Introduction
Pemphigus vulgaris (PV) is a bullous skin disease
mediated by autoantibodies, primarily desmoglein (Dsg) 3 or/and
Dsg1 antibodies, and is considered a Th2 cell predominant
autoimmune disease. The balance of Th1/Th2 cells in the peripheral
blood plays an important role in the PV immunopathogenesis
(1). It has been widely
demonstrated that levels of Th1 cells and Th1 cytokines
(interferon-gamma (IFN-γ), IL-2) are decreased, whereas levels of
Th2 cells and Th2-type cytokines (IL-4, IL-10) are significantly
increased in the peripheral blood of PV patients (2,3). The
mean frequency of Th2 CD4+ T cells significantly elevates in active
disease (2). In contrast, Th1
cells show decreased levels in the acute stages of PV (1). Titers of Dsg3-reactive IgG are
directly related to the ratio of autoreactive Th1/Th2 cells
(1). Altogether, these results
suggest that the onset and extent of disease are related to an
imbalance of Th1/Th2 cells. However, to date, the exact mechanisms
underpinning this phenomenon remain unclear.
Currently, numerous studies have demonstrated that
altered expression of miRNAs also play roles in various autoimmune
diseases, including multiple sclerosis (4), systemic lupus erythematosus (SLE)
(5), rheumatoid arthritis
(6), and psoriasis (7). miRNAs are non-coding RNAs and their
aberrant expression is involved in various cellular processes,
including differentiation, apoptosis and immune response, by
suppressing target gene expression. However, it is no report
whether miRNAs also play a role in PV. Previously, we demonstrated
that there were 124 miRNAs aberrantly expressed in the peripheral
blood mononuclear cells (PBMCs) from PV patients after miRNA array
analysis (8). miR-338-3p has been
listed as one of the most significantly increased miRNAs with more
than a 500-fold change between PV patients and healthy control
samples. As reported, miR-338-3p was considered as a
tumor-suppressor and showed to play a role in various diseases,
including nasopharyngeal (9),
non-small cell lung (10) and
hepatocellular carcinoma (11),
gastric cancer (12) and breast
cancer (13), and esophageal
squamous cell carcinoma (14).
miR-338-3p also contributed to formation of basolateral polarity in
epithelial cells (15) as well as
differentiation of odontoblasts (16) and oligodendrocytes (17). In addition, decreased expression of
miR-338-3p was shown to increase expression of innate and adaptive
immune proteins in celiac disease (18). Thus, miR-338-3p can not only
regulate differentiation and apoptosis, but also possess
immunomodulatory functions. In PV, however, the impact of the
increased expression of miR-338-3p remains to be elucidated. In
this study, we investigated the role of miR-338-3p in PV and the
immune response.
Materials and methods
Patients and peripheral blood
samples
This study was approved by Research Center Ethics
Committee, Nanfang Hospital, The Southern Medical University
(Guangzhou, China) and the informed consent was obtained from all
participants. PV patients were recruited from Nanfang Hospital and
only new-onset patients or recurrent patients without
immunosuppressant and steroid hormone treatment for at least three
months were included in this study. Peripheral blood samples were
collected using EDTA anticoagulant tubes when patients were
admitted to our hospital. PBMCs from freshly drawn blood of
patients and healthy volunteers were purified by gradient
centrifugation with Ficoll-Paque Plus, and collected for RNA
isolation or transfection experiments.
RNA isolation, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
RNA was isolated from PBMCs using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and reversely transcribed into cDNA
using All-in-One First-Strand cDNA Synthesis kit (GeneCopoeia Inc.,
Germantown, MD, USA). qRT-PCR was performed with primers specific
for miR-338-3p or TNFR1-associated death domain protein (TRADD)
(Table I). 18srRNA and U6 gene
regions were used as controls. The qPCR was run on an ABI
PRISM® 7500 Sequence Detection System using SYBR-Green
PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan). The ΔΔCt method
was used to normalize transcripts to 18srRNA and U6 and to
calibrate the fold changes.
 | Table I.Polymerase chain reaction primers used
in this study. |
Table I.
Polymerase chain reaction primers used
in this study.
| miR-338-3p | RT primer |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAACAA-3′ |
|
| Forward |
5′-GTCAGTTCCAGCATCAGTGATT-3′ |
|
| Reverse |
5′-GTGCAGGGTCCGAGGT-3′ |
| TRADD | Forward |
5′-GGACCCTGAAACTCCACTTG-3′ |
|
| Reverse |
5′-GATGAAGTCCAGGACACCAA-3′ |
Transfection of miRNA into PBMCs
Oligonucleotides of miR-338-3p mimics, miR-338-3p
inhibitors, and negative control (NC or inhibitor NC) were
synthesized by RiboBio (Shanghai, China). For their transfection,
purified PBMCs were suspended in Opti-MEM (Invitrogen) and then
transfected with 50 nM of miR-338-3p mimics, miR-338-3p inhibitors
or negative control oligonucleotides using Lipofectamine™ RNAiMAX
(Invitrogen) for 4 h and after that, the medium was replaced with
RPMI-1640 (HyClone, Logan, UT, USA). Transfected cells were
harvested at different time points for analysis. Each transfection
experiment was carried out in triplicate.
ELISA detection of cytokine
levels
The blood samples were centrifuged for 5 min at
2,000 rpm, and the serum was saved for anti-Dsg3 antibody detection
using MESACUP Desmoglein TEST ‘Dsg3’ (Medical and Biological
Laboratories, Nagano-ken, Japan). In addition, 72 h after gene
transfection, PBMC culture medium was collected for detection of
T-type cytokines and levels of IFN-γ, IL-4 and IL-10 were also
measured by ELISA according to the instructions of manufacture of
human ELISA Kits (RayBiotech, Norcross GA, USA).
Cell viability CCK-8 assay
Transfected cells were seeded into 96-well plates at
1×104 cells/well and grown for 0, 24, 48 or 72 h,
respectively. At the end of each experiment, 10 µl of CCK-8
solution (dilution 1:10; CCK-8; Beyotime Institute of
Biotechnology, Shanghai, China) was added and incubated for 4 h.
The optical density of each well was then measured at the
wavelength of 450 nm (OD=450).
Identification of miRNA targets
The target genes of miR-338-3p were predicted using
three microRNA target databases (MiRanda, PITA and TargetScan). The
selected target genes were first validated by dual-luciferase assay
and then confirmed using qRT-PCR and western blot assays.
Dual-luciferase assay
HEK293 cells were cultured in 24-well plates for a
dual-luciferase reporter assay. Briefly, cells were co-transfected
with 50 nM of miRNA or miRNA NC and 500 ng of wild or mutant type
reporter plasmid (psi-CHECK2) using Lipofectamine 2000 reagent
(Invitrogen). Forty-eight hours later, cells were harvested and
lysed to measure Firefly and Renilla luciferase activities
using the Dual-Glo Luciferase Reporter Assay Kit (Promega, Madison,
WI, USA).
Western blotting
Cells were harvested and lysed for western blot
analysis after 48 gene transfection. Total crude proteins were
extracted from PBMC lysates and separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
electrophoresis. Proteins were then electrophoretically transferred
on to polyvinylidene fluoride (PVDF) membranes and the membranes
were incubated with the rabbit monoclonal antibody against human
TRADD at a dilution of 1:1,000 (Abcam, Cambridge, UK) at 4°C
overnight followed by horseradish peroxidase-conjugated secondary
Goat Anti-Rabbit IgG (1:5,000; Southern Biotech, Birmingham, AL,
USA) for 1 h at the room temperature. The immuno-complexes were
detected by chemiluminescence. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as a protein-loading control.
Statistical analysis
SPSS 21.0 software was used for all statistical
analyses. The data were presented as means ± standard deviation
(SD). Group comparisons were analyzed by Student's t-test or a
one-way analysis of variance (ANOVA) followed by LSD test. Pearson
method was used to analyze the correlation between mikR-338-3p and
PAAS or anti-Dsg3 antibody. A value of P<0.05 was considered
statistically significant.
Results
Association of elevated miR-338-3p
expression with PV severity
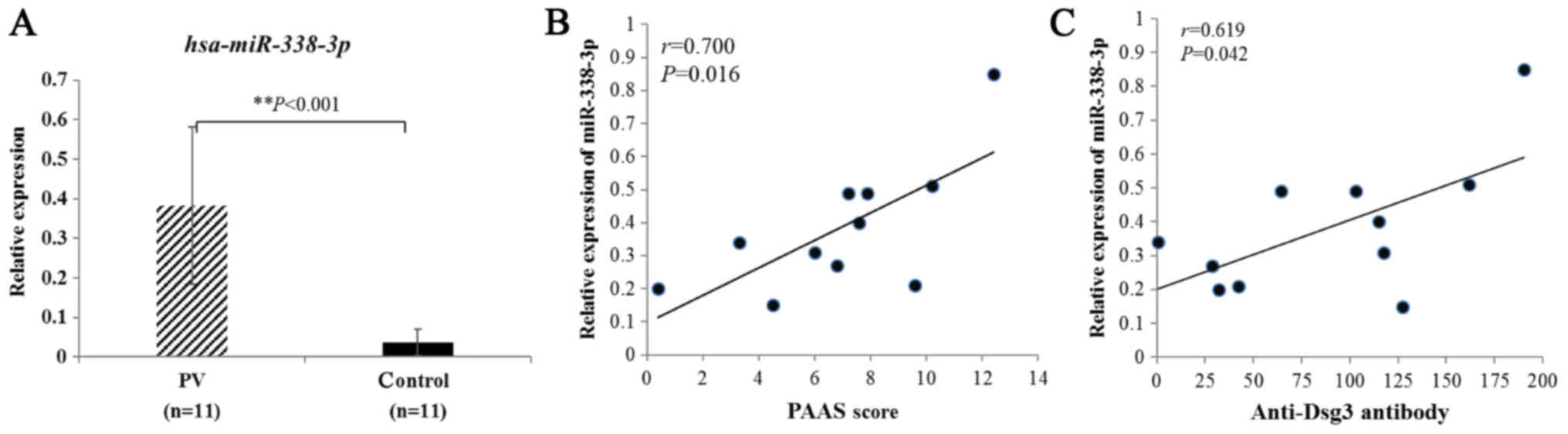
In this study, we first analyzed level of miR-338-3p
expression in PV vs. control sera using RT-qPCR. The results showed
that the average level of miR-338-3p relative expression from PV
patients was substantially higher than that from healthy controls
(Fig. 1A). We also found that
miR-338-3p levels positively correlated with Pemphigus Area and
Activity Score (PAAS) (Fig. 1B)
and anti-Dsg3 antibody titers (Fig.
1C). Seven initial cases and four recurrent cases were included
and no significance was found with respect to sex and age (Table II).
 | Table II.Baseline demographics and clinical
characteristic of PV patients and healthy volunteers. |
Table II.
Baseline demographics and clinical
characteristic of PV patients and healthy volunteers.
| Characteristic | Patients with PV
(n=11) | Healthy volunteers
(n=11) | P-value |
|---|
| Sex |
|
| 0.68 |
|
Female | 5 (45.5%) | 4 (36.4%) |
|
| Male | 6 (54.5%) | 7 (63.6%) |
|
| Age, years |
|
| 0.90 |
| Mean ±
SD | 44.27±12.65 | 43.64±11.35 |
|
| Median
(range) | 42 (26–64) | 45 (28–65) |
|
| Disease stages |
|
|
|
| Initial
stage | 7 (63.6%) | – |
|
|
Recurrent stage | 4 (36.4%) | – |
|
| Anti-Dsg3
antibodies, mean ± SD, | 87.48±57.07, 103
(0.63–170.3) | – |
|
| PAAS, median
(range), IQR |
|
|
|
| Cutaneous
score | 3.6 (0.4–12.4),
(1.2–5.95) | – |
|
| Mucus
membrane score | 6 (0–9), (0–6) | – |
|
| Total
scorea | 7.2 (0.4–12.4),
(5.25–8.25) | – |
|
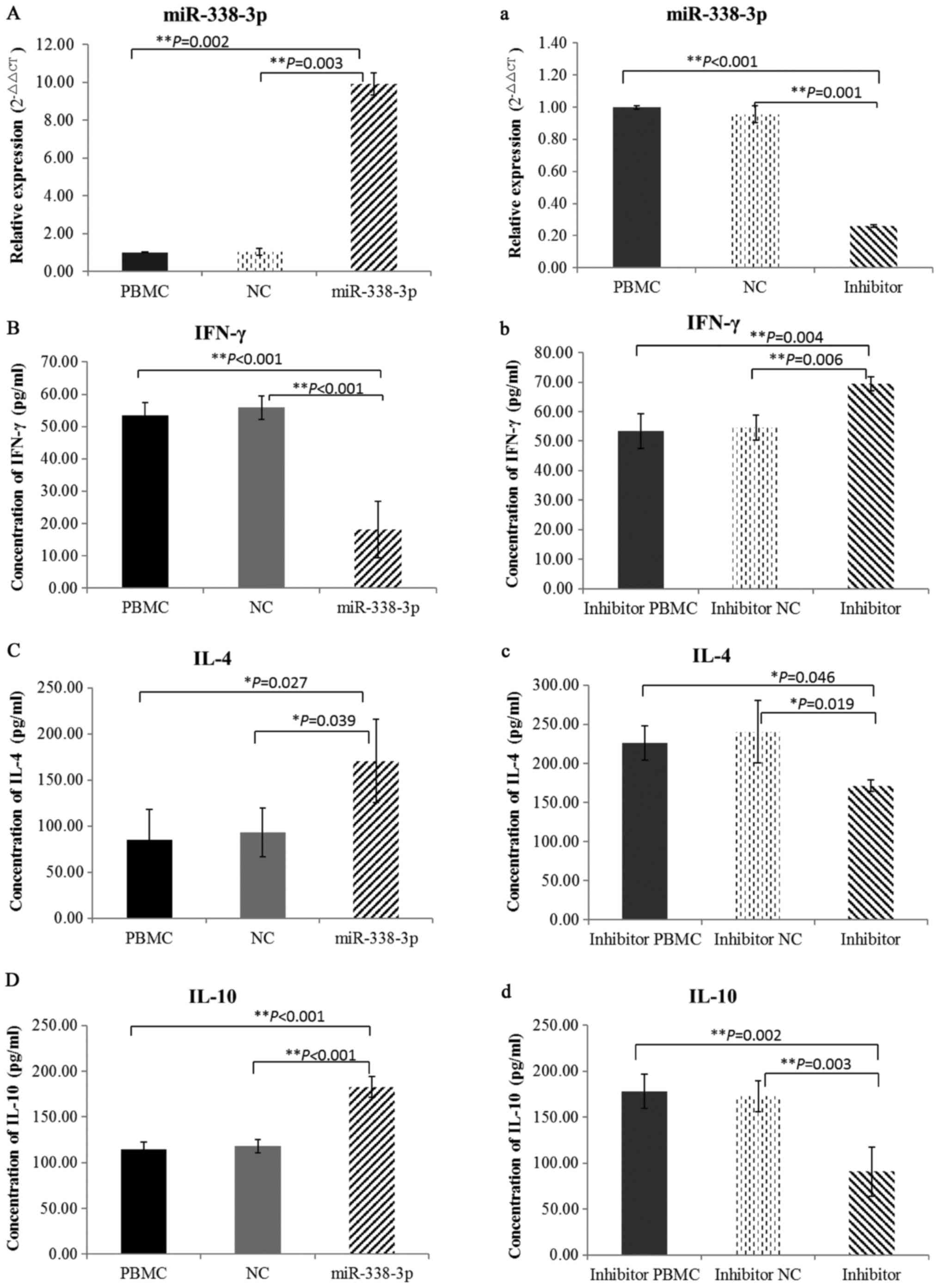
Effect of miR-338-3p on regulation of
Th1/Th2 cell functional balance in cultured cells
To ensure whether miR-338-3p was successfully
upregulated or downregulated in cultured cells, the expression of
miR-338-3p was detected by qRT-PCR after transfection with
miR-338-3p mimics, miR-338-3p inhibitor or negative control
oligonucleotides (Fig. 2A and
a).
To examine the levels of T lymphocyte cytokines, we
detected IFN-γ, IL-4 and IL-10 by ELISA. miR-388-3p overexpression
in cultured PBMCs from healthy individuals lead to significantly
decreased levels of IFN-γ (Fig.
2B) and the Th2 cytokines, IL-4 and IL-10, were markedly
increased (Fig. 2C and D).
However, when miR-388-3p was inhibited in cultured PBMCs from
patients, opposing findings were observed (Fig. 2b-d). Taken together, these results
suggested that miR-338-3p could regulate the balance of Th1/Th2
cells in PV patients.
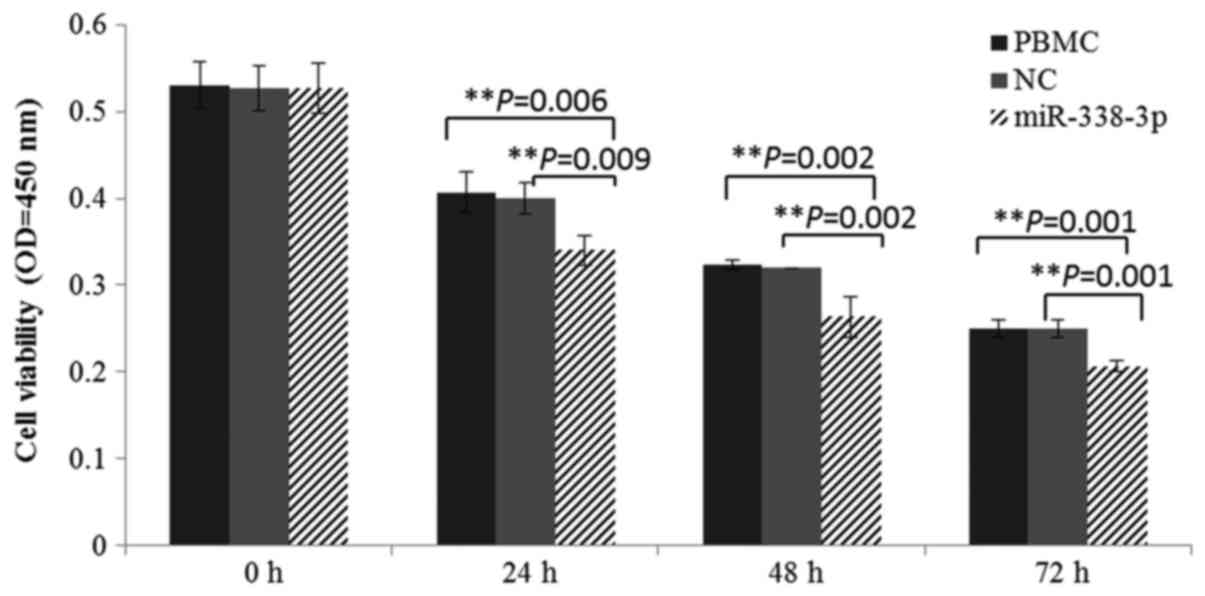
Effect of miR-338-3p on survival of
the cultured cells
To the best of our knowledge, increased expression
of miR-338-3p suppresses proliferation and differentiation in
tumorigenesis. During in vitro culture, cell activity was
markedly decreased after miR-338-3p mimic transfection. Cultured
cells, with a significantly lower viability in miR-338-3p group,
were also confirmed by CCK-8 analysis (Fig. 3). In summary, the overexpression of
miR-338-3p could suppress the survival of PBMCs.
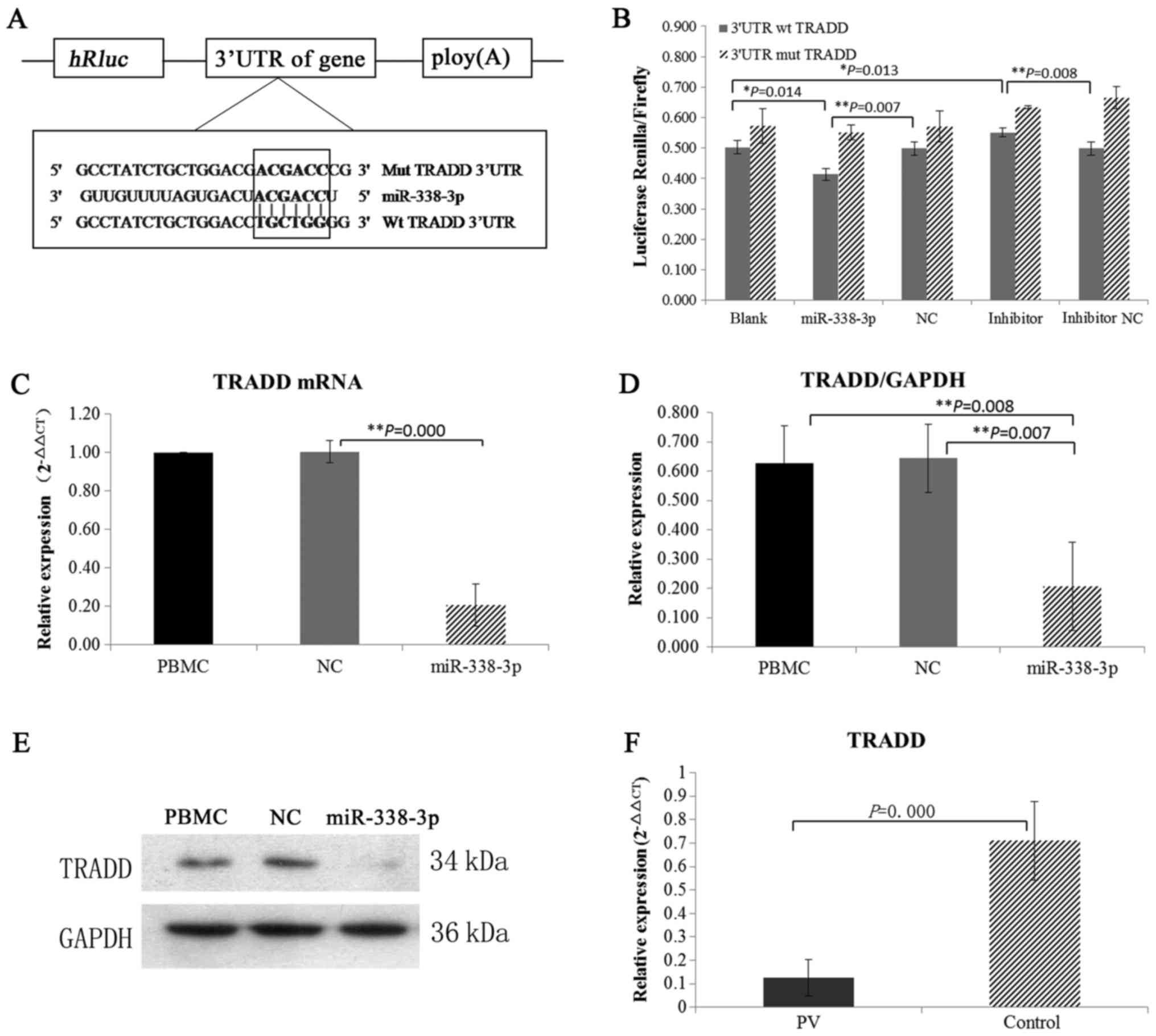
Identification of TRADD as a direct
and functional target of miR-338-3p
Target genes of miR-338-3p were predicted through
three databases (MiRanda, PITA and TargetScan), and TRADD, which
regulates cell proliferation and apoptosis, was identified as a
putative target. Mutation and wild type 3′UTR of TRADD were
conducted in Dual-Glo luciferase reporter assay and the results of
co-transfected of wt/mut 3′UTR with miRNAs demonstrated that
miR-338-3p directly targeted TRADD (Fig. 4A and B).
miR-338-3p directly targets TRADD in
PV
The changes of TRADD protein expression were
detected when miR-338-3p was overexpressed in vitro. The
levels of TRADD mRNA and protein were found to be significantly
lower in the miR-338-3p group compared with PBMC group and control
group (Fig. 4C-E). We also
examined the expression of TRADD in vivo. We found that mRNA
expression of TRADD was decreased in PV patients when miR-338-3p
was overexpressed (Fig. 4F).
Altogether, these findings suggested that miR-338-3p directly
targets TRADD in PV.
Discussion
PV is an intractable autoimmune bullous disease, and
the mechanism of the main pathogenic antibody Dsg3, remains
elusive. As mentioned earlier, miR-338-3p has been previously
suggested to play a role in the pathogenesis of PV. Firstly,
increased miR-338-3p expression in PV compared to controls was
demonstrated (Fig. 1A). In
addition, the expression of miR-338-3p showed a significant
positive correlation with PAAS scores (Fig. 1B) and anti-Dsg3 antibody titers
(Fig. 1C), which are known to be
positively associated with disease extent in PV. Hence, we
speculated that miR-338-3p may play a role in the synthesis of Dsg3
antibody.
To study the functions of miR-338-3p in the
production of Dsg3 antibody, the experiments of miR-338-3p
overexpressed in healthy PBMCs and inhibited in PV PBMCs were
conducted, respectively. Results showed that increased expression
of miR-338-3p downregulated IFN-γ production, and upregulated IL-4
and IL-10 (Fig. 2b-d). However,
opposing findings were demonstrated when miR-338-3p was inhibited
in PV PBMCs (Fig. 2b-d). These
results imply that altered expression of miR-338-3p may be a
trigger factor in the imbalance of Th1/Th2 cells in autoimmunity.
As previously demonstrated, IFN-γ insufficiency and over secretion
of IL-4 and IL-10, usually representing the altered proportion of
Th1 and Th2 respectively, has been confirmed in peripheral blood of
patients with PV (1–3). The balance of Th1/Th2 cells in an
immune response plays a pivotal regulative role in the production
of Dsg3 antibody, though it is directly secreted by B cells
(19). Taken together, elevated
expression of miR-338-3p contributes to the production of Dsg3
antibody by mediating an abnormal balance of Th1/Th2 cells in
PV.
miRNA-338-3p is known to be a suppressor in tumor
cell proliferation. In miR-338-3p overexpression experiments, the
declined cultured cell activity was observed in miR-338-3p group
(Fig. 3), suggesting that
increased expression of miR-338-3p suppressed in vitro
survival. The imbalance of Th1/Th2 cells in PV may result from an
unbalanced inhibition by elevated miR-338-3p between Th1 and Th2
cells. However, miR-338-3p cannot directly mediate cell
proliferation or apoptosis. Thus, the potential and functional
target genes of miR-338-3p were further predicted through three
databases (MiRanda, PITA and TargetScan), and TRADD was identified
as a putative target (Fig. 4A and
b). TRADD was initially identified as an adaptor molecule,
transducing the signal downstream of tumor necrosis factor receptor
1 (TNFR1) that induces either apoptosis or proliferation (20). The death domain of TRADD can
recruit FADD or RIP, interacting with TRAF2, leading to apoptosis
or the activation of NF-κB pathway protecting against cell death.
TRADD is the key transduction molecule for apoptosis or
proliferation, but may not be required for the induction of
TNF-induced apoptosis (21). It
may play a protective role against apoptosis in TRAIL/TRAIL-R
signaling (22). In addition,
TRADD contributes to the formation of the membrane survival
TRADD-RIP1-TRAF2 complex I leading to proliferation. As a result,
insufficiency of TRADD induces apoptosis but not proliferation
(23). In miR-338-3p
overexpression in vitro expression, we observed that the
mRNA and protein expression of TRADD was significantly
downregulated in response to miR-338-3p overexpression (Fig. 4C-E). In addition, mRNA expression
of TRADD was also downregulated in PV patients in vivo
(Fig. 4F). These results imply
that increased miR-338-3p regulates the imbalance of Th1/Th2 cells
by directly suppressing the function of TRADD.
In conclusion, we found that miR-338-3p was
significantly elevated in PV patients and positively correlated
with disease severity. Increased expression of miR-338-3p
contributed to the production of Dsg3 antibody by inhibiting the
expression of TRADD to induce an imbalance of Th1/Th2 cells. Taken
together, we, for the first time, revealed the novel mechanism of
miR-338-3p to further the understanding of the pathogenesis of
PV.
Acknowledgements
This work was supported in part by a grant from the
National Natural Science Foundation of China (grant no.
81171627).
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PV
|
pemphigus vulgaris
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
Th
|
T helper cells
|
|
IFN-γ
|
interferon-gamma
|
|
IL
|
interleukin
|
|
TRADD
|
TNFR1-associated death domain
protein
|
|
NC
|
negative control
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
Dsg
|
desmoglein
|
|
miRNA
|
microRNA
|
|
CCK-8
|
Cell-Counting Kit-8 assay
|
|
PVDF
|
polyvinylidene fluoride
|
|
ANOVA
|
one-way analysis of variance
|
|
PAAS
|
pemphigus area and activity score
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
TRAF2
|
TNF receptor-associated factor 2
|
|
TNFR1
|
tumor necrosis factor receptor 1
|
References
|
1
|
Hertl M and Veldman C: T-cellular
autoimmunity against desmogleins in pemphigus, an
autoantibody-mediated bullous disorder of the skin. Autoimmun Rev.
2:278–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizzo C, Fotino M, Zhang Y, Chow S,
Spizuoco A and Sinha AA: Direct characterization of human T cells
in pemphigus vulgaris reveals elevated autoantigen-specific Th2
activity in association with active disease. Clin Exp Dermatol.
30:535–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satyam A, Khandpur S, Sharma VK and Sharma
A: Involvement of T(H)1/T(H)2 cytokines in the pathogenesis of
autoimmune skin disease-Pemphigus vulgaris. Immunol Invest.
38:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandhi R: miRNA in multiple sclerosis:
Search for novel biomarkers. Mult Scler. 21:1095–1103. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stagakis E, Bertsias G, Verginis P, Nakou
M, Hatziapostolou M, Kritikos H, Iliopoulos D and Boumpas DT:
Identification of novel microRNA signatures linked to human lupus
disease activity and pathogenesis: miR-21 regulates aberrant T cell
responses through regulation of PDCD4 expression. Ann Rheum Dis.
70:1496–1506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Churov AV, Oleinik EK and Knip M:
MicroRNAs in rheumatoid arthritis: Altered expression and
diagnostic potential. Autoimmun Rev. 14:1029–1037. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Langkilde A, Raaby L, Johansen C and
Iversen L: MicroRNA normalization candidates for quantitative
reverse-transcriptase polymerase chain reaction in real time in
lesional and nonlesional psoriatic skin. Br J Dermatol.
169:677–681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang M, Liang L, Li L, Han K, Li Q, Peng
Y, Peng X and Zeng K: Increased miR-424-5p expression in peripheral
blood mononuclear cells from patients with pemphigus. Mol Med Rep.
15:3479–3484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shan Y, Li X, You B, Shi S, Zhang Q and
You Y: MicroRNA-338 inhibits migration and proliferation by
targeting hypoxia-induced factor 1α in nasopharyngeal carcinoma.
Oncol Rep. 34:1943–1952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun J, Feng X, Gao S and Xiao Z:
microRNA-338-3p functions as a tumor suppressor in human
non-small-cell lung carcinoma and targets Ras-related protein 14.
Mol Med Rep. 11:1400–1406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang G and Sun Y, He Y, Ji C, Hu B and Sun
Y: MicroRNA-338-3p inhibits cell proliferation in hepatocellular
carcinoma by target forkhead box P4 (FOXP4). Int J Clin Exp Pathol.
8:337–344. 2015.PubMed/NCBI
|
|
12
|
Huang N, Wu Z, Lin L, Zhou M, Wang L, Ma
H, Xia J, Bin J, Liao Y and Liao W: MiR-338-3p inhibits
epithelial-mesenchymal transition in gastric cancer cells by
targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget.
6:15222–15234. 2015.PubMed/NCBI
|
|
13
|
Jin Y, Zhao M, Xie Q, Zhang H, Wang Q and
Ma Q: MicroRNA-338-3p functions as tumor suppressor in breast
cancer by targeting SOX4. Int J Oncol. 47:1594–1602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Li Z, Yang G and Pan Z:
MicroRNA-338-3p suppresses tumor growth of esophageal squamous cell
carcinoma in vitro and in vivo. Mol Med Rep.
12:3951–3957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuchiya S, Oku M, Imanaka Y, Kunimoto R,
Okuno Y, Terasawa K, Sato F, Tsujimoto G and Shimizu K:
MicroRNA-338-3p and microRNA-451 contribute to the formation of
basolateral polarity in epithelial cells. Nucleic Acids Res.
37:3821–3827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Q, Liu H, Lin H, Yuan G, Zhang L and
Chen Z: MicroRNA-338-3p promotes differentiation of mDPC6T into
odontoblast-like cells by targeting Runx2. Mol Cell Biochem.
377:143–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebrahimi-Barough S, Massumi M,
Kouchesfahani HM and Ai J: Derivation of pre-oligodendrocytes from
human endometrial stromal cells using overexpression of microRNA
338. J Mol Neurosci. 51:337–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magni S, Comani Buoli G, Elli L, Vanessi
S, Ballarini E, Nicolini G, Rusconi M, Castoldi M, Meneveri R,
Muckenthaler MU, et al: miRNAs affect the expression of innate and
adaptive immunity proteins in celiac disease. Am J Gastroenterol.
109:1662–1674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan M, Zhu H and Xu R: Immune cellular
regulation on autoantibody production in pemphigus. J Dermatol.
42:11–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pobezinskaya YL and Liu Z: The role of
TRADD in death receptor signaling. Cell Cycle. 11:871–876. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin Z and El-Deiry WS: Distinct signaling
pathways in TRAIL-versus tumor necrosis factor-induced apoptosis.
Mol Cell Biol. 26:8136–8148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao X, Pobezinskaya YL, Morgan MJ and Liu
ZG: The role of TRADD in TRAIL-induced apoptosis and signaling.
FASEB J. 25:1353–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Micheau O and Tschopp J: Induction of TNF
receptor I-mediated apoptosis via two sequential signaling
complexes. Cell. 114:181–190. 2003. View Article : Google Scholar : PubMed/NCBI
|