Introduction
Diabetic cardiomyopathy (DC) results in the
development of cardiac microvascular lesions and myocardial
structural disruption caused by metabolic disorder (1). DC is one of the most common diabetic
complications and is pathologically characterized by the apoptosis
and hypertrophy of myocardial cells, myocardial interstitial
fibrosis and inflammation. DC may lead to heart failure, a major
cause of death among patients with DM (2,3).
However, the pathogenic mechanisms underlying the development of DC
have not been fully elucidated.
Previous studies reported that cell apoptosis is
involved in the occurrence and development of DC (4–6).
Myocyte apoptosis caused by the long-term effects of high blood
glucose comprises a cascade amplification reaction of caspase
hydrolysate that is regulated as the genetic level; the B-cell
lymphoma (Bcl) family and caspase-3 serve important roles in
apoptosis (4). Calpain, a member
of the caspase superfamily, is a calcium-activated neutral
protease. The earliest known members of the calpain family,
calpain-1 and calpain-2, are the most extensively studied (7). Under certain pathological conditions,
particularly a high-calcium environment, calpains are critical
factors in inducing cell hypertrophy and/or death (8). Additionally, previous studies have
revealed that upregulation of calpains in myocardial cells can lead
to cell apoptosis (9,10).
Angiotensin-converting enzyme inhibitors (ACEIs) can
inhibit myocardial cell apoptosis in diabetic rats, significantly
improve cardiac function and effectively reverse ventricular
remodeling in DC (11,12). However, whether ACEI regulates
calpain-mediated apoptosis of myocardial cells and affects cardiac
function in patients with DM remains unknown. It has been reported
that angiotensin 2 receptor density increases in type 2 diabetic
patients, and activation of the renin-angiotensin-aldosterone
system can improve left ventricular function in patients with type
2 DM (13). In the present study,
the ACEI captopril was investigated in streptozotocin (STZ)-induced
diabetic rats for its effects on the apoptosis of myocardial cells
in DM, the expression of apoptotic proteins and left ventricular
function.
Materials and methods
Ethical approval of the study
protocol
The present study was approved by the Ethics
Committee of Wenzhou Medical University (Wenzhou, China). All
experimental procedures conformed to the guidelines for the Animal
Care and Use Committee of Wenzhou Medical University (Wenzhou,
China).
Reagents and instruments
Captopril was purchased as 25 mg tablets from
Changzhou Pharmaceutical Factory Co., Ltd. (Changzhou, China). STZ
and sodium citrate were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
assay kit was purchased from Wuhan Boster Biological Technology,
Ltd. (Wuhan, China). Antibodies against calpain-1 and calpain-2 and
GAPDH were purchased from Genetimes Technology, Inc. (Shanghai,
China); rabbit anti-mouse Bcl-2, Bcl-2 associated protein X (Bax),
caspase-3 polyclonal antibodies and horseradish peroxidase
(HRP)-tagged goat anti-rabbit secondary antibodies were obtained
from OriGene Technologies, Inc. (Beijing, China). A bicinchoninic
acid (BCA) protein assay kit was obtained from Pierce (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Polyvinylidene fluoride
(PVDF) membranes were purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA) and a BeyoECL Plus Western Blotting
chemiluminescence kit was purchased from Beyotime Institute of
Biotechnology (Shanghai, China). The Johnson Sure Step®
blood glucose meter and test strips were purchased from Johnson
& Johnson (New Brunswick, NJ, USA). The GB303 automatic
electronic balance was obtained from Inesa Instrument Co., Ltd.
(Shanghai, China) and the DH-140B animal ventilator was obtained
from the Experimental Instrument Factory of Zhejiang Medical
University (Hangzhou, China). The BL-420E data acquisition and
processing system for bio-functional experiments was purchased from
Chengdu Techman Software Co., Ltd. (Chengdu, China); the MUVB-20
gel imaging system was purchased from Ultra-Lum, Inc. (Claremont,
CA, USA); the H-600 transmission electron microscope (TEM) was
obtained from Hitachi, Ltd. (Tokyo, Japan); and the light
microscope was obtained from Olympus Corporation (Tokyo,
Japan).
Animal model and experimental
protocol
A total of 30 healthy male specific pathogen-free
(SPF) Sprague-Dawley rats (180–220 g; age, 2 months) were obtained
from the Experimental Animal Center of Wenzhou Medical University
(Wenzhou, China) and housed in an air-conditioned room at 23±2°C
under a 12-h light/dark cycle. Rats were randomly assigned to the
normal control group (NC; n=10) and the diabetes group (n=20). All
animals were kept in an SPF environment and had access to food and
water ad libitum. After a 12 h fast, rats of the diabetes group
were intraperitoneally administered STZ (65 mg/kg) and the NC group
rats received the equivalent volume of saline. At 72 h after the
STZ injection, blood glucose was measured. Rats (n=20) with a blood
glucose level >13.8 mmol/l were selected and assigned to the
diabetes mellitus group (DM; n=10) and the other half of the
diabetic rats were treated with captopril (Cap; n=10). Animals in
the Cap group were intragastrically administered captopril daily
(50 mg/kg) for 12 weeks; DM and NC groups were given normal saline
at an equivalent volume. Additionally, the mental state, behavior,
coat color/luster, consumption of feed and water, paving wetness
degree by the urine were observed to analyze polydipsia, polyphagia
and polyuria; body weight of the rats was monitored daily for 12
weeks. Postprandial blood glucose was measured every second week
using a Johnson Sure Step® blood glucose meter, with
blood sampled from the tail vein. At the end of week 12, left
ventricular function was assessed using the BL-420E bio-functional
experiment system. Following this, all animals were sacrificed,
hearts were harvested and stored in an ice bath. The hearts and
left ventricles were weighed. Subsequently, the left ventricular
tissues were used for making paraffin sections (4–6 µm) and
electron microscope specimens or stored immediately in −70°C for
western blotting.
Left ventricular function and left
ventricular mass index (LVMI)
The animals were intraperitoneally anesthetized with
10% chloral hydrate (350 mg/kg). A tracheal cannula was inserted
into the rats and connected to a DH-140B animal ventilator; the
tidal volume was 5 ml and the respiratory rate was 55 breaths per
minute, with an inspiration/expiration ratio of 1.5:1. A cardiac
catheter was subsequently inserted into the left ventricle to
determine the left ventricular systolic pressure (LVSP), left
ventricular end-diastolic pressure (LVDEP), maximal rate of left
ventricular pressure increase (+dp/dtmax) and maximal rate of left
ventricular pressure decrease (-dp/dtmax). Following this, rats
were sacrificed to harvest the heart and the left ventricle was
isolated. The mass of the left ventricle was measured. The LVMI was
calculated as the mass of the left ventricle/mass of the heart.
Western blot analysis
The expression of calpain-1, calpain-2, Bcl-2, Bax
and caspase-3 was examined by western blotting. Briefly, total
protein of the heart tissue was extracted with ice-cold
radioimmunoprecipitation assay lysis buffer supplemented with PMSF
for 30 min, the whole-cell lysate and the concentration of total
protein was determined by the BCA method (14). Equal amounts of protein (20 µg)
were separated by SDS-PAGE (5% stacking gel and 12% separating gel)
and transferred to PVDF membranes. The membranes were blocked in 5%
non-fat dry milk for 1 h at room temperature and subsequently
incubated overnight at 4°C with calpain-1 large-subunit antibody
(1:400), calpain-2 large-subunit antibody (1:400), rabbit
anti-mouse Bcl-2 (1:250), Bax polyclonal antibody (1:400) and
caspase-3 polyclonal antibody (1:600). The membrane was rinsed in
Tris-buffered saline with 0.05% Tween 20 prior to incubation with
HRP-labeled secondary antibody (1:5,000; cat. no. 111-035-006) at
room temperature for 2 h. Protein bands were visualized with the
BeyoECL Plus Western Blotting chemiluminescence kit and analyzed
with Quantity One software (version 4.52, Bio-Rad Laboratories,
Inc.). GAPDH (1:1,000; cat. no. 5174) was included in as an
internal reference for the quantification of relative protein
expression.
Myocardial apoptosis and apoptotic
index (AI)
Apoptotic myocardial cells were detected by a TUNEL
assay kit according to the manufacturer's instructions (Wuhan
Boster Biological Technology, Ltd.). Paraffin sections of the heart
tissues were dewaxed, rinsed in water and PBS, and treated with
protease K solution at 37°C for 15 min. Tagging buffer (20 µl) was
added at 37°C. After 60 min, samples were rinsed with PBS, stained
with 0.05% 3′3′-Diaminobenzidine for 10 min at room temperature and
counterstained with 0.025% hematoxylin for 1 min at room
temperature. Positive cells (apoptotic) were characterized by brown
granules inside the nucleus. Apoptotic cells in five random
high-power visual fields (magnification, ×400) were counted under a
light microscope. AI (%) was determined as the apoptotic cell
count/100 cells (15).
Myocardial ultrastructure
observation
Myocardial tissue (1×1×1 mm3) was
collected from the anterior wall of the left ventricle, pre-fixed
in 2.5% glutaraldehyde and post-fixed in 1% osmic acid at 37°C for
60 min. Samples were subsequently dehydrated with a gradient of
acetone (50% acetone for 10 min, 70% acetone 10 for min, 80%
acetone for 10 min, 90% acetone for 10 min and twice with 100%
acetone for 10 min), embedded in Epon812 (45°C for 6 h then 65°C
for 48 h) and sliced into ultrathin sections (1 µm). Samples were
double stained with 4% lead nitrate for 10 min at room temperature
and uranyl acetate 30 min at room temperature and observed by
TEM.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform the statistical analyses. All data are
expressed as the mean ± standard deviation. The data in each group
were subjected to normality testing and homogeneity of variance was
analyzed. Inter-group comparisons were performed by one-way
analysis of variance. Pairwise comparisons between groups with
homogeneous variance were performed with LSD method, whereas
comparisons between groups with homogeneous variance were performed
with Dunnett's T3 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
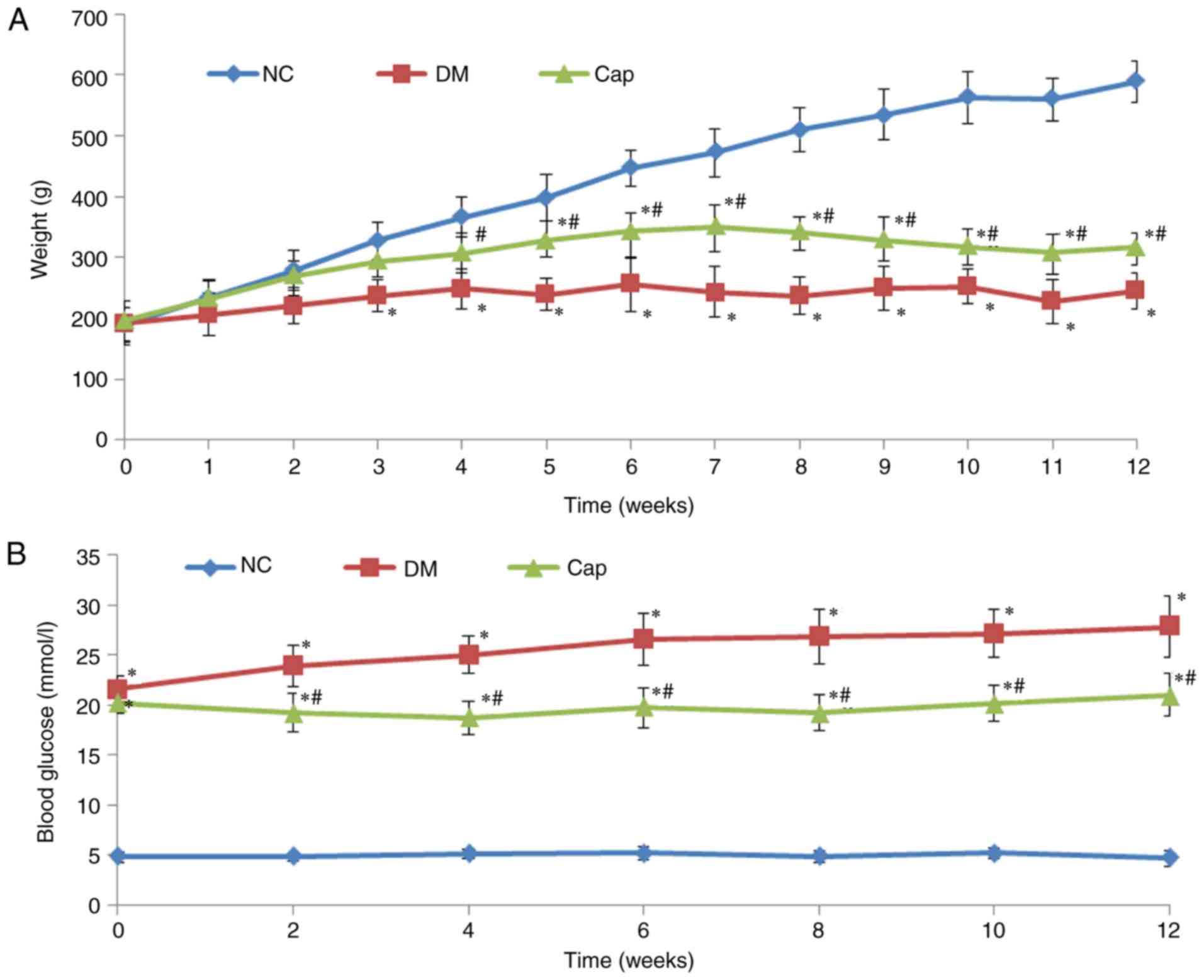
Captopril treatment increases body
weight and reduces glucose levels in diabetic rats
Compared with the DM group, animals in the Cap group
exhibited significant improvement in DM-associated symptoms,
including polydipsia, polyphagia and polyuria. Cap rats were
relatively active and their coats regained luster. The body weights
in the DM and Cap groups were significantly decreased compared with
those in the NC group. Furthermore, the body weights in the Cap
group were significantly increased compared with the DM group
(P<0.05; Fig. 1A). Blood
glucose at 12 weeks was significantly increased in the DM and Cap
groups, compared with the NC group (P<0.05). Cap group glucose
levels were significantly decreased compared with those in the DM
group (P<0.05; Fig. 1B).
Captopril treatment improves cardiac
function in diabetic rats
Compared with the NC group, DM rats had
significantly increased LVDEP and LVMI. LVSP, +dp/dtmax and
-dp/dtmax were significantly decreased in the DM group, compared
with the NC group. Furthermore, compared with the DM group,
Cap-treated rats had significantly lower LVDEP and LVMI. LVSP,
+dp/dtmax and -dp/dtmax significantly increased compared with the
DM group (Table I).
 | Table I.Comparison of cardiac function among
different groups in rats. |
Table I.
Comparison of cardiac function among
different groups in rats.
| Group | LVSP (mmHg) | LVDEP (mmHg) | +dp/dtmax
(mmHg/s) | -dp/dtmax
(mmHg/s) | LVMI (mg/g) |
|---|
| NC | 118.20±10.83 | 3.57±1.19 |
5,382.43±693.10 |
5,310.53±696.23 | 1.53±0.16 |
| DM |
90.20±8.87a |
10.80±3.37a |
3,783.52±863.27a |
3,725.95±864.76a |
2.25±0.30a |
| Cap |
103.41±9.36b |
5.10±1.21b |
47,89.99±707.06b |
5,024.43±573.60b |
1.82±0.21b |
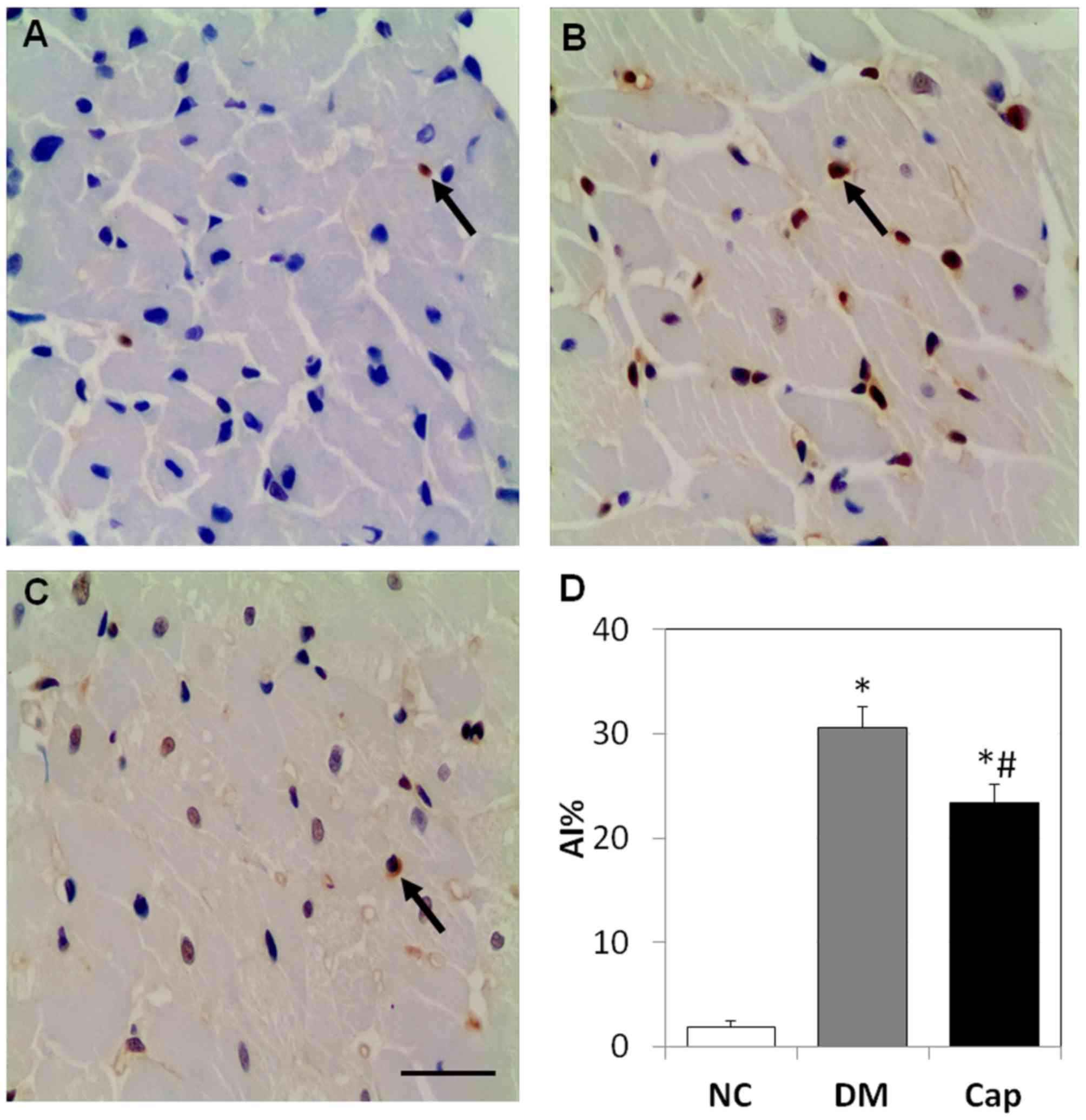
Captopril reduces cell apoptosis in
myocardial tissue
In the NC group, cell apoptosis was rarely observed.
Compared with the NC group, apoptosis in the DM group increased by
~15-fold (P<0.05). Captopril treatment decreased the number of
apoptotic cells by 24%, compared with the DM group (P<0.05;
Fig. 2).
Calpain-1, calpain-2, Bcl-2, Bax and
total caspase-3 expression alterations in heart tissue in response
to captopril
Compared with the NC group, Bcl-2 expression was
significantly decreased in the DM group, while calpain-1,
calpain-2, Bcl-2, Bax and total caspase-3 expression significantly
increased (P<0.05). By contrast, captopril treatment
significantly increased Bcl-2 expression and significantly reduced
calpain-1, calpain-2, Bax and total caspase-3 expression compared
with the DM group (P<0.05; Fig.
3).
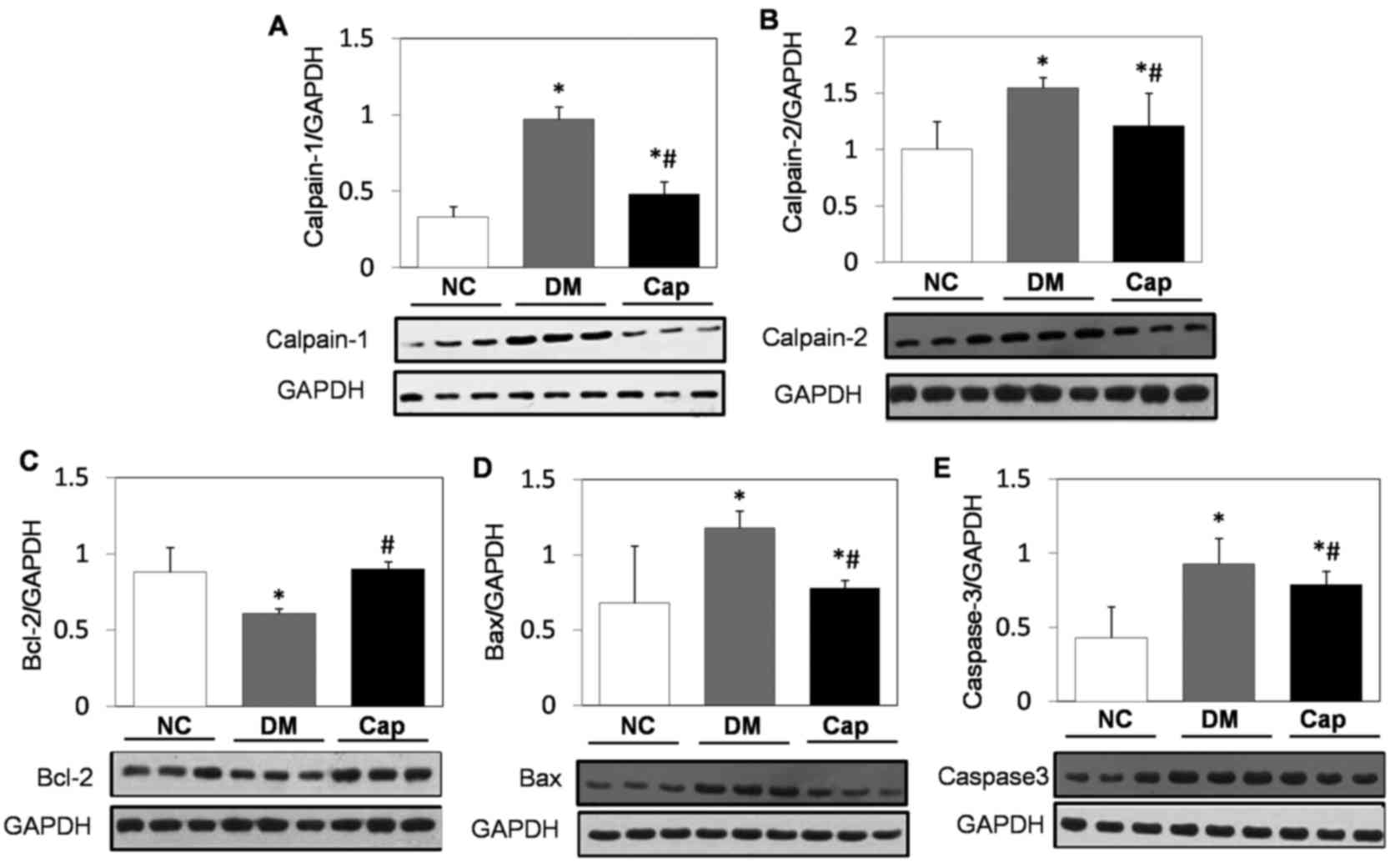 | Figure 3.Western blot analysis of apoptotic
protein expression. Protein expression of (A) calpain-1, (B)
calpain-2, (C) Bcl-2, (D) Bax and (E) caspase-3 in cardiomyocytes
from NC, DM and Cap-treated rats. GAPDH was used as the internal
reference protein. Data are expressed as the mean ± standard
deviation. *P<0.05 vs. NC group, #P<0.05 vs. DM
group. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated protein X;
NC, negative control; DM, diabetes mellitus; Cap, captopril. |
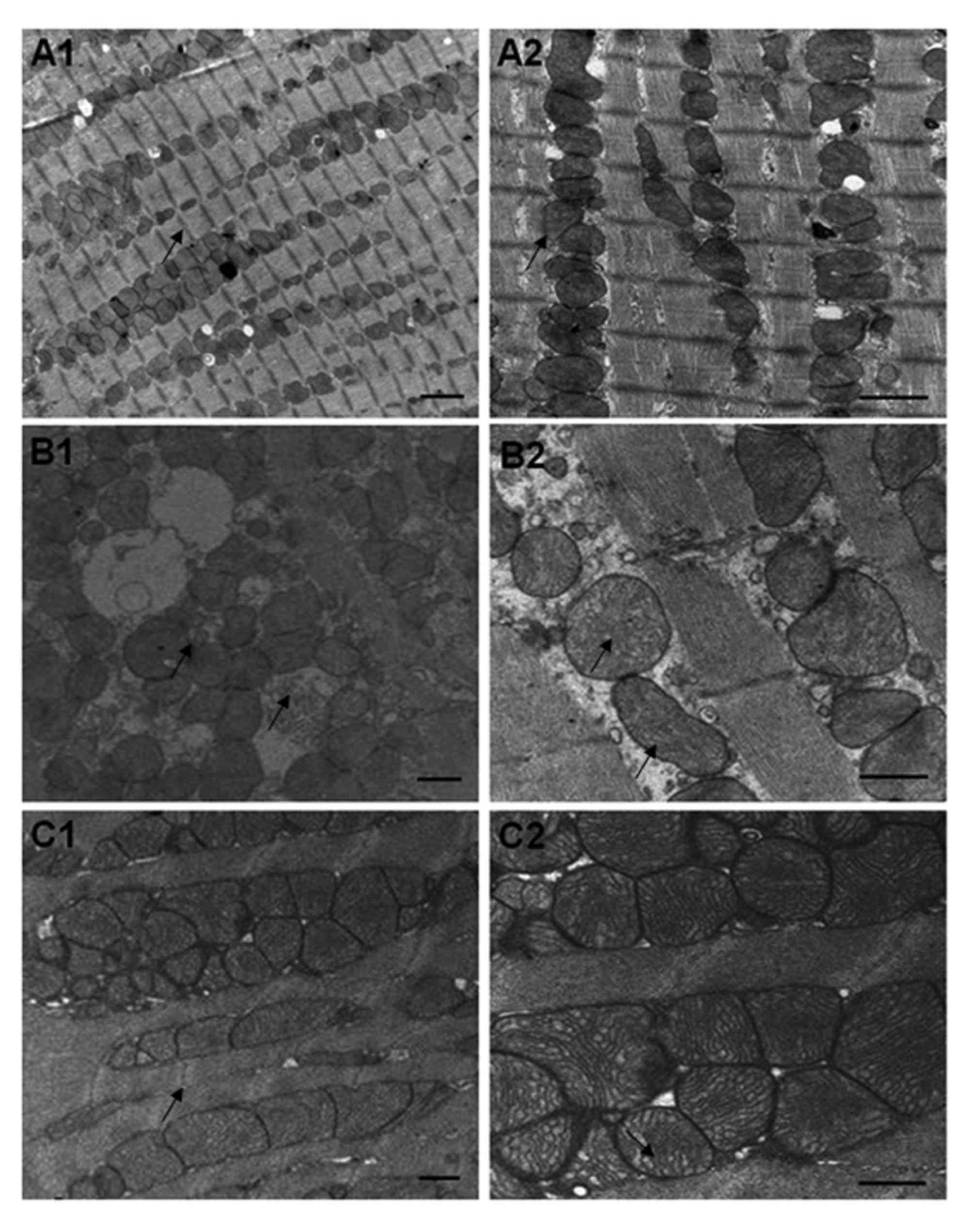
Alterations in myocardial
ultrastructure in response to captopril
The myocardium of rats in the NC group exhibited
solid myofibrils and regularly distributed myofilaments. The
mitochondrial membranes were intact and the arrangement of the
ridges was regular. The basilar membranes of the microvessels were
continuous and intact (Fig.
4A).
In the myocardium of the DM group, the myocytes were
swollen and punctiform myofibril dissolution was observed. The
arrangement of the myofilaments was irregular and myofilaments were
partially disrupted. The mitochondria were enlarged and the ridge
arrangement was disrupted, with the presence of vacuoles.
Interstitial collagen hyperplasia was observed and the basement
membranes of the microvessels were thickened (Fig. 4B).
In the Cap group, the myofibrils were partially
disrupted. Mitochondrial enlargement was alleviated. Minor
disruptions were observed in the ridges and the basement membrane
thickening of the microvessels was attenuated (Fig. 4C).
Discussion
In the present study, it was demonstrated that
treatment with captopril increased body weight and reduced blood
glucose in diabetic rats. This result was consistent with a
previous study reporting that captopril can protect islet function,
prevent diabetes occurrence and improve diabetic rat weight loss
(16,17). In the present study, captopril
improved cardiac function, inhibited myocardial cell apoptosis and
protected myocardial structure, thereby improving ventricular
function. This was likely achieved by reducing the activation of
calpain-1, calpain-2 and Bax, as well as upregulating the
expression of Bcl-2, leading to the inhibition of
caspase-3-dependent apoptosis.
At week 12, STZ-induced diabetic rats in the current
study exhibited abnormalities in the systole and diastole of the
left ventricle and structural damage in the myocardium, including a
significant increase in myocardial cell apoptosis. This was
consistent with a previous study demonstrating that left
ventricular systolic and diastolic dysfunction in DC is associated
with myocardial cell apoptosis (18). The mechanism underlying the
diabetes-induced apoptosis of myocytes is complex, involving the
Bcl-2 and caspase gene families (19,20).
Bcl-2 was the first anti-apoptotic gene discovered in humans
(21). Although Bax is also a
member of the Bcl-2 family, it functions as a pro-apoptotic gene.
High Bcl-2 expression may lead to the formation of Bcl-2/Bcl-2
homodimers and Bcl-2/Bax heterodimers, both of which have
anti-apoptotic effects; however, high expression of Bax may yield
Bax/Bax homodimers, which are pro-apoptotic (21). Whether apoptosis occurs in a
certain group of cells and how much they are affected is determined
by the ratio of Bcl-2 and Bax expression (22). In the present study, it was
demonstrated that Bcl-2 was significantly decreased and Bax
significantly increased in the DM group, compared with the NC
group. This is in accordance with the results reported by Kumar
et al (23), which revealed
that the number of apoptotic myocardial cells increases in DM,
along with a decrease in Bcl-2 expression and increase in Bax
expression.
The caspase family is a group of proteins critical
for regulating and executing cell apoptosis. Caspase-3 is referred
to as the ‘death protease’, as its activated form catalyzes the
hydrolysis of specific proteins to promote apoptosis (24,25).
In the present study, apoptosis induced by hyperglycemia is
regulated by the caspase-3-dependent mitochondrial pathway. Cai
et al (26) demonstrated
that high glucose-induced cell apoptosis occurs at least partially
via a caspase-3-dependent mitochondrial pathway.
Calpains are a family of calcium-dependent cysteine
proteases in the cytoplasm that are distributed widely in most
mammals. Calpain-1 and calpain-2 are the most extensively studied
members of this family; both are heterodimers composed of 28 and 80
kDa subunits and may be expressed in myocytes. Their sequences
share 55–65% homology. Calpain-1 and −2 differ in that they require
different calcium concentrations for their activation; µmol levels
for calpain-1 and mmol levels for calpain-2. Typically,
Ca2+ concentration in myocytes is not sufficient to
activate these calpains (27).
However, high glucose may increase the calcium load in myocytes,
which is associated with a decrease in the activity of the
Na+/Ca2+ exchanging ATPase and the
Ca2+ATPase, and subsequently inhibits the calcium
concentration resurge in the sarcoplasmic reticulum (28). As revealed by a previous report
(27), high glucose may lead to
the production of reactive oxygen species, causing the activation
of L-type calcium channels and ryanodine receptors and increase of
in intracellular calcium concentrations, which subsequently leads
to the activation of calpain. Activated calpain mediates the
apoptosis of myocytes under high-glucose conditions via the
caspase-3 pathway. An in vitro experiment demonstrated that
pro-apoptotic Bax maybe cleaved by calpain to yield an 18 kDa
active fragment, which induces the release of mitochondrial
cytochrome c to mediate cell apoptosis (29). Therefore, the Bcl-2 family is
involved in the pro-apoptotic effects of calpain, and several
members of the Bcl-2 family are substrates of calpain (30). In the present research, compared
with the control group, Bcl-2 expression was downregulated in
myocardial tissue, whereas calpain-1, calpain-2, Bax and total
caspase-3 expression was increased in the DM group, indicating that
cell apoptosis in DC may be associated with the alterations in the
expression of these proteins. This is consistent with previous
reports (21–23). Furthermore, activation of calpain-1
and calpain-2 may mediate caspase-3-dependent apoptosis by
downregulating Bcl-2 and upregulating Bax to further induce the
occurrence of DC.
ACEIs, including captopril, are considered
protective agents against pancreatic dysfunction and diabetes
(16). In the present study,
captopril treatment alleviated the symptoms of DM rats. Local
overactivation of the renin/angiotensin system (RAS) and
dysfunction of angiotensin II (Ang II) are thought to be involved
in DC apoptosis (31). Captopril
may inhibit cell apoptosis by blocking local or systemic activation
of RAS and inhibiting the bio-synthesis of Ang II (32). In the present study, increased
expression of Bcl-2 and decreased expression of calpain-1,
calpain-2, Bax and caspase-3 were observed in the myocardial
tissue. Additionally, cell apoptosis was significantly inhibited,
LVMI was decreased and systolic function was significantly
improved. Furthermore, the ultrastructural damage in the myocardium
was alleviated, suggesting that captopril may improve ventricular
function and protect the myocardium by inhibiting the activation of
calpain-1 and calpain-2, upregulating Bcl-2 and downregulating Bax
to inhibit caspase-3-dependent myocyte apoptosis. A previous study
reported similar functions of ACEI and calcium antagonists
(33), both of which can
ameliorate calcium overload. Therefore, ACEIs may inhibit calpain
activation through the attenuation of calcium overload. However,
further confirmation of this hypothesis is required.
In conclusion, the present study demonstrated that
captopril may preserve myocardial function in diabetic rats via
inhibition of cardiac cell apoptosis, suggesting that ACEIs,
including captopril, maybe considered as a therapeutic option in
DC.
Acknowledgements
We thank Professor Jianmin Li, a pathological
specialist from Wenzhou Medical University (Wenzhou, China) for
technical assistance in this study. We are deeply grateful to
Fanyan Wang and Lei Ying, Pathophysiological doctors from Wenzhou
Medical University for their kind assistance in proofreading the
manuscript.
Funding
This investigation was supported by a grant from
National Nature Science Foundation of China (grant no. 81170204)
and College Students' Science and Technology Innovation Foundation
of Zhejiang Province (grant no. 2010R413002).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
LYD, LPY and XXQ designed the study, drafted the
manuscript and approved its final version. JZ acquired data and
revised the article for important intellectual content. KKJ was
involved in generating the idea and gave final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Wenzhou Medical University. All experimental
procedures conformed to the guidelines for the Animal Care and Use
Committee of Wenzhou Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACEI
|
angiotensin converting enzyme
inhibitor
|
|
DM
|
diabetes mellitus
|
|
STZ
|
streptozotocin
|
|
LVSP
|
left ventricular systolic pressure
|
|
LVDEP
|
left ventricular end-diastolic
pressure
|
|
+dp/dtmax
|
maximal rate of left ventricular
pressure increase
|
|
-dp/dtmax
|
maximal rate of left ventricular
pressure decrease
|
|
LVMI
|
left ventricular mass index
|
|
AI
|
apoptosis index
|
|
TUNEL
|
terminal
deoxynucleotidyltransferase-mediated dUTP nick-end labeling
|
|
DC
|
diabetic cardiomyopathy
|
|
RAS
|
renin angiotensin system
|
|
Ang II
|
angiotensin II
|
References
|
1
|
Mazzone T, Chait A and Plutzky J:
Cardiovascular disease risk in type 2 diabetes mellitus: Insights
from mechanistic studies. Lancet. 371:1800–1809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miettinen H, Lehto S, Salomma V, Mähönen
M, Niemelä M, Haffner SM, Pyörälä K and Tuomilehto J: The FINMONICA
Myocardial Infarction Register Study Group: Impact of diabetes on
mortality after the first myocardial infarction. Diabetes Care.
21:69–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Groote P, Lambin N, Mouquet F, Plichon
D, McFadden E, Van Belle E and Bauters C: Impact of diabetes
mellitus on long-term survival in patients with congestive heart
failure. Eur Heart J. 25:656–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai L and Kang YJ: Cell death and diabetic
cardiomyopathy. Cardiovasc Toxicol. 3:219–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun
Y, Zhang Y and Ge Z: Endoplasmic reticulum stress is involved in
myocardial apoptosis of streptozocin-induced diabetic rats. J
Endocrinol. 196:565–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ou HC, Tzang BS, Chang MH, Liu CT, Liu HW,
Lii CK, Bau DT, Chao PM and Kuo WW: Cardiac contractile dysfunction
and apoptosis in streptozotocin-induced diabetic rats are
ameliorated by garlic oil supplementation. J Agric Food Chem.
58:10347–10355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inserte J, Garcia-Dorado D, Hernando V and
Soler-Soler J: Calpain-mediated impairment of Na+/K+-ATPase
activity during early reperfusion contributes to cell death after
myocardial ischemia. Circ Res. 97:465–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galvez AS, Diwan A, Odley AM, Hahn HS,
Osinska H, Melendez JG, Robbins J, Lynch RA, Marreez Y and Dorn GW
II: Cardiomyocyte degeneration with calpain deficiency reveals a
critical role in protein homeostasis. Circ Res. 100:1071–1078.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bajaj G and Sharma RK: TNF-alpha-mediated
cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem
Biophys Res Commun. 345:1558–1564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang CH, Lu J, Yu XJ, Sun L and Zang WJ:
Ameliorative effect of Captopril and Valsartan on an animal model
of diabetic cardiomyopathy. Biol Pharm Bull. 31:2045–2049. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan SJ, Ma ZH, Wu YL, Zhang JP, Liang F,
Weiss JW, Guo QY, Wang JY, Ji ES and Chu L: Long-term
administration of fasudil improves cardiomyopathy in
streptozotocin-induced diabetic rats. Food ChemToxicol.
50:1874–1882. 2012. View Article : Google Scholar
|
|
13
|
Symeonides P, Koulouris S, Vratsista E,
Triantafyllou K, Ioannidis G, Thalassinos N and Katritsis D: Both
ramipril and telmisartan reverse indices of early diabetic
cardiomyopathy. A comparative study. Eur J Echocardiogr. 8:480–486.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akins RE and Tuan RS: Measurement of
protein in 20 seconds using a microwave BCA assay. Biotechniques.
12:496–499. 1992.PubMed/NCBI
|
|
15
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solski LV and Longyhore DS: Prevention of
type 2 diabetes mellitus with angiotensin-converting-enzyme
inhibitors. Am J Health Syst Pharm. 65:935–940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Araujo Rodrigues G, de Faria Granato K,
Lima WG, Bda Pádua C, Rossoni JV Jr, Souza AA, Chianca-Júnior D,
Silva ME, Pedrosa ML, Chaves MM and Costa DC: Effect of captopril
and the bradykinin-PKC pathway on ROS production in type 1 diabetic
rats. Can J Physiol Pharmacol. 89:923–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okoshi K, Guimarães JF, Di Muzio BP,
Fernandes AA and Okoshi MP: Diabetic cardiomyopathy. Arq Bras
Endocrinol Metabol. 51:160–167. 2007.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bojunga J, Nowak D, Mitrou PS, Hoelzer D,
Zeuzem S and Chow KU: Antioxidative treatment prevents activation
of death-receptor- and mitochondrion-dependent apoptosis in the
hearts of diabetic rats. Diabetologia. 47:2072–2080. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li CJ, Zhang QM, Li MZ, Zhang JY, Yu P and
Yu DM: Attenuation of myocardial apoptosis by alpha-lipoic acid
through suppression of mitochondrial oxidative stress to reduce
diabetic cardiomyopathy. Chin Med J (Engl). 122:2580–2586.
2009.PubMed/NCBI
|
|
21
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walensky LD: Bcl-2 in the crosshairs:
Tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar S, Prasad S and Sitasawad SL:
Multiple antioxidants improve cardiac complications and inhibit
cardiac cell death in streptozotocin-induced diabetic rats. PLoS
One. 8:e670092013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boatright KM and Salvesen GS: Caspase
acrivation. Biochem Soc Symp. 233–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Donovan M and Cotter TG: Control of
mitochondrial integrity by Bcl-2 family members and
caspase-independent cell death. Biochim Biophys Acta. 1644:133–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
Mitochondrial cytochrome C-mediated caspase-3 activation pathway.
Diabetes. 51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cesario DA, Brar R and Shivkumar K:
Alterations in ion channel physiology in diabetic cardiomyopathy.
Endocrinol Metab Clin North Am. 35:601–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Li Y, Feng Q, Arnold M and Peng T:
Calpainactivation contributes to hyperglycaemia-induced apoptosis
in cardiomyocytes. Cardiovasc Res. 84:100–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao G and Dou QP: N-terminal cleavage of
bax by calpain generates a potent proapoptotic 18-kDa fragment that
promotes bcl-2-independent cytochrome C release and apoptotic cell
death. J Cell Biochem. 80:53–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gil-Parrado S, Fernández-Montalván A,
Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, Knoch TA,
Auerswald EA, Welsh K, Reed JC, et al: Ionomycin-activated calpain
triggers apoptosis. A probable role for Bcl-2 family members. J
Biol Chem. 277:27217–27226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang CH, Lu J, Yu XJ, Sun L and Zang WJ:
Ameliorative effect of Captopril and Valsartan on an animal model
of diabetic cardiomyopathy. Biol Pharm Bull. 31:2045–2049. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Connelly KA, Boyle AJ and Kelly DJ:
Angiotensin II and the cardiac complications of diabetes mellitus.
Curr Pharm Des. 13:2721–2729. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Z, Tepel M, Neusser M, Mehring N and
Zidek W: Effect of captopril on vasoconstriction and Ca2+ fluxes in
aortic smooth muscle. Hypertension. 22:806–811. 1993. View Article : Google Scholar : PubMed/NCBI
|