Introduction
Thyroid carcinoma is the most common endocrine
malignant tumor in the world, which accounts for 94.5% of all
endocrine tumors. The incidence of thyroid cancer has been
increasing since the end of last century and has ranked the top of
the list of head and neck cancers (1,2).
Papillary thyroid cancer (PTC) is the most common pathology type in
thyroid cancer, ~90% of thyroid carcinoma. 85–90% incidence of
thyroid cancer was caused by PTC. More women are involved in it
than men, and most of them are accompanied by cervical lymph node
metastasis. PTC is a low-grade malignancy, the main clinical
symptoms of which are the slow growth of thyroid mass and
multifocal occurrence, tendency of regional lymph nodes metastasis.
The prognosis of PTC is good after proper effective treatment, with
5-year survival rate of 95%, and 10-year survival rate of above 90%
(3). However, some PTC is of high
invasion ability, and some of them has the tendency of
dedifferentiation to form low-differentiated or non-differentiated
cancers and result in the decreasing of survival rate and life
quality (4).
The occurrence and development of thyroid cancer is
a complicated process including a variety of oncogenes, signaling
pathway and aberrant proteins, resulting in abnormal proliferation
and mutation. Therefore, study on PTC molecular mechanism will help
looking for new biomarkers for PTC early diagnosis, lymph nodes
metastasis prediction, treatment and prognosis.
Telomerase is a self-templated reverse
transcriptase, containing two subunits of TERC (telomerase RNA
component) and TERT (telomerase reverse transcriptase). As the core
subunit of telomerase, TERT catalyzes TERC reverse transcription to
regulate telomerase activity and maintain telomere length (5–7).
Over-expression of TERT could promote the proliferation of
mesenchymal stem cells, epithelial cells and nerve cells (8,9). For
a long time, studies on TERT were mainly focused on its maintaining
telomere length function to promote cell proliferation ceaselessly.
However, TERT has also been found non-telomere dependent functions
in recent years (10–12), including regulating gene expression
(13,14), cell signal pathway (15) or cell cycle (16), protecting mitochondrial DNA
(17), and regulating DNA injury
reaction (18). Researches before
discovered that TERT could regulate >300 downstream factors,
which were related to many kinds of cell signaling, cell
proliferation and cell cycle regulation (19).
TERT could regulate cell proliferation and cell
cycle by different signal pathways, to excert functions in tumors
and different tissues. As important signal pathways, Rb/E2F,
Wnt/β-catenin, and phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT) pathways were all reported to be related to TERT regulation
in different cells (20). However,
the mechanism of TERT function on PTC cells is still not clear now.
In order to illuminate the exact molecular mechanism, we performed
TERT over-expression and TERT silencing respectively in PTC cells,
to study function of TERT on PTC cells proliferation and related
signal pathways. It will provide new thoughts for the treatment of
PTC.
Materials and methods
Cell culture
Human PTC K1 cells were used in the present study.
Though we lately discovered K1 cells were actually cells mixed with
GLAG-66 cells, of the thyroid gland papillary carcinoma, it did not
affect studying function of the target gene on PTC, which was
verified by numerous researches before (21–30).
K1 cells (mixed thyroid gland papillary carcinoma cells) were
purchased from ATCC (Guangzhou, China) were cultured in Dulbecco's
modified Eagle's medium (DMEM) of 10% fetal bovine serum (FBS; both
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C
with 5% CO2. Cells of logarithm phase were used in our
research.
Recombined plasmid construction and
cell transfection
TERT siRNA (siTERT) sequence was synthesized by
Genepharma Company (Shanghai, China). Recombined TERT
over-expression plasmid and negative control (NC) were constructed
before cell transfection too. siTERT, TERT over-expression plasmid
and NC were transfected to mixed thyroid gland papillary carcinoma
cells respectively. Briefly, cells were first seeded in 6-well
plates at the initial concentration of 5×104 cells/well
and cultured in DMEM with 10% FBS and without antibiotic for 24 h,
Then, cells were washed by serum-free DMEM twice and cultured for
30 min in it. When cells were sufficient confluent, they were
transfected with siTERT group, recombined TERT plasmid (rTERT
group) and NC group by Lipofectamin 2000 (Invitrogen) respectively
and cultured for 6 h, according to the manufacturer's instructions.
Finally, cells were cultured in 10% FBS-containing DMEM for another
48 h, and the transfection efficiency was detected by RT-qPCR and
western blot.
Methyl thiazolyl tetrazolium (MTT)
assay
Cell proliferation of living cells in different
groups (Control, NC, siTERT, rTERT) was determined by MTT assay at
24, 48 and 72 h respectively, according to the manufacturer's
protocols (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells
(5×103/well) and 10 µl 5 mg/ml MTT reagents were added
and mixed in 96-well plates, with the incubation of 4 h in 5%
CO2-containing incubator at 37°C. Supernatant was
removed and 150 µl DMSO was added to dissolve the blue crystals
(formazan) to be measured under 490 nm by microplate reader
(Syngene Europe, Cambrige, UK). The higher the OD value was, the
more living cells and higher activity existed.
Carboxyfluorescein diacetate
succinimidyl ester (CFSE) assay
Cell proliferation of all cells including living
cells and dead cells in different groups (Control, NC, siTERT,
rTERT) was detected by CSFE cell proliferation kit (Invitrogen),
according to the manufacturer's protocols. Cells after 24 h
transfection were resuspended in 1 ml preheating phosphate buffer
solution (PBS) in sterile centrifuge tubes, at the final
concentration of 1×106/ml. 2 µl CFSE (5 mM) stocking
reagent was added into cell suspension to the final concentration
of 10 µM, and incubated for 10 min at 37°C after sufficient mixing.
Then cells were cultured in 10 ml icy DMEM with 10% bovine serum
for 5 min on ice in the dark. After centrifuged for 7 min at 1,000
rpm, cells were suspended and washed in 5 ml DMEM containing 10%
bovine serum for two times. After that, cells were inoculated in
24-well plates (1×105/cell) and incubated with 5%
CO2 at 37°C. After being washed with PBS for two times,
cells were digested, collected and detected by flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA), with the whole process
avoiding light.
Cell cycle analysis
Cell cycle status in different groups (Control, NC,
siTERT, rTERT) was measured by propidium iodide (PI) staining after
cell transfection. Cells after 24 h transfection were trypsinized,
washed twice using PBS and fixed by ice-cold 70% ethanol for 4 h.
After being washed twice with PBS, 400 µl PI was added in and
incubated for 30 min reaction at room temperature. Thereafter, cell
cycle status was immediately measured by flow cytometer (BD
Biosciences). The proportion of cells in G0/G1, S and G2/M phases
was detected.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from different cell groups
(Control, NC, siTERT, rTERT) respectively, and cDNA was acquired
using a first strand cDNA kit (Sigma-Aldrich; Merck KGaA),
according to the manufacturer's protocols. PCR amplification
process included: predenaturation at 95°C for 30 sec, followed by
40 cycles reaction: Denaturation at 95°C for 5 sec,
annealing/extension at 60°C for 30 sec in ABI 7300 Thermocycler
using the SYBR-Green Master Mix (Applied Biosystems; Thermo Fisher
Scientific Inc.). The primer sequences were displayed in Table I.
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Name | Type | Sequence
(5′-3′) |
|---|
| β-actin | Forward |
GTGGACATCCGCAAAGAC |
|
| Reverse |
GAAAGGGTGTAACGCAACT |
| TERT | Forward |
CTTCCTCTACTCCTCAGGCG |
|
| Reverse |
CAAGCAGCTCCAGAAACAGG |
| P27 | Forward |
CTCTGAGGACACGCATTTGG |
|
| Reverse |
GTTTGACGTCTTCTGAGGCC |
| P53 | Forward |
GCCCCTCCTCAGCATCTTAT |
|
| Reverse |
AAAGCTGTTCCGTCCCAGTA |
| PTEN | Forward |
ACCCACCACAGCTAGAACTT |
|
| Reverse |
CGCCTCTGACTGGGAATAGT |
| CDK2 | Forward |
GCTTTTGGAGTCCCTGTTCG |
|
| Reverse |
ACAAGCTCCGTCCATCTTCA |
| Cyclin D1 | Forward |
CCCTCGGTGTCCTACTTCAA |
|
| Reverse |
CTTAGAGGCCACGAACATGC |
| CDC25A | Forward |
CTGTTTGACTCCCCTTCCCT |
|
| Reverse |
GGGGAAGATGCCAGGGATAA |
Western blot analysis
Total proteins were extracted from different cell
groups (Control, NC, siTERT, rTERT). The concentrations of proteins
were determined by BCA assay. Then proteins were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electroblotted to a polyvinylidene fluoride membrane
(PVDF; GE Healthcare, Life Sciences, Little Chalfont, UK). After
being blocked by 5% nonfat dry milk, the membranes were reacted
with specific primary antibodies respectively overnight at 4°C,
including: rabbit anti-TERT (ab191523; 1:1,000), anti-P27 KIP1
(ab75908; 1:1,000), anti-P53 (ab38497; 1:1,000), anti-PTEN
(ab31392; 1:1,000), anti-CDK2 (ab32147; 1:5,000), anti-Cyclin D1
(ab226977; 1:5,000), anti-CDC25A (ab75743; 1:1,000), anti-β-catenin
(ab16051; 1:4,000), anti-Rb (ab47763; 1:1,000), anti-p-AKT
(ab38449; 1:1,000), anti-AKT (ab18785; 1:1,000) and anti-β-actin
(ab8227; 1:2,000; loading control). then they were conjugated with
the appropriate HRP-conjugated secondary antibodies (ab205718;
1:5,000; all Abcam, Cambridge, UK). The PVDF membranes were exposed
to X-ray film and detected by adding enhanced chemiluminescense
(ECL) reagent (GE Healthcare, Life Sciences,). Lab Works Image
Acquisition and Analysis Software (UVP, Inc., Upland, CA, USA) were
used to quantify band intensities.
Statistical analysis
Data were expressed as mean ± standard deviations of
three independent experiments. Statistical analysis was conducted
by SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA).
Differences were analyzed by one-way analysis of variance and a
Tukey test. P<0.05 was considered to indicate a statistically
significant difference.
Results
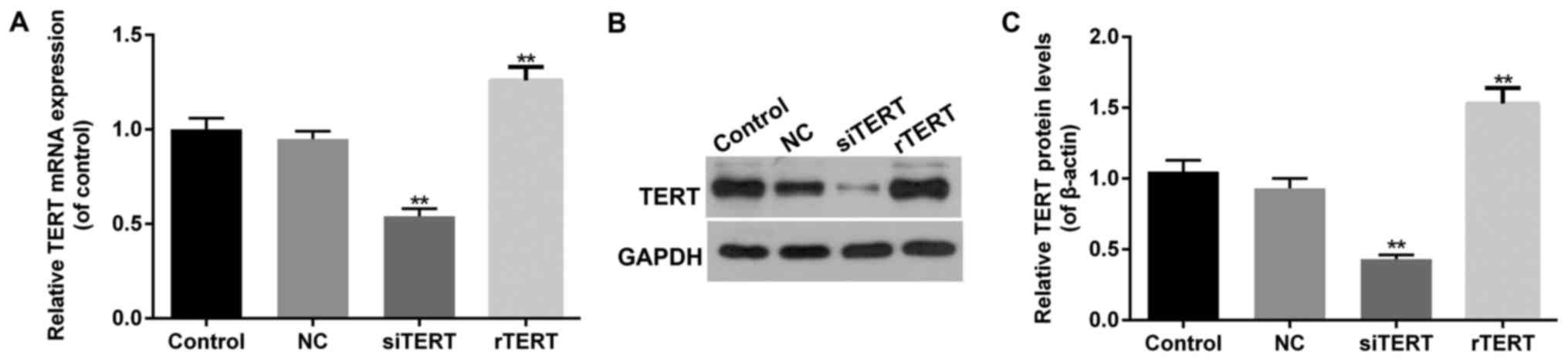
The transfection efficiency of TERT
over-expression and interference in mixed PTC cells
RT-qPCR and western blot were performed to detect
TERT transfection efficiency in different groups of mixed PTC
cells. It showed that both mRNA and protein levels of TERT
increased significantly in rTERT group, and decreased significantly
in siTERT group (P<0.05), compared with NC group. Besides, there
was no significant difference of TERT expression between NC and
control groups (P>0.05) (Fig.
1).
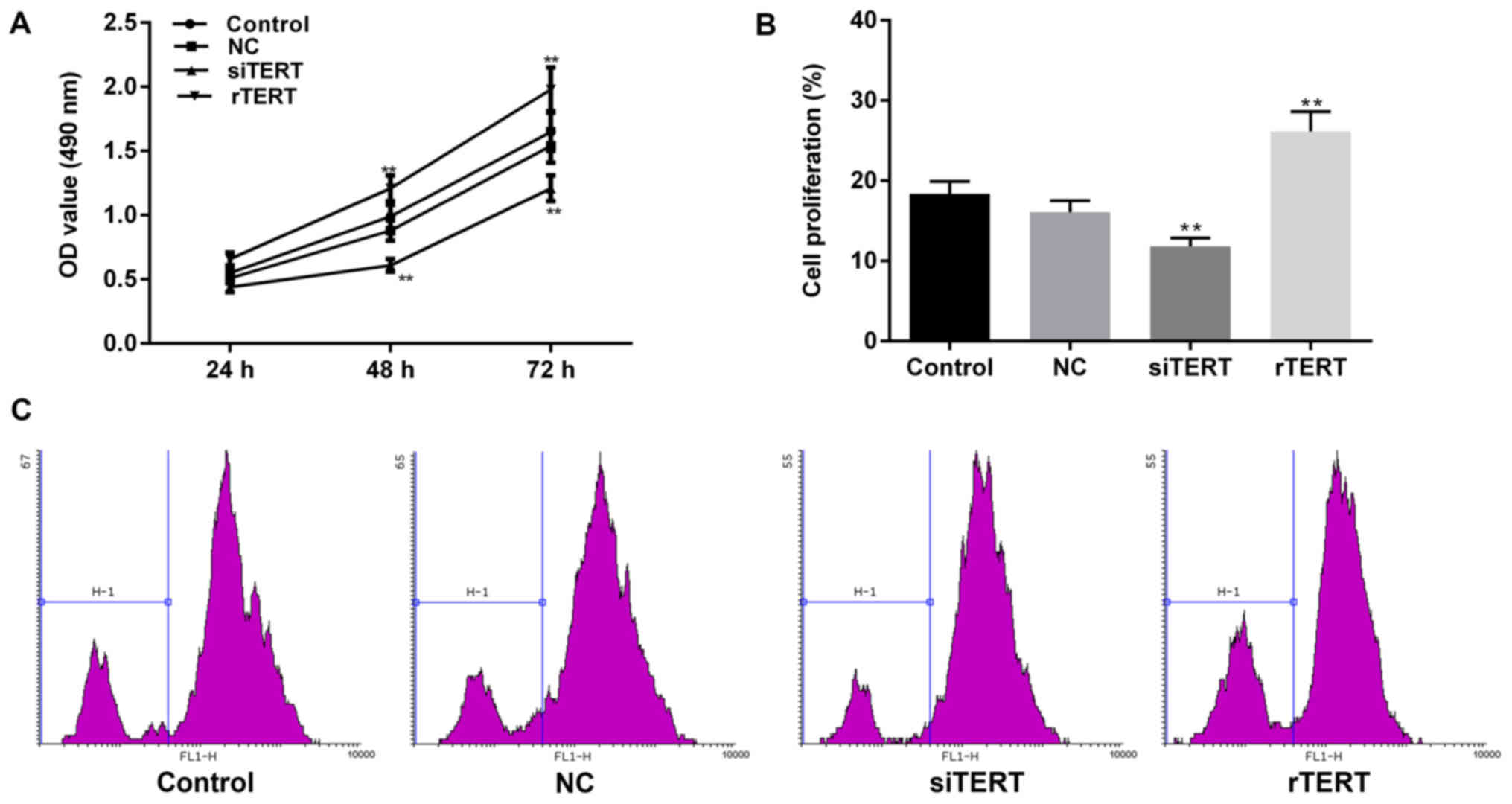
TERT promoted cell proliferation in
mixed PTC cells
Cell proliferation of living cells after
transfection is determined by MTT assay, because the NADP-related
dehydrogenases in mitochondria of living cells could reduce yellow
MTT to blue crystal. Hence dead cells couldn't be detected by MTT
for lacking of these dehydrogenases. However, CFSE assay can detect
not only living cells, but also cells having divided and died. It
attributes to the evenly distribution of CFSE fluorescence when
CFSE labeled cells divide to two daughter cells. The fluorescence
intensity remains the same level in a few days after division to
help fully analyzing cell proliferation.
The results of MTT indicated that TERT
over-expression significantly promoted cell proliferation of mixed
PTC cells in time-dependent manners (24, 48 and 72 h), compared
with NC group (P<0.05). Cell activity of NC group was of no
statistically difference with control group (P>0.05). The living
cell number in rTERT group was significantly higher than NC and
control group after transfection for 24 h. At the same time, TERT
interference dramatically inhibited cell proliferation of mixed PTC
cells in time-dependent manners (24, 48 and 72 h), compared with NC
and control groups (P<0.05). The living cell numbers in siTERT
group were significantly lower than NC and control groups after
transfection for 24 h (Fig.
2A).
CFSE was used to verify these results after 24 h of
transfection. The results of flow cytometry showed that, M1 value
decreased significantly in siTERT group, compared with NC group
(P<0.05), which represented TERT interference inhibited cell
proliferation of mixed PTC cells. The M1 value of rTERT group
increased significantly, compared to NC group (P<0.05), meaning
TERT over-expression promoted cell proliferation of mixed PTC cells
(Fig. 2B, C).
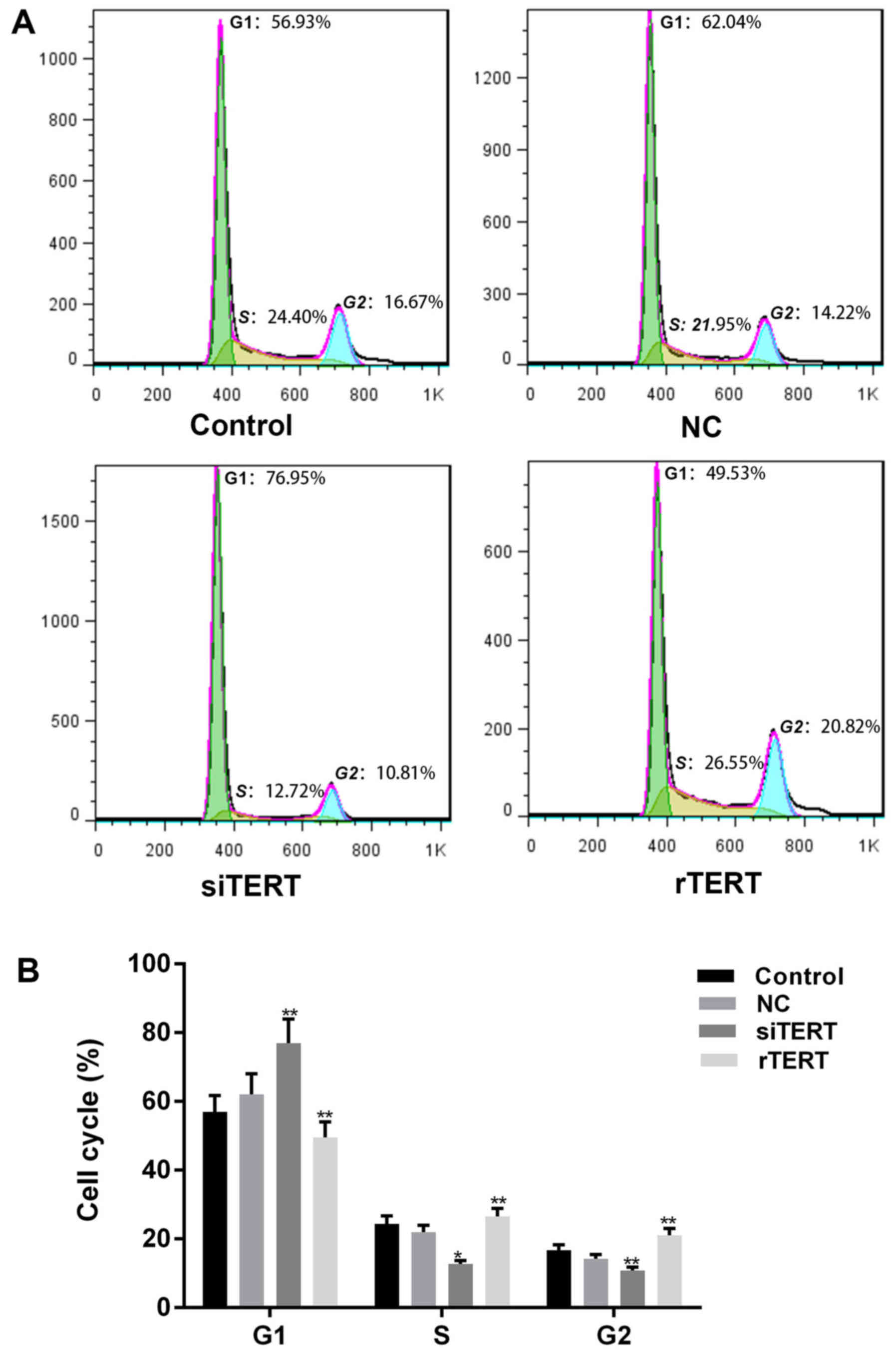
TERT promoted cell cycle progression
in mixed PTC cells
PI was used to determine cell cycle progression of
different cells. More cells of siTERT group stayed in G1 phase, and
less cells stayed in S and G2 phases, compared with NC and control
groups (P<0.05). The results showed that TERT interference could
inhibit cell proliferation of mixed PTC cells by blocking cell
cycle from G1/S transition. Otherwise, less cells in G1 phase and
more cells in S and G2 phases were observed in rTERT group than NC
and control groups (P<0.05), which indicated that TERT promoted
cell proliferation of mixed PTC cells by accelerating cell cycle
progression (Fig. 3).
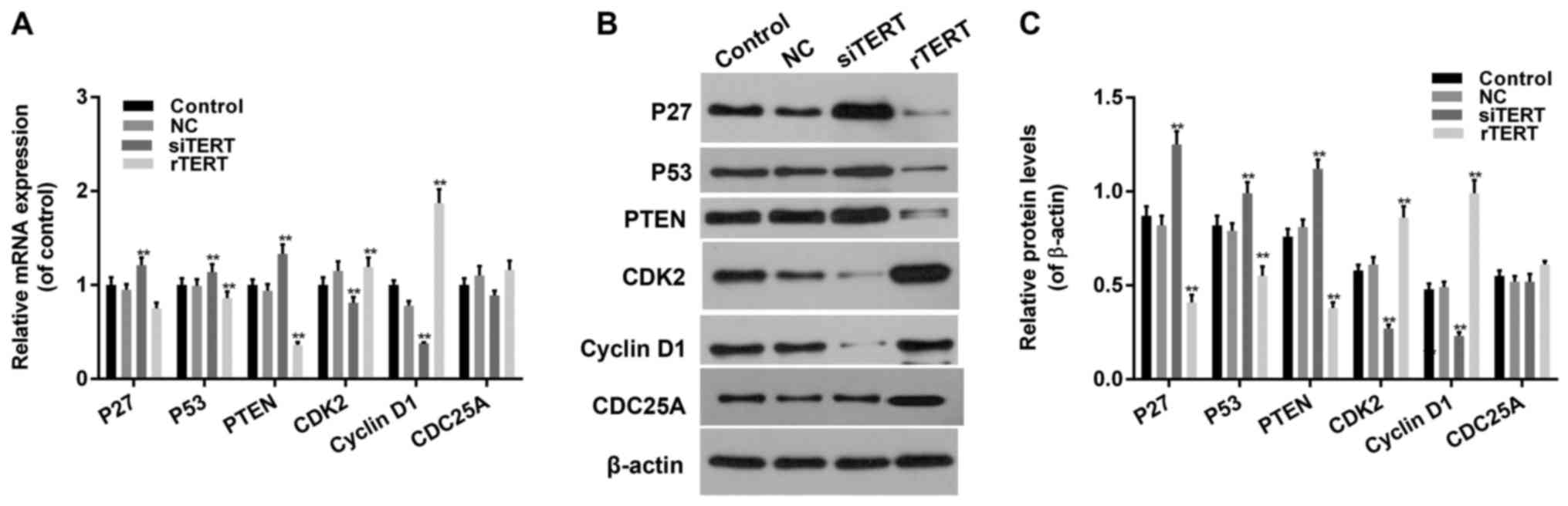
TERT promoted cell cycle progression
by regulating expression levels of cell cycle related factors in
mixed PTC cells
RT-qPCR and western blot were conducted to detect
cell cycle related factors expression in different groups. The
expression levels of P27, P53 and PTEN decreased significantly in
rTERT group and increased significantly in siTERT group, both in
mRNA and protein manners (P<0.05). The expression levles of CDK2
and Cyclin D1 increased significantly in rTERT group and decreased
significantly in TERT silencing group, both in mRNA and protein
manners (P<0.05). The expression level of CDC25A didn't change
too much in different groups, both in mRNA and protein manners
(P>0.05) (Fig. 4).
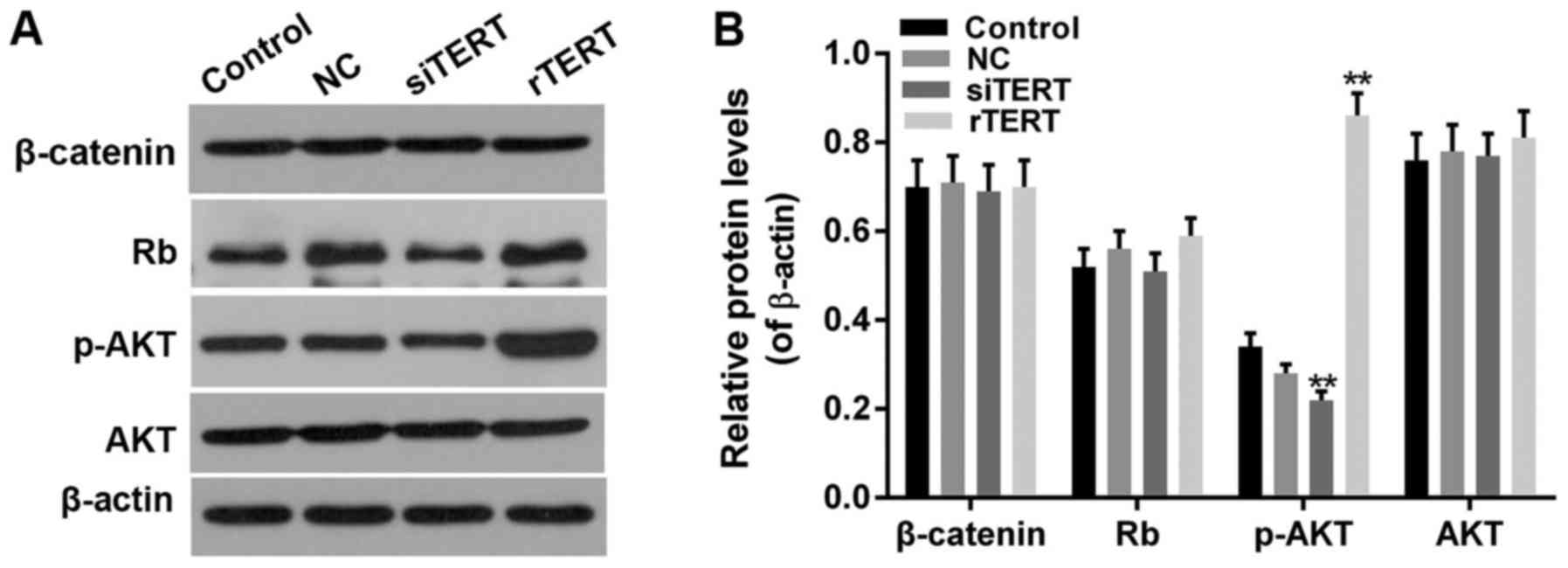
TERT promoted cell cell proliferation
by activating AKT signaling pathway in mixed PTC cells
As P27 and P53 were the upstream factors of Rb
pathway, Cyclin D1 was the critical regulator and effector of Rb
and Wnt/β-catenin pathway, and PTEN inactivation could abnormally
activate PI3K/AKT pathway, we further detected function of TERT on
Rb, β-catenin, AKT and phosphorylated AKT (p-AKT) in PTC cells.
Results showed that the protein levels of p-AKT increased
significantly in rTERT group and decreased significantly in siTERT
group (P<0.05), with no significant change on total AKT protein
expression (P>0.05). However, the protein levels of Rb and
β-catenin had no significant change both in rTERT and siTERT
groups, compared with NC and control groups (P>0.05) (Fig. 5).
Discussion
Thyroid carcinoma is the most common endocrine
malignant tumor in the world, and the incidence has ranked top of
the list of head and neck cancers. The development of thyroid
cancer is a complex process including many signaling pathways, with
abnormal cell proliferation.
Except maintaining telomere length, TERT has also
been found non-telomere dependent functions including cell signal
pathway or cell cycle regulation and so on. Previous researchers
found that over-expression of TERT could promote the proliferation
of many cells like epidermal hair follicle stem cells in mice,
without significant telomere extension observed (31). But whether and how TERT effect on
PTC cells still needs further research.
In our study, we used liposome transfection to
acquire TERT over-expression and TERT silencing PTC cells (actually
mixed thyroid gland papillary carcinoma cells) successfully.
RT-qPCR and western blot were performed to detect TERT levels
significantly promoted in TERT over-expressed cells, and
dramatically decreased in TERT silencing cells, verifying good
transfection effect. Then function of TERT on cell proliferation of
mixed PTC cells was evaluated. MTT assay detected living cells
proliferation, while CFSE assay determined all cells including
living cells and dead cells proliferation. It indicated that TERT
could promote cell proliferation of mixed PTC cells, for not only
living cells but also dead cells proliferation was detected
promoted by TERT over-expression, and inhibited by TERT silencing
in mixed PTC cells.
More cells in S and G2 phases and fewer cells in G1
phase were observed by flow cytometer in TERT over-expressed cells,
meaning more cells went into cell cycle to promote cell
proliferation of mixed PTC cells. Otherwise, TERT silencing could
block cell cycle at G1/S transition. It indicated that TERT
accelerated cell proliferation by causing more cells into cell
cycle progression, while TERT silencing inhibited cell
proliferation by inducing cell cycle arrestment. Thereafter,
RT-qPCR and western blot were conducted to detect the expression
changes of cell cycle and proliferation related factors, to
preliminarily illustrate the mechanism of TERT promoting cell
proliferation and cell cycle progression of mixed PTC cells.
Cell cycle dependent proteins have special functions
in the terminal differentiation of eukaryotic cells and the
regulation of cell cycle. They play a dual role in maintaining cell
cycle progression and keeping cells in a stationary phase after
mitosis. Cell cycle progression is regulated by cyclin dependent
kinases (CDKs). The activity of CDKs is also mediated by positive
regulators such as Cyclin D1 (32–34)
and CDK inhibitors (CDKIs) including retinoblastoma protein (Rb)
and Rb upstream molecules like P27 and P53. Phosphatase and tensin
homolog (PTEN) was reported to downregulate the expression of P27
to promote cell proliferation (35). Cyclin D1 is a critical positive
regulating factor in cell cycle on G1 phase progression. The
activation of Cyclin D1 could accelerate G1/S transition by
promoting downstream gene expression (36,37).
Increased Cyclin D1 is reported in many tumors like hepatocarcinoma
cell and so on (38). Our study
showed that TERT could modulate cell proliferation of mixed PTC
cells by positive regulating Cyclin D1 expression. Cell division
cycle 25A (CDC25A) play important roles to fully activate CDKs, by
removing the inhibitory phosphorylation on CDKs. But the expression
of CDC25A was of no significant differences among TERT
over-expression or silencing mixed-PTC cells.
Otherwise, gene mutation induces the downregulation
of cancer gene suppressor and abnormal persistent activation of
some signaling pathways, including MAPK signaling pathway,
Wnt/β-catenin, and PI3K/AKT pathway and so on, which induce tumor
development, invasion, metastasis and recurrence. P53 gene point
mutation and Wnt/β-catenin activation are considered the definite
markers of PTC transition from differentiated to undifferentiated
carcinoma (39–41). P27 or P53-coding transcription
factors significantly regulate some cell functions of great
importance, such as cell growth, proliferation, cell cycle,
apoptosis and DNA repairing and so on. P27 inhibit cell cycle
transition from G1 to S phage by inhibiting the activation of CDKs
(42,43). P53 gene point mutation makes it
lose tumor suppressor function and is involved in the initiation of
multiple tumors (44). P53
inactivation often occurs in late stages of thyroid cancer or
poorly differentiated pathological thyoid cancer (44,45),
and promotes cell proliferation and persistently differentiate. In
our study, P27 and P53 decreased significantly in TERT
over-expressed mixed-PTC cells and increased in TERT interfering
cells. It indicated TERT promoted cell proliferation of mixed PTC
cells in a way of inactivating P27 and P53. As P27 and P53 are the
upstream factors of Rb pathway, and Cyclin D1 is the critical
regulator and effector of Rb and Wnt/β-catenin pathway, we further
detected function of TERT on Rb and β-catenin expression in mixed
PTC cells. Rb is the first found tumor suppressor gene in human,
also a negative cell cycle regulatory factor. It is considered to
be the main regulator of all tissue cell growth and development,
even cancer occurrence. Rb can prevent cell cycle progression,
promote cell differentiation, and inhibit cell over-growth, which
depends on its interaction with transcription factor E2F and DP
(45). However, both
over-expression or interference of TERT were found nothing to do
with Rb expression, indicating TERT had no effect on Rb to regulate
PTC cell proliferation. The abnormal activation of Wnt/β-catenin
pathway could induce endothelium tumor development. As a
cytoplasmic protein, β-catenin plays important roles in cell
adherence to induce epithelial-mesenchymal transition. Though
function of β-catenin as promoting thyroid tumor proliferation and
dedifferentiation is definite (46), further researches are needed for
the mechanism of it in early stage of thyroid carcinoma. In our
study, β-catenin was found of no relationship with TERT involved
thyroid carcinoma development mechanism, even when TERT
over-expressed or interfered.
PI3K/AKT signaling pathway is the critical way of
regulating cell growth and proliferation (47). The abnormal activation of PI3K/AKT
pathway participates in tumor formation of thyroid carcinoma. The
activation of AKT could stimulate PI3K/AKT pathway to phosphorylate
and activate mTOR to regulate cell growth critical factors
translation like Cyclin D1 and so on, so to regulate cell growth
and proliferation. PTEN participated in inhibiting PI3K/AKT
signaling pathway as a cancer suppressor gene, which could inhibit
tumor cell proliferation, metastasis and invasion. Researches
before found that PTEN inactivation and RAS persistent activation
were also the reason of PI3K/AKT abnormal activation in thyroid
carcinoma. In our study, PTEN expression was found significantly
downregulated in TERT over-expressed cells, and upregulated in TERT
interfering cells. p- and activated AKT were detected increased
significantly in TERT over-expressed cells, and decreased in TERT
interfering cells. It definitely demonstrated that TERT regulated
cell proliferation of mixed-PTC cells through PTEN/AKT pathway.
In conclusion, our study preliminarily illustrated
the mechanism of TERT promoting cell proliferation and cell cycle
progression of mixed PTC cells, which mainly functioned through
PTEN/AKT pathway. Though it is a pity that the PTC K1 cell line
used in this study is mixed with thyroid gland papillary carcinoma
cells, it still provides a novel molecular target for thyroid
carcinoma diagnosis, treatment and prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
NH conceived the research. HZ performed the
experiments and wrote the paper. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y and Wang W: Increasing incidence of
thyroid cancer in Shanghai, China, 1983–2007. Asia Pac J Public
Health. 27:NP223–NP229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Albores-Saavedra J, Henson DE, Glazer E
and Schwartz AM: Changing patterns in the incidence and survival of
thyroid cancer with follicular phenotype-papillary, follicular, and
anaplastic: A morphological and epidemiological study. Endocr
Pathol. 18:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wynford-Thomas D: Origin and progression
of thyroid epithelial tumours: Cellular and molecular mechanisms.
Horm Res. 47:145–157. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomez DE, Armando RG, Farina HG, Menna PL,
Cerrudo CS, Ghiringhelli PD and Alonso DF: Telomere structure and
telomerase in health and disease (review). Int J Oncol.
41:1561–1569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beier F, Foronda M, Martinez P and Blasco
MA: Conditional TRF1 knockout in the hematopoietic compartment
leads to bone marrow failure and recapitulates clinical features of
dyskeratosis congenita. Blood. 120:2990–3000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Autexier C and Lue NF: The structure and
function of telomerase reverse transcriptase. Annu Rev Biochem.
75:493–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen H and Sinclair DA:
Recombination-mediated lengthening of terminal telomeric repeats
requires the Sgs1 DNA helicase. Proc Natl Acad Sci USA.
98:3174–3179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarin KY, Cheung P, Gilison D, Lee E,
Tennen RI, Wang E, Artandi MK, Oro AE and Artandi SE: Conditional
telomerase induction causes proliferation of hair follicle stem
cells. Nature. 436:1048–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith LL, Coller HA and Roberts JM:
Telomerase modulates expression of growth-controlling genes and
enhances cell proliferation. Nat Cell Biol. 5:474–479. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cong Y and Shay JW: Actions of human
telomerase beyond telomeres. Cell Res. 18:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allsopp RC, Morin GB, DePinho R, Harley CB
and Weissman IL: Telomerase is required to slow telomere shortening
and extend replicative lifespan of HSCs during serial
transplantation. Blood. 102:517–520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirkpatrick KL, Newbold RF and Mokbel K:
The mRNA expression of hTERT in human breast carcinomas correlates
with VEGF expression. J Carcinog. 3:12004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma GG, Gupta A, Wang H, Scherthan H,
Dhar S, Gandhi V, Iliakis G, Shay JW, Young CS and Pandita TK:
hTERT associates with human telomeres and enhances genomic
stability and DNA repair. Oncogene. 22:131–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh A, Saginc G, Leow SC, Khattar E,
Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, et al: Telomerase
directly regulates NF-κB-dependent transcription. Nat Cell Biol.
14:1270–1281. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang H, Wang J, Mao Y, Liu M, Reddy VN
and Li DW: Human telomerase accelerates growth of lens epithelial
cells through regulation of the genes mediating RB/E2F pathway.
Oncogene. 21:3784–3791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmed S, Passos JF, Birket MJ, Beckmann T,
Brings S, Peters H, Birch-Machin MA, von Zglinicki T and Saretzki
G: Telomerase does not counteract telomere shortening but protects
mitochondrial function under oxidative stress. J Cell Sci.
121:1046–1053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee J, Sung YH, Cheong C, Choi YS, Jeon
HK, Sun W, Hahn WC, Ishikawa F and Lee HW: TERT promotes cellular
and organismal survival independently of telomerase activity.
Oncogene. 27:3754–3760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perrault SD, Hornsby PJ and Betts DH:
Global gene expression response to telomerase in bovine
adrenocortical cells. Biochem Biophys Res Commun. 335:925–936.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu XQ, Huang C, He X, Tian YY, Zhou DX, He
Y, Liu XH and Li J: Feedback regulation of telomerase reverse
transcriptase: New insight into the evolving field of telomerase in
cancer. Cell Signal. 25:2462–2468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng L, Jin Y, Liu M, Ruan M and Chen L:
HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation
in papillary thyroid cancer harboring BRAFV600E. Oncotarget.
8:19843–19854. 2017.PubMed/NCBI
|
|
22
|
Kolanowska M, Wójcicka A, Kubiak A,
Świerniak M, Kotlarek M, Maciag M, Gaj P, Koperski Ł, Górnicka B
and Jażdżewski K: Functional analysis of a novel,
thyroglobulin-embedded microRNA gene deregulated in papillary
thyroid carcinoma. Sci Rep. 7:99422017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Huang Q, Sun D, Zhang G and Tan J:
RDM1 gene overexpression represents a therapeutic target in
papillary thyroid carcinoma. Endocr Connect. 6:700–707. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Sun W, Dong W, Wang Z, Qin Y, Zhang
T and Zhang H: HSP90 inhibitor NVP-AUY922 induces cell apoptosis by
disruption of the survivin in papillary thyroid carcinoma cells.
Biochem Biophys Res Commun. 487:313–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu K, Huang W, Yan DQ, Luo Q and Min X:
Overexpression of long intergenic noncoding RNA LINC00312 inhibits
the invasion and migration of thyroid cancer cells by
down-regulating microRNA-197-3p. Biosci Rep. 37:pii: BSR20170109.
2017. View Article : Google Scholar
|
|
26
|
Ni J, Wang F, Yue L, Xiang GD, Zhao LS,
Wang Y, Ye LZ and Dong J: The effects and mechanisms of berberine
on proliferation of papillary thyroid cancer K1 cells induced by
high glucose. Zhonghua Nei Ke Za Zhi. 56:507–511. 2017.(In
Chinese). PubMed/NCBI
|
|
27
|
Stasiołek M, Adamczewski Z, Śliwka PW,
Puła B, Karwowski B, Merecz-Sadowska A, Dedecjus M and Lewiński A:
The molecular effect of diagnostic absorbed doses from 131I on
papillary thyroid cancer cells in vitro. Molecules. 22:pii: E993.
2017. View Article : Google Scholar
|
|
28
|
Yang D, Wang C, Luo Y, Li X, Song Q, Zhang
J and Xin S: Activated E2F activity induces cell death in papillary
thyroid carcinoma K1 cells with enhanced Wnt signaling. PLoS One.
12:e01789082017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin Y, Hong S, Yu S, Huang Y, Chen S, Liu
Y, Zhang Q, Li Y and Xiao H: MiR-195 inhibits tumor growth and
metastasis in papillary thyroid carcinoma cell lines by targeting
CCND1 and FGF2. Int J Endocrinol. 2017:61804252017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang MM, Sun F, Cui B, Zhang LL, Fang Y,
Li Y, Zhang RJ, Ye XP, Ma YR, Han B and Song HD: Tumor-suppressive
function of UNC5D in papillary thyroid cancer. Oncotarget.
8:96126–96138. 2017.PubMed/NCBI
|
|
31
|
Flores I, Cayuela ML and Blasco MA:
Effects of telomerase and telomere length on epidermal stem cell
behavior. Science. 309:1253–1256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malgrange B, Belachew S, Thiry M, Nguyen
L, Rogister B, Alvarez ML, Rigo JM, Van De Water TR, Moonen G and
Lefebvre PP: Proliferative generation of mammalian auditory hair
cells in culture. Mech Dev. 112:79–88. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Wang F, Gao X, Zha D, Xue T, Cheng
X, Zhong C, Han Y and Qiu J: Decreased level of cyclin A2 in rat
cochlea development and cochlear stem cell differentiation.
Neurosci Lett. 453:166–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laine H, Sulg M, Kirjavainen A and Pirvola
U: Cell cycle regulation in the inner ear sensory epithelia: Role
of cyclin D1 and cyclin-dependent kinase inhibitors. Dev Biol.
337:134–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun C, Zhao J, Jin Y, Hou C, Zong W, Lu T,
Li H and Gao J: PTEN regulation of the proliferation and
differentiation of auditory progenitors through the
PTEN/PI3K/Akt-signaling pathway in mice. Neuroreport. 25:177–183.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cattani P, Hohaus S, Bellacosa A, Genuardi
M, Cavallo S, Rovella V, Almadori G, Cadoni G, Galli J, Maurizi M,
et al: Association between cyclin D1 (CCND1) gene amplification and
human papillomavirus infection in human laryngeal squamous cell
carcinoma. Clin Cancer Res. 4:2585–2589. 1998.PubMed/NCBI
|
|
37
|
Calbó J, Parreño M, Sotillo E, Yong T,
Mazo A, Garriga J and Grana X: G1 cyclin/cyclin-dependent
kinase-coordinated phosphorylation of endogenous pocket proteins
differentially regulates their interactions with E2F4 and gene
expression. J Biol Chem. 277:50263–50274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmidt VA, Chiariello CS, Capilla E,
Miller F and Bahou WF: Development of hepatocellular carcinoma in
Iqgap2-deficient mice is IQGAP1 dependent. Mol Cell Biol.
28:1489–1502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Omur O and Baran Y: An update on molecular
biology of thyroid cancers. Crit Rev Oncol Hematol. 90:233–252.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Wang Z, Liu J, Tang C, Duan C and Li
C: Proteomic analysis of differentially expressed proteins in
normal human thyroid cells transfected with PPFP. Endocr Relat
Cancer. 19:681–694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cahill S, Smyth P, Finn SP, Denning K,
Flavin R, O'Regan EM, Li J, Potratz A, Guenther SM, Henfrey R, et
al: Effect of ret/PTC 1 rearrangement on transcription and
post-transcriptional regulation in a papillary thyroid carcinoma
model. Mol Cancer. 5:702006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gardner LB, Li Q, Park MS, Flanagan WM,
Semenza GL and Dang CV: Hypoxia inhibits G1/S transition through
regulation of p27 expression. J Biol Chem. 276:7919–7926. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kuo MY, Hsu HY, Kok SH, Kuo RC, Yang H,
Hahn LJ and Chiang CP: Prognostic role of p27(Kip1) expression in
oral squamous cell carcinoma in Taiwan. Oral Oncol. 38:172–178.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Donghi R, Longoni A, Pilotti S, Michieli
P, Della Porta G and Pierotti MA: Gene p53 mutations are restricted
to poorly differentiated and undifferentiated carcinomas of the
thyroid gland. J Clin Invest. 91:1753–1760. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim CS and Zhu X: Lessons from mouse
models of thyroid cancer. Thyroid. 19:1317–1331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miyake N, Maeta H, Horie S, Kitamura Y,
Nanba E, Kobayashi K and Terada T: Absence of mutations in the
beta-catenin and adenomatous polyposis coli genes in papillary and
follicular thyroid carcinomas. Pathol Int. 51:680–685. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mian C, Barollo S, Pennelli G, Pavan N,
Rugge M, Pelizzo MR, Mazzarotto R, Casara D, Nacamulli D, Mantero
F, et al: Molecular characteristics in papillary thyroid cancers
(PTCs) with no 131I uptake. Clin Endocrinol (Oxf). 68:108–116.
2008. View Article : Google Scholar : PubMed/NCBI
|