Introduction
Osteoporosis is a common clinical disease exerting
tremendous emotional, economic and social repercussions on patients
and their families (1).
Traditional pharmacological agents either promoting bone formation
(e.g., parathyroid hormone) or inhibiting bone resorption (e.g.,
calcitonin, estrogen and bisphosphonate) may contribute to the
prevention and reversal of osteoporosis to a certain extent;
however, undesirable side effects, including metrorrhagia,
esophagitis and mammary cancer, may occur simultaneously (2,3).
Despite extensive research in experimental studies and preclinical
trials of other novel pharmacological agents (e.g., denosumab,
odanacatib and saracatinib), the potential mechanisms and possible
side effects are not fully understood (4–6).
Bonnick et al (7) found
that odanacatib is a selective cathepsin K inhibitor being
developed for the treatment of osteoporosis, but it increased the
risk of atrial fibrillation and stroke. Furthermore, certain drugs
are too expensive for patients to afford due to the long courses of
drug treatment required.
Since the first application of pulsed
electromagnetic fields (PEMFs) in accelerating clinical bone
fracture healing in 1974 (8,9), the
biological effects of PEMFs have gained considerable attention in
orthopaedic research. Over the past four decades, studies regarding
electromagnetic fields on different cells and animals have been
extensively described. Accumulating evidence has revealed that PEMF
stimulation potently promotes osteogenesis and enhances bone
mineralization both in vivo and in vitro (10–12).
Other studies have indicated that electromagnetic field treatment
exerts protective effects on human bone marrow mesenchymal stem
cells (13). Kang et al
(14) suggested that the
osteogenic differentiation of adipose-derived stem cells and bone
regeneration is accelerated by PEMF stimulation. He et al
(15) demonstrated that PEMFs
significantly reduce the number of osteoclast-like cells in the
culture with macrophage colony stimulating factor (M-CSF) and
receptor activator of nuclear factor-κB ligand (RANKL), which
indicates the potential role of PEMFs in osteoporosis. Despite the
considerable beneficial effects of PEMF on osteoporosis (16,17),
the underlying mechanism remains to be fully elucidated, which may
impose restrictions for the clinical application of PEMFs.
The pivotal role of osteoclasts in bone defects and
osteoporosis have resulted in their becoming a key therapeutic
target in osteoporosis (18).
Previous studies have investigated the effects of PEMFs on
osteoblasts and osteoclasts. The results have demonstrated that
PEMFs inhibit the differentiation of osteoclasts and facilitate the
formation of osteoblasts, the underlying mechanisms of which are
different (19,20). The promotion of osteoblast
differentiation by PEMFs is primarily focused on the bone
morphogenetic protein and the Wnt/β-catenin signaling pathways, the
activation of which facilitates osteoblast differentiation, and
improves bone microstructure and strength (21,22).
Various studies have suggested that PEMF may suppress osteoclast
differentiation by regulating certain pathways in the
RANK/RANKL/osteoprotegerin (OPG) signaling system (23), of which the protein kinase B
(Akt)/mammalian target of rapamycin (mTOR) signaling pathway may be
key (24). The present study aimed
to elucidate the effects of PEMFs on RANKL-induced osteoclast
differentiation from RAW264.7 macrophages and to explore the
potential mechanisms involving the intracellular Akt/mTOR signaling
pathway during this process.
Materials and methods
Cell culture and reagents
RAW264.7 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA; 4.500 mg/l D-glucose) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) for
maintaining cell growth or in α-minimum essential medium (α-MEM;
Gibco; Thermo Fisher Scientific, Inc., 1.000 mg/l D-glucose)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific Inc.)
for inducing cell differentiation, at 37°C in an environment
containing 5% CO2. Recombinant mouse RANKL was obtained
from R&D Systems (Minneapolis, MN, USA). Cell Counting Kit-8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
(Kumamoto, Japan) and a tartrate-resistant acid phosphatase (TRAP)
staining kit was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Bovine bone slices were obtained from Third
Military Medical University (Chongqing, China). cDNA Synthesis kits
and All-in-One qPCR Mix were purchased from GeneCopoeia (Rockville,
MD, USA). Antibodies against Akt, mTOR, ribosome S6 protein kinase
(p70S6K), phosphorylated (p)-Akt, p-mTOR, p-p70S6K, nuclear factor
of activated T-cells 1 (NFATc1), GAPDH and anti-rabbit horseradish
peroxidase-conjugated antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies against matrix
metallopeptidase (MMP)-9, TRAP and cathepsin K (CTSK) were
purchased from Proteintech (Rocky Hill, NJ, USA). Inhibitors
perifosine and rapamycin were purchased from Selleck Chemicals
(Houston, TX, USA).
PEMF stimulation
To explore the effects of PEMFs on osteoclast
differentiation, an electromagnetic field device was used, as
previously described (25). Based
on previous studies and the preliminary work of the present study,
the appropriate parameters of PEMFs with 50 Hz and 1 mT were
selected. Briefly, RAW264.7 macrophages were exposed to PEMFs in a
system formed by Helmholtz coils (inner diameter of ~30 cm) that
oriented to produce a sinusoidal PEMF with 50 Hz and 1 mT. All
studies were conducted in a humidified incubator at 37°C under 5%
CO2 for 4 h per day. The control group was cultured in a
separate incubator under the same conditions without exposure to
PEMFs. Medium was changed every 2 days.
CCK-8 assay for cell viability
Cell viability was tested using a CCK-8 assay.
RAW264.7 macrophages were seeded at a density of 2×103
cells/well onto a 96-well culture plate in DMEM. Following the
overnight incubation for attachment to the wall, the culture medium
was changed to α-MEM and cells were cultured further in a
humidified incubator with RANKL (50 ng/ml), RANKL (50 ng/ml) +
PEMF, perifosine (2.5 µM), rapamycin (1 µM) and PEMFs for 4 days. A
CCK-8 assay was carried out every 24 h (24, 48, 72 and 96 h)
according to the manufacturer's protocol. CCK-8 reagent was added
to each well, followed by incubation for 4 h, prior to absorbance
being measured at a wavelength of 450 nm using a microplate
reader.
TRAP staining and activity
RAW264.7 macrophages were seeded at a density of
1.5×104 cells/well onto a 24-well culture plate in DMEM
overnight. Then, the medium was changed to α-MEM with the addition
of RANKL (50 ng/ml) or processed with RANKL + PEMF (50 Hz, 1 mT)
for 4 days to induce osteoclast differentiation. Then, cells were
fixed with 3.7% formaldehyde for 30 sec and were stained with TRAP
staining solution (45 ml deionized water, 0.5 ml Fast Garnet GBC
Base solution, 0.5 ml sodium nitrite solution, 0.5 ml naphthol
AS-BI phosphate solution, 2 ml acetate solution and 1 ml tartrate
solution) according to the manufacturer's protocol. TRAP-positive
osteoclasts containing three or more nuclei were counted in 12
wells/plate. This counting was repeated five times. TRAP activity
was measured from osteoclast culture supernatants using a TRAP
Staining kit. In brief, supernatants (30 µl per well) were
incubated with the chromogenic substrates (170 µl) in a
tartrate-containing buffer for 3 h at 37°C. Then, absorbance was
measured to determine TRAP activities at a wavelength of 540 nm
(26).
Bone resorption assay
Cells were seeded at a density of 1.5×104
cells/well onto a 48-well plate covered with bovine bone slices.
RAW264.7 macrophages were then induced with RANKL (50 or 100 ng/ml)
for 8 days with or without PEMF exposure. Following 8 days of
culture, the bovine bone slices were washed with 5% sodium
hypochlorite solution and toluidine blue staining was performed. To
quantify the osteoclastic bone resorption, the resorbed pit areas
were confirmed under a light microscope and identified by Image
pro-plus (version 6.0).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Cells were washed with DPBS, and total RNA was
extracted using 1 ml TRIzol reagent. Samples homogenized in TRIzol
reagent were then extracted using 0.2 ml chloroform. Following
centrifugation at 10,000 rpm at 4°C for 15 min, the supernatant
containing RNA was transferred into a new vial and RNA was
precipitated by adding 500 µl isopropanol. The supernatant was
discarded following incubation for 10 min and centrifugation at
10,000 rpm for 10 min. The pellet was washed with 1 ml 75% ethanol
and centrifuged for 5 min at 7,000 rpm. The supernatant was then
discarded and the pellet was dried. After adding 30 µl
diethylpyrocarbonate (DEPC)-treated water, the pellet was dissolved
at 85°C for 10 min. Total RNA (1 µg) was then reverse transcribed
with the cDNA synthesis kit to obtain cDNA, using the following
temperature protocol: 37°C for 60 min, followed by 98°C for 5 min.
qPCR was conducted in an ABI StepOnePlus Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using
ALL-in-One qPCR Mix. The thermocycling conditions were as follows:
95°C for 10 min followed by 95°C for 10 sec, then 45 cycles of 60°C
for 20 sec and 72°C for 15 sec. The relative expression of genes
was calculated using the 2−ΔΔCq method and all results
were normalized to GAPDH (27).
The sequences of the forward and reverse primers were as follows:
GAPDH, ACCACAGTCCATGCCATCAC and TCCACCACCCTGTTGCTGTA; NFATc1,
GGTAACTCTGTCTTTCTAACCTTAAGCTCandGTGATGACCCCAGCATGCACCAGTCACAG;
MMP-9, CGCTCATGTACCCGCTGTAT and TGTCTGCCGGACTCAAAGAC; TRAP,
CTGGAGTGCACGATGCCAGCGACA and TCCGTGCTCGGCGATGGACCAGA; and CTSK,
AGGCAGCTAAATGCAGAGGGTACA and ATGCCGCAGGCGTTGTTCTTATTC.
Western blotting
Cells were treated for the indicated time with
various treatments. Then, western blotting was performed according
to standard procedures. Cells were rinsed with PBS and harvested in
radioimmunoprecipitation assay buffer containing protease and
phosphatase inhibitors. Following incubation on ice for 30 min, the
cell lysates were centrifuged at 12,000 rpm for 20 min and protein
precipitations were collected. Proteins (20 ug) were separated on
SDS-PAGE (8–12% gels) and devolved to nitrocellulose membranes. The
membranes were blocked with 5% bovine serum albumin in TBST
containing 0.1% Tween-20 at room temperature for an hour and probed
successively with rabbit primary antibodies against the following:
MMP-9 (1:1,000), TRAP (1:1,000), CTSK (1:500), NFATc1 (1:1,000),
Akt (1:1,000), p-Akt (1:2,000), mTOR (1:1,000), p-mTOR (1:1,000),
p70S6K (1:1,000), p-p70S6K (1:1,000), β-actin (1:1,000) and GAPDH
(1:2,000) primary antibodies overnight at 4°C. Horseradish
peroxidase-conjugated goat anti-rabbit IgG antibodies were used as
secondary antibodies at room temperature for an hour. The signals
were detected by exposure in an enhanced chemiluminscence system
system and then analyzed using ImageJ software (version 1.46).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using Student's
t-tests or one-way analysis of variance, followed by
Student-Newman-Keuls post hoc test, using SPSS software, version
20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
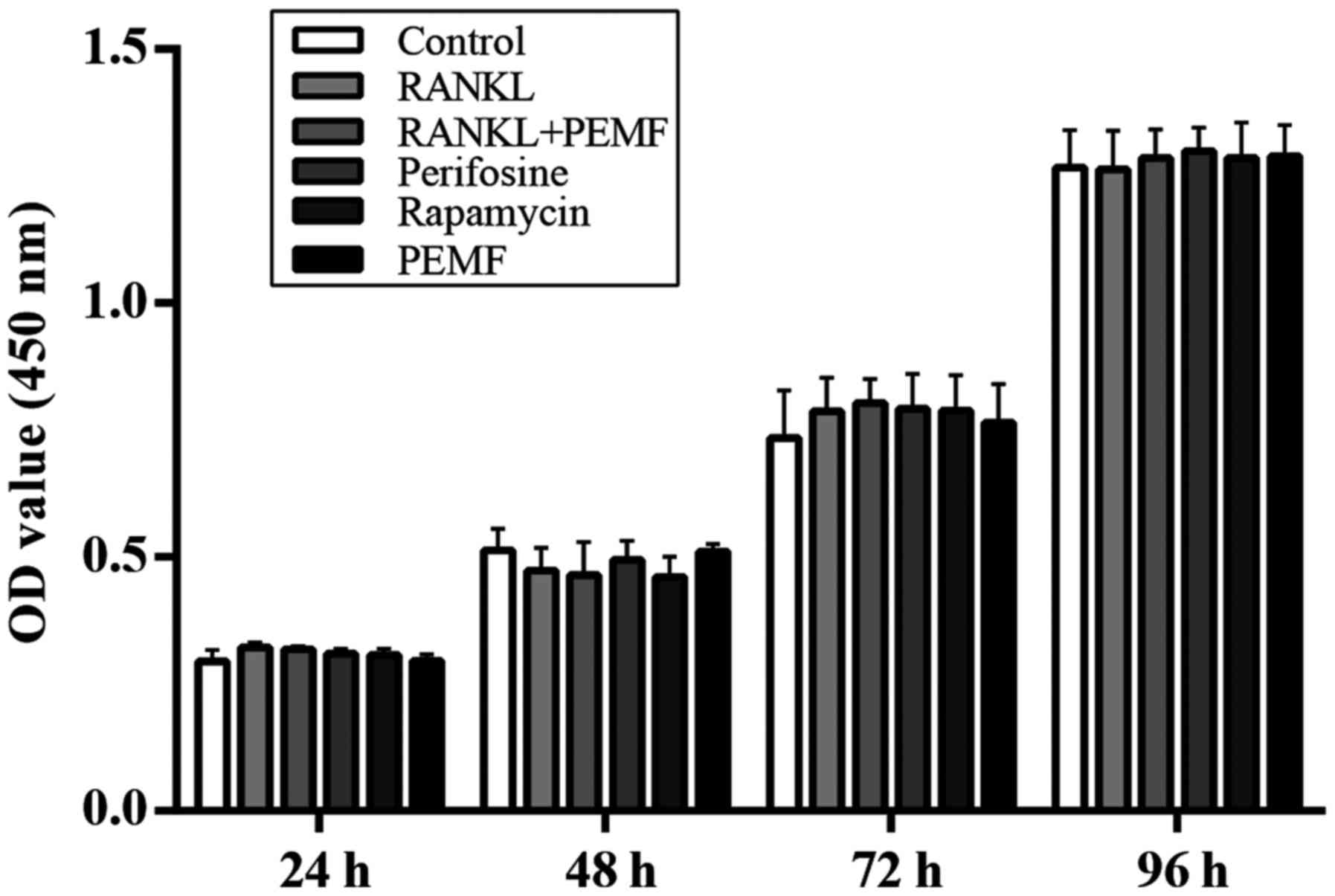
Effect of PEMF on cell viability
To investigate whether RANKL, PEMF or
pharmacological inhibitors influenced cell viability, a CCK-8 assay
was performed. RAW264.7 macrophages were cultured with different
stimuli for 4 days. The 450 nm absorbance was detected every 24 h
to assess cell viability post-incubation with the CCK-8 reagent. As
presented in Fig. 1, over the four
day period, there was no significant difference observed among the
different groups, with respect to cell viability, suggesting that
RANKL, PEMF and pharmacological inhibitors had no cytotoxic effects
on osteoclast precursor cells.
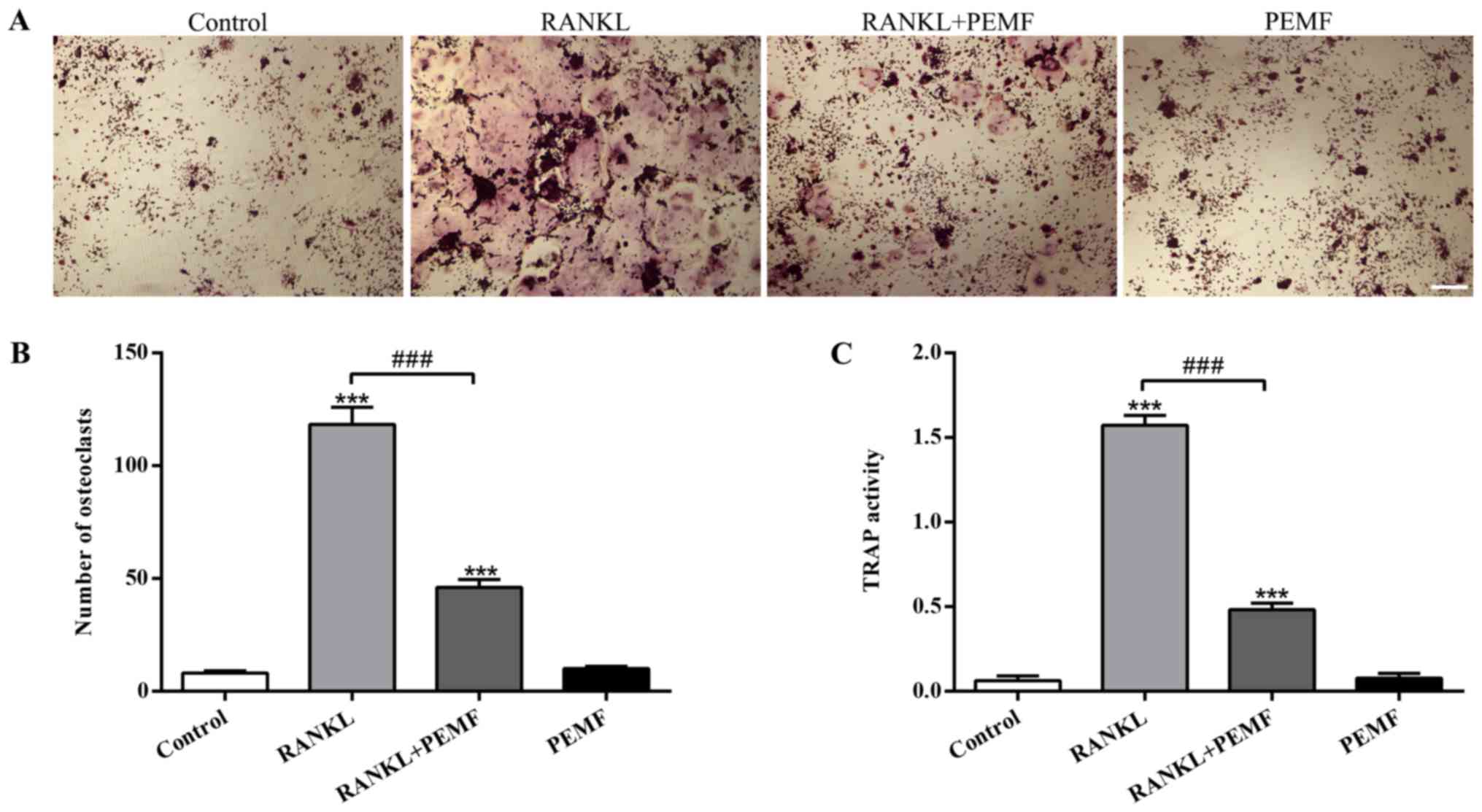
Effect of PEMF on osteoclast formation
and TRAP activity
TRAP enzyme is highly expressed and secreted in
mature osteoclasts, and functions as a secure indicator for
osteoclast formation (28). To
explore the influence of PEMF on osteoclast formation, TRAP
staining and measurement of TRAP activity was conducted.
Osteoclasts containing three or more nuclei were categorized as
TRAP-positive cells. When treated with RANKL, the number of giant
osteoclasts containing multiple nuclei was markedly increased
compared with the control (Fig.
2A). However, the number of multinuclear osteoclasts induced by
RANKL was significantly decreased by PEMF application (Fig. 2B). Results of the TRAP activity
assay also demonstrated that RANKL treatment resulted in
enhancement of TRAP activity, while this facilitating effect was
attenuated by PEMF application (Fig.
2C). The suppression of multinucleated osteoclast formation and
TRAP activity during osteoclast differentiation suggested that PEMF
decreased osteoclastogenesis via osteoclast precursor (RAW264.7
macrophages) fusion inhibition.
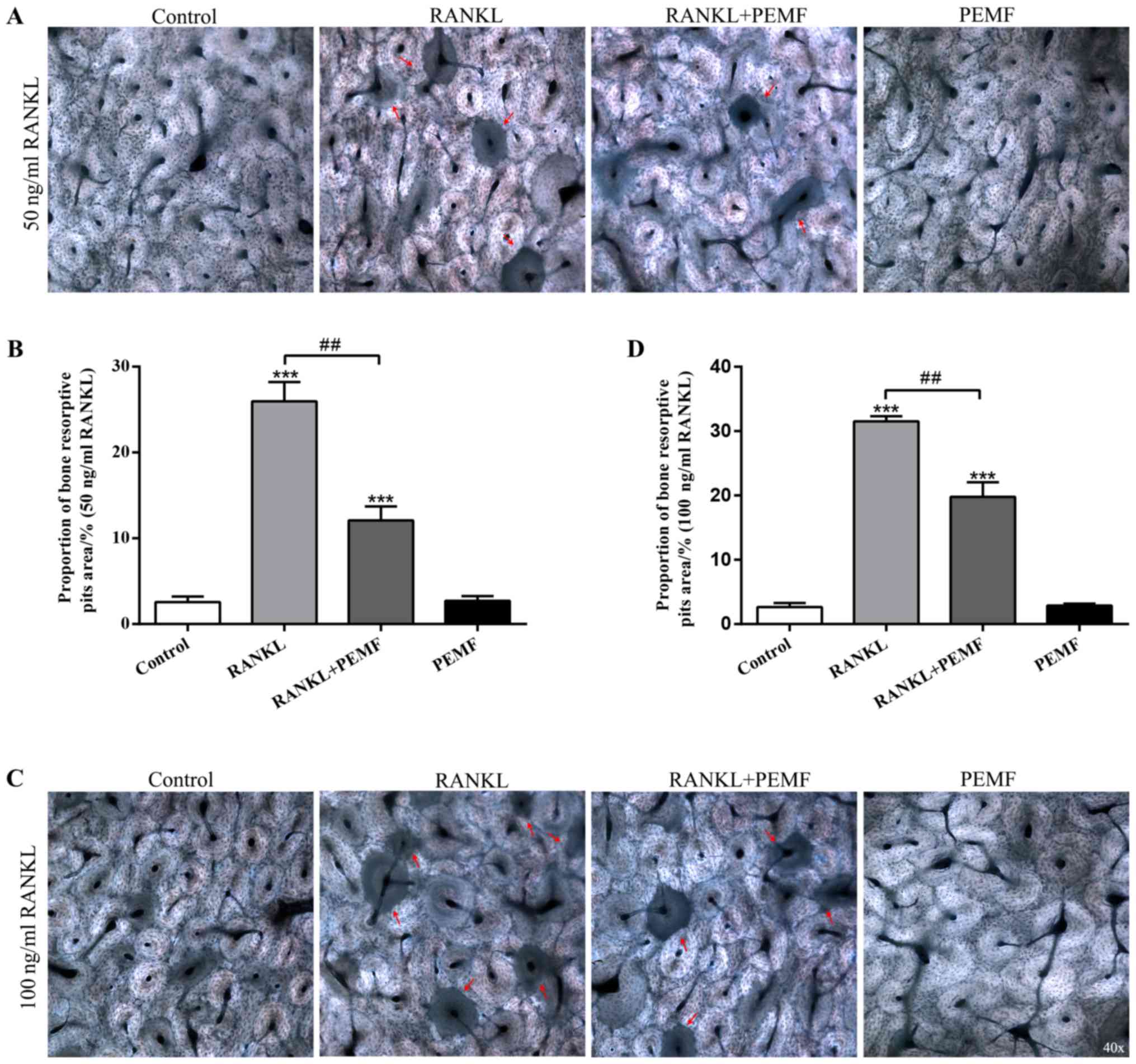
Effect of PEMF on osteoclastic bone
resorption in vitro
Mature osteoclasts function to absorb bone matrix,
resulting in an irregular surface of the matrix (29). The present study investigated
whether PEMF could inhibit osteoclastic bone resorption in
vitro (Fig. 3). RANKL-induced
osteoclasts resulted in resorption pits on bovine bone slices
(Fig. 3A). The data also revealed
that bone resorptive pit areas increased with a higher
concentration of RANKL (100 ng/ml; Fig. 3C). However, the bone resorption of
osteoclasts induced by RANKL was markedly attenuated by exposure to
50 Hz PEMFs (Fig. 3B and D), which
was consistent with the inhibitory effects of PEMFs on osteoclast
formation, and in turn implied the active bone resorption activity
of multinucleated osteoclasts. These results indicated that PEMF
repressed the bone resorption activity of osteoclasts in
vitro.
Effect of PEMF on osteoclastic
differentiation
To further elucidate the role of PEMF in osteoclast
differentiation, the present study examined the expression of
osteoclastic marker genes during osteoclastogenesis using RT-qPCR.
The expression of NFATc1, one of the osteoclast-specific
transcription factors, as well as that of three other osteoclastic
specific genes, MMP-9, TRAP and CTSK, was upregulated upon
treatment with RANKL. However, this was significantly decreased by
the addition of PEMF (Fig. 4A-D).
This PEMF-regulated expression of osteoclastogenic markers were
further confirmed by western blotting (Fig. 4E and F). Collectively, these data
supported the inhibition of osteoclast formation by PEMFs.
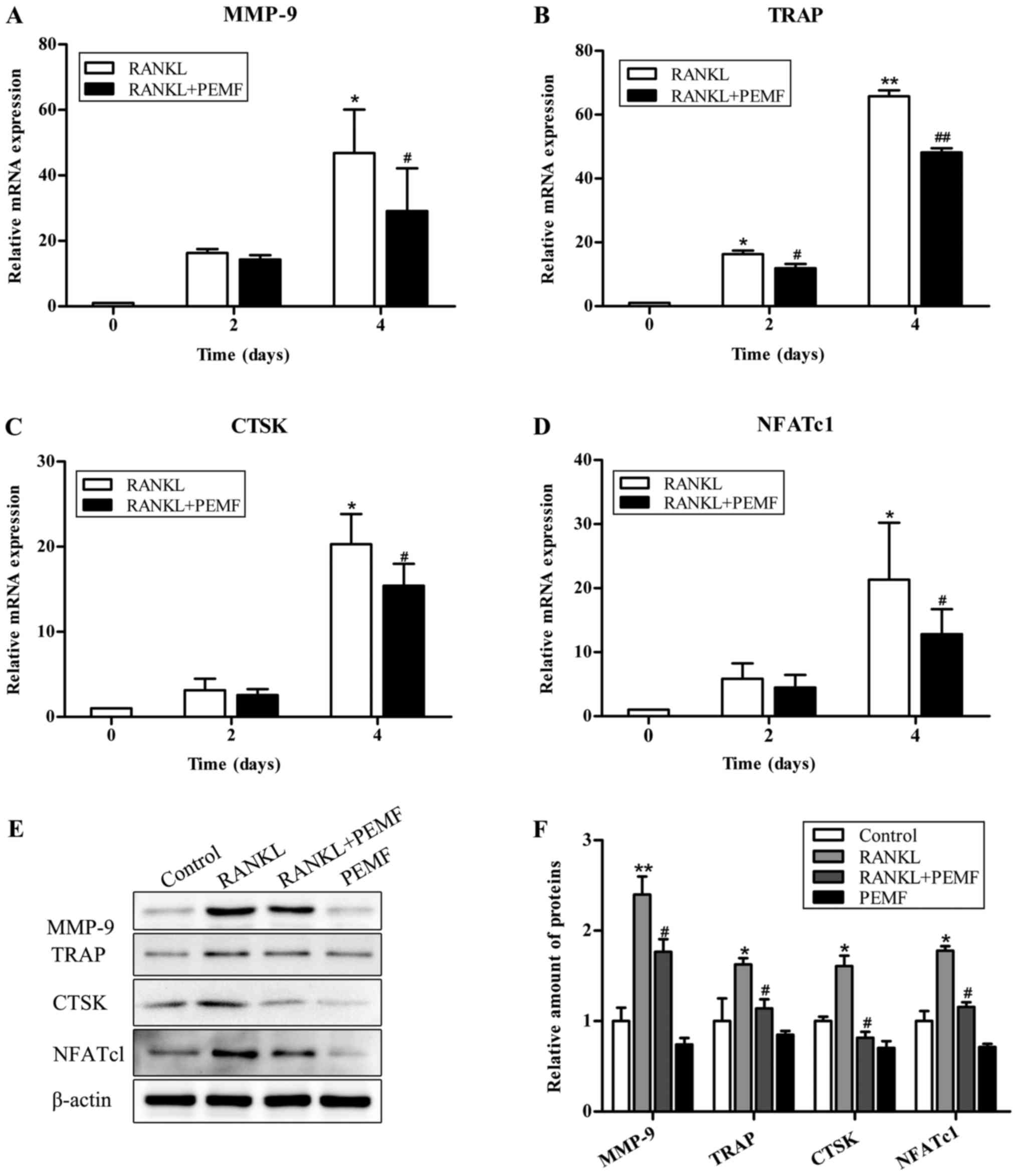 | Figure 4.PEMF suppresses RANKL-induced
osteoclastic specific gene and protein expression. Cells were
exposed to RANKL (50 ng/ml) alone or in combination with PEMF
exposure for 2 or 4 days. Gene expression levels of osteoclastic
markers (A) MMP-9, (B) TRAP, (C) CTSK and (D) NFATc1 were
determined by reverse transcription-quantitative polymerase chain
reaction. GAPDH served as a loading control. (E) On day 4, lysates
were immunoblotted with MMP-9, TRAP, CTSK and NFATc1 antibodies.
β-actin served as a loading control. (F) Relative protein
expression levels were measured using ImageJ software. Data are
presented as the mean ± standard deviation of three independent
experiments. *P<0.05, **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. RANKL group,
based on Student's t-tests and one-way analysis of variance. PEMF,
pulsed electromagnetic fields; TRAP, tartrate-resistant acid
phosphatase; RANKL, receptor activator of nuclear factor-κB ligand;
MMP-9, matrix metallopeptidase-9; CTSK, cathepsin K. |
PEMFs inhibit RANKL-induced
osteoclastogenesis via regulation of the Akt/mTOR signaling
pathway
To evaluate the effect of PEMF on RANKL signaling
activation following RANKL treatment in RAW264.7 cells, proteins
associated with the Akt/mTOR signaling pathway, including Akt, mTOR
and p70S6K, were examined by western blotting. As demonstrated in
Fig. 5A, the data revealed that
there was no significant difference among groups in the total
protein expression levels of Akt, mTOR and p70S6K. However, the
expression of p-Akt, p-mTOR and p-p70S6K, the type of modified
protein with a crucial role in this signal transduction, was
increased in response to RANKL treatment (Fig. 5B). Conversely, the upregulation of
these proteins induced by RANKL was suppressed by pharmacological
inhibitors, perifosine (Akt inhibitor) and rapamycin (mTOR and
p70S6K inhibitor). In accordance with the inhibitory effects of
antagonists, PEMF application also distinctly decreased
RANKL-induced upregulation of these phosphorylated proteins
(Fig. 5C and D), suggesting the
possibility that PEMF may inhibit RANKL-induced osteoclastogenesis
by suppression of the Akt/mTOR signaling pathway./
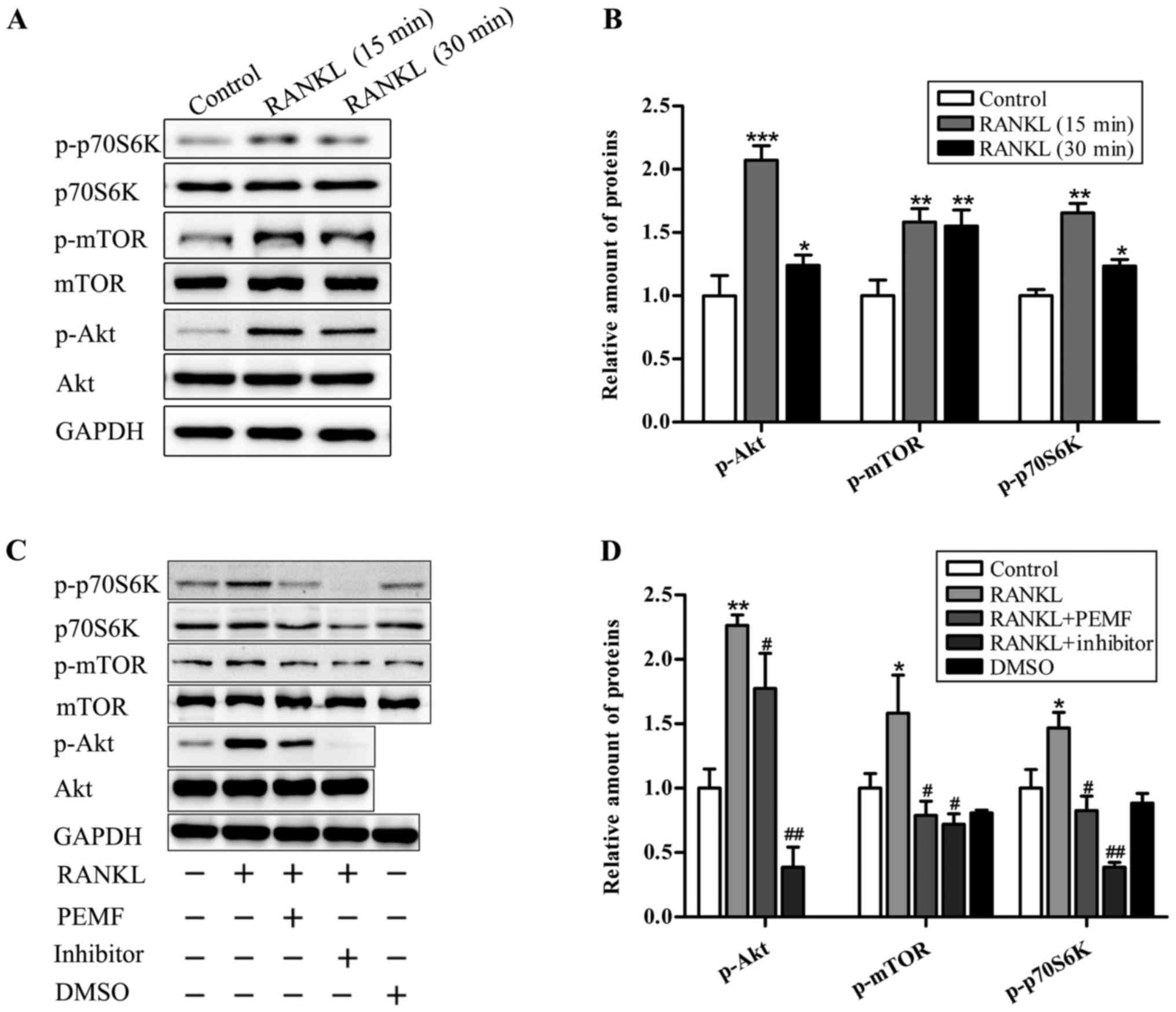 | Figure 5.PEMF reduces RANKL-induced
osteoclastogenesis via inhibition of the Akt/mTOR signaling
pathway. (A) Following serum-starvation for 6 h, RAW264.7 cells
were stimulated with RANKL (50 ng/ml) for 15 or 30 min. Cell
lysates were prepared for immunoblotting with antibodies, as
indicated. (B) Relative protein expression levels were measured
using ImageJ software. GAPDH served as a loading control. (C)
Following serum-starvation for 6 h, RAW264.7 cells were incubated
for 15 min with RANKL (50 ng/ml), RANKL (50 ng/ml) + PEMF or RANKL
(50 ng/ml) with pharmacological inhibitors [perifosine (Akt
inhibitor, 2.5 µM, dissolved in water) and rapamycin (mTOR and
p70S6K inhibitor, 1 µM, dissolved in dimethyl sulfoxide)]. Total
protein was extracted from each group and evaluated by western
blotting. (D) Relative protein expression levels were measured
using ImageJ software. GAPDH served as a loading control. Data are
presented as the mean ± standard deviation of three independent
experiments. *P<0.05, **P<0.01, ***P<0.001 vs. control
group; #P<0.05, ##P<0.01 vs. RANKL
group, based on Student's t-tests and one-way analysis of variance.
PEMF, pulsed electromagnetic fields; RANKL, receptor activator of
nuclear factor-κB ligand; Akt, protein kinase B; mTOR, mammalian
target of rapamycin. |
Discussion
Osteoclasts, the specialized cells derived from
monocyte/macrophage haematopoietic lineage, develop and adhere to
bone matrix, and subsequently secrete acid and lyase that degrade
it in an extracellular compartment (24). Due to excess osteoclastic activity,
bone resorption is accelerated resulting in an imbalance of bone
remodeling, leading to osteoporosis and other associated diseases,
particularly in the elderly population (30). Osteoporosis is an important issue
in orthopedics. PEMF has been successfully applied to various
diseases in several basic research experiments, most commonly
including senile osteoporosis, osteoarthritis and cancer bone
metastasis. However, few studies have reported the effects of PEMF
on osteoclast differentiation and formation, and the associated
signaling pathway mechanisms (31,32).
A previous study demonstrated that PEMF exposure markedly inhibited
RANKL-induced osteoclast formation from RAW264.7 macrophages, and
that this effect resulted from suppression of intracellular Akt and
mTOR protein phosphorylation.
RANKL and M-CSF are necessary for the
differentiation and maturation of osteoclasts, which then activate
the RANK receptor on the surface of haematopoietic precursor cells
(33). Macrophage RAW264.7 cells
are capable of secreting adequate M-CSF independently to support
their osteoclast differentiation. This cell line is known to be
suitable for investigating RANKL-induced osteoclast formation in
vitro due to the high levels of RANK it produces (34). The present study demonstrated that
RANKL led to a significant increase in the number of osteoclasts,
and the mRNA and protein expression of osteoclastic markers
throughout the 4 day experimental period. However, the marked
increases in the number of multinucleated giant osteoclasts and the
expression of osteoclastic-specific marker were suppressed by PEMF
exposure during osteoclastogenesis. Furthermore, it was
demonstrated that PEMFs inhibited RANKL-induced Akt and mTOR
activation. Therefore, these results revealed that PEMF exposure
reversed RANKL-induced osteoclast differentiation and formation via
suppression of the Akt/mTOR signaling pathway.
RANKL, a tumor necrosis factor (TNF) family member,
serves an important role in osteoclast survival, cytoskeletal
reorganization, bone resorption and osteoclast differentiation.
Activation of RANK by its ligand is crucial for osteoclastogenesis.
Wong et al (35)
demonstrated that RANKL activates the anti-apoptotic
serine/threonine kinase Akt through a signaling complex involving
TNF receptor-associated factor (TRAF) 6 and c-Src in osteoclasts.
Akt, also known as protein kinase B, has been demonstrated to be
vital in osteoclast differentiation (36). Furthermore, Akt functions to
phosphorylate mTOR, a protein involved in cell growth, homeostasis
and cell differentiation via the phosphatidylinositol 3-kinase
(PI3K)/Akt signaling pathway (37). Inhibition of mTOR by rapamycin, an
mTOR inhibitor, decreases the number of multinucleated
TRAP-positive osteoclasts in the chondro-osseous junction in rats
(38). Following the activation of
Akt and mTOR protein, the downstream signaling pathways are
activated and osteoclast differentiation is promoted.
Despite the future potential of arising therapeutic
strategies revealed by these studies, there are also limitations.
The specific mechanism by which PEMF inhibits the Akt/mTOR
signaling pathway remains unclear. One possibility is that PEMF
influences the Src signaling pathway, which is a significant RANK
signaling network in osteoclasts. Src, a signaling protein required
for osteoclast activation, has also been revealed to bind to TRAF6
and allow RANK-mediated signaling to proceed through lipid kinase
phosphatidylinositol 3 OH kinase and serine/threonine protein
kinase Akt (39). Src has an
important role in the signal transduction of the RANK/RANKL/OPG
signaling network. The activation or inhibition of Src signaling
pathways may influence cell proliferation, differentiation,
motility and cytoskeletal rearrangement (24). However, further investigation is
required to elucidate the specific underlying mechanisms and
associations.
In conclusion, the results of the present study
demonstrated that PEMF (50 Hz, 1 mT) inhibited osteoclast
differentiation from RAW264.7 macrophages by inhibiting the
Akt/mTOR signaling pathway. Therefore, PEMF may be an effective
non-invasive method in the treatment of a wide range of
osteoclast-associated diseases. Understanding the
anti-osteoclastogenesis mechanism of PEMF may be helpful for the
efficient use of PEMF in osteoporosis-associated diseases, both in
animal experiments and as a future therapeutic strategy.
Acknowledgements
The authors gratefully acknowledge the technical
assistance of Dr Hui Zhou and Mr Zhonghao Li of the Department of
Pathophysiology, Key Laboratory for Shock and Microcirculation
Research of Guangdong, Southern Medical University (Guangzhou,
China). The authors would also like to thank the Department of
Pathophysiology, Southern Medical University (Guangzhou, China) for
allowing the use of the laboratory.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL, JS, HX, QY, MZ, JT designed the experiments. YL
performed the major experiments and data analysis. JS, HX performed
a part of the experiments. YL drafted the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu W, Yang LH, Kong XC, An LK and Wang R:
Meta-analysis of osteoporosis: Fracture risks, medication and
treatment. Minerva Med. 106:203–214. 2015.PubMed/NCBI
|
|
2
|
Musette P, Brandi ML, Cacoub P, Kaufman
JM, Rizzoli R and Reginster JY: Treatment of osteoporosis:
Recognizing and managing cutaneous adverse reactions and
drug-induced hypersensitivity. Osteoporos Int. 21:723–732. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gartlehner G, Patel SV, Feltner C, Weber
RP, Long R, Mullican K, Boland E, Lux L and Viswanathan M: Hormone
therapy for the primary prevention of chronic conditions in
postmenopausal women: Evidence report and systematic review for the
US preventive services task force. JAMA. 318:2234–2249. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hadji P, Papaioannou N, Gielen E, Tepie
Feudjo M, Zhang E, Frieling I, Geusens P, Makras P, Resch H, Möller
G, et al: Persistence, adherence, and medication-taking behavior in
women with postmenopausal osteoporosis receiving denosumab in
routine practice in Germany, Austria, Greece and Belgium: 12-month
results from a European non-interventional study. Osteoporosis Int.
26:2479–2489. 2015. View Article : Google Scholar
|
|
5
|
Bone HG, Dempster DW, Eisman JA, Greenspan
SL, McClung MR, Nakamura T, Papapoulos S, Shih WJ, Rybak-Feiglin A,
Santora AC, et al: Odanacatib for the treatment of postmenopausal
osteoporosis: Development history and design and participant
characteristics of LOFT, the long-term Odanacatib fracture trial.
Osteoporosis Int. 26:27212015. View Article : Google Scholar
|
|
6
|
Hannon RA, Clack G, Rimmer M, Swaisland A,
Lockton JA, Finkelman RD and Eastell R: Effects of the Src kinase
inhibitor saracatinib (AZD0530) on bone turnover in healthy men: A
randomized, double-blind, placebo-controlled,
multiple-ascending-dose phase I trial. J Bone Miner Res.
25:463–471. 2013. View Article : Google Scholar
|
|
7
|
Bonnick S, De Villiers T, Odio A, Palacios
S, Chapurlat R, DaSilva C, Scott BB, Le Bailly De Tilleghem C,
Leung AT and Gurner D: Effects of odanacatib on BMD and safety in
the treatment of osteoporosis in postmenopausal women previously
treated with alendronate: A randomized placebo-controlled trial. J
Clin Endocrinol Metab. 98:4727–4735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bassett CA, Pawluk RJ and Pilla AA:
Augmentation of bone repair by inductively coupled electromagnetic
fields. Science. 184:575–577. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bassett CA, Pawluk RJ and Pilla AA:
Acceleration of fracture repair by electromagnetic fields. A
surgically noninvasive method. Ann N Y Acad Sci. 238:242–262. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang T, Wang P, Cao Z, Wang X, Wang D,
Shen Y, Jing D, Luo E and Tang W: Effects of BMP9 and pulsed
electromagnetic fields on the proliferation and osteogenic
differentiation of human periodontal ligament stem cells.
Bioelectromagnetics. 38:63–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, He H, Yang L, Chen S, Guo H, Xia
L, Liu H, Qin Y, Liu C, Wei X, et al: Effects of pulsed
electromagnetic fields on bone mass and Wnt/β-catenin signaling
pathway in ovariectomized rats. Arch Med Res. 274–282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jing D, Zhai M, Tong S, Xu F, Cai J, Shen
G, Wu Y, Li X, Xie K, Liu J, et al: Pulsed electromagnetic fields
promote osteogenesis and osseointegration of porous titanium
implants in bone defect repair through a Wnt/β-catenin
signaling-associated mechanism. Sci Rep. 24:320452016. View Article : Google Scholar
|
|
13
|
Urnukhsaikhan E, Cho H, Mishig-Ochir T,
Seo YK and Park JK: Pulsed electromagnetic fields promote survival
and neuronal differentiation of human BM-MSCs. Life Sci.
151:130–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang KS, Hong JM, Seol YJ, Rhie JW, Jeong
YH and Cho DW: Short-term evaluation of electromagnetic field
pretreatment of adipose-derived stem cells to improve bone healing.
J Tissue Eng Regen Med. 9:1161–1171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He J, Zhang Y, Chen J, Zheng S, Huang H
and Dong X: Effects of pulsed electromagnetic fields on the
expression of NFATc1 and CAII in mouse osteoclast-like cells. Aging
Clin Exp Res. 27:13–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei T, Liang Z, Li F, Tang C, Xie K, Wang
P, Dong X, Shan S, Jiang M, Xu Q, et al: Pulsed electromagnetic
fields (PEMF) attenuate changes in vertebral bone mass,
architecture and strength in ovariectomized mice. Bone. 108:10–19.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin HY and Lin YJ: In vitro effects of low
frequency electromagnetic fields on osteoblast proliferation and
maturation in an inflammatory environment. Bioelectromagnetics.
32:552–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang D, Huang Y, Huang Z, Zhang R, Wang H
and Huang D: FTY-720P suppresses osteoclast formation by regulating
expression of interleukin-6 (IL-6), interleukin-4 (IL-4) and matrix
metalloproteinase 2 (MMP-2). Med Sci Monitor. 22:2187–2194. 2016.
View Article : Google Scholar
|
|
19
|
Yang J, Zhang J, Ding C, Dong D and Shang
P: Regulation of osteoblast differentiation and iron content in
MC3T3-E1 cells by static magnetic field with different intensities.
Biol Trace Elem Res. Oct 19–2017.(Epub ahead of print). doi:
10.1007/s12011-017-1161-5.
|
|
20
|
Barnaba SA, Ruzzini L, Di Martino A,
Lanotte A, Sgambato A and Denaro V: Clinical significance of
different effects of static and pulsed electromagnetic fields on
human osteoclast cultures. Rheumatol Int. 32:1025–1031. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie YF, Shi WG, Zhou J, Gao YH, Li SF,
Fang QQ, Wang MG, Ma HP, Wang JF, Xian CJ, et al: Pulsed
electromagnetic fields stimulate osteogenic differentiation and
maturation of osteoblasts by upregulating the expression of BMPRII
localized at the base of primary cilium. Bone. 93:22–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jing D, Li F, Jiang M, Cai J, Wu Y, Xie K,
Wu X, Tang C, Liu J, Guo W, et al: Pulsed electromagnetic fields
improve bone microstructure and strength in ovariectomized rats
through a Wnt/Lrp5/β-catenin signaling-associated mechanism. PLoS
One. 8:e793772013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang K, Chang WH, Huang S, Huang S and
Shih C: Pulsed electromagnetic fields stimulation affects
osteoclast formation by modulation of osteoprotegerin, RANK ligand
and macrophage colony-stimulating factor. J Orthop Res.
23:1308–1314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H, Zhang J, Lei Y, Han Z, Rong D, Yu Q,
Zhao M and Tian J: Low frequency pulsed electromagnetic field
promotes C2C12 myoblasts proliferation via activation of MAPK/ERK
pathway. Biochem Biophys Res Commun. 479:97–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park KH, Park B, Yoon DS, Kwon SH, Shin
DM, Lee JW, Lee HG, Shim JH, Park JH and Lee JM: Zinc inhibits
osteoclast differentiation by suppression of
Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun
Signal. 11:742013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kapetanakis NI, Uzan C, Jimenez-Pailhes
AS, Gouy S, Bentivegna E, Morice P, Caron O, Gourzones-Dmitriev C,
Le Teuff G and Busson P: Plasma miR-200b in ovarian carcinoma
patients: Distinct pattern of pre/post-treatment variation compared
to CA-125 and potential for prediction of progression-free
survival. Oncotarget. 6:36815–36824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Halleen JM, Tiitinen SL, Ylipahkala H,
Fagerlund KM and Vӓӓnӓnen HK: Tartrate-resistant acid phosphatase
5b (TRACP 5b) as a marker of bone resorption. Clin Lab. 52:499–509.
2006.PubMed/NCBI
|
|
29
|
Liu H, Li D, Liu S, Liu Z and Li M:
Histochemical evidence of IGF2 mRNA-binding protein 2-mediated
regulation of osteoclast function and adhesive ability. Histochem
Cell Biol. 149:343–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu HF, He HC, Yang L, Yang ZY, Yao K, Wu
YC, Yang XB and He CQ: Pulsed electromagnetic fields for
postmenopausal osteoporosis and concomitant lumbar osteoarthritis
in southwest China using proximal femur bone mineral density as the
primary endpoint: Study protocol for a randomized controlled trial.
Trials. 16:2652015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muramatsu Y, Matsui T, Deie M and Sato K:
Pulsed electromagnetic field stimulation promotes anti-cell
proliferative activity in doxorubicin-treated mouse osteosarcoma
cells. In Vivo. 31:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Visagie A, Kasonga A, Deepak V, Moosa S,
Marais S, Kruger MC and Coetzee M: Commercial Honeybush
(cyclopia spp.) tea extract inhibits osteoclast formation
and bone resorption in RAW264.7 murine macrophages-an in vitro
study. Int J Environ Res Public Health. 12:13779–13793. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Collin-Osdoby P and Osdoby P:
RANKL-mediated osteoclast formation from murine RAW 264.7 cells.
Methods Mol Biol. 816:187–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong BR, Besser D, Kim N, Arron JR,
Vologodskaia M, Hanafusa H and Choi Y: TRANCE, a TNF family member,
activates Akt/PKB through a signaling complex involving TRAF6 and
c-Src. Mol Cell. 4:1041–1049. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Sapkota M, Gao M, Choi H and Soh Y:
Macrolactin F inhibits RANKL-mediated osteoclastogenesis by
suppressing Akt, MAPK and NFATc1 pathways and promotes
osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling
pathway. Eur J Pharmacol. 815:202–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Navé BT, Ouwens M, Withers DJ, Alessi DR
and Shepherd PR: Mammalian target of rapamycin is a direct target
for protein kinase B: Identification of a convergence point for
opposing effects of insulin and amino-acid deficiency on protein
translation. Biochem J. 344:427–431. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsieh CJ, Kuo PL, Hou MF, Hung JY, Chang
FR, Hsu YC, Huang YF, Tsai EM and Hsu YL: Wedelolactone inhibits
breast cancer-induced osteoclastogenesis by decreasing Akt/mTOR
signaling. Int J Oncol. 46:555–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takeshita S: SHIP-deficient mice are
severely osteoporotic due to increased numbers of hyperresorptive
osteoclasts. Nat Med. 9:943–949. 2002. View
Article : Google Scholar
|