Introduction
Recently, degradable materials developed as
orthopaedic implants have attracted much attention since their use
could avoid the necessity for a secondary operation to remove the
implants. Among the possible materials, magnesium and its alloys
are the most promising due to their degradability, suitable
mechanical properties and good biocompatibility (1,2).
Several magnesium alloys, such as WE43 (3), AZ91 (4), Mg-Zn (5), Mg-Ca (2) and Mg-Mn-Zn (3) show great potential in clinical
application. More specifically, previous in vivo experiments
have identified the gradual degradability of Mg alloys in bone
tissue. In addition, the degradation products induced an
appropriate level of inflammatory response (6). However, the rapid corrosion rate of
Mg alloys is still a significant obstacle in the process of
clinical applications (5).
Researchers have tried various ways to deal with
this challenge. Among these, it has been demonstrated that alloying
is the most effective approach to manipulate the corrosion
resistance and mechanical properties of Mg alloys. For the sake of
safety and human body tolerance, only a small number of alloying
elements are suitable for inclusion in biodegradable Mg alloys,
such as Zn, Nd, Ca, Sr, Mn and several rare earth elements
(7,8).
Zn is one of the essential elements in the human
body (9). Mg-Zn-based alloys are
very promising because not only are they the second strongest
ductile alloy system, but their corrosion rates can also be greatly
reduced by utilizing certain strategies. More importantly,
Mg-Zn-based alloys may be RE (rare earth) free. It has been shown
that Mg-Zn-based alloys are the second strongest alloying system
with varying corrosion rates. They could be RE-free systems which
compete with the Mg-RE-based alloys and which are used in
RE-sensitive implants (10).
Meanwhile, it is reported that Mg-2Nd alloys have
characteristically high elongation ratios, and they improve the
yield strength and degradation rate (11). The addition of light RE elements to
a magnesium alloy can not only improve its corrosion resistance and
mechanical properties, but also help to improve the
anti-coagulation behaviour of biological implants. The element Nd
is a rare earth element with minimal toxicity. A small amount of Nd
can be added to a magnesium alloy, without causing any significant
cytotoxicity in experiments (12).
The skeletal muscle as the dynamical device of the motor system, is
attached to the skeleton, which is of great significance to the
movement of the joints. We may implant materials to repair injuries
of the motor system. Whether it is suture or other internal
fixation materials, it is inevitable that the muscle is contacted.
But the biocompatibility of magnesium alloys to skeletal muscle is
not clear, and it is not sure whether magnesium alloys has any
effect on the adhesion and proliferation of skeletal muscle cells.
So the skeletal muscle biocompatibility of magnesium alloys is of
great significance in the research process of implant
materials.
Bone morphogenetic protein 2 (BMP-2), which is a
member of the transforming growth factor (TGF)-β superfamily, has
profound effects on the osteoblast activity (13,14).
Many studies have found that BMP-2 not only exists in the bone
matrix, but it is also present in other tissues (15). In recent years, other researchers
have found that BMP-2 is involved in the regulation of the
proliferation, differentiation and apoptosis of many types of
cells, thus affecting their biological behaviour (16,17).
Intracellular kinase signalling plays an important
role in many biological functions including cell differentiation
(18,19). Adenosine monophosphate-activated
protein kinase (AMPK) is a principal intracellular energy sensor
which activates energy-producing pathways (20). Moreover, AMPK activation can
mediate the downstream signalling response of the phosphoinositide
3-kinase (PI3K)/Akt, mitogen-activated protein kinases (MAPK) and
the mammalian target of the rapamycin (mTOR) pathway (21). mTOR as a serine/threonine protein
kinase can regulate cell proliferation (22,23),
and also plays an important role in cell apoptosis and survival
(24,25).
These beneficial effects of Zn and Nd prompted us to
investigate the feasibility of alloying Zn-Nd with Mg and the
corresponding effects on the corrosion properties and
biocompatibility of the resulting alloy. In this study, a
Mg-2Zn-0.5Nd alloy was designed and prepared. To date there have
been no systematic researches on Mg-2Zn-0.5Nd alloy systems for
biomedical applications. The purpose of the present study was to
investigate the effect of Mg-2Zn-0.5Nd on the expression of BMP-2-
and mTOR-related signalling proteins. The purpose of this study is
to clarify the effect of Mg-2Zn-0.5Nd alloy on the proliferation of
skeletal muscle cells, and to explore the effect of Mg-2Zn-0.5Nd on
the expression of BMP-2 in skeletal muscle cells and mTOR related
signal proteins.
Materials and methods
Material preparation
Alloys of 317L, Ti-6Al-4V and Mg-2Zn-0.5Nd were
prepared in the Institute of Metal Research (Chinese Academy of
Science, Shenyang, China). Plate samples with a diameter of 10 mm
and a thickness of 1 mm were prepared. Cylindrical rods with a
diameter of 1 mm were machined for implantation into mice. All
samples went through ultrasonic cleaning in acetone, absolute
ethanol and distilled water for 10 min each and then sterilization
with ethylene oxide.
The leaching solution was prepared in accordance
with the ISO 10993-5: 2009 standard (26). Specifically, plate samples were
immersed in complete DMEM with 10% foetal bovine serum (FBS), 100
U/ml penicillin and 100 µg/ml streptomycin and incubated at 37°C
for the indicated duration. The extracts were analysed using
inductively coupled plasma optical emission spectroscopy (ICP-OES;
VISTAPRO; Agilent Technologies, Inc., Santa Clara, CA USA) to
determine the elemental concentrations of Mg, Zn and Nd.
Culture of L6 cells
The L6 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and maintained in complete
Dulbecco's modified Eagle's medium (DMEM) with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. The cells were grown in a
humidified atmosphere containing 5% CO2 at 37°C. Before
the experiment, cells (5×105 cells/well in 6-well
plates) were grown for 24 h. The next day, cells were treated with
different concentrations of extraction medium. The biological
morphology of skeletal muscle cells in each group was observed by
an inverted microscope after 72 h.
Cell proliferation assay
The proliferative effect of the leaching solution on
L6 cells was determined using the CCK-8 kit (Dojindo Molecular
Technology, Kumamoto, Japan). Cells were plated in 96-well plates
at 5×103 cells/well in triplicate. After 1, 3 and 5 days
of culture, 90 µl of culture medium and 10 µl of CCK-8 solution
were added to each well at each time-point and incubated at 37°C
for another 4 h. The optical density (OD) was measured using an
ELX800 absorbance microplate reader (Bio-Tek Instruments Inc.,
Winooski, VT, USA) at 450 nm (650 nm reference).
Western blot analysis
Aliquots containing 2×106 cells per well
were plated into 6-well plates and cultured in various leaching
solutions for the periods indicated. Then the L6 cells were
harvested and washed with cold PBS, lysed for 30 min on ice and
then centrifuged for 10 min at 12,000 × g at 4°C. The supernatants
were collected, mixed with loading buffer, and boiled for 10 min.
Electrophoresis was performed on 12% SDS-PAGE for 3 h and then
proteins were transferred onto PVDF membranes in transfer buffer
(containing 20 mM Tris, 20% methanol, and 150 mM glycine) at 200 mA
for 70 min. The membrane was incubated in non-fat dried milk for 2
h. After washing with TBST three times the membrane was incubated
with primary antibodies against BMP-2, p-mTOR, p-AKT, FoxO1 and p38
overnight at 4°C. Membranes were incubated with the appropriate
secondary antibodies conjugated with IRDye 800CW (molecular weight,
1,166 kDa), and antibody reactivity was detected by exposure in an
Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE,
USA). Each group was repeated 10 times and the gray value was
calculated by Image J method. The gray value of BMP-2, t-mTOR,
t-AKT, t-FoxO1 and t-P38 to GAPDH protein was used as the protein
expression in BMP-2, t-mTOR, t-AKT, t-FoxO1 and t-P38 groups. The
gray value of p-mTOR, p-AKT, p-FoxO1, and p-P38 to t-mTOR, t-AKT,
t-FoxO1, and t-P38 is the relative expression of p-mTOR, p-AKT,
p-FoxO1, and p-P38.
Statistical analysis
The data are presented as the mean ± standard error
mean of three independent experiments. One-way analysis of variance
was performed with a Bonferroni post hoc test to analyse the
results using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
The biological morphology of skeletal
muscle cells
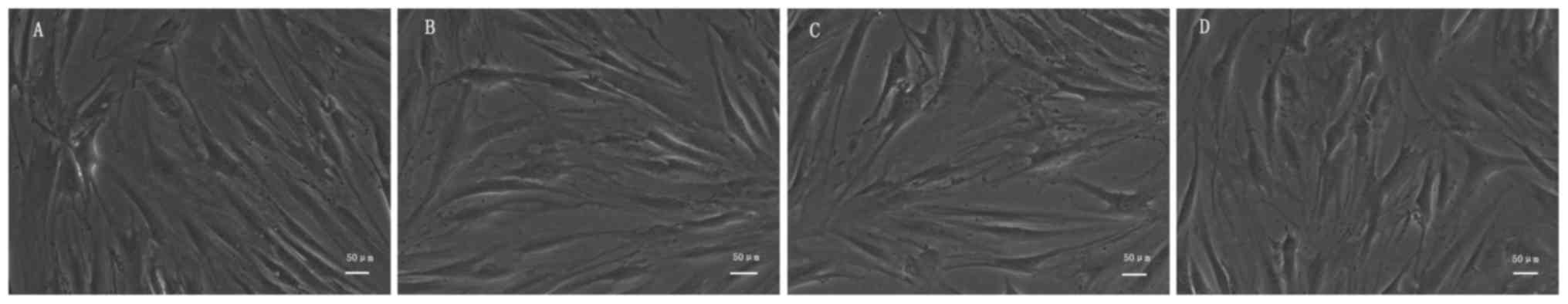
As shown in Fig. 1,
we found that after being cultured 72 h with different extracts,
the cells of each group adherent growth, appears cell clusters, the
number of long spindle skeletal muscle cells increased
significantly, gradually becoming slender and interconnected to a
network. The growth state of the cells in each group was good, but
no difference was observed between the different groups.
The effect of Mg-2Zn-0.5Nd on cell
proliferation
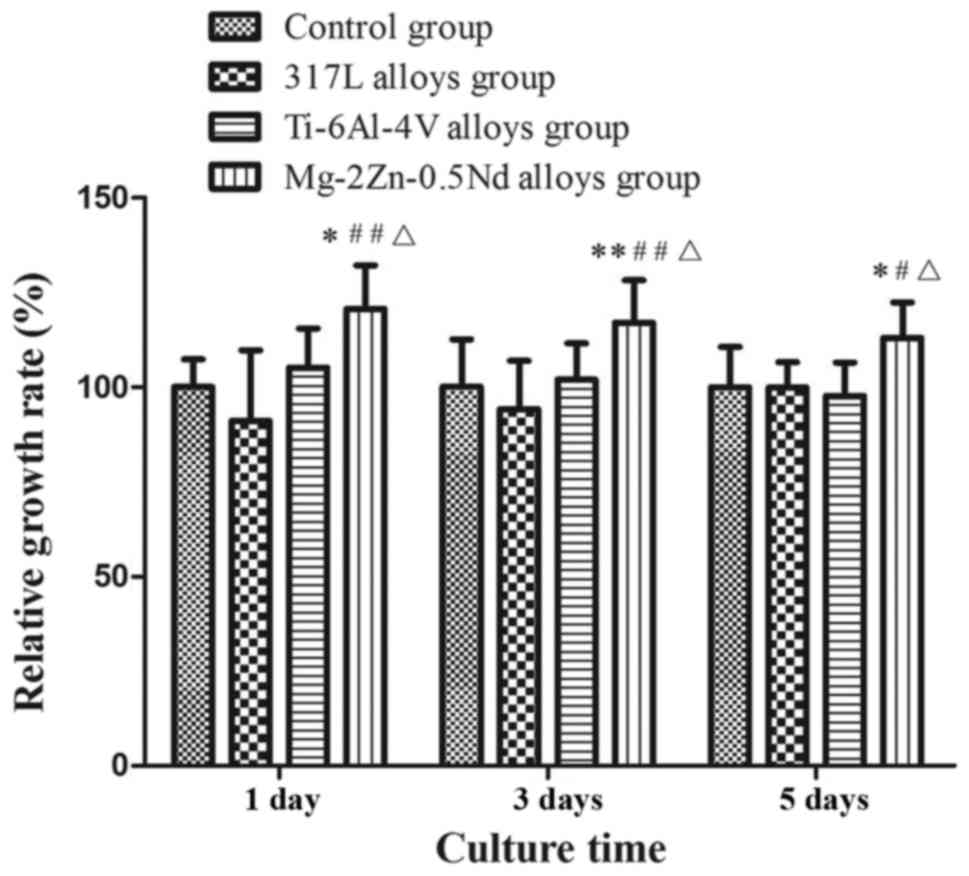
Cell proliferation was determined by CCK-8 assay
after incubating Mg-2Zn-0.5Nd with L6 cells for 24 h (Fig. 2). Cell proliferation is expressed
as relative growth rates (RGR) as determined by RGR (%)=(OD
sample/OD negative control) ×100%. The CCK-8 values were calculated
based on means ± standard deviations from 5 wells (SD, n=5). The
differences between the groups were considered statistically
significant at P<0.05. With Mg-2Zn-0.5Nd, we observed an
increase in cell proliferation, indicating that Mg-2Zn-0.5Nd
promoted cell growth and proliferation. In contrast to
Mg-2Zn-0.5Nd, incubation with 317L alloys and the Ti-6Al-4V groups
resulted is no significant increase in cell proliferation,
indicating that Mg-2Zn-0.5Nd has significantly better bioactivity
than the other two alloys.
Mg-2Zn-0.5Nd stimulates the
phosphorylation of BMP-2 in L6 cells
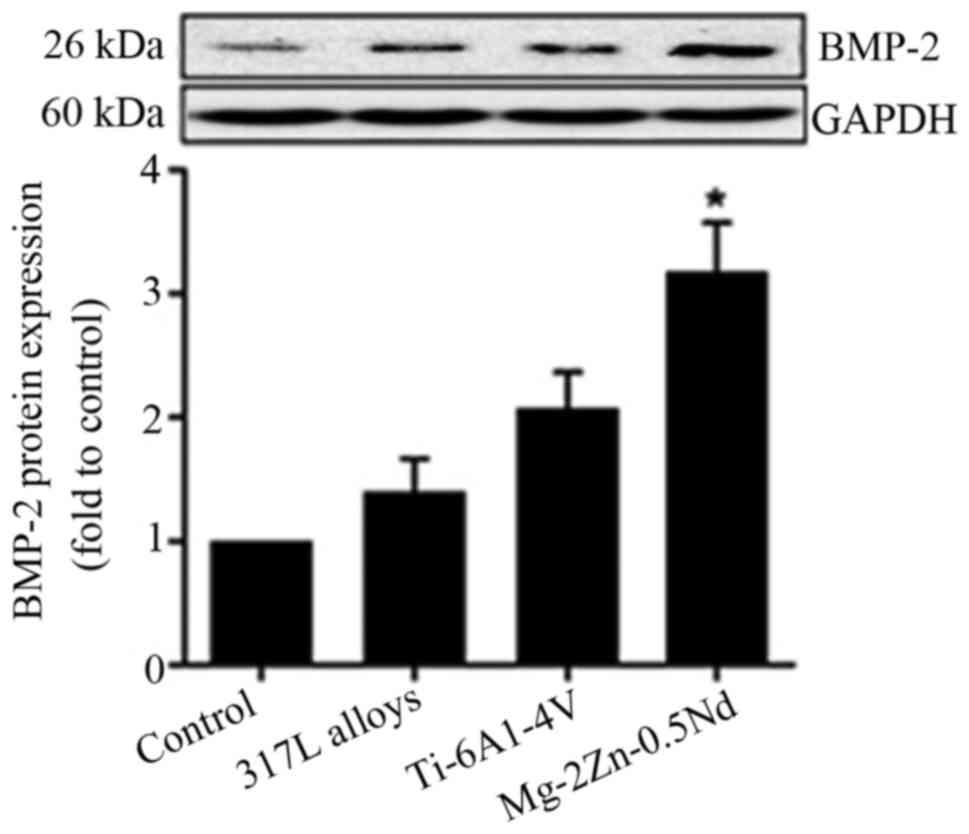
In this study, the BMP-2 protein content of L6 cells
cultured for 24 h with leaching solution from the indicated alloys
was determined by the western blot analysis. As shown in Fig. 3, cells cultured with Mg-2Zn-0.5Nd
exhibited the highest phosphorylation level of BMP-2. No increase
in BMP-2 phosphorylation was observed when the L6 cells were
cultured with 317L alloys and Ti-6Al-4V alloys.
Mg-2Zn-0.5Nd stimulates the activity
of p-mTOR in L6 cells
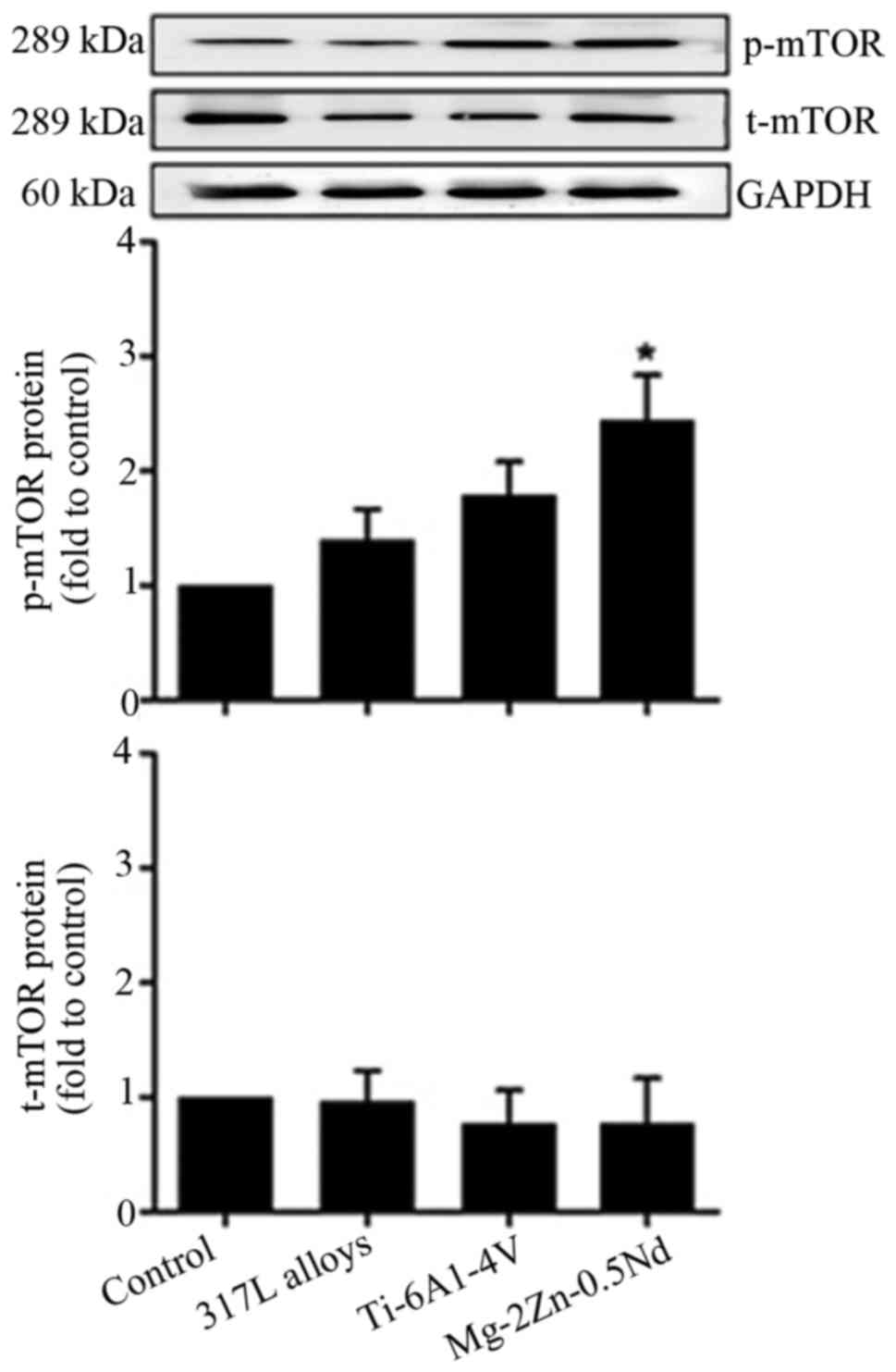
In order to identify whether Mg-2Zn-0.5Nd promoted
the proliferation of skeletal muscle cells via mTOR-related
signalling pathway, the western blot analysis was performed to
examine mTOR protein expression in vitro. As shown in
Fig. 4, in comparison with the
control group, Mg-2Zn-0.5Nd treatment significantly upregulated
p-mTOR expression, while 317L alloys and Ti-6Al-4V alloys caused no
significant change in p-mTOR expression.
Mg-2Zn-0.5Nd stimulates the activity
of AKT in L6 cells
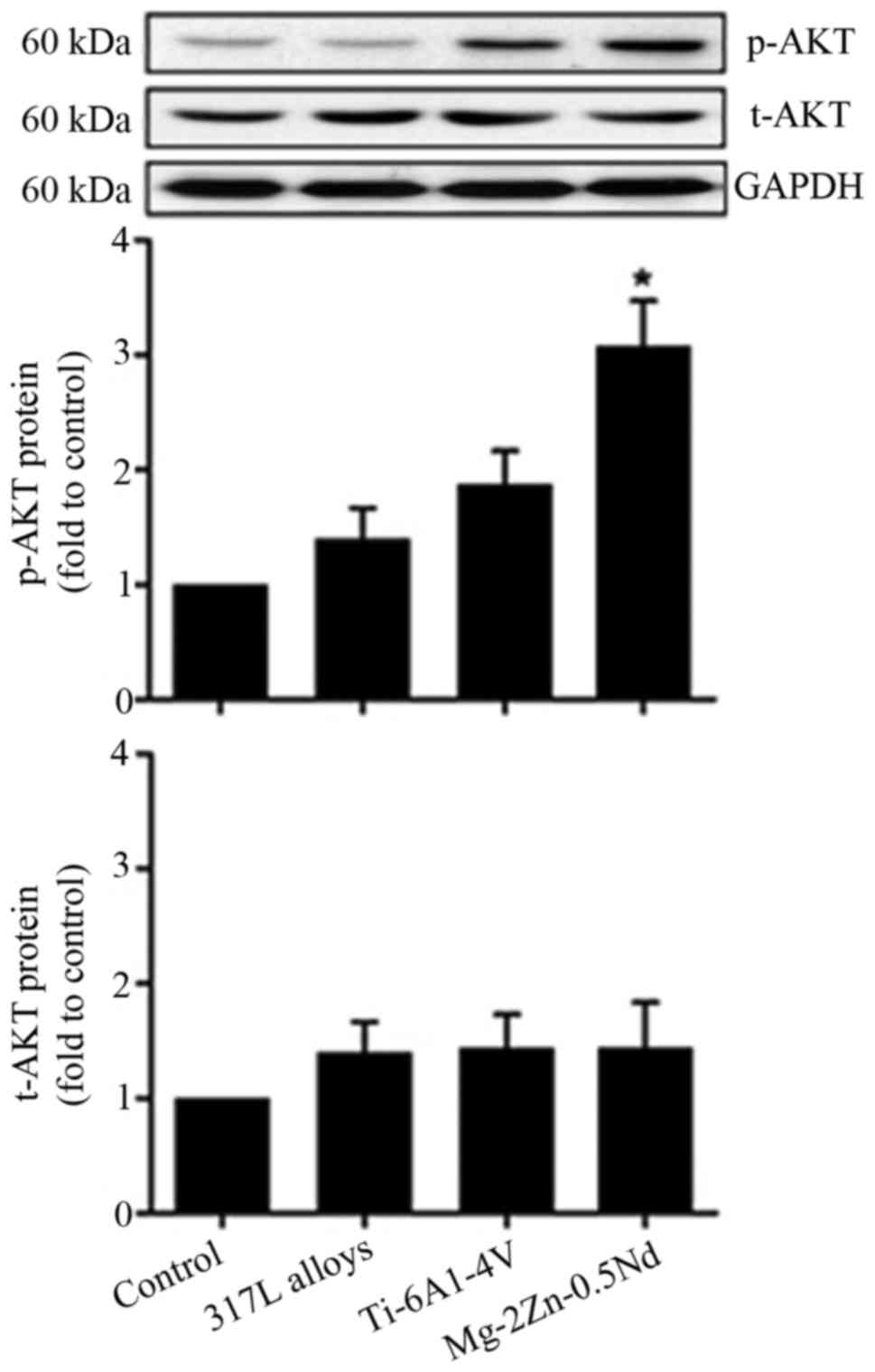
In this present study, our results show that the
activation of p-AKT proteins is significantly increased in the
Mg-2Zn-0.5Nd group, while co-culture with 317L alloys and Ti-6Al-4V
alloys does not affect the expression of p-AKT proteins (Fig. 5). These results suggest that
Mg-2Zn-0.5Nd affects mTOR activity of L6 cells partly through the
AKT-mTOR axis.
Mg-2Zn-0.5Nd stimulates the activity
of FoxO1 in L6 cells
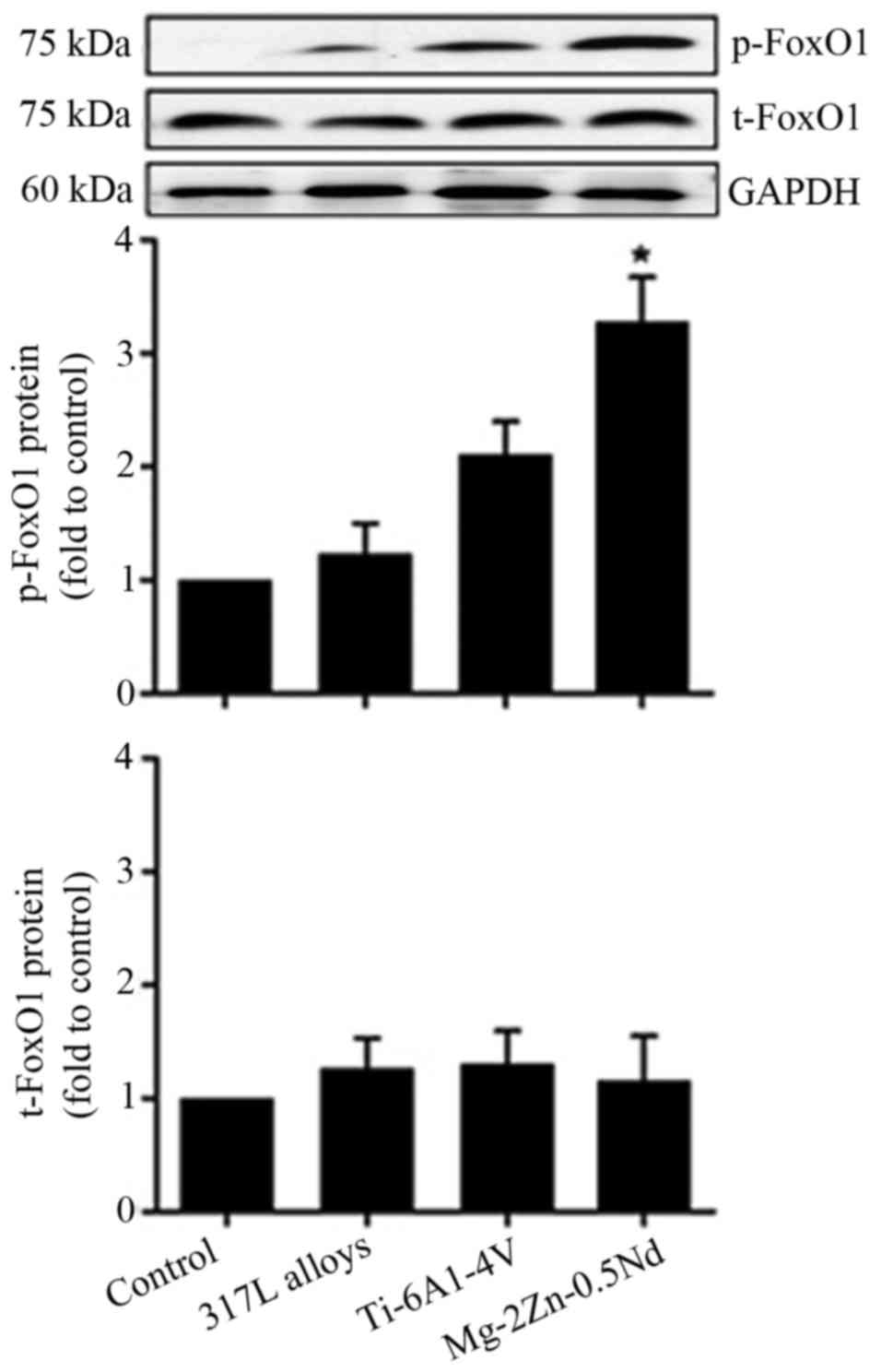
In order to identify whether FoxO1 is involved in
the Mg-2Zn-0.5Nd-induced proliferation of skeletal muscle cells
in vitro. We undertook western blot experiments to study the
effects of Mg-2Zn-0.5Nd on the activation of FoxO1 proteins. As
shown in Fig. 6, our results show
that the expression of p-FoxO1 proteins is significantly increased
in both the Mg-2Zn-0.5Nd and Ti-6Al-4V alloy groups, while
co-culture with 317L alloy does not affect the expression of
p-FoxO1 proteins.
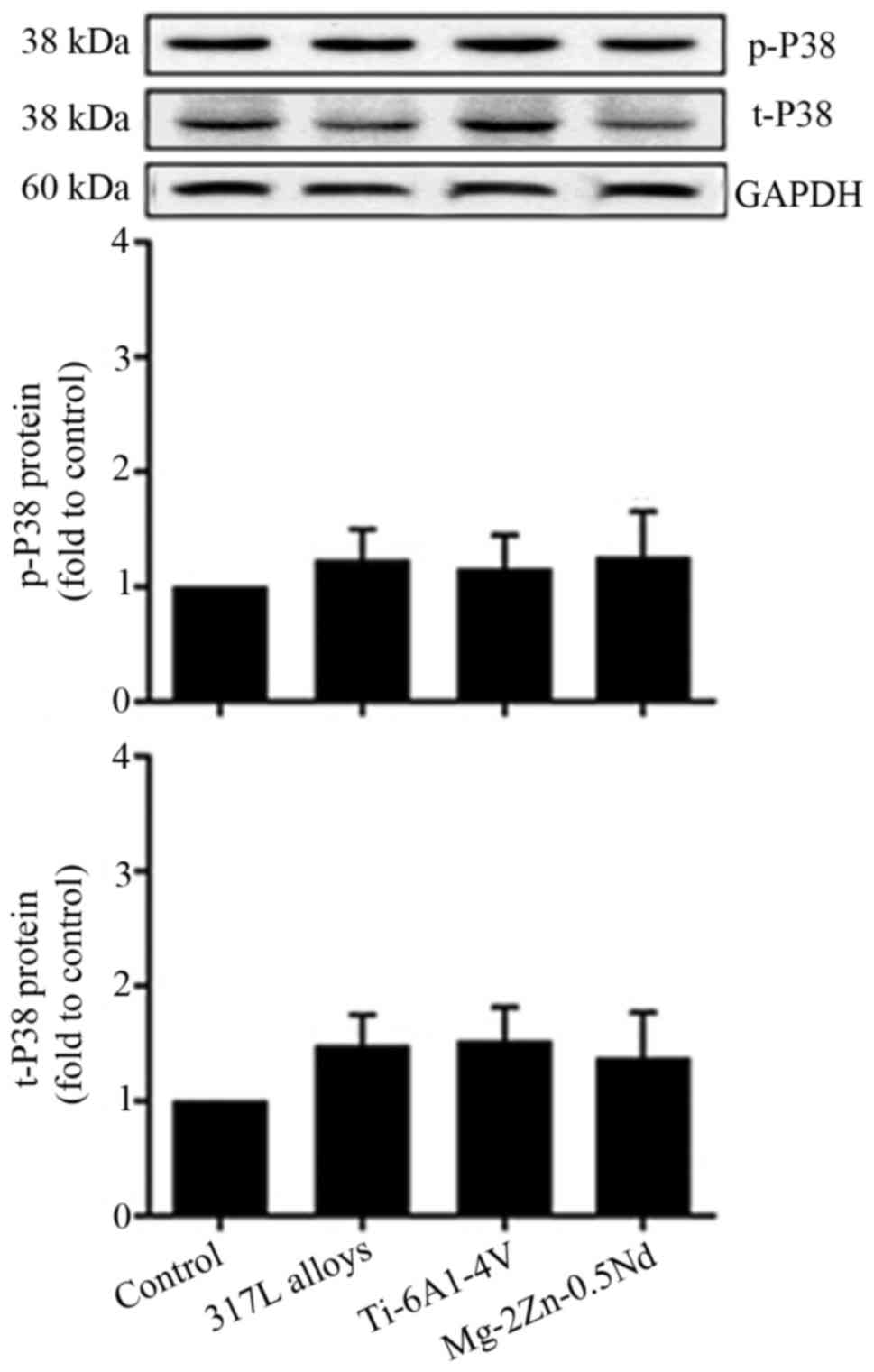
Mg-2Zn-0.5Nd has no effect on P38
activity in L6 cells
To gain further insight into the molecular
mechanisms by which Mg-2Zn-0.5Nd participates in cell
proliferation, the phosphorylation of p38/MAPK in L6 cells was
examined. As shown in Fig. 7, the
p38 protein expression could be identified in all groups, however
there was no significant difference between the groups. These data
suggest that p38/MAPK might not participate in Mg-2Zn-0.5Nd-induced
changes in cell proliferation.
Discussion
Extensive studies have been carried out on Mg alloys
as biodegradable materials. Mg alloy has been regarded as a
promising candidate for bone implants because of its
biodegradability and special mechanical properties (27). However the fast corrosion rate,
release of hydrogen gas and the lack of long-term mechanical
integrity of the implants are the most critical obstacles for the
clinical applications of Mg alloys. Previous research provided
various solutions such as polymer coatings, composition
optimization, corrosion potential optimization and so on, in an
attempt to retard the rapid corrosion reaction of Mg alloys
(10).
Current research has mainly focused on reducing the
degradation rate, and promoting the mechanical and biological
properties (8,28,29).
Specifically, researchers hope to develop a new magnesium alloy of
the indicated ratios, one proved to be an ideal alloy system with
appropriate mechanical performance, stable degradation speed and
favourable biocompatibility. Since Mg-based alloys are
biodegradable, Mg alloy degradation induces dynamic
micro-environmental changes. Therefore, much effort is needed to
fully understand how the alloy degradation process evokes
physiological reactions, especially for the sake of safety in
considering potential usage in humans (30,31).
Zn is one of the essential elements in the human
body, acting in a pivotal role in mediating the activity of
hundreds of enzymes (9). In its
ionic form, Zn is also involved in the cell metabolism (32,33).
As implant-related material, Mg-Zn-based alloys exhibit the lowest
strength and ductility with varying corrosion rates. More
importantly, Mg-Zn-based alloys could be RE-free systems which
could compete with the Mg-RE-based alloys and could be used in
RE-sensitive implants (10).
Meanwhile, the addition of light rare earth elements
to a magnesium alloy can not only improve the corrosion resistance
and mechanical properties of the alloy, but also help to improve
the anti-coagulation behaviour of biological implants. The element
Nd is a rare earth element with minimal toxicity. When a small
amount of Nd is added to a magnesium alloy in an experimental
situation, no significant cytotoxicity is found (12). More importantly, it has been
reported that Mg-2Nd alloys have the characteristic of a high
elongation ratio, which improves the yield strength and degradation
rate (11).
As a new kind of magnesium alloy, the
microstructure, mechanical properties and degradation properties of
Mg-2Zn-0.5Nd alloy have been previously studied. It showed
excellent plastic deformation properties and moderate strength, and
the corrosion resistance was significantly higher (34), so it has a good prospect of
clinical application. But before the clinical application, it is
necessary to evaluate its biocompatibility. At present; the
research of magnesium alloy materials is mainly based on the study
of bone tissue and osteoblasts. There are few studies related to
the skeletal muscle. But without the study of the effect of
magnesium alloy on skeletal muscle, the biocompatibility of
magnesium alloy materials is not comprehensive. The skeletal muscle
as the dynamical device of the motor system is attached to the
skeleton, which is of great significance to the maintenance of the
posture of the human body and the movement of the joints. We may
implant materials to repair injuries of the motor system. Whether
it is suture or other internal fixation materials, it is inevitable
that the muscle is contacted. The implant material is good, not
only need to meet the excellent biocompatibility of osteoblasts,
but also to ensure the good biocompatibility of skeletal muscle
cells. The skeletal muscle biocompatibility of implanted materials
is of great significance in the research process of implant
materials.
We attempted to study the effect of Mg-2Zn-0.5Nd
alloy on the proliferation of L6 cells, by culturing different
alloy extracts with rat skeletal muscle cells. The results showed
that in the Mg-2Zn-0.5Nd alloy group, the relative cell
proliferation rate was higher than in the 317L group or the
Ti-6Al-4V alloy group, and the difference was significant. Our
study showed that Mg-2Zn-0.5Nd alloy has the ability to improve the
proliferation of L6 cells. In order to clarify the mechanism of
Mg-2Zn-0.5Nd alloy involved in improving the adhesion and
proliferation of L6 cells, we analysed the expression of
intracellular related proteins in the experimental groups.
BMP is a member of the TGF family. Early studies
have shown that BMP-2 can induce bone and cartilage formation in
vivo and play an important role in bone regeneration and
repair. Later it was found that BMP-2 not only exists in the bone
matrix, but it is also found in other tissues. Musgrave et
al (35) found that skeletal
muscle satellite cells can also express BMP-2. In recent years,
other researchers have found that BMP-2 is involved in the
regulation of proliferation, differentiation and apoptosis of many
types of cells, thus affecting their biological behaviour (36–38).
In vitro experiments, Wei et al (39) found that BMP-2 could promote the
adhesion and proliferation of skeletal muscle satellite cells.
Our experiment found that the expression of BMP-2 in
the Mg-2Zn-0.5Nd alloy group was higher than that in the 317L alloy
group or the Ti-6Al-4V alloy group, suggesting that Mg-2Zn-0.5Nd
alloy can effectively promote the expression of BMP-2 and may play
an important role in promoting proliferation via the action of
BMP-2.
How does Mg-2Zn-0.5Nd promote the expression of
BMP-2? Studies have shown that the BMP-2 receptor (serine/threonine
kinase) is regulated by PI3K/AKT and the MAPK pathway (40). AKT kinase, which is activated by
growth factors, hormones and drugs, regulates cell proliferation
and survival (41). It has been
proved that magnesium ions can activate the PI3K/Akt signaling
pathway (42). In the process of
degradation, magnesium alloys can release magnesium ions to
regulate the expression of Akt and activate the downstream target
proteins, and then affect cell adhesion, proliferation and
differentiation. mTOR is a downstream factor of AKT which induces
cell differentiation (22,43). mTOR as a serine/threonine protein
kinase plays an important role in BMP2-induced changes in cell
metabolism (44). A previous study
demonstrated that the AKT-mTOR signalling axis plays a vital role
in mediating proliferation and apoptosis (45). To be more specific, mTOR negatively
regulates autophagy, which is manipulated by several upstream
activators such as PI3K-AKT and MAPK (46). Phosphorylation refers to the
addition of a phosphate group to a protein or other type of
molecule, thereby changing its activity. p-mTOR is the active form
of mTOR, and the activation of AKT is dependent on the
phosphorylation of AKT. Therefore, we measured the levels of both
p-AKT and p-mTOR, and found that both p-AKT and p-mTOR were
increased in the Mg-2Zn-0.5Nd alloy group. Furthermore, we
speculated that Mg-2Zn-0.5Nd can activate mTOR via the PI3K/AKT
pathway, and thus increase the expression of BMP-2.
FoxO1 is also one of the essential transcription
factors in the regulation of cell proliferation and differentiation
(47). Specifically, FoxO1, which
is expressed in skeletal muscle cells, Inhibit the proliferation of
skeletal muscle cells (48).
Yamashita et al (49) found
that an increased expression of FoxO1 reduced the proliferation of
muscle cells, and suggested that FoxO1 could inhibit the
proliferation of skeletal muscle cells in vitro. In this
study, p-FoxO1 levels were increased, suggesting that Mg-2Zn-0.5Nd
alloy can activate p-FoxO1, thereby inhibiting the excessive growth
of cells. In our experiments, we found that the expression of p-P38
was not increased. p-P38 is the active form of P38 and the upstream
factor of the MAPK pathway. We therefore speculate that
Mg-2Zn-0.5Nd alloy does not affect the differentiation of L6 cells
through the p38/MAPK pathway.
Above all, in the present study we demonstrate that
the novel alloy Mg-2Zn-0.5Nd shows no cytotoxicity in vitro
and even exhibits a stimulatory effect on cell proliferation.
Meanwhile, further studies into the molecular mechanisms suggests
that Mg-2Zn-0.5Nd may affect BMP-2 protein expression through the
PI3K/AKT/mTOR pathway and thus promote the proliferation of L6
cells.
Acknowledgements
The authors would like to thank the First Affiliated
Hospital Laboratory Centre of China Medical University (Liaoning,
China) for kindly providing the equipment required during the
present study.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81470998, 81071460
and 81271996) and the Natural Science Foundation of Liaoning
Province (grant no. 20170541033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL performed the experiments and wrote the article.
LG contributed to the design of the study and revised the
manuscript. TJ and SN performed the data analysis and revised the
manuscript. YZ performed the western blot analysis and revised the
manuscript. All the authors read and approved the final version to
be published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gu X, Zheng Y, Zhong S, Xi T, Wang J and
Wang W: Corrosion of, and cellular responses to Mg-Zn-Ca bulk
metallic glasses. Biomaterials. 31:1093–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Gu X, Lou S and Zheng Y: The
development of binary Mg-Ca alloys for use as biodegradable
materials within bone. Biomaterials. 29:1329–1344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu L, Yu G, Zhang E, Pan F and Yang K: In
vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant
application. J Biomed Mater Res A. 83:703–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Witte F, Fischer J, Nellesen J, Crostack
HA, Kaese V, Pisch A, Beckmann F and Windhagen H: In vitro and in
vivo corrosion measurements of magnesium alloys. Biomaterials.
27:1013–1018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang S, Zhang X, Zhao C, Li J, Song Y,
Xie C, Tao H, Zhang Y, He Y, Jiang Y and Bian Y: Research on an
Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 6:626–640.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duygulu O, Kaya RA, Oktay G and Kaya AA:
Investigation on the potential of magnesium alloy AZ31 as a bone
implant. Materials Science Forum. 546–549:421–424. 2007. View Article : Google Scholar
|
|
7
|
Rosalbino F, De Negri S, Saccone A,
Angelini E and Delfino S: Bio-corrosion characterization of Mg-Zn-X
(X=Ca, Mn, Si) alloys for biomedical applications. J Mater Sci
Mater Med. 21:1091–1098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song G: Control of biodegradation of
biocompatable magnesium alloys. Corrosion Science. 49:1696–1701.
2007. View Article : Google Scholar
|
|
9
|
Watt NT, Taylor DR, Kerrigan TL, Griffiths
HH, Rushworth JV, Whitehouse IJ and Hooper NM: Prion protein
facilitates uptake of zinc into neuronal cells. Nat Commun.
3:11342012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Xu Z, Smith C and Sankar J: Recent
advances on the development of magnesium alloys for biodegradable
implants. Acta Biomater. 10:4561–4573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seitz JM, Eifler R, Stahl J, Kietzmann M
and Bach FW: Characterization of MgNd2 alloy for potential
applications in bioresorbable implantable devices. Acta Biomater.
8:3852–3864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feyerabend F, Fischer J, Holtz J, Witte F,
Willumeit R, Drücker H, Vogt C and Hort N: Evaluation of short-term
effects of rare earth and other elements used in magnesium alloys
on primary cells and cell lines. Acta Biomater. 6:1834–1842. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Zhang R, Chen D, Oyajobi BO and
Zhao M: Functional redundancy of type II BMP receptor and type IIB
activin receptor in BMP2-induced osteoblast differentiation. J Cell
Physiol. 227:952–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tachi K, Takami M, Sato H, Mochizuki A,
Zhao B, Miyamoto Y, Tsukasaki H, Inoue T, Shintani S, Koike T, et
al: Enhancement of bone morphogenetic protein-2-induced ectopic
bone formation by transforming growth factor-β1. Tissue Eng Part A.
17:597–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: A critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang B, Lin X, Yang C, Tan J, Li W and
Kuang H: Sambucus williamsii hance promotes mc3t3-e1 cells
proliferation and differentiation via bmp-2/smad/p38/jnk/runx2
signaling pathway. Phytother Res. 29:1692–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei L, Lei GH, Yi HW and Sheng PY: Bone
formation in rabbit's leg muscle after autologous transplantation
of bone marrow-derived mesenchymal stem cells expressing human bone
morphogenic protein-2. Indian J Orthop. 48:347–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SY, Auh QS, Kang SK, Kim HJ, Lee JW,
Noh K, Jang JH and Kim EC: Combined effects of dentin sialoprotein
and bone morphogenetic protein-2 on differentiation in human
cementoblasts. Cell Tissue Res. 357:119–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SK, Chung JH, Choi SC, Auh QS, Lee YM,
Lee SI and Kim EC: Sodium hydrogen sulfide inhibits nicotine and
lipopolysaccharide-induced osteoclastic differentiation and
reversed osteoblastic differentiation in human periodontal ligament
cells. J Cell Biochem. 114:1183–1193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hardie DG: AMP-activated/SNF1 protein
kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han D, Li SJ, Zhu YT, Liu L and Li MX:
LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer.
Asian Pac J Cancer Prev. 14:4033–4039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pantovic A, Krstic A, Janjetovic K, Kocic
J, Harhaji-Trajkovic L, Bugarski D and Trajkovic V: Coordinated
time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy
controls osteogenic differentiation of human mesenchymal stem
cells. Bone. 52:524–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeh LC, Ma X, Ford JJ, Adamo ML and Lee
JC: Rapamycin inhibits BMP-7-induced osteogenic and lipogenic
marker expressions in fetal rat calvarial cells. J Cell Biochem.
114:1760–1771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grozinsky-Glasberg S, Rubinfeld H,
Nordenberg Y, Gorshtein A, Praiss M, Kendler E, Feinmesser R,
Grossman AB and Shimon I: The rapamycin-derivative RAD001
(everolimus) inhibits cell viability and interacts with the
Akt-mTOR-p70S6K pathway in human medullary thyroid carcinoma cells.
Mol Cell Endocrinol. 315:87–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grozinsky-Glasberg S, Franchi G, Teng M,
Leontiou CA, de Oliveira Ribeiro A Jr, Dalino P, Salahuddin N,
Korbonits M and Grossman AB: Octreotide and the mTOR inhibitor
RAD001 (everolimus) block proliferation and interact with the
Akt-mTOR-p70S6K pathway in a neuro-endocrine tumour cell Line.
Neuroendocrinology. 87:168–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fischer J, Pröfrock D, Hort N, Willumeit R
and Feyerabend F: Reprint of: Improved cytotoxicity testing of
magnesium materials. Materials Science and Engineering: B.
176:1773–1777. 2011. View Article : Google Scholar
|
|
27
|
Ding W: Opportunities and challenges for
the biodegradable magnesium alloys as next-generation biomaterials.
Regen Biomater. 3:79–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hornberger H, Virtanen S and Boccaccini A:
Biomedical coatings on magnesium alloys-a review. Acta Biomater.
8:2442–2455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seal CK, Vince K and Hodgson MA:
Biodegradable surgical implants based on magnesium alloys-A Review
of Current ResearchIOP Conference Series: Materials Science and
Engineering. IOP Publishing; Bristol: pp. p0120112009
|
|
30
|
Virtanen S: Biodegradable Mg and Mg
alloys: Corrosion and biocompatibility. Materials Science and
Engineering: B. 176:1600–1608. 2011. View Article : Google Scholar
|
|
31
|
Witte F, Hort N, Vogt C, Cohen S, Kainer
KU, Willumeit R and Feyerabend F: Degradable biomaterials based on
magnesium corrosion. Current Opinion in Solid State and Materials
Science. 12:63–72. 2008. View Article : Google Scholar
|
|
32
|
Seo HJ, Cho YE, Kim T, Shin HI and Kwun
IS: Zinc may increase bone formation through stimulating cell
proliferation, alkaline phosphatase activity and collagen synthesis
in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 4:356–361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Zhang JC, Chen Y, Xiao PG and Yang
MS: Effect of zinc ion on the osteogenic and adipogenic
differentiation of mouse primary bone marrow stromal cells and the
adipocytic trans-differentiation of mouse primary osteoblasts. J
Trace Elem Med Biol. 21:84–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J, Tan L, Peng W, Yu X and Ke Y: Study
on microstructure and properties of extruded Mg-2Nd-0.2Zn alloy as
potential biodegradable implant material. Mater Sci Eng C Mater
Biol Appl. 49:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Musgrave DS, Pruchnic R, Wright V, Bosch
P, Ghivizzani SC, Robbins PD and Huard J: The effect of bone
morphogenetic protein-2 expression on the early fate of skeletal
muscle-derived cells. Bone. 28:499–506. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng J, Yang G, Yuan G, Gluhak-Heinrich J,
Yang W, Wang L, Chen Z, McDaniel Schulze J, Donly KJ, Harris SE, et
al: Abnormalities in the Enamel in Bmp2-Deficient Mice. Cells
Tissues Organs. 194:216–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL,
Kim JS and Yoo YA: Metastatic function of BMP-2 in gastric cancer
cells: The role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9
expression. Exp Cell Res. 317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakase T and Yoshikawa H: Potential roles
of bone morphogenetic proteins (BMPs) in skeletal repair and
regeneration. J Bone Miner Metab. 24:425–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei K, Pei G and Dan JI: Effects of
recombinant human bone morphogenetic protein-2 on the proliferation
and adhension of skeletal muscle satellite cells. Chin J Rehab Med.
18:416–417. 2003.
|
|
40
|
Ghosh-choudhury N, Abboud SL, Nishimura R,
Celeste A, Mahimainathan L and Choudhury GG: Requirement of
BMP-2-induced phosphatidylinositol 3-kinase and Akt
serine/threonine kinase in osteoblast differentiation and
Smad-dependent BMP-2 gene transcription. J Biol Chem.
277:333612002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng W, Wang H, Zeng Z, Lin J, Little PJ,
Srivastava LK and Quirion R: The possible role of the Akt signaling
pathway in schizophrenia. Brain Res. 1470:145–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akca E and Gursel A: The Effect of
Diffusion Welding Parameters on the Mechanical Properties of
Titanium Alloy and Aluminum Couples. Metals. 7:222017. View Article : Google Scholar
|
|
43
|
Foster KG and Fingar DC: Mammalian target
of rapamycin (mTOR): Conducting the cellular signaling symphony. J
Biol Chem. 285:14071–14077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang MH, Zhou XM, Zhang MY, Shi L, Xiao
RW, Zeng LS, Yang XZ, Zheng XFS, Wang HY and Mai SJ: BMP2 promotes
proliferation and invasion of nasopharyngeal carcinoma cells via
mTORC1 pathway. Aging (Albany NY). 9:1326–1340. 2017.PubMed/NCBI
|
|
45
|
Park KR, Nam D, Yun HM, Lee SG, Jang HJ,
Sethi G, Cho SK and Ahn KS: β-Caryophyllene oxide inhibits growth
and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1
pathways and ROS-mediated MAPKs activation. Cancer Lett.
312:178–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McGonnell IM, Grigoriadis AE, Lam EW,
Price JS and Sunters A: A specific role for phosphoinositide
3-kinase and AKT in osteoblasts? Front Endocrinol (Lausanne).
3:882012.PubMed/NCBI
|
|
47
|
Van der Vos KE and Coffer PJ: FOXO-binding
partners: It takes two to tango. Oncogene. 27:2289–2299. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Behl Y, Siqueira M, Ortiz J, Li J, Desta
T, Faibish D and Graves DT: Activation of the acquired immune
response reduces coupled bone formation in response to a
periodontal pathogen. J Immunol. 181:8711–8718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamashita A, Hatazawa Y, Hirose Y, Ono Y
and Kamei Y: FOXO1 delays skeletal muscle regeneration and
suppresses myoblast proliferation. Biosci Biotechnol Biochem.
80:1531–1535. 2016. View Article : Google Scholar : PubMed/NCBI
|