Introduction
Cervical cancer has become the leading cause of
cancer-associated mortality in the female population (1); the etiopathogenesis of cervical
cancer is complex. Thus, improving understanding of the molecular
mechanisms underlying cervical cancer is of primary concern
(2). Chronic inflammation has been
reported to be a promoting factor in the majority of human
malignancies and has been directly associated with various steps
involved in tumorigenesis (3).
Inflammatory cytokines, interleukins (ILs), interferons,
transforming growth factors, chemokines and adhesion molecules from
stromal cells, such as fibroblasts, have been associated with
chronic inflammation and the promotion of cervical cancer (4).
Cancer-associated fibroblasts (CAFs) were reported
to support tumorigenesis by stimulating angiogenesis, cancer cell
proliferation and invasion; CAFs may also mediate tumor-promoting
inflammation (5). Additionally,
normal dermal fibroblasts may be affected by carcinoma cells to
induce the expression of proinflammatory genes (5). Thus, inhibiting the activation of
CAFs may be a potential strategy for cancer management. Long
non-coding RNAs (lncRNAs) have been reported to contribute to the
tumor-promoting phenotype of CAFs (6,7). For
example, LINC00092 binds a glycolytic enzyme,
6-phophofructo-2kinase/fructose-2,6,-bisphosphatase, thereby
promoting metastasis by altering glycolysis and sustaining the
local supportive function of CAFs (8); however, the role of lncRNA
homeodomain-interacting protein kinase 1 antisense RNA (HIPK1-AS)
on CAF activation remains unknown.
Sipi soup (SPS), the aqueous extract derived from
the root bark of Sophora japonica L, Salix babylonica L., Morus
alba L., as well as Amygdalus davidiana (Carr.) C. de
Vos, is a traditional Chinese medicine frequently used to
prevent and treat infection and inflammation (9–11).
The extract from the leaves of M. alba L. have been reported
to exhibit a highly inhibitory effect against acute inflammation,
which may be associated with the presence of chlorogenic acid and
flavonoids (12); however, its
role in CAFs remains unknown. In the present study, the effects of
SPS on fibroblasts and the potential underlying mechanism of
SPS-regulated fibroblast activation were investigated.
Materials and methods
Tissues
Cervical samples (n=184) were collected from women
(age, 25–76-years-old) who underwent routine cervical cancer
investigation at the Longyan First Hospital from May 2015 to April
2016 (Fujian, China). The subjects with any other types of cancer
and systemic inflammatory diseases, including diabetes, sepsis,
nephritis, hepatitis and lupus erythematosus were excluded. Written
informed consent was obtained from all participants in the present
study. Additionally, the present study was approved by the Ethics
Committee of the Longyan First Hospital. The specimens were grouped
according to the histological diagnosis. Samples analyzed in the
present study consisted of normal cervical (n=30), cervicitis
(n=40), cervical intraepithelial neoplasia-I (CIN I; n=34), CIN
II–III (n=38) and cervical squamous cell carcinoma tissues (SCC;
n=42), which were graded using the World Health Organization
grading system (13).
Classification of tissue samples was conducted in a blind manner by
two pathologists. Samples were stored at −80°C until use.
Preparation of SPS
Dried forms of root bark of S. japonical L, S.
babylonica L., M. alba L., as well as A. davidiana (Carr.)
C. de Vos were purchased from a Traditional Chinese pharmacy
(Baicaotang, Putian, China). SPS was prepared by adding 20 g of
each dried plant into 100 ml distilled water, which was heated for
20–30 min until it started to boil. After 30 min of boiling, the
aqueous extract was filtered with filter screen (10×10-cm mesh) to
remove any solid particles. SPS was then cooled down and used for
subsequent analysis.
Cell culture and treatment
HeLa cells and human normal cervical fibroblasts
were purchased from the Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China). The cell lines were
cultured routinely for 48 h prior to passaging in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and cultured in a 37°C
humidified atmosphere containing 5% CO2.
For activation, the fibroblasts
(1×107/ml) were treated with the DMEM culture media (CM,
2 ml) from the HeLa cell line for 48 h at 37°C. Knockdown of
homeodomain-interacting protein kinase 1 antisense RNA (HIPK1-AS)
in fibroblasts was achieved via transfection with lentivirus
containing short hairpin RNA (shRNA) against HIPK1-AS:
5′-TGCTGTACAGCGGCAGTCTGTTCAACGTTTTGGCCACTGACTGACGTTGAACACTGCCGCTGTA-3′
(multiplicity of infection=10) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The plasmid
construction and lentivirus package were performed by Shanghai
Genechem Co. Ltd. (Shanghai, China). The untreated cells were used
as control. Cells were plated in 6-well clusters and transfected
for 48 h. Transfected cells were used in further assays; qPCR was
performed to measure HIPK1-AS expression to determine whether
transfection was successful.
SPS treatment
Fibroblasts (1×107/ml) were treated with
SPS (0.1, 0.5, 1, 2, 3 and 4 mg/ml) for 48 h or 2 mg/ml SPS for 0,
24, 48, 72 and 96 h at 37°C. As the proliferation rate of the
control group was similar to that of cells treated with SPS (2
mg/ml), this concentration was selected for further analysis. Cells
untreated with SPS served as the control.
Long non-coding (lnc)RNA array
The Arraystar Human LncRNA Microarray v4.0
(Affymetrix; Thermo Fisher Scientific, Inc.) was used for the
global scanning of lncRNA expression in total RNA samples, which
were extracted with TRIzol® (Thermo Fisher Scientific,
Inc.) from untreated fibroblasts (control), HeLa-CM-treated
fibroblasts and HeLa-CM together with SPS treated fibroblasts.
Total RNA was analyzed by Kangchen BioTech Inc. (Shanghai, China)
using Arraystar Human LncRNA Microarray v4.0 (Affymetrix; Thermo
Fisher Scientific, Inc.). A total of 600 ng total RNA from each
sample was employed; sample labeling (cyanine 3; Quick Amp Labeling
kit, cat no. 5190-0442, Agilent Technologies, Santa Clara, CA,
USA), microarray hybridization (Agilent Gene Expression
Hybridization kit, cat no. 5188-5242, Agilent Technologies) and
washing (Gene Expression Wash Buffer 1 and 2, cat no. 5188-5325 and
5188–5326, respectively, Agilent Technologies) were performed based
on the manufacturer's standard protocols. The raw data were
normalized with the quantile algorithm (Kangchen BioTech).
Differentially expressed lncRNAs were then identified by analyzing
fold change, as well as the P-value. The threshold set for
significantly up- and downregulated genes was a fold change >2.0
and P<0.05.
Reverse transcription-quantitative
polymerase chain (RT-qPCR) analysis
Total RNA was extracted from cells, including
control (untreated) cells, cells treated with HeLa CM, cells
treated with HeLa-CM plus SPS or HIPK1 shRNA, and the samples
obtained from patients using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The expression levels
of HIPK1-AS, fibroblast activation protein (FAP), IL-6, α-smooth
muscle actin (α-SMA), programmed cell death protein 4 (PDCD4) were
measured with a OneStep RT-PCR kit (Qiagen, Inc., Valencia, CA,
USA) according to the manufacturers' protocols on CFX96 Touch™ Deep
Well Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The expression of β-actin served as an
endogenous control. The primer sequences were as follows: HIPK1-AS,
sense 5′-GCCTCTACCAGAAGGAAGGC-3′, antisense,
5′-CCAGCACTTGTGGGATGGAA-3′; FAP, sense 5′-TTGAAACTTGGCACGGTATTC-3′,
antisense, 5′-CCGATCAGGTGATAAGCCGTAA-3′; IL-6, sense
5′-TCTCAACCCCCAATAAATATAGGAC-3′, antisense,
5′-GATGCCGTCGAGGATGTACC-3′; α-SMA, sense
5′-TCCGCTTCAATTCCTGTCCG−3′, antisense, 5′-CAGGATTCCCGTCTTAGTCCC-3′;
PDCD4, sense 5′-ACCCTGCAGATCCTGATAACT-3′, anti-sense,
5′-TTTGGACTGGTTGGCACAGT-3′ and β-actin, sense
5′-TTGTTACAGGAAGTCCCTTGCC-3′, anti-sense,
5′-ATGCTATCACCTCCCCTGTGTG-3′. qPCR was performed as follows: 95°C
for 3 min, and 39 cycles of 95°C for 10 sec and 60°C for 30 sec.
The experiment was repeated in triplicate. Data were processed
using the 2−ΔΔCq method (14).
CCK-8 cell proliferation assay
Cell proliferation rates of fibroblasts were
measured using a Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Hangzhou, China). A total of 0.5×104
cells treated with SPS (2 mg/ml for 0, 24, 48, 72 and 96 h, or 0.1,
0.5, 1, 2, 3 and 4 mg/ml for 48 h) were seeded in each 96-well
plate for 24 h, and further incubated for 24, 48 and 72 and 96 h at
37°C, respectively. CCK-8 reagent (10 µl) was added to each well at
1 h at 37°C prior to the endpoint of each respective incubation.
The optical density value in each well was determined at a
wavelength of 490 nm using a microplate reader. Proliferation rate
was calculated as (optical density value of SPS treatment
group-optical density value of control group)/optical density value
of control group ×100%.
Annexin V-fluorescein isothiocyanate
(FITC) staining and flow cytometry
Staining was performed with Annexin V-FITC kit
according to the manufacturer's protocols (Nanjing KeyGen Biotech,
Co., Ltd., Nanjing, China). Briefly, 2×105 cells were
harvested by centrifugation at 1,000 × g for 5 min at room
temperature and resuspended in 100 µl binding buffer (contained in
the kit), followed by a 15 min incubation with 5 µl Annexin V-FITC
in the dark at 37°C. Subsequently, 10 µl propidium iodide was added
with gentle agitation for 10 min in the dark at 37°C. A flow
cytometer (BD Biosciences, San Jose, CA, USA) was employed for
detecting apoptotic events and FlowJo (version 10.4.2, FlowJo LLC,
Ashland, OR, USA) was used to analyze the apoptotic rate.
Immunofluorescence assays
Cells, including control (untreated), cells treated
with HeLa CM and cells treated with HeLa-CM plus HIPK1 shRNA,
cultured on coverslips (1×104/ml) for 48 h at 37°C were
fixed with 4% formaldehyde for 30 min at room temperature and then
permeabilized with 0.5% Triton-X-100 in PBS for 20 min.
Subsequently, cells were blocked with non-fat 5% milk in
tris-buffered saline and 0.1% Tween-20 for 60 min at 37°C,
incubated with anti-α-SMA antibody (cat no. ab5694, 1:200, Abcam,
Cambridge UK) at 4°C overnight. The cells were incubated with Cy3
Goat Anti-Rabbit IgG (cat. no. C2821, 1:200, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C for 1 h. The coverslips were
stained with DAPI (1:2,000, SC-3598, Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) for 2 min at room temperature and mounted on
slides using anti-fade mounting medium (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). Immunofluorescence
images were acquired using a fluorescence microscope (Nikon Eclipse
80i, Nikon Corporation, Tokyo, Japan). An excitation wavelength of
552 nm and ×100 magnification were applied for immunofluorescence
analysis. ImageJ (version 1.8, National Institutes of Health,
Bethesda, MD, USA) was used to analyze fluorescence intensity
Statistical analysis
In the present study, all experiments were repeated
at least three times, and all data were expressed as the mean ±
standard error of the mean. SPSS software, version 18.0 (SPSS,
Inc., Chicago, IL, USA) was used to perform statistical analysis.
Differences between two groups were compared with an
independent-samples t-test. Differences among three or more groups
were compared with one-way analysis of variance with Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of SPS on fibroblast
proliferation and apoptosis
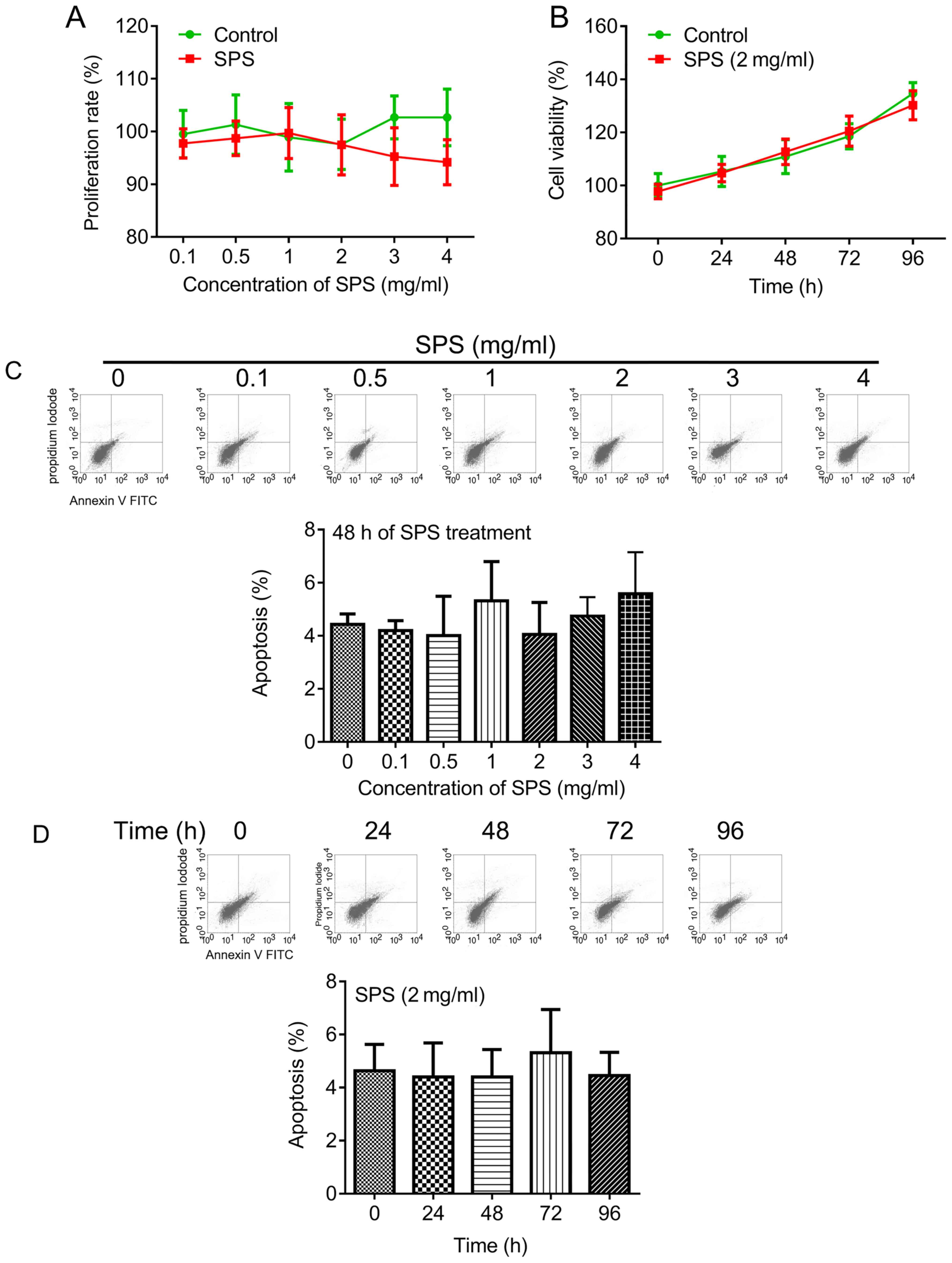
To investigate the role of SPS on the proliferation
and apoptosis of fibroblasts, cells treated with different
concentrations SPS and the effects of various durations of
treatment with 2 mg/ml SPS were analyzed. Fibroblasts were treated
with SPS at 0.1 to 4 mg/ml for 48 h. The results of the present
study revealed that SPS did not induce significant inhibition of
fibroblast proliferation; however, a notable reduction with 4 mg/ml
SPS was observed (Fig. 1A). Cells
were treated with SPS (2 mg/ml) for various durations. The results
revealed that there were no significant differences in cell
viability between the control and SPS-treated groups (Fig. 1B). In addition, the role of SPS in
fibroblast apoptosis was investigated in the present study. The
results demonstrated that treatment with serial concentrations of
SPS and treatment of varying durations of 2 mg/ml SPS did not
significantly induce cell apoptosis (Fig. 1C and D, respectively).
SPS inactivates fibroblasts to reduce
the release of proinflammatory factors
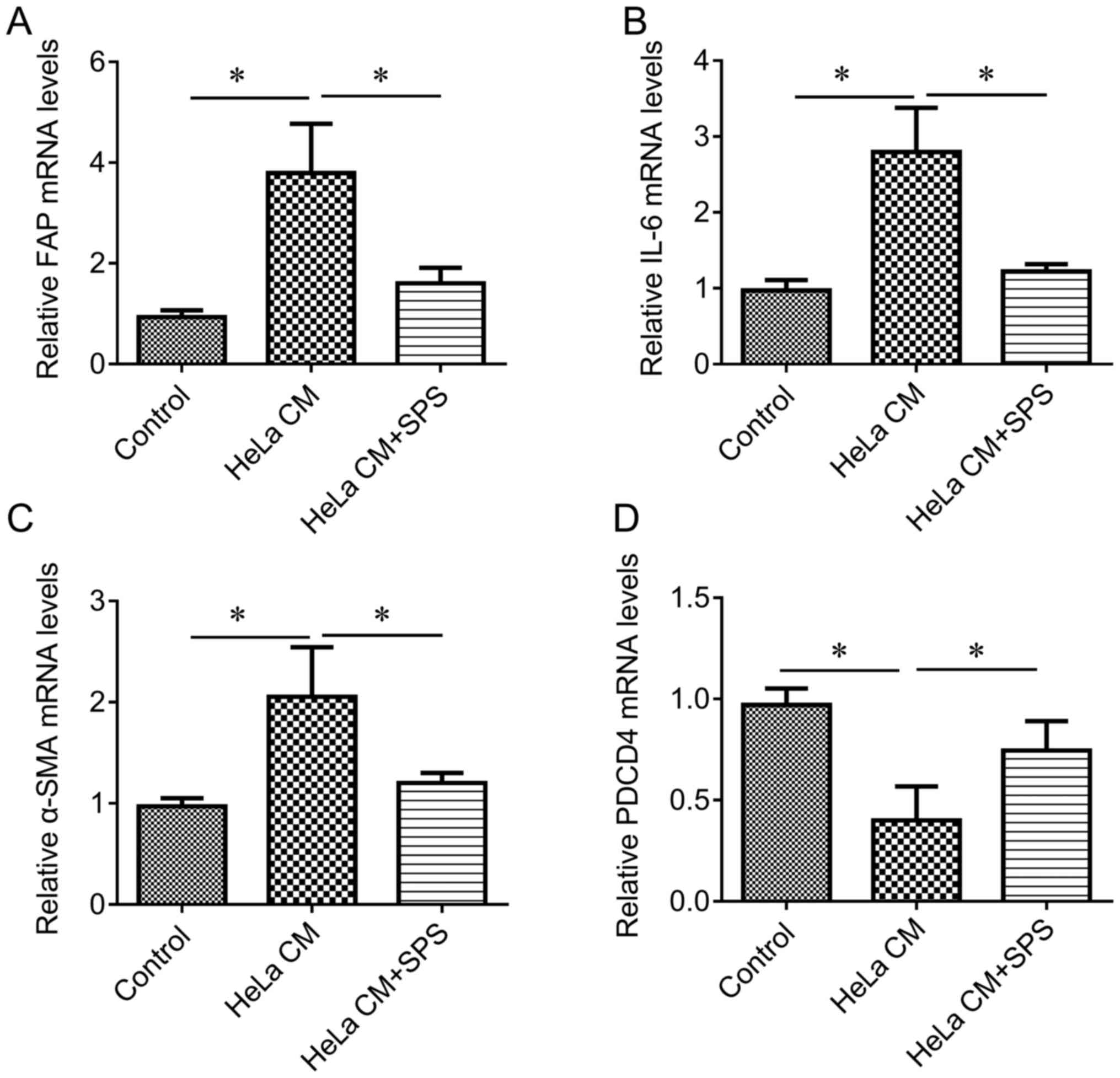
As significant effects of SPS on fibroblast
proliferation and apoptosis were not observed, the present study
investigated whether SPS may regulate the activation of
fibroblasts. The fresh CM obtained from 48 h of culture from HeLa
cells was used to induce the activation of fibroblasts. The results
of the present study revealed that HeLa CM induced a significant
increase in the expression levels of FAP, IL-6 and α-SMA (Fig. 2A-C, respectively), but
significantly reduced the expression of PDCD4 (Fig. 2D) compared with in the control
group, indicating that HeLa CM induced the activation of
fibroblasts. Combined treatment of HeLa CM and SPS (2 mg/ml) for 48
h indicated that SPS treatment significantly reduced HeLa
CM-mediated upregulation of FAP, IL-6 and α-SMA expression, and
significantly increased the expression of PDCD4 (Fig. 2) compared with the HeLa CM group,
suggesting that SPS may reverse HeLa CM-induced activation of
fibroblasts.
SPS regulates lncRNA expression in
fibroblasts
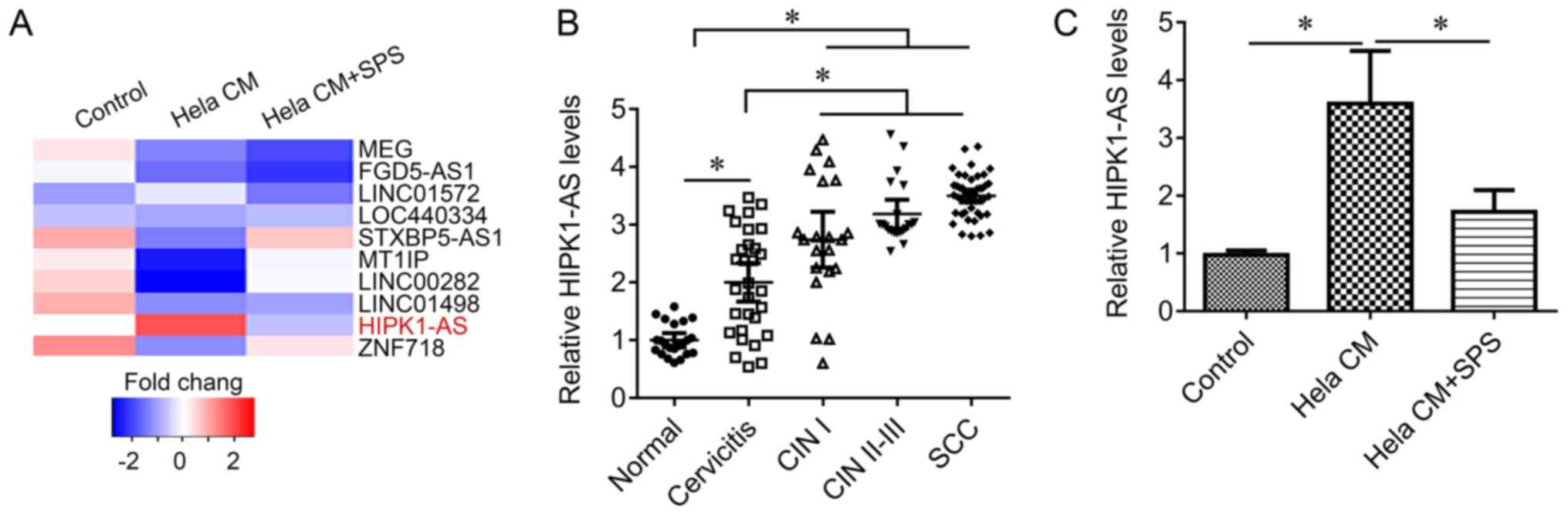
To investigate the underlying mechanism by which SPS
may inactivate and prevent activation of fibroblasts, an lncRNA
array was performed to screen genes and lncRNAs that may be
associated with the underlying molecular mechanism in cells.
Numerous differentially expressed lncRNAs were analyzed in HeLa
CM-treated cells and HeLa CM together with SPS-treated cells
compared with the control group. The results of the present study
revealed that lncRNA HIPK1-AS expression may be induced by HeLa CM,
whereas treatment with SPS was associated with the reduction in
HIPK1-AS expression (Fig. 3A). To
further investigate this difference, cervical tissue samples were
obtained, including cervicitis, CIN I, CIN II–III and cervical
squamous cell carcinoma (SCC). HIPK1-AS expression levels were
significantly increased in cervicitis tissues compared with in
normal cervical tissues; the expression levels of HIPK1-AS were
significantly higher in SCC tissues and CIN II–III tissues than in
cervicitis tissues (Fig. 3B). In
addition, the results of the present study indicated that HIPK1-AS
expression levels were significantly increased in fibroblasts in
response to HeLa CM compared with in the control; this upregulation
was also significantly inhibited by treatment with SPS (Fig. 3C), suggesting that HIPK1-AS may
mediate the effects of SPS on fibroblast activation.
Knockdown of HIPK1-AS by shRNA
inactivates fibroblasts and reduces the release of proinflammatory
factors
The role of HIPK1-AS in fibroblast activation was
investigated in the present study. HIPK1-AS expression was
significantly downregulated via lentiviral infection with HIPK1-AS
compared with in the control (Fig.
4A). The present study reported that the downregulation of
HIPK1-AS significantly inhibited HeLa CM-mediated upregulation of
FAP, IL-6 and α-SMA expression levels, and the downregulation of
PDCD4 (Fig. 4B-E). The
immunofluorescence results also indicated that HIPK1-AS knockdown
reduced the number of HeLa CM-activated fibroblasts (Fig. 4F). These results indicate that
HIPK1-AS knockdown may inhibit HeLa CM-mediated activation of
fibroblasts and the release of proinflammatory factors.
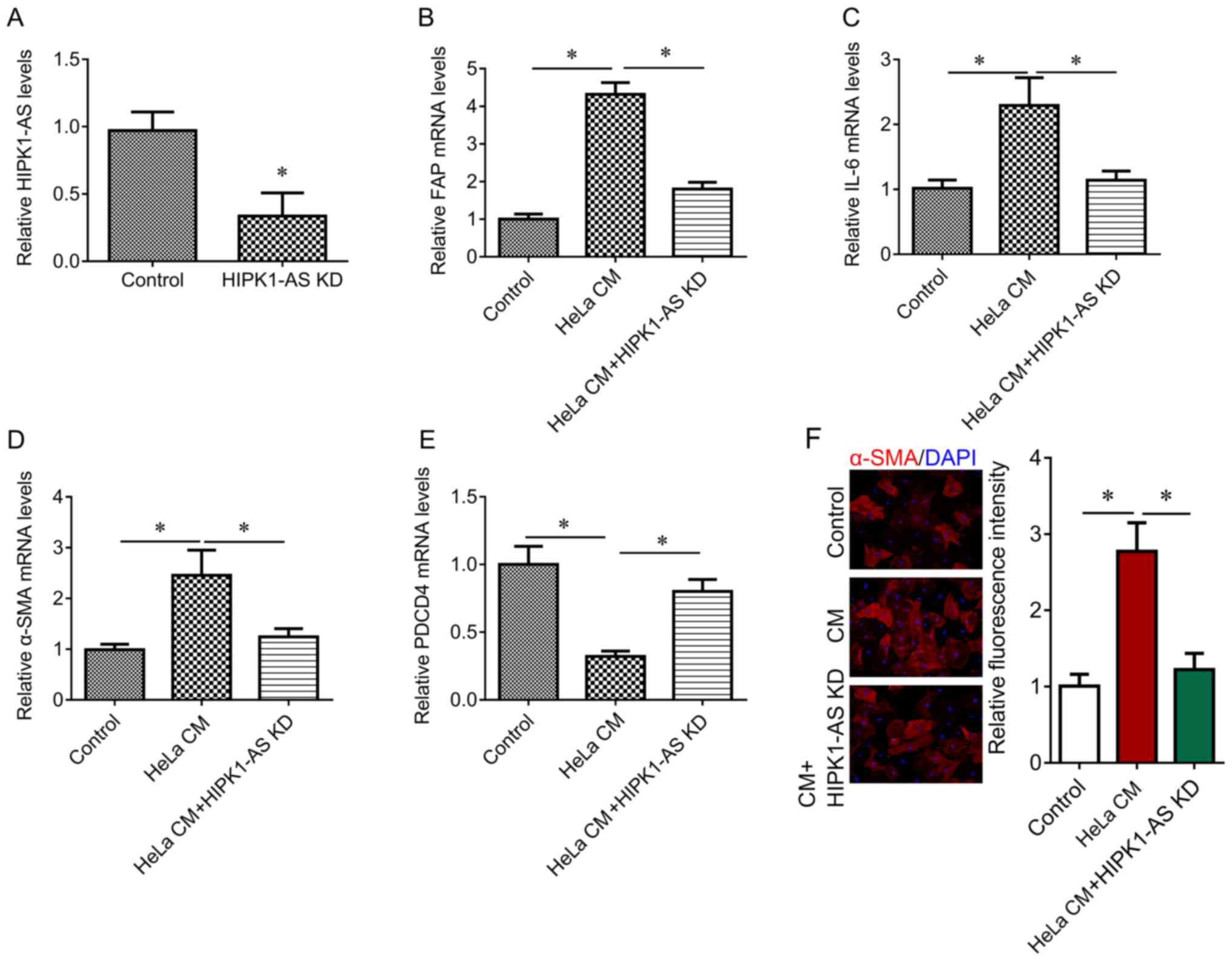 | Figure 4.Effects of HIPK1-AS on fibroblast
activation. (A) RT-qPCR was performed to measure the expression of
HIPK1-AS after HIPK1-AS shRNA lentivirus infection. The fibroblasts
were treated with HeLa CM alone, or together with HIPK1-AS short
hairpin RNA. RT-qPCR was performed to measure the expression of (B)
FAP, (C) IL-6, (D) α-SMA and (E) PDCD4. The untreated cells served
as the control. (F) Representative images for α-SMA
immunofluorescent staining (red) and DAPI (blue), and fluorescent
intensity quantification. Magnification, ×100. *P<0.05. α-SMA,
α-smooth muscle actin; CM, culture medium; FAP,
fibroblast-associated protein; PDCD4, programmed cell death 4;
HIPK1-AS, homeodomain-interacting protein kinase 1 antisense RNA;
KD, knockdown; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
Discussion
In the present study, the proliferative and
apoptotic abilities of fibroblasts were not markedly altered under
treatment with serial concentrations of SPS, or treatment with SPS
for various durations; however, SPS may reverse the activation of
fibroblasts and release of proinflammatory factors mediated by HeLa
CM.
The use of S. japonica (Fabaceae), also known
as Huai (Chinese), has been recorded in classical medicinal
treatises of ancient China, and it is currently recorded in the
Chinese Pharmacopoeia and European Pharmacopoeia (10). Numerous flavonoids and
isoflavonoids comprise the active constituents of S.
japonica. These chemical compounds exhibit a wide range of
biological activities in vitro and in vivo, including
anti-inflammatory, antioxidant and antitumor properties (15,16).
Oxymatrine, a potent monosomic alkaloid extracted from S.
japonica, may inhibit the production of tumor necrosis
factor-α, IL-1β and IL-6, and may also inhibit the extracellular
signal-regulated kinase, p38 and c-Jun N-terminal kinase signaling
pathways in lipopolysaccharide-stimulated microglial cells
(17). In addition, oxymatrine was
reported to exhibit anti-inflammatory properties in septic
shock-induced myocardial injury via the inhibition of the Janus
kinase 2/signal transducer and activator of transcription 3
signaling pathway (18).
Sophoricoside was isolated from immature fruits of S.
japonica and was reported to inhibit the bioactivity of IL-6
(19). UP1306, a proprietary
extract of M. alba, was demonstrated to reduce bone and
cartilage degradation via the reported inhibition of catabolic
proinflammatory signaling pathways (20). Morusin, a prenylated flavonoid
isolated from the root bark of M. alba, may reduce tissue
damage in an animal model of 2′4′6-trinitrobenzene-induced colitis
(21). In the present study, it
was revealed that SPS may attenuate HeLa CM-mediated fibroblast
activation and the release of proinflammatory factors; however, the
specific chemical components in SPS that serve a role in fibroblast
activation require further validation.
Furthermore, the present study investigated the
potential mechanism underlying fibroblast inactivation and
prevention of proinflammatory factor release mediated by SPS. By
using an lncRNA array, the expression levels of HIPK1-AS were
observed be induced by HeLa CM and inhibited by SPS in fibroblasts.
In addition, the findings of the present study demonstrated that
HIPK1-AS expression levels were increased in cervicitis, CIN I–III
and SCC tissues. Loss of function experiments revealed that
knockdown of HIPK1-AS inhibited the activation of fibroblasts and
proinflammatory factor release mediated by HeLa CM. HIPK1-AS is a
novel lncRNA that has been located on the host gene HIPK1
(chromosome 1: 113,924,001–113,929,258 reverse strand); the
transcript contains 5 exons and maps to 276 oligo probes (22,23).
HIPKs regulate cell differentiation, proliferation and apoptosis
(24). HIPK1 was identified as a
oncoprotein in lung adenocarcinoma cells (25). Additionally, HIPK1 mRNA was
reported to be a translational target of PDCD4 (26). HIPK1 stimulates the translation of
its own mRNA; PDCD4 may suppress the translation of HIPK1 mRNA by
interfering with this auto-regulatory feedback mechanism (26). In the present study, it was
reported that HIPK1-AS knockdown increased the expression of PDCD4;
however, the underlying mechanism requires further investigation.
IL-6 has been associated with several stages of tumor development
by mediating epithelial-stromal interactions (27). The upregulation of IL-6 has been
reported to serve a significant role in the pathogenesis of
cervical cancer, and has been frequently detected in the stromal
region of cervical cancer tissues (28). Additionally, IL-6 was reported to
be co-expressed with α-SMA in fibroblasts (29,30);
combined with transforming growth factor-β, α-SMA and IL-6 have
been suggested to interact with PDCD4 in the tumor stroma (27).
As a possible limitation of the present study, the
dried components of SPS were not ground during the preparation of
SPS. Further investigation may be performed in the future to
investigate whether more notable effects occur with various methods
of SPS preparation; the effects of SPS in vivo require
further study.
In conclusion, the present study demonstrated that
SPS attenuated HeLa CM-mediated fibroblast activation and the
release of proinflammatory factors by inhibiting HIPK1-AS
expression. HIPK1-AS may be associated with cervical lesions, as
well as cervicitis; the upregulation of HIPK1-AS in the tumor
stroma may serve a role in the inflammatory process and the
progression of cervical cancer. Therefore, HIPK1-AS and SPS may be
considered as a therapeutic target and agent, respectively in the
treatment of cervical cancer; however, further investigation is
required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author contributions
RQ and LY made substantial contributions to the
design of the study. BZ and YY analyzed and interpreted the patient
data. BQ and YY performed cell biological experiments. BZ and BQ
performed quantitative polymerase chain reaction and
immunofluorescence staining. All authors contributed to writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Longyan First Hospital (Fujian, China). Written
informed consent was obtained from all patients.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Senba M and Mori N: Mechanisms of virus
immune evasion lead to development from chronic inflammation to
cancer formation associated with human papillomavirus infection.
Oncol Rev. 6:e172012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pandey S and Chandravati: Autophagy in
cervical cancer: An emerging therapeutic target. Asian Pac J Cancer
Prev. 13:4867–4871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma D, Chang LY, Zhao S, Zhao JJ, Xiong YJ,
Cao FY, Yuan L, Zhang Q, Wang XY, Geng ML, et al: KLF5 promotes
cervical cancer proliferation, migration and invasion in a manner
partly dependent on TNFRSF11a expression. Sci Rep. 7:156832017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walch-Rückheim B, Mavrova R, Henning M,
Vicinus B, Kim YJ, Bohle RM, Juhasz-Böss I, Solomayer EF and Smola
S: Stromal fibroblasts induce CCL20 through IL6/C/EBPβ to support
the recruitment of Th17 cells during cervical cancer progression.
Cancer Res. 75:5248–5259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang D, Ding L, Li Y, Ren J, Shi G, Wang
Y, Zhao S, Ni Y and Hou Y: Midkine derived from cancer-associated
fibroblasts promotes cisplatin-resistance via up-regulation of the
expression of lncRNA ANRIL in tumour cells. Sci Rep. 7:162312017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vafaee F, Colvin EK, Mok SC, Howell VM and
Samimi G: Functional prediction of long non-coding RNAs in ovarian
cancer-associated fibroblasts indicate a potential role in
metastasis. Sci Rep. 7:103742017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Ji G, Le X, Wang C, Xu L, Feng M,
Zhang Y, Yang H, Xuan Y, Yang Y, et al: Long noncoding RNA
LINC00092 acts in cancer-associated fibroblasts to drive glycolysis
and progression of ovarian cancer. Cancer Res. 77:1369–1382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yimam M, Lee YC, Wright L, Jiao P, Horm T,
Hong M, Brownell L and Jia Q: A botanical composition mitigates
cartilage degradations and pain sensitivity in osteoarthritis
disease model. J Med Food. 20:568–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He X, Bai Y, Zhao Z, Wang X, Fang J, Huang
L, Zeng M, Zhang Q, Zhang Y and Zheng X: Local and traditional
uses, phytochemistry, and pharmacology of Sophora japonica
L.: A review. J Ethnopharmacol. 187:160–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren Y, Qiao W, Fu D, Han Z, Liu W, Ye W
and Liu Z: Traditional Chinese medicine protects against cytokine
production as the potential immunosuppressive agents in
atherosclerosis. J Immunol Res. 2017:74243072017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oliveira AM, Nascimento MF, Ferreira MR,
Moura DF, Souza TG, Silva GC, Ramos EH, Paiva PM, Medeiros PL,
Silva TG, et al: Evaluation of acute toxicity, genotoxicity and
inhibitory effect on acute inflammation of an ethanol extract of
Morus alba L. (Moraceae) in mice. J Ethnopharmacol.
194:162–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumours of Female Reproductive
Organs (Fourth edition). WHO Press; 2014
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang T, Miao M, Bai M, Li Y, Li M, Li C
and Xu Y: Effect of sophora japonica total flavonoids on pancreas,
kidney tissue morphology of streptozotocin-induced diabetic mice
model. Saudi J Biol Sci. 24:741–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu JS, Lee D, Lee SR, Lee JW, Choi CI,
Jang TS, Kang KS and Kim KH: Chemical characterization of cytotoxic
indole acetic acid derivative from mulberry fruit (Morus
alba L.) against human cervical cancer. Bioorg Chem. 76:28–36.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong XQ, Du Q, Yu WH, Zhang ZY, Zhu Q, Che
ZH, Chen F, Wang H and Chen J: Anti-inflammatory effects of
oxymatrine through inhibition of nuclear factor-kappa B and
mitogen-activated protein kinase activation in
Lipopolysaccharide-induced BV2 microglia cells. Iran J Pharm Res.
12:165–174. 2013.PubMed/NCBI
|
|
18
|
Zhang M, Wang X, Wang X, Hou X, Teng P,
Jiang Y, Zhang L, Yang X, Tian J, Li G, et al: Oxymatrine protects
against myocardial injury via inhibition of JAK2/STAT3 signaling in
rat septic shock. Mol Med Rep. 7:1293–1299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim BH, Chung EY, Ryu JC, Jung SH, Min KR
and Kim Y: Anti-inflammatory mode of isoflavone glycoside
sophoricoside by inhibition of interleukin-6 and cyclooxygenase-2
in inflammatory response. Arch Pharm Res. 26:306–311. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalman DS and Hewlings SJ: The effects of
Morus alba and Acacia catechu on quality of life and overall
function in adults with osteoarthritis of the knee. J Nutr Metab.
2017:48931042017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vochyánová Z, Pokorná M, Rotrekl D, Smékal
V, Fictum P, Suchý P, Gajdziok J, Šmejkal K and Hošek J: Prenylated
flavonoid morusin protects against TNBS-induced colitis in rats.
PLoS One. 12:e1824642017. View Article : Google Scholar
|
|
22
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soibam B: Super-lncRNAs: Identification of
lncRNAs that target super-enhancers via RNA:DNA:DNA triplex
formation. RNA. 23:1729–1742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rey C, Soubeyran I, Mahouche I, Pedeboscq
S, Bessede A, Ichas F, De Giorgi F and Lartigue L: HIPK1 drives p53
activation to limit colorectal cancer cell growth. Cell Cycle.
12:1879–1891. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee D, Park SJ, Sung KS, Park J, Lee SB,
Park SY, Lee HJ, Ahn JW, Choi SJ, Lee SG, et al: Mdm2 associates
with Ras effector NORE1 to induce the degradation of oncoprotein
HIPK1. EMBO Rep. 13:163–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohnheiser J, Ferlemann E, Haas A, Muller
JP, Werwein E, Fehler O, Biyanee A and Klempnauer KH: Programmed
cell death 4 protein (Pdcd4) and homeodomain-interacting protein
kinase 2 (Hipk2) antagonistically control translation of Hipk2
mRNA. Biochim Biophys Acta. 1853:1564–1573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao Q, Cao S, Li C, Mengesha A, Kong B and
Wei M: Micro-RNA-21 regulates TGF-β-induced myofibroblast
differentiation by targeting PDCD4 in tumor-stroma interaction. Int
J Cancer. 128:1783–1792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kinoshita H, Hirata Y, Nakagawa H,
Sakamoto K, Hayakawa Y, Takahashi R, Nakata W, Sakitani K, Serizawa
T, Hikiba Y, et al: Interleukin-6 mediates epithelial-stromal
interactions and promotes gastric tumorigenesis. PLoS One.
8:e609142013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren C, Cheng X, Lu B and Yang G:
Activation of interleukin-6/signal transducer and activator of
transcription 3 by human papillomavirus early proteins 6 induces
fibroblast senescence to promote cervical tumourigenesis through
autocrine and paracrine pathways in tumour microenvironment. Eur J
Cancer. 49:3889–3899. 2013. View Article : Google Scholar : PubMed/NCBI
|