Introduction
Breast cancer is the second most common cancer
worldwide and the most common cancer among women (1). Triple-negative breast cancer (TNBC),
which comprises ~10–15% of all breast cancer cases (2), is characterized by the absence of the
estrogen receptor, progesterone receptor, and the human epidermal
growth factor receptor-2 (3).
Management of TNBC is challenging due to a current lack of targeted
therapies, aggressive behavior and relatively poor prognosis
(4). There are currently few
therapeutic options, and conventional chemotherapy is one of the
treatments, which may be effective for patients following surgery
(5); however, it is associated
with severe side effects. Therefore, it is important to identify
novel and effective therapeutic agents with low levels of toxicity
for the treatment of patients with TNBC. Traditional Chinese
medicine has been used for the treatment of patients with cancer,
either alone or in combination with western medicines (6). It is a promising field for research
and development.
Xi Huang pills (XHP) are a Chinese formula first
mentioned in the Life-saving Manual of Diagnosis and Treatment of
External Diseases, written by Hongxu Wang in the year 1740
(7). XHP contains Niu Huang
(Calculus bovis), She Xiang (Moschus berezovskii), Ru
Xiang (Resina olbani) and Moyao (Commiphora myrha).
The primary function of XHP is detoxification, as well as relieving
swelling and pain. It is used primarily for the treatment of
furunculosis, scrofula and neoplasms. XHP is a well-known
traditional Chinese medicine that demonstrates anticancer
properties. It has been reported that XHP may be efficacious
against breast cancer, liver cancer, leukemia and additional
malignancies (8). However,
evidence for the antitumor effects of XHP against TNBC is scare.
Pan et al (9) used the
MDA-MB-231 TNBC cell line in 2013, and demonstrated the cytotoxic
effects of aqueous XHP extracts on these cells. The potential
underlying mechanisms were thought to have been associated with
apoptosis; however, Pan et al (9) did not investigate the molecular
mechanisms further or study the effects in vivo. Therefore,
the aim of the present study was to investigate the antitumor
effects of XHP on MDA-MB-231 cells in vitro and in
vivo, and elucidate the potential underlying molecular
mechanisms.
Materials and methods
Chemicals and antibodies
XHP was purchased from Tong Ren Tang Technologies
Co., Ltd. (Beijing, China). Dimethyl sulphoxide and MTT reagent
were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Human insulin, epidermal growth factor, cholera toxin and
hydrocortisone were purchased from Gibco; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). The human caspase-3 monoclonal antibody
(cat. no. ab32351; 1:3,000 dilution) was purchased from Abcam
(Cambridge, UK). The human cyclin A (cat. no. 4656; 1:5,000
dilution), p21Cip1 (cat. no. 2947; 1:5,000 dilution),
caspase-8 (cat. no. 9746; 1:4,000 dilution), Bcl-2-associated X
protein (Bax; cat. no. 5023; 1:5,000 dilution) and B-cell lymphoma
2 (Bcl-2; cat. no. 2870; 1:5,000 dilution) monoclonal antibodies
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). The β-actin rabbit monoclonal antibody (cat. no. TDY051;
1:5,000 dilution) was purchased from Beijing TDY Biotech Co., Ltd.
(Beijing, China). The horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibodies (cat. no. ZB-2306; 1:5,000
dilution) and goat anti-mouse IgG secondary antibodies (cat. no.
ZF-0312; 1:5,000 dilution) were purchased from OriGene
Technologies, Inc. (Beijing, China).
Drug preparation
A total of 3 g XHP was dissolved in 15 ml cold
distilled water, and mixed by Test Tube Rotary Mixer (Thermo Fisher
Scientific, Inc.) for 2 h at 4°C. XHP was then fragmented using an
ultrasound oscillator (40 kHz) for 2 h at 37°C, and the sample was
centrifuged at 1,500 × g for 5 min at 4°C. The supernatant was
centrifuged again at 4,800 × g for 15 min at 4°C, and the final
supernatant was filtered through a sterile microporous membrane
(0.45-µm in diameter) before storing at −20°C. XHP was diluted in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) to the
desired concentrations prior to treatment of the cells.
For the in vivo experiments, a total of 3 g
XHP was dissolved in 22.5 or 45 ml cold distilled water, rotated
for 2 h at 4°C. XHP was then fragmented with an ultrasound
oscillator (40 kHz) for 2 h at 37°C, and stored at −20°C until
required. XHP was warmed to room temperature and manually agitated
prior to intragastric administration of nude mice with the XHP
solution.
Cell culture
The MDA-MB-231 human breast cancer cell line was
purchased from the Cell Resource Center of the Peking Union Medical
College (Beijing, China). The cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin (Solarbio Science & Technology Co., Ltd., Beijing,
China). Cells were incubated in a humidified chamber at 37°C and 5%
CO2.
MCF-10A human breast epithelial cells were a
generous gift from Professor Liu Zhihua (Cancer Hospital Chinese
Academy of Medical Sciences, Beijing, China). The cells were
cultivated, maintained and treated in Dulbecco's modified Eagle's
medium/F-12 (1:1; Gibco; Thermo Fisher Scientific, Inc.),
supplemented with human insulin (10 µg/ml), epidermal growth factor
(20 ng/ml), cholera toxin (100 ng/ml), hydrocortisone (0.5 µg/ml),
5% horse serum (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin.
In vivo tumor xenograft model
Female BALB/c nude mice (n=30, weight 18–20 g, mean
19 g; 5–8 weeks old) were obtained from Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China). The animals were
housed in laminar airflow cabinets under pathogen-free conditions
with a 12-h light/dark cycle, and were fed autoclaved standard food
and water ad libitum. The animal experiment protocol was
approved by Peking University Animals Research Committee (Beijing,
China) and was conducted in accordance with the recommendations in
the Guide for the Care and Use of Laboratory Animals (10). Human MDA-MB-231 cells
(4×106) were diluted in 0.1 ml RPMI-1640 medium, mixed
at a 1:1 ratio with Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) to make the percentage of Matrigel 50%. The cells were
inoculated at 2×106 cells/mouse subcutaneously into the
right flank of the mice. Tumor growth was measured, and tumor
volume was calculated using the following formula: Tumor volume
(mm3)=0.5 × length × width2. When the tumor
volume had reached 50–100 mm3, the mice were randomly
divided into three different treatment groups (n=10/each group):
Distilled water/control group, 20 mg/day group and 40 mg/day group.
The mice and administrated with distilled water, 20 mg/day XHP and
40 mg/day XHP intragastrically, respectively. The total dose of the
drug was administrated twice per 12 h for 15 days. The body weight
and tumor volume of the mice were measured every 5 days following
XHP administration. The mice were sacrificed 15 days after XHP
administration under 1.5% pentobarbital sodium (1 ml/kg;
Lianshuoinc, Shanghai, China) and tumor tissues were stored at
−80°C for protein expression analysis.
MTT assay
MDA-MB-231 cells (1×104) were seeded in
96-well plates (Corning Incorporated, Corning, NY, USA) and treated
on the following day with different concentrations of XHP (0, 4, 8,
12, 16 mg/ml) for 6, 12, 24 and 48 h, respectively. MCF-10A cells
(1×104) were seeded in 96-well plates and treated on the
following day with XHP (12 mg/ml) for 12 h. The MTT assay was
performed using the methods described previously (11), and the optical density (OD) was
read at 570 nm using a 96-well microplate reader (Bio-Rad
Laboratories, Hercules, CA, USA). As reduction of MTT only occurs
in metabolically active cells, the OD values were used to provide a
measure of cell viability. The percentage cell viability was
calculated according to the following formula:
(ODtreatment/ODcontrol)×100.
Apoptosis assay
MDA-MB-231 cells (3.2×105) were seeded on
30-mm culture dishes (Corning Incorporated). The following day,
cells were treated with 0, 4, 8 or 12 mg/ml XHP for 24 h. Cells
were subsequently trypsinized (Gibco; Thermo Fisher Scientific,
Inc.), washed with cold phosphate-buffered saline (PBS), and
stained using an Annexin V/fluorescein isothiocyanate/propidium
iodide (PI) staining kit (BestBio Company, Shanghai, China). The
cells were detected by flow cytometry analysis using a BD
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and the results were analyzed by ModFit software version 6.0
(BD Biosciences).
Mitochondrial membrane potential
assay
The loss of mitochondrial membrane potential was
detected using a mitochondrial membrane potential assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Briefly,
MDA-MB-231 cells (3.2×105) were seeded on 30-mm culture
dishes. The following day, the cells were treated with 0, 4, 8 and
12 mg/ml XHP for 24 h. The cells were then trypsinized and stained
with JC-1 at 37°C for 15 min. The cells were subsequently washed
with JC-1 staining buffer twice and analyzed immediately by flow
cytometry using a BD FACSCalibur flow cytometer (BD Biosciences),
results of which were analyzed by ModFit software version 6.0 (BD
Biosciences).
Cell cycle distribution assay
MDA-MB-231 cells (8×105) were seeded on
50-mm culture dishes. The following day, cells were treated with 0,
4, 8 and 12 mg/ml XHP for 48 h. The cells were then trypsinized,
washed with PBS and fixed in 1 ml ice-cold 70% ethanol overnight at
4°C. The cells were centrifuged (1,000 × g, 5 min, 4°C), washed
with cold PBS and treated with PI/RNase Staining buffer (BD
Pharmingen, San Diego, CA, USA) according to the manufacturer's
protocols for 15 min at room temperature in the dark. The cell
cycle distribution was determined by flow cytometry using a BD
FACSCalibur flow cytometer. The percentage of cells in
G1, S or G2/M phases was calculated using
ModFit software version 6.0 (BD Biosciences).
Western blot analysis
MDA-MB-231 cells (3.06×106) were seeded
on 100-mm culture dishes. The following day, the cells were treated
with 0, 4, 8 and 12 mg/ml XHP for 24 or 48 h. Cells were then
harvested, washed with cold PBS and homogenized with
radioimmunoprecipitation assay lysis buffer (TDY Biotech Co., Ltd,
Beijing, China). In the mice tumor xenograft model, following the
sacrifice of the mice, tumor tissues were stored at −80°C for
protein expression analysis. Upon protein extraction, tumor tissues
were homogenized with radioimmunoprecipitation assay lysis buffer
(TDY Biotech Co., Ltd, Beijing, China). The concentration of total
protein extracts was determined using a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). The protein samples
were subsequently mixed with 5X loading buffer (CWBIO, Beijing,
China) and were boiled for 5 min. Protein samples (35 µg) were
separated by 10% SDS-PAGE, and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked for 2 h at room temperature with 5% fat-free milk or
bovine serum albumin in Tris-buffered saline-Tween-20 (TBST)
containing 10 mM Tris-HCl, 0.1 M NaCl and 0.1% Tween-20 (pH 7.4).
The membranes were then incubated with specific primary antibodies,
as detailed above in ‘chemicals and antibodies’ at 4°C overnight
with gentle agitation. Following washing with cold TBST, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies for 1 h at room temperature. Visualization of
the protein bands was accomplished using the Immobilon Western
Chemiluminescent HRP substrate (EMD Millipore, Billerica, MA, USA).
The protein band intensities were measured using ImageJ software
version 2.0 (National Institutes of Health, Bethesda, MD, USA),
normalized to that of β-actin and compared to the control.
Statistical analysis
The results are presented as the mean ± standard
deviation of at least three independent experiments. The data were
analyzed by one-way analysis of variance and Tukey as the post hoc
test with SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
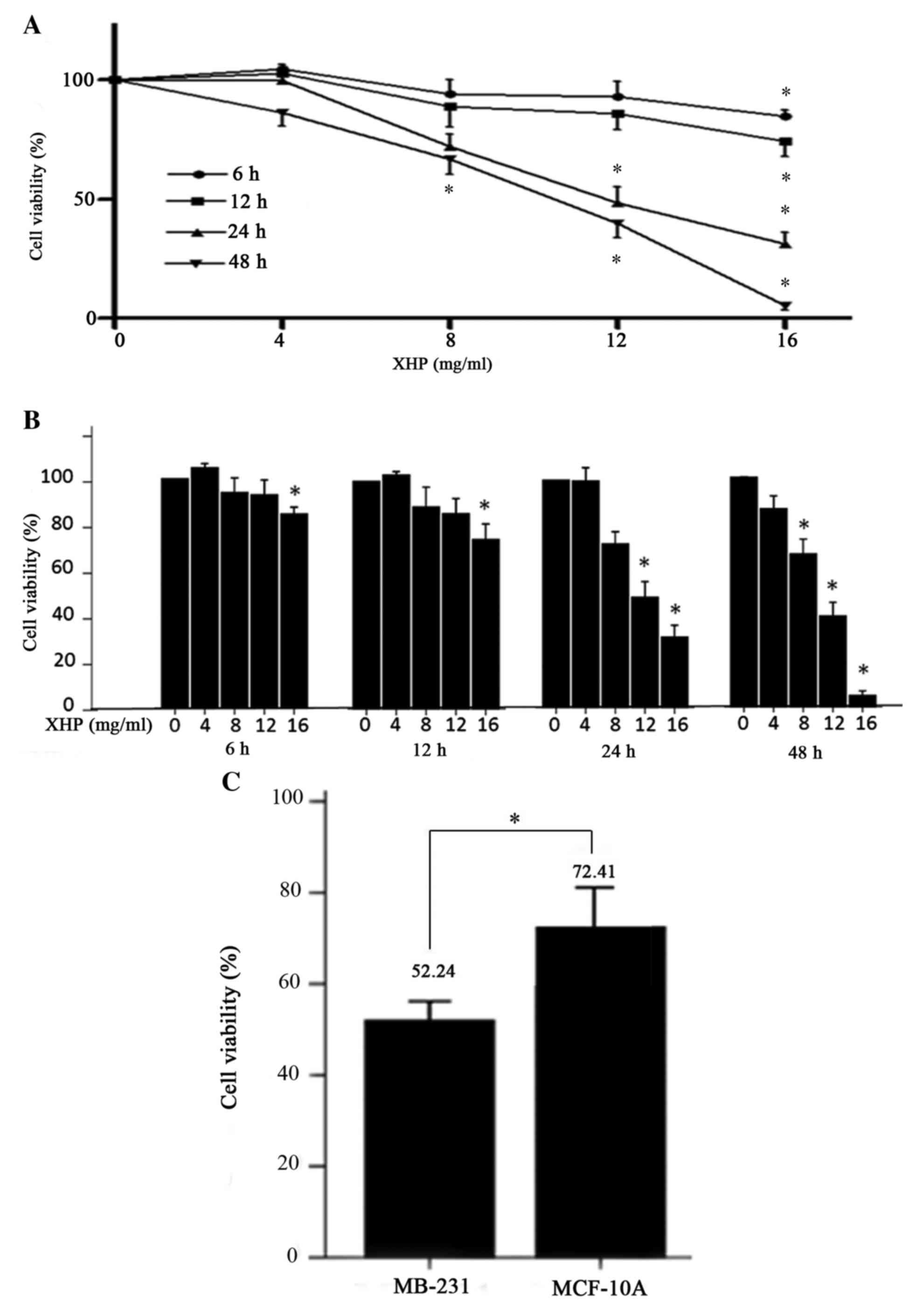
XHP inhibited the viability of
MDA-MB-231 cells
To evaluate the cytotoxic effects of XHP on
MDA-MB-231 cells, the viability of cells treated with different
concentrations (from 0 to 16 mg/ml) of XHP was measured using an
MTT assay. The results demonstrated that XHP reduced cell viability
in a dose- and time-dependent manner (Fig. 1A and B). The inhibition of
MDA-MB-231 cell viability following exposure to XHP was observed as
early as 6 h following exposure (Fig.
1A). The percentage cell viability following exposure to XHP
for 6, 12, 24 and 48 h at the highest concentration was
significantly decreased from 100% to 84.66±5.03%, 74.49±11.53%,
31.01±9.03% and 5.15±3.28%, respectively. By contrast, no
statistical difference between the control and the 4 mg/ml
XHP-treated group was observed at all time points (Fig. 1B).
The MCF-10A cell line was used to represent normal
human breast epithelial cells, while the MDA-MB-231 cell line was
used to represent human TNBC cells for the purposes of the present
study. These cell lines were treated with 12 mg/ml XHP for 24 h,
and the viability of MDA-MB-231 and MCF-10A cells significantly
decreased to 52.24±3.63% and 72.41±9.79% respectively, compared
with untreated controls, (Fig.
1C). The results indicated that XHP reduced cell viability in a
cell-selective manner.
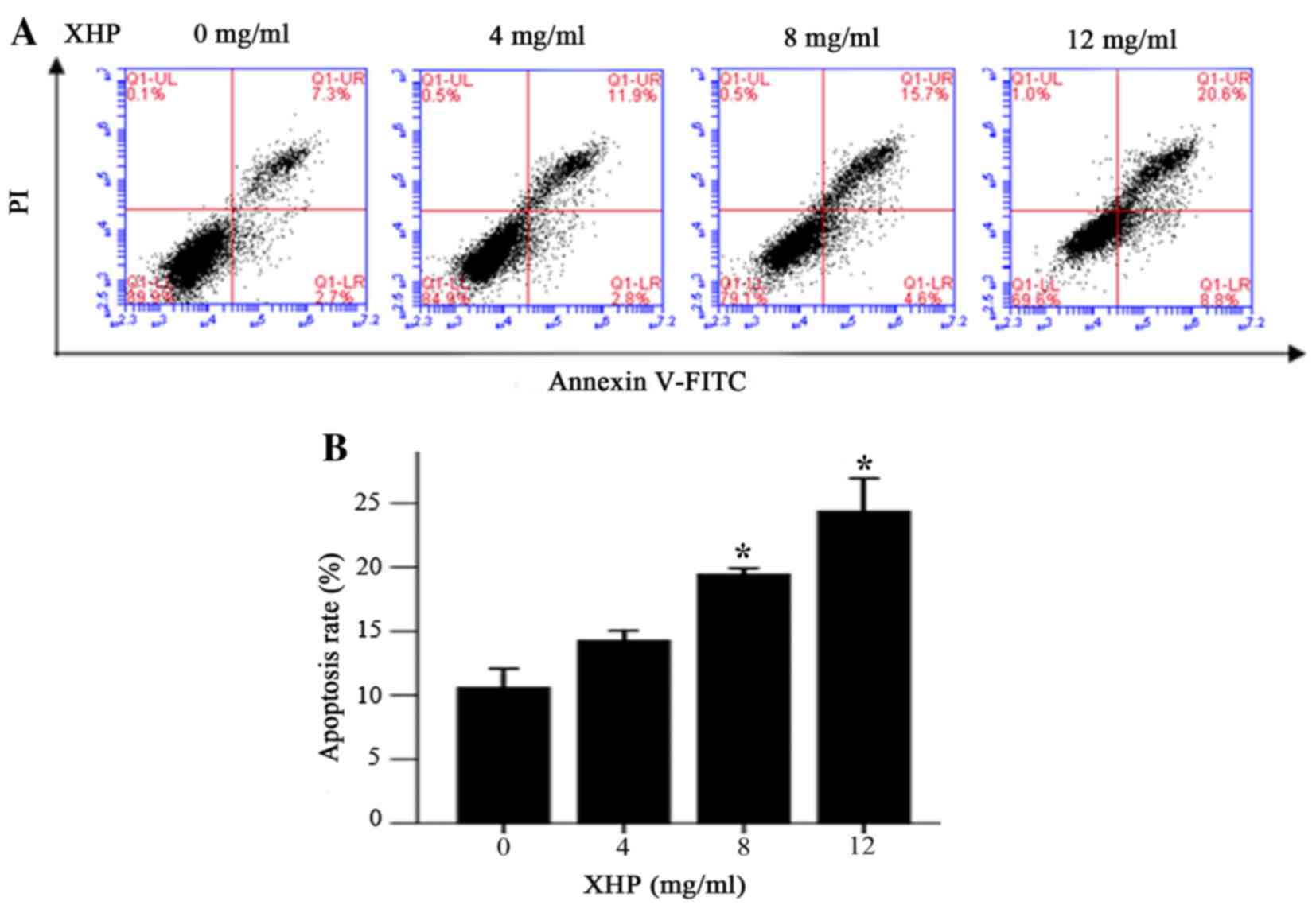
XHP induced apoptosis in MDA-MB-231
cells
In order to determine whether the antiproliferative
effects of XHP on MDA-MB-231 cells may involve induction of
apoptosis, the Annexin V/propidium iodide double-staining method
combined with flow cytometry analysis was employed. Following
treatment with 4, 8 and 12 mg/ml XHP for 24 h, the percentage of
cells in early apoptosis increased from 2.7% (untreated cells) to
2.8, 4.6 and 8.8%, respectively, whereas the percentage of cells in
late apoptosis increased from 7.3% (untreated cells) to 11.9, 15.7
and 20.6%, respectively (Fig. 2A).
A statistically significant increase in the rate of apoptosis was
observed following treatment of cells with 8 and 12 mg/ml XHP when
compared with the controls (Fig.
2B). Therefore, XHP induced apoptosis in MDA-MB-231 cells in a
dose-dependent manner.
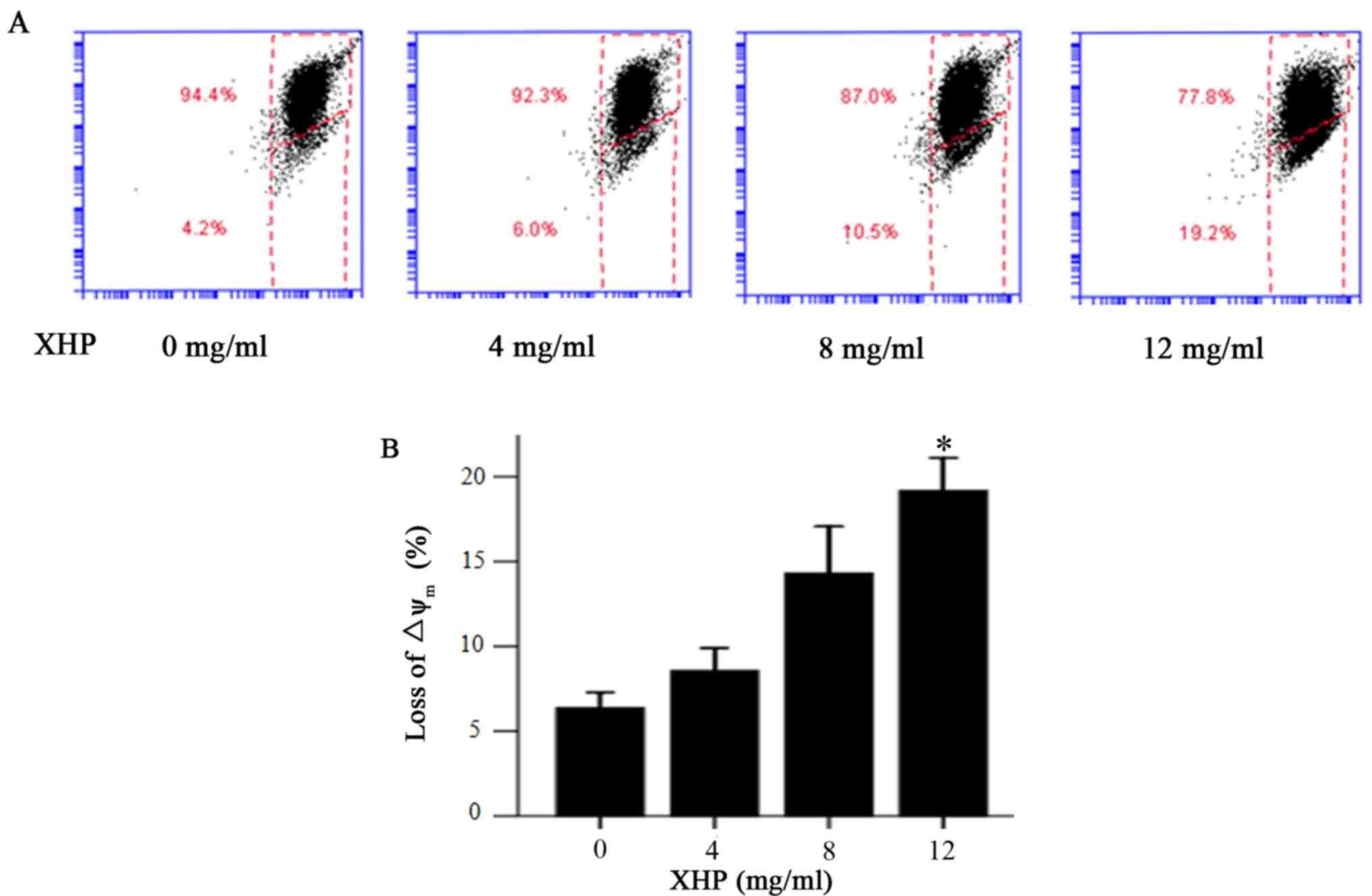
XHP induced the depletion of
mitochondrial membrane potential in MDA-MB-231 cells
In order to determine whether the increased
apoptosis rate of MDA-MB-231 cells was associated with a depletion
of mitochondrial membrane potential, flow cytometry analysis of
MDA-MB-231 cells treated with different concentrations of XHP was
performed to measure alterations in the mitochondrial membrane
potential. Following treatment with XHP (4, 8 and 12 mg/ml) for 24
h, the percentage of cells with a low mitochondrial membrane
potential increased from 4.2% (untreated cells) to 6.0, 10.5 and
19.2%, respectively (Fig. 3A). XHP
induced significant mitochondrial membrane potential depletion in
MDA-MB-231 cells following treatment with 12 mg/ml XHP (Fig. 3B). The results indicate that XHP
induced a dose-dependent reduction in mitochondrial membrane
potential.
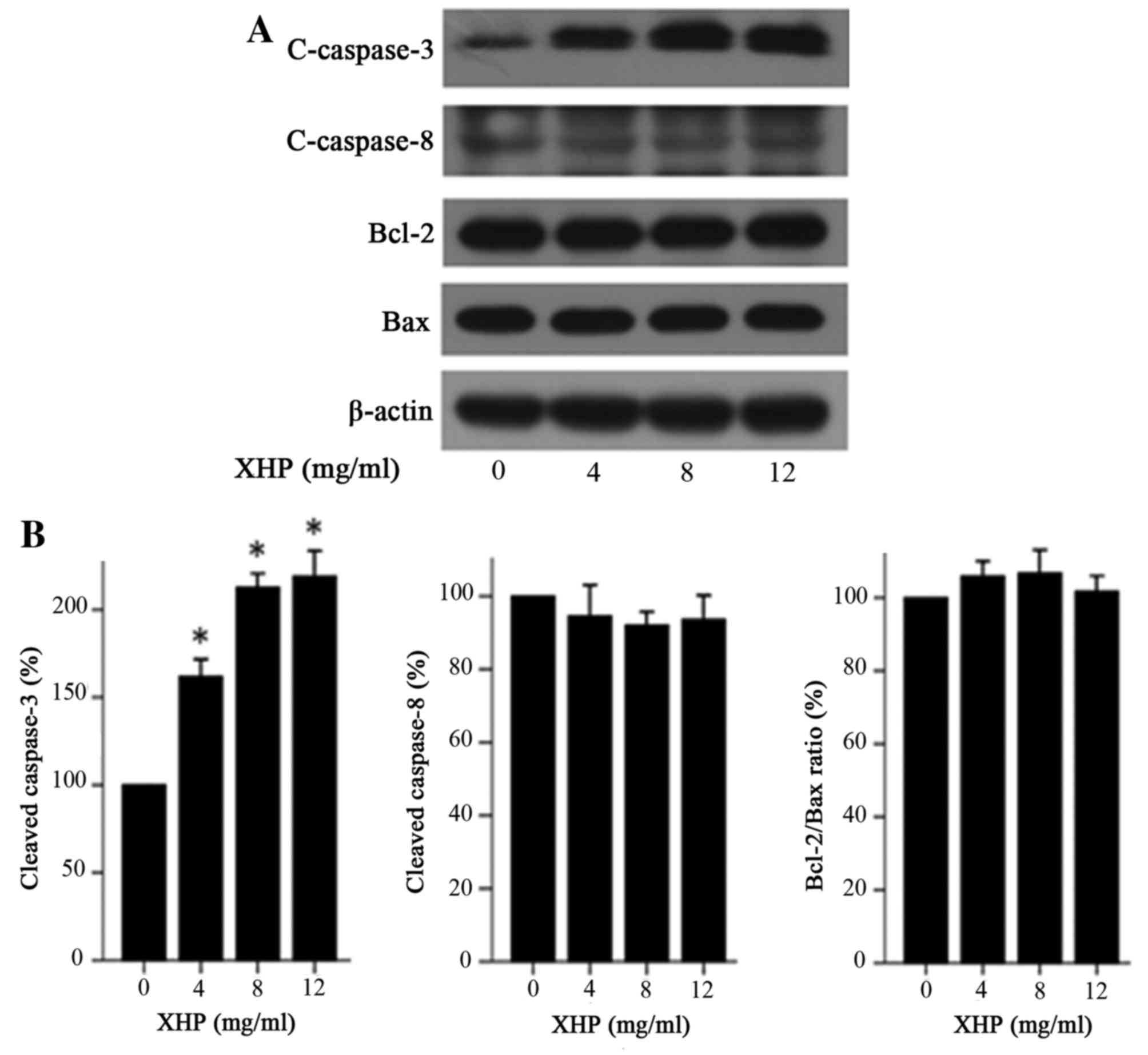
XHP induced apoptosis via the
intrinsic pathway
Apoptosis is executed via two major pathways, known
as the intrinsic and extrinsic pathways (12). These pathways lead to caspase-3
activation. The caspase family is the most prominent protease
family involved in apoptosis (13), and is divided into two functional
groups; the apoptosis initiators (caspase-8, −9 and −10) and the
apoptosis executors (caspase-3, −6 and −7) (14).
Bax is a well-known proapoptotic protein and Bcl-2
is an anti-apoptotic protein, and the Bcl-2/Bax ratio serves a
decisive role in apoptosis induction (15,16).
To determine whether caspase-3, caspase-8, Bax and Bcl-2 proteins
were involved in apoptosis induction, the authors of the present
study examined the expression of these proteins in MDA-MB-231 cells
treated with XHP by western blot analysis. The results indicated
that the protein expression levels of the cleaved caspase-3 were
increased by 1.62-, 2.13- and 2.19-fold, when compared to the
control following 4, 8 and 12 mg/ml XHP treatment for 24 h,
respectively (Fig. 4A and B).
However, the expression levels of cleaved caspase-8, Bcl-2, Bax and
the Bcl2/Bax ratio in MDA-MB-231 cells were not significantly
different among cells treated with 0, 4, 8 and 12 mg/ml XHP
(Fig. 4). These results
demonstrated that XHP may induce apoptosis via the intrinsic
pathway and not the extrinsic pathway, which is associated with
Bcl-2/Bax ratio.
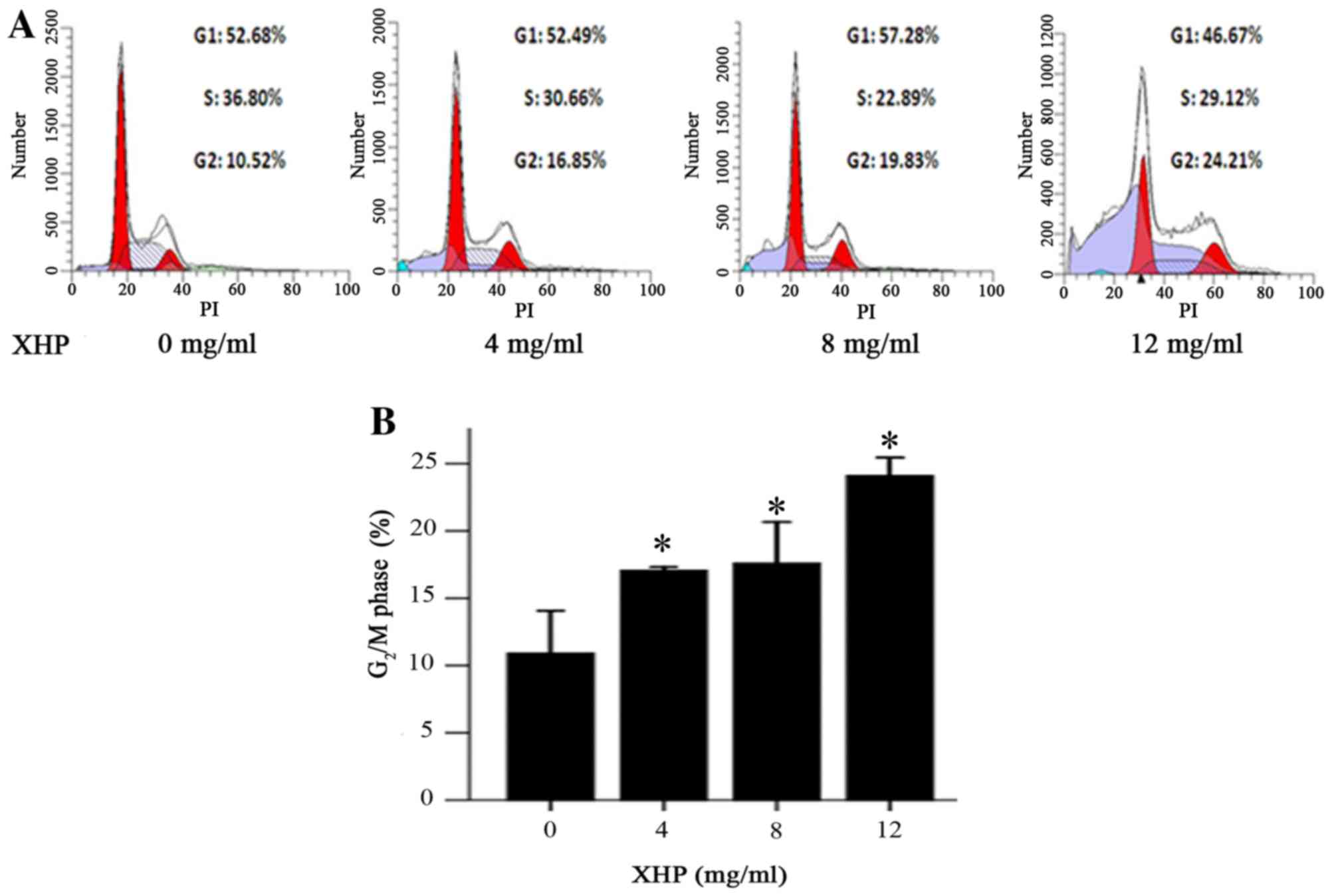
XHP induced cell cycle arrest at the
G2/M phase
Cell cycle regulation is important for cell
proliferation. Numerous antitumor drugs induce cell cycle
inhibition by impeding cell proliferation (17,18).
In order to examine whether the antiproliferative effects of XHP
was associated with cell cycle arrest in the present study, the
cell cycle distribution of XHP-treated MDA-MB-231 cells was
examined. Cells in the G2/M phase increased from 10.52%
in the untreated group to 16.85, 19.83 and 24.21% following
treatment with 4, 8 and 12 mg/ml XHP, respectively (Fig. 5). Consistent with these
alterations, the percentages of cells in the G1 or S
phases were concomitantly decreased (Fig. 5A). The results indicated that XHP
treatment significantly affected the cell cycle distribution of
MDA-MB-231 cells, leading to cell cycle arrest at G2/M
phase in a dose-dependent manner (Fig.
5B).
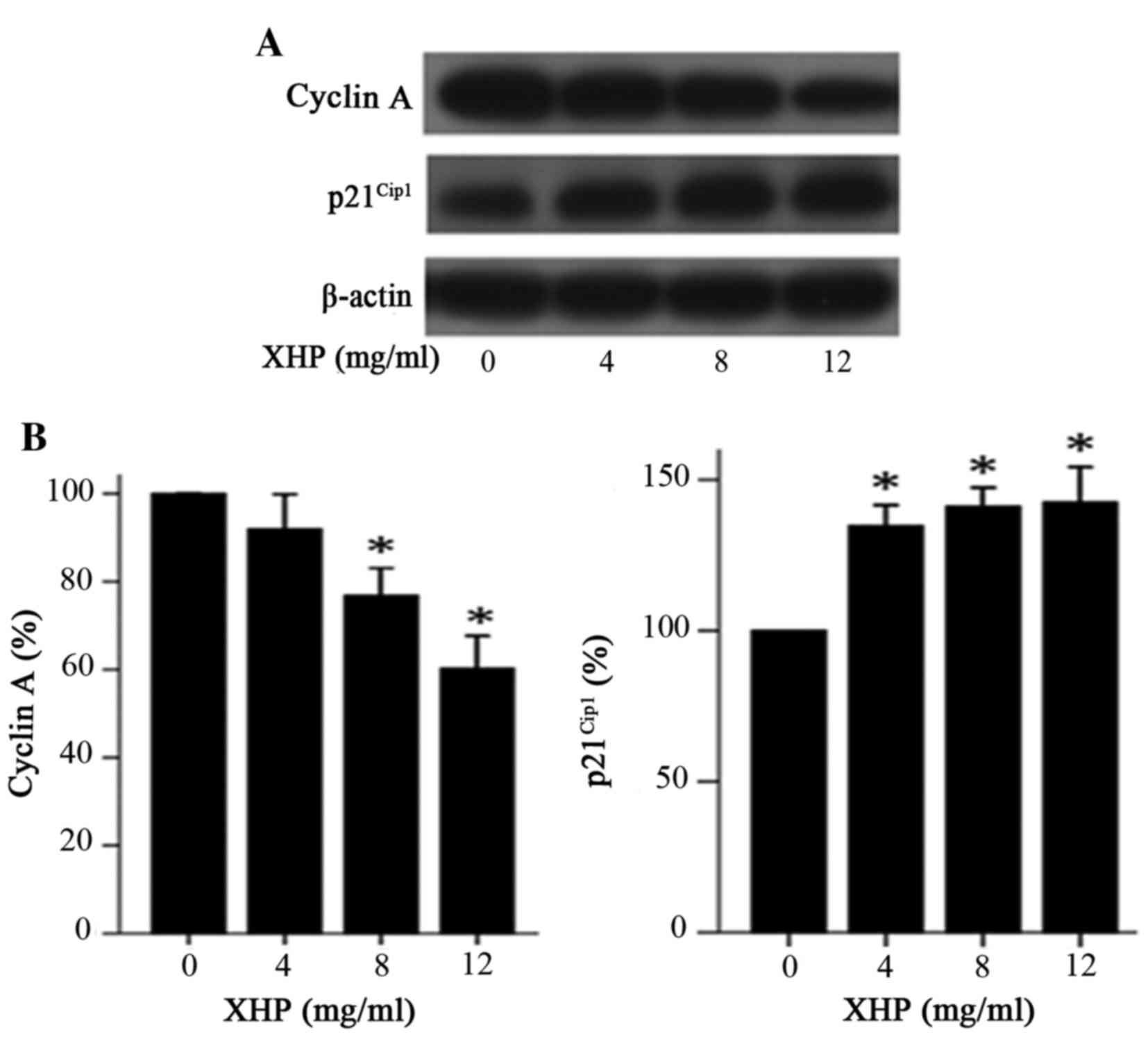
Effect of XHP on the expression levels
of cell cycle regulatory proteins
To explore the mechanism by which XHP induced cell
cycle arrest at G2/M phase in MDA-MB-231 cells, western
blot analysis was performed to determine whether XHP modulates the
expression of cell cycle regulatory molecules in the present study.
The results demonstrated that treatment with different
concentrations of XHP (4, 8 and 12 mg/ml) for 48 h resulted in a
decrease in the expression of cyclin A and an increase in the
expression of p21Cip1 when compared with the control
group (Fig. 6A and B). These
results suggested that the decreased expression of cyclin A and the
increased expression of p21Cip1 may have contributed to
the cell cycle arrest of MDA-MB-231 cells in the G2/M
phase following exposure to XHP.
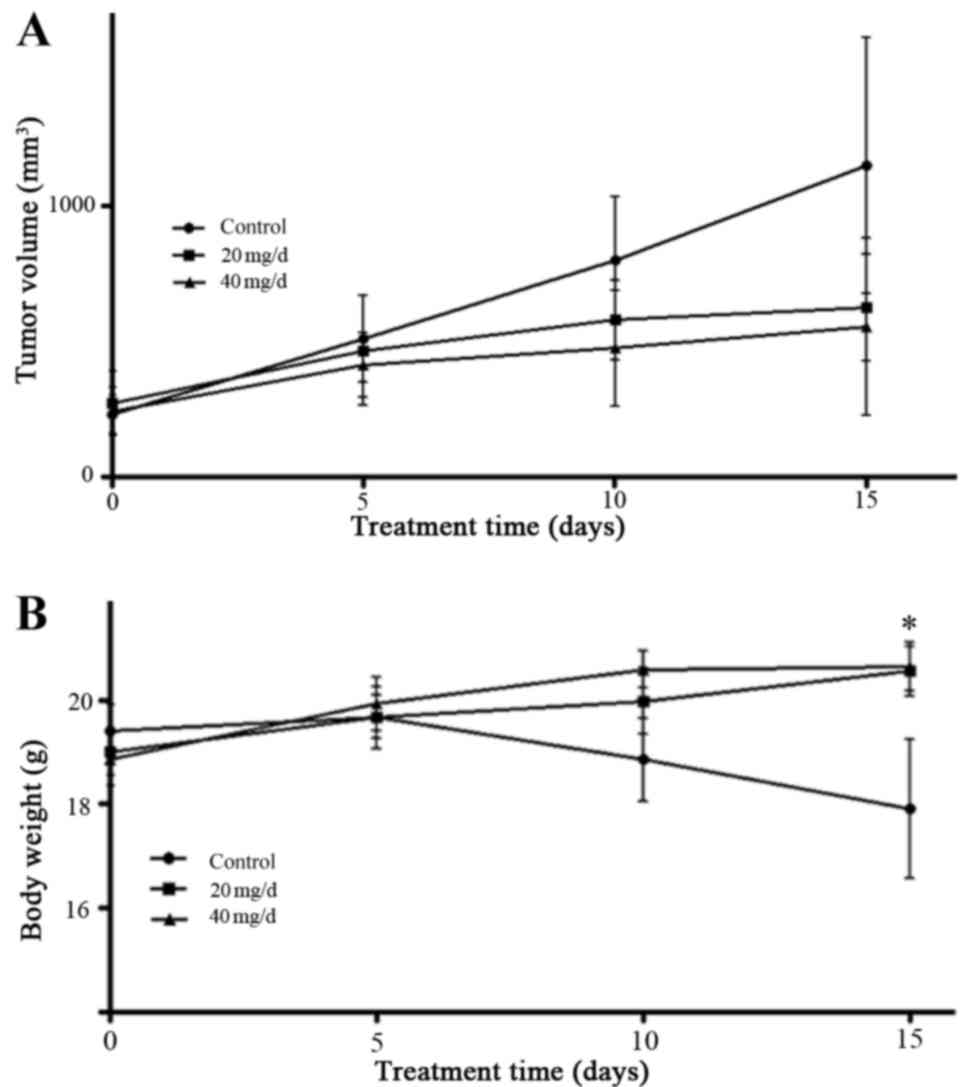
XHP inhibited the tumor growth in vivo
without body weight loss
The results presented so far indicate that XHP may
reduce cell viability in vitro. Therefore, the next aim of
the present study was to determine whether XHP inhibits the growth
of xenograft tumors in vivo. MDA-MB-231 cells were
subcutaneously inoculated into nude mice, which were subsequently
administered with a total dose of 20 and 40 mg/day XHP
administrated intragastrically twice every 12 h for 15 days when
the tumor volume had reached ~50–100 mm3. The body
weight and tumor volume were recorded every 5 days. The results
indicated that, despite the lack of statistical significance, 20
and 40 mg/day XHP inhibited the growth of xenograft tumors in nude
mice when compared with the controls (Fig. 7A). A 7.7% decrease in the weight of
mice in the control group was observed compared with the weight at
the commencement of treatment, whereas an increase in body weight
of 8.1 and 9.4% in mice treated with 20 and 40 mg/ml XHP,
respectively, was observed compared with the weight at the
commencement of treatment (Fig.
7B).
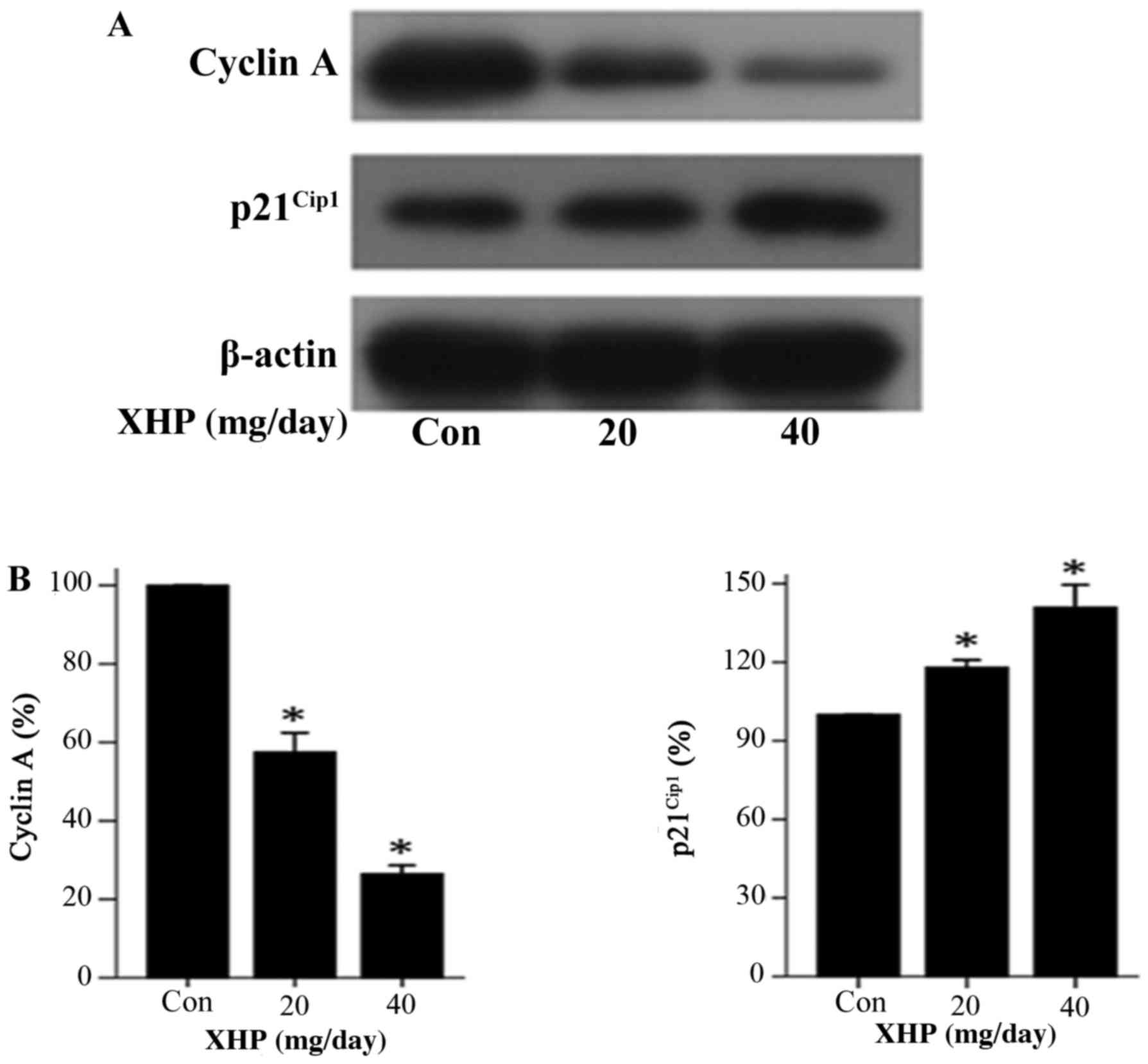
Expression of apoptosis-associated
proteins and cell cycle regulatory proteins in vivo
The results of in vitro analysis demonstrated
that XHP affected the expression of apoptosis-associated proteins
and cell cycle regulatory proteins (Figs. 4 and 6). Therefore, the authors of the present
study investigated whether XHP demonstrated the same effect on the
expression of these molecules in vivo. The results indicated
that, following treatment with 20 and 40 mg/day XHP, the expression
of cleaved caspase-3 was increased by 1.35- and 2.08-fold,
respectively, when compared with the control group (Fig. 8). By contrast, the expression
levels of cleaved caspase-8, Bcl-2, Bax and the Bcl-2/Bax ratio
were not significantly different among cells treated with different
concentrations of XHP (Fig. 8A and
B). The expression levels of cyclin A decreased by 0.58- and
0.26-fold following treatment with 20 and 40 mg/day XPA,
respectively when compared with the control (Fig. 9). The expression levels of
p21Cip1 increased by 1.18- and 1.41-fold following
treatment with 20 and 40 mg/day XHP, respectively, when compared
with the control (Fig. 9). These
results demonstrated that similar alterations in the expression of
apoptosis and cell cycle regulatory proteins were observed in
vivo and in vitro.
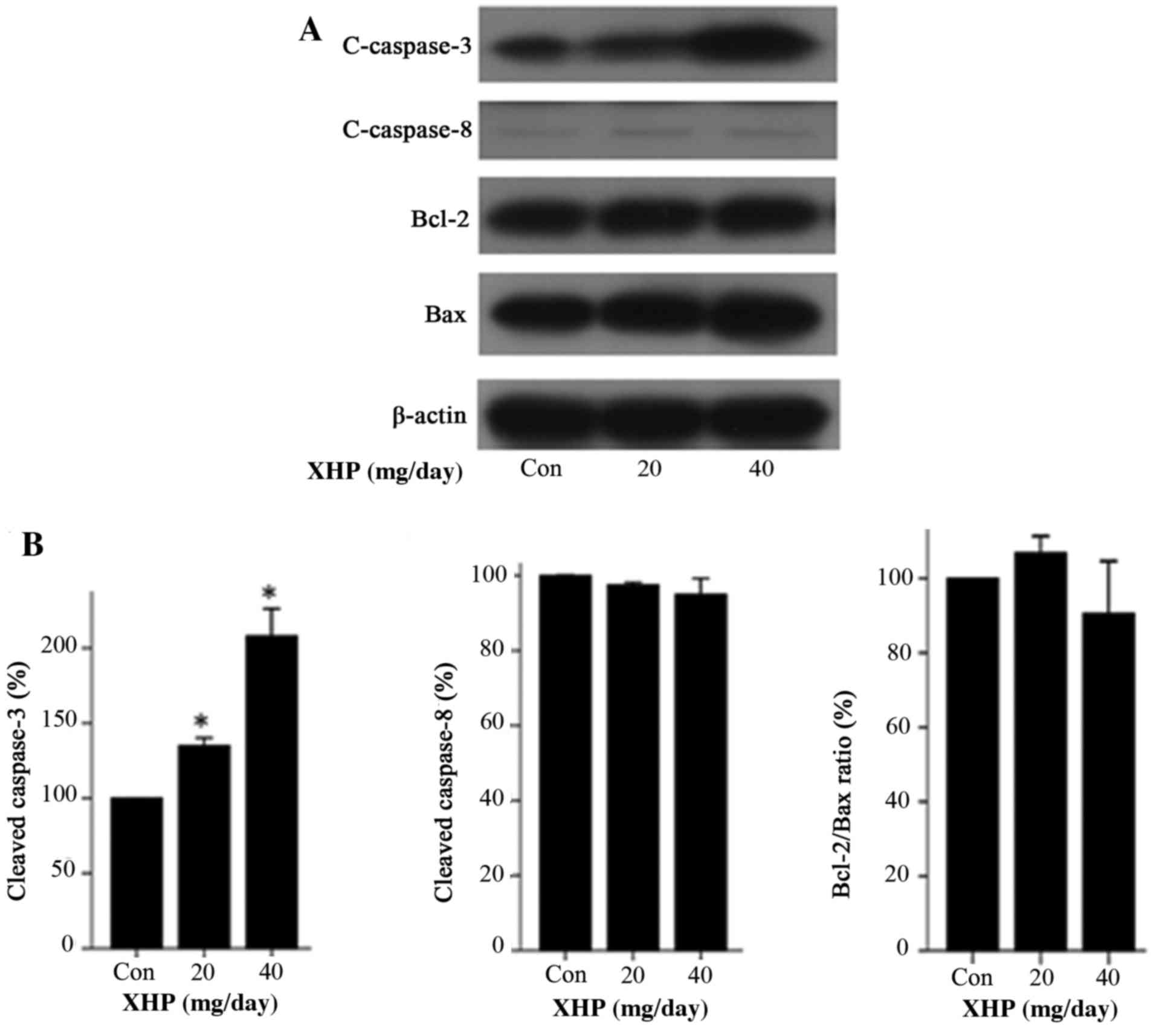 | Figure 8.Expression of apoptosis-associated
proteins in mouse MDA-MB-231 xenograft tumor tissues. (A) Nude mice
with tumor xenografts were treated with different concentrations of
XHP (20 and 40 mg/day) for 15 days, and the tumor tissues from mice
in each group were collected for western blot analysis of
c-caspase-3, c-caspase-8, Bcl-2 and Bax expression. The con group
was treated with distilled water only. (B) A histogram showing the
normalized band densities of c-caspase-3, c-caspase-8 and the
Bcl-2/Bax ratio, relative to the con group. Values represent the
mean ± standard deviation (n=3). *P<0.05 vs. control. XHP, Xi
Huang pills; con, control; c-caspase, cleaved caspase; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
Discussion
The aim of the present study was to investigate the
antitumor effects of XHP on MDA-MB-231 cells in vitro and
in vivo, as well as the potential underlying molecular
mechanisms involved. Therefore, MTT, apoptosis, cell cycle
distribution and western blot assays were conducted, and a
xenograft tumor model in nude mice was established.
The results of the MTT assay demonstrated that XHP
inhibited the viability of MDA-MB-231 cells in a dose- and
time-dependent manner. Based on the difference in MDA-MB-231 and
MCF-10A cell viability following XHP treatment, it is possible that
XHP inhibited the viability of MDA-MB-231 cells in a cell-selective
manner, which is a significant factor in cancer treatment.
In order to understand the mechanisms underlying the
antiproliferative effects of XHP in vitro, the level of
apoptosis and the cell cycle distribution of MDA-MB-231 cells
treated with different concentrations of XHP were determined. The
results demonstrated that XHP induced apoptosis and cell cycle
arrest at the G2/M phase in MDA-MB-231 cells, which was
consistent with the results of the MTT assay. A previous study
involving the Hs578T human TNBC cell line, demonstrated similar
effects of XHP in vitro, whereby XHP inhibited the viability
of Hs578T cells, induced apoptosis and cell cycle arrest in
S-phase, not the G2/M phase (19). Previous studies have demonstrated
the anti-tumor effect of XHP, in which XHP inhibited the
proliferation of human tumor cell lines SMMC7721 (liver cancer cell
line), T24 (bladder cancer cell line), A549 (lung cancer cell
line), LoVo (colorectal cancer cell line) and LAC (human lung
cancer stem cell line) in vitro (20–22),
and XHP could induce H22 cell (mouse liver cancer cell line) and
Bel-7402 cell (human liver cancer cell line) apoptosis by
downregulating Bcl-2 expression in tumor-bearing mice (23,24).
All these studies, including the present study, indicated that XHP
possessed anti-tumor activity in a wide range of cancer types. In
order to elucidate the mechanisms underlying the antiproliferative
effects of XHP, further studies have been performed, and beside the
apoptosis and cell cycle arrest noted in the present study, the
anti-tumor mechanisms elucidated included the suppression of the
invasion, migration and metastasis of tumor cells (21,25,26),
inhibition of angiogenesis (26,27)
and modulation of the tumor immune microenvironment (26,28–30).
However, there remains further studies to be performed to elucidate
the anti-tumor mechanisms of XHP treatment on MDA-MB-231.
In the present study, the protein expression levels
of caspase-8 and caspase-3 were detected by western blot analysis,
in order to elucidate the mechanism by which XHP induces apoptosis
in MDA-MB-231 cells in vitro. A significant increase in the
expression of cleaved caspase-3 was observed, whereas there were no
significant alterations in the expression of cleaved caspase-8.
Therefore, XHP may induce apoptosis via the intrinsic apoptotic
pathway and not the extrinsic pathway. In addition, it is possible
that the observed decrease in mitochondrial membrane potential
following XHP treatment may have induced the intrinsic pathway and
led to apoptosis.
In human cells, Bax is a constituent of the ion
channel in the mitochondrial membrane which causes the loss of
Δψm, and Bcl-2, an oncogene with many anti-apoptotic
functions, and which combines with Bax to prevent formation of the
ion channel (31). Thus, the
Bcl-2/Bax ratio serves an important role in the induction of
apoptosis. The results of western blot assay demonstrated there
were no alterations in the expression of Bax, Bcl-2 or the
Bcl-2/Bax ratio in MDA-MB-231 cells in vitro. Therefore, the
authors of the present study concluded that the depletion of
mitochondrial membrane potential induced by XHP was not associated
to alterations in the Bcl-2/Bax ratio, and deduced that it may
instead be associated with factors that damage the mitochondrial
membrane directly, or activate Bax or additional Bcl-2 family
members. This requires further investigation in future studies.
Cell cycle progression is orchestrated by a complex
network of interactions between proteins, among which are cyclins,
cyclin-dependent kinases (CDKs), E3 ubiquitin ligase complexes, CDK
activating kinase, cell division cycle 25 phosphatases and CDK
inhibitors (32). In order to
explore the mechanisms by which XHP induces cell cycle arrest at
the G2/M phase in MDA-MB-231 cells, a western blot assay
was used to determine whether XHP modulates the expression of cell
cycle regulatory proteins. The results indicated that XHP treatment
was associated with decreased expression of cyclin A and increased
expression of p21Cip1. The G2/M phase arrest
may be explained by the decreased expression of cyclin A, as cyclin
A is a regulatory protein specific to the late stage of the S phase
and the early stage of G2 phase (18). As p21Cip1 forms a
heterotrimeric complex with cyclin D-, cyclin E- and cyclin
A-dependent kinases, this leads to the inhibition of their
activities (33), and the increase
in the expression of p21Cip1 may therefore arrest the
cell cycle at all stages.
In order to investigate the antitumor effect of XHP
on MDA-MB-231 cells in vivo in the present study, a mouse
xenograft tumor model was established. The results indicated that,
despite the lack of statistical significance, treatment with 20 and
40 mg/day XHP inhibited the growth of xenograft tumors in nude mice
when compared with controls, which was in accordance with the in
vitro MTT assay results. In addition, weight loss was observed
in the untreated control group. By comparison, a significant
increase in the weight of mice treated with 40 mg/day XHP was
observed, which suggested that XHP may be safe and non-toxic. This
is consistent with the results of previous studies that have
examined the clinical use of XHP in cancer treatment (34,35).
The expression levels of apoptosis-associated and cell cycle
regulatory proteins in xenograft tumor tissues were analyzed by
western blotting in the present study. The results demonstrated
that the expression of these molecules was altered in a similar
manner in vitro and in vivo. Therefore, the observed
alterations in protein expression in vivo provides further
evidence of the molecular mechanisms of apoptosis and cell cycle
arrest induced by XHP in vitro. In addition, the authors
detected the expression levels of CDK1 and cyclin B in vitro
and in vivo (data not shown). The CDK1 and cyclin B
expression were increased when the XHP concentration increased
in vivo, while in the in vitro experiment the CDK1
expression was decreased and the cyclin B expression was unchanged
when the XHP concentration increased. The explanation for this
observation may be due to the complex composition of XHP which has
numerous of compounds to be determined in the future. All these
data, in vitro and in vivo demonstrated the complex
effects of XHP, which merits further study.
In conclusion, MDA-MB-231 cell viability was
significantly inhibited by XHP treatment in a dose-dependent,
time-dependent and cell-selective manner in vitro. The
potential underlying mechanisms may involve induction of apoptosis
and cell cycle arrest at the G2/M phase. XHP may induce
apoptosis of MDA-MB-231 cells via the intrinsic pathway, which is
not associated with alterations in the Bcl-2/Bax ratio. The
observed cell cycle arrest in G2/M phase may have been
due to the integrated action of decreased cyclin A expression and
increased p21Cip1 expression. In addition, XHP inhibited
the growth of xenograft tumors in nude mice without decreasing body
weight in vivo. Therefore, the results of the present study
indicated that XHP demonstrates antitumor effects on the MDA-MB-231
TNBC cell line, which provides evidence that XHP may be a useful
antitumor agent for the treatment of patients with TNBC.
Acknowledgements
The authors thank Dr Chen Duo for his kind help in
the data analysis. We thank Professor An Guo for the suggestion of
animal experiment and Professor Liu Xijuan (all from the Key
Laboratory of Carcinogenesis and Translational Research, Ministry
of Education, Central Laboratory, Peking University Cancer Hospital
and Institute, Beijing, China) for help in the conduct of flow
cytometry.
Funding
The present study was supported by the Key Program
Foundation of Beijing Administration of Traditional Chinese
Medicines (grant no. 2004-IV15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
WZ, MC, SJ, XH and XL performed cell culture, MTT
assay, apoptosis detection, cell cycle detection and western-blot
respectively. WZ performed the in vivo experiment and wrote
the manuscript. SH and PL designed the experiment and led the team.
HD performed the flow cytometry.
Ethics approval and consent to
participate
The animal experiment protocol was approved by
Peking University Animals Research Committee (Beijing, China) and
was conducted in accordance with the recommendations in the Guide
for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kolak A, Kamińska M, Sygit K, Budny A,
Surdyka D, Kukiełka-Budny B and Burdan F: Primary and secondary
prevention of breast cancer. Ann Agric Environ Med. 24:549–553.
2017.PubMed/NCBI
|
|
2
|
Liedtke C, Bernemann C, Kiesel L and Rody
A: Genomic profiling in triple-negative breast cancer. Breast Care
(Basel). 8:408–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosch A, Eroles P, Zaragoza R, Viña JR and
Lluch A: Triple-negative breast cancer: Molecular features,
pathogenesis, treatment and current lines of research. Cancer Treat
Rev. 36:206–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yadav BS, Sharma SC, Chanana P and Jhamb
S: Systemic treatment strategies for triple-negative breast cancer.
World J Clin Oncol. 5:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiao Q, Wu A, Shao G, Peng H, Wang M, Ji
S, Liu P and Zhang J: The latest progress in research on triple
negative breast cancer (TNBC): Risk factors, possible therapeutic
targets and prognostic markers. J Thorac Dis. 6:1329–1335.
2014.PubMed/NCBI
|
|
6
|
Meng Z, Garrett CR, Shen Y, Liu L, Yang P,
Huo Y, Zhao Q, Spelman AR, Ng CS, Chang DZ and Cohen L: Prospective
randomised evaluation of traditional Chinese medicine combined with
chemotherapy: A randomised phase II study of wild toad extract plus
gemcitabine in patients with advanced pancreatic adenocarcinomas.
Br J Cancer. 107:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YC: A Concise Dictionary of Traditional
Chinese Medicine. People's Medical Publishing Press; Beijing: pp.
9001979
|
|
8
|
Jin SR, Zhu BD and Qin XH: Comparative
study of anti-tumors effects of Xi huang pellet by different
processing methods. Shi Zhen Guo Yi Guo Yao. 19:1735–1737.
2008.
|
|
9
|
Pan G, Wang W, Wang L, Zhang F, Yin X,
Wang J and Liang R: Anti-breast cancer effects and mechanisms of
Xihuang pill on human breast cancer cell lines. J Tradit Chin Med.
33:770–778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You S, Li W and Guan Y: Tunicamycin
inhibits colon carcinoma growth and aggressiveness via modulation
of the ERK-JNK-mediated AKT/mTOR signaling pathway. Mol Med Rep.
17:4203–4212. 2018.PubMed/NCBI
|
|
11
|
Wang W, Li N, Luo M, Zu Y and Efferth T:
Antibacterial activity and anticancer activity of Rosmarinus
officinalis L. essential oil compared to that of its main
components. Molecules. 17:2704–2713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strasser A, Huang DC and Vaux DL: The role
of the bcl-2/ced-9 gene family in cancer and general implications
of defects in cell death control for tumourigenesis and resistance
to chemotherapy. Biochim Biophys Acta. 1333:F151–F178.
1997.PubMed/NCBI
|
|
16
|
Gao N, Budhraja A, Cheng S, Yao H, Zhang Z
and Shi X: Induction of apoptosis in human leukemia cells by grape
seed extract occurs via activation of c-Jun NH2-terminal kinase.
Clin Cancer Res. 15:140–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shapiro GI and Harper JW: Anticancer drug
targets: Cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi EJ, Oh HM, Wee H, Choi CS, Choi SC,
Kim KH, Han WC, Oh TY, Kim SH and Jun CD: Eupatilin exhibits a
novel anti-tumor activity through the induction of cell cycle
arrest and differentiation of gastric carcinoma AGS cells.
Differentiation. 77:412–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng W, Han S, Jiang S, Pang L, Li X, Liu
X, Cao M and Li P: Multiple effects of Xihuang pill aqueous extract
on the Hs578T triple-negative breast cancer cell line. Biomed Rep.
5:559–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen JR, Zhu BD, Qin XH and Zhang XS:
Antitumous effects of Xi Huang Pellet on diverse human malignant
tumor cell strains (MDA-MB-231, SMMC7721, T24, HL-60, A549). J
Sichuan Traditional Chin Med. 24:10–13. 2006.
|
|
21
|
Wang M, Meng JY and He SF: Xihuang Pill ()
induces mesenchymal-epithelial transition and inhibits loss of
apicalbasal polarity in colorectal cancer cell through regulating
ZEB1-SCRIB loop. Chin J Integr Med. 20:751–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao H, Qin XH, Lai Y, Shen JR and Lai L:
Containing Xihuang Pill drug serum regulates growth of lung cancer
stem cells by controlling cyclin D1 of Wnt signaling pathway. Chin
J Exp Trad Med Form. 20:172–176. 2014.
|
|
23
|
Xu H, Cui LR and Liu JC: Study on the
effects of Xihuang Pill on the expression of Bcl-2 mRNA of mice
bearing H22. Modern Preventive Med. 38:2120–2121. 2011.
|
|
24
|
Li LF, Chen RS, Liu XM, Jin QW and Deng
XG: Mechanism of Xihuang Pill on inducing liver cancer cell
apoptosis. Chin Arch Trad Chin Med. 22:125–126. 2004.
|
|
25
|
Li-na SUN, Jing-yan MENG, Wei WANG,
Sen-lin YING, Dan LI, Zuo-ying MA and Yan-tao JIA: Effect of
Xihuang Pills on protein expressions of MMP-2 and MMP-9 in human
colorectal carcinoma LoVo cell. Tianjin J Traditional Chin Med.
29:378–380. 2012.
|
|
26
|
Wang YY, Ren YZ, Jiao Z, Zeng CQ, Gao WB
and Liang WB: The influence of Xihuang Pill on the formation of con
tumor-bearing mice. Pharmacol Clin Chin Materia Med. 30:11–13.
2014.
|
|
27
|
Si-feng WANG, Ke-chun LIU, Xi-min WANG,
Qiu-xia HE, Xue WANG, Xi-qiang CHEN and Yan-qiang YUAN: Effect of
Xihuang Pill on angiogenesis in Zebrafish embryo. Chin J Hospital
Pharm. 30:821–823. 2010.
|
|
28
|
Ma Jie, Guan Shuo, Yang Wei, Hu Junxia,
Zeng Changqian, Gao Wenbin and Liang Wengbo: Experimental study on
the effect of Xihuang Pill ethanol extract on immune function of
tumorbearing rats. Pharmacol Clinics of Chin Materia Medica.
29:124–126. 2013.
|
|
29
|
Guan S, Yang W, Hu JX, Ma J, Gao WB and
Liang WB: Effect of chloroform extract of Xihuang Pill on the
immune clearance function of tumor-bearing rats. Chin J Modern
Applied Pharm. 31:144–148. 2014.
|
|
30
|
Yang Wei, Guan Shuo, Hu Junxia, Zeng
Changqian, Liang Wenbo, Ma Jie and Gao Wenbin: Experimental study
on antitumor effect of volatile oil of Xihuang Pill and its immune
mechanism. World Sci Technol-Modern of Trad Chin Med. 16:68–72.
2014.
|
|
31
|
Rebecca SY and Wong: Apoptosis in cancer:
From pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diaz-Moralli S, Tarrado-Castellarnau M,
Miranda A and Cascante M: Targeting cell cycle regulation in cancer
therapy. Pharmacol Ther. 138:255–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo Q, Lin J, Liu R, Gao Y, He S, Xu X,
Hua B, Li C, Hou W, Zheng H and Bao Y: Review on the applications
and molecular mechanisms of xihuang pill in tumor treatment. Evid
Based Complement Alternat Med. 2015:8543072015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu D and An GY: Clinical effects of
xihuang pill combined with chemotherapy in patients with advanced
colorectal cancer. Evid Based Complement Alternat Med.
2017:59360862017. View Article : Google Scholar : PubMed/NCBI
|