
(Tokyo, Japan). Cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences; Logan, UT, USA), 2 mM L-glutamine, 1 mM
non-essential amino acids (Gibco; Thermo Fisher Scientific, Inc.),
antibiotics [100 U/ml penicillin, 100 µg/ml streptomycin, 2.5 µg/ml
amphotericin B (all Beyotime Institute of Biotechnology, Shanghai,
China)] at 37°C in humidified air with 5% CO2. All
experiments were performed using cells in the phase of logarithmic
growth.
Drug treatment
Paclitaxel (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved in dimethylsulphoxide (DMSO; Sigma-Aldrich;
Merck KGaA) at a concentration of 50 mg/ml and protected from
exposure to light and stored at −80°C. Prior to use, the stock
paclitaxel solution was further diluted with serum-free DMEM into
the concentrations of 0, 0.01, 0.1 and 1 µM. The final
concentrations of DMSO in each sample was <0.05%.
Cell viability assays
CHMm cells were collected during the exponential
growth phase by trypsinization (0.25% trypsin; Beyotime Institute
of Biotechnology) and cell densities were adjusted to
1×104/ml suspensions. The cells were seeded into 96-well
culture plates at a volume of 200 µl/well and incubated at 37°C in
5% CO2 for 24 h. The experimental groups were treated
with different concentrations of paclitaxel (0, 0.01, 0.1 and 1 µM)
and other treatments to assess synergistic effects, including 20 µM
LY294002 or SB203850 (PI3K/AKT inhibitor and P38 inhibitor,
respectively; both Beyotime Institute of Biotechnology) for 24 h.
MTT (100 µl; 5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each
well and incubated for another 4 h. The supernatants were discarded
and 150 µl DMSO was added while the cells were incubated in the
dark for 10 min. Optical density (OD) values was measured at a
reference wavelength of 490 nm, and a detection wavelength of 570
nm. Cell viability values were calculated using the following
formula: Cell viability rate (%)=(ODexperimental
group-ODblank group)/(ODnormal control
group-ODblank group) ×100.
Detection of lactate dehydrogenase
activity (LDH)
The activity of LDH was measured using the LDH
release assay kit (Beyotime Institute of Biotechnology), according
to the manufacturer's protocol. Cells (3×104; 200
µl/well) were treated with various concentrations of paclitaxel for
24 h and cell supernatants were then harvested by centrifugation at
400 × g for 5 min at room temperature. The resulting culture
supernatants were added to black to 96-well culture plates (120
µl/well). Absorbance value was measured at a wavelength of 490 nm
in a microplate reader.
Morphological observation of apoptotic
cells
Paclitaxel-treated and untreated cells were removed
from culture plates by digestion with 0.25% trypsin. The cells were
washed with PBS and cell densities were adjusted to
1×106/ml in PBS, 20 µl acridine orange/ethidium bromide
(AO/EB; 1:1; Sigma-Aldrich; Merck KGaA) staining fluid was added to
each milliliter of PBS at room temperature for 2–5 min. Apoptosis
was assessed using a fluorescent microscope (Nikon Corporation,
Tokyo, Japan). A subset of the collected cells was fixed in 2.5%
glutaraldehyde overnight at 4°C, washed thrice with PBS (pH 7.2)
and stained with 2% osmium tetroxide at room temperature for 1.5 h.
The samples stained with osmium tetroxide were subsequently
dehydrated with graded ethanol and immersed in pure acetone.
Subsequently, the samples were embedded in an epoxy resin mixture.
The blocks were sectioned using Ultracut E ultra-thin slicer, with
a section thickness of 5–7 µm. The sections were subsequently
stained with 3% uranyl acetate and lead citrate at 25°C for 15–20
min, washed with double distilled water and observed by
transmission electron microscopy (TEM; Philips Medical Systems
B.V., Eindhoven, The Netherlands).
Cell cycle analysis
CHMm cells were treated paclitxel for 24 h, as
described above. Following the treatment period, cells were
dissociated from the culture plates using 0.25% trypsin, collected
and rinsed with cold PBS. Subsequently, the cells were fixed with
ice-cold 70% ethanol and incubated at 4°C overnight. The cells were
subsequently re-suspended in cold PBS and incubated with 1 ml
propidium iodide (PI) staining liquid (containing 50 µg/ml
propidium iodide and 50 µg/ml RNase A; Beyotime Institute of
Biotechnology) at room temperature for 1 h in the dark. The
distribution of the cell cycle phase was evaluated by flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using Modfit LT
4.0 software (Verity Software House, Inc., Topsham, ME, USA).
Analysis of cell apoptosis
CHMm cells were seeded in 6-well plates at a density
of 3×104/well and incubated overnight at 37°C. Culture
medium was replaced with one of the described above paclitaxel
doses and incubated for 24 h. The cells were dissociated from the
culture plate and collected by centrifugation at room temperature
1,000 × g for 5 min, the cell pellets were re-suspended in 500 µl
binding buffer (containing 5 µl FITC Annexin V and 5 µl PI; Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), and incubated for 20 min
at room temperature in the dark. Apoptotic cells were analyzed by
flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) using
FACSDiva Version 6.1.3 software (BD Biosciences) after 1 h.
Determination of intracellular ROS
levels
CHMm cells were treated with (0, 0.01, 0.1 and 1 µM)
paclitaxel, with or without 5 mM N-acetyl-L-cysteine (NAC). After a
treatment period of 24 h, cells were digested with 0.25% trypsin.
Levels of ROS were measured using a dichlorodihydrofluorescein
diacetate (DCFH-DA) detection kit, according to the manufacturer's
protocol (Beyotime Institute of Biotechnology). Briefly, cells were
collected (1×106/ml), washed with serum free DMEM and
incubated with 10 mM DCFH-DA at 37°C for 20 min. Stained cells were
washed three times and re-suspended in serum-free DMEM.
Intracellular ROS oxidized the non-fluorescent DCFH resulting in
conversion to the fluorescent DCF molecule. The levels of ROS were
measured using a fluorescent microplate reader using a reference
wavelength of 490 nm and a detection wavelength of 570 nm.
Measurement of superoxide dismutase
(SOD) activity and malondialdehyde (MDA) content
CHMm cells were dissociated from the culture plates
using 0.25% trypsin, collected and rinsed with cold PBS. Cells were
lysed with 0.05 M Tris-HCl (Beijing Biotopped Science &
Technology Co., Ltd., Being, China) extraction buffer on ice. The
cell lysates were centrifuged at 4°C 12,000 × g for 10 min. The
resulting cell lysates were used to assess the SOD activity and MDA
content, which were measured according to the manufacturer's
protocols (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Western blotting
Western blot analysis was used to evaluate the
expression level of cytochrome c (cyt-c; cat. no. WL02410; 1:500),
cyclin B1 (cat. no. WL02310; 1:500), Bcl-2 (cat. no. WL01556;
1:500), apoptosis regulator BAX (Bax, (cat. no. WL01637; 1:500),
cyclin dependent kinase inhibitor 1 (P21; cat. no. WL0362; 1:500;
Wanleibio Co., Ltd., Shenyang, China), tumor protein 53 (P53; cat.
no. 9282; 1:750) and cleaved-caspase-3 (cat. no. 9664; 1:750; CST
Biological Reagents Co., Ltd., Shanghai, China); AKT (cat. no.
E1A6259; 1:500), p-AKT (ser-124; cat. no. E1A3260; 1:500), P38
(cat. no. E1A6456; 1:500), p-p38 (tyr-182; cat. no. E1A3455;
1:500), p-ribosomal protein S6 kinase (P70S6K; ser-229; cat. no.
E1A3226; 1:500), P70S6K (cat. no. E1A6226; 1:500), p-90 kDa
ribosomal protein S6 kinase 1 (P90RSK; ser-352; cat. no. E011113;
1:500) and P90RSK (cat. no. E110536; 1:500; Enjing Biotech Co.,
Ltd., Nanjing, China) in CHMm cells following treatment with
paclitaxel. The synergistic effects of paclitaxel and inhibitors of
cell signaling molecules in CHMm cells. CHMm cells were incubated
in serum-free medium with LY294002 (20 µM) or SB203580 (20 µM) for
1 h and subsequently incubated with paclitaxel (1 µM) for 30 min at
37°C. Then, the cells were lysed with cell RIPA lysis buffer
(Beyotime Institute of Biotechnology) to obtain a total protein
lysate. For the detection of cyt-c, the collected cells were
processed using a mitochondria isolation kit (Beyotime Institute of
Biotechnology) to obtain the cytosolic component and mitochondrial
pellet. Protein concentration from each culture was determined
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Proteins were denatured by boiling for 10 min and
separated using 10–15% SDS-PAGE. Subsequently, the resolved protein
bands were transferred onto nitrocellulose membranes and blocked
with 5% skim milk at room temperature for 1 h. The membranes were
incubated with the primary antibodies at 4°C overnight. The
membranes were subsequently washed three times for 10 min each in
TBST buffer (1% Tween-20) and incubated with horseradish
peroxidase-labeled secondary antibodies (goat anti-rabbit, cat. no.
ZB2301, 1:2,000; goat anti-mouse, cat. no. ZB2305, 1:2,000; OriGene
Technologies, Inc., Beijing, China) at 37°C for 1 h. The
immunoreactive bands were visualized using Tanon™ High-sig ECL
Substrate (Tanon Science and Technology Co., Ltd., Shanghai, China)
and images of membranes were captured using Chemidoc XRS system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total protein and
β-actin protein (cat. no. TA09; 1:1,000; OriGene Technologies,
Inc.) served as internal controls. Relative protein levels were
quantified using ImageJ 1.48 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Analyses of differences between groups were carried out using
one-way analysis of variance followed by Tukey's multiple
comparison test in the GraphPad Prism 5.0 software package
(GraphPad Software, Inc., La Jolla, CA, USA). All experiments were
replicated at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of paclitaxel on growth and
cytotoxicity in CHMm cells
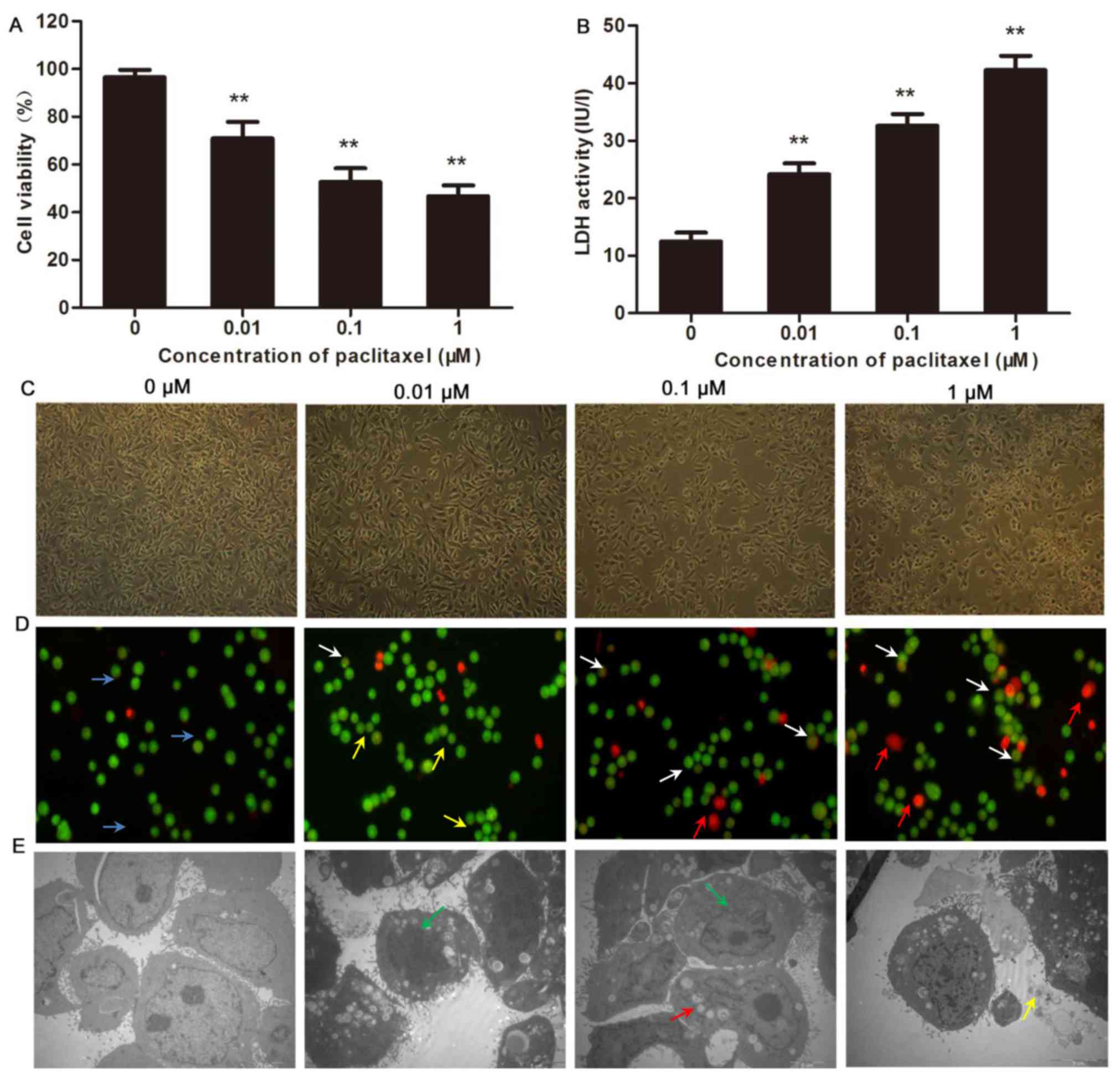
The efficacy of paclitaxel-mediated inhibition of
CHMm cell growth was assessed using MTT assay. The data indicated
that with increasing concentration of paclitaxel (0, 0.01, 0.1 and
1 µM), cell viability decreased in a dose-dependent manner,
compared with the control group (P<0.01; Fig. 1A). Furthermore, treatment with
paclitaxel enhanced LDH activity in CHMm cells in a dose-dependent
manner (P<0.01; Fig. 1B). These
data indicate that paclitaxel effectively suppressed CHMm cell
proliferation.
Morphological alterations
characteristic of apoptosis induced by paclitaxel in CHMm
cells
To further investigate the effects of paclitaxel on
CHMm cells, the morphological alterations were observed using an
inverted phase contrast microscope. In the paclitaxel treatment
group at 24 h, irregularities in cell growth and development were
observed. Specifically, the cells appeared oval or round with no or
one nucleus. Detached or necrotic cells, reductions in the numbers
of adherent cells and poor cell refraction were observed in a dose
dependent manner (Fig. 1C). The
results of AO/EB staining demonstrated that the control group cells
stained light green with few apoptotic cell present. Following
response to paclitaxel, a notable increase in cells with intense
green, orange and red staining were observed, which represented
cells undergoing early apoptosis, late apoptosis and necrosis,
respectively (Fig. 1D). The
morphology of apoptotic cells was observed by TEM. The observed
characteristics included marginalization and condensation of
chromatin, nuclear fragmentation, intracellular vacuolization and
emergence of apoptotic bodies (Fig.
1E).
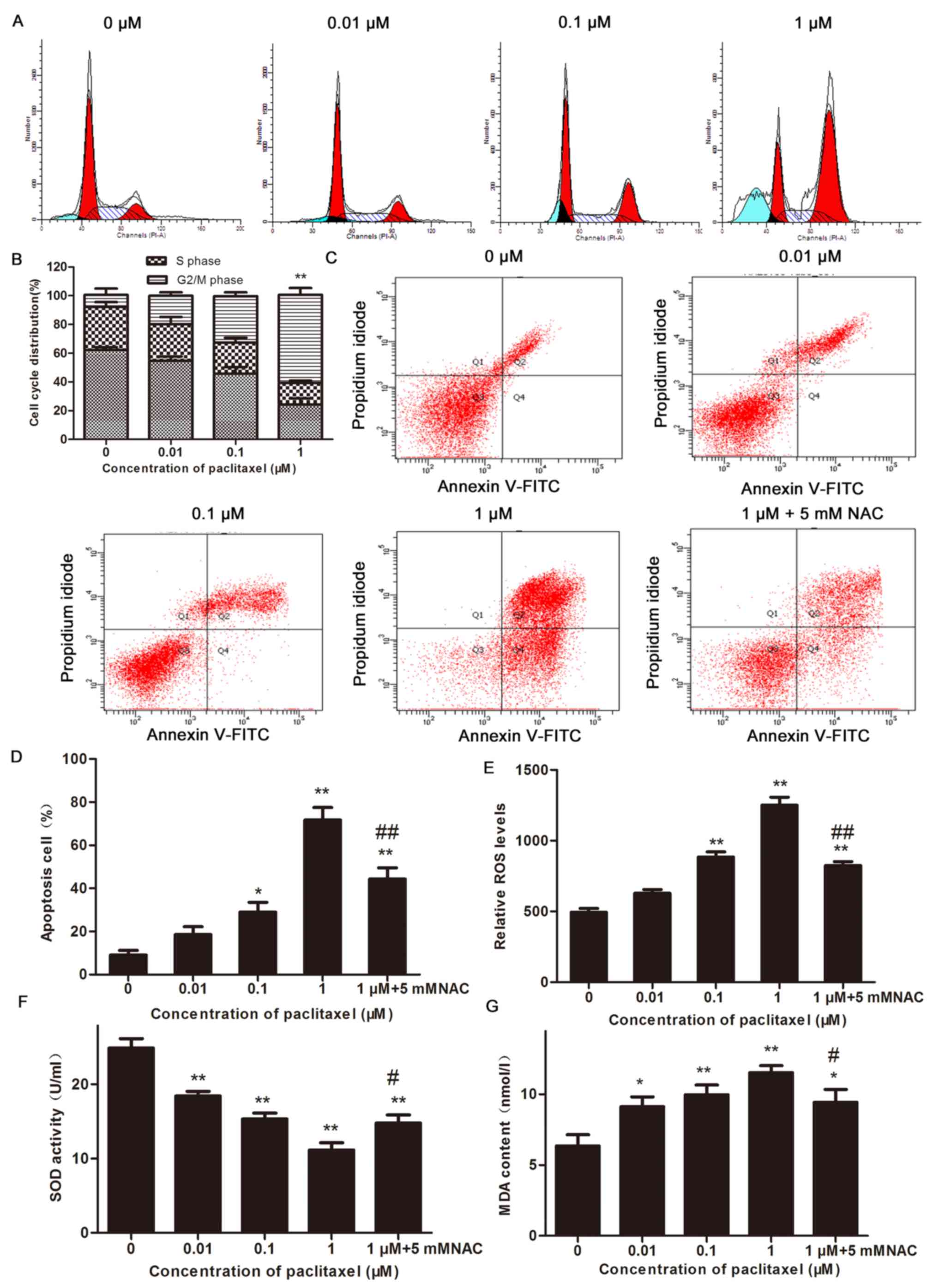
The effect of paclitaxel on cell-cycle
arrest and apoptosis in CHMm cells by flow cytometry
The data collected using flow cytometry demonstrated
that the percentage of cells arrested in G2/M-phase
significantly increased in the groups treated with 1 µM paclitaxel,
compared with the untreated controls, after 24 h (P<0.01). The
effect of 1 µM paclitaxel was more pronounced compared with 0.1 µM
paclitaxel (Fig. 2A and B). The
number of apoptotic CHMm cells increased in a dose dependent manner
after 24 h of treatment with paclitaxel. The proportion of
apoptotic cells reached the greatest value when cells were treated
with 1 µM paclitaxel. Treatment with 5 mM NAC had significant
inhibitory effect the paclitaxel-induced apoptosis in CHMm cells.
These results indicate that paclitaxel likely promoted apoptosis in
the CHMm cells, through a process that may be associated with
increased oxidative stress, ultimately arresting the cell cycle in
the G2/M-phase (Fig. 2C and
D).
Effect of paclitaxel on the ROS, SOD
and MDA in CHMm cells
Excess ROS production is an early event that
triggers cell apoptosis. To determine the involvement of ROS, SOD
and MDA during paclitaxel-induced apoptosis on CHMm cells. ROS
levels were evaluated using a DCFH-DA probe, compared with the
control group, the levels of ROS and MDA in the CHMm cells treated
with paclitaxel were significantly increased (P<0.05 and
P<0.01), whereas SOD activity decreased significantly in a
dose-dependent manner (P<0.01; Fig.
2E-G). However, compared with the 1 µM group, the ROS
(P<0.01) and MDA (P<0.05) contents in the 5 mM NAC-treated
cells decreased (Fig. 2E and G).
By contrast, compared with the 1 µM group, SOD activity in 5 mM
NAC-treated cells was increased (P<0.05; Fig. 2F). These results indicate that
paclitaxel may lead to increased generation of ROS in CHMm cells
and excessive accumulation of intracellular ROS can cause oxidative
stress damage and promote apoptosis.
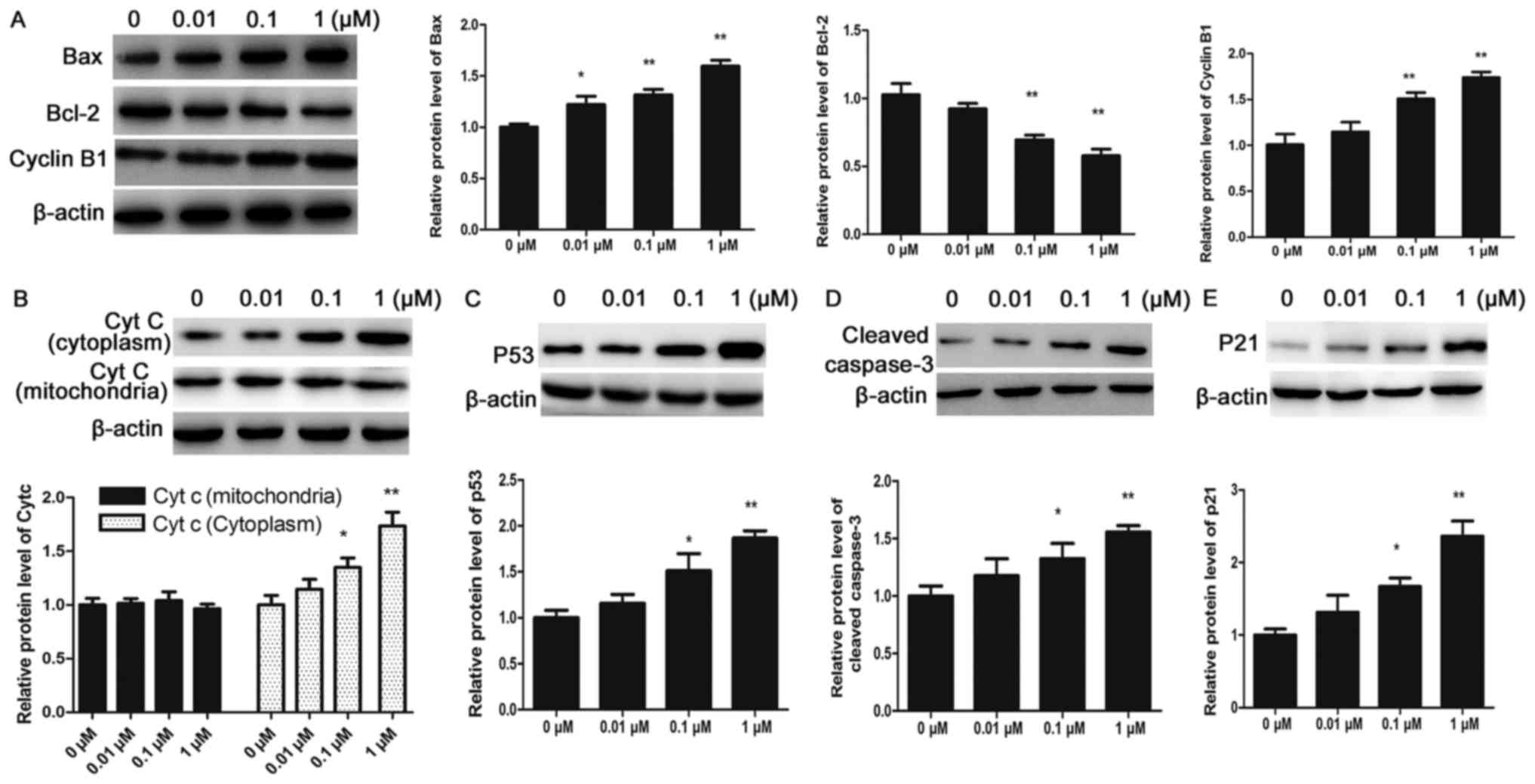
Effect of paclitaxel on the expression
of apoptosis-associated proteins in CHMm cells
To further determine the cellular basis of the
apoptotic response observed in CHMm cells, the expression of
apoptosis-associated proteins was detected by western blot
analysis. P53, a tumor suppressor gene, is upregulated in response
to several cellular processes, including DNA damage and oxidative
stress, which directly or indirectly regulate mitochondrial
physiology (23,24). The results of the present study
demonstrated that paclitaxel may promote expression of P53 protein
in CHMm cells. Bcl-2 family members serve regulatory roles in the
mitochondrial dysfunction during programed cell death (25). Treatment with paclitaxel decreased
the expression of Bcl-2 and increased the expression of Bax in a
dose-dependent manner in CHMm cells, as determined by western blot
analysis. The results indicated that expression levels of cyt-c
(cytoplasmic), Bax and cleaved caspase-3 were upregulated whereas
the expression of Bcl-2 was downregulated following a 24 h
paclitaxel treatment in CHMm cells, and these expression patterns
were dose-dependent, and differences were statistically significant
compared with the control group (P<0.05 and P<0.01; Fig. 3). These results indicate that
paclitaxel-induced cell apoptosis is medicated by increased
activation of pro-apoptotic protein Bax, release of cyt-c to
cytosol and increased levels of cleaved caspase-3 in CHMm
cells.
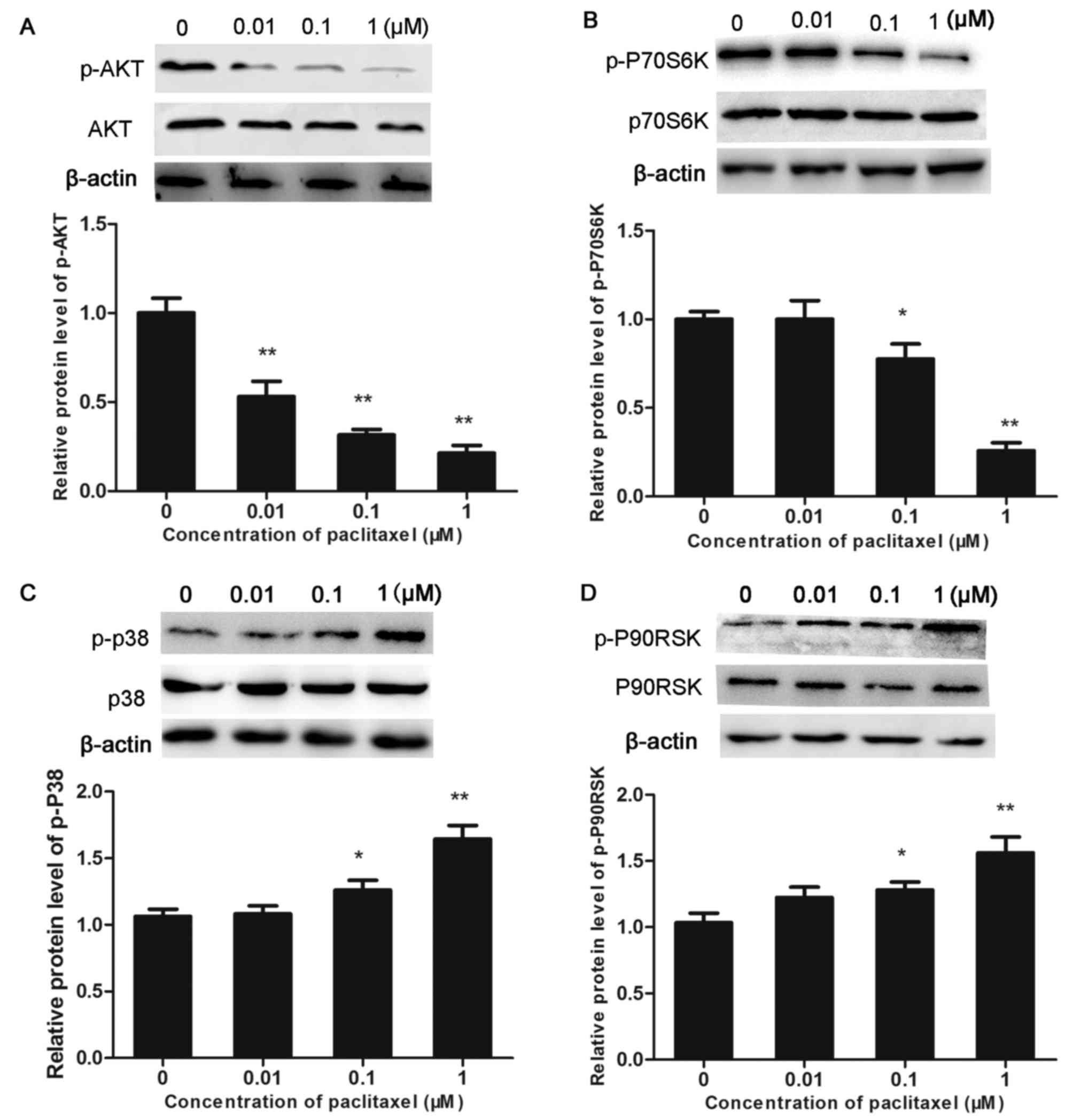
Effects of paclitaxel on the AKT/MAPK
signaling pathway in CHMm cells
The aim of subsequent experiments was to confirm
whether the effect of paclitaxel on the viability of CHMm cells was
associated with alterations in the AKT/MAPK signal transduction
pathways. Western blot analysis indicated that the level of p-P38
and p-P90RSK was significantly increased in the paclitaxel-treated
groups, in a dose-dependent manner (P<0.05 and P<0.01). By
contrast, expression levels of total P38 and P90RSK proteins
remained unaltered. In addition, treatment with paclitaxel resulted
in decreased levels of p-AKT and p-P70S6K in a dose-dependent
manner (P<0.05 and P<0.01; Fig.
4). The above results indicate that paclitaxel-induced
apoptosis is likely mediated the AKT/MAPK signaling transduction
pathway in CHMm cells.
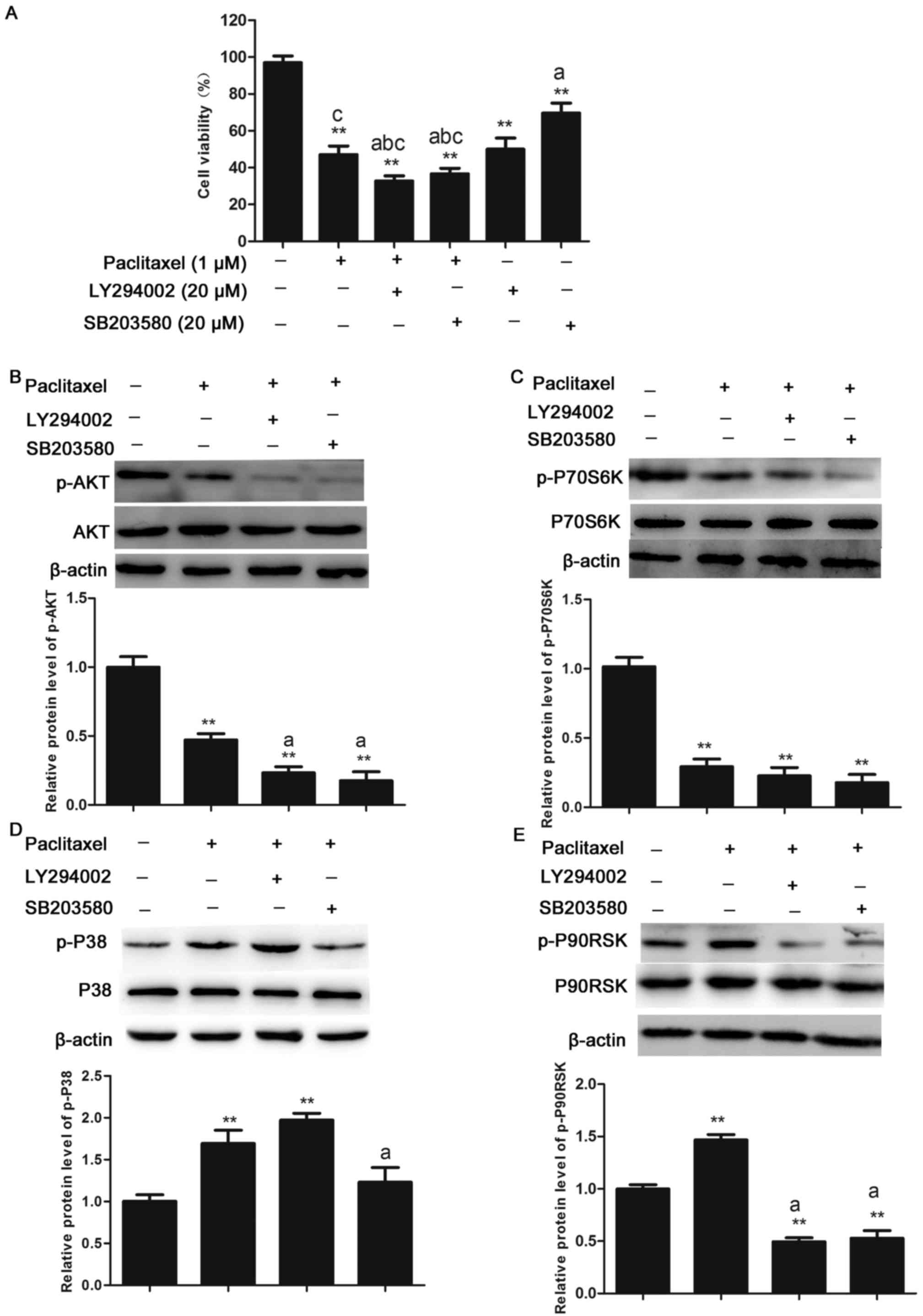
Effects of treatment with paclitaxel
in combination with inhibitors of cell signaling proteins on the
AKT/MAPK signal transduction in CHMm cells
To determine whether the reduction in viability of
CHMm cells following treatment with paclitaxel was mediated by the
AKT/MAPK pathway, cell viability was measured in CHMm cells treated
with 1 µM paclitaxel alone or in combination with pharmacological
inhibitors including LY294002 (PI3K/AKT inhibitor; 20 µM) or
SB203850 (P38 inhibitor; 20 µM). Compared with control cells,
viability of CHMm cells to 45% (P<0.01) when treated with
paclitaxel alone. Compared with cells either treated with
paclitaxel alone or the pharmacological inhibitors alone, CHMm
cells treated in combination exhibited a significant reduction in
viability (P<0.05; Fig. 5A).
Furthermore, the expression levels of relevant signaling proteins
in CHMm cells following treatment with paclitaxel or
pharmacological inhibitors were determined by western blot
analysis. The AKT phosphorylation following paclitaxel treatment
alone was further inhibited using SB203580 and LY294002 in CHMm
cells, and expression of p-P70S6K was inhibited by SB203580 and
LY294002 in CHMm cells. Compared with the paclitaxel treatment
group, activation of p-P38 was completely suppressed by SB203580
(P<0.05). The 90 kDa ribosomal S6 kinases are a family of
Ser/Thr kinases that regulate various cellular growth processes, as
downstream molecule of the Ras-MAPK and ERK1/2 signaling pathway
(26), p-P90RSK was inhibited by
SB203580 and LY294002 (Fig. 5B-E),
The above results suggest that the molecular mechanisms underlying
the inhibition of CHMm cells proliferation by paclitaxel was
mediated by inhibition of the PI3K/AKT signaling and activation of
MAPK signaling pathway.
Discussion
Although numerous studies have revealed the
anticancer effect of paclitaxel in a number of human cancer cells,
including breast cancer, to the best of the authors' knowledge, the
role of paclitaxel in canine mammary gland tumor has not been
studied before (8). In the present
study, the effect of paclitaxel on the viability of CHMm cells was
assessed by MTT and LDH release assay in vitro. The results
demonstrated that paclitaxel inhibited proliferation of CHMm cells
in vitro, in a dose-dependent manner. In addition, as the
paclitaxel-mediated activity of LDH increased, cellular viability
was inhibited in a dose-dependent manner.
Apoptosis is a proactive cell death process in which
cells undergo a certain stimulus to maintaining the stability of
the internal environment, The morphological alterations of
apoptosis are characterized by cell shrinkage, chromatin
condensation, nuclear fragmentation, DNA degradation, and apoptotic
body formation (27). Paclitaxel
may arrest the division of cancer cells in the
G2/M-phase and elevate the expression of cyclinB1
(28,29). In the present study,
paclitaxel-treated CHMm cells exhibited morphological alterations
characteristic of apoptosis, including cell shrinkage,
intracellular vacuolization and chromatin condensation. Flow
cytometry demonstrated that with increasing concentration of
paclitaxel, the rates of apoptosis increased and cell cycle arrest
in the G2/M-phase was promoted. The P53 protein is a
multifunctional transcription factor, associated with the
occurrence and progression of numerous human tumors, mainly
responsible for tumor cell apoptosis and cell cycle arrest
(23,24). Activation of P53 induces the
expression of P21 and cyclinB1. Taken together, the above results
suggest that paclitaxel-induced apoptosis and cell-cycle arrest in
the G2/M-phase in CHMm cells are likely to be associated
with activation of P53 by DNA damage.
The Bcl-2 family of proteins, including Bcl-2,
Bcl-like 1, Bax and BH3-interacting domain death agonist, have
emerged as regulators of mitochondria-mediated apoptosis. An
increase in the levels of pro-apoptosis proteins and/or a decrease
in anti-apoptosis proteins can lead to a decrease in mitochondrial
membrane potential and an opening of mitochondrial permeability
transition pores, leading to cyt-c release from mitochondria into
cytoplasm (30). A previous study
revealed that paclitaxel can induce apoptosis of NB-1 cells, which
may be mediated by downregulation of Bcl-2 and upregulation of Bax
(31).
In the present study, treatment of CHMm cells with
paclitaxel resulted in a dose-dependent decrease in the levels of
anti-apoptotic proteins Bcl-2 and a simultaneous increase in
pro-apoptotic protein Bax. This alteration is known to be
responsible for the concomitant execution phase of apoptosis,
including the disruption of mitochondrial membrane potential (MMP),
increased release of cyt-c into cytoplasm and cleavage of caspase-3
protein (32,33). Regulation of intrinsic apoptosis by
Bcl-2 family proteins occurs through ROS production and is followed
by reduction in the MMP level, which stimulates mitochondria to
release proapoptotic molecules and results in activation of
caspase-9 and −3 (34,35). The results of the present study
demonstrated that levels of ROS and MDA were elevated, whereas SOD
activity decreased following exposure to paclitaxel, when
paclitaxel was administered with NAC, the levels of ROS and MDA
were reduced, and SOD activity was increased. These results
indicated that paclitaxel induced alterations in expression of
apoptosis-associated proteins and generation of ROS. However, it
remains to be elucidated whether mitochondria-dependent apoptosis
induced by paclitaxel is mediated by ROS; the mechanism in which
paclitaxel selectively induced apoptosis of CHMm cells has been
defined but requires further investigation.
Paclitaxel induces apoptosis in various tumor types
and regulates different signaling mechanisms in each (12,17,36).
The AKT/MAPK signaling pathway is hypothesized to have a role in
sensitivity to paclitaxel and serve as a potential therapeutic
target for treatment of gastric cancer (37). Previous results indicate that
regulation of AKT/MAPK signaling pathways is closely associated
with proliferation, migration and invasion of cancer cells during
carcinogenesis (38). Based on the
data presented in the present study, level of p-AKT and p-P70S6K
signaling proteins decreased, whereas expression of the p-P38 and
p-P90RSK signaling proteins increased in response to paclitaxel
treatment in a dose-dependent manner. Furthermore, the present
study demonstrated a synergistic effect when inhibitors LY294002
and SB203580 were administered in together with paclitaxel,
significantly decreasing the viability of CHMm cells.
Phosphorylation levels of P90RSK and P70S6K proteins were reduced
by treatment with LY294002 and SB203580, the level of p-P38 was
decreased significantly by treatment with SB203580 and p-AKT was
decreased by LY294002. These results indicate that the
paclitaxel-mediated suppression of proliferation of CHMm cells was
likely the result of alterations to the AKT/MAPK pathway
signaling.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that paclitaxel
effectively suppresses proliferation of CHMm cells, induces cell
cycle arrest in G2/M-phase, increases the levels of MDA,
ROS and reduces levels of SOD. Based on the results of the present
study, treatment with paclitaxel induced cell apoptosis which may
be mediated by downregulation of Bcl-2 and upregulation of Bax, and
is likely associated with the inhibition of the PI3K/AKT signaling
and activation of MAPK signaling pathways (Fig. 6). Collectively, the present study
provides a theoretical basis for the clinical use of paclitaxel for
treatment of canine mammary gland tumors.
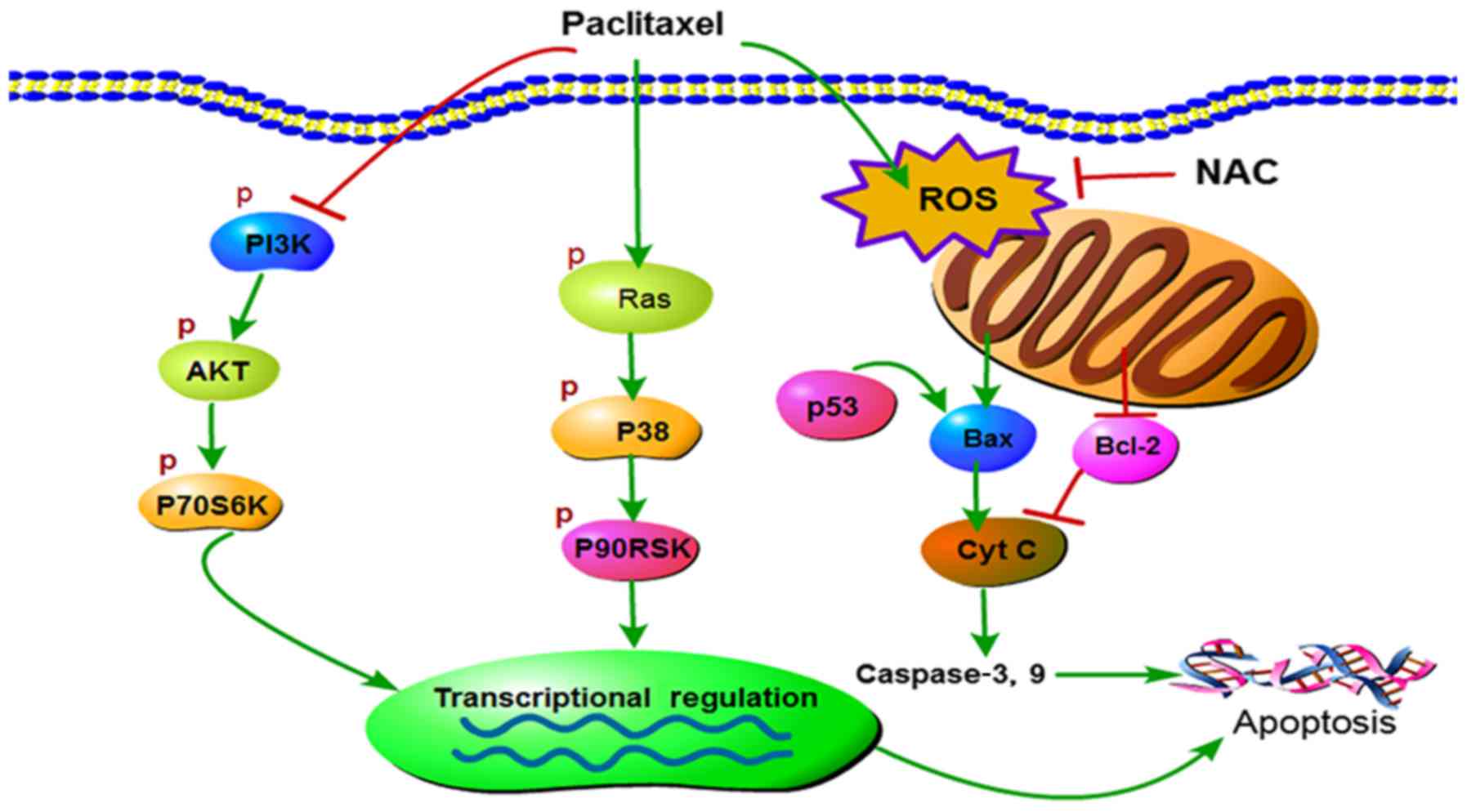 | Figure 6.Graphical representation of the
current working hypothesis regarding anticancer mechanisms induced
by paclitaxel targeting multiple signaling pathways in CHMm cells.
P, phospho; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase;
AKT, RAC-α serine/threonine-protein kinase; P70S6K, ribosomal
protein S6 kinase; P90RSK, 90 kDa ribosomal protein S6 kinase 1;
ROS, reactive oxygen species; NAC, N-acetyl-L-cysteine; Bax,
apoptosis regulator BAX; Cyt c, cytochrome c; Bcl-2, apoptosis
regulator Bcl-2; p53, tumor protein 53. |
Acknowledgements
The authors are grateful to Professor N. Sasaki,
University of Tokyo for kindly providing the CHMm cell lines and
thanks to the help of the Veterinary Surgery Laboratory at the
College of Veterinary Medicine, Northeast Agricultural
University.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 31372492) and by the
National Key Research Projects, China (grant no.
2016YFD0501008).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL conceived and designed the experiments. XR, BZ,
HC, MX and YW performed the experiments. XR analyzed the data and
wrote the paper. YW and YL assisted in critically revised the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Laboratory
Animal Ethical Committee of Northeast Agricultural University,
Harbin, China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorenmo K: Canine mammary gland tumors.
Vet Clin North Am Small Anim Pract. 33:573–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vail DM and MacEwen EG: Spontaneously
occurring tumors of companion animals as models for human cancer.
Cancer Invest. 18:781–792. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chauhan A, Sharma MM and Kumar K:
Evaluation of surgical outcomes of oncoplasty breast surgery in
locally advanced breast cancer and comparison with conventional
breast conservation surgery. Indian J Surg Oncol. 7:413–419. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shamsi M and Islamian Pirayesh J: Breast
cancer: Early diagnosis and effective treatment by drug delivery
tracing. Nucl Med Rev Cent East Eur. 20:45–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong JY, Kim KS, Moon JS, Song JA, Choi
SH, Kim KI, Kim TH and An HJ: Targeted inhibition of phosphatidyl
inositol-3-kinase p110β, but not p110α, enhances apoptosis and
sensitivity to paclitaxel in chemoresistant ovarian cancers.
Apoptosis. 18:509–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGuire WP, Rowinsky EK, Rosenshein NB,
Grumbine FC, Ettinger DS, Armstrong DK and Donehower RC: Taxol: A
unique antineoplastic agent with significant activity in advanced
ovarian epithelial neoplasms. Ann Intern Med. 111:273–279. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quispe-Soto ET and Calaf GM: Effect of
curcumin and paclitaxel on breast carcinogenesis. Int J Oncol.
49:2569–2577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holmes FA, Walters RS, Theriault RL,
Forman AD, Newton LK, Raber MN, Buzdar AU, Frye DK and Hortobagyi
GN: Phase II trial of taxol, an active drug in the treatment of
metastatic breast cancer. J Natl Cancer Inst. 83:1797–1805. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Wang R, Zhou K, Wang S, Wang J,
Shi H, Dou Y, Yang D, Chang L, Shi X, et al: JD enhances the
anti-tumour effects of low-dose paclitaxel on gastric cancer MKN45
cells both in vitro and in vivo. Cancer Chemoth Pharm. 78:971–982.
2016. View Article : Google Scholar
|
|
11
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2008.PubMed/NCBI
|
|
12
|
Hu J, Zhang NA, Wang R, Huang F and Li G:
Paclitaxel induces apoptosis and reduces proliferation by targeting
epidermal growth factor receptor signaling pathway in oral cavity
squamous cell carcinoma. Oncol Lett. 10:2378–2384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donaldson KL, Goolsby GL, Kiener PA and
Wahl AF: Activation of p34cdc2 coincident with taxol-induced
apoptosis. Cell Growth Differ. 5:1041–1050. 1994.PubMed/NCBI
|
|
14
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren L, Li Z, Dai C, Zhao D, Wang Y, Ma C
and Liu C: Chrysophanol inhibits proliferation and induces
apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade
in breast cancer cell lines. Mol Med Rep. 17:4376–4382.
2018.PubMed/NCBI
|
|
16
|
Kim GM, Kim S, Park HS, Kim JY, Nam S,
Park S, Kim SI, Kim D and Sohn J: Chemotherapy-induced irreversible
alopecia in early breast cancer patients. Breast Cancer Res Treat.
163:527–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okano J and Rustgi AK: Paclitaxel induces
prolonged activation of the Ras/MEK/ERK pathway independently of
activating the programmed cell death machinery. J Biol Chem.
276:19555–19564. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu R, Sato N, Yanai K, Akiyoshi T, Nagai
S, Wada J, Koga K, Mibu R, Nakamura M and Katano M: Enhancement of
paclitaxel-induced apoptosis by inhibition of mitogen-activated
protein kinase pathway in colon cancer cells. Anticancer Res.
29:261–270. 2009.PubMed/NCBI
|
|
19
|
Tserga A, Chatziandreou I, Michalopoulos
NV, Patsouris E and Saetta AA: Mutation of genes of the PI3K/AKT
pathway in breast cancer supports their potential importance as
biomarker for breast cancer aggressiveness. Virchows Arch.
469:35–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways? J Signal
Transduct. 2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang C, Lim W, Bazer FW and Song G:
Myricetin suppresses invasion and promotes cell death in human
placental choriocarcinoma cells through induction of oxidative
stress. Cancer Lett. 399:10–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morales-Cano D, Calviño E, Rubio V,
Herráez A, Sancho P, Tejedor MC and Diez JC: Apoptosis induced by
paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4
human leukaemia cells is not modulated by ERK inhibition. Exp
Toxicol Pathol. 65:1101–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakashita F, Osada S, Takemura M, Imai H,
Tomita H, Nonaka K, Takahashi T and Seishima M: The effect of p53
gene expression on the inhibition of cell proliferation by
paclitaxel. Cancer Chemother Pharmacol. 62:379–385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Norbury CJ and Zhivotovsky B: DNA
damage-induced apoptosis. Oncogene. 23:2797–2808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Festjens N, van Gurp M, van Loo G, Saelens
X and Vandenabeele P: Bcl-2 family members as sentinels of cellular
integrity and role of mitochondrial intermembrane space proteins in
apoptotic cell death. Acta Haematol. 111:7–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anjum R and Blenis J: The RSK family of
kinases: Emerging roles in cellular signalling. Nat Rev Mol Cell
Biol. 9:747–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ban JO, Hwang CJ, Park MH, Hwang IK, Jeong
HS, Lee HP, Hyun BK, Kim JY, Youn HS, Ham YW, et al: Enhanced cell
growth inhibition by thiacremonone in paclitaxel-treated lung
cancer cells. Arch Pharm Res. 38:1351–1362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia RL, Lu Y, Zhu LN, Zhang SF, Zhao FK
and Fu CY: Different regulatory pathways are involved in the
proliferative inhibition of two types of leukemia cell lines
induced by paclitaxel. Oncol Rep. 30:1853–1859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nonaka M, Ikeda H, Fujisawa A, Uehara M
and Inokuchi T: Induction of apoptosis by paclitaxel in human oral
carcinoma cells. Int J Oral Maxillofac Surg. 35:649–652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suh DH, Kim MK, Kim HS, Chung HH and Song
YS: Mitochondrial permeability transition pore as a selective
target for anti-cancer therapy. Front Oncol. 3:412013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang M, Wang B, Gao J, Zhang Y, Xu W and
Tao L: Spinosad induces programmed cell death involves
mitochondrial dysfunction and cytochrome C release in Spodoptera
frugiperda Sf9 cells. Chemosphere. 169:155–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azad N, Iyer AK, Wang L, Lu Y, Medan D,
Castranova V and Rojanasakul Y: Nitric oxide-mediated bcl-2
stabilization potentiates malignant transformation of human lung
epithelial cells. Am J Respir Cell Mol Biol. 42:578–585. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meshkini A and Yazdanparast R: Involvement
of oxidative stress in taxol-induced apoptosis in chronic
myelogenous leukemia K562 cells. Exp Toxicol Pathol. 64:357–365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akiyama M, Sowa Y, Taniguchi T, Watanabe
M, Yogosawa S, Kitawaki J and Sakai T: Three combined treatment, a
Novel HDAC inhibitor OBP-801/YM753, 5-Fluorouracil and paclitaxel,
induces G2 phase arrest through the p38 pathway in human
ovarian cancer cells. Oncol Res. 25:1245–1252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu G, Qin XQ, Guo JJ, Li TY and Chen JH:
AKT/ERK activation is associated with gastric cancer cell
resistance to paclitaxel. Int J Clin Exp Pathol. 7:1449–1458.
2014.PubMed/NCBI
|
|
38
|
Lim W, Park S, Bazer FW and Song G:
Apigenin reduces survival of choriocarcinoma cells by inducing
apoptosis via the PI3K/AKT and ERK1/2 MAPK pathways. J Cell
Physiol. 231:2690–2699. 2016. View Article : Google Scholar : PubMed/NCBI
|