Introduction
Breast cancer is the most common life-threatening
cancer type among women worldwide (1). In spite of numerous improvements in
early diagnosis, surgical treatment and endocrine therapy, ~30% of
patients with breast cancer do not respond to these standard
treatments (2). Depending on the
subtype (2,3), the average survival time of these
unresponsive patients is 1–4 years, which thus represents a
significant unsolved medical challenge. The development of more
effective therapeutic drugs is urgently needed. As a family of
transcription factors, nuclear factor (NF)-κB plays critical roles
in cell proliferation, differentiation and survival, and
inflammation and immunity (4). The
NF-κB subunits, including RelA (p65), RelB, c-Rel, NF-κB1, and
NF-κB2, are held in the cytoplasm by the specific inhibitors IκBs
(IκBα, IκBβ, and IκBγ). Inflammation in the tumor microenvironment
can activate IκB kinases (IKKs), which phosphorylate and degrade
IκB. As a result, NF-κB is released to the cell nucleus and may
promote cancer invasion and metastasis. Many tumors, including
breast cancer (5,6), have been observed to have aberrant
NF-κB activation. Therefore, inhibition of the NF-κB pathway is
expected to prevent and treat cancer.
As a traditional folk medicine, Salicornia
plants have been used to treat hypertension, cephalalgia, scurvy
and cancer (7). Bigelovii A is a
30-nortriterpenoid glycoside, isolated from Salicornia
bigelovii Torr (8). Yan et
al (9) reported that Bigelovii
A could also mitigate LPS-induced acute lung injury by suppressing
NF-κB and CCAAT/enhancer-binding protein δ pathways. Our previous
studies indicated that Bigelovii A exhibited potential cytotoxicity
against HL-60, MCF7, HepG2, A549, Lovo and LN229 cells (8,10,11).
In particular in HL-60 cells, Bigelovii A decreased the expression
of apoptosis regulator Bcl-2 (Bcl-2), activated caspase-3 and
induced cell apoptosis (10).
However, the anti-tumor mechanism of Bigelovii A in human breast
cancer cells has not been examined. The current study initially
compared the cytotoxicity of Bigelovii A in three human cancer cell
types (MCF7, MDA-MB-231 and MDA-MB-468). Subsequently, the
apoptotic effects mediated by IKK/NF-κB signaling pathways were
investigated.
Materials and methods
Reagents
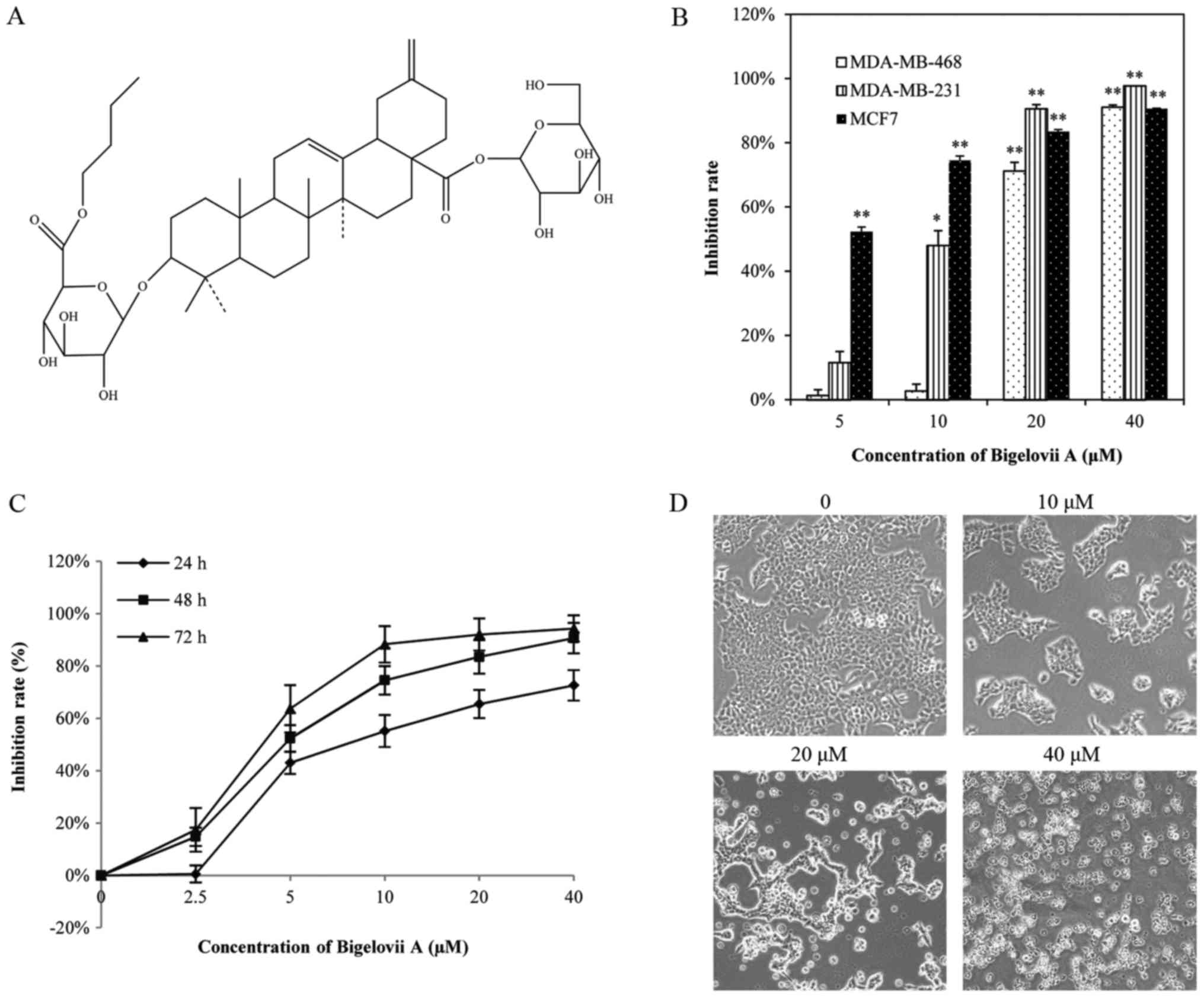
Bigelovii A (Fig.
1) was isolated from Salicornia bigelovii Torr. and
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to a 100 mM stock solution. The final DMSO
concentration in the medium did not exceed 0.1% (v/v) throughout
the study. Fetal bovine serum and modified Eagle's medium (MEM)
were provided by Gibco™ (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Antibiotics, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl
tetrazolium bromide (MTT) and RIPA lysis buffer were purchased from
Nanjing Sunshine Biotechnology Co., Ltd. (Nanjing, China). An
Annexin V-PE apoptosis kit was provided by BD Biosciences (Franklin
Lakes, NJ, USA). Primary antibodies against NF-κB pathway sampler
kit (cat. no. 9936), Cyclin D1 (cat. no. 2978), COX-2 (cat. no.
4482), p-Bcl-2 (cat. no. 2827), Bcl-2 (cat. no. 2870), B-cell
lymphoma-extra large (Bcl-xl; cat. no. 2764), cleaved caspase-3
(cat. no. 9936), cleaved caspase-7 (cat. no. 8438), cleaved
caspase-9 (cat. no. 9505), cleaved PARP (cat. no. 5625), cleaved
caspase-8 (cat. no. 9496) and β-actin (cat. no. 4970), horseradish
peroxidase (HRP)-conjugated secondary antibody and an enhanced
chemiluminescence (ECL) kit were supplied by Cell Signaling
Technology, Inc. (Danvers, MA, USA). NE-PER™ Nuclear and
Cytoplasmic Extraction Reagents, Halt™ Protease Inhibitor Cocktail
(EDTA-Free) and a LightShift Chemiluminescent EMSA kit were
purchased from Thermo Fisher Scientific, Inc. Hoechst 33258 and a
Cellular NF-κB Translocation kit were obtained from Beyotime
Institute of Biotechnology (Haimen, China).
Cell culture
Three human breast cancer cell lines (MCF7,
MDA-MB-231 and MDA-MB-468) were purchased from the Cell Bank of
Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China). They were propagated using
the methods provided. MCF7 cells were cultured in MEM supplemented
with 10% fetal bovine serum, 10 µg/ml insulin, 100 U/ml penicillin
and 100 µg/ml streptomycin, while MDA-MB-231 and MDA-MB-468 cells
were cultured without insulin.
Cell viability assays
The effect of Bigelovii A on cell viability was
determined by MTT assay. Cells were seeded at a density of 1×104
cells/well in 96-well plates and treated with Bigelovii A at
various concentrations. Following incubation for the indicated
time, 5 mg/ml MTT was added to each well and incubated in the CO2
incubator for 4 h. The formazan crystals were dissolved in DMSO and
absorbance was recorded on an ELISA reader (Tecan Group, Ltd.,
Mannedorf, Switzerland) at a test wavelength of 570 nm and a
reference wavelength of 690 nm. Inhibition rate was calculated by
the following formula: Inhibition rate (%)=(1-ODt/ODc)×100%. ODt
and ODc denoted the average absorbance of treated groups and
control groups, respectively. IC50 was considered to be the
concentration that caused 50% inhibition rate.
Annexin V-PE/7-AAD analysis and
Hoechst 33258 staining for cell apoptosis
MCF7 cells were plated at a density of 4×105
cells/well. Following treatment with 5, 10 or 20 µM Bigelovii A for
24 h, cells were washed twice with PBS, resuspended in binding
buffer, then incubated with 7-AAD and Annexin V-PE in the dark for
15 min. The samples were analyzed using an Accuri C6 flow cytometer
(BD Biosciences) for 1 h. Then, the apoptotic morphology was
studied using the Hoechst 33258 dye. Cells (1×106/ml) were seeded
on glass slides in 6-well tissue culture plates. After treatment
with 20 µM Bigelovii A for 24 h, cells were fixed with 4%
formaldehyde for 10 min, washed with PBS twice and stained with
Hoechst 33258 dye for 5 min. Stained nuclei were observed at
magnification, ×100 under an IX51 fluorescence microscope.
Propidium iodide (PI) staining for
cell cycle analysis
MCF7 cells (1×106/well) were treated with different
doses of Bigelovii A and incubated for 24 h. Floating and adherent
cells were harvested and washed with cold PBS. The cell pellet was
fixed in 70% ethanol overnight at 4°C, incubated with 100 µg/ml
RNaseA for 30 min at 37°C and stained with PI (50 µg/ml) for 30 min
at 4°C. Cell cycle distribution was analyzed in an Accuri C6 flow
cytometer (BD Biosciences).
Mitochondrial membrane potential
Following treatment with Bigelovii A (0, 5, 10 and
20 µM) for 24 h, MCF7 cells were washed twice with cold PBS and
incubated with Rh123 in a CO2 incubator for 30 min. The stained
cells were collected by centrifugation, washed twice with PBS, then
analyzed by flow cytometry.
Isolation of total cellular proteins,
cytosolic and nuclear proteins
MCF7 cells were cultured to 80% confluence and
treated with various concentrations of Bigelovii A for the
indicated times. Total cellular proteins were isolated with RIPA
lysis buffer, with the lysates centrifuged for 15 min at 12,000 × g
and 4°C. Cytosolic and nuclear proteins were separated according to
the manufacturer's recommended protocol. After washing, cells were
trypsinized and centrifuged at 500 × g for 5 min. Cells were lysed
with ice-cold CER I, vigorously vortexed on the highest setting for
15 sec, then incubated on ice for 10 min. Ice-cold CER II was added
to the tube, then the tube was vortexed for 5 sec on the highest
setting, and centrifuged for 5 min at 16,000 × g. The supernatants
were saved as the cytoplasmic fractions and the nuclear pellets
were resuspended in ice-cold NER. The tubes were vortexed on the
highest setting for 15 sec, then placed on ice. Vortexing was
continued for 15 sec every 10 min, for a total of 40 min. The tube
was then centrifuged at 16,000 × g for 10 min and the nuclear
supernatant was immediately transferred to a clean, pre-chilled
tube.
Western blot analysis
The concentrations of total cellular proteins,
cytosolic and nuclear proteins were measured by the Bradford assay.
Equal amounts of proteins (50 µg) were fractionated on 10% SDS-PAGE
gels and transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% milk,
the blots were probed with specific primary antibodies at 4°C
overnight, followed by HRP-labeled secondary antibody at 37°C for 1
h. Immunoreactive proteins were visualized with the enhanced
chemiluminescence reagents. Anti-β-actin antibody was used to
ascertain equal loading of proteins.
Electrophoretic mobility shift assay
(EMSA)
EMSA was performed to investigate the inhibitory
effect of Bigelovii A on NF-κB activation. The nuclear extracts,
isolated as described above, were incubated with biotin-labeled
oligonucleotide probes (5′-AGTTGAGGGGACTTTCCCAGGC-3′ and
3′-TCAACTCCCCTGAAAGGGTCCG-5′) for 20 min at room temperature.
Unlabeled oligonucleotide was used to confirm the specificity of
binding. The formed DNA-protein complex was electrophoresed on 6%
non-denaturing polyacrylamide gel, transferred onto a nylon
membrane, and cross-linked for 10–15 min under 312 nm bulbs. The
nylon membrane was visualized using the Chemiluminescent EMSA kit
(Thermo Fisher Scientific, Inc.).
Immunocytochemistry for NF-κB p65
localization
The effect of Bigelovii A on NF-κB nuclear
translocation was examined by immunofluorescence confocal
microscopy according to the kit's protocol. MCF7 cells were plated
on glass coverslips in 6-well plates and treated with 10 µM
Bigelovii A for 24 h. Following washing, cells were incubated with
a blocking buffer at room temperature for 1 h and the primary NF-κB
p65 antibody at 4°C overnight. The slides were washed again in
washing buffer and incubated with Cy3-labeled secondary antibody
for 1 h. The nuclei were counterstained with DAPI for 5 min. The
stained slides were observed using a laser confocal microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of mRNA levels
RT-qPCR was performed as described in previous
reports (12,13). Briefly, total RNA was extracted
with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. First-strand cDNA was
synthesized using HiScript II Q RT SuperMix for qPCR (Vazyme
Biotech Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. The primers were designed using the software Primer
Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA) and
are listed in Table I. The
quantified expression levels of the tested genes were normalized
against the housekeeping gene β-actin. qPCR was performed using
SYBR-Green Master mix (Vazyme Biotech Co., Ltd.) and run on a
qTOWER2.2 Real-Time PCR system (Analytik Jena AG, Jena, Germany).
The conditions for quantitative analysis were as follows: 95°C for
5 min; 40 cycles of 95°C for 15 sec, 56°C for 15 sec, and 72°C for
20 sec; 72°C for 5 min. The specificity of each PCR reaction was
determined by melting curve analysis. Data for each sample were
calculated using the 2−ΔΔCq method (14).
 | Table I.Sequences of primers used for PCR and
RT-qPCR. |
Table I.
Sequences of primers used for PCR and
RT-qPCR.
| Gene | Primer sequence (5′
to 3′) |
|---|
| Bcl-2 |
|
|
Forward |
GGTCATGTGTGTGGAGAGCG |
|
Reverse |
CAGGGTGATGCAAGCTCCCA |
| Cyclin D1 |
|
|
Forward |
TGAACCTGAGGAGCCCCAAC |
|
Reverse |
GCCTTGGGGTCCATGTTCTGCT |
| COX-2 |
|
|
Forward |
GCAGTACAGAAAGTATCACAGGC |
|
Reverse |
CGATGTCACCATAGAGTGCTTCC |
| Bcl-xl |
|
|
Forward |
ATGGGGTAAACTGGGGTCGC |
|
Reverse |
GCATTGTTCCCATAGAGTTCCAC |
| β-actin |
|
|
Forward |
CCGACAGGATGCAGAAGGAG |
|
Reverse |
CTCGTCATACTCCTGCTTGCTG |
Statistical analysis
Data are presented as the mean ± standard deviation
from triplicate experiments. The statistical significance was
evaluated using Student's t-test to compare the difference between
two groups, and one-way analysis of variance followed by a
Newman-Keuls post-hoc test was used to perform multiple comparisons
(GraphPad Software, Inc., San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Bigelovii A inhibits the proliferation
of human breast cancer cells
To measure the cytotoxic effect of Bigelovii A,
three human breast cancer cell lines (MCF-7, MDA-MB-231 and
MDA-MB-468) were treated with 5, 10, 20 and 40 µM of Bigelovii A
for 48 h. As presented in Fig. 1B,
the inhibitory effect of Bigelovii A occurred in a
concentration-dependent manner, particularly in MCF7 cells (IC50:
4.10±1.19 µM). The inhibitory effect on MCF7 cells was also in a
time-dependent manner (Fig. 1C).
Microscopy observations indicated that MCF7 cells with treatment
with Bigelovii A became round and floated, while untreated cells
exhibited a epithelial and adherent cytoskeleton (Fig. 1D).
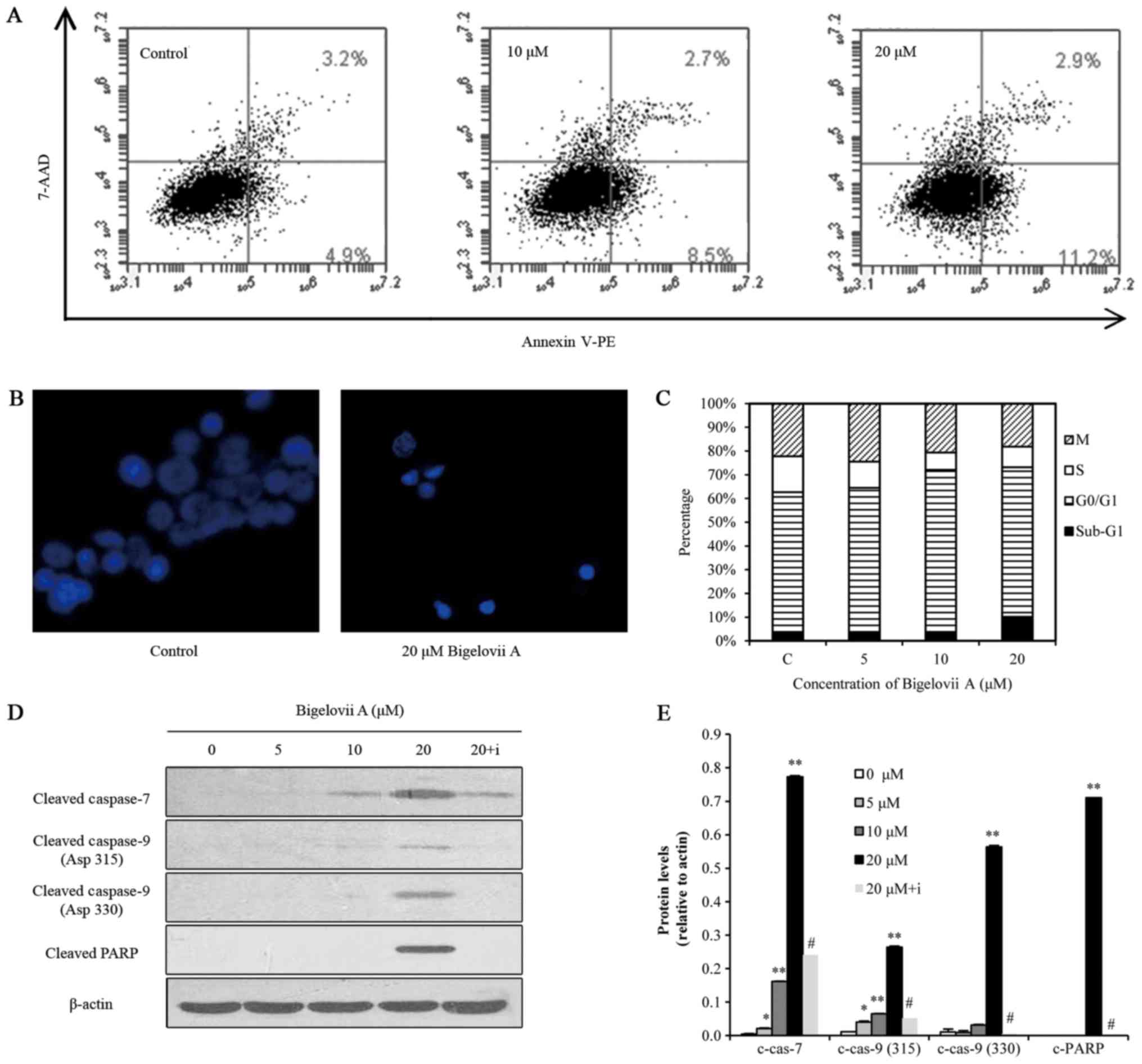
Bigelovii A induces apoptosis in MCF7
cells
To evaluate whether Bigelovii A induced apoptosis in
MCF7 cells, Annexin V-FITC, Hoechst 33258 staining, sub-G1 DNA
content, caspase and PARP cleavage assays were conducted (Fig. 2). Flow cytometric analysis of
Annexin V/7-AAD staining suggested that Bigelovii A increased the
percentage of Annexin V-positive MCF7 cells in a dose-dependent
manner (Fig. 2A), indicating the
activation of apoptosis. Hoechst 33258 staining (Fig. 2B) indicated that Bigelovii A at 20
µM induced chromatin fragmentation and condensation in MCF7 cells
following 24 h of treatment. Cell cycle analysis revealed that
treatment with this compound at 5 and 10 µM for 24 h resulted in
cell cycle arrest at G1 phase, while treatment at 20 µM induced the
hypodiploid sub-G1 phase, confirming an apoptotic effect (Fig. 2C). As indicated in Fig. 2D, treatment of MCF7 cells with
Bigelovii A (5, 10 or 20 µM) led to activation of caspase-7 and
caspase-9, and cleavage of PARP. Z-VAD-FMK, a pan caspase
inhibitor, reversed Bigelovii A-induced caspase and PARP
activation. These data indicated that Bigelovii A killed MCF7 cells
by inducing apoptosis. However, activation of caspase-3 and
caspase-8 was not detected. Thangaiyan and Sipra (15) reported that MCF-7 cells do not
express caspase-3. Bigelovii A activated caspase-9 instead of
caspase-8, indicating mitochondrion-mediated apoptosis.
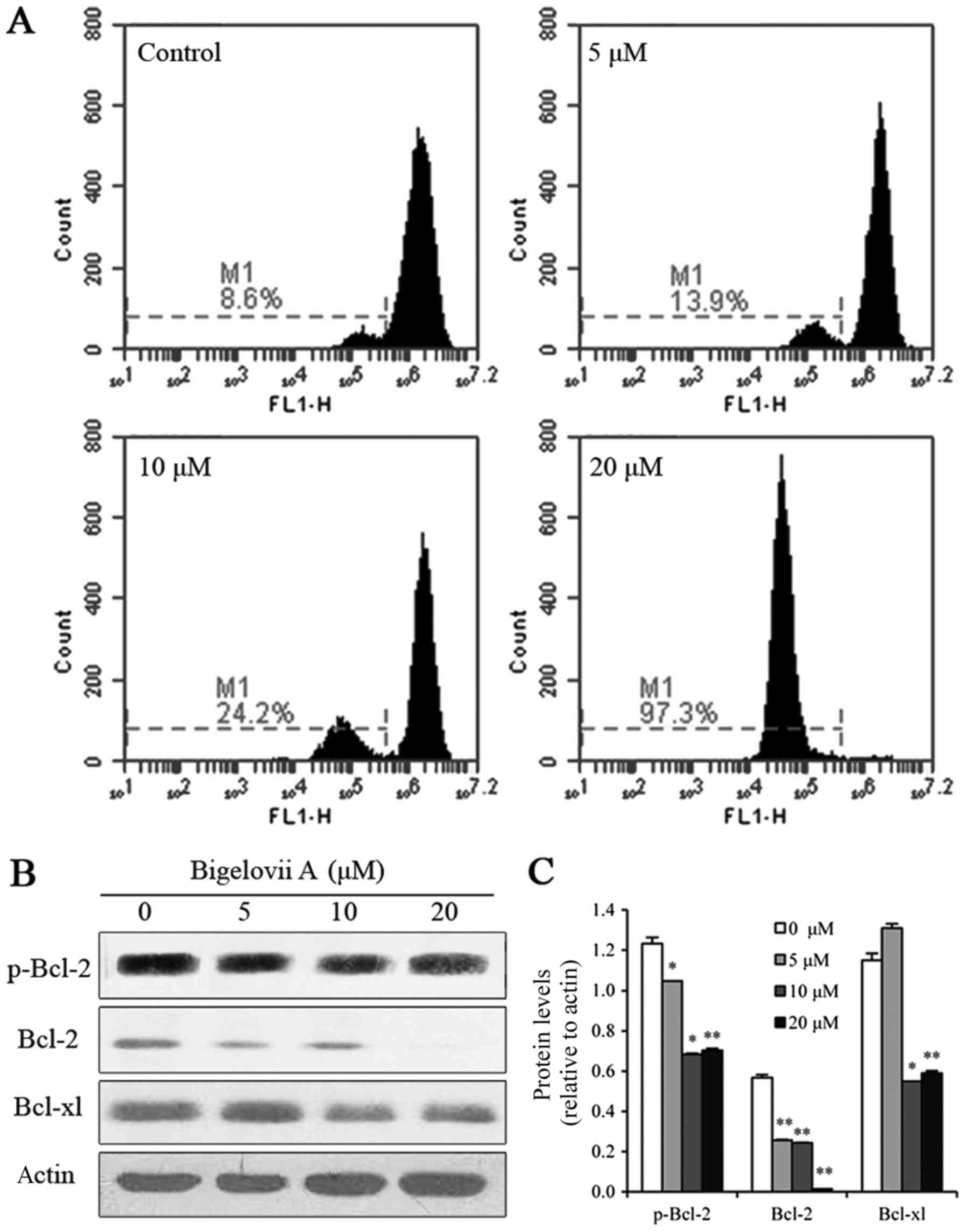
Bigelovii A induces apoptosis via
mitochondrial pathways
To confirm whether the mitochondrial pathway
contributed to the induction of apoptosis by Bigelovii A,
mitochondrial membrane potential was examined in MCF7 cells. As
indicated in Fig. 3, loss of
mitochondrial membrane potential was observed following treatment
with Bigelovii A for 24 h (8.6, 13.9, 24.2 and 97.3% at 0, 5, 10
and 20 µM Bigelovii A, respectively). To further confirm that
Bigelovii A induced mitochondrial damage, the levels of Bcl-2
family proteins (p-Bcl-2, Bcl-2 and Bcl-xl) were measured by
western blot analysis. These anti-apoptotic Bcl-2 proteins
(p-Bcl-2, Bcl-2 and Bcl-xl) were all markedly decreased following
exposure to Bigelovii A for 24 h. In addition, as indicated in
Fig. 2D, caspase-9 instead of
caspase-8 was activated. Therefore, the apoptosis induced by
Bigelovii A was mediated via the mitochondrial pathway.
Apoptosis induced by Bigelovii A
involves inhibition of NF-κB
Bcl-2 and Bcl-xl are negative regulators of
apoptosis and their overexpression can be induced by NF-κB
(16,17). Significantly decreased expression
levels of Bcl-2 and Bcl-xl in MCF7 cells were induced by Bigelovii
A, suggesting that its pro-apoptotic effect may lead to NF-κB
inactivation. In order to investigate the effect of Bigelovii A on
the NF-κB pathway, the effect of Bigelovii A on the translocation
and phosphorylation at serine residue 536 of p65 were analyzed,
since phosphorylation is necessary for the transcriptional activity
of p65. Cytoplasmic total p65 level was not altered, while
cytoplasmic phosphorylated p65 was decreased. Meanwhile, nuclear
total p65 expression and nuclear phosphorylated p65 expression were
both reduced (Fig. 4A and B).
These results indicated that Bigelovii A inhibited the
translocation and phosphorylation of p65. Furthermore,
immunocytochemical analysis demonstrated that Bigelovii A inhibited
the translocation of p65 to the nucleus in MCF7 cells (Fig. 4C). For cells without any treatment,
some p65 was translocated to the nucleus, whereas for cells treated
with Bigelovii A, nearly all p65 was fixed in the cytoplasm. These
results supported the notion that Bigelovii A inhibited the
translocation of p65. Next, in order to investigate the role of
Bigelovii A in regulating NF-κB DNA binding activity in MCF-7
breast cancer cells, cells were treated with 5, 10 or 20 µM
Bigelovii A for 24 h and EMSA was performed. The results indicated
that DNA binding activity of NF-κB decreased as the concentration
of Bigelovii A increased (Fig.
4D). Phosphorylation of the inhibitor IκB by IKKs is a vital
step for the nuclear translocation and activity of NF-κB (18). Fig. 4E
and F indicated that Bigelovii A treatment for 4 h decreased
phosphorylation levels of IKKα, IKKβ and IκBα, and increased the
expression levels of IκBα, while there was no effect on the
expression levels of IKKα and IKKβ. In addition to Bcl-2 and
Bcl-xl, COX-2 and Cyclin D1 are also NF-κB-regulated genes
(19,20). It was identified that in MCF7
cells, Bigelovii A decreased protein expression of COX-2 and Cyclin
D1 (Fig. 5A). RT-qPCR analysis was
performed to further confirm Bcl-2, Bcl-xl, COX-2 and Cyclin D1
gene alterations. As indicated in Fig.
5B, Bigelovii A suppressed Bcl-2, Bcl-xl, COX-2 and Cyclin D1
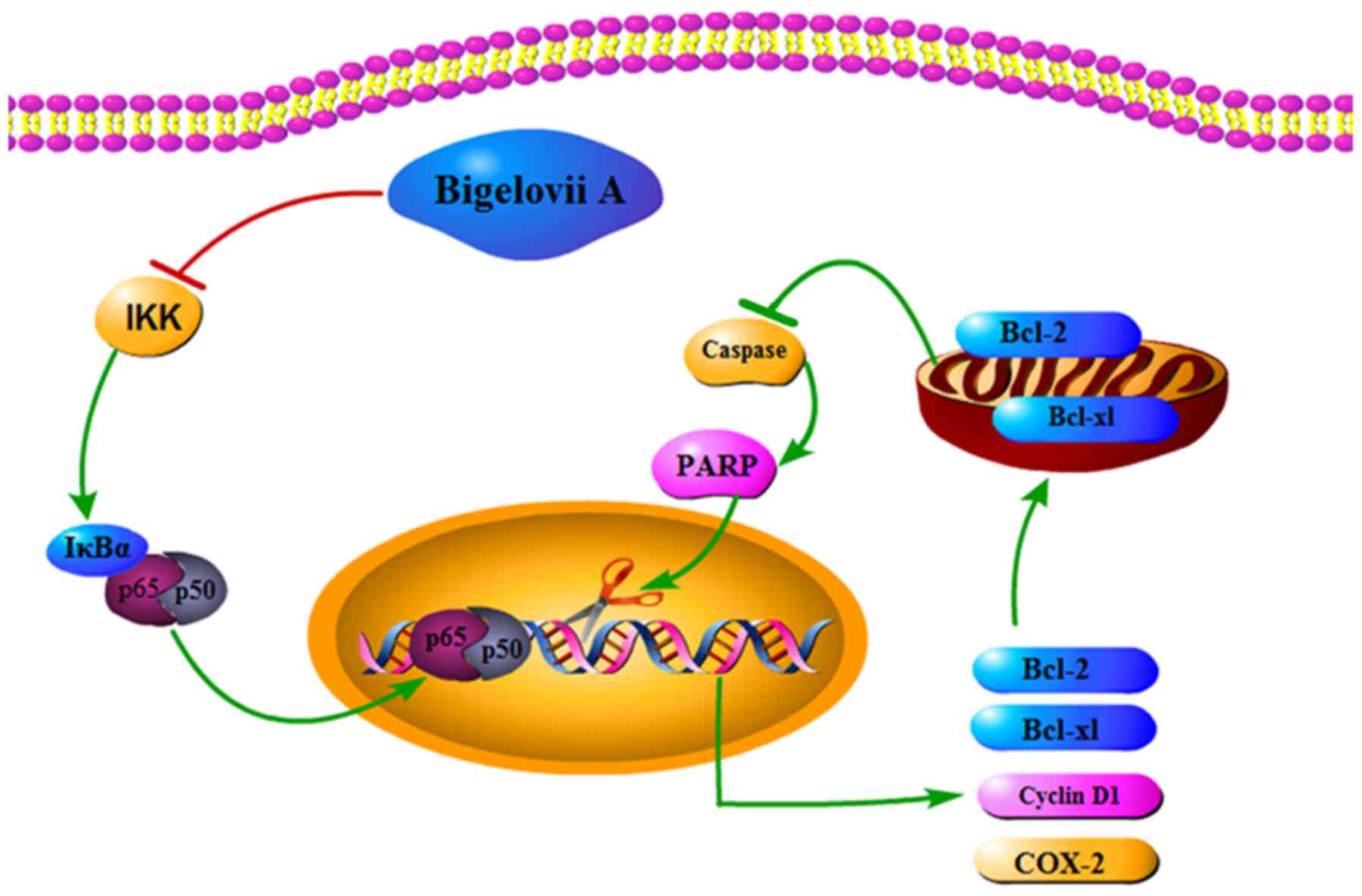
mRNA levels. Fig. 6 demonstrated
the role of Bigelovii A in blocking NF-κB activation and mediating
cell apoptosis.
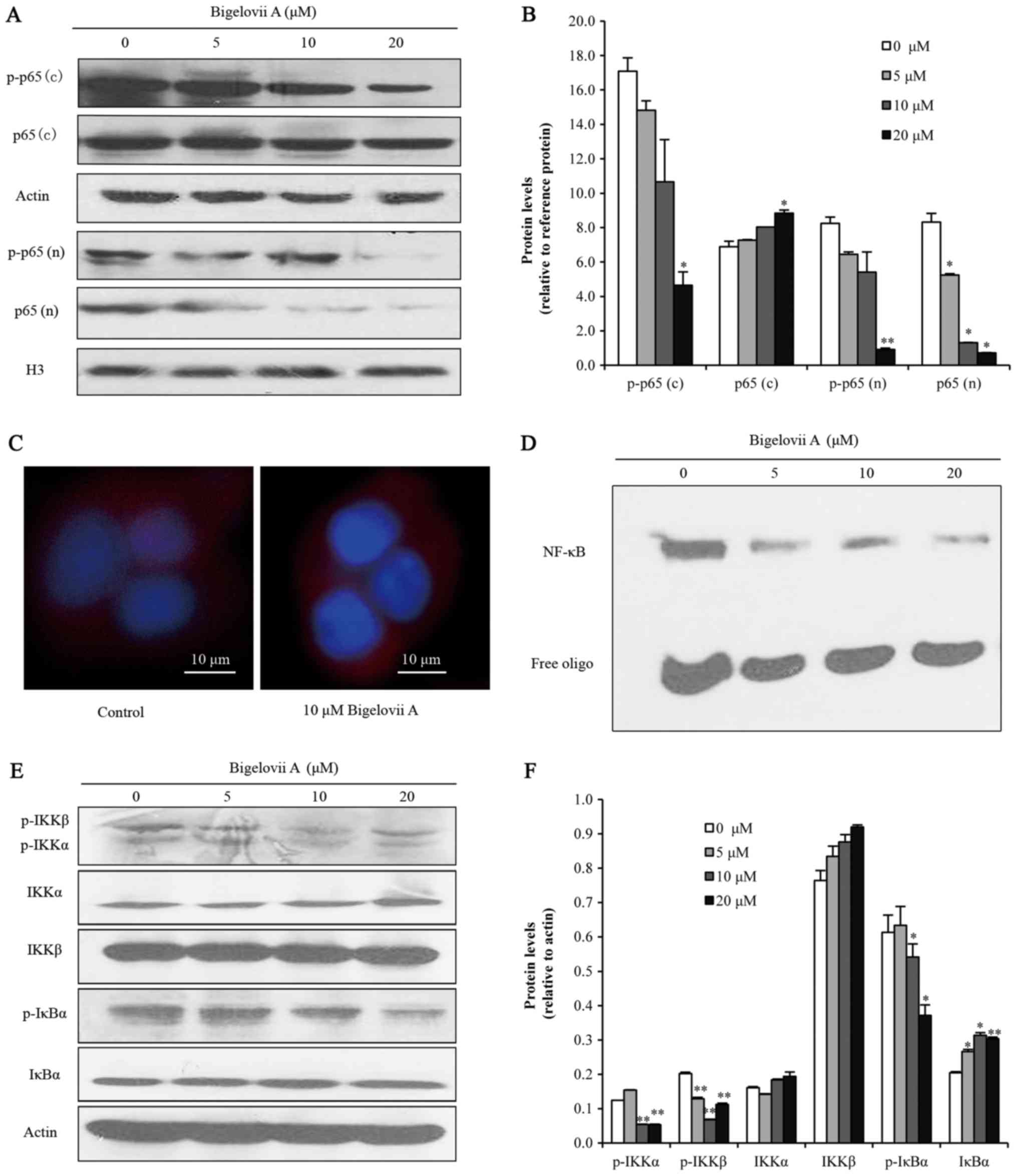 | Figure 4.Bigelovii A blocked the IKK/NF-κB
pathway. (A and B) Protein levels of p65 and phosphorylated p65 in
the cytoplasm (C) and nucleus (N) were analyzed by western blotting
and quantified by Image J. For loading control of cytoplasmic and
nuclear proteins, the membranes were reblotted with β-actin and H3
antibody, respectively. (C) Nuclear translocation of p65 was
analyzed by immunocytochemistry. Blue indicates nuclei and red
indicates p65. (D) NF-κB DNA binding activity was assayed by EMSA
with nuclear extracts. (E and F) Following treatment with Bigelovii
A for 4 h, total protein extracts were analyzed and quantified for
the expression levels of p-IKK, IKKα, IKKβ, p-IκBα and IκBα.
*P<0.05, **P<0.01 vs. 0 µM. EMSA, electrophoretic mobility
shift assay; NF, nuclear factor; IKK, IκB kinase. |
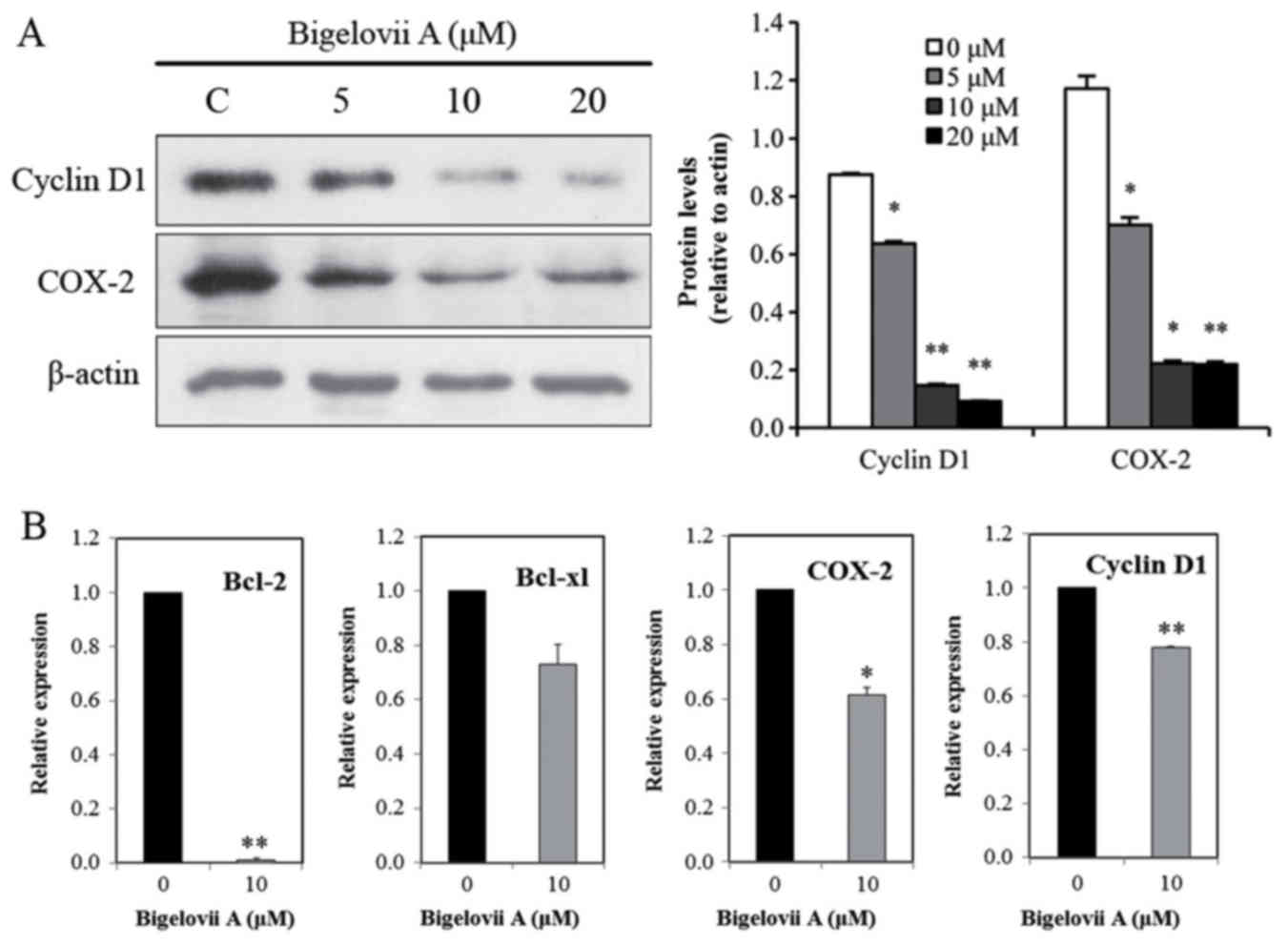 | Figure 5.Effects on NF-κB-dependent gene
expression of Bigelovii A. (A) Western blotting analysis. Following
treatment with Bigelovii A for 24 h, total protein extracts were
isolated and subjected to western blotting for Cyclin D1 and COX-2.
(B) Effects of Bigelovii A on levels of Bcl-2, Bcl-xl, COX-2 and
Cyclin D1 mRNA. Cells were treated with 10 µM Bigelovii A. Total
RNA was extracted at 24 h, and was examined by RT-qPCR for Bcl-2,
Bcl-xl, COX-2 and Cyclin D1. β-actin was used as an internal
control. *P<0.05, **P<0.01 vs. 0 µM. NF, nuclear factor;
Bcl-xl, B-cell lymphoma-extra large; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Discussion
Breast cancer is the leading cause of
cancer-associated cases of mortality in women worldwide (21). Plant-derived triterpenoids are
considered to be promising agents in treating breast cancer
(22). In the current study,
Bigelovii A, a new triterpenoid isolated from Salicornia
bigelovii Torr., was shown to inhibit the growth of MCF7 cells
in dose-dependent and time-dependent manners. MCF-7 cells were
arrested in the G1 phase of the cell cycle and underwent apoptosis
in a dose-dependent manner following treatment with Bigelovii A, as
indicated by chromatin condensation, externalization of
phosphatidylserine on the plasma membrane, hypodiploid DNA, caspase
activation and PARP cleavage.
Two apoptotic pathways have been identified in
cells, regulated by either the death receptor or mitochondria
(23). The mitochondrion
transduction pathway is regulated by the Bcl-2 protein family
(15). Pro-apoptotic protein Bax
is translocated to the mitochondrial outer membrane, after which
cytochrome c is released and MMP is disrupted. By contrast,
mitochondrial integrity is preserved by anti-apoptotic Bcl-2 in
order to inhibit the process. Caspase-9 is recruited and cleaved by
mitochondria-dependent death signal, while activation of caspase-2,
−8, or −10 is induced by death receptors. These caspases then
activate caspase-3, −6 and −7 (24), and PARP is cleaved into p24 and
p89, leading to DNA fragmentation and inducing apoptosis (25,26).
The current results suggested that Bigelovii A disrupted the
mitochondrial membrane potential. Western blotting indicated that
Bcl-2 anti-apoptotic protein was decreased, and caspase-7 and
caspase-9, instead of caspase-8, were activated following Bigelovii
A treatment. Therefore, Bigelovii A induced apoptosis via the
mitochondrion-mediated pathway.
Aberrant NF-κB activation is responsible for the
development of various cancer types (5). Inflammatory cytokines, including
tumor necrosis factor-α and interleukin-1β, are abundant in breast
cancer and activate NF-κB pathway in cancer cells (5,27).
The current results indicated that MCF7 cells had the ability to
constitutively express active NF-κB, which was consistent with two
recent reports by Liu et al (28) and Wang et al (29), which indicated constitutive NF-κB
activation in MCF7 cells on western blot analysis. In the current
study, Bigelovii A was demonstrated to inhibit constitutive NF-κB
activation in MCF7 cells. These results were in accordance with
previous reports from our laboratory (9) and other groups, that triterpenoids
are potent inhibitors of NF-κB activation (30). By using antibodies that
specifically detect the phosphorylated form of IκBα, we showed that
Bigelovii A blocks consitutive phosphorylation of IκBα. The
phosphorylation of IκBα is regulated by a large number of kinases
including IKK-α, IKK-β, IKK-γ, NIK, TAK1, Akt, and
mitogen-activated protein kinase kinase kinase 1 (31). Akt and NIK are primarily known to
activate IKK-α, whereas mitogen-activated protein kinase kinase
kinase 1 and atypical protein kinase C activate IKK-β. Bigelovii A
inhibited IKK activity without directly interfering with the IKK
protein. Thus, more detailed studies are warranted to identify one
or more of the upstream kinases responsible for IKK activation.
Inhibition of NF-κB by Bigelovii A significantly decreased
expression of several gene products regulated by NF-κB. The
expression of Bcl-2, Bcl-xl, cyclin D1 and COX-2, are known to be
regulated by NF-κB, whose synthesis process was inhibited by
Bigelovii A. Therefore, it was not surprising to identify that
Bigelovii A induced G1 arrest and apoptosis.
In conclusion, Bigelovii A decreased IKK kinase
activity, suppressed constitutive IκBα phosphorylation and nuclear
p65 expression, and reduced expression of the NF-κB-regulated gene
products Bcl-2, Bcl-xl, cyclin D1 and COX-2. These effects
inhibited the proliferation of MCF7 cells, arrested cells at the G1
phase boundary of the cell cycle and induced apoptosis.
Acknowledgements
The authors would like to thank Dr Sheng Xu, Dr Lu
Yan and Dr Xiaoqin Ding at the Institute of Botany, Jiangsu
Province and Chinese Academy of Sciences for performing RT-qPCR and
statistical analysis.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81402829, 31470425 and
31570359) and the Jiangsu Provincial Natural Science Foundation of
China (grant no. BK20131338).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FQG, YS and XF designed the study. FQG, MY, FL, YYZ
and JHZ performed the experiments. FQG, QZW, MW and YC analyzed the
data. FQG, MY and FL wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santa-Maria CA and Gradishar WJ: Changing
treatment paradigms in metastatic breast cancer: Lessons learned.
JAMA Oncol. 1:528–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Shaughnessy J: Extending survival with
chemotherapy in metastatic breast cancer. Oncologist. 10:20–29.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruland J: Return to homeostasis:
Downregulation of NF-κB responses. Nat Immunol. 12:709–714. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaturvedi MM, Sung B, Yadav VR, Kannappan
R and Aggarwal BB: NF-κB addiction and its role in cancer: ‘One
size does not fit all’. Oncogene. 30:1615–1630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fornier MN, Rathkopf D, Shah M, Patil S,
O'Reilly E, Tse AN, Hudis C, Lefkowitz R, Kelsen DP and Schwartz
GK: Phase I dose finding study of weekly docetaxel followed by
flavopiridol for patients with advanced solid tumors. Clin Cancer
Res. 13:5841–5846. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan FQ, Shan Y, Zhao YY, Wang QZ, Wang M,
Sun H, Chen Y, Yin M and Feng X: Research development on
pharmacological activities and mechanism of Salicornia
plants. Chin Pharm Bull. 29:1188–1191. 2013.
|
|
8
|
Wang QZ, Liu XF, Shan Y, Guan FQ, Chen Y,
Wang XY, Wang M and Feng X: Two new nortriterpenoid saponins from
Salicornia bigelovii Torr. and their cytotoxic activity.
Fitoterapia. 83:742–749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan C, Guan F, Shen Y, Tang H, Yuan D, Gao
H and Feng X: Bigelovii a protects against
lipopolysaccharide-induced acute lung injury by blocking NF-κB and
CCAAT/enhancer-binding protein δ pathways. Mediators Inflamm.
2016:92016042016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan FQ, Wang HT, Shan Y, Zhang DM, Zhao
YY, Chen Y, Wang QZ, Wang M and Feng X: Bigelovii A induces
apoptosis of HL60 human acute promyelocytic leukaemia cells. Mol
Med Rep. 7:1585–1590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan F, Wang Q, Wang M, Shan Y, Chen Y,
Yin M, Zhao Y, Feng X, Liu F and Zhang J: Isolation, identification
and cytotoxicity of a new noroleanane-type triterpene saponin from
Salicornia bigelovii Torr. Molecules. 20:6419–6431. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Cai HY, Zhao Q, Xing T, Li JH and
Wang NS: Epinephrine evokes renalase secretion via
α-adrenoceptor/NF-κB pathways in renal proximal tubular epithelial
cells. Kidney Blood Press Res. 39:252–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Zhang G, Lu Z, Geurts A M, Usa K,
Jacob HJ, Cowley AW, Wang N and Liang M: Antithrombin III/SerpinC1
insufficiency exacerbates renal ischemia/reperfusion injury. Kidney
Int. 88:796–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rabi T and Banerjee S: Novel Synthetic
triterpenoid methyl 25-hydroxy-3-oxoolean-12-en-28-oate induces
apoptosis through JNK and p38 MAPK pathways in human breast
adenocarcinoma MCF-7 cells. Mol Carcinog. 47:415–423. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sevilla L, Zaldumbide A, Pognonec P and
Boulukos KE: Transcriptional regulation of the bcl-x gene encoding
the antiapoptotic Bcl-xL protein by Ets, Rel/NF-κB, STAT and AP1
transcription factor families. Histol Histopathol. 16:595–601.
2001.PubMed/NCBI
|
|
18
|
Zandi E and Karin M: Bridging the gap:
Composition, regulation and physiological function of the IκB
kinase complex. Mol Cell Biol. 19:4547–4551. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pahl HL: Activators and target genes of
Rel/NF-κB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Plummer SM, Holloway KA, Manson MM, Munks
RJ, Kaptein A, Farrow S and Howells L: Inhibition of
cyclo-oxygenase 2 expression in colon cells by the chemopreventive
agent curcumin involves inhibition of NF-κB activation via the
NIK/IKK signalling complex. Oncogene. 18:6013–6020. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bishayee A, Ahmed S, Brankov N and Perloff
M: Triterpenoids as potential agents for the chemoprevention and
therapy of breast cancer. Front Biosci (Landmark Ed). 16:980–996.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashe PC and Berry MD: Apoptotic signaling
cascades. Prog Neuropsychopharmacol Biol Psychiatry. 27:199–214.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gambi N, Tramontano F and Quesada P: Poly
(ADPR)polymerase inhibition and apoptosis induction in cDDP-treated
human carcinoma cell lines. Biochem Pharmacol. 75:2356–2363. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scovassi AI and Poirier GG: Poly
(ADP-ribosylation) and apoptosis. Mol Cell Biochem. 199:125–137.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldberg JE and Schwertfeger KL:
Proinflammatory cytokines in breast cancer: Mechanisms of action
and potential targets for therapeutics. Curr Drug Targets.
11:1133–1146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A cytoplasmic NF-κB interacting
long noncoding RNA blocks IκB phosphorylation and suppresses breast
cancer metastasis. Cancer Cell. 27:370–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Zhao B, Yi Y, Zhang W, Wu X, Zhang
L and Shen Y: Mycoepoxydiene, a fungal polyketide inhibits MCF-7
cells through simultaneously targeting p53 and NF-κB pathways.
Biochem Pharmacol. 84:891–899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Oleanolic acid and its synthetic
derivatives for the prevention and therapy of cancer: Preclinical
and clinical evidence. Cancer Lett. 346:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: Critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012. View Article : Google Scholar : PubMed/NCBI
|