Introduction
Pancreatic cancer is a malignant tumor that exhibits
a high degree of malignancy, a low cure rate and very poor
prognosis. In western countries, pancreatic cancer is the fourth
leading cause of cancer-associated mortalities (1). Pancreatic cancer incidence ranked the
eighth and the mortality ranked the sixth in China in 2013
(2). Although the technologies
used for diagnosis and treatment of pancreatic cancer have been
greatly improved, the majority of patients are diagnosed at the
later stages, owing to a lack of specific clinical manifestations
of pancreatic cancer and therefore the five-year survival rate is
currently <5% (3–5). Gemcitabine (GEM) is a standard
first-line treatment for advanced pancreatic cancer. However, its
strong side effects and the drug resistance of tumor cells affect
the efficacy of treatment and the quality of life of patients
(6).
During the process of malignant tumor development,
tumor cells are often in a state in which there is a lack of oxygen
and nutrients owing to excessive growth (7). In solid tumors, hypoxia is a common
characteristic that may be powerful driving force that promotes
tumor progression and is one of the main reasons for failure of
treatment (8,9). Hypoxia may induce alterations in the
biological characteristics and microenvironment of tumor cells,
which may lead to faster tumor growth and stronger invasive
abilities of the tumoral cells (10). Pancreatic cancer is hypoxic and
hypoxia impacts on the ability of pancreatic cancer cells to invade
and metastasize (11). The
expression level of HIF-1α in pancreatic cancer is linked to tumor
progression, angiogenesis, invasion, and metastasis (12,13).
Epithelial mesenchymal transition (EMT) serves a key
role in tumor invasion and metastasis. EMT can make tumor cells
lose their epithelial cell-like polarity characteristics, and
obtain interstitial cell characteristics and accordingly increase
the metastatic and invasive potential of tumor cells. E-cadherin is
an important intercellular adhesion molecule, mainly expressed on
the surface of cell membranes and serves an important role in
maintaining intercellular adhesion. As the decrease in the
intercellular adhesion can increase the migration ability of cells
and promote tumor cells to invade surrounding tissues, a decrease
in E-cadherin is considered to serve an important role in tumor
metastasis (14). As a major
member of the intermediate filament protein family, vimentin is
expressed in almost all normal interstitial cells, and serves an
important role in maintaining cell integrity and resisting external
emergency injury (15). A decrease
in the expression of E-cadherin and an abnormal increase in the
expression of vimentin are considered as major molecular markers
for the initiation of the EMT program (16). In previous years, certain scholars
have proposed that, p53 is also involved in the regulation of the
EMT process, loss of p53 function can occur in tumor cells with a
mutation in the p53 gene, which facilitates the occurrence of EMT
(17).
Through pre-clinical trials, it has been confirmed
that obatoclax (OBX) may be used to inhibit tumor cells and the
growth of transplanted tumors (12). Therefore, a better therapeutic
effect for treatment may be obtained with therapeutic strategies
against cellular characteristics under hypoxic conditions. In the
present study, pancreatic cancer BxPC-3 was used as a study
subject, to investigate the effect of OBX combined with gemcitabine
on the proliferation, migration and invasion of BxPC-3 cells under
hypoxia, and the effects on EMT-related molecular markers including
E-cadherin, vimentin and p53, in order to provide a theoretical
basis for improving the curative effect of GEM in the treatment of
pancreatic cancer.
Materials and methods
Main reagents
RPMI-1640 culture medium was purchased from HyClone
(GE Healthcare Life Sciences, Logan, UT, USA). Fetal bovine serum
(FBS) was purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). GEM and OBX were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Penicillin, streptomycin and Cell
Counting kit-8 (CCK-8) were obtained from Beyotime Institute of
Biotechnology (Haimen, China). The hypoxia-inducible factor
(HIF)-1α antibody was obtained from BD Biosciences (Franklin Lakes,
NJ, USA; cat. no. 610958), GAPDH antibody was obtained from
Sigma-Aldrich (Merck KGaA; cat. no. G9545), and antibodies against
vimentin (cat. no. sc-6260), E-cadherin (cat. no. sc-71009) and p53
(cat. no. sc-126) were purchased from Santa Cruz Biotechnology,
Inc., (Dallas, TX, USA).
Cell culture
The human pancreatic cancer cell line BxPC-3 was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in RPMI-1640
medium supplemented with 10% FBS, 100 U/l penicillin and 100 µg/ml
streptomycin. Cells were cultured in an incubator with 5%
CO2 at 37°C, and passaged every three days. Cells in the
logarithmic growth phase were used in subsequent experiments.
Cell groupings
The concentrations of OBX (18) and GEM (19) were determined according to these
studies. The cells were first cultured at 37°C under normal oxygen
conditions until they covered 50% of the bottom, then, divided into
four groups under different conditions of treatment. Cells were
separated into the following groups: i) Normoxia group, the cells
continued to cultured at 37°C under normal oxygen condition; ii)
hypoxia group, the cells were cultured under hypoxic conditions
(induction conditions of 37°C, 1% O2, 5% CO2,
and 94% N2); iii) OBX group, the cells were added with
1.25 µM of OBX, then cultured at 37°C under hypoxia condition; and
the fourth group; and iv) OBX + GEM group, the cells were added
with 1.25 µM of OBX and 0.3 µM of GEM, then cultured at 37°C under
hypoxia conditions.
Proliferation in the four groups
detected by CCK-8
The cells in the four groups were inoculated into
96-well plates at the dose of 1,000/well, continued to culture for
24 and 48 h at 37°C, then the cells were harvested, respectively.
CCK-8 solution (100 µl) was added into each well and incubated at
37°C for 2 h; optical density was detected by a microplate reader
at 450 nm.
Scratch test for cell migration
Cells at a density of 5×105/well in 2 ml
were inoculated in a 6-well plate and incubated for 12 h at 37°C. A
straight line was scratched onto the bottom of Petri dish using a
syringe needle. Cells were incubated in serum-free medium for 48 h
at 37°C, and cell migration was observed under a microscope and
images were captured.
Detection of cell invasive ability by
Matrigel assay
The cells in each group were incubated in serum-free
culture medium at 37°C for 24 h. The surface of the upper chamber
of the Transwell plate was coated with Matrigel diluted with
serum-free culture medium (8:1), and the chamber was incubated at
37°C for 24 h. The cells in each group (5×104 cells/100
µl) were inoculated with serum-free culture medium in the upper
chamber of the Transwell chamber for 24 h. The cells in the upper
chamber were wiped off with a cotton swab, whereas cells on the
lower membrane were washed twice with PBS and fixed with 4%
paraformaldehyde at 4°C for 30 min. The membrane was stained with
0.1% crystal violet for 20 min, and washed with PBS twice.
Subsequently, cells were observed under a microscope and images
were captured. The number of cells that had migrated was quantified
by counting them in five distinct randomly chosen fields using a
light microscope. The number of invading cells was calculated.
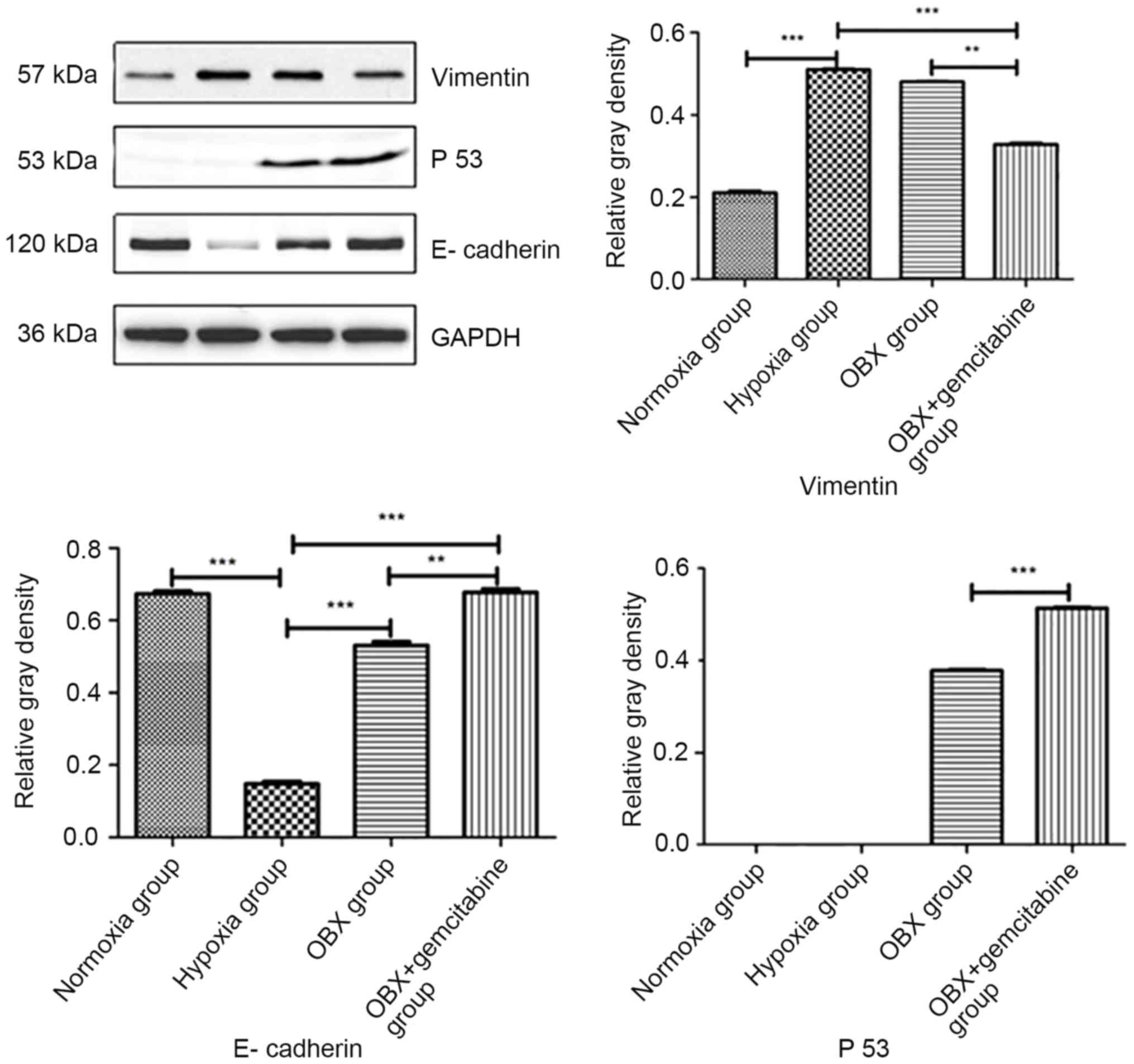
Western blot assay
The western blotting method was used to detect the
HIF-1α protein expression in the hypoxia group and normoxia group,
and the vimentin, E-cadherin and p53 protein expression levels in
each group. Following incubation, the culture medium was discarded,
Cells were lysed on ice for 30 min in 500 µl RIPA lysis solution
[150 mM NaCl, 1% Nonidet P-40, 1% deoxycholate, 0.1% SDS, 10 mM
Tris-HCl, pH 8.0, 1 mM EDTA, (pH 8.0)] and the total protein was
extracted supplemented with protease inhibitors (1 mM
phenylmethylsulfonyl fluoride (PMSF), 0.2 mM sodium orthovanadate
and 1 µg/ml aprotinin). Lysates were centrifuged at 12,000 × g for
20 min at 4°C. The protein concentration was determined using the
Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) with bovine serum albumin (BSA; Wako Pure Chemical Industries,
Ltd., Osaka, Japan) as the standard. Equal amounts of proteins (30
µg) were separated on 12% acrylamide gels (Bio-Rad Laboratories,
Inc.) and transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA) and the membranes were blocked with
5% skimmed milk at room temperature for 1 h, followed by incubation
with antibodies against HIF-1α (1:500; cat. no. 610958; BD
Biosciences, Franklin Lakes, NJ, USA), vimentin (1:500; cat. no.
sc-6260), E-cadherin (1:500; cat. no. sc-71009), p53 (1:500; cat.
no. sc-126; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and GAPDH (1:10,000; cat. no. G9545; Sigma-Aldrich, Merck KGaA) at
4°C overnight. Membranes were subsequently incubated with
horseradish peroxidase-conjugated goat anti-rabbit (1:5,000; cat.
no. sc-2004) or goat anti-mouse (1:5,000; cat. no. sc-2005)
secondary antibodies (all Santa Cruz Biotechnology, Inc.) at room
temperature for 1.5 h. Odyssey film scanning was performed by the
Odyssey Fc Imaging System (LI-COR Biosciences; Lincoln, NE, USA)
and Image J Software, version 1.48 (National Institutes of Health,
Bethesda, MD, USA) was used for densitometric analysis.
Statistical analysis
SPSS 13.0 software system (SPSS, Inc., Chicago, IL,
USA) was used for statistical evaluation. Data are presented as the
mean ± standard deviation of at least three independent
experiments. Statistical significance was evaluated by analysis of
variance followed by Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of HIF-1α under hypoxic
conditions
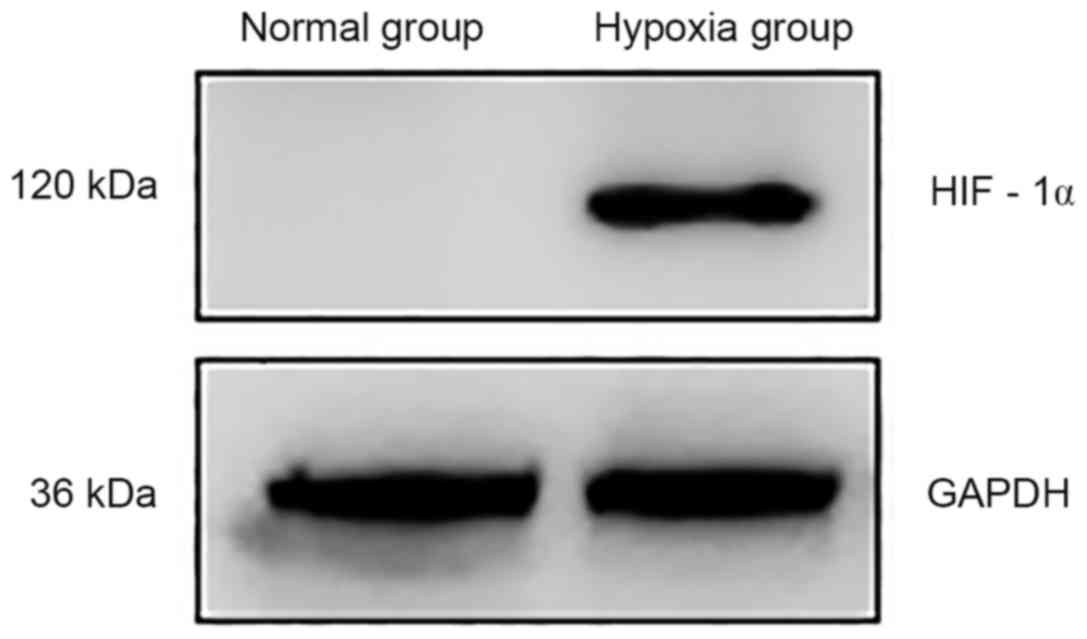
Western blotting results revealed that HIF-1α
protein expression in BxPC-3 cells grown under normoxic conditions
was almost undetectable, whereas a notable increase in expression
was observed in cells grown under hypoxic conditions (Fig. 1).
Effects of OBX and OBX + GEM
treatments on cell proliferation under hypoxic conditions
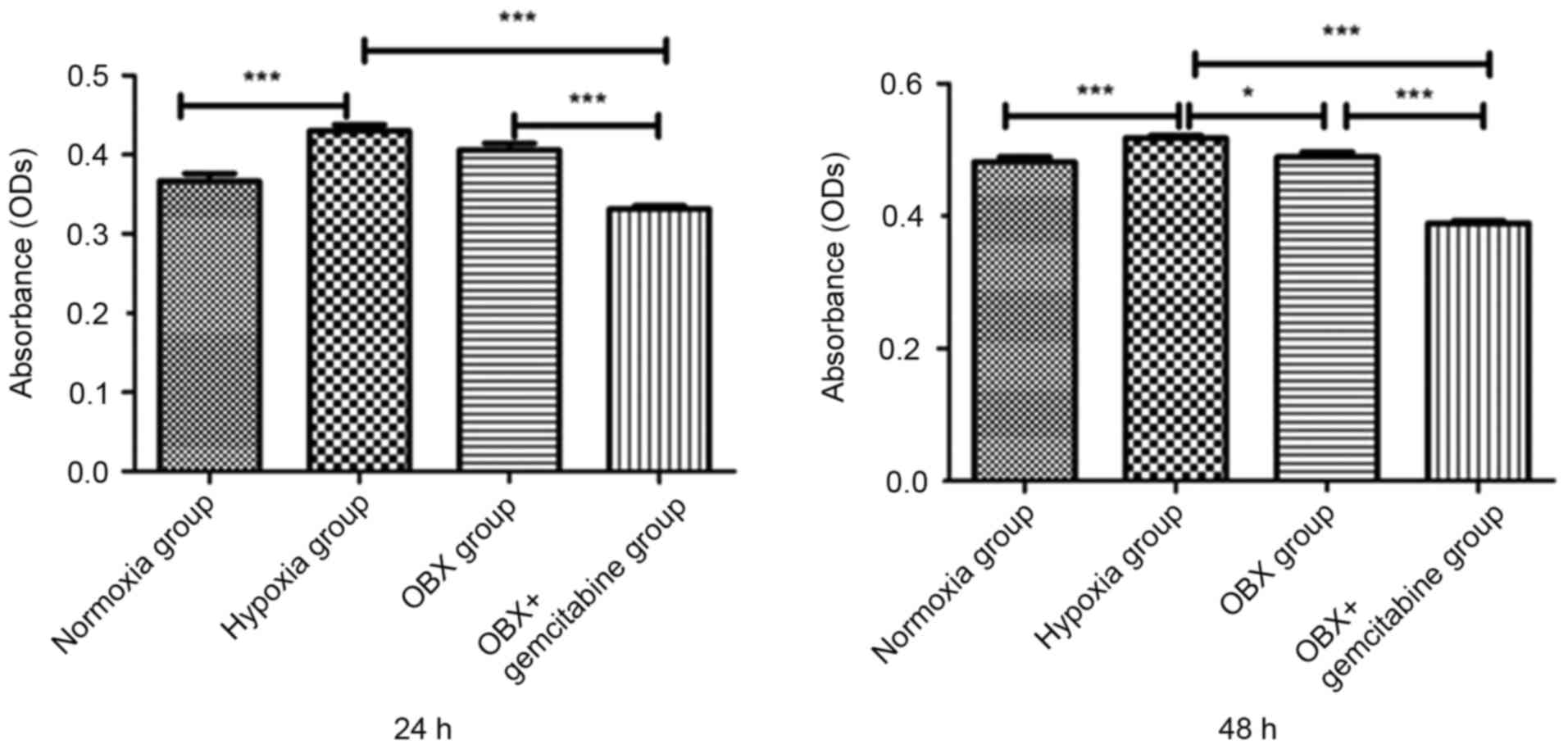
Results of CCK-8 detection identified that, compared
with normal oxygen group, the proliferation ability of BxPC-3 cells
in the hypoxia group was significantly increased (P<0.001); at
24 h, the cell proliferation ability of OBX group was not altered
compared with the hypoxia group, but at 48 h, cell proliferation
decreased; in the OBX + GEM group, cell proliferation markedly
decreased from 24 h compared with not only hypoxia group but also
OBX group (Fig. 2).
Effects of OBX and OBX + GEM
treatments on cell migration ability under hypoxic conditions
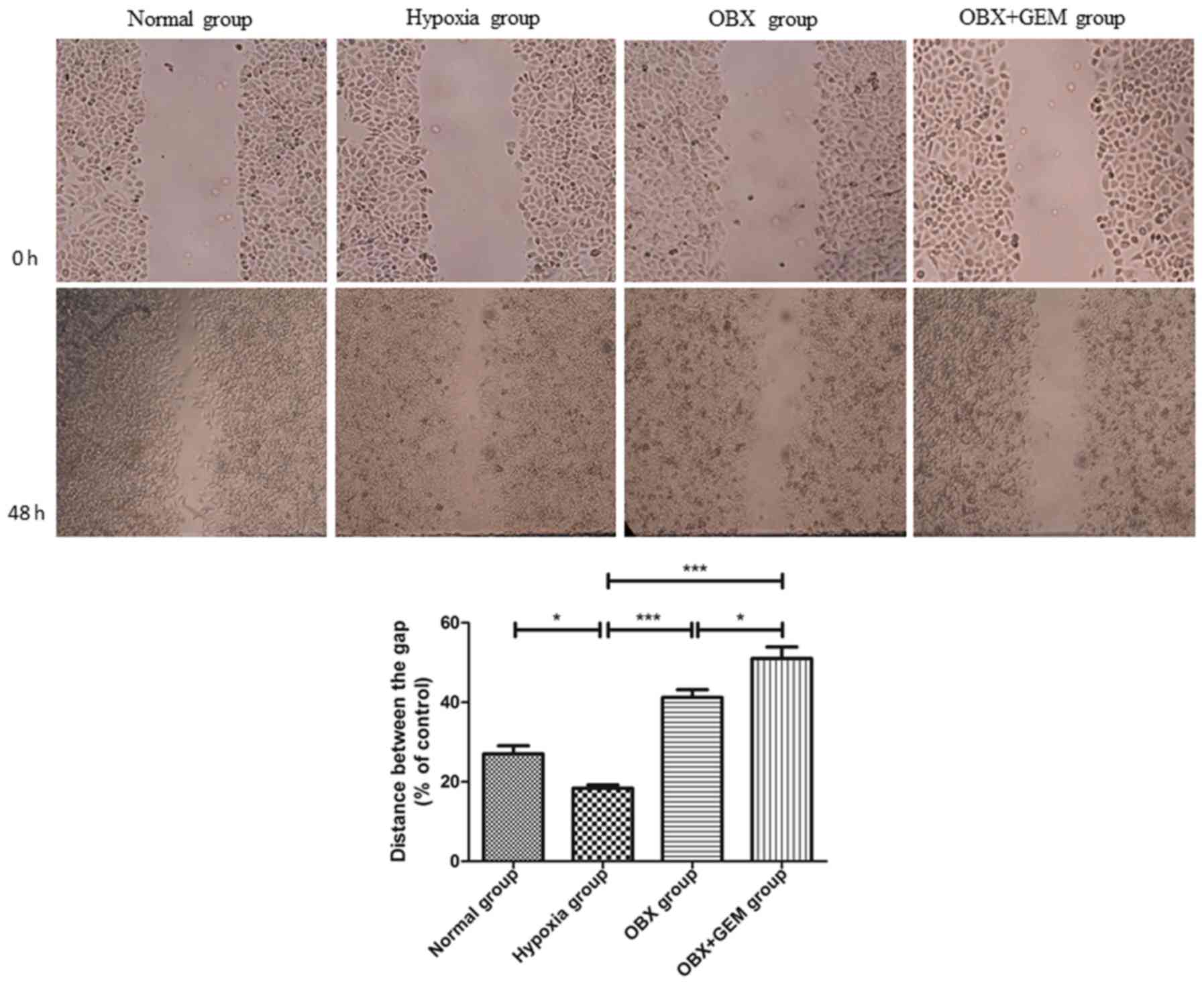
Scratch test results revealed that the healing rate
of BxPC-3 cells in the hypoxia group was higher compared with the
normoxic group, and the difference was statistically significant
(P<0.05). Compared with the hypoxia group, the migratory ability
of cells in the OBX group and OBX + GEM group decreased
(P<0.001); and the migratory ability of cells in the OBX + GEM
group decreased more significantly (P<0.05), which indicated
that co-treatment with OBX and GEM may reduce markedly the ability
of BxPC-3 cells to migrate under hypoxic conditions (Fig. 3).
Effects of OBX and OBX + GEM
treatments on the invasiveness of cells under hypoxic
conditions
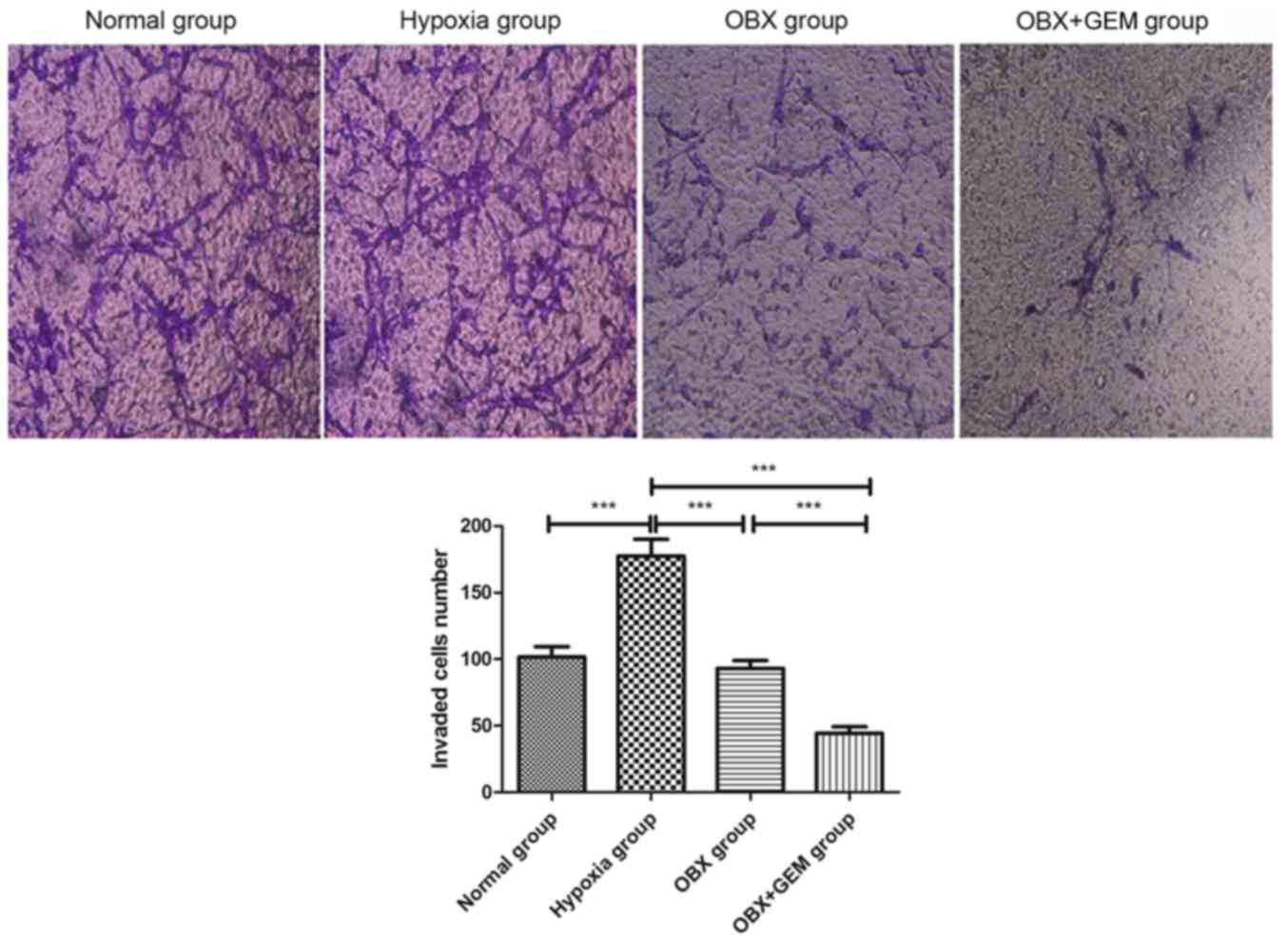
Results from the Matrigel invasion assay revealed
that the number of invading BxPC-3 cells significantly increased in
the hypoxia group (P<0.001; Fig.
4). Compared with cells in the hypoxia group, the number of
invading cells in the OBX group and in the OBX + GEM group
decreased significantly (P<0.001); Futhermore, compared with the
OBX group, the number of invasive cells in the OBX + GEM group
decreased significantly (P<0.001), which indicated that
co-treatment with OBX and GEM may reduce markedly the invasive
ability of BxPC-3 cells under hypoxic conditions (Fig. 4).
Effects of OBX and OBX + GEM
treatments on EMT related proteins expression under hypoxic
conditions
Compared with BxPC-3 cells in the normoxia group,
the protein expression level of vimentin in cells in hypoxia group
was significantly increased (Fig.
5). However, the expression of E-cadherin was significantly
decreased, and no significant changes were found in the protein
expression of p53. Compared to the hypoxia group, the expression of
E-cadherin and p53 was upregulated in the OBX group, and the
expression of vimentin did not change. Compared with the hypoxia
group and OBX group, the expression of E-cadherin and p53 was
upregulated in the OBX + GEM group, and the expression of vimentin
was significantly downregulated (P<0.01). These data indicated
that when BxPC-3 cells were treated with OBX combined with GEM in
hypoxic conditions, the EMT related proteins altered markedly.
Discussion
Pancreatic cancer is a fatal disease with poor
prognosis; in China, as in other countries worldwide, the health
burden of pancreatic cancer continues to grow (13). Furthermore, the annual mortality
rate is almost equal to its incidence rate, and the annual
incidence and mortality rates in China have exceeded those of the
United States (13). GEM is a
standard first-line treatment for advanced pancreatic cancer;
however, whether treated with GEM monotherapy or combination
therapy, the survival periods of patients is <9.8 months
(20,21).
The characteristics of pancreatic cancer include
reduced blood supply and the continuous exposure of cells to
hypoxic conditions (11). A
previous study determined the oxygen state of tumor cells in
patients with pancreatic cancer during surgery, and demonstrated
that all seven of the collected tumor tissues were in an hypoxic
state, whereas the adjacent normal pancreatic tissues were in a
normoxic state (22). The lack of
oxygen is usually due to excessive growth in tumor volume. The
inner tumor cells, particularly the tumor center, do not obtain
enough blood supply, which results in insufficient oxygen supply to
meet the growth needs of the tumor (22). In mouse models of pancreatic ductal
adenocarcinoma (PDAC), measurement of hypoxia following systemic
administration of pimonidazole (a chemical that becomes reduced in
low oxygen environments and binds to thiol-containing molecules
inside cells, forming adducts that can be detected by antibodies)
revealed the presence of frequent intratumoral hypoxic areas
(23). A similar result was
observed in orthotopic implants of human PDAC samples into mice
supporting the idea that intratumoral hypoxia is an important
component of the PDAC microenvironment (24). Hypoxia may induce alterations in
the biological characteristics of tumor cells, as well as changes
in the tumor microenvironment, the occurrence of drug resistance in
tumor cells. These alterations may lead to faster growth of tumor
cells and the enhanced invasiveness of tumor cells (25–27).
Therefore, a better therapeutic effect may be obtained using
therapeutic strategies against cellular characteristics under
hypoxic conditions. A potential reason for the failure of the
classical therapeutic approach may be explained by pancreatic
cancer's high metastatic potential (28). The occurrence of EMT can alter the
composition of the extracellular matrix, facilitating tumor
invasion and metastasis. EMT is one of the important mechanisms of
cell invasion, migration and secondary metastasis. The molecular
indicators for EMT are the decrease of epithelial markers,
including E-cadherin, and the increase in the levels of mesenchymal
markers, such as N-cadherin and vimentin (29). Presently, certain scholars consider
p53 to also be involved in the occurrence of EMT (30).
Hypoxia may induce changes in the biological
characteristics of tumor cells, as well as changes in the tumor
microenvironment, and the occurrence of drug resistance in tumor
cells. These changes may lead to faster growth of tumor cells and
the enhanced invasiveness of tumor cells (15–17).
Therefore, a better therapeutic effect may be obtained using
therapeutic strategies against cellular characteristics under
hypoxic conditions.
It was previously reported in preclinical
experiments that OBX is a BH3 peptide analogue that may be used as
to inhibit the growth of tumor cells and transplantation of tumors
(9). Other studies have
demonstrated that the BH3 analogue ABT-737 was able to exert its
effect on tumor cells more efficiently under hypoxic conditions
compared with cells treated in normoxic conditions (31,32).
OBX was reported to reduce the expression level of HIF-1α under
hypoxic conditions, thereby enhancing the sensitivity of colon
cancer cells to 5-fluorouracil treatment (33). However, whether OBX combined with
GEM was able to change the invasive phenotype of BxPC-3 pancreatic
cancer cells under hypoxic conditions and improve the efficiency of
GEM treatment remained unreported.
Results from the present study revealed that under
hypoxic conditions, OBX combined with a small dose of GEM was able
to promote the expression of E-cadherin and p53, reduce the
expression of vimentin, decrease the migratory and invasive
ability, thereby improving the efficiency of GEM.
In conclusion, the present experimental results
revealed that under hypoxic conditions, OBX combined with a small
dose of GEM may be able to inhibit the growth, migration and
invasion of pancreatic cancer cells, possibly via inhibition of EMT
process. However, identification of the specific mechanisms of
action requires further study.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng R, Zeng H, Zhang S and Chen W:
Estimates of cancer incidence and mortality in China, 2013. Chin J
Cancer. 36:662017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lau MK, Davila JA and Shaib YH: Incidence
and survival of pancreatic head and body and tail cancers: A
population-based study in the United States. Pancreas. 39:458–462.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borowa-Mazgaj B: Pancreatic
cancer-mechanisms of chemoresistance. Postepy Hig Med Dosw
(Online). 70:169–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol. 33:207–214. 2012. View Article : Google Scholar
|
|
8
|
Cosse JP and Michiels C: Tumour hypoxia
affects the responsiveness of cancer cells to chemotherapy and
promotes cancer progression. Anticancer Agents Med Chem. 8:790–797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaupel P, Thews O and Hoeckel M: Treatment
resistance of solid tumors: Role of hypoxia and anemia. Med Oncol.
18:243–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bendinelli P, Maroni P, Matteucci E and
Desiderio MA: Cell and signal components of the microenvironment of
bone metastasis are affected by hypoxia. Int J Mol Sci.
17:E7062016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuen A and Díaz B: The impact of hypoxia
in pancreatic cancer invasion and metastasis. Hypoxia (Auckl).
16:91–106. 2014.
|
|
12
|
Shore GC and Viallet J: Modulating the
bcl-2 family of apoptosis suppressors for potential therapeutic
benefit in cancer. Hematology Am Soc Hematol Educ Program.
2005.226–230. PubMed/NCBI
|
|
13
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geiger T, Sabanay H, Kravchenko-Balasha N,
Geiger B and Levitzki A: Anomalous features of EMT during
keratinocyte transformation. PLoS One. 3:e15742008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim T, Veronese A, Pichiorri F, Lee TJ,
Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, et al:
p53 regulates epithelial-mes-enchymal transition through microRNAs
targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang S, Okumura K and Sinicrope FA: BH3
mimetic obatoclax enhances TRAIL-mediated apoptosis in human
pancreatic cancer cells. Clin Cancer Res. 15:150–159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morgan MA, Meirovitz A, Davis MA, Kollar
LE, Hassan MC and Lawrence TS: Radiotherapy combined with
gemcitabine and oxaliplatin in pancreatic cancer cells. Transl
Oncol. 1:36–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vogel A, Römmler-Zehrer J, Li JS, McGovern
D, Romano A and Stahl M: Efficacy and safety profile of
nab-paclitaxel plus gemcitabine in patients with metastatic
pancreatic cancer treated to disease progression: A subanalysis
from a phase 3 trial MPACT). BMC Cancer. 16:8172016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gresham GK, Wells GA, Gill S, Cameron C
and Jonker DJ: Chemotherapy regimens for advanced pancreatic
cancer: A systematic review and network meta-analysis. BMC Cancer.
14:4712014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koong AC, Mehta VK, Le QT, Fisher GA,
Terris DJ, Brown JM, Bastidas AJ and Vierra M: Pancreatic tumors
show high levels of hypoxia. Int J Radiat Oncol Biol Phys.
48:919–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guillaumond F, Leca J, Olivares O, Lavaut
MN, Vidal N, Berthezène P, Dusetti NJ, Loncle C, Calvo E, Turrini
O, et al: Strengthened glycolysis under hypoxia supports tumor
symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma.
Proc Natl Acad Sci USA. 110:3919–3924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang Q, Jurisica I, Do T and Hedley DW:
Hypoxia predicts aggressive growth and spontaneous metastasis
formation from orthotopically grown primary xenografts of human
pancreatic cancer. Cancer Res. 71:3110–3120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YJ, Li L, Liang CQ, Sun XL, Jiang TF
and Ji RX: Expression of hypoxia-inducible factor-1 alpha and
apoptosis and proliferation in pancreatic cancer. Acta Aacademiae
Medical Qingdao Universitatis. 40:11–14. 2004.
|
|
26
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smith KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Copple BL: Hypoxia stimulates hepatocyte
epithelial to mesenchymal transition by hypoxia-inducible factor
and transforming growth factor-β-dependent mechanisms. Liver Int.
30:669–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keane MG, Bramis K, Pereira SP and Fusai
GK: Systematic review of novel ablative methods in locally advanced
pancreatic cancer. World J Gastroenterol. 20:2267–2278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rogers CD, Saxena A and Bronner ME: Sip1
mediates an E-cadherin-to-N-cadherin switch during cranial neural
crest EMT. J Cell Biol. 203:835–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Díaz-López A, Moreno-Bueno G and Cano A:
Role of microRNA in epithelial to mesenchymal transition and
metastasis and clinical perspectives. Cancer Manag Res. 6:205–216.
2014.PubMed/NCBI
|
|
31
|
Klymenko T, Brandenburg M, Morrow C, Dive
C and Makin G: The novel Bcl-2 inhibitor ABT-737 is more effective
in hypoxia and is able to reverse hypoxia-induced drug resistance
in neuroblastoma cells. Mol Cancer Ther. 10:2373–2383. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harrison LR, Micha D, Brandenburg M,
Simpson KL, Morrow CJ, Denneny O, Hodgkinson C, Yunus Z, Dempsey C,
Roberts D, et al: Hypoxic human cancer cells are sensitized to BH-3
mimetic-induced apoptosis via downregulation of the Bcl-2 protein
Mcl-1. J Clin Invest. 121:1075–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gariboldi MB, Taiana E, Bonzi MC,
Craparotta I, Giovannardi S, Mancini M and Monti E: The BH3-mimetic
obatoclax reduces HIF-1α levels and HIF-1 transcriptional activity
and sensitizes hypoxic colon adenocarcinoma cells to
5-fluorouracil. Cancer Lett. 364:156–164. 2015. View Article : Google Scholar : PubMed/NCBI
|