Introduction
According to the World Health Organization (WHO),
cardiovascular disease has become one of the most dangerous
diseases endangering human health. It is predicted that
cardiovascular diseases will progress to become the leading cause
of human mortality by 2020, thus is an important public health
problem (1). There are 230 million
people with cardiovascular disease in China and there are ~3
million cases of cardiovascular disease-associated mortality per
year, accounting for ~50% of all causes of mortality in China
(2). Someone dies due to
cardiovascular complications every 13 sec (2) and it is predicted that by 2030, the
morbidity and mortality of cardiovascular disease in China will
increase by 73% (2). At present,
cardiovascular disease costs up to 300 billion yuan (~£35 billion)
in China every year, in addition its growth rate is almost twice
that of China's GDP, thus will cause a large economic burden on
society (2). Atherosclerosis (AS)
accounts for the largest proportion of cardiovascular disease,
along with the highest morbidity and mortality (3). Chronic vascular inflammatory reaction
serves a significant role in the occurrence and development of AS
(4). In addition, vascular
inflammatory injury is an important pathogenic mechanism leading to
the occurrence and development of hypertension, aneurysm, in
addition to percutaneous transluminal coronary angioplasty
(5). Previous studies have
demonstrated that tumor necrosis factor (TNF)-α-induced
inflammation in vascular endothelial cells is a common model for
studying vascular inflammation (5).
MicroRNAs (miRNAs) are small non-coding RNAs that
are approximately 19–22 bases in length. They function normally as
regulators of gene expression, and have been demonstrated to serve
key regulatory roles in biological processes (6). This regulation is also present in the
self-renewal of stem cells and the differentiation of a variety of
cell lineages (7). It is confirmed
that the generation of miRNAs can interfere with the process of
angiogenesis and the function of vascular endothelial cells
(8).
Inflammation is a common cause of endothelial
dysfunction. Under physiological conditions, the endothelium will
affect the vascular inflammation through the release of nitric
oxide (9). However, endothelial
dysfunction will produce excessive reactive oxygen species and
aggravate inflammation of the blood vessels, thereby damaging the
blood vessels (10). Various
inflammatory factors are associated with endothelial dysfunction
and atherosclerosis (11).
Inflammation is also associated with overexpression of TNF-α and
interleukin (IL)-6, which promotes the adhesion and migration of
monocytes (12). In addition,
these inflammatory factors can also cause expression of adhesion
molecules, including vascular cell adhesion molecules,
intracellular adhesion molecules and monocyte chemotactic factor 1
in endothelial cells and monocytes. Thus, this causes more severe
endothelial dysfunction. Upon reaching the intima, the monocytes
are transformed into giant cells, which are conductive to the
expression of receptors that facilitate lipid uptake and
accumulation (12). In this way,
the macrophages can be transformed into foam cells. Subsequently,
smooth muscle cell migration occurs, which together with foam
cells, form necrotic nuclei, leading to the formation of
atherosclerotic plaques (13).
Therefore, the endothelial dysfunction caused by inflammation
additionally has a direct association with the occurrence and
development of vascular diseases.
A previous study identified that PTEN is closely
associated with tumor angiogenesis (14). It can inhibit the angiogenesis by
activating the phosphoinositide 3-kinase (PI3K) signaling pathway
and regulating hypoxia-inducible factor 1 in addition to vascular
endothelial growth factor (15).
The present study aimed to investigate the role of miRNA (miR)-214
on inflammation and apoptosis in the vascular system and to examine
its potential mechanism.
Materials and methods
Cell culture and transfection
Human umbilical vein endothelial cells (HUVECs) were
obtained from the Shanghai Cell Bank of Chinese Academy of Sciences
(Shanghai, China) and cultured in M199 medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal
bovine serum (Hyclone; GE Healthcare Life Sciences) at 37°C in a
humidified 5% CO2 environment. Anti-miR-214 mimics were
obtained from Shanghai Sangon Biotech Co., Ltd. (Shanghai, China).
Anti-miR-214 mimics were transfected with Lipofectamine®
2000 Reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) into the HUVECs. After transfection at 48 h, HUVECs were
induced by 500 ng/ml TNF-α (cat. no. sc-4564; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 24 h.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA isolation was conducted using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of 1 µg total
RNA was reverse transcribed into cDNA using the PrimeScript™ RT
reagent kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. The RT-PCR reaction was conducted using an
AB7300 thermal cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using Platinum SYBR Green qPCR SuperMix-UDG
(Invitrogen; Thermo Fisher Scientific, Inc.). The primers used were
as follows: miR-214, forward 5′-CACCGCATCCGCTCACCTGTACAGC-3′ and
reverse 5′-AAACGCTGTACAGGTGAGCGGATGC-3′; U6, forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′. The
amplification conditions were as follows: Initial denaturation at
95°C for 10 min, followed by 40 cycles for 30 sec at 95°C and 1 min
in 60°C. Data were analyzed using the 2−ΔΔCq method
(16).
Cell proliferation
Cell viability was determined by the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
assay. MTT was added (5.0 mg/ml) into a plate and incubated at 37°C
in 5% CO2 for 4 h. Dimethyl sulfoxide was added to each
well and shaken for 20 min at 37°C. The optical density was
determined (Stat Fax 2100 Microplate Reader; Awareness Technology,
Inc., Palm City, FL, USA) at 490 nm.
Apoptosis assay
Cells were then washed with PBS and pelleted by
centrifugation at 500 × g for 5 min at 4°C and resuspended in
binding buffer (FACS Vantage Buffer; BD Biosciences, San Jose, CA,
USA). Cells were stained with 5 µg Annexin-V-fluorescein
isothiocyanate and 5 µg propidium iodide at room temperature in the
dark for 15 min. The samples were examined by flow cytometry (FACS
Vantage; BD Biosciences).
ELISA
Cells were harvested by scraping from the wells,
proteins were obtained with radioimmunoprecipitation assay lysis
buffer (RIPA; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
protein concentrations were determined using Coomassie protein
reagent (Bio-Rad Laboratories, Inc.). Proteins (5 µg) were used to
measure TNF-α, IL-1β, IL-6 and IL-18 levels using ELISA kits. The
optical density was determined (Stat Fax 2100 Microplate Reader) at
450 nm. Caspase-3 and caspase-9 activity were measured using
Caspase-3 and caspase-9 activity kits (Beyotime Institute of
Biotechnology, Jiangsu, China). The optical density was determined
(Stat Fax 2100 Microplate Reader) at 405 nm.
Western blotting
Cells were harvested by scraping from the wells,
proteins were obtained with RIPA. The protein concentrations were
determined using Coomassie protein reagent. Protein (50 µg) was
loaded per lane and separated by 8–10% SDS-PAGE and then
transferred to nitrocellulose membranes (GE Healthcare Life
Sciences, Arlington Heights, IL, USA). Membranes were blocked in
Tris-buffered saline containing 0.1% Tween-20 and 5% skimmed milk
for 1 h at 37°C and incubated with primary antibodies: Bax (cat.
no. sc-6236; 1:1,000; Santa Cruz Biotechnology, Inc.), PTEN (cat.
no. sc-9145; 1:1,000; Santa Cruz Biotechnology, Inc.), NF-κB (cat.
no. sc-109; 1:2,000; Santa Cruz Biotechnology, Inc.), p-Akt (cat.
no. sc-7985-R; 1:500; Santa Cruz Biotechnology, Inc.) and GAPDH
(cat. no. sc-25778; 1:5,000; Santa Cruz Biotechnology, Inc.) at 4°C
overnight, followed by incubation with anti-rabbit IgG
peroxidase-conjugated secondary antibodies (cat. no. 7054; 1:5,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 h at 37°C.
The detection of specific proteins was carried out with an ECL
Western blotting and quantified using a G: Box gel imaging system
by Syngene (Syngene, Frederick, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis of data was performed using one-way analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
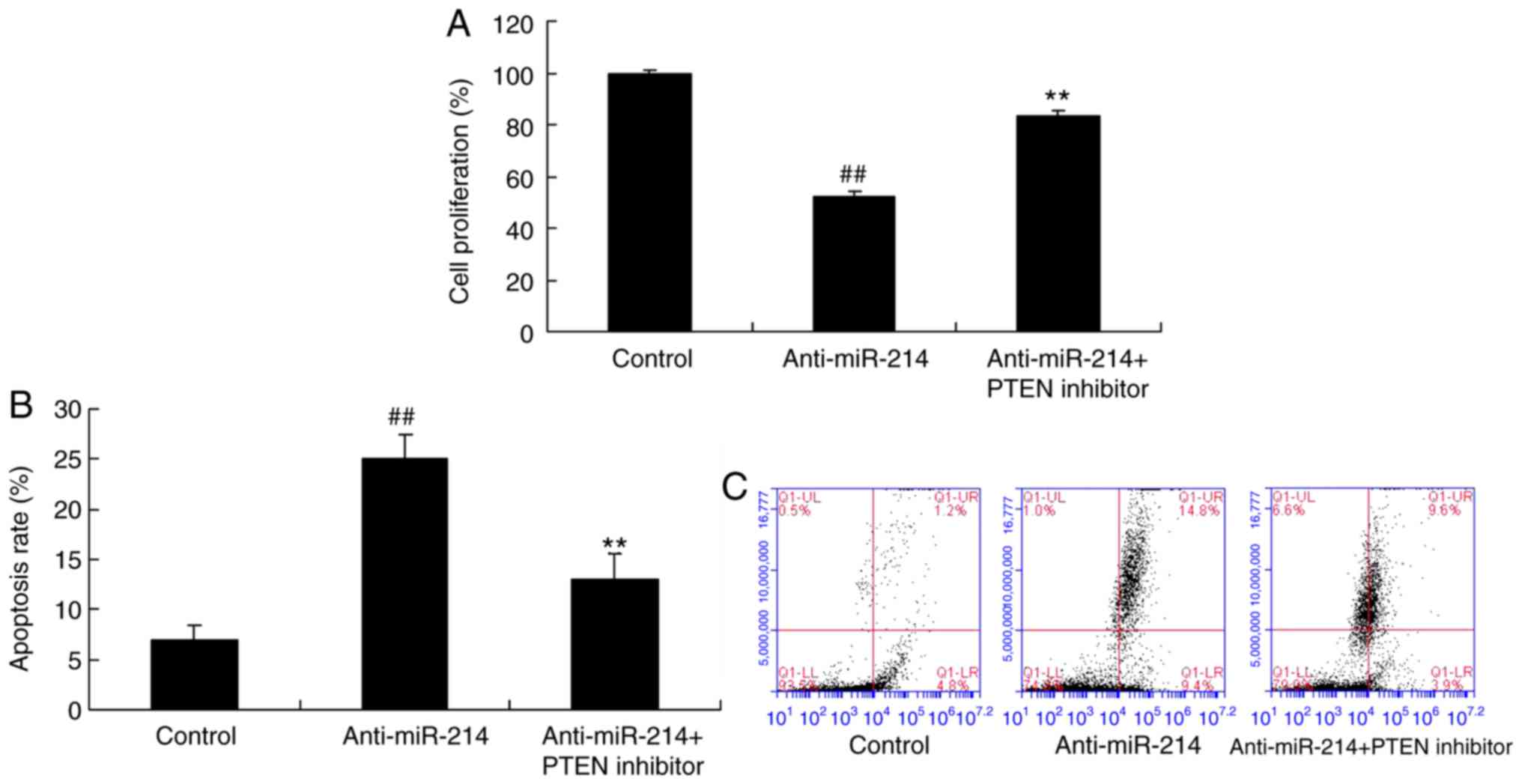
Cell proliferation and cell apoptosis
in vascular endothelial cells with miR-214 downregulation
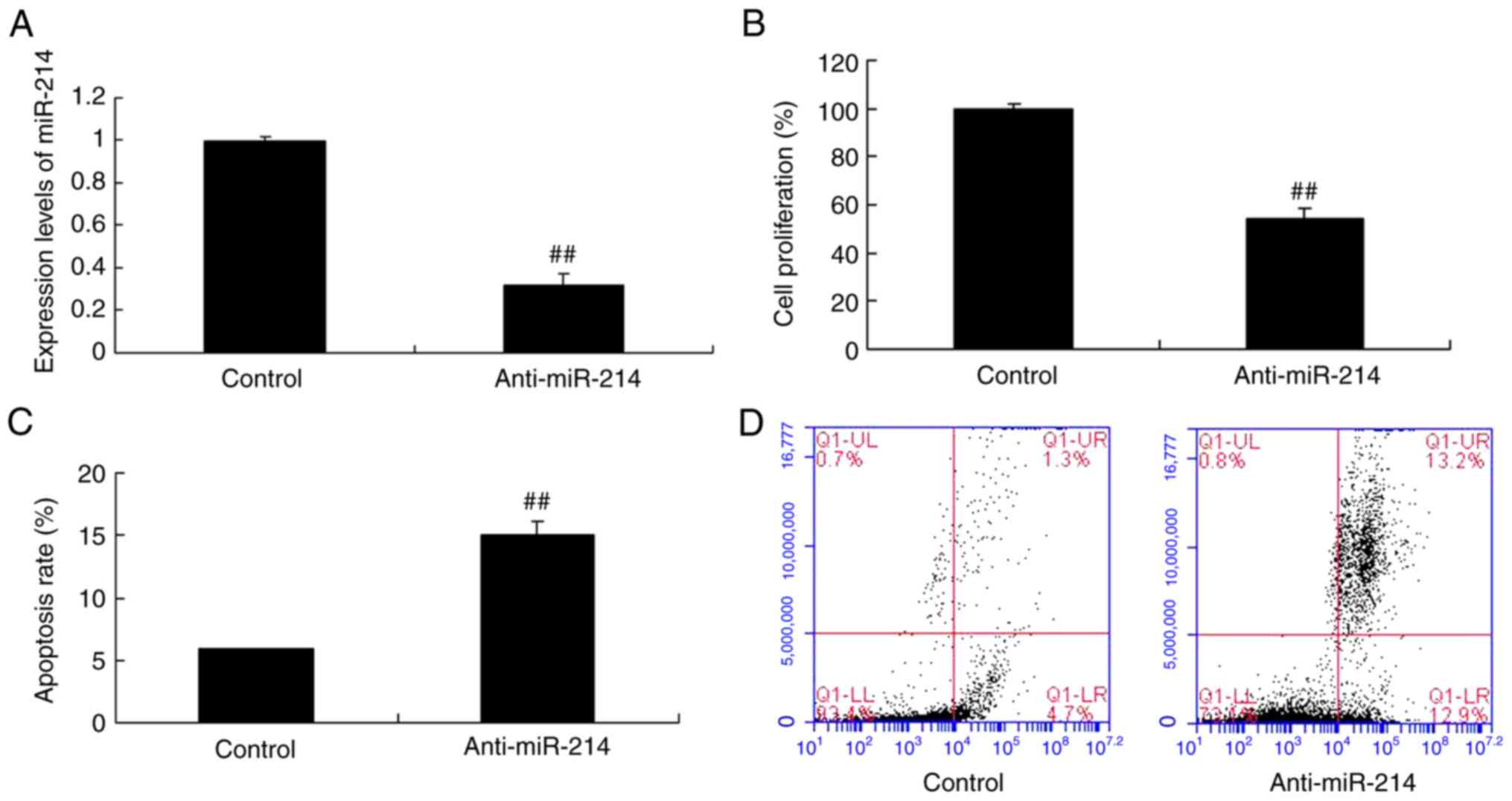
In order to explore the role of miR-214 on cell
growth and apoptosis in TNF-α-induced inflammation in vascular
endothelial cells, the cell proliferation and cell apoptosis were
analyzed. As presented in Fig. 1A,
anti-miR-214 mimics reduced miR-214 expression in vascular
endothelial cells induced by TNF-α, compared with the control
group. However, miR-214 downregulation inhibited cell proliferation
and induced apoptosis of TNF-α-induced inflammation vascular
endothelial cells, compared with the control group (Fig. 1B-D).
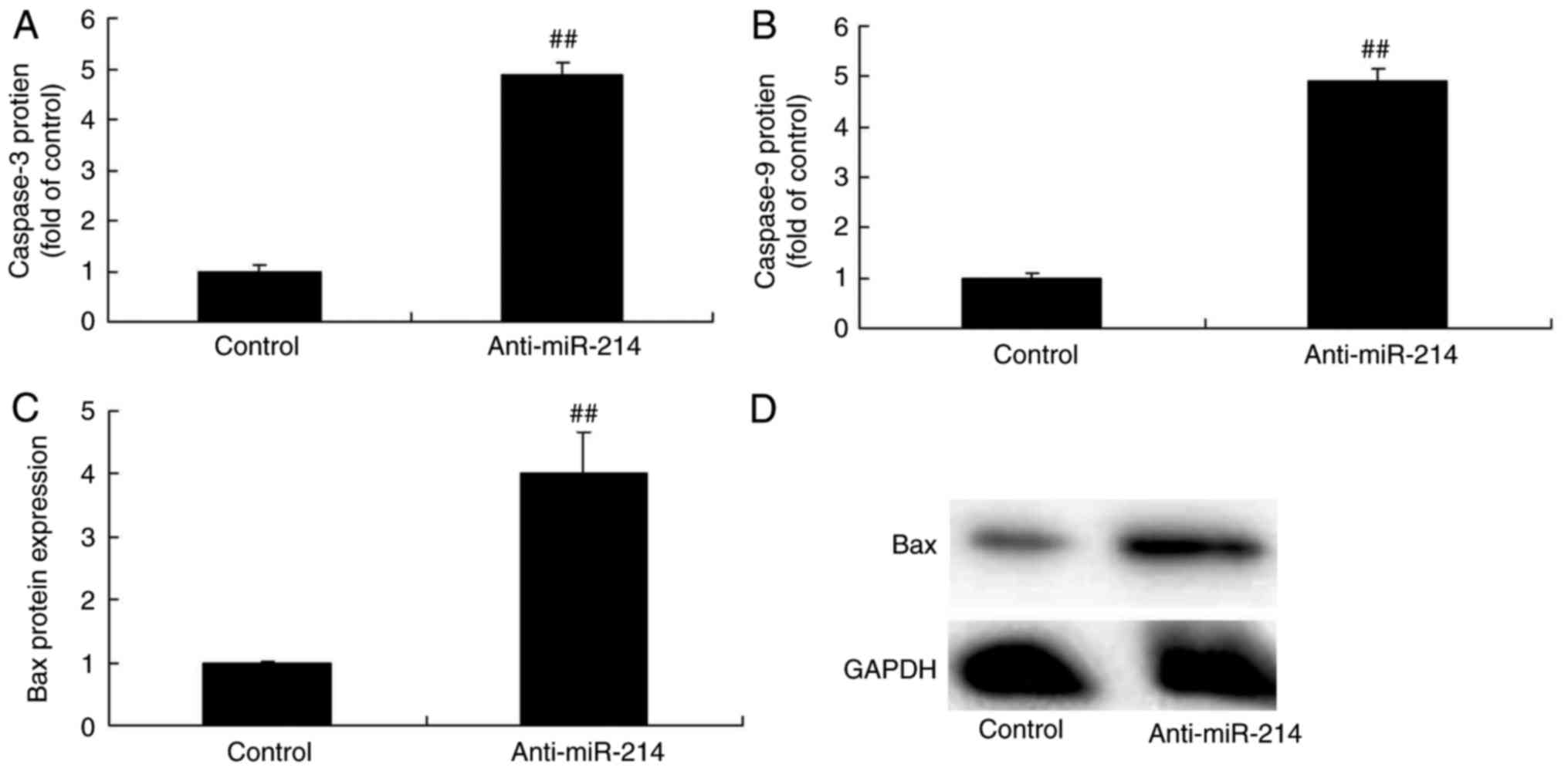
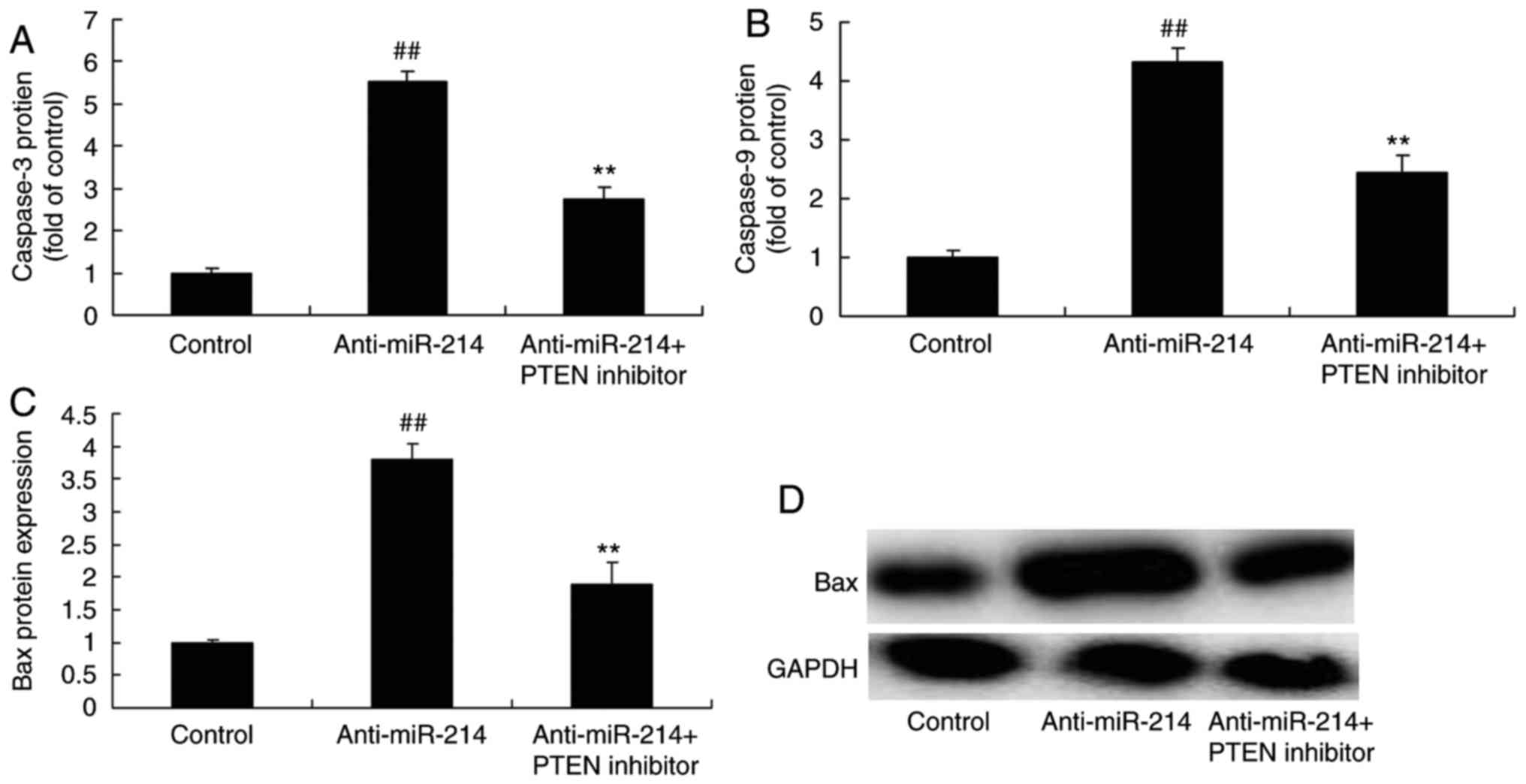
Caspase-3/9 and Bax protein expression
in vascular endothelial cells with miR-214 downregulation
Subsequently, the function of miR-214 in apoptosis
in TNF-α-induced inflammation in vascular endothelial cells was
investigated, with caspase-3/9 activity and Bax protein expression
as markers of apoptosis. There were significant increases of
caspase-3/9 activity and Bax protein expression in TNF-α-induced
inflammation in vascular endothelial cells with miR-214
downregulation, which indicated that miR-214 downregulation induced
vascular endothelial cell apoptosis (Fig. 2).
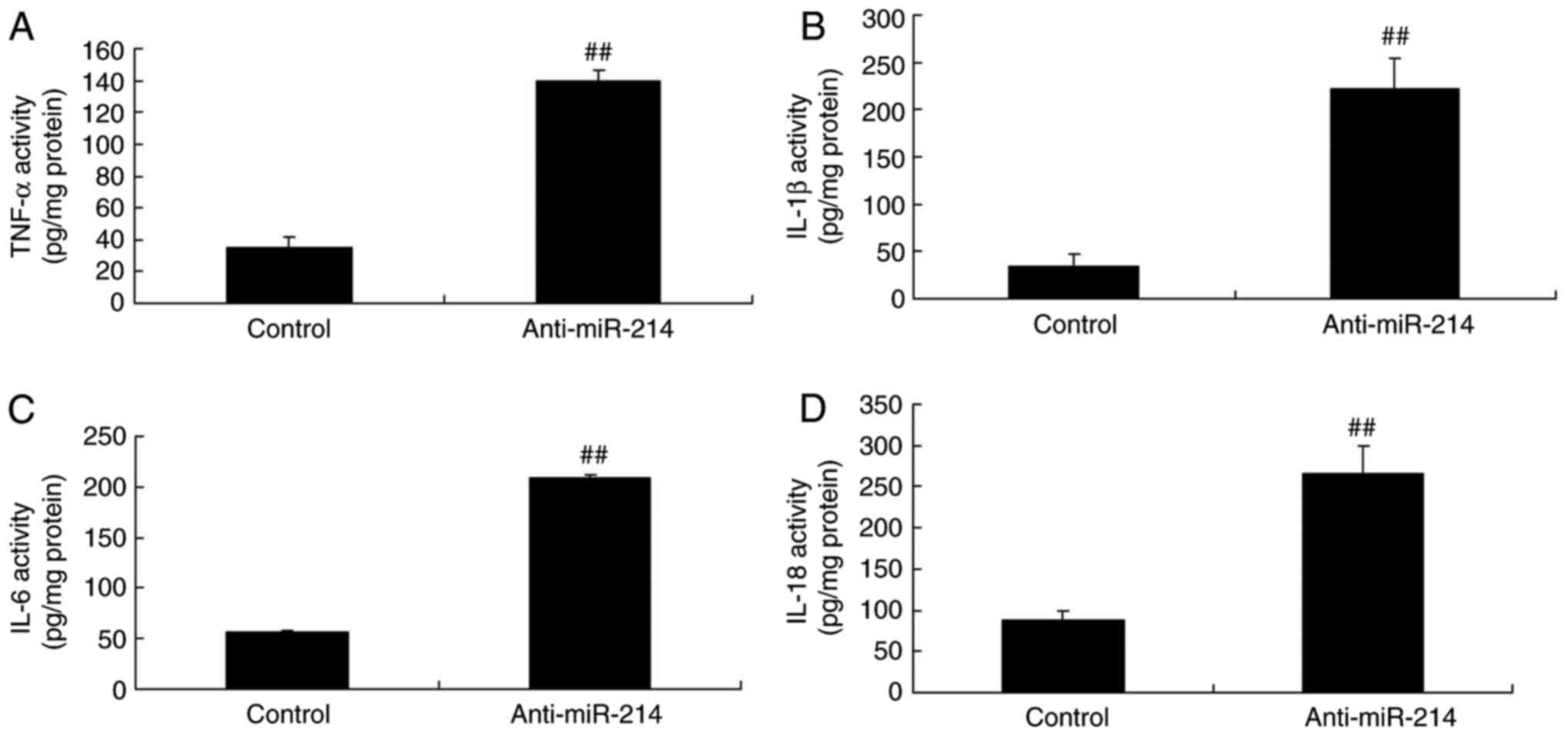
Inflammation changes in vascular
endothelial cells with miR-214 downregulation
To determine the function of anti-miR-214 on
inflammation changes in vascular endothelial cells, TNF-α, IL-1β,
IL-6 and IL-18 levels were determined using ELISA kits. Following
transfection with miR-214 mimics for 48 h, TNF-α, IL-1β, IL-6 and
IL-18 levels were significantly increased in TNF-α-induced
inflammation in vascular endothelial cells with miR-214
down-expression (Fig. 3).
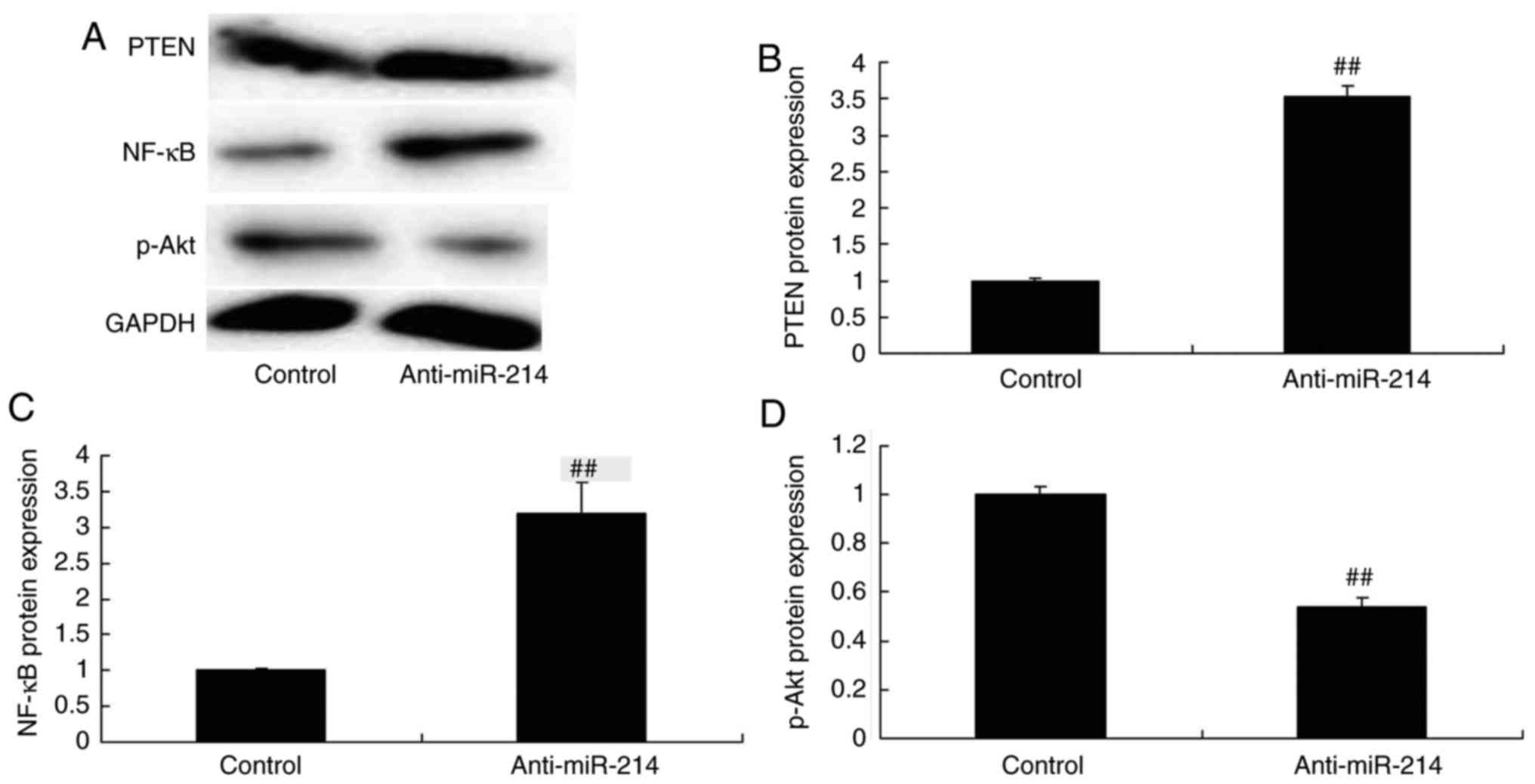
PTEN and NF-κB protein expression in
vascular endothelial cells with miR-214 downregulation
In order to examine the mechanism of anti-miR-214 on
inflammation and apoptosis in TNF-α-induced inflammation in
vascular endothelial cells, PTEN and NF-κB protein expression
levels were measured in vascular endothelial cells. Fig. 4 indicates that PTEN and NF-κB
protein expression were significantly induced, and p-Akt protein
expression was significantly reduced in TNF-α-induced inflammation
in vascular endothelial cells by miR-214 downregulation.
PTEN inhibitor inhibited the function
of miR-214 on PTEN and NF-κB protein expression in vascular
endothelial cells
According to the above results, the PTEN inhibitor
was used to adjust PTEN expression in TNF-α-induced inflammation in
vascular endothelial cells by miR-214 downregulation. As presented
in Fig. 5, the PTEN inhibitor
could inhibit PTEN and NF-κB protein expression, and induced p-Akt
protein expression in TNF-α-induced inflammation in vascular
endothelial cells by miR-214 downregulation.
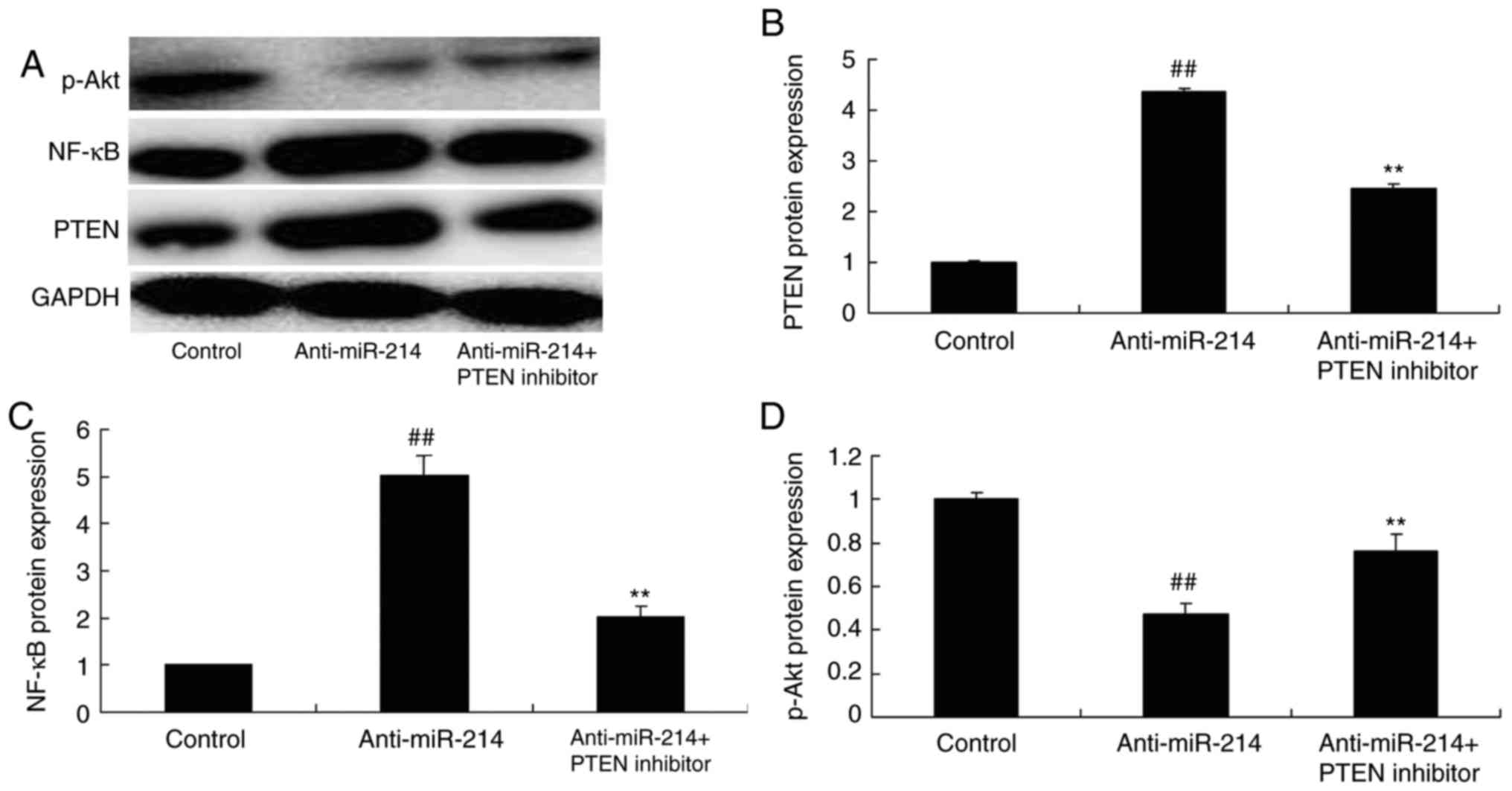 | Figure 5.PTEN inhibitor, inhibited the function
of miR-214 on PTEN and NF-κB protein expression in vascular
endothelial cells. the PTEN inhibitor inhibited the function of
miR-214 on PTEN, NF-κB and p-Akt protein expression observed by (A)
western blotting and statistical analysis of (B) PTEN, (C) NF-κB
and (D) p-Akt in vascular endothelial cells by miR-214
downregulation. ##P<0.01 vs. control group,
**P<0.01 vs. anti-miR-214 mimics group. PTEN, phosphatase and
tensin homolog; miR, microRNA; PTEN inhibitor, VO-Ohpic trihydrate
+ anti-miR-214 group; NF-κB, nuclear factor κB; p-Akt,
phosphorylated protein kinase B; control, control group;
Aanti-miR-214, anti-miR-214 mimics group. |
PTEN inhibitor inhibited the function
of anti-miR-214 on apoptosis in vascular endothelial cells
Subsequently, it was investigated whether the PTEN
inhibitor decreased the function of miR-214 on apoptosis in
vascular endothelial cells. The inhibition of PTEN expression
promoted cell proliferation and inhibited apoptosis of
TNF-α-induced inflammation in vascular endothelial cells by miR-214
downregulation (Fig. 6).
PTEN inhibitor inhibited the function
of miR-214 on caspase-3 and Bax protein expression in vascular
endothelial cells
In addition, it was identified that the inhibition
of PTEN expression decreased the function of miR-214 on caspase-3
and Bax protein expression in vascular endothelial cells (Fig. 7).
PTEN inhibitor inhibited the function
of miR-214 on inflammation in vascular endothelial cells
To investigate the role of anti-miR-214 on
inflammation in vascular endothelial cells, TNF-α, IL-1β, IL-6 and
IL-18 levels were measured. The inhibition of PTEN expression
significantly reduced TNF-α, IL-1β, IL-6 and IL-18 levels in
TNF-α-induced inflammation in vascular endothelial cells by miR-214
downregulation (Fig. 8).
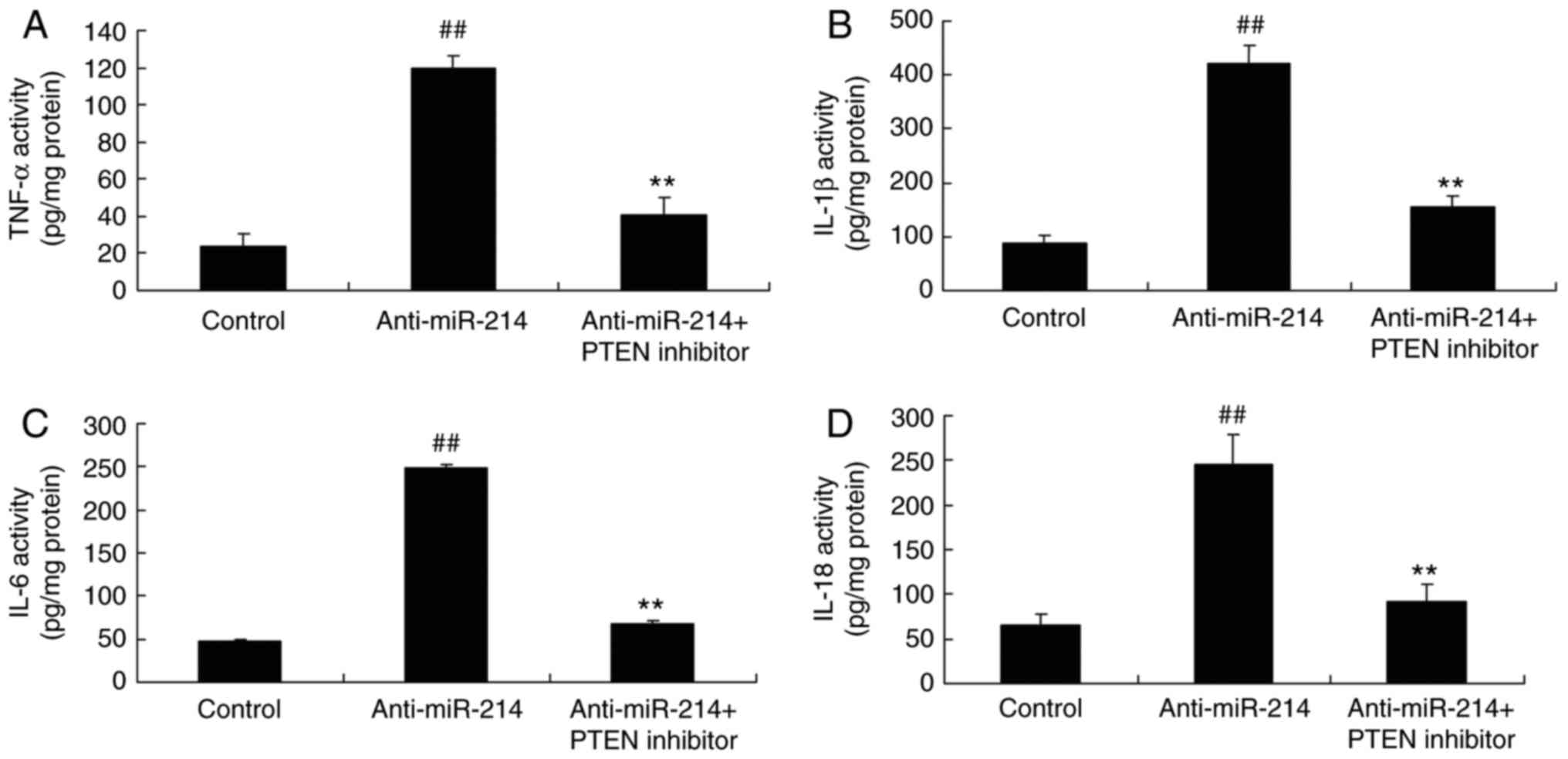 | Figure 8.PTEN inhibitor, inhibited the function
of miR-214 on inflammation in vascular endothelial cells. The PTEN
inhibitor inhibited the function of miR-214 on (A) TNF-α, (B)
IL-1β, (C) IL-6 and (D) IL-18 levels. ##P<0.01 vs.
control group, **P<0.01 vs. anti-miR-214 mimics group. PTEN,
phosphatase and tensin homolog; miR, microRNA; PTEN inhibitor,
VO-Ohpic trihydrate + anti-miR-214 group; TNF-α, tumor necrosis
factor α; IL, interleukin; control, control group; anti-miR-214,
anti-miR-214 mimics group. |
Discussion
A clinical epidemiological survey has indicated that
the morbidity and mortality of cardiovascular disease in China is
increasing, it is predicted that the cases of cardiovascular
disease will increase by 73% by 2030 in China (17). Chronic inflammation serves an
important role in the occurrence and development of cardiovascular
diseases (18). However, at
present, the traditional anti-inflammatory therapies have not
achieved satisfactory efficacy (19). Therefore, it is of significance to
study the role and mechanism of the inflammatory reaction in
cardiovascular disease. Full elucidation of the association between
inflammation and cardiovascular disease in addition to exploring
novel targets for intervention of vascular inflammation is
important (19). In the present
study, it was demonstrated that miR-214 downregulation inhibited
cell proliferation and induced apoptosis in TNF-α-induced vascular
endothelial cells. Yang et al (20) has suggested that miR-214 inhibited
left ventricular remodeling through suppressing cell apoptosis in
acute myocardial infarction.
A large number of inflammatory markers and
inflammatory mediators demonstrate the role of inflammatory
responses in hypertensive disorders. In addition, innate and
adaptive immune system disorders are important factors in the
development of hypertension (21).
In animal models with hypertensive diseases, the vessel wall is
infiltrated by a large number of inflammatory cells. In addition,
target organs including the heart and kidney are accompanied by
varying degrees of inflammatory injury (22). Vascular inflammation is also
involved in the pathophysiology of other hypertension-associated
cardiovascular diseases, and vascular inflammation is suggested to
be a bridge between hypertension and atherosclerosis (23). Specifically, it was observed that
miR-214 downregulation increased TNF-α, IL-1β, IL-6 and IL-18
levels of TNF-α induces inflammation in vascular endothelial cells.
Chen et al (24)
demonstrated that the upregulated microRNA-214 enhances cardiac
injury-induced inflammation. Future studies should investigate the
role of miR-214 in vascular inflammation further by examining the
effects of miR-214 overexpression in vascular endothelial
cells.
PTEN expression is predominantly regulated by miRNA
(miR-21), phosphorylation, acetylation and ubiquitination after
transcription (15). A number of
studies have demonstrated that the deletion of the PTEN gene serves
an important role in the development and progression of tumors
(25). In addition, its
angiogenesis can be affected by PI3K/Akt signaling. The main
process is that of PTEN reversing the phosphorylation of PI3K and
removing the phosphatidylinositol-3 phosphate (PIP3) phosphate
group through its lipid phosphatase activity. In this way, it is
reduced to phosphatidylinositol-2-phosphate, maintaining low
intracellular PIP3 levels (25,26).
At present, multiple experimental studies have reported that PTEN
serves an important role in the regulation of key signals in tumor
angiogenesis (25,26). Notably, it was observed that
miR-214 downregulation induced PTEN and NF-κB protein expression
and suppressed p-Akt protein expression in TNF-α-induced
inflammation in vascular endothelial cells. Zhao et al
(27) demonstrated that miR-214
promotes osteoclastogenesis by targeting the PTEN/PI3K/Akt pathway.
Chu et al (28) reported
that microRNA-214 suppresses NF-κB-mediated inflammatory responses
in fish, further studies will aim to investigate this association
anti-miR-214-mediated regulation of NF-κB in further detail.
PTEN additionally regulates the behavior of
endothelial cells. Certain experiments have demonstrated that the
PTEN expression is disrupted in most cell matrix and endothelial
cells, thus reducing the activation of PTEN signaling in PI3K in
endothelial cells (29). In
addition, it leads to increased endothelial cell proliferation,
survival and migration. These changes are often important factors
and markers of tumor angiogenesis (29). It was additionally observed that
the PTEN inhibitor inhibited the function of miR-214 on apoptosis
and inflammation in TNF-α-induced inflammation in vascular
endothelial cells through PTEN/Akt signaling. Wang et al
(30) suggested that microRNA-214
protects against hypoxia/reoxygenation-induced cell damage through
suppression of PTEN and Bim1 expression. The present study lacked
specificity of the PTEN inhibitor, future experiments should use
siRNA to knockdown PTEN expression in order to investigate its role
in miR-214-induced vascular inflammation.
In summary, the data of the present study
demonstrated that the downregulation of miR-214 increased vascular
inflammation and apoptosis via the PTEN/Akt signaling pathway in
TNF-α-induced inflammation in vascular endothelial cells. Thus,
miR-214 may serve a role in vascular inflammation and apoptosis
that may be harnessed for use in clinical applications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
MW designed the study and wrote the manuscript; ML,
TN and QL performed the experiments; MW and ML analyzed the
data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mateos MV, Oriol A, Martínez-López J,
Teruel AI, Bengoechea E, Palomera L, de Arriba F, Esseltine DL,
Cakana A, Pei L, et al: Outcomes with two different schedules of
bortezomib, melphalan, and prednisone (VMP) for previously
untreated multiple myeloma: Matched pair analysis using long-term
follow-up data from the phase 3 VISTA and PETHEMA/GEM05 trials. Ann
Hematol. 95:2033–2041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang J, Wang Y, Li H, Liu X, Qiu Q and Qi
L: Neck circumference and early stage atherosclerosis: The
cardiometabolic risk in Chinese (CRC) study. Cardiovasc Diabetol.
13:1072014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gómez-Pardo E, Fernández-Alvira JM,
Vilanova M, Haro D, Martínez R, Carvajal I, Carral V, Rodríguez C,
de Miguel M, Bodega P, et al: A comprehensive lifestyle peer
group-based intervention on cardiovascular risk factors: The
randomized controlled fifty-fifty program. J Am Coll Cardiol.
67:476–485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan DC, Pang J, McQuillan BM, Hung J,
Beilby JP, Barrett PH and Watts GF: Plasma proprotein convertase
subtilisin kexin type 9 as a predictor of carotid atherosclerosis
in asymptomatic adults. Heart Lung Circ. 25:520–525. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi R, Tamura K, Wakui H, Ohsawa M,
Azushima K, Haku S, Uneda K, Ohki K, Haruhara K, Kinguchi S and
Umemura S: Effect of single-pill irbesartan/amlodipine
combination-based therapy on clinic and home blood pressure
profiles in hypertension with chronic kidney diseases. Clin Exp
Hypertens. 38:744–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dissanayake E and Inoue Y: MicroRNAs in
allergic disease. Curr Allergy Asthma Rep. 16:672016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao J, Ma X, Zhang Y, Guo M and Shi D: The
role of microRNAs in prethrombotic status associated with coronary
artery disease. Thromb Haemost. 117:429–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orlicka-Płocka M, Gurda D,
Fedoruk-Wyszomirska A, Smolarek I and Wyszko E: Circulating
microRNAs in cardiovascular diseases. Acta Biochim Pol. 63:725–729.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranković G, Milicić B, Savić T, Dindić B,
Mancev Z and Pesić G: Effects of physical exercise on inflammatory
parameters and risk for repeated acute coronary syndrome in
patients with ischemic heart disease. Vojnosanit Pregl. 66:44–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Longenecker CT, Jiang Y, Yun CH, Debanne
S, Funderburg NT, Lederman MM, Storer N, Labbato DE, Bezerra HG and
McComsey GA: Perivascular fat, inflammation, and cardiovascular
risk in HIV-infected patients on antiretroviral therapy. Int J
Cardiol. 168:4039–4045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugimoto M, Ichio A and Kondo M: Short
pulse duration high-power laser photocoagulation during vitrectomy
for diabetic retinopathy reduces postoperative inflammation. PLoS
One. 10:e01351262015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Calzón S, Zalba G, Ruiz-Canela M,
Shivappa N, Hébert JR, Martínez JA, Fitó M, Gómez-Gracia E,
Martínez-González MA and Marti A: Dietary inflammatory index and
telomere length in subjects with a high cardiovascular disease risk
from the PREDIMED-NAVARRA study: Cross-sectional and longitudinal
analyses over 5 y. Am J Clin Nutr. 102:897–904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruiz-Canela M, Zazpe I, Shivappa N, Hébert
JR, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Fitó M,
Lamuela-Raventós RM, Rekondo J, et al: Dietary inflammatory index
and anthropometric measures of obesity in a population sample at
high cardiovascular risk from the PREDIMED (PREvención con DIeta
MEDiterránea) trial. Br J Nutr. 113:984–995. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X,
Yu H, Miao J, Kao R, Kalbfleisch J, et al: Attenuation of cardiac
dysfunction and remodeling of myocardial infarction by
microRNA-130a are mediated by suppression of PTEN and activation of
PI3K dependent signaling. J Mol Cell Cardiol. 89:87–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling S, Birnbaum Y, Nanhwan MK, Thomas B,
Bajaj M and Ye Y: MicroRNA-dependent cross-talk between VEGF and
HIF1alpha in the diabetic retina. Cell Signal. 25:2840–2847. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Tian F, Wang J, Yang JJ, Zhang T,
Jing J and Chen YD: Efficacy study of olmesartan medoxomil on
coronary atherosclerosis progression and epicardial adipose tissue
volume reduction in patients with coronary atherosclerosis detected
by coronary computed tomography angiography: Study protocol for a
randomized controlled trial. Trials. 17:102016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia P, Jin W, Teng J, Zhang H, Zou J, Liu
Z, Shen B, Cao X and Ding X: Acute effects of hemodiafiltration
versus conventional hemodialysis on endothelial function and
inflammation: A randomized crossover study. Medicine (Baltimore).
95:e34402016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neufcourt L, Assmann KE, Fezeu LK, Touvier
M, Graffouillère L, Shivappa N, Hébert JR, Wirth MD, Hercberg S,
Galan P, et al: Prospective association between the dietary
inflammatory index and cardiovascular diseases in the
SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX)
Cohort. J Am Heart Assoc. 5:e0027352016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang X, Qin Y, Shao S, Yu Y, Zhang C, Dong
H, Lv G and Dong S: MicroRNA-214 inhibits left ventricular
remodeling in an acute myocardial infarction rat model by
suppressing cellular apoptosis via the phosphatase and tensin
homolog (PTEN). Int Heart J. 57:247–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Riso P, Klimis-Zacas D, Del Bo' C, Martini
D, Campolo J, Vendrame S, Møller P, Loft S, De Maria R and Porrini
M: Effect of a wild blueberry (Vaccinium angustifolium) drink
intervention on markers of oxidative stress, inflammation and
endothelial function in humans with cardiovascular risk factors.
Eur J Nutr. 52:949–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reichert V, Xue X, Bartscherer D, Jacobsen
D, Fardellone C, Folan P, Kohn N, Talwar A and Metz CN: A pilot
study to examine the effects of smoking cessation on serum markers
of inflammation in women at risk for cardiovascular disease. Chest.
136:212–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Høgh Kølbæk Kjær AS, Brinkmann CR,
Dinarello CA, Olesen R, Østergaard L, Søgaard OS, Tolstrup M and
Rasmussen TA: The histone deacetylase inhibitor panobinostat lowers
biomarkers of cardiovascular risk and inflammation in HIV patients.
AIDS. 29:1195–1200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen ZG, Liu H, Zhang JB, Zhang SL, Zhao
LH and Liang WQ: Upregulated microRNA-214 enhances cardiac injury
by targeting ITCH during coxsackievirus infection. Mol Med Rep.
12:1258–1264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang QG, Wu DN, Han D and Zhang GY:
Critical role of PTEN in the coupling between PI3K/Akt and JNK1/2
signaling in ischemic brain injury. FEBS Lett. 581:495–505. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boosani CS and Agrawal DK: PTEN
modulators: A patent review. Expert Opin Ther Pat. 23:569–580.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao C, Sun W, Zhang P, et al: miR-214
promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA
Biol. 12:343–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu Q, Sun Y, Cui J and Xu T: Inducible
microRNA-214 contributes to the suppression of NF-kappaB-mediated
inflammatory response via targeting myd88 gene in fish. J Biol
Chem. 292:5282–5290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kar S, Samii A and Bertalanffy H:
PTEN/PI3K/Akt/VEGF signaling and the cross talk to KRIT1, CCM2, and
PDCD10 proteins in cerebral cavernous malformations. Neurosurg Rev.
38:229–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Ha T, Hu Y, et al: MicroRNA-214
protects against hypoxia/reoxygenation induced cell damage and
myocardial ischemia/reperfusion injury via suppression of PTEN and
Bim1 expression. Oncotarget. 7:86926–86936. 2016. View Article : Google Scholar : PubMed/NCBI
|