Introduction
Intrauterine adhesions (IUA) frequently occur as a
result of trauma to the basal layer of the endometrium that may
lead to a range of symptoms, such as amenorrhea, hypomenorrhea,
infertility or recurrent pregnancy loss (1,2).
Additionally, it may lead to a variety of subsequent sequelae and
severely affect women's health and fertility (3). Despite the wide use of diagnostic and
operative hysteroscopy that have improved the functional outcome of
patients with IUA, advances in the management of IUA remain
limited. Endometrial fibrosis is the primary pathological feature
of IUA characterized by excessive deposition and reorganization of
extracellular matrix (ECM) replacing the normal endometrium. The
prevention of endometrial fibrosis may potentially lead to
endometrial repair and promote the formation of fibrous scar
adhesions (4). However, the
specific mechanisms of underlying endometrial fibrosis remain to be
elucidated.
Transforming growth factor (TGF)-β1 is a secreted
protein expressed in most cell types, which has been implicated in
endometrial fibrosis, by inducing fibroblast synthesis and by
induction of epithelial mesenchymal transition (EMT) (5). It has been previously established
that TGF-β1 exert its biological activity by binding to its
receptor through two critical downstream mediators, SMAD family
member 2 (Smad2) and Smad3, receptor-regulated Smads (6,7).
Previous studies have reported that TGF-β1 has an important role in
the development of fibrosis diseases (8–10).
Increasing TGF-β1 expression in the mouse lung leads to severe
interstitial and pleural fibrosis (11). Previous studies demonstrated that
TGF-β1/Smad3 pathway mediated fibrosis by directly binding to
various ECM promoters, including collagen I, II, and III (12–15).
It has also been demonstrated the expression of TGF-β in the
endometrial tissue of IUA was significantly increased compared with
normal endometrium and uterine septum (5). However, whether the TGF-β1/Smad3
pathway is involved in endometrial fibrosis remains to be
determined.
MicroRNAs (miRNAs) are a class of endogenous small
(19–24 nucleotide) non-coding RNAs that suppress translation of
target messenger RNAs (mRNAs) or induce degradation of target mRNAs
by binding to the 3′-untranslated region (UTR) of target mRNAs
(16). Previous studies revealed
that aberrant expression or dysfunction of miRNAs has an important
role in various types of tissue fibrosis, such as in the heart
(17,18), kidney (19,20),
liver (21) and lung (22). However, to the best of our
knowledge, at present, there have only been preliminary studies on
the biological roles of miRNAs in IUA. MiR-29b was determined to be
downregulated in TGF-β1-treated endometrial stromal cells (ESCs)
and inhibited TGF-β1-induced fibrosis via regulation of the
TGF-β1/Smad pathway in ESCs (23).
Therefore, more extensive investigation on the identification and
the functions of miRNAs involved in the development of IUA are
required.
The present study focused on the expression level of
miR-326 in IUA and investigated its role in endometrial fibrosis.
The present findings revealed that overexpression of miR-326 may
inhibit endometrial fibrosis by inactivating the TGF-β1/Smad3
pathway. These findings suggest that miR-326 may be a novel
therapeutic target for IUA.
Materials and methods
Clinical subjects
All the subjects were recruited from Hainan Branch
of PLA General Hospital (Sanya, China) between January 2015 and
July 2016. Written informed consent was obtained prior to inclusion
and the present study was approved by the Ethics Committee of
Hainan branch of PLA General Hospital. A total of 30 endometrial
tissues were collected from IUA patients diagnosed by hysteroscopy,
with mean age 28.06 years (range 19–34). Normal endometrial tissues
from individuals without IUA (n=15) in the same period were used as
controls. All samples were flash-frozen in liquid nitrogen, and
stored at −80°C until subsequent molecular analysis.
MicroRNA expression profiling
Total RNA was extracted from endometrial tissues
using the miRcute miRNA Isolation kit (Tiangen Biotech Co., Ltd.,
Beijing, China). RNA quality was determined using an Agilent
Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA).
Samples were labeled using a miRCURY
Hy3™/Hy5™ Power labeling kit and hybridized
to the miRCURY LNA™ Array version 18.0 (Agilent
Technologies, Inc.). The chips were scanned with the Axon Gene Pix
4000B Microarray Scanner (Axon Instruments, Foster City, CA, USA).
The procedure and image processing method was performed as
previously described (24). The
miRNA expressions of all differentially expressed samples were
clearly displayed by a hierarchical clustering heat map.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from endometrial tissues
using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
For the detection of miR-326, RT-qPCR assays were performed using
the miRcute miRNA qPCR Detection kit (Tiangen Biotech Co., Ltd.)
following the manufacturer's protocol. For detection of the TGF-β1,
α-smooth muscle actin (α-SMA), collagen type I α 1 chain (COL1A1)
and fibronectin (FN) mRNA levels, 1 µg of total RNA was reverse
transcribed at 37°C for 60 min using the miRcute miRNA First-Strand
cDNA Synthesis kit (Tiangen Biotech Co., Ltd.), then amplified on
an Applied Biosystems 7900HT cycler using SuperReal PreMix Plus
(Tiangen Biotech Co., Ltd.). The PCR conditions consisted of 5 min
at 94°C for one cycle followed by 40 cycles of 94°C for 20 sec and
60°C for 20 sec. U6 and GAPDH were used as normalization controls
for miRNA qPCR and qPCR, respectively. The relative expression was
calculated using the 2−ΔΔCq method (25). Each reaction was conducted in
triplicate. The primers for RT-qPCR analysis were as follows:
miR-326 forward primer: 5′-GGCGCCCAGAUAAUGCG-3′; miR-326 reverse
primer: 5′-CGTGCAGGGTCCGAGGTC-3′; U6 forward primer:
5′-TGCGGGTGCTCGCTTCGCAGC-3′; U6 reverse primer:
5′-CCAGTGCAGGGTCCGAGGT-3′; TGF-β1 forward primer:
5′-TGGACCGCAACAACGCCATCTATGAGAAAACC-3′, reverse primer:
5′-TGGAGCTGAAGCAATAGTTGGTATCCAGGGCT-3; COL1A1 forward primer:
5′-GAGGGCCAAGACGAAGACATC-3, reverse primer:
5′-CAGATCACGTCATCGCACAAC-3; α-SMA forward primer:
5′-GGCTCTGGGCTCTGTAAGG-3, reverse primer:
5′-CTCTTGCTCTGGGCTTCATC-3; fibronectin forward primer:
5′-GAGAGATCTGGAGGTCAT-3′, reverse primer:
5′-GGGTGACACCTGAGTTGAA-3′; GAPDH reverse primer:
5′-CGGAGTCAACGGATTTGGTCGTAT-3′, reverse primer:
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
Primary ESC isolation and culture
ESCs were isolated from the endometrial tissues as
previously described (26). ESCs
were maintained in Dulbecco's modified Eagle's medium/Nutrient
Mixture F-12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, 100 µg/ml streptomycin at
37°C in a humidified atmosphere of 5% CO2. The cells
reached confluence in 2–3 days, and the passage 3–6 were used for
subsequent experiments.
Cell transfection
MiR-326 mimics, miR-326 inhibitor and controls were
purchased from Shanghai GenePharma Co., Ltd., (Shanghai, China).
ESCs were seeded at 2×105 cells/well in 6-well plates
and cultured in antibiotic-free DMEM at 37°C and 5% CO2.
Next, the cells were incubated overnight to a confluence of 30–50%,
cell transfection was performed using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cells were collected for further
experiments 48 h after the transfection.
Luciferase reporter assay
A cDNA fragment of the TGF-β1 3′-UTR mRNA containing
the seed sequence of the miR-326-binding site or a mutated binding
site was cloned into the pmirGLO dual-luciferase vector (Promega
Corporation, Madison, WI, USA). The constructed dual-luciferase
vector was co-transfected with miR-326 mimics, miR-326 inhibitor or
NC into ESCs isolated from patients with IUA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
relative firefly luciferase activity normalized with Renilla
luciferase was measured 48 h after transfection by using the
Dual-Luciferase Reporter system (Promega Corporation) following the
manufacturer's protocol. All of the dual-luciferase reporter assays
were performed in triplicate for each experiment and three
independent experiments were conducted.
Western blotting
Cells were lysed using radioimmunoprecipitation
assay protein extraction reagent (Beyotime Institute of
Biotechnology, Haimen, China). The concentration of proteins was
determined using a bicinchoninic acid assay kit (Pierce
Biotechnology, Rockford, IL, USA). Protein extracts (50 µg per
lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis, then transferred to nitrocellulose membranes
(Sigma-Aldrich, Merck Millipore; Darmstadt, Germany) and incubated
with the following primary antibodies: TGF-β1 monoclonal antibody
(cat. no. sc-52892; 1:300; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-phosphorylated (p)-Smad3 monoclonal antibody (cat.
no. sc-517575; 1:1,000; Santa Cruz Biotechnology, Inc.) and Smad3
monoclonal antibody (cat. no. sc-101154; 1:1,000; Santa Cruz
Biotechnology, Inc.). Subsequently, the membranes were incubated
with peroxidase conjugated goat anti-rabbit IgG (cat. no. TA130023;
1:5,000; OriGene Technologies, Inc., Beijing, China) conjugated to
horseradish peroxidase for 1 h at 37°C. ECL chromogenic substrate
(Thermo Fisher Scientific, Inc.) was used to visualize the bands
and the intensity of the bands was quantified by densitometry using
ImageJ software (version 2.1.4.7; National Institutes of Health,
Bethesda, MD, USA). Control antibody used was anti-β-actin (cat.
no. sc-8432; 1:1,000; Santa Cruz Biotechnology, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Differences were analyzed with Student's t-test between two groups
or with one-way analysis of variance among four groups. Correlation
analyses were performed using Spearman's correlation coefficient
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
MiR-326 is downregulated in
endometrial tissues from patients with IUA
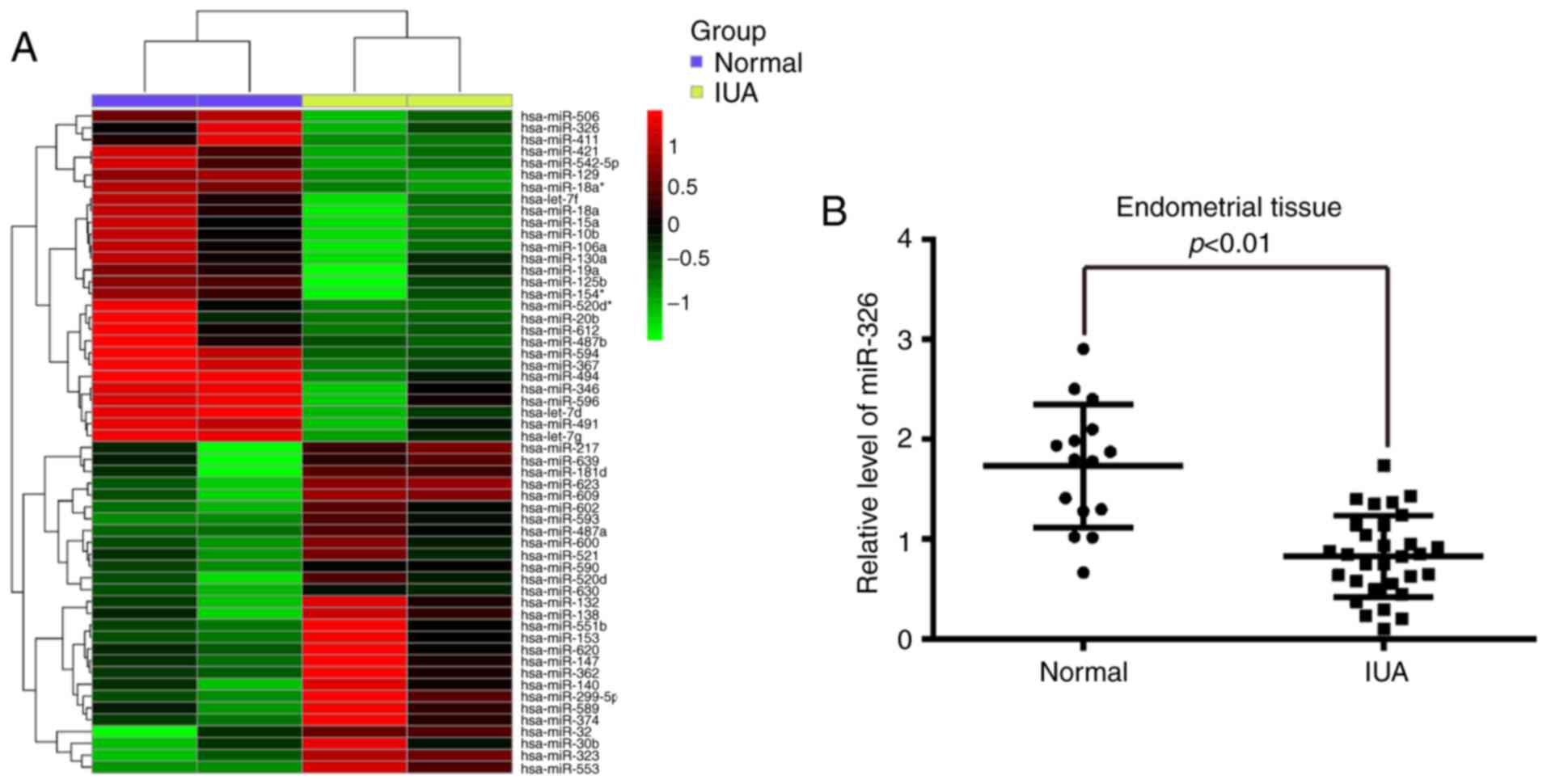
To determine the expression of miRNAs associated
with IUA, the present study performed a miRNA microarray on
endometrial tissues from pairs of patients with or without IUA. The
current findings revealed that the expression of 56 miRNAs was
significantly different in the IUA group compared with the normal
group and from these miRNAs, 28 miRNAs were downregulated, whereas
28 miRNAs were upregulated (Fig.
1A). From these aberrantly expressed miRNAs, miR-326 was one
which was significantly downregulated. In addition, a previous
study reported that miR-326 had a key role in alleviating lung
fibrosis by regulating TGF-β1 expression and other pro-fibrotic
genes (27). Therefore, the
present study selected miR-326 for further investigation.
To validate the expression trend identified for
miR-326 in endometrial tissues obtained from miRNA microarray assay
RT-qPCR was performed to detect miR-326 expression levels in 30 IUA
endometrial tissues and 15 normal endometrial tissues. As presented
in Fig. 1B, miR-326 was
significantly downregulated in the IUA group compared with the
normal group. These findings indicated that miR-326 may be involved
in IUA progression.
Correlation between miR-326 and the
level of fibrotic markers in endometrial tissues
The present study examined whether abnormal
expression of miR-326 was associated with the endometrial fibrosis
of patients with IUA following confirmation of the reduced
expression of miR-326 in endometrial tissues. Previous studies
revealed that TGF-β1 may promote the endometrial fibrosis, and
α-SMA, COL1A1 and FN are the primary fibrotic marker genes
(9,20,28).
Thus, the miR-326 expression level and the fibrotic markers (TGF-β1
α-SMA, COL1A1, and FN) mRNA expression level in 30 IUA endometrial
tissues were determined. Using Spearman's correlation coefficient,
a negative correlation was identified between TGF-β1, α-SMA,
COL1A1, and FN mRNA expression levels and the miR-326 expression
level (Fig. 2). These findings
indicate that miR-326 may potentially serve as an effective
biomarker for the prognosis of patients with IUA. Additionally,
miR-326 may be relevant to fibrotic processes in IUA pathology.
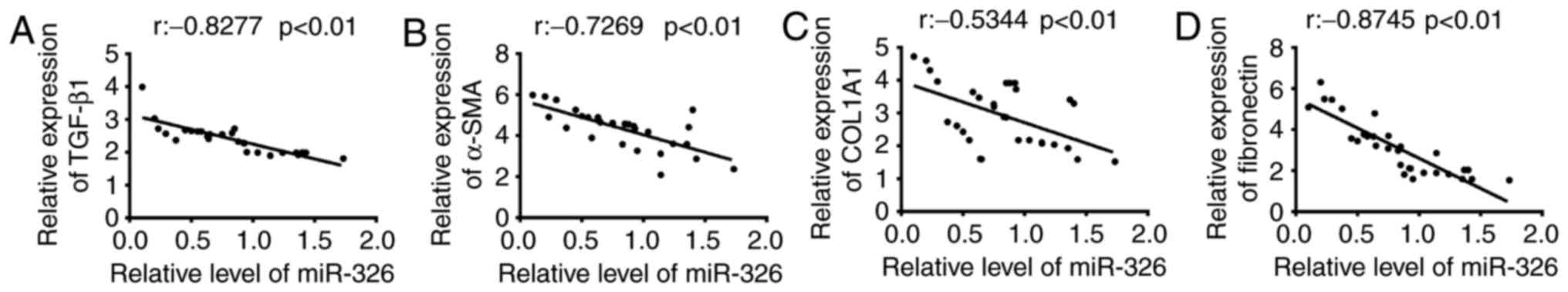 | Figure 2.Correlation between miR-326 and
fibrotic markers expression in endometrial tissues. The expression
levels of miR-326 and the mRNA expression of fibrotic markers were
measured by reverse transcription-quantitative polymerase chain
reaction in 30 endometrial tissues collected from patients with
IUA. Correlation between miR-326 level and (A) TGF-β1 (r=−0.8277,
P<0.01), (B) α-SMA (r=−0.7269, P<0.01), (C) COL1A1
(r=−0.5344, P<0.01) and (D) fibronectin (r =−0.8745, P<0.01)
expression was analyzed using Spearman's correlation coefficient.
IUA, intrauterine adhesion; miR, microRNA; TGF-β1, transforming
growth factor-β1; α-SMA, α-smooth muscle actin; COL1A1, collagen
type I α 1 chain. |
miR-326 inhibits the fibrosis of
endometrial stromal cells (ESCs)
Previous studies have reported that deregulated
proliferation and differentiation of ESCs are important factors
contributing to the development of endometrial fibrosis (29,30).
Therefore, the present study used the primary ESCs isolated from
patients with IUA to investigate function of miR-326 in endometrial
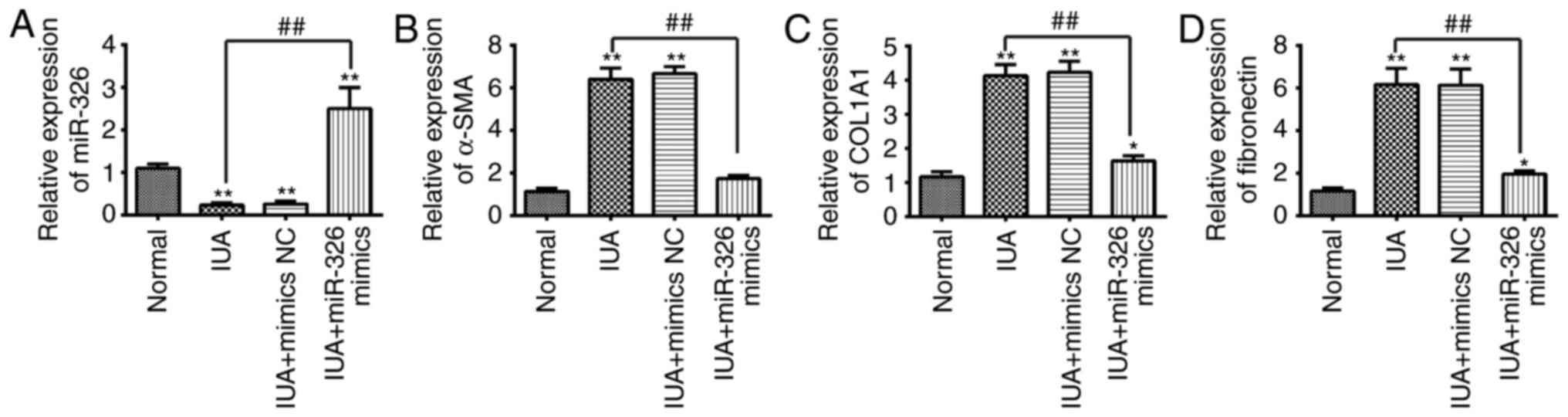
fibrosis. ESCs were transfected with miR-326 mimics to overexpress
miR-326. The miR-326 mimic group had a significantly increased the
expression level of miR-326 (Fig.
3A). The alteration of the expression of fibrotic-associated
markers in ESCs were investigated following miR-326 overexpression.
As presented in Fig. 3B-D, the
mRNA expression levels of α-SMA, COL1A1 and FN in ESCs isolated
from patients with IUA was increased when compared with normal
subjects, whereas overexpression of miR-326 was able to suppress
the mRNA expression levels of α-SMA, COL1A1 and FN. These findings
suggest that miR-326 may inhibit the fibrosis of endometrial
stromal cells.
TGF-β1 is a direct target of miR-326
in ESCs
TGF-β1, an important pro-fibrogenic mediator
(31), was previously reported to
be involved in the development of fibrosis and organ dysfunction,
by upregulating the extracellular matrix (ECM) protein expression
and α-SMA (8). As miR-326
regulates the TGF-β1 level and attenuates bleomycin-induced lung
fibrosis mice (27), it was
hypothesized that TGF-β1 may also be involved in the anti-fibrotic
role of miR-326 in IUA. In order to investigate the association
between miR-326 and TGF-β1, a luciferase reporter assay was
performed in ESCs isolated from patients with IUA. The findings
revealed that overexpression of miR-326 significantly reduced the
luciferase activity of TGF-β1 with wild-type (wt) 3′-UTR, whereas
miR-326 inhibition increased the luciferase activity of TGF-β1 with
wt 3′-UTR (Fig. 4A). Cells
co-transfected with miR-326 mimics, miR-326 inhibitor, and
TGF-β1-mut-3′UTR showed no obvious change in their luciferase
activity (Fig. 4A). These findings
indicate that miR-326 directly targeted the 3′-UTR of TGF-β1. The
present study further examined whether miR-326 may modulate the
expression of TGF-β1 in ESCs by RT-qPCR and western blotting. The
findings revealed that the mRNA and protein expression levels of
TGF-β1 in ESCs were significantly downregulated following
transfection with miR-326 mimics, compared with the negative
control, whereas transfection with the miR-326 inhibitor increased
TGF-β1 expression (Fig. 4B and C).
Collectively, these findings suggest that TGF-β1 is a direct
downstream target of miR-326 in ESCs.
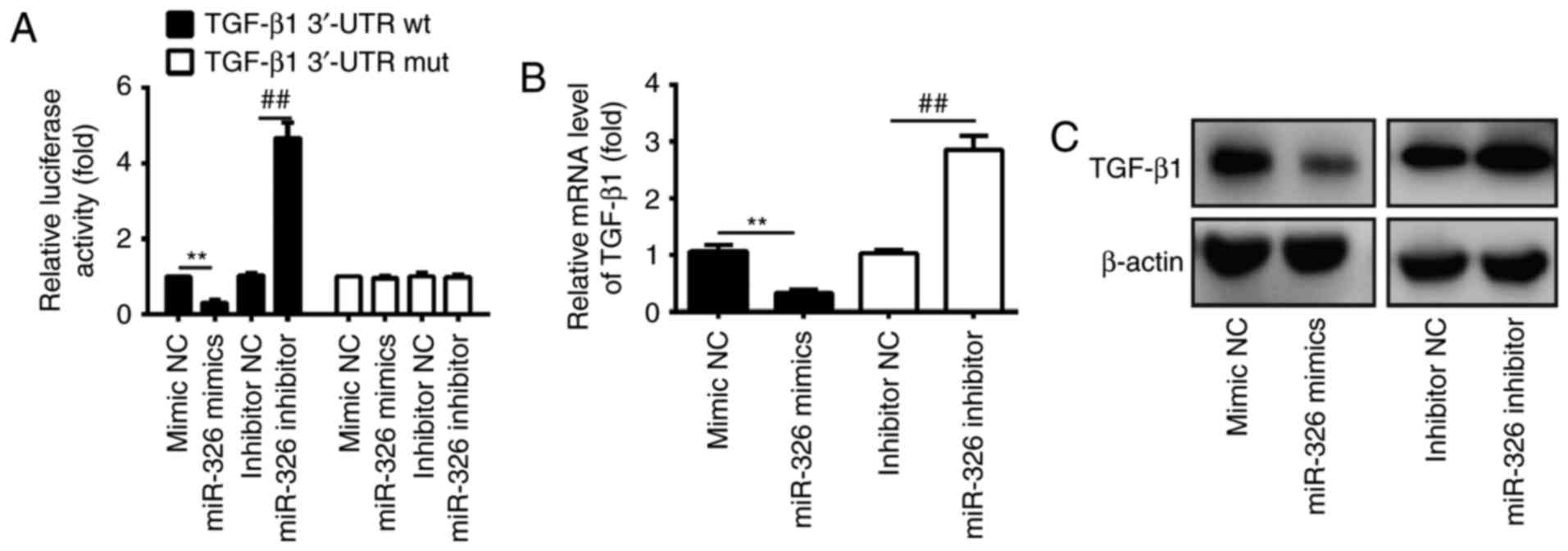 | Figure 4.TGF-β1 was a direct target of miR-326
in ESCs. (A) Luciferase activity in ESCs isolated from patients
with intrauterine adhesion, subsequently co-transfected with
miR-326 mimics, miR-326 inhibitor and luciferase reporters
containing TGF-β1 wt or mut 3′-UTR. Histograms indicate the values
of luciferase determined 48 h after transfection. miR-326 mimics,
miR-326 inhibitor and controls were transfected into ESCs, then the
(B) mRNA and (C) protein levels of TGF-β1 were detected by reverse
transcription-quantitative polymerase chain reaction and western
blot assays. Data are presented as the mean ± standard deviation of
three independent experiments. **P<0.01 vs. mimic NC;
##P<0.01 vs. inhibitor NC group. NC, negative
control; miR, microRNA; wt, wild-type; mut, mutation; 3′-UTR,
3′-untranslated region; ESCs, endometrial stromal cells; TGF-β1,
transforming growth factor-β1. |
miR-326 protects ESCs from fibrosis by
regulating the TGF-β1/Smad3 pathway
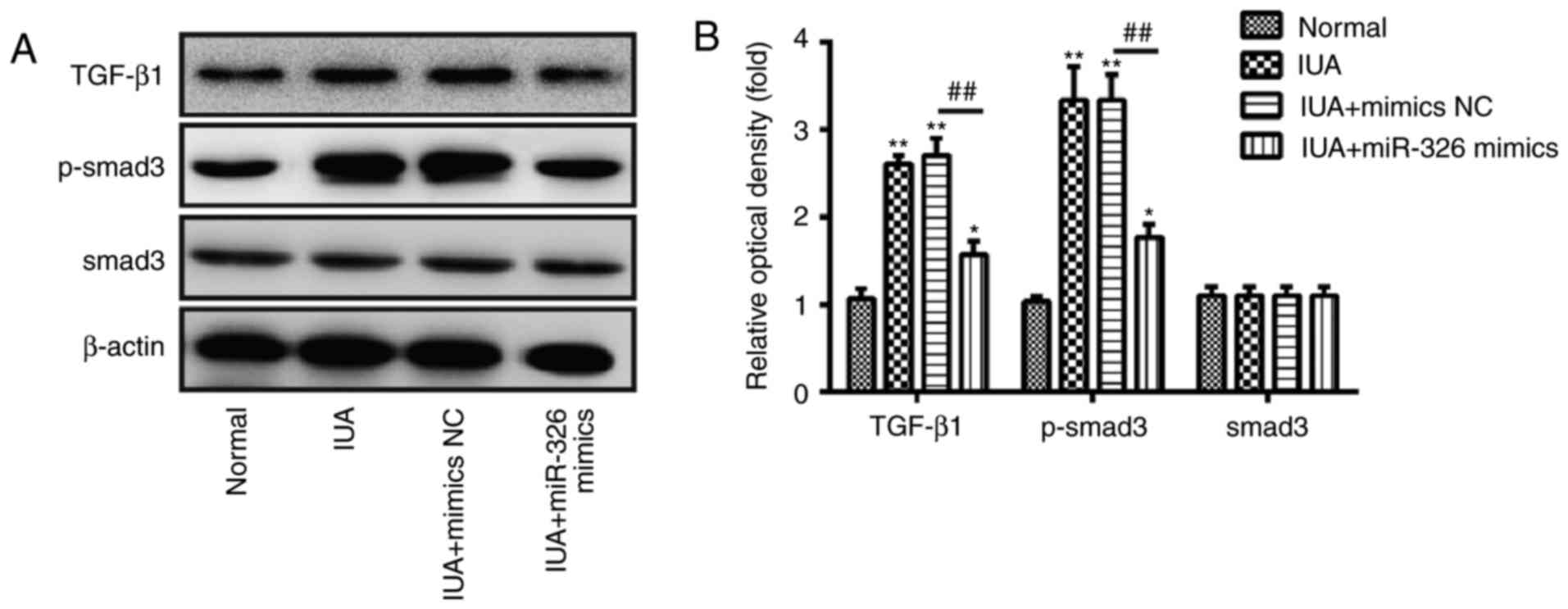
Due to the important role of the TGF-β1/Smad3
pathway in organ fibrosis, including endometrial fibrosis (23,32,33),
subsequent experiments were designed to investigate the effects of
miR-326 on the activity of the TGF-β1/Smad3 pathway in ESCs.
Western blotting was performed to determine the protein expression
levels of TGF-β1, p-Smad3 and Smad3 in ESCs. The present study
confirmed that TGF-β1 and p-Smad3 protein expression levels were
increased in ESCs from patients with IUA (Fig. 5A and B). However, overexpression of
miR-326 effectively downregulated TGF-β1 and p-Smad3 protein
expression levels (Fig. 5A and B).
In addition, no change was observed in the expression levels of
Smad3. These findings suggest that miR-326 may inhibit endometrial
fibrosis via inactivating the TGF-β1/Smad3 pathway.
Discussion
The present study identified that miR-326 was
downregulated in endometrial tissues and ESCs isolated from
patients with IUA. Additionally, miR-326 expression levels were
inversely correlated with the expression levels of fibrotic markers
including TGF-β1, α-SMA, COL1A1 and FN. The present findings also
demonstrated that miR-326 exerted an anti-fibrotic effect by
inhibiting TGF-β1, thus negatively regulating the TGF-β1/Smad3
signaling pathway in IUA. Therefore, the miR-326/TGF-β1/Smad3 axis
may be a potential target for the prevention and treatment of
IUA.
Previous studies suggest that miRNAs have important
roles in fibrosis diseases. For example, Pandit et al
described a novel role for miR-21 in the context of pulmonary
fibrosis by regulating EMT TGF-β signaling activity (34). Additionally, Tao et al have
determined the involvement of miR-433 in the regulation of cardiac
fibrosis through TGF-β and the mitogen-activated protein
kinase/extracellular-signal regulated kinase/P38 pathways (35). However, the role of miRNAs in
endometrial fibrosis remains to be determined. In order to identify
miRNAs which could potentially regulate endometrial fibrosis in the
present study, a miRNA array in endometrial tissues from patients
with IUA was performed and determined that miR-326 was
significantly downregulated. Additionally, it was observed that an
inverse relationship between miR-326 expression and the expression
levels of fibrotic markers including TGF-β1, α-SMA, COL1A1 and FN
in endometrial tissues and ESCs. These findings indicated that
miR-326 have an important role in endometrial fibrosis.
Previous studies have reported the involvement of
the TGF-β1/Smad pathway in fibrosis diseases. For example, Zhou
et al revealed that casticin attenuated liver fibrosis by
blocking the TGF-β/Smad signaling pathway (36). A study by Choi et al
reported that capsaicin was able to ameliorate hepatic fibrosis by
inhibiting the TGF-β1/Smad pathway via peroxisome
proliferator-activated receptor-γ activation (37). Additionally, Gao et al
determined that anoctamin-1 inhibited cardiac fibrosis after
myocardial infraction via the TGF-β/Smad3 pathway (32). Previous studies have made
significant progress in identifying the core signaling pathways in
endometrial fibrosis, including the wnt/β-catenin, nuclear
factor-κB and TGF-β1/Smad pathways (5,23,28,38).
These pathways are frequently constitutively activated in subsets
of endometrial tissues and cell lines, particularly in the
TGF-β1/Smad pathway. Therefore, understanding the molecular
mechanisms involved in the TGF-β1/Smad pathway in endometrial
fibrosis is necessary. Das et al reported that miR-326
physiologically reduced TGF-β1 expression and attenuated the
fibrotic process in a bleomycin-induced lung fibrosis model
(27). However, it remains to be
determined whether miR-326 is also involved in endometrial fibrosis
via TGF-β1/Smad pathway. To the best of our knowledge, the present
study is the first to report that miR-326 regulates TGF-β1
expression post-transcriptionally and overexpression of miR-326 may
inhibit TGF-β1 and p-Smad3 protein expression in ESCs. These
findings indicated that miR-326 protects ESCs from fibrosis by
inactivating the TGF-β1/Smad3 pathway.
In conclusion, the findings of the current study
suggest that miR-326 inhibits endometrial fibrosis by negatively
regulating the TGF-β1/Smad3 pathway via direct targeting of TGF-β1,
confirming that miR-326 may be an anti-fibrotic element in
endometrial fibrosis. Therefore, the present study proposed that
the miR-326/TGF-β1/Smad3 axis may represent a promising therapeutic
target for the future treatment of IUA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Planning and performance of experiments,
contribution of reagents or other essential materials and writing
the paper: JN. Data analysis and manuscript review: JN, HZ and
HY.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hainan branch of PLA General Hospital.
Consent for publication
Written informed consent was obtained prior to
inclusion to the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asherman JG: Traumatic intra-uterine
adhesions. J Obstet Gynaecol Br Emp. 57:892–896. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu D, Li TC, Xia E, Huang X, Liu Y and
Peng X: Factors affecting reproductive outcome of hysteroscopic
adhesiolysis for Asherman's syndrome. Fertil Steril. 89:715–722.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans-Hoeker EA and Young SL: Endometrial
receptivity and intrauterine adhesive disease. Semin Reprod Med.
32:392–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diegelmann RF and Evans MC: Wound healing:
An overview of acute, fibrotic and delayed healing. Front Biosci.
9:283–289. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue X, Chen Q, Zhao G, Zhao JY, Duan Z and
Zheng PS: The overexpression of TGF-β and CCN2 in intrauterine
adhesions involves the NF-κB signaling pathway. PLoS One.
10:e01461592015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayashi H, Abdollah S, Qiu Y, Cai J, Xu
YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL
and Falb D: The MAD-related protein Smad7 associates with the
TGFbeta receptor and functions as an antagonist of TGFbeta
signaling. Cell. 89:1165–1173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeisberg M and Kalluri R: Cellular
mechanisms of tissue fibrosis. 1. Common and organ-specific
mechanisms associated with tissue fibrosis. Am J Physiol Cell
Physiol. 304:C216–C225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu GX, Li YQ, Huang XR, Wei L, Chen HY,
Shi YJ, Heuchel RL and Lan HY: Disruption of Smad7 promotes ANG
II-mediated renal inflammation and fibrosis via
Sp1-TGF-β/Smad3-NF.κB-dependent mechanisms in mice. PLoS One.
8:e535732013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Li H, Liu X, Xu D and Wang F:
MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal
transition of retinal pigment epithelial cells by targeting AKT2.
Exp Cell Res. 345:115–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sime PJ, Xing Z, Graham FL, Csaky KG and
Gauldie J: Adenovector-mediated gene transfer of active
transforming growth factor-beta1 induces prolonged severe fibrosis
in rat lung. J Clin Invest. 100:768–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verrecchia F, Vindevoghel L, Lechleider
RJ, Uitto J, Roberts AB and Mauviel A: Smad3/AP-1 interactions
control transcriptional responses to TGF-beta in a
promoter-specific manner. Oncogene. 20:3332–3340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vindevoghel L, Lechleider RJ, Kon A, de
Caestecker MP, Uitto J, Roberts AB and Mauviel A: SMAD3/4-dependent
transcriptional activation of the human type VII collagen gene
(COL7A1) promoter by transforming growth factor beta. Proc Natl
Acad Sci USA. 95:14769–14774. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen SJ, Yuan W, Mori Y, Levenson A,
Trojanowska M and Varga J: Stimulation of type I collagen
transcription in human skin fibroblasts by TGF-beta: Involvement of
Smad 3. J Invest Dermatol. 112:49–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Koka V and Lan HY: Transforming
growth factor-beta and Smad signalling in kidney diseases.
Nephrology (Carlton). 10:48–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao W, Shi P and Ge JJ: miR-21 enhances
cardiac fibrotic remodeling and fibroblast proliferation via
CADM1/STAT3 pathway. BMC Cardiovasc Disor. 17:882017. View Article : Google Scholar
|
|
18
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et
al: MicroRNA-21 contributes to myocardial disease by stimulating
MAP kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Komers R, Carew R, Winbanks CE, Xu
B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K,
Gregorevic P, et al: Suppression of microRNA-29 expression by
TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc
Nephrol. 23:252–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung AC, Huang XR, Meng X and Lan HY:
miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc
Nephrol. 21:1317–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hall C, Ehrlich L, Meng F, Invernizzi P,
Bernuzzi F, Lairmore TC, Alpini G and Glaser S: Inhibition of
microRNA-24 increases liver fibrosis by enhanced menin expression
in Mdr2−/− mice. J Surg Res. 217:160–169. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balderas-Martinez YI, Rinaldi F, Contreras
G, Solano-Lira H, Sánchez-Pérez M, Collado-Vides J, Selman M and
Pardo A: Improving biocuration of microRNAs in diseases: A case
study in idiopathic pulmonary fibrosis. Database (Oxford).
2017:2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Cen B, Chen S and He Y: MicroRNA-29b
inhibits TGF-β1-induced fibrosis via regulation of the TGF-β1/Smad
pathway in primary human endometrial stromal cells. Mol Med Rep.
13:4229–4237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu H, Wu Y, Li L, Yuan W, Zhang D, Yan Q,
Guo Z and Huang W: MiR-344b-1-3p targets TLR2 and negatively
regulates TLR2 signaling pathway. Int J Chron Obstruct Pulmon Dis.
12:627–638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuzaki S and Darcha C: In vitro effects
of a small-molecule antagonist of the Tcf/ß-catenin complex on
endometrial and endometriotic cells of patients with endometriosis.
PLoS One. 8:e616902013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Das S, Kumar M, Negi V, Pattnaik B,
Prakash YS, Agrawal A and Ghosh B: MicroRNA-326 regulates
profibrotic functions of transforming growth factor-β in pulmonary
fibrosis. Am J Respir Cell Mol Biol. 50:882–892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsuzaki S and Darcha C: Involvement of
the Wnt/β-catenin signaling pathway in the cellular and molecular
mechanisms of fibrosis in endometriosis. PLoS One. 8:e768082013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Götte M, Wolf M, Staebler A, Buchweitz O,
Kelsch R, Schüring AN and Kiesel L: Increased expression of the
adult stem cell marker Musashi-1 in endometriosis and endometrial
carcinoma. J Pathol. 215:317–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maruyama T, Masuda H, Ono M, Kajitani T
and Yoshimura Y: Human uterine stem/progenitor cells: Their
possible role in uterine physiology and pathology. Reproduction.
140:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muro AF, Moretti FA, Moore BB, Yan M,
Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D,
et al: An essential role for fibronectin extra type III domain A in
pulmonary fibrosis. Am J Respir Crit Care Med. 177:638–645. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao Y, Zhang YM, Qian LJ, Chu M, Hong J
and Xu D: ANO1 inhibits cardiac fibrosis after myocardial
infraction via TGF-β/smad3 pathway. Sci Rep. 7:23552017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao TT, Zhang HJ, Lu XG, Huang XR, Zhang
WK, Wang H, Lan HY and Li P: Chaihuang-Yishen granule inhibits
diabetic kidney disease in rats through blocking TGF-β/Smad3
signaling. PLoS One. 9:e908072014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pandit KV, Corcoran D, Yousef H,
Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh
M, Handley D, et al: Inhibition and role of let-7d in idiopathic
pulmonary fibrosis. Am J Respir Crit Care Med. 182:220–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tao L, Bei Y, Chen P, Lei Z, Fu S, Zhang
H, Xu J, Che L, Chen X, Sluijter JP, et al: Crucial role of miR-433
in regulating cardiac fibrosis. Theranostics. 6:2068–2083. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Dong X, Wang L, Shan L, Li T, Xu
W, Ding Y, Lai M, Lin X, Dai M, et al: Casticin attenuates liver
fibrosis and hepatic stellate cell activation by blocking
TGF-β/Smad signaling pathway. Oncotarget. 8:56267–56280.
2017.PubMed/NCBI
|
|
37
|
Choi JH, Jin SW, Choi CY, Kim HG, Lee GH,
Kim YA, Chung YC and Jeong HG: Capsaicin inhibits
dimethylnitrosamine-induced hepatic fibrosis by inhibiting the
TGF-β1/Smad pathway via peroxisome proliferator-activated receptor
gamma activation. J Agric Food Chem. 65:317–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sadasivan SK, Siddaraju N, Khan KM,
Vasamsetti B, Kumar NR, Haridas V, Reddy MB, Baggavalli S, Oommen
AM and Rao Pralhada R: Developing an in vitro screening assay
platform for evaluation of antifibrotic drugs using precision-cut
liver slices. Fibrogenesis Tissue Repair. 8:12014. View Article : Google Scholar : PubMed/NCBI
|