Introduction
The transmission of hepatitis B virus (HBV) is a
major issue threatening public health worldwide, particularly in
China. It is reported that in China, up to 10–20% of the population
carry HBV (1). HBV infection leads
to damage to multiple organs and in vivo lesions, with the
kidney being among the susceptible vital organs. The morbidity of
HBV-associated glomerulonephritis (HBV-GN) is a leading cause of
secondary nephropathy in China (2). Nephron loss induced by HBV infection
and the consequent imbalance of cell cycle progression are
considered to be major factors in the pathogenesis of HBV-GN. Cell
cycle progression is strictly regulated by genes and proteins that
have been investigated extensively, including cyclin A, cyclin E,
p16 and p21 proteins. When cell cycle progression is disturbed, the
consequent cellular apoptosis or non-programmed cell death have an
important role in renal injury.
The HBV gene is comprised of four open reading
frames, which include the preS/S, P, C and X genes (3). The HBV X (HBx) gene guides the
synthesis of the HBx protein, which is a unique non-structural
protein of HBV. The HBx protein is a type of functional protein
possessing various regulatory effects, such as transactivation, and
it has an important regulatory role in virus replication, cell
infection, cellular apoptosis induction and the triggering of
inflammatory responses (4–10). Although HBx has been demonstrated
to exert effects on the regulation of cell proliferation, the
detailed regulatory mechanisms of the HBx protein are yet to be
established.
Previous research conclusions concerning HBx have
been based on transformed or immortalized cell lines, and the
genetic and regulatory mechanisms of the cell cycle of these cell
lines are often altered. This may misguide the understanding of the
mechanisms underlying the effects of the HBx protein on the
physiology of renal tubular epithelial cells and HBV replication.
In order to exclude the variation from the transformed and
immortalized cell lines and investigate the effects of HBx protein
on the cell cycle progression of renal cells, primary renal tubular
epithelial cells are more suitable model cells. However, to the
best of our knowledge, no previous reports have employed primary
renal tubular epithelial cells as model cells to investigate the
effects of HBx in renal cells.
Therefore, the current study investigated the
specific mechanisms underlying the effects of HBx protein in the
regulation of the cell cycle progression of primary renal tubular
epithelial cells by determining the expression levels of cell
cycle-associated proteins following transfection of rat primary
renal tubular epithelial cells with a HBx gene eukaryotic
expression vector.
Materials and methods
Materials
Cellulose acetate membrane was purchased from EMD
Millipore (Billerica, MA, USA). Collagenase type I,
penicillin-streptomycin and epithelial cell growth factor were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Rabbit anti-Cyclin A monoclonal antibody (cat. no. ab181591;
1:2,000), rabbit anti-Cyclin D1 monoclonal antibody (cat. no.
ab134175; 1:10,000), rabbit anti-Cyclin E polyclonal antibody (cat.
no. ab71535; 1:2,000), rabbit anti-cytokeratin 18 monoclonal
antibody (cat. no. ab32118; 1:400) and rabbit anti-HBx polyclonal
antibody (cat. no. ab39716; 1:2,000) were all obtained from Abcam
(Cambridge, MA, USA). Mouse anti-β actin (cat. no. TA-09; 1:2,000),
horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (cat.
no. ZB-2305; 1:2,000), HRP goat anti-rabbit IgG (ZB-2301; 1:2,000)
secondary antibodies and concentrated DAB kit (cat. no. ZLI-9017)
were purchased from ZSGB-Bio, Inc. (Beijing, China). HRP conjugated
polymer anti-rabbit IgG antibody (cat. no. SV0002; 1:100) was
purchased from Boster Biological Technology (Pleasanton, CA, USA).
Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS)
and trypsin were purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). KpnI and EcoRV endonucleases, T4 DNA
ligase and TOP10 competent cells were from Beijing Transgen Biotech
Co., Ltd. (Beijing, China). SuperSignal West Pico Chemiluminescent
Substrate was purchased from Thermo Fisher Scientific, Inc..
Epithelial cell medium (EpiCM) was obtained from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, Jiangsu, China). The HiFiScript cDNA
Synthesis kit was purchased from CW Biotech Co., Ltd. (Beijing,
China). TRNzol and Mini and Maxi Plasmid kits were from Tiangen
Biotech Co., Ltd. (Beijing, China). TaKaRa Ex Taq was purchased
Takara Biotechnology Co., Ltd. (Dalian, China). Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
kit (cat. no. KGA105) was purchased from Nanjing KeyGen Biotech
Co., Ltd. (Nanjing, China).
Animals
A total of 2 male Sprague Dawley (SD) rats (weight,
220 and 240 g; aged 8 weeks old) were purchased from the
Experimental Animal Center of the Medical College of Zhejiang
University (Hangzhou, China). The rats were raised with free access
to water and food in a sterile environment of 25–28°C, 40–60%
relative humidity and a 12/12 h light/dark cycle. Prior to the
experiment, the rats were fasted for 12 h with free access to
water. The study protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Zhejiang University
and was in accordance with the guidelines established by the
Chinese Council of Animal Care (11).
Separation and culture of primary
renal tubular epithelial cells
Rats were euthanized by cervical vertebra
dislocation and immersed in 70% (v/v) ethanol for 2–3 min. Two
kidneys were removed. Renal cortexes were cut into pieces (~1 mm)
and ground on 80 mesh sieves. Following 2–3 washes with PBS,
residue on the sieves was collected, pipetted repeatedly and
centrifuged at 1,000 × g and room temperature for 5 min. The
supernatant was discarded. The pellet was resuspended in 2 ml
collagenase type-I (1 mg/ml) and incubated under vibration in a
37°C water bath for 30 min. The mixture was centrifuged at 1,000 ×
g at room temperature for 3 min. The supernatant was discarded
again and the pellet was resuspended in 4 ml DMEM containing 10%
(v/v) FBS and 1% (v/v) penicillin-streptomycin (10 mg/ml penicillin
and 10 mg/ml streptomycin). Subsequently, primary cells were
routinely cultured for 3 days at 37°C and 5% CO2,
followed by washing with PBS three times. Trypsin buffer (2 ml,
0.25%), and a mixture of trypsin (0.25%) and EDTA (0.02%), were
dropwise added to immerse the cells. Cells were subsequently
trypsinized in a 37°C water bath for 1–2 min. 2X EpiCM containing
10% (v/v) FBS, 1% (v/v) epithelial cell growth factor (1 µg/l) and
1% (w/v) penicillin-streptomycin (10 mg/ml penicillin and 10 mg/ml
streptomycin) was immediately added. Cells were subsequently
subcultured at a ratio of 1:2.
Morphology characterization by
immunohistochemical analysis
Cells (2.5×105) were seeded into 24-well
plates and covered by coverslips. Following the culturing of cells
for 36 h and 7 days time intervals, they were removed and washed
with PBS three times. Cells were subsequently fixed in 4% (w/v)
cold paraformaldehyde at 4°C for 30 min, permeabilized in 0.5%
(w/v) Triton X-100 at 37°C for 5 min and incubated in deionized
water containing 3% (w/v) H2O2 at room
temperature for 10 min. Cells were subsequently blocked using 5%
bovine serum albumin (Beyotime Institute of Biotechnology, Jiangsu,
China) at 37°C for 30 min. After rinsing with PBS, cells were
incubated in anti-cytokeratin 18 primary antibody (1:400) buffer at
4°C overnight. Cells were incubated in an equal volume of PBS in
the control group. After rinsing with PBS, cells were incubated in
HRP conjugated polymer anti-rabbit IgG antibody (1:100) buffer at
37°C for 1.5 h. Subsequently, development using a concentrated DAB
kit, rinsing, counterstaining using 0.1% hematoxylin at room
temperature for 3 min, dehydration using ethanol,
transparentization using xylol and mounting were performed in
sequence. The coverslips were observed under an optical microscope
(XDZ-103; Shanghai Tiancheng Medical Flow Technology Co., Ltd.,
Shanghai, China) with Image Analysis 11.0 software (Beijing
Changheng Rongchuang Technology Co., Ltd., Beijing, China).
Construction of HBx gene pcDNA3.1/myc
vector
Primers were designed according to HBV genomic
sequence of ayr subtype in GenBank (12) (https://www.ncbi.nlm.nih.gov/pubmed/6300776) and the
HBx gene was amplified via polymerase chain reaction (PCR) using
TaKaRa Ex Taq according to the manufacturer's protocol. The
thermocycling conditions used were as follows: Pre-denaturation for
3 min at 96°C; followed by 23 cycles of denaturation for 20 sec at
95°C, annealing for 20 sec at 58°C, elongation for 30 sec at 72°C;
and final extension for 1 min at 72°C. The sequences of primers
were as follows: Forward, 5′-GGGGTACCGTGGCAGAGGTGAAAAAGTTGC-3′ and
reverse, 5′-GCCGATATCCTAACATTGAGATTCCCGAG-3′. The forward and
reverse primers contained KpnI and EcoRV endonuclease cutting
sites, respectively. The pcDNA3.1/myc plasmid (Miaolingbio
Bioscience & Technology Co., Ltd., Wuhan, China) and the
amplified HBx segments were managed by double enzyme digestion with
restriction endonucleases KpnI and EcoRV. Electrophoresis was
performed and the enzyme-digested products were recovered and
purified using an agarose gel DNA recovery kit (Tiangen Biotech
Co., Ltd., Beijing, China), according to the manufacturer's
protocol. Subsequently, ligation was performed with T4 DNA ligase.
The ligated products were transformed into TOP10 competent cells
and the cells were cultured based on ampicillin screening. Finally,
DNA sequencing was performed by Sangon Bioetch Co., Ltd. (Shanghai,
China) for identification.
Extraction of the HBx gene vector
Saved strains were inoculated into 200 ml LB media
(Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China) containing
100 mg/l ampicillin at 37°C for 14 h. Colonies containing plasmids
on the LB plates were removed and added into 2 ml LB media
containing ampicillin (100 µg/ml). When the optical density value
of the bacterial solution reached ~2, the solution was centrifuged
at 10,000 × g at room temperature for 1 min to collect the bacteria
pellets. The pcDNA3.1/myc plasmids were extracted using Mini and
Maxi Plasmid kits plasmid according to the manufacturer's
protocol.
HBx gene transfection
When primary renal tubular epithelial cells,
cultured in 4 ml DMEM containing 10% (v/v) FBS and 1% (v/v)
penicillin-streptomycin (10 mg/ml penicillin and 10 mg/ml
streptomycin), in 6-well plates reached 90% confluency
(1.2×106), the culture medium was replaced by serum-free
DMEM medium and cells were incubated again at 37°C for 48 h. The
plasmid was gently mixed with Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) and culture medium
according to the manufacturer's protocol. The mixture was added
into the 6-well plates (5 µg plasmid/well). Subsequently, cells
were incubated at 37°C for 4 h followed by the replacement of the
culture medium with medium containing serum at 37°C for another 48
h.
Determination of HBx and cell
cycle-associated gene expression by reverse
transcription-quantitative PCR (RT-qPCR)
Cells were divided into three groups: Control, empty
and HBx. Cells transfected with empty plasmid pcDNA3.1(+) and
recombinant plasmid pcDNA3.1(+)-HBx were designated as the empty
and HBx groups, respectively. Cells without transfection served as
the control group. Total RNA was extracted from cells
(1.2×106) with TRNzol, according to the manufacturer's
protocol. Primers sequences are presented in Table I. Reverse transcription was
performed with a HiFiScript cDNA Synthesis kit, according to the
manufacturer's protocol. qPCR was performed using UltraSYBR Mixture
(cat. no. CW0957M; CWBIO, Inc., Beijing, China). Thermocycling
parameters were set as follows: Pre-denaturation for 10 min at
95°C, followed by 40 cycles of denaturation for 10 sec at 95°C,
annealing for 30 sec at 51.2°C and elongation for 30 sec at 72°C.
β-actin served as internal control and the relative level to
β-actin was calculated accordingly using classical 2-ΔΔCq method
(13).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence,
5′-3′ |
|---|
| Cyclin E | F:
ATCTGGCGGTTGACTTT |
|
| R:
GGCAGCGATGAAGAGTG |
| Cyclin A | F:
ACGAACATGTCGCTAGTGGT |
|
| R:
GTGGGATTTTCTGGGTGTCCT |
| Cyclin D1 | F:
CAGAAGTGCGAAGAGGAA |
|
| R:
GAAGCCAGGAACATACAAG |
| HBx | F:
CACTTCGCTTCACCTCTGC |
|
| R:
TCGGTCGTTGACATTGCTG |
| β-actin | F:
ATCGTCCACCGTAAATGC |
|
| R:
TGAAGTGGTAGTCGGGTG |
Determination of HBx and cell
cycle-associated protein expression by western blot analysis
Cells were also divided into three groups for
western blot analysis: Control, empty and HBx. Following
transfection, the culture medium was discarded and cells were
washed two times with ice-precooled PBS. Subsequently, cells
(1.2×106) were scraped and centrifuged at 2,000 × g and
4°C for 5 min. Cell pellets were collected and resuspended in RIPA
lysis buffer (cat. no. C1053; Applygen Technologies, Inc., Beijing,
China) containing 10% (w/v) phenylmethanesulfonyl fluoride and 1%
(w/v) phosphatase inhibitors for 20 min. This mixture was
centrifuged at 10,000 × g at room temperature for 10 min and the
supernatant was collected to obtain total cell protein. Gel loading
buffer (5X) was added into total protein and the mixture was boiled
for 5 min to denature proteins. Subsequently, proteins (2 µg per
lane) were loaded to perform 10% SDS-PAGE electrophoresis. Proteins
were transferred to a polyvinylidene difluoride membrane at 4°C.
The membrane was incubated in blocking buffer that consisted of TBS
with 0.1% Tween-20 (TBST) containing 5% (w/v) nonfat-dried milk on
a horizontal shaker at room temperature for 1.5 h. Subsequently,
the membrane was immersed in 3–5 ml with rabbit anti-Cyclin A
antibody, rabbit anti-Cyclin D1, rabbit anti-Cyclin E, rabbit
anti-HBx, and mouse anti-β actin primary antibodies at 4°C
overnight. TBST was used to rinse the membrane three times for 10
min each time. The membrane was incubated with HRP conjugated goat
anti-mouse IgG and goat anti-rabbit IgG secondary antibodies at
room temperature for 1.5 h. The membrane was rinsed with TBST three
times for 10 min each time. Following the addition of ECL solution,
the membrane was exposed on a gel imaging system (ChemiDoc XRS;
Bio-Rad Laboratories, Inc.). Gray values were determined using
Quantity One software (v4.6.2; Bio-Rad Laboratories, Inc.).
Evaluation of cell apoptosis and the
cell cycle by flow cytometry
Renal tubular epithelial cells in logarithmic phase
were divided into three groups: Control, empty and HBx. Following
transfection, cells were cultured for a further 24 h
(1.2×106) and trypsinized for 5 min. Cells were
centrifuged at 1,200 × g at room temperature for 5 min, washed and
resuspended in 500 µl binding buffer (1X). Cells were gently mixed
with Annexin V-FITC (5 µl) and incubated at 4°C in the dark for 30
min. After gentle mixing with PI (5 µl), the mixture was incubated
at 4°C in the dark for another 5 min. Finally, the cell suspension
was immediately evaluated on a flow cytometer (BD FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA). Data were analyzed using
CellQuest software (v.17; BD Biosciences). In order to investigate
the cell cycle, cells were cultured following transfection at 37°C
for a further 24 h (1.2×106) and subsequently
trypsinized for 5 min. Cell were fixed in pre-cooled 70% ethanol at
4°C for 3 h. Cells were then centrifuged at 1,200 × g at room
temperature for 5 min, washed with PBS and then resuspended in 1 ml
PI (50 µg/ml) containing 0.5% Triton X-100 at room temperature in
the dark for 30 min. Finally, the cell suspension was evaluated
using a flow cytometer (BD FACSCalibur; BD Biosciences), and data
were subsequently analyzed using CellQuest software (v.17; BD
Biosciences).
Statistical analysis
All experiments were independently repeated three
times and data are presented as the mean + standard deviation.
Statistical analysis was performed using SPSS 18.0 software (SPSS,
Inc., Chicago, IL, USA). Statistical comparisons among groups were
performed by one way analysis of variance followed by Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphology characterization of primary
renal tubular epithelial cells
In the present study, cells that exhibited various
intensities of non-uniform, scattered brown staining in the
cytoplasm and perinuclear region, and with a shape resembling
multilateral cobblestones with mutually tight arrangements, were
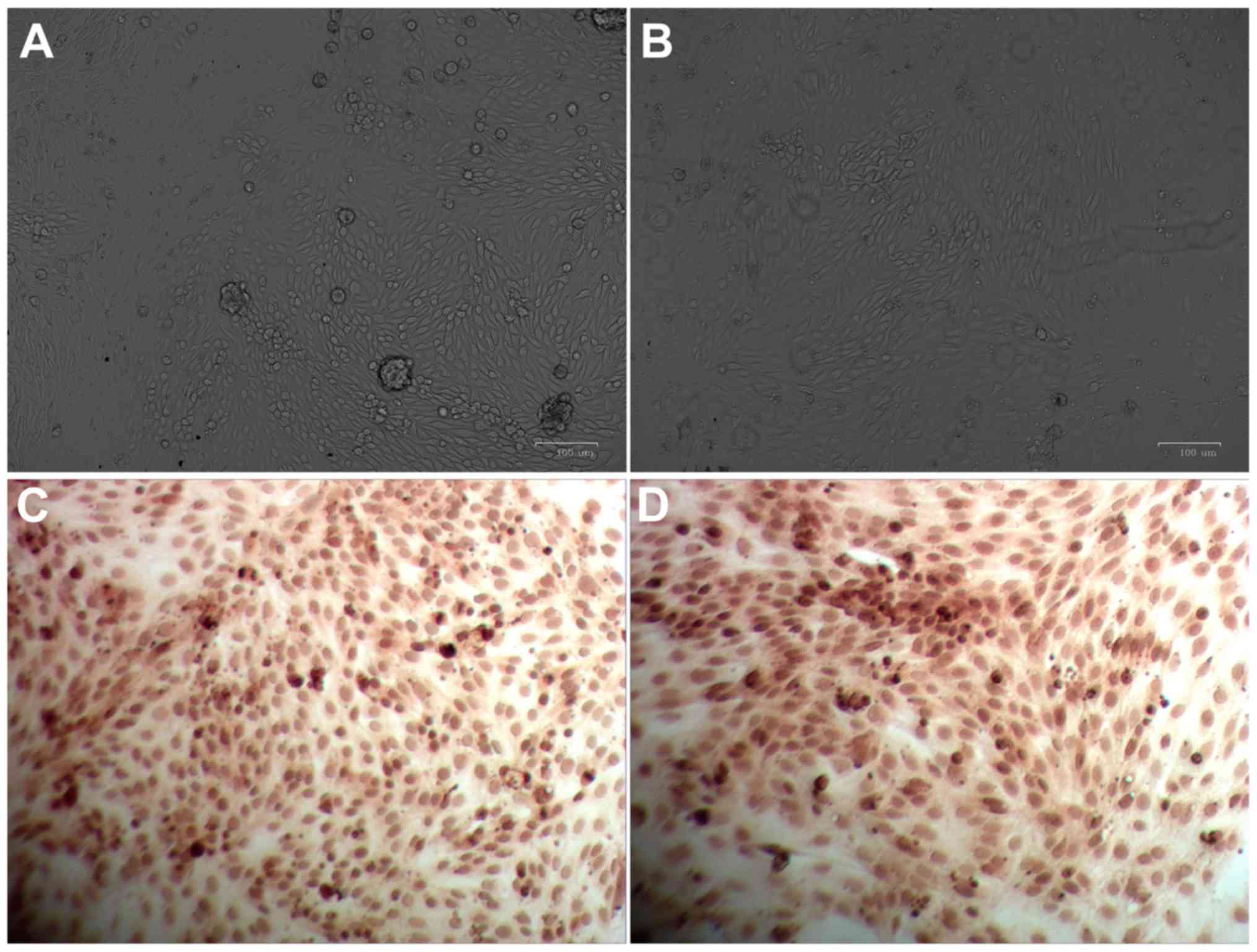
considered to be verified as renal tubular epithelial cells. Images
of primary renal tubular epithelial cells are presented in Fig. 1. When primary cells were cultured
for 12 h, their anchorage growth was observed. After 24–36 h,
epithelioid cells emerged from the periphery of the anchorage area
and the whole cell population exhibited an ‘island-like’
distribution (Fig. 1A). A total of
6–7 days post-primary culture, cells grew all over the bottom of
the culture bottle. At this time-point, cells were observed to
resemble the shape of typical multilateral cobblestones with tight
arrangements and high transparency (Fig. 1B). The present study employed
immunohistochemical analysis to determine the levels of cytokeratin
18 specifically expressed in renal tubular epithelial cells and the
results demonstrated the presence of brown granules with a
non-uniform and scattered distribution in the cytoplasm and
perinuclear region (Fig. 1C and
D). These results indicated that these cells were verified to
be renal tubular epithelial cells.
mRNA expression levels of HBx and cell
cycle-associated genes
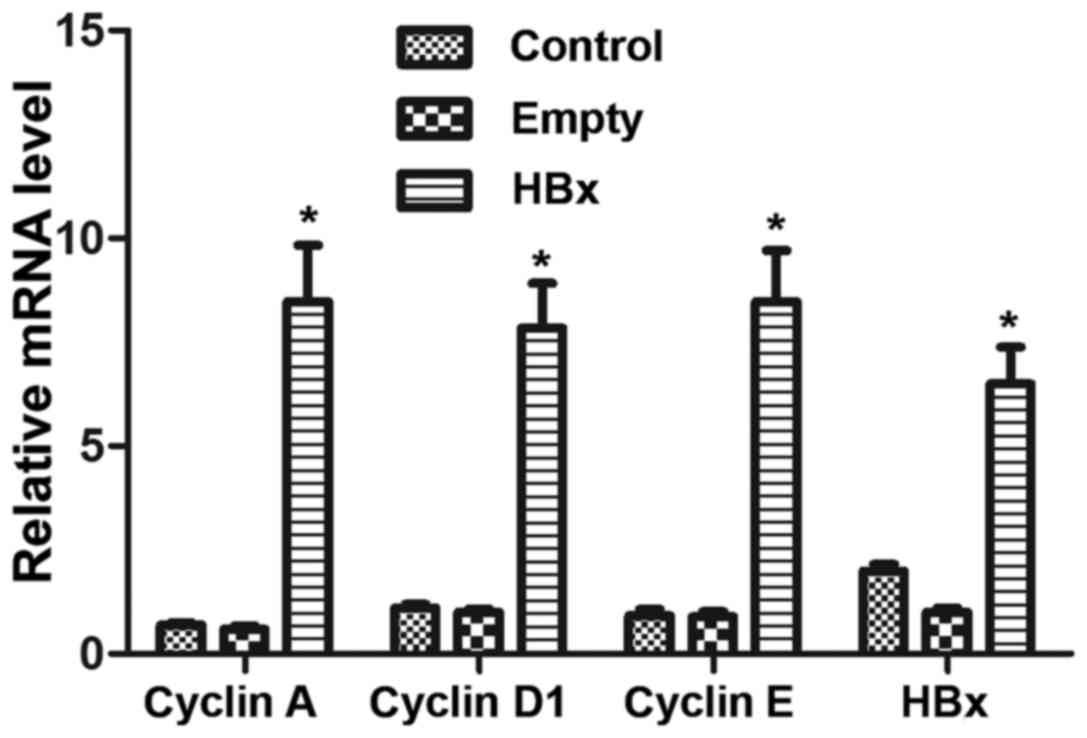
The mRNA levels of HBx and the cell cycle-associated
genes cyclin A, cyclin D1 and cyclin E in renal tubular epithelial
cells was determined by RT-qPCR. No significant differences were
observed between the mRNA levels in the control and empty groups
(P>0.05; Fig. 2). However,
renal tubular epithelial cells in the HBx group exhibited
significantly higher mRNA levels of HBx, cyclin A, cyclin D1 and
cyclin E compared with the empty group (P<0.05; Fig. 2).
HBx and cell cycle-associated protein
levels
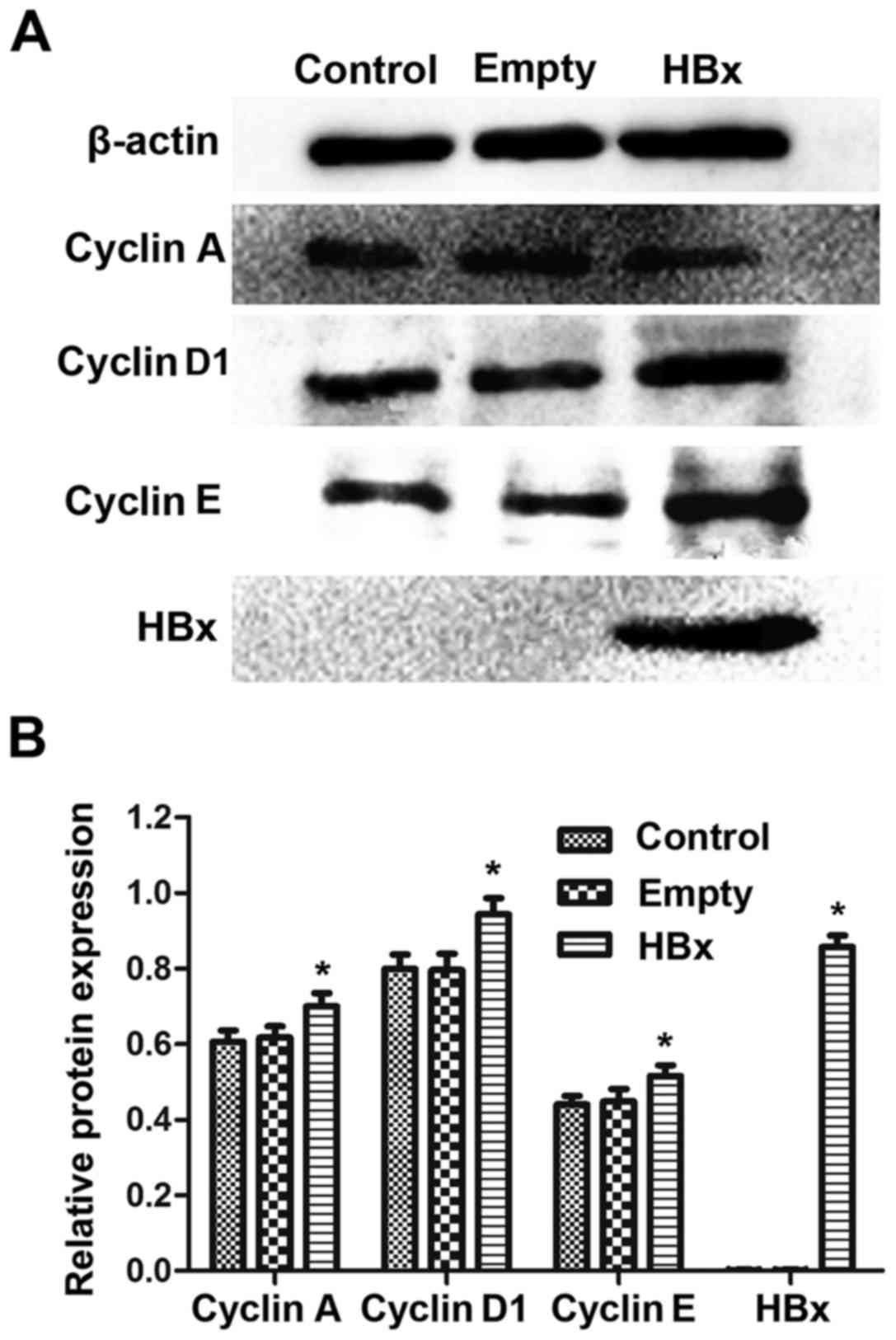
The protein levels of HBx and the cell
cycle-associated proteins cyclin A, cyclin D1 and cyclin E in renal
tubular epithelial cells were assessed by western blot analysis. As
demonstrated in Fig. 3A, similar
blots for β-actin were observed among the control, empty and HBx
groups, indicating that the western blotting method was reliable.
An obvious dark blot for HBx was observed in the HBx group, but no
blot traces of HBx were observed in the control and empty groups.
The quantified results demonstrated similar protein levels of
cyclin A, cyclin D1 and cyclin E between the control and empty
groups (P>0.05; Fig. 3B).
Notably, in the HBx group, the protein expression of cyclin A,
cyclin D1 and cyclin E were significantly upregulated compared with
the empty group (P<0.05; Fig.
3B). Quantitatively, HBx protein was scarcely detected in the
control and empty groups, while following HBx gene transfection,
renal tubular epithelial cells exhibited significantly increased
expression of HBx protein (Fig.
3B).
Cell apoptosis
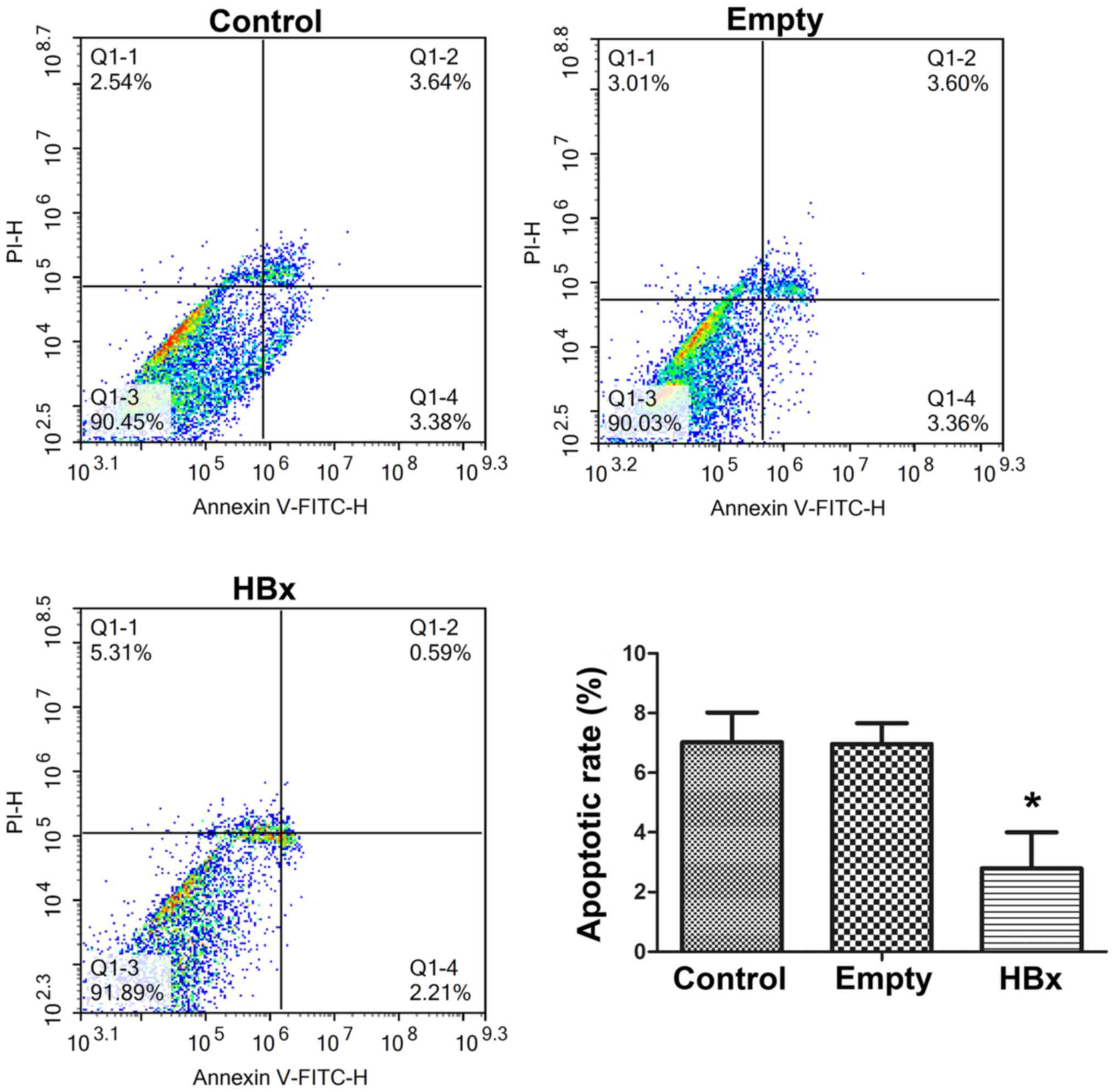
The cell apoptosis of renal tubular epithelial cells
in the control, empty and HBx groups was evaluated by Annexin
V-FITC/PI double staining and flow cytometry results are presented
in Fig. 4. The apoptotic rates of
cells in the control, empty and HBx groups were 7.02±1.02,
6.96±0.86 and 2.80±0.24%, respectively. Cells in the control and
empty groups exhibited similar apoptotic rates, demonstrating that
the transfection of the empty plasmid did not affect the apoptosis
of renal tubular epithelial cells. Compared with the empty group,
the apoptotic rate was markedly reduced by >60% following the
transfection of the plasmid containing the HBx gene (P<0.05;
Fig. 4), indicating that HBx gene
transfection was able to effectively suppress the apoptosis of
renal tubular epithelial cells.
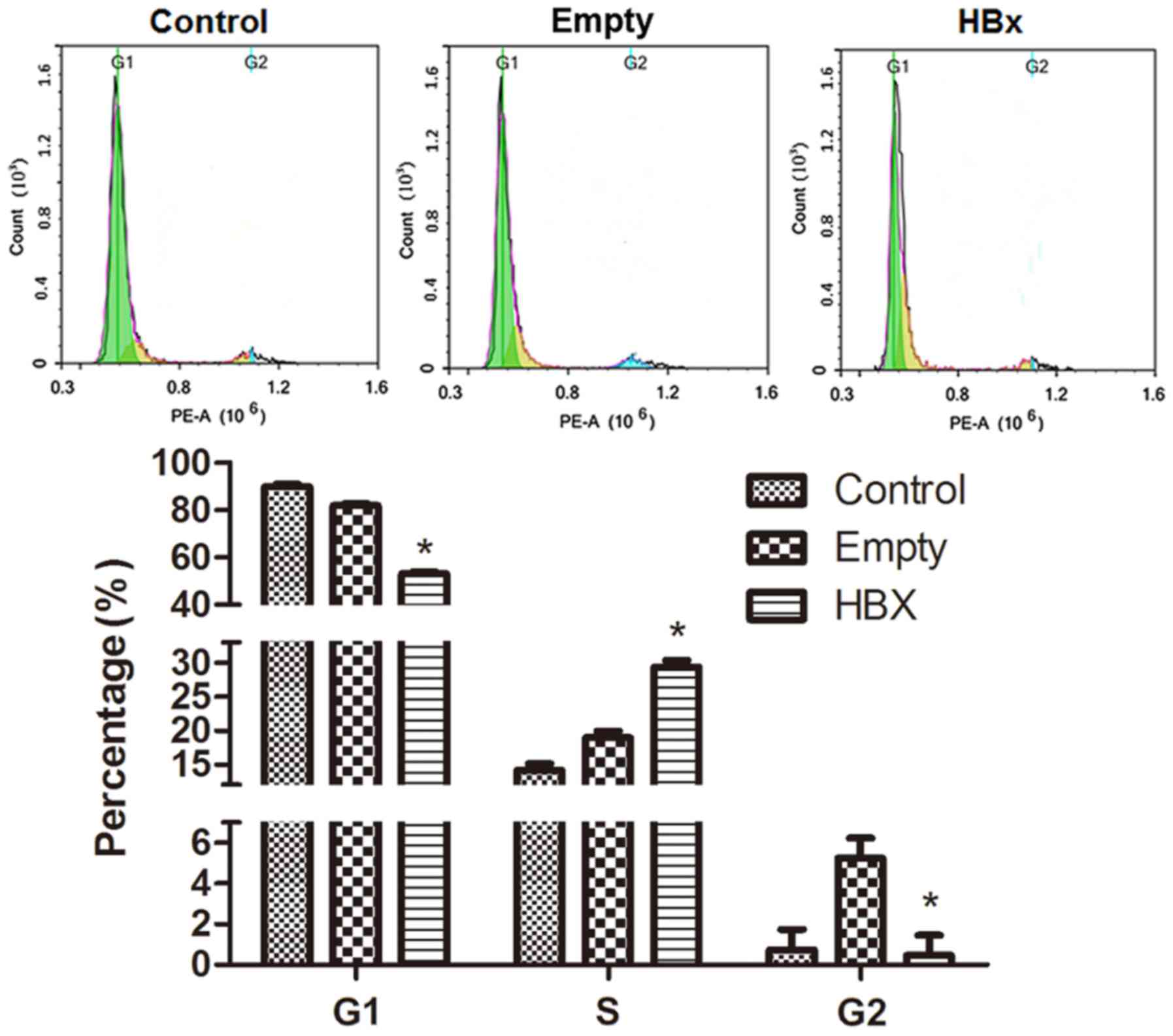
Cell cycle
The cell cycle of renal tubular epithelial cells in
the control, empty and HBx groups was evaluated by flow cytometry
and results are presented in Fig.
5. The green peak area on the left, yellow peak area in the
middle and blue peak area on the right represent cells in G1, S and
G2 phases, respectively. The distribution of the cell cycle in the
control group was comprised of G1 89.38% (Freq G1), S 14.15% (Freq
S) and G2 0.74% (Freq G2); the majority of cells were in the
presynthetic phase, a few cells were in the synthetic phase and a
limited number of cells were in the postsynthetic phase. In the
empty group, cells numbers in the S and G2 phases were marginally
augmented. However, when cells were transfected with the HBx gene,
G1 and S phase cell percentages were markedly decreased and
increased, respectively, while the percentage in the G2 phase was
similar to the control group. The results indicated that HBx gene
transfection promoted the progression of renal tubular epithelial
cells from the G1 to S phase.
Discussion
Chronic hepatitis B in patients is usually
associated with various types of extrahepatic damage, and HBV-GN is
one of the most common forms of extrahepatic damage. However, an
understanding of the risk factors and pathogenesis of HBV-GN remain
unclear, and effective preventive and therapeutic strategies are
also yet to be established (14).
At present, direct infection of the kidney by HBV and subsequent
immunological dysfunction induced by the infection are considered
to be the major pathogenic causes of HBV-GN (15,16).
In the nephridial tissues of patients with HBV-GN, obvious
tubulointerstitial lesions were identified, which presented as
inflammatory cell infiltration and interstitial fibrosis (17). The severity of interstitial lesions
was reported to be positively associated with the infiltration
degree of inflammatory cells and atrophy of renal tubular
epithelial cells (HK-2 cells) was observed in the pathological
tissues (17). The results of
previous studies indicated that human renal tubular epithelial
cells cultured in vitro were susceptible to HBV and HBV
enhanced the secretion of transforming growth factor-β1 by human
renal tubular epithelial cells (18,19).
Other reports revealed that HBx protein was expressed in the renal
tubular epithelial cells of patients with HBV-GN and was primarily
distributed in the cytoplasm (20,21).
Furthermore, its expression level in the renal tubular epithelial
cells was demonstrated to be higher compared with in glomerular
cells (20,21). Therefore, HBx may be principally
replicated and transcribed in situ within renal tubular
epithelial cells and the damage of renal tubular epithelial cells
may be generated in this process.
In order to elucidate the damage mechanism of HBx on
renal tubular epithelial cells, a renal tubular epithelial cell
model with HBx transfection was established. It was previously
reported that HBx transfection promoted the transdifferentiation of
HK-2 cells, indicating that HBx may alter the microenvironment of
HK-2 cells and facilitate epithelial-mesenchymal transition
(22,23). Although the evolution from the
damage of renal tubular epithelial cells to the final reduction of
cell number was regulated by multiple mechanisms, the ultimate
effect was altered cell cycle progression (24). The cell cycle is composed of a
resting phase (G0), DNA presynthetic phase (G1), DNA synthetic
phase (S), DNA postsynthetic phase (G2) and mitotic phase (M).
Intervention in cell cycle progression influences the replication
of HBV and, in addition, HBx protein was demonstrated to alter the
cell apoptosis process of renal tubular epithelial cells during the
pathological process of renal tubular interstitial fibrosis induced
by HBV (25). Therefore, analysis
of alterations in the expression of cell cycle-associated proteins
and confirmation of the association with HBV replication level
in vivo are required to investigate the mutual effects
between HBx protein and the cell cycle.
Cyclins are a family of proteins with cell
cycle-specific or temporal expression and catabolism. The cell
cycle is regulated by various cyclins. Cyclin A regulates the
transition from G1 to S phase, which is associated with DNA
replication (26), while cyclin D1
and cyclin E are associated with G1 phase. Upregulation of cyclin
A, cyclin D1 and cyclin E will facilitate the cell transition from
G1 to S phase (27). The tumor
suppressor genes p16 and p21 compete with multiples cyclins to bind
to various cyclin-dependent kinase sites, inhibit the activation of
cyclin and arrest the cell cycle in the G1 phase, which
consequently regulates cells division and differentiation, and
prevents the excessive proliferation of cells (28,29).
As a type of trans-acting factor with extensive
biological functions, the HBx protein has been reported to activate
intracellular signal transduction systems and regulate the cell
proliferation through binding to cell transcription factors
(30). In the past, it was widely
considered that HBx may accelerate cell cycle progression and cell
proliferation through shortening the G0/G1 phase. However,
Clippinger et al (6)
demonstrated that the effect of HBx on cell apoptosis was
bidirectional in hepatocytes. Zhai et al (4) further revealed that the effect of HBx
on the cell cycle was dose-dependent, but the specific mechanisms
of this effect remain unclear (31).
Therefore, in the present study, HBx was transfected
into primary renal tubular epithelial cells. Following HBx
transfection, the mRNA expression of cyclin A, cyclin D1 and cyclin
E were markedly increased, and the corresponding protein levels
were also upregulated. These results indicated that HBx may
influence the cell cycle progression of renal tubular epithelial
cells by regulating the expression of cyclins and causing a rapid
transition of cells from G0 to S phase via G1 phase. Thus, the
present study also investigated the levels of cell apoptosis and
cell cycle distribution, and the results demonstrated that HBx
transfection markedly promoted the progression of renal tubular
epithelial cells from the G1 to S phase and suppressed the cell
apoptosis, which was consistent with the results of RT-qPCR and
western blot analysis of cyclin expression. These results indicated
that HBx protein may function in altering cell cycle
progression.
Although the current study demonstrated that HBx
protein may act on cyclin A, cyclin E and cyclin D1 to regulate the
cell cycle of renal tubular epithelial cells, the further specific
regulation mechanism requires future exploration. For example, the
results of the present study do not indicate whether the HBx
protein affects the expression of cyclin A, cyclin E and cyclin D1
by competitive binding or altering upstream signaling. However,
dysfunction of the tumor suppressor gene p53 was reported to be
associated with the overexpression of HBx (32,33).
Our future studies will focus on the effects of p53, p21 and p16
genes on the HBx protein and HBV replication in primary renal
tubular epithelial cells.
The present study revealed the effects of HBx on the
cell cycle progression of primary renal tubular epithelial cells by
transfection with HBx gene pcDNA3.1/myc vector. We also
hypothesized that the HBx gene may integrate into the genomic DNA
of hosts by recombination. As a multifunctional protein, HBx may
function in the proliferation or apoptosis of host cells through
stimulating signal transduction pathways in the cytoplasm and the
activation of transcription factors in the nucleus (34). However, further studies are
required to confirm whether the Hbx gene is able to integrate into
the genomic DNA of host cells.
In conclusion, primary renal tubular epithelial
cells of rats were separated and cultured to investigate the effect
of HBx gene transfection on the expression of cyclins, cell
apoptosis and the cell cycle. Consequently, HBx gene transfection
was demonstrated to regulate the apoptosis and cell cycle of
primary renal tubular epithelial cells by influencing the
expression of cyclins. These results may improve the understanding
of the pathogenesis of HBV-GN and may also provide insight and
theoretical support for the design and development of future
hepatitis B virus drugs.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
2015 General Medical and Health Research Program of Zhejiang
Province (Class A), Health and Family Planning Commission of
Zhejiang Province, China (grant no. 2015KYA043).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
WH and ML designed the study and wrote the
manuscript. WH, ML, MH, YZhu, YZho, HD, GH, LL, QC and YL performed
the experiments. MH, YZhu, YZho and HD collected and analyzed
data.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Zhejiang University
(Hangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Q and Xue C: Research progress in
pathogenesis of hepatitis B virus associated nephritis. Med Recap.
2543–2545. 2013.
|
|
2
|
Yi Z, Jie YW and Nan Z: The efficacy of
anti-viral therapy on hepatitis B virus-associated
glomerulonephritis: A systematic review and meta-analysis. Ann
Hepatol. 10:165–173. 2011.PubMed/NCBI
|
|
3
|
Seeger C and Mason WS: Hepatitis B virus
biology. Microb Mol Biol Rev. 64:51–68. 2000. View Article : Google Scholar
|
|
4
|
Zhai LL, Liu J and Xie YH: Dose-dependent
modulation of cell apoptosis by hepatitis B virus X protein. J
Microb Infect. 72–77. 2011.
|
|
5
|
Xia LM, Huang WJ, Wu JG, Yang YB, Zhang Q,
Zhou ZZ, Zhu HF, Lei P, Shen GX and Tian DA: HBx protein induces
expression of MIG and increases migration of leukocytes through
activation of NF-kappa B. Virology. 385:335–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clippinger AJ, Gearhart TL and Bouchard
MJ: Hepatitis B virus X protein modulates apoptosis in primary rat
hepatocytes by regulating both NF-kappa B and the mitochondrial
permeability transition pore. J Virology. 83:4718–4731. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan C, Guo H, Zheng M, Chen Y and Huang W:
Involvement of mitochondrial permeability transition in hepatitis B
virus replication. Virus Res. 145:307–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He P, Shang H, Li D and Feng G: Effects of
HBx gene on proliferation and apoptosis of human renal tubular
epithelial cells. Chin J Nephrol. 29:380–381. 2013.
|
|
9
|
Chen HY, Tang NH, Lin N, Chen ZX and Wang
XZ: Hepatitis B virus X protein induces apoptosis and cell cycle
deregulation through interfering with DNA repair and checkpoint
responses. Hepatol Res. 38:174–182. 2008.PubMed/NCBI
|
|
10
|
Huang YQ, Wang LW, Yan SN and Gong ZJ:
Effects of cell cycle on telomerase activity and on hepatitis B
virus replication in HepG22.2.15 cells. Hepatobiliary Pancreat Dis
Int. 3:543–547. 2006.
|
|
11
|
General Administration of Quality
Supervision, Inspection and Quarantine of China: Laboratory
animal-Guideline of welfare ethical review (Draft for approval).
Mar 18–2017.
|
|
12
|
Ono Y, Onda H, Sasada R, Igarashi K,
Sugino Y and Nishioka K: The complete nucleotide sequences of the
cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids
Res. 11:1747–1757. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He P, Li H, Li D and Feng G: HBx gene
promotes apoptosis in HK-2 cells through regulating
apoptosis-relatedprotein ratio of Bax/Bcl-2. J Chin Med Univer.
43:38–44. 2014.
|
|
15
|
An S, Zhang R, Yang W, Zhou H, Zhang Z,
Yang Y and Guo X: Research advances in hepatitis B virus-associated
glomerulonephritis. J Clin Hepatol. 32:366–369. 2016.
|
|
16
|
Jiang W, Xu Y, Guan G, Ma R and Dong H:
Renal amyloidosis and hepatitis B virus-associated
glomerulonephritis in a patient with nephrotic syndrome. Chin Med J
(Engl). 127:11992014.PubMed/NCBI
|
|
17
|
Deng CL, Song XW, Liang HJ, Feng C, Sheng
YJ and Wang MY: Chronic hepatitis B serum promotes apoptotic damage
in human renal tubular cells. World J Gastroenterol. 12:1752–1756.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu N, Zhou Y, Yuan WJ, Liu J, Shang MH,
Wang L and Gu LJ: Toll-like receptor 4 deposition and its
significance in hepatitis B virus associated nephropathy. Zhonghua
Nei Ke Za Zhi. 50:1008–1012. 2011.(In Chinese). PubMed/NCBI
|
|
19
|
Han WL and Huang ZX: Experimental study of
direct pathogenic effects of hepatitis B virus on human tubular
kidney cell. J Wenzhou Med College. 38:144–147. 2008.
|
|
20
|
Han WL and Huang ZX: Infection of
hepatitis B virus on human tubular kidney cells in vitro. J
Zhejiang Med Univer. 32:1148–1150. 2010.
|
|
21
|
Hong L, Zhang J, Li Q, Min J, Lu J, Li F,
Li H and Guo S: Role of NF-κB activiation by MHBst167/HBx in human
renal tubular cells. Chin J Nephrol Dial Transplant. 19:135–141.
2010.
|
|
22
|
Hong L, Zhang J, Min J, Lu J, Li F, Li H,
Guo S and Li Q: A role for MHBst167/HBx in hepatitis B
virus-induced renal tubular cell apoptosis. Nephrol Dial
Transplant. 25:2125–2133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Wang X, Yuan W and Zhu N: Effect
of hepatitis B virus X gene on transdifferentiation of human
proximal tubular epithelial cells. Chin J Nephrol. 28:956–960.
2012.
|
|
24
|
Zhou Y, Zhu N, Wang X, Wang L, Gu LJ and
Yuan WJ: The role of the toll-like receptor TLR4 in hepatitis B
virus-associated glomerulonephritis. Arch Virol. 158:425–433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marshall CB and Shankland SJ: Cell cycle
regulatory proteins in podocyte health and disease. Nephron Exp
Nephrol. 106:e51–e59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Madden CR and Slagle BL: Stimulation of
cellular proliferation by hepatitis B virus X protein. Dis Markers.
17:153–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhan SD and Huang JX: Progression of
Cyclin A, PTEN, Survivin and p27 expression in esophageal cancer. J
Modern Oncol. 2245–2248. 2014.
|
|
28
|
Li YJ and Li YY: Advances in the research
of cyclin E and p27 in gynecological. Foreign Med Sci (Obstet
Gynecol Fascicle). 177–180. 2007.
|
|
29
|
Lin T: Expression and significance of Cell
cycle regulatory proteins in the hepatitis B virus associated
nephritsFujian Med Univer. Fuzhou: pp. 522011
|
|
30
|
Wu RS: Cell cycle regulation of p16, cdk4
and its role in the formation of middle ear cholesteatoma. J Basic
Med Shandong Univer. 53–55. 2004.
|
|
31
|
Lu HZ, Liu D and Zhou JH: Effects of HBV X
protein on glomerular mesangial cell proliferation and
proinflammation factor expression. Chongqing Med. 40:2227–2230.
2011.
|
|
32
|
Matsuda Y and Ichida T: Impact of
hepatitis B virus X protein on the DNA damage response during
hepatocarcinogenesis. J Med Mol Morphol. 42:138–142. 2009.
View Article : Google Scholar
|
|
33
|
Park SG, Min JY, Chung C, Hsieh A and Jung
G: Tumor suppressor protein p53 induces degradation of the
oncogenic protein HBx. Cancer Lett. 282:229–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bouehard MJ and Sehneider RJ: The
enigmatic X gene of hepatitis B virus. J Virol. 78:12725–12734.
2004. View Article : Google Scholar : PubMed/NCBI
|