Introduction
One of the leading factors causing individuals to
live for years with disability is depression, the incidence of
which is increasing worldwide (1).
When combined with a chronic medical condition, depression has a
marked effect on compromised health (2). In America, >17,000,000 adults have
survived an acute coronary syndrome (ACS) event as at 2010, and the
number of new survivors each year is 1,200,000 (3). In every five of these individuals,
two report marked symptoms of depression, with symptoms persisting
for a long duration following discharge (4,5).
Therefore, up to 7,000,000 individuals in America with coronary
artery disease (CAD) suffer from clinically significant depression,
of which there are 500,000 new cases each year, placing a burden on
public health (6). There is high
possibility that the two often occur in combination and the
occurrence of either signals the increased possibility of the onset
of the other. Therefore, the focus has shifted to the complicated
association between depression and ACS. For example, the presence
of depression and/or minimally increased symptoms of depression
appear to be a reliable risk factor and prognostic factor of CAD
relapse and mortality rates from all causes (4).
Individuals suffering from occasional depression are
distinct from those who have a history of depression, as
demonstrated in a previous study of CAD severity performed in a
population exhibiting CAD comorbidities with depression. In a study
performed in 39 patients recently diagnosed with CAD and who met
the criteria for major depressive disorder (MDD), patients who had
history of depression exhibited less severe CAD compared with
patients without history of depression (7). The direct visualization of coronary
arteries via angiogram was used to assess the severity of CAD,
which was expressed as the number of occluded coronary arteries
with >50% stenosis. There appeared to be an association between
the incidence of MDD and severity of CAD, whereas no association
was found between a new MDD event and the severity of CAD in
patients with a history of MDD. However, the samples in the study
did not include the full spectrum of patients with ACS, and the
significance level of the difference in severity of CAD between
patients with recurrent and incident MDD was only P=0.07.
MicroRNAs (miRNAs) have a crucial regulatory role in
stress responses and cardiac homeostasis. miRNAs have ‘nodal’
control on important biological processes via the destabilizing of
messenger RNAs encoding proteins involved in cell growth,
metabolism, programmed death pathways and calcium signaling
(8). Generally, individual
miRNA-mRNA interactions marginally decrease the levels of target
mRNAs, suggesting that miRNAs are ‘fine tuners’ in the functioning
of cells (9). However, when
certain miRNA levels are deliberately controlled, particularly when
all types of a specific miRNA are genetically removed, notable
phenotypes can be triggered (10,11).
Considering the fact that NOS1 is functionally
involved in the pathogenesis of depression in patients with CAD
(12,13), NOS1 is a potential target of
miR-146a, as the presence of rs2910164 reduces the expression of
miR-146a (14). The present study
aimed to verify the miR-146a/NOS1 association in U251 cells and
examine the association between the miR-146a rs2910164 polymorphism
and the development of depression in patients with CAD.
Materials and methods
Sample collection
Peripheral blood samples from 865 patients with CAD,
including 412 patients who experienced depression following CAD and
453 who did not, were collected at the Department of Cardiology of
The First People's Hospital (Jining, China) between June 2013 and
December 2014. Patients experiencing current alcohol and/or
substance abuse, current psychotic disorder, active suicidal or
homicidal thoughts, or cognitive impairment were excluded from the
study. The study was performed according to the latest version of
the Declaration of Helsinki. The patients provided signed informed
consent for participation in the study following explanation of
potential risks. The Ethics and Research Committees of the First
People's Hospital at Jining (Jining, China) approved the study.
Target prediction and functional
analysis
By scanning the mostly used target gene prediction
databases online miRNA database (www.mirdb.org), the putative target genes of the
miR-146a were pooled from the three databases in total.
Experimental validation on the majority of the target genes was
performed.
Genotyping by direct sequencing
The TaqMan genotyping kit (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
determine the genotype of rs2910164 according to the manufacturer's
protocol in each participant.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RT-qPCR analyses were performed to determine the
expression level of miR-146a and NOS1 mRNA in U251 cells. The
Absolutely RNA™ RT-PCR miniprep kit (Qiagen, Inc., Valencia, CA,
USA) was used to purify the total RNA from U251 cells in accordance
with the manufacturer's protocol. A total NanoDrop
ND-3300® fluorospectrometer (Thermo Fisher Scientific,
Inc.) was used to estimate the density of the RNA at a final
concentration of 2 ng/µg. For further experiments, the RNA was
placed in microcentrifuge tube and stored at −80°C.
For detecting the expression levels of mature
miR-146a, TaqMan miRNA assays (ABI PRISM; Thermo Fisher Scientific,
Inc.) with the stem-loop method were used. The High-Capacity cDNA
reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to perform the RT reaction to synthesize
cDNA (NOS1), according to the manufacturer's protocol, with a
mixture of RT primer (3 µl) and 10 ng total RNA. The RT reactions
were performed at 16°C for 30 min (primer binding), 42°C for 30 min
(cDNA synthesis) and 85°C for 5 min (inactivation of enzyme),
followed by maintenance at 4°C. TaqMan primers (2 µl; Qiagen, Inc.)
and 1.5 µl cDNA were used for the subsequent PCR analysis,
according to the manufacturer's protocol. The sequences of the
primers were: miR-146a (forward:
5′-ACACTCCAGCTGGGTGAGAACTGAATTCC-3′; reverse:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAACCCATGG-3′); NOS1
(forward: 5′-AGACGCACGAAGATAGTTGAC-3′; reverse:
5′-CCGAAGCTCCAGAACTCAC-3′); β-actin (forward:
5′-CTCTTCCAGCCTTCCTTCCT-3′; reverse: 5′-TCATCGTACTCCTGCTTGCT-3′);
U6 (forward: 5′-CTCGCTTCGGCAGCACA-3′; reverse:
5′-AACGCTTCACGAATTTGCGT-3′). A TaqMan® miRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
detect the expression of NOS1 and miR-146a. The 2−ΔΔCq
value (15) was used to calculate
the relative gene expression levels between the different treatment
groups. The expression of U6 was used as the internal control of
the reaction. All experiments were performed in triplicate.
Cell culture and transfection
The U251 cells (ATCC, Manassas, VA, USA) were
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 100 U/ml penicillin, 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
100 µg/ml streptomycin, in an atmosphere of 5% CO2/95%
air at 37°C until use. When confluence reached 80%,
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to perform the transfection procedures
according to the manufacturer's protocol.
Luciferase assay
The 3′-untranslated region (UTR) of NOS1 containing
the binding site of miR-146a was amplified via PCR. A Dual
Luciferase Reporter Assay system (Promega Corporation, Madison, WI,
USA) was used to measure the activity of luciferase. The 3′-UTR of
NOS1 and the mutated type were inserted into the empty TOP-FLASH,
FOP-FLASH plasmids (EMD Millipore, MA, USA) to construct the
luciferase reporter, according to the manufacturer's protocol.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to co-transfect U251 cells with
miRNA-481, and Renilla was used as an internal control in
accordance with the manufacturer's protocol. At 48 h
post-transfection, the Dual-Luciferase Reporter Assay system
(Promega Corporation) was used to detect the luciferase activity of
the cells, compared with the activity of Renilla (internal
control). Each independent experiment was performed in triplicate
and the average of the report data was accepted.
Western blot analysis
Following all treatments, the U251 cells were
harvested and washed twice using ice cold PBS. RIPA buffer
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) containing 50
mM Tris Cl, 50 mM NaF, 150 mM NaCl, 1% sodium deoxycholate 5 mM
EDTA, 0.1% SDS/1% aprotinin, 1% Nonidet P-40 and 0.1 mM
Na3VO4 (pH 7.4), with a protease inhibitor
cocktail (Promega Corporation) was used to lyse U251 cells, and the
cellular lysates were centrifuged at 13,000 × g for 15 min at 48°C.
A BCA assay kit (BioTeke Corporation, Beijing, China) was used to
measure the concentration of the supernatant protein in accordance
with the manufacturer's protocol, following which the proteins (40
µg) were heated in boiling water for 5 min with loading buffer.
Subsequently, a 10% sodium dodecyl sulfate polyacrylamide gel was
used to isolate the proteins, followed by semi-dry transfer onto a
polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The blot was then incubated with mouse antibody
against β-actin (sc-418965; 1:2,000; Santa Cruz Biotechnology,
Inc., Shanghai, China) and mouse antibodies against NOS1
(sc-136006; 1:1,000; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. In order to enhance chemiluminescence (Boster Systems,
Inc., Pleasanton, CA, USA), the blot was incubated with goat
anti-mouse secondary antibody conjugated to horseradish peroxidase
(BA1075; 1:3,000; Boster Systems, Inc.) for 90 min at room
temperature. ImageJ software version 1.46r (National Institutes of
Health, Bethesda, MA, USA) was used to quantify the results.
Following normalization to individual β-actin levels, the
proportions of the expression of NOS1 and miR-146a were determined.
All samples were run in triplicate.
Statistical analysis
All data are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference. The differences in the demographic and
clinicopathological parameters between the two groups were analyzed
using Student's t-test or χ2 test. Logistic regression
analysis was used to determine the difference in genotype between
the two groups with the potential confounding factors adjusted. All
statistical analyses were performed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Demographic, clinicopathological and
genotypic parameters of participants
A total of 412 patients with CAD with depression and
453 patients with CAD without depression were recruited for the
present study. Student's t-test was used to analyze the gender,
age, age of onset, cigarette smoking, family history, levels of
total cholesterol, triglyceride and fasting glucose, blood pressure
and body mass index between these two groups, the details of which
are shown in Table I. All
participants were divided into three groups by rs2910164 genotype:
CC, CG and GG, and logistic regression analysis was performed. The
95% confidence interval (CI) was 0.42–0.73 and odds ratio (OR) was
0.56 (P=0.001). All details are shown in Table II. The participants were also
divided into two groups by frequency: C and G, and logistic
regression analysis revealed a 95% CI of 0.46–0.70 and OR of 0.57
(P=0.001). All details are shown in Table II.
 | Table I.Demographic and genotypic
characteristics of patients with CAD with or without
depression. |
Table I.
Demographic and genotypic
characteristics of patients with CAD with or without
depression.
| Characteristic | CAD with depression
(n=412) | CAD without
depression (n=453) | P-value |
|---|
| Gender
(male/female) | 157/255 | 166/287 |
|
| Age in years
(range) | 48.55±11.2
(18–75) | 47.35±8.2
(20–78) | 0.44 |
| Single/recurrent
episode | 115/297 | – |
|
| Family history
(+/-) | 218/194 | – |
|
| Age of onset
(years) |
35.2±11.4 | – |
|
| Cigarette smoking n
(%) | 35 (22.56) | 38 (24.16) | 0.71 |
| Total cholesterol
(mg dl−1) | 214.21±45.3 | 208.28±41.3 | 0.08 |
| Triglyceride (mg
dl−1) | 133.24±70.2 | 113.26±55.2 | 0.25 |
| Fasting glucose (mg
dl−1) |
95.64±21.6 |
93.24±11.5 | 0.81 |
| Blood
pressuresys (mmHg) | 128.23±14.3 | 120.15±12.1 | 0.15 |
| Blood
pressuredias (mmHg) | 76.14±8.3 | 71.12±4.6 | 0.26 |
| Body mass index (kg
m−2) | 25.24±3.3 | 22.31±4.1 | 0.95 |
 | Table II.Association between genotypes and
allele frequencies of rs2910164 polymorphisms and the risk of major
depression in patients with CAD. |
Table II.
Association between genotypes and
allele frequencies of rs2910164 polymorphisms and the risk of major
depression in patients with CAD.
| rs2910164
polymorphism | CAD with depression
(n=412) n (%) | CAD without
depression (n=453) n (%) | Significance |
|---|
| Genotype |
|
|
|
| GG | 160 (39) | 240 (53) | OR=0.56 |
|
|
|
| 95%
CI=0.42–0.73 |
| GC | 185 (45) | 185 (41) |
|
| CC | 67
(16) | 28 (6) |
|
|
GC/CC | 252 (61) | 213 (47) | P<0.001 |
| Allele
frequency |
|
|
|
| G | 505 (61) | 665 (73) | OR=0.57 |
|
|
|
| 95%
CI=0.46–0.70 |
| C | 319 (39) | 241 (27) | P<0.001 |
NOS1 is a virtual target of
miR-146a
miR-146a has been found to be commonly involved in
the pathogenesis of various human diseases, including skin cancer,
hepatocellular carcinoma and CAD (16–18).
The present study aimed to investigate the molecular mechanism,
including the signaling pathways and potential regulator of NOS1,
which may be functionally involved in the regulation of depression
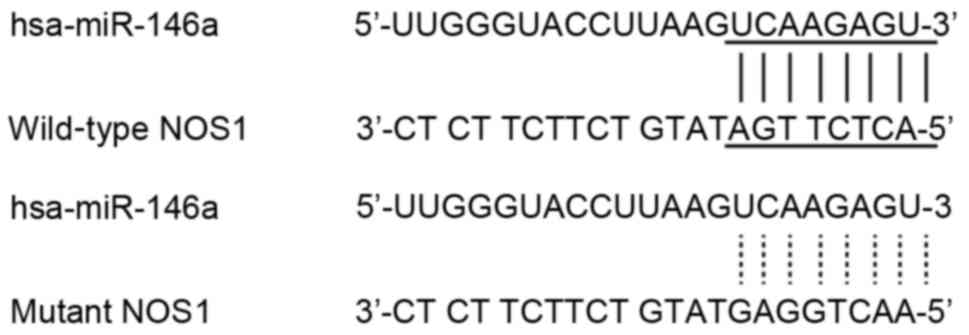
in the patients with CAD. A prediction algorithm revealed that
there was complementarity between the NOS1 3′-UTR and the seed
sequence of miR-146a. To validate whether miR-146a was able to
target NOS1, reporter constructs were generated; these contained
either a wild-type or a mutant NOS1, reporter constructs were
generated; genes (Fig. 1).
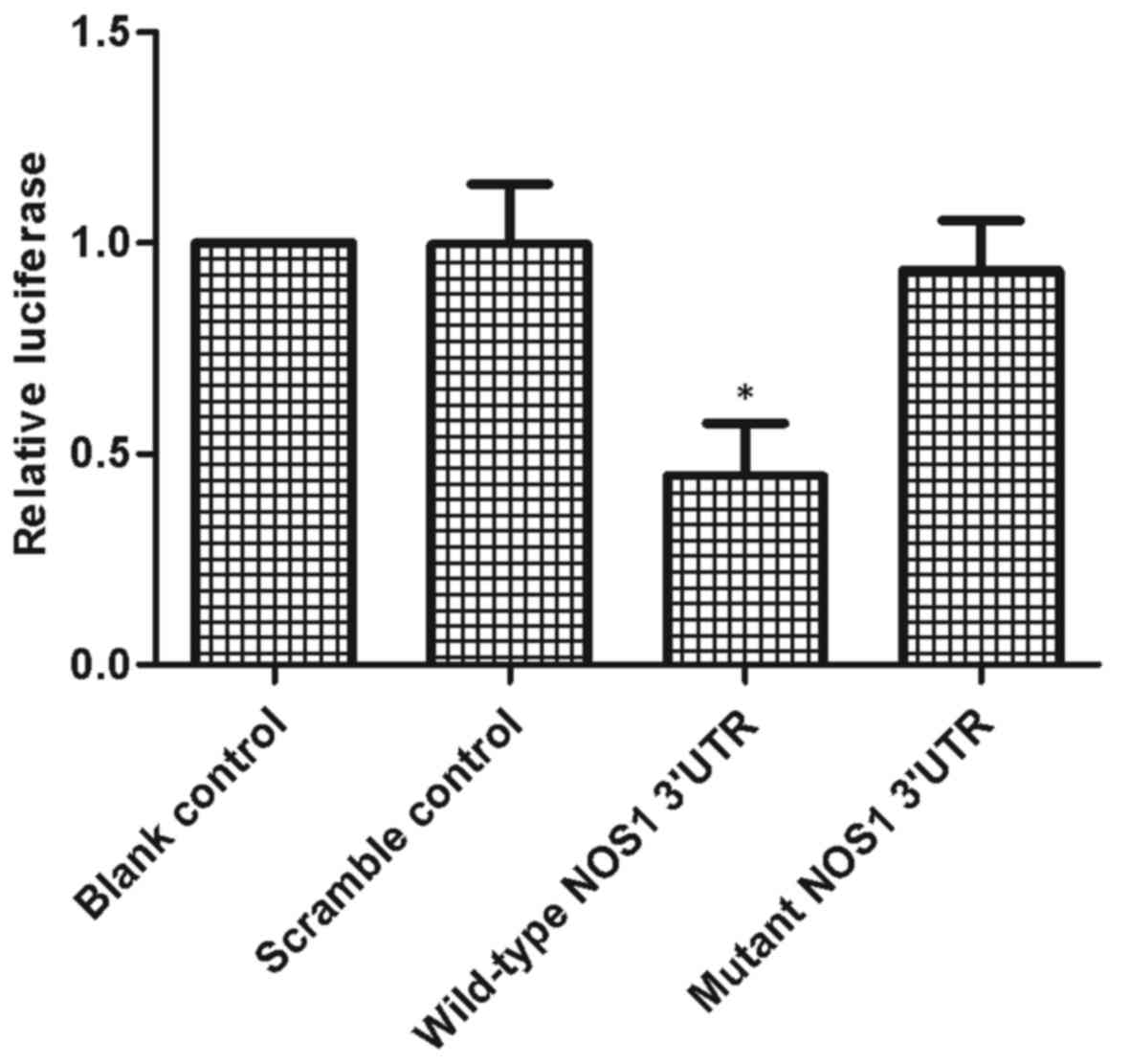
In order to confirm the regulatory association
between NOS1 and miR-146a, a luciferase reporter assay system was
used. Luciferase reporter plasmids were constructed harboring the
wild-type 3′-UTR and mutant 3′-UTR (Fig. 2). The reporter luciferase activity
of the wild-type 3′-UTR was significantly inhibited by miR-146a in
the miR-146a mimic group, compared with the scramble and blank
control (Fig. 2), indicting the
negative regulatory association between miR-146a and NOS1.
Comparing the luciferase activity of the scramble control, blank
control and cells carrying the mutant NOS1 3′-UTR constructs
indicated NOS1 as the direct target gene of miR-146a, with the
binding site located at the segment which was mutated. Therefore,
NOS1 was a direct target of miR-146a, and the binding site was
located at the 3′-UTR of NOS1.
Expression levels of miR-146a and NOS1
differ between treatment groups
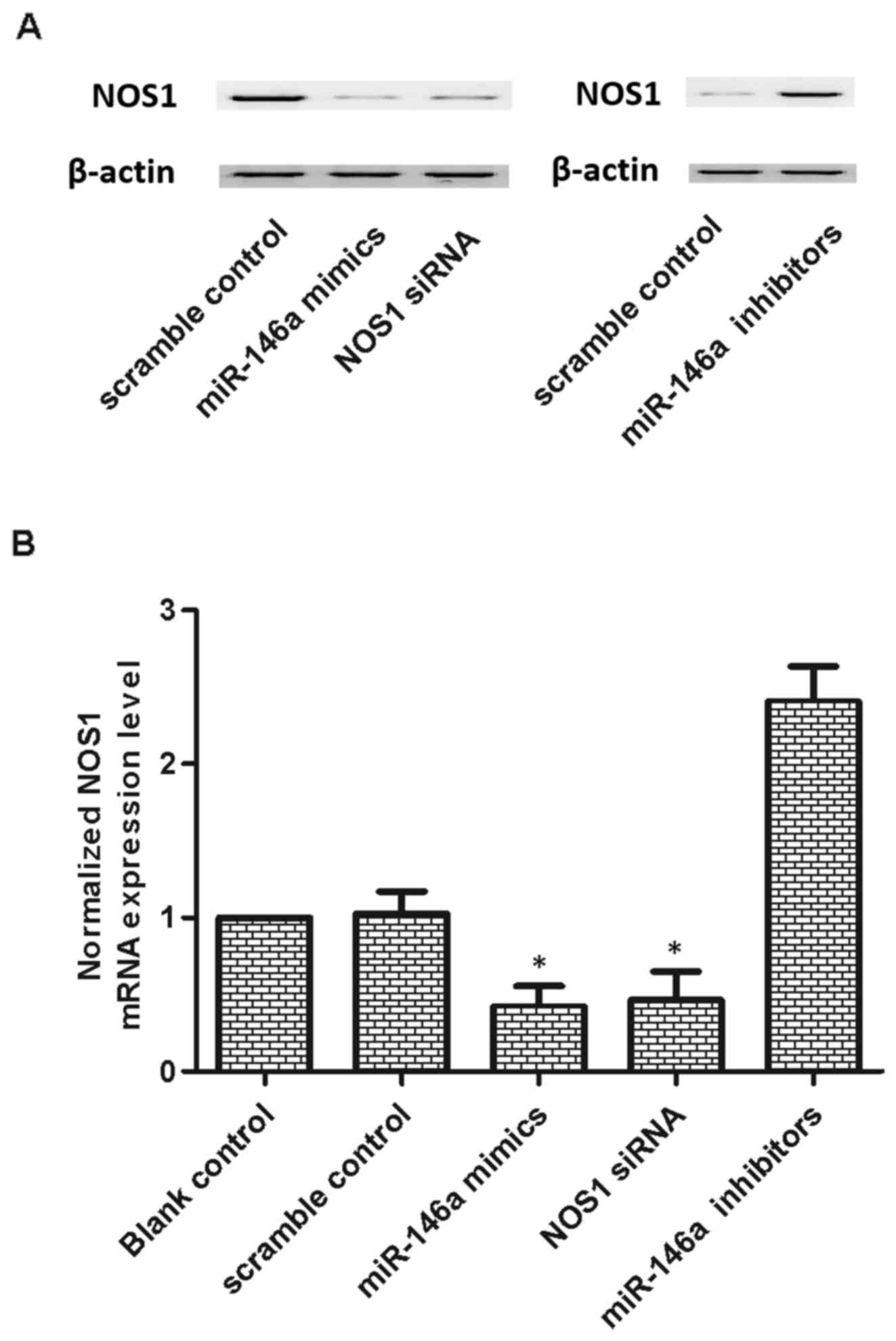
In order to determine the role of miR-146a in
mediating the mechanism in patients with CAD and depression, the
expression of miR-146a in U251 cells was reduced using an miR-146a
inhibitor to inhibit its expression. The expression levels of
miR-146a and NOS1 were determined using RT-qPCR and western blot
analyses. As shown in Fig. 3A, the
protein expression levels of NOS1 in U251 cells treated with
miR-146a mimics and NOS1 siRNA were lower, compared with that in
the scramble control, indicating a negative regulatory association
between miR-146a and NOS1. The miR-146a inhibitor was used to
inhibit its expression, and the miR-146a inhibitor treatment group
exhibited higher protein expression of NOS1, confirming the
negative regulatory association between miR-146a and NOS1.
As shown in Fig.
3B, the mRNA expression levels of NOS1 in U251 cells treated
with miR-146a mimics and NOS1 siRNA were lower, compared with that
in the scramble control group, indicating a negative regulatory
association between miR-146a and NOS1 mRNA. The miR-146a inhibitor
was used to suppress its expression, and the cells in the miR-146a
inhibitor treatment group exhibited a markedly increased mRNA level
of NOS1. Transfection with miR-146a inhibitor reversed this effect;
therefore, the results revealed there was a negative regulatory
association between miR-146a and NOS1.
Discussion
Accumulating evidence has shown that depression is a
risk factor for cardiac morbidity and mortality rates in patients
who have had CAD in the previous three decades (19). However, several questions remain to
be elucidated and there are also debates regarding this finding.
Whether depression is not only a risk factor but also a causal risk
factor for adverse CAD results, and which biobehavioral mechanisms
are involved remain significant scientific questions regarding
patients with CAD and depression (20,21).
In clinical settings, it remains to be elucidated whether
depression in patients with CAD is treatable and whether cardiac
event-free-survival can be improved by treatment (22).
Nitric oxide (NO) directly modifies protein thiols
(S-nitrosylation) at the post-translational level and activates
signaling pathways dependent on cyclic guanosine monophosphate,
thereby having a crucial regulatory role in cardiac function
(23). NO originating from active
neuronal NOS1 is associated with S-nitrosylation of crucial
sarcoplasmic reticulum (SR) Ca2+ handling proteins
(24). It is not surprising that
the dysfunction of NOS is an important contributor to vascular
pathophysiology due to the pivotal role of NO signaling in
inflammation, thrombosis, smooth muscle proliferation and
endothelial function (25).
Previous loss of function and gain of function genetic studies have
shown the significance of NOS3 in vascular homeostasis, with
deletion aggravating vascular remodeling and neointimal medial
thickening following disruption (26); whereas overexpressed
endothelial-targeted NOS3 decreases atherosclerosis in ApoE-null
mice and inhibits vascular remodeling (27,28).
Of note, oxidant-coupled hyperpolarization and NO-dependent
vasorelaxation can be inhibited by the deletion of NOS3 (29). Although less NO is generated by
uncoupled NOS3, its synthesis of O2−, which becomes
H2O2 by Cu, Zn-superoxide dismutase, leads to
vasodilation (30). The latter is
coupled to oxidated C46 residues in protein kinase G1K to generate
an internal disulfide bond and a cGMP-independently activated
kinase (31). In the brain, the
richest form is NOS1, which has an extensive effect in synaptic
signaling, and is involved in neuronal plasticity, memory,
learning, and a variety of psychiatric conditions, including
schizophrenia and depression (32,33).
NOS1 serves a more specific regulatory role in the
hypothalamic-pituitary-adrenal axis and the serotonin pathway
(34,35). A study by Pogun and Kuhar (35) demonstrated that, via their mutual
PDZ-binding motifs, they are coupled to Ca2+-permeable
N-methyl-D-aspartate receptors at the postsynaptic density
(PSD-95). In several regions of the brain, including the
hippocampus in animal models, there is a stress response in
addition to the increased expression of NOS1 caused by chronic
stress (34). A number of animal
experiments have shown that, in stress models, drugs, which
suppress NOS exhibit antidepressant-analog behavioral actions
(36).
It has been shown that miR-146a has a critical
effect on tumorigenesis, cell differentiation, proliferation and
apoptosis (37). In 2008,
Jazdzewski et al (14)
showed that a common polymorphism, known as rs2910164, in the
pre-miR-146a sequence, altered the expression of mature miR-146a
and had an important effect on tumorigenesis. The correlation
between the rs2910164 G>C polymorphism and cancer risk,
including gastrointestinal tumors, have been investigated
extensively in substantial meta-analyses and reviews over the last
3 years (38). The association
between the rs2910164 polymorphism and the risk of gastric cancer
has been assessed in nine of these (39). The findings were consistent with no
significant correlation between gastric cancer and the rs2910164
polymorphism. Whereas risk and decreased survival rates in patients
with glioma are associated with the CC genotype, miR-146a
(rs2910164) GC polymorphisms are important in papillary thyroid
carcinoma (40). A meta-analysis
examined conflicting studies regarding predisposition to cancer.
There was no pattern between tumor type and the SNP. By contrast, a
correlation was reported between elevated risk of cancer in an
Asian population and GG variant genotypes (41), which may indicate the heterogeneity
of the disorder. It has also been reported that the G-allele is
correlated with pulmonary tuberculosis in various directions in
Tibetan (risk) and Han (protection) populations regarding
mycobacterial infections (42).
Data were compiled, which showed evidence of an association between
the GC miRSNP-146a and leprosy in a Brazilian population.
Family-based and case control study designs were used, and a
correlation between the C-allele and predisposition to leprosy was
demonstrated, with age-at-diagnosis being a critical factor
adjusting this correlation, which was also described previously in
leprosy (43,44). In the present study, NOS1 was
identified as a direct target of miR-146a using computational
analysis and a luciferase assay, which was further confirmed by the
observation that the mRNA and protein expression levels of NOS1 in
U251 cells treated with miR-146a mimics and NOS1 siRNA were
substantially downregulated, compared with those in the scramble
control, whereas cells treated with miR-146a inhibitors showed
increased expression of NOS1. In the present association study,
patients with CAD, including 412 patients with depression and 453
patients without depression, were recruited, who were further
divided into three groups by rs2910164 genotype: CC, CG and GG.
Logistic regression analysis was performed, which revealed that the
presence of the minor allele of the polymorphism was significantly
associated with the risk of depression in patients with CAD (95%
CI, 0.42–0.73; OR, 0.56; P<0.001). It was also shown that the
allele frequency was significantly associated with the risk of
depression in CAD (95% CI, 0.46–0.7; OR, 0.57; P<0.001).
In conclusion, the results of the present study
indicated a decreased risk of depression in patients with CAD who
are carriers of the miR-146a rs2910164 C allele, and this
association may be attributed to its ability to compromise the
expression of miR-146a, and thereby increase the expression of its
target gene, NOS1.
Acknowledgements
Not applicable.
Funding
This study was sponsored by the Shandong Province
Medical Science and Technology Development Plan Youth Projects
(grant no. 2013WS0054).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ: Study planning, data analysis and
interpretation, and preparation of the manuscript; QH: Study
planning, data collection, preparation of the manuscript, and
literature analysis; WS: Study planning, data collection, and
preparation of the manuscript; CZ: Data collection, data analysis,
preparation of the manuscript, and literature analysis; ZW: Data
interpretation, preparation of the manuscript, and literature
analysis; BX: Data analysis and interpretation, and preparation of
the manuscript; and, QL: Data collection and sourcing of funds.
Ethics approval and consent to
participate
The study was performed according to the latest
version of the Declaration of Helsinki. The Ethics and Research
Committees of the First People's Hospital at Jining (Jining, China)
approved the study.
Consent for publication
The patients provided signed informed consent for
participation in the study following explanation of potential
risks.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: Systematic analysis of population health data.
Lancet. 367:1747–1757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moussavi S, Chatterji S, Verdes E, Tandon
A, Patel V and Ustun B: Depression, chronic diseases and decrements
in health: Results from the World Health Surveys. Lancet.
370:851–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Writing Group Members, . Lloyd-Jones D,
Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB,
Ford E, Furie K, et al: Heart disease and stroke statistics-2010
update: A report from the American Heart Association. Circulation.
121:e46–e215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carney RM and Freedland KE: Depression in
patients with coronary artery disease. Am J Med. 121 11 Suppl
2:S20–S27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bush DE, Ziegelstein RC, Patel UV, Thombs
BD, Ford DE, Fauerbach JA, McCann UD, Stewart KJ, Tsilidis KK,
Patel AL, et al: Post-myocardial infarction depression. Evid Rep
Technol Assess (Summ). 1–8. 2005.PubMed/NCBI
|
|
6
|
Enas EA, Kuruvila A, Khanna P, Pitchumoni
CS and Mohan V: Benefits & risks of statin therapy for primary
prevention of cardiovascular disease in Asian Indians-a population
with the highest risk of premature coronary artery disease &
diabetes. Indian J Med Res. 138:461–491. 2013.PubMed/NCBI
|
|
7
|
Freedland KE, Carney RM, Lustman PJ, Rich
MW and Jaffe AS: Major depression in coronary artery disease
patients with vs. without a prior history of depression. Psychosom
Med. 54:416–421. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dorn GW II: MicroRNAs: Redefining
mechanisms in cardiac disease. J Cardiovasc Pharmacol. 56:589–595.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon C, Han Z, Olson EN and Srivastava D:
MicroRNA1 influences cardiac differentiation in Drosophila and
regulates Notch signaling. Proc Natl Acad Sci USA. 102:18986–18991.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Ransom JF, Li A, Vedantham V, von
Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ and
Srivastava D: Dysregulation of cardiogenesis, cardiac conduction
and cell cycle in mice lacking miRNA-1-2. Cell. 129:303–317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Y, Zhou Y, Xi Q, Cui H, Luo T, Song H,
Nie X, Wang L and Ying B: Genetic variations in microRNA processing
genes are associated with susceptibility in depression. DNA Cell
Biol. 31:1499–1506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruenig D, Morris CP, Mehta D, Harvey W,
Lawford B, Young RM and Voisey J: Nitric oxide pathway genes
(NOS1AP and NOS1) are involved in PTSD severity, depression,
anxiety, stress and resilience. Gene. 625:42–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarginson JE, Deakin JF, Anderson IM,
Downey D, Thomas E, Elliott R and Juhasz G: Neuronal nitric oxide
synthase (NOS1) polymorphisms interact with financial hardship to
affect depression risk. Neuropsychopharmacology. 39:2857–2866.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forloni M, Dogra SK, Dong Y, Conte D Jr,
Ou J, Zhu LJ, Deng A, Mahalingam M, Green MR and Wajapeyee N:
miR-146a promotes the initiation and progression of melanoma by
activating Notch signaling. Elife. 3:e014602014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang S, He R, Rong M, Dang Y and Chen G:
Synergistic effect of MiR-146a mimic and cetuximab on
hepatocellular carcinoma cells. Biomed Res Int. 6:3841212014.
|
|
18
|
Wang J, Yan Y, Song D and Liu B: Reduced
plasma miR-146a is a predictor of poor coronary collateral
circulation in patients with coronary artery disease. Biomed Res
Int. 2016:42859422016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charlson FJ, Stapelberg NJ, Baxter AJ and
Whiteford HA: Should global burden of disease estimates include
depression as a risk factor for coronary artery disease? BMC Med.
9:472011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuper H, Nicholson A, Kivimaki M,
Aitsi-Selmi A, Cavalleri G, Deanfield JE, Heuschmann P, Jouven X,
Malyutina S, Mayosi BM, et al: Evaluating the causal relevance of
diverse risk markers: Horizontal systematic review. BMJ.
339:b42652009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carney RM, Freedland KE, Miller GE and
Jaffe AS: Depression as a risk factor for cardiac mortality and
morbidity: A review of potential mechanisms. J Psychosom Res.
53:897–902. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carney RM and Freedland KE:
Treatment-resistant depression and mortality after acute coronary
syndrome. Am J Psychiatry. 166:410–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamargo J, Caballero R, Gómez R and Delpón
E: Cardiac electrophysiological effects of nitric oxide. Cardiovasc
Res. 87:593–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lima B, Forrester MT, Hess DT and Stamler
JS: S-nitrosylation in cardiovascular signaling. Circ Res.
106:633–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heitzer T, Schlinzig T, Krohn K, Meinertz
T and Münzel T: Endothelial dysfunction, oxidative stress and risk
of cardiovascular events in patients with coronary artery disease.
Circulation. 104:2673–2678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moroi M, Zhang L, Yasuda T, Virmani R,
Gold HK, Fishman MC and Huang PL: Interaction of genetic deficiency
of endothelial nitric oxide, gender and pregnancy in vascular
response to injury in mice. J Clin Invest. 101:1225–1232. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Haperen R, de Waard M, van Deel E,
Mees B, Kutryk M, van Aken T, Hamming J, Grosveld F, Duncker DJ and
de Crom R: Reduction of blood pressure, plasma cholesterol and
atherosclerosis by elevated endothelial nitric oxide. J Biol Chem.
277:48803–48807. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawashima S, Yamashita T, Ozaki M, Ohashi
Y, Azumi H, Inoue N, Hirata K, Hayashi Y, Itoh H and Yokoyama M:
Endothelial NO synthase overexpression inhibits lesion formation in
mouse model of vascular remodeling. Arterioscler Thromb Vasc Biol.
21:201–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matoba T, Shimokawa H, Nakashima M,
Hirakawa Y, Mukai Y, Hirano K, Kanaide H and Takeshita A: Hydrogen
peroxide is an endothelium-derived hyperpolarizing factor in mice.
J Clin Invest. 106:1521–1530. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morikawa K, Shimokawa H, Matoba T, Kubota
H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi
S and Takeshita A: Pivotal role of Cu, Zn-superoxide dismutase in
endothelium-dependent hyperpolarization. J Clin Invest.
112:1871–1879. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burgoyne JR, Madhani M, Cuello F, Charles
RL, Brennan JP, Schröder E, Browning DD and Eaton P: Cysteine redox
sensor in PKGIa enables oxidant-induced activation. Science.
317:1393–1397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weber H, Klamer D, Freudenberg F,
Kittel-Schneider S, Rivero O, Scholz CJ, Volkert J, Kopf J, Heupel
J, Herterich S, et al: The genetic contribution of the NO system at
the glutamatergic post-synapse to schizophrenia: further evidence
and meta-analysis. Eur Neuropsychopharmacol. 24:65–85. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Steinert JR, Chernova T and Forsythe ID:
Nitric oxide signaling in brain function, dysfunction and dementia.
Neuroscientist. 16:435–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou QG, Zhu LJ, Chen C, Wu HY, Luo CX,
Chang L and Zhu DY: Hippocampal neuronal nitric oxide synthase
mediates the stress-related depressive behaviors of glucocorticoids
by downregulating glucocorticoid receptor. J Neurosci.
31:7579–7590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pogun S and Kuhar MJ: Regulation of
neurotransmitter reuptake by nitric oxide. Ann N Y Acad Sci.
738:305–315. 1994.PubMed/NCBI
|
|
36
|
Silva M, Aguiar DC, Diniz CR, Guimarães FS
and Joca SR: Neuronal NOS inhibitor and conventional antidepressant
drugs attenuate stress-induced fos expression in overlapping brain
regions. Cell Mol Neurobiol. 32:443–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hou Z, Xie L, Yu L, Qian X and Liu B:
MicroRNA-146a is down-regulated in gastric cancer and regulates
cell proliferation and apoptosis. Med Oncol. 29:886–892. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Srivastava K and Srivastava A:
Comprehensive review of genetic association studies and
meta-analyses on miRNA polymorphisms and cancer risk. PLoS One.
7:e509662012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu Z, Zhang L, Cao H and Bai B: MiR-146a
rs2910164 G/C polymorphism and gastric cancer susceptibility: A
meta-analysis. BMC Med Genet. 15:1172014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Permuth-Wey J, Thompson RC, Nabors Burton
L, Olson JJ, Browning JE, Madden MH, Chen Ann Y and Egan KM: A
functional polymorphism in the pre-miR-146a gene is associated with
risk and prognosis in adult glioma. J Neurooncol. 105:639–646.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Bi J, Liu X, Li K, Di J and Wang
B: Has-miR-146a polymorphism (rs2910164) and cancer risk: A
meta-analysis of 19 case-control studies. Mol Biol Rep.
39:4571–4579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li D, Wang T, Song X, Qucuo M, Yang B,
Zhang J, Wang J, Ying B, Tao C and Wang L: Genetic study of two
single nucleotide polymorphisms within corresponding microRNAs and
susceptibility to tuberculosis in a Chinese Tibetan and Han
population. Hum Immunol. 72:598–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alcaïs A, Quintana-Murci L, Thaler DS,
Schurr E, Abel L and Casanova JL: Life-threatening infectious
diseases of childhood: Single-gene inborn errors of immunity? Ann N
Y Acad Sci. 1214:18–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alter A, Fava VM, Huong NT, Singh M,
Orlova M, Van Thuc N, Katoch K, Thai VH, Ba NN, Abel L, et al:
Linkage disequilibrium pattern and age-at-diagnosis are critical
for replicating genetic associations across ethnic groups in
leprosy. Hum Genet. 132:107–116. 2013. View Article : Google Scholar : PubMed/NCBI
|