Introduction
Preeclampsia (PE) is a pregnancy-specific clinical
disorder that affects approximately 3–8% of all pregnant women
(1). PE is defined by new-onset
hypertension and proteinuria after 20 weeks of gestation and is a
leading cause of maternal and perinatal mortality (2). A successful pregnancy depends on
well-regulated trophoblast invasion into the uterine decidua and
moderate uterine spiral artery remodeling. To date, the
pathophysiology of PE has not been fully elucidated.
Serum retinol-binding protein 4 (RBP4) is a binding
partner of retinol, which is delivered into the circulation by the
carrier protein transthyretin (TTR) (3); the formation of this complex
increases the molecular weight of RBP4, thus preventing its
glomerular filtration and subsequent catabolism in the kidneys.
Under physiological conditions, the liver is the major source of
RBP4, producing approximately 80% of this protein. However, RBP4
can also be produced in adipose tissues (4). Chen et al (5) showed that RBP4 is not only a carrier
of retinol but also acts as a circulating cytokine. Our previous
study using surface-enhanced laser desorption ionization
time-of-flight mass spectrometry (SELDI-TOF-MS) revealed that RBP4
is downregulated in PE (6). Serum
samples were analyzed using a peptide ligand library conjugated to
beads and liquid chromatography-mass spectrometry/mass
spectrometry; RBP4 concentrations were found to be significantly
lower in women with severe PE than in women with a healthy
pregnancy. Immunohistochemistry (IHC) demonstrated significantly
lower RBP4 expression (brown) in preeclamptic placental tissues
than in normal placental tissues (7).
During placental development, trophoblasts with
reduced invasive ability fail to deeply invade the myometrium and
to appropriately remodel the uterine spiral arteries, resulting in
a shallow placental bed and ultimately leading to PE. In our study,
we hypothesized that RBP4 participates in the regulation of
trophoblastic cell invasion and migration. The aim of the present
study was to investigate the effect of RBP4 on the biological
behavior of trophoblasts and to explore the potential signaling
pathways involved in this process.
Materials and methods
Patients and clinical samples
The study protocol was approved by the Ethics
Committee of Beijing Chao-Yang Hospital (Beijing, China). All women
enrolled in the present study were Chinese patients at the
Department of Obstetrics and Gynecology in Chao-Yang Hospital,
Capital Medical University in Beijing, China, and all patients
provided written informed consent before inclusion. Thirty-five
patients with PE and thirty healthy pregnant women were recruited
for enzyme-linked immunosorbent assay (ELISA) analysis. PE was
defined as the onset of hypertension (systolic blood pressure ≥140
mmHg and/or diastolic blood pressure ≥90 mmHg on at least two
occasions, 4 h to 1 week apart) after 20 weeks of gestation with
proteinuria (≥300 mg in 24-h urine collection or at least one
dipstick measurement ≥2+). The control patients were pregnant women
who underwent cesarean section because of malposition and premature
rupture of membranes. None of the participants had any history of
hypertension, diabetes, cardiovascular disease, kidney disease,
hyperthyroidism, smoking, alcoholism, chemical dependency,
intrauterine fetal death, fetal congenital or chromosomal
abnormalities or pregnancies conceived by in vitro
fertilization. Blood was drawn via venipuncture and collected in a
serum-separator tube. Serum was separated by centrifugation at
1,300 × g and 4°C for 10 min within 2 h of collection and was
stored at −80°C until analysis.
ELISA
ELISAs were conducted according to the
manufacturer's instructions (Cloud-Clone Corp., Katy, TX, USA). In
brief, 100 µl of diluted standards was added to each well
containing the quality control and the samples, and the plate was
incubated on an orbital microplate shaker at room temperature for 1
h. After the wells were washed three times, 100 µl of conjugate
solution was added, and the plate was then incubated for 1 h at
room temperature while shaking at 300 rpm. The plate was washed
three times with wash buffer, 100 µl of the substrate solution was
added to each well, and the plate was incubated for approximately
10 min to enable the reaction to develop. Absorbance at 450 nm was
measured using an ELISA plate reader.
IHC
RBP4 expression in placenta tissue was assessed
using PV-9000 (standard polymer detection system) for
immunohistological staining. IHC was performed to detect RBP4
expression and localization in the placenta. Tissue samples were
fixed with sodium phosphate buffer containing 10% formalin,
embedded in paraffin and sliced into 5-µm continuous sections. The
sections were deparaffinized, rehydrated, and incubated with 3%
H2O2 in methanol for 30 min to quench
endogenous peroxidase activity. After a short rinse, the sections
were heated in a 37°C water bath for 15–20 min in citrate buffer.
Following cooling and rinsing, the sections were incubated with a
rabbit polyclonal anti-RBP4 antibody (1:50) for 30 min at room
temperature, overnight at 4°C, and for 30 min at room temperature
while shaking. Antibody binding was amplified for 10 min using
biotin and horseradish peroxidase-conjugated streptavidin. The
slides were counterstained with hematoxylin, dehydrated in gradient
ethanol, cleared in dimethylbenzene, and observed under a
microscope. Normal controls for IHC were prepared under the same
conditions, 5 placental specimens for each group. The immunostained
sections were examined using a Leica DMLA light microscope (Leica
Microsystems, Wetzlar, Germany) to assess the prevalence of
DAB-positive staining and immunostaining localization within the
tissues. All sections were assessed on a microscope for positive
DAB staining. Trophoblast cells with unequivocal staining of
granular cytoplasm were considered positive. RBP4 expression was
evaluated via a computer assessment and scored based on the
cytoplasmic staining intensity throughout the entire section.
Immunohistochemical staining pictures were taken with the same
exposure time by microscope (MOTIC BA400) coupled with camera
device (MOTICCAM 2306) in the same microscope environment and
conditions. The expressions of target proteins were quantified and
the average optical density (AOD) was analyzed by Motic Image
Advanced v.3.2 software (MOTIC, Xiamen, China).
Cell culture
The human immortalized trophoblast cell line
HTR8/SVneo was obtained from ATCC. The cells were transiently
transfected to overexpress or knockout RBP4, and unaltered
HTR8/SVneo cells served as the control. The cells were cultured in
RPMI 1640 medium (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin,
and 100 mg/ml streptomycin in a 37°C humidified incubator (5%
CO2). The cells were subcultured at a 1:3 ratio when
they reached 80–90% confluence (8).
Lentivirus infection
cDNA containing the human RBP4 sequence was cloned
into a pLVX-mCMV-ZsGreen-PGK-Puro vector using EcoRI and BamHI
digestion. A sequencing analysis was conducted to confirm correct
insertion of the DNA fragment. 293T cells (Xi Bei Hong Cheng
Biological Technology, Co., Beijing, China) were used to generate
packaging lentiviruses for transduction. HTR cells were infected
with the pLVX-RBP4-mCMV-ZsGreen-PGK-Puro construct. Finally, stable
cell lines were screened.
We used pLVX-shRNA2-Puro lentiviral vectors carrying
short hairpin RNAs (shRNAs). The shRNA vectors were obtained from
Health and Biological Company, and the lentiviruses were obtained
via overnight triple co-transfection of 293T cells. Knockdown
efficiency was determined by reverse transcription-polymerase chain
reaction (RT-PCR). We found that the best interference effect was
achieved using shRNA2-RBP4-2. HTR cells were infected with the
pLVX-shRNA2-RBP4-Puro construct, followed by puromycin selection to
establish stable cell lines.
Western blotting
Total cell protein was extracted from cells in each
group. After centrifugation at 12,000 rpm and 4°C for 15 min, the
protein concentration in the supernatant was determined with a BCA
protein assay. Equal amounts of total protein (30 µg) were loaded
and separated on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose
(NC) membranes using standard procedures.
The primary antibodies rabbit polyclonal anti-MMP2
(1:1,000; Abcam, Cambridge, UK), rabbit polyclonal anti-MMP9
(1:1,000; Abcam), rabbit polyclonal anti-AKT (1:1,000; Abcam),
rabbit polyclonal anti-p-AKT (Thr308, Ser473) (1:1,000; Abcam),
mouse polyclonal anti-PI3K (1:1,000; Abcam), rabbit polyclonal
anti-p-PI3K (1:1,000; Abcam), rabbit polyclonal anti-Bcl-2
(1:2,000; Abcam), rabbit polyclonal anti-GAPDH (1:2,000; Abcam),
and rabbit polyclonal anti-RBP4 were used. GAPDH served as the
internal loading control.
The membranes were probed with primary antibodies
diluted in 5% SBS/PBS-Tween 20 (PBST-20) overnight at 4°C, rinsed
three times with PBST-20 and then incubated with secondary
antibodies (1:3,000) for 2 h at room temperature. Excess secondary
antibody was removed by four washes in PBST-20. The targeted
protein bands were visualized and imaged using an ECL western
blotting kit (CWBiotech, Beijing, China), and densitometry was
performed using ImageJ (v.1.49e; National Institutes of Health,
Bethesda, MD, USA).
In vitro invasion assay with
Matrigel
Cell invasion was assessed using transwell chambers
(24-well inserts; 8-µm pore size) precoated with Matrigel (200
µg/ml; BD Biosciences, Franklin Lakes, NJ, USA). Briefly, 24 h
after transfection, HTR8-SVneo cells (RBP4 knockdown, RBP4
overexpression, and control) were trypsinized and seeded into the
upper chambers (5×105 cells/chamber) in serum-free
medium, and the lower chambers were filled with 600 µl of RPMI 1640
medium containing 10% FBS as a chemoattractant. After incubation of
the plates for 48 h, the cells on the upper surface of the membrane
were gently removed, and the invasive cells on the lower surface of
the membrane were fixed in methanol and stained with crystal
violet. The invasive cells in four randomly selected fields of view
were imaged using a light microscope and counted.
Cell treatment
RBP4 knockdown, RBP4 overexpression and control
HTR8/SVneo cells (5,000/well) were seeded into 96-well plates. A
CCK8 Assay Kit was used to assess cell survival at different time
points (0, 24, 48, 72, 96 and 144 h). Optical density (OD) was
measured at 450 nm. These experiments were performed three
times.
Statistical analysis
Statistical analysis was performed using SPSS v.20.0
software (IBM Corp., Armonk, IL, USA). The data were tested for
normality using unpaired t-tests, one-way analysis of variance with
a least significant difference post hoc test and nonparametric
tests (median test). Normally distributed data are presented as the
mean ± standard deviation, and non-normally distributed data, as
the median (interquartile range). P<0.05 was considered to
indicate a statistically significant difference.
Results
Subject characteristics
Blood samples from thirty-five patients with PE and
thirty healthy pregnant women were analyzed via ELISA. The patient
characteristics are listed in Table
I.
 | Table I.Subject characteristics for ELISA. |
Table I.
Subject characteristics for ELISA.
| Characteristics | Controls (n=30) | PE (n=35) | P-value |
|---|
| Age (years) | 33.3±2.4 | 33±3.6 | 0.328 |
| Gestational age
(weeks) | 37±6.5 | 34.7±7.4 | 0.066 |
| Blood pressure
(mmHg) |
|
|
|
| SBP | 119.2±15.3 | 166.4±13.2 | <0.0001 |
| DBP | 75.5±9.3 | 96.8±12.6 | <0.0001 |
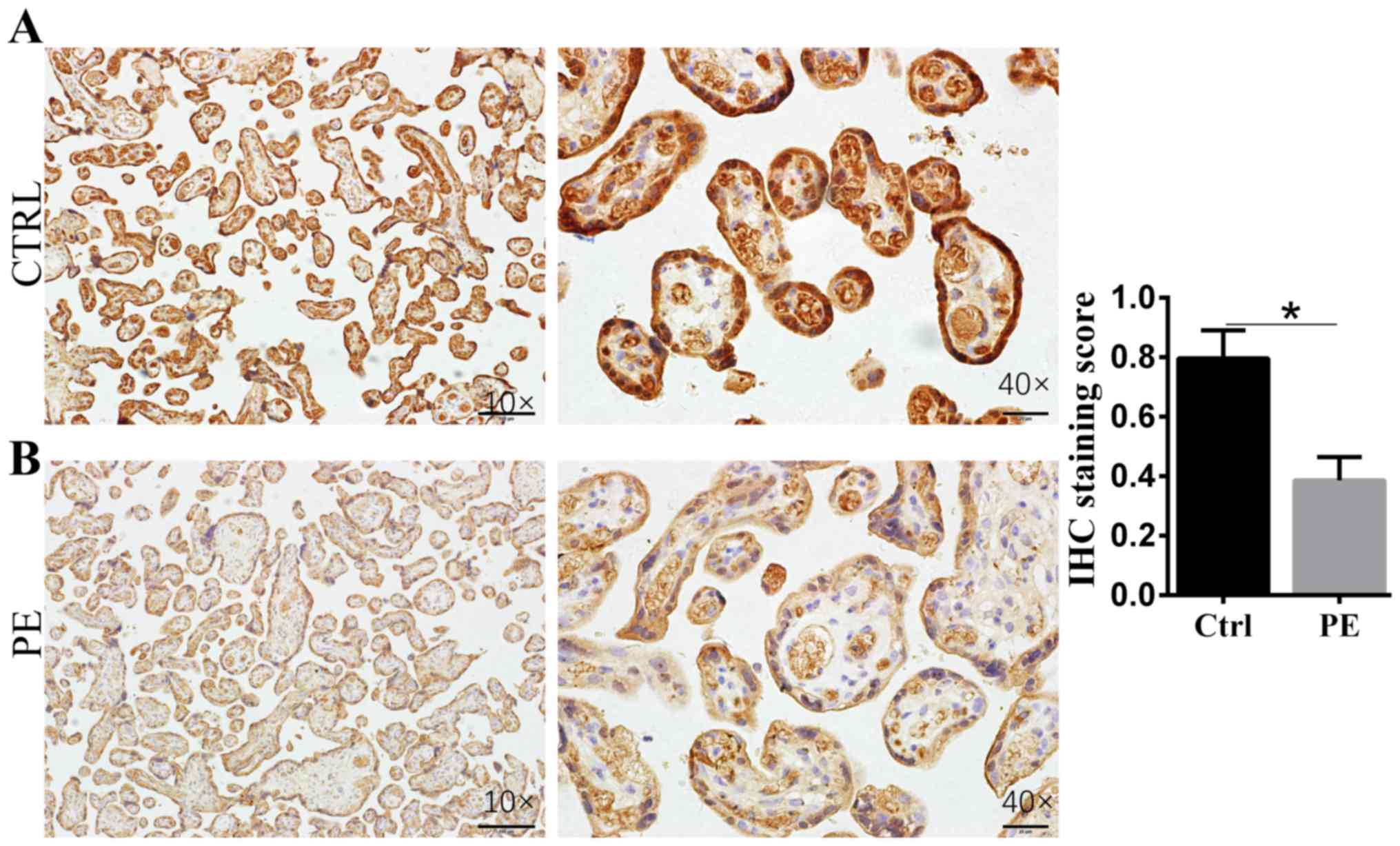
IHC to assess RBP4 expression in the
placenta
IHC analysis showed that RBP4 localized to
syncytiotrophoblasts (STBs). RBP4 expression in PE placenta tissue
(n=5) was significantly decreased compared with that in normal
controls (n=5; Fig. 1). Normal
placenta tissue stained for RPB4 at 0.79±0.05; PE placenta tissue
stained for RPB4 at 0.39±0.02.
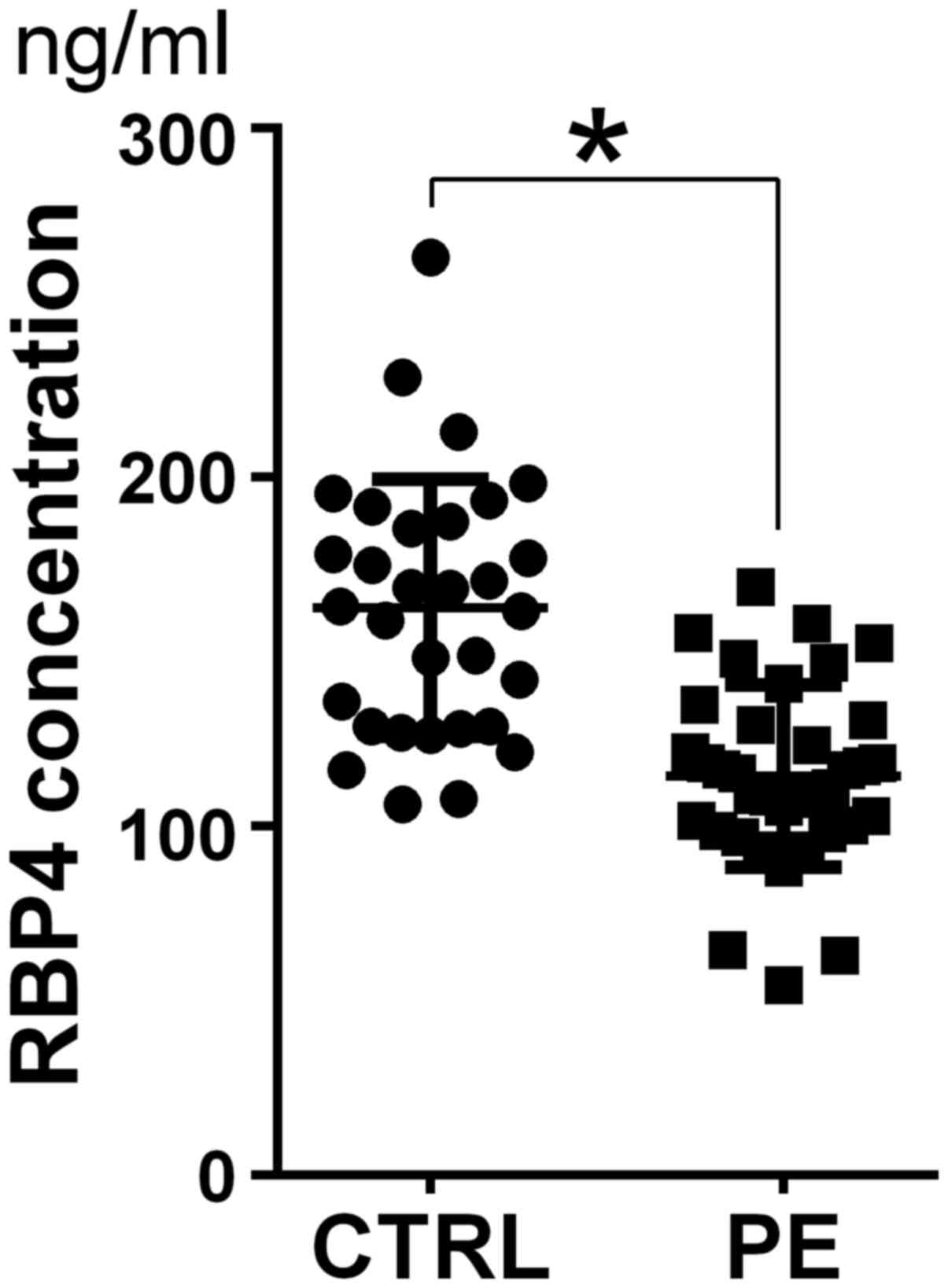
ELISA
Serum RBP4 expression was compared between patients
with PE and healthy pregnant women using ELISA (PE median, 129.3
ng/ml vs. control median, 166.7 ng/ml; P=0.046; standard curve line
y=0.0176× + 0.1724 (R2=0.9527); Fig. 2).
RBP4 regulates HTR8/SVneo cell
proliferation in vitro
We investigated whether RBP4 affects the
proliferation of trophoblasts. We found that overexpression of RBP4
increased cell proliferation after 120 and 144 h, whereas
siRNA-mediated knockdown of RBP4 significantly decreased HTR8/SVneo
cell proliferation at 24, 48 and 72 h (Fig. 3). Two bands for RBP4 should be
present in the western blot of the RBP4 overexpression group.
However, RBP4 was cloned into the vector without including vsgreen
in the frame, resulting in overexpression of RBP4 without any tags.
Therefore, only one band was observed when blotting with the
anti-RBP4 antibody. Western blot analysis of RBP4 to confirm
overexpression and knock down was successful.
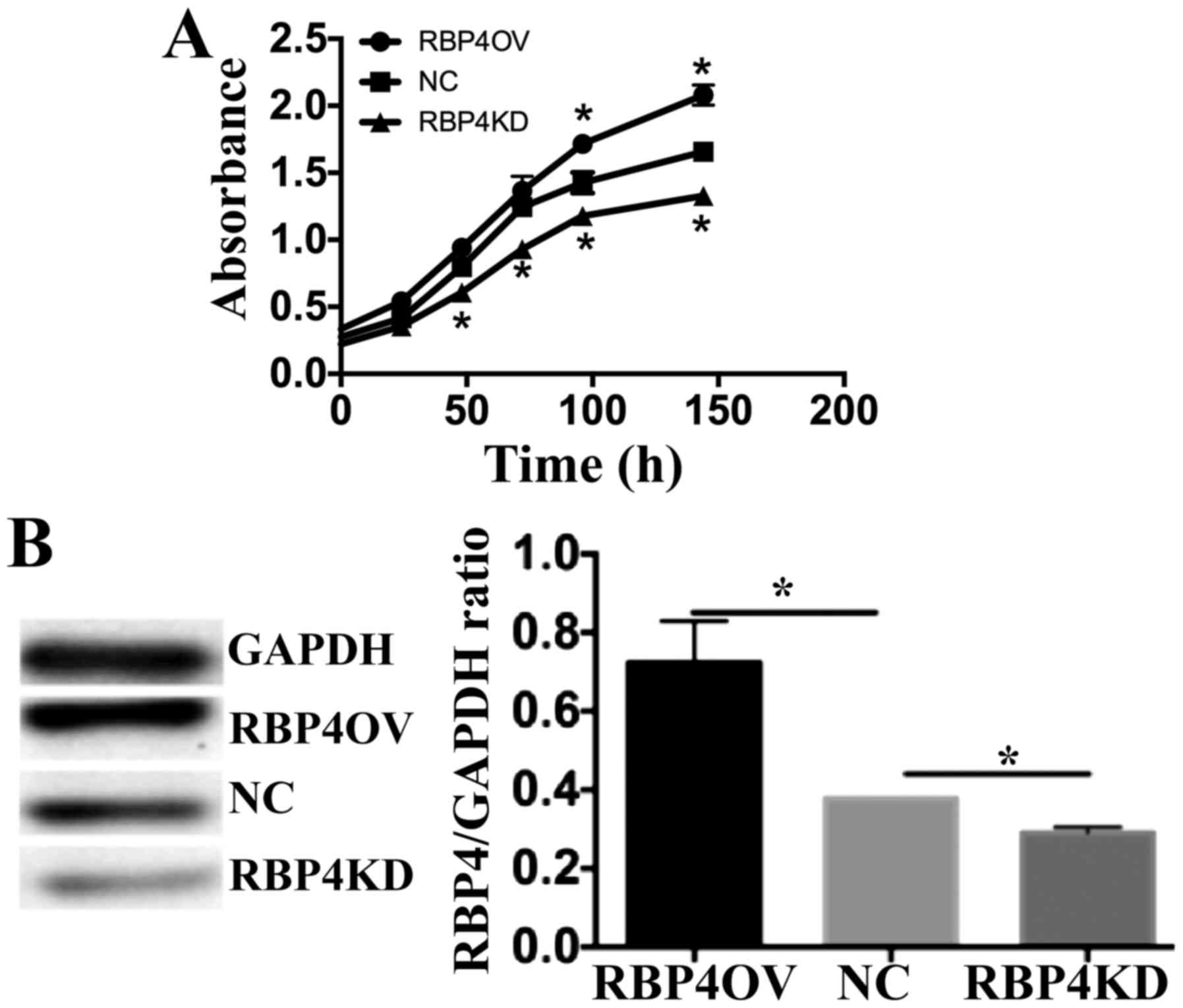 | Figure 3.RBP4 regulates HTR8/SVneo cell
proliferation in vitro. (A) RBP4 overexpression increased
cell proliferation, and RBP4 knockdown inhibited HTR8/SVneo cell
proliferation. The proliferation of control cells,
RBP4-overexpressing cells and RBP4 knockdown cells at 0, 24, 48,
72, 120 and 144 h was determined by Cell Counting Kit-8 assays. The
absorbance of viable cells was determined at 450 nm (optical
density 450). Proliferation of RBP4-overexpressing cells was
significantly higher than that of the control cells at 96 and 144
h. Proliferation of RBP4-knockdown cells was significantly lower
than that of the control cells at 48, 72, 96 and 144 h. *P<0.05
vs. NC. (B) Western blot analysis of RBP4 protein expression to
confirm that overexpression and knockdown were successful.
*P<0.05, as indicated. All quantitative data are presented as
the mean ± standard deviation of three independent experiments.
RBP4, Retinol-binding protein 4; NC, normal control; RBP4OV, RBP4
overexpression group; RBP4KD, RBP4 knockdown group. |
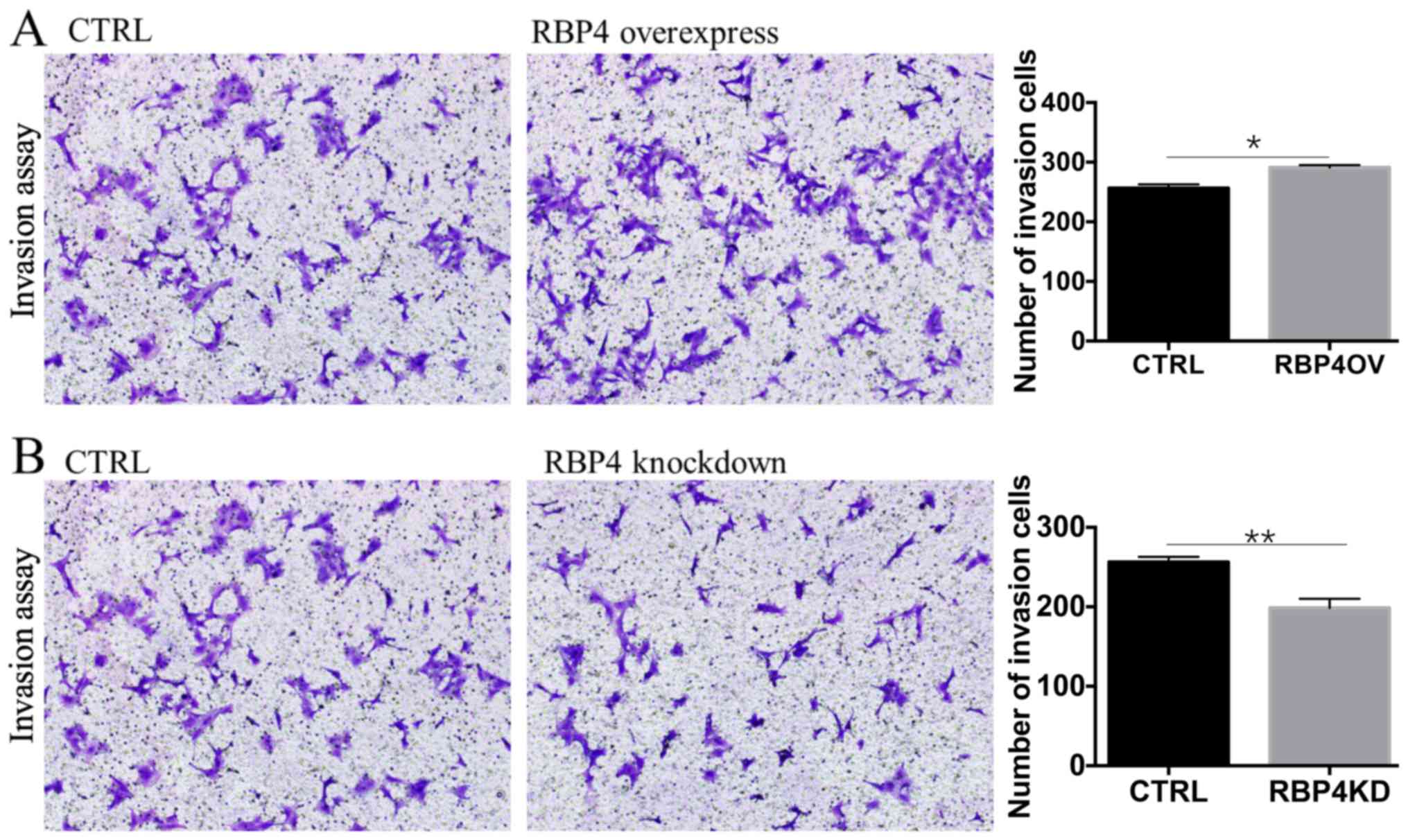
In vitro invasion assay
Cell invasion assays were employed to investigate
the effect of RBP4 expression on HTR8/SVneo cell invasion (Fig. 4). RBP4 overexpression promoted the
invasion of HTR8/SVneo cells (A group), and RBP4 knockdown
decreased the invasion of HTR8/SVneo cells (B group).
Effect of RBP4 on MMP2, MMP9 and Bcl-2
expression
MMP2, MMP9, and Bcl-2 are associated with invasion
and apoptosis. Therefore, we measured the protein levels of MMP2,
MMP9 and Bcl-2 via western blotting. Overexpression of RBP4
significantly increased MMP2, MMP9 and Bcl-2 expression in
HTR8/SVneo cells (B group), and knockdown of RBP4 decreased MMP2,
MMP9 and Bcl-2 expression in HTR8/SVneo cells (A group). Each
experiment was repeated three times (Fig. 5).
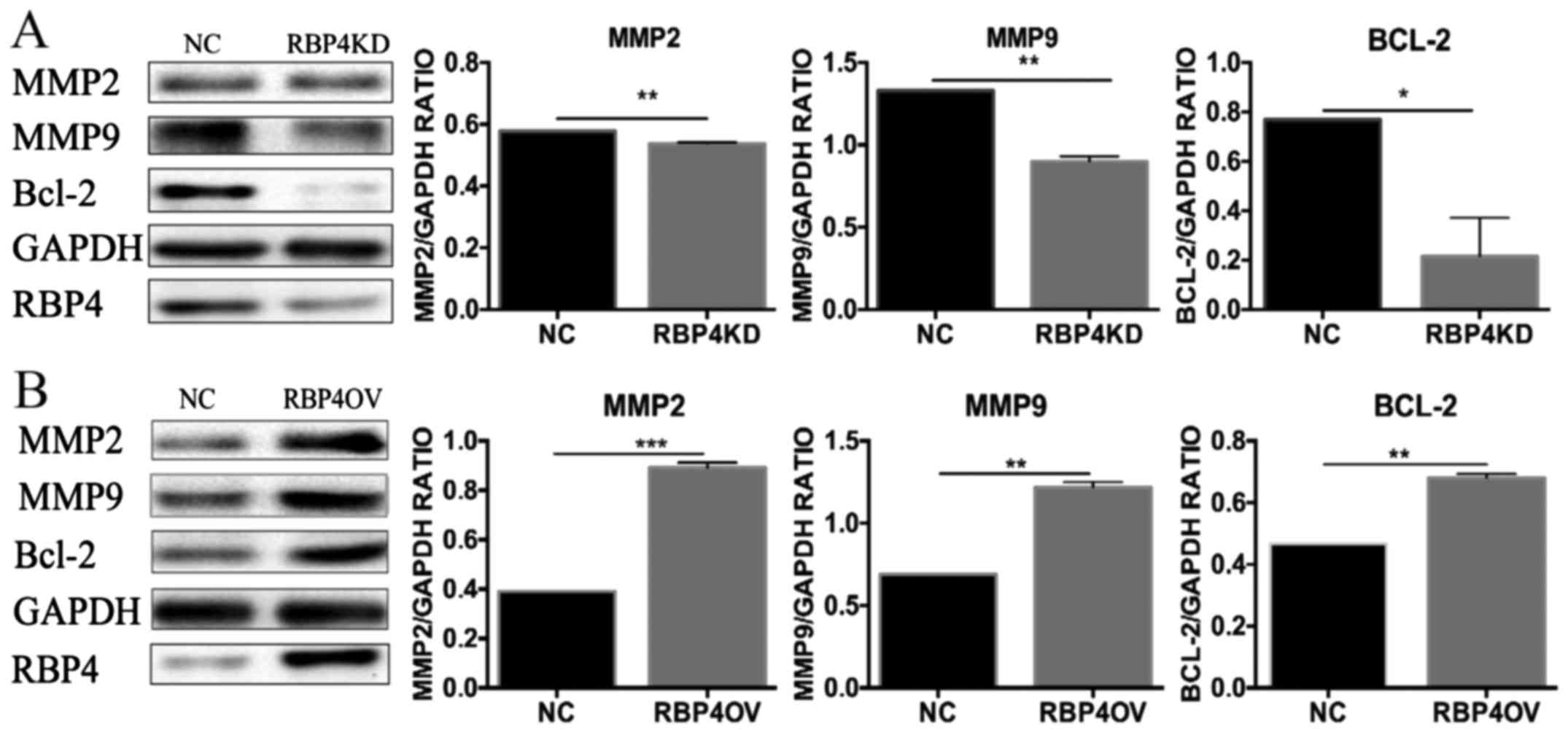 | Figure 5.Effect of RBP4 on MMP2, MMP9 and Bcl-2
expression. Effects of RBP4 knockdown and overexpression on the
expression of proteins associated with invasion. (A) MMP2, MMP9 and
Bcl-2 protein expression was downregulated in the RBP4KD group when
compared with the NC group. (B) MMP2, MMP9 and Bcl-2 expression was
upregulated in the RBP4OV group when compared with the NC group.
*P<0.05, **P<0.01 and ***P<0.001, as indicated. RBP4,
Retinol-binding protein 4; NC, normal control; RBP4OV, RBP4
overexpression group; RBP4KD, RBP4 knockdown group; MMP, matrix
metalloproteinase; Bcl-2, B-cell lymphoma 2. |
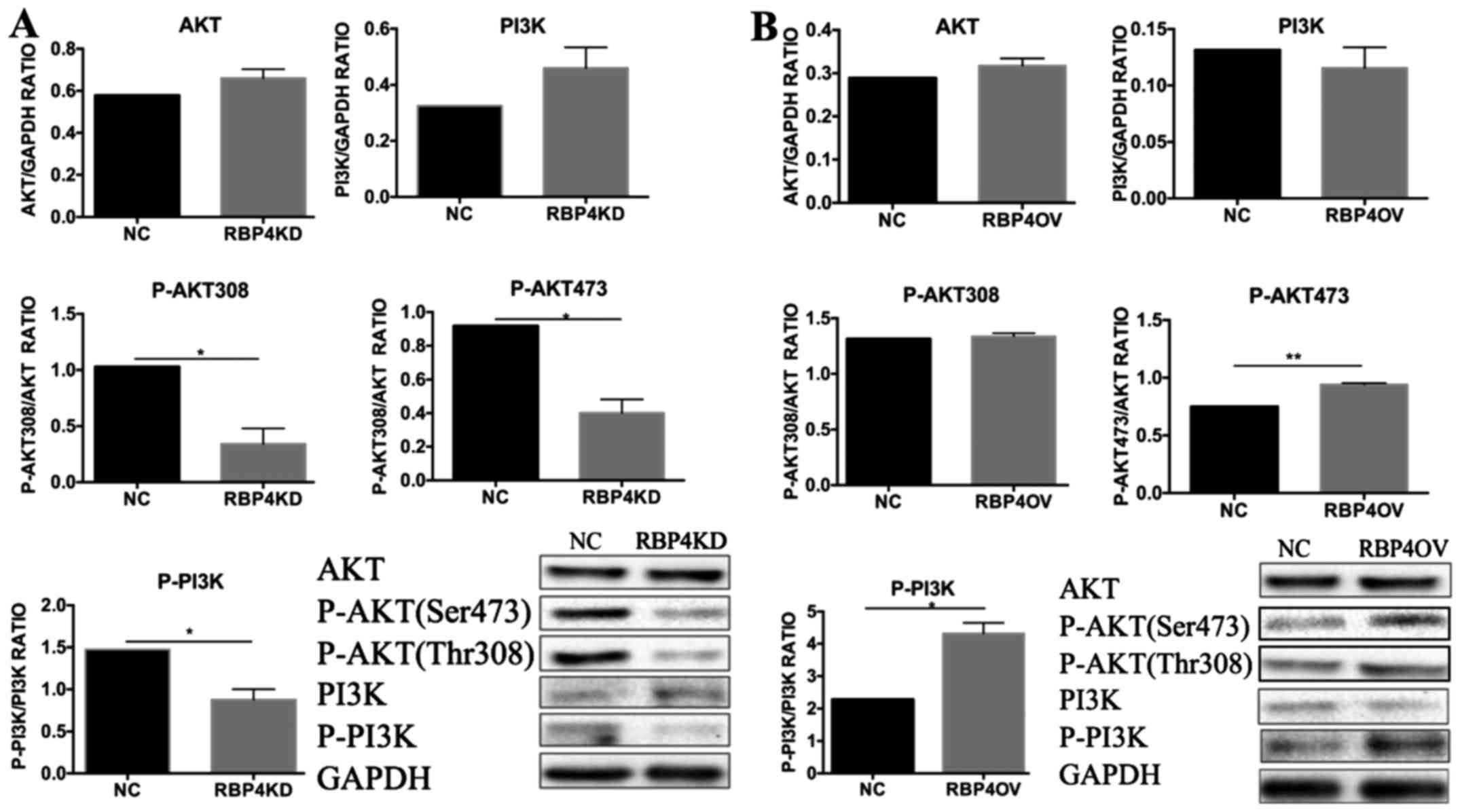
Effect of RBP4 on the PI3K/AKT
signaling pathway in HTR8/SVneo cells
To elucidate the mechanisms by which RBP4 promotes
trophoblast invasion, we evaluated PI3K/AKT signaling in
trophoblast cells. We examined PI3K, AKT, p-PI3K and p-AKT
expression via western blotting. Each experiment was repeated three
times (Fig. 6). p-AKT (Ser473) and
p-PI3K were significantly upregulated in the RBP4 overexpression
group compared with the normal control group, and there were no
differences in AKT and PI3K levels between the groups (B group).
p-AKT (Ser473), p-AKT (Thr308) and p-PI3K were significantly
downregulated in the RBP4 knockdown group compared with the normal
control group (A group).
Discussion
PE is generally defined as new-onset hypertension
accompanied by proteinuria or complications in multiple organs at
or after the 20th week of gestation (9,10).
Although extensive studies have demonstrated that immunologic,
antigenic, genetic, metabolic, and environmental factors are
associated with PE (11), the
pathophysiology of PE has not yet been fully elucidated. Moreover,
there are no reliable biomarkers for predicting PE during early
pregnancy.
Maintenance of a normal pregnancy depends on
placental development and function. Moderate invasion of human
extravillous trophoblast cells into the maternal decidual stroma
and spiral arteries is essential for establishing a successful
pregnancy. Similar to tumor cells, trophoblasts have invasive
ability; however, unlike tumor invasion, trophoblast invasion is a
strictly controlled physiological event. Abnormal trophoblast
differentiation is associated with a variety of
pregnancy-associated diseases. Insufficient trophoblast invasion
and proliferation are correlated with PE development (12,13).
Recent studies have suggested that RBP4, an integral
member of a trimer with retinol and TTR that is transported to
specific tissues and organs in the body, may be linked to insulin
resistance, type 2 diabetes mellitus, renal dysfunction,
atherosclerosis and hypertension in women (14). RBP4 is a 21-kDa protein and a
specific carrier of retinol (vitamin A) from the liver to
peripheral tissues (15). In the
present study, IHC analysis showed that RBP4 is localized in STBs,
and RBP4 expression in PE placenta tissue was significantly
decreased compared with that in normal controls. In the present
study, serum RBP4 concentration as determined by ELISA was found to
be significantly lower in patients with PE than in healthy pregnant
women, consistent with the results of our previous study (7).
The trophoblast invasion process requires
degradation and remodeling of the decidual extracellular matrix.
Because the MMP family is involved in breakdown of the
extracellular matrix, to gain a greater understanding of the
mechanisms underlying RBP4-regulated cell invasion, we examined the
expression of MMP2 and MMP9, which were previously found to
increase the invasiveness of trophoblasts (16–18).
We found that RBP4 knockdown decreased cell invasion, and RBP4
overexpression increased the invasion of HTR8/SVneo cells.
Moreover, the production of MMP2 and MMP9 was decreased in
RBP4-knockdown HTR8/SVneo cells and dramatically increased in
RBP4-overexpressing cells. These results indicated that MMP2 and
MMP9 likely contribute to RBP4-induced trophoblast invasion. Thus,
it is worthwhile to ascertain whether RBP4 acts directly on MMP2
and MMP9 promoters or functions indirectly through some other
factor.
The PI3K/AKT signaling pathway plays important roles
in cell growth, proliferation, migration and invasion (19). We found that RBP4 overexpression
did not increase HTR8/SVneo cell proliferation at 24, 48 or 72 h,
and thus, we cultivated the cells for longer periods of time. After
96 and 144 h (20,21), we found that the overexpression of
RBP4 increased cell proliferation, whereas siRNA-mediated RBP4
knockdown significantly decreased HTR8/SVneo cell proliferation at
24, 48, 72, 96 and 144 h. Activation of PI3K/AKT signaling inhibits
apoptosis (22) through Bcl-2
(23), an anti-apoptotic protein
that is downstream of the PI3K/AKT pathway. Bcl-2 is associated
with or may be necessary for cell growth and survival. In our
study, Bcl-2 was increased in RBP4-overexpressing cells and
decreased in RBP4-knockdown cells. RBP4 is known to activate
PI3K/AKT signaling in HTR8/SVneo cells. We found that RBP4
knockdown decreased AKT (Ser473 and Thr308) and PI3K
phosphorylation, although the total protein expression of AKT and
PI3K remained constant. Therefore, blockade of the PI3K/AKT pathway
may be one reason for the cell growth inhibition after RBP4
knockdown.
In summary, our data show that RBP4 is involved in
the proliferation and invasion of trophoblastic cells.
Additionally, RBP4 can activate the PI3K/AKT signaling pathway and
upregulate MMP2 and MMP9 expression. Based on the results of the
current study, we hypothesize that RBP4 regulates trophoblastic
cell proliferation and invasion by activating the PI3K/AKT pathway.
Decreased RBP4 expression in the placenta may contribute to PE
development by decreasing the invasive ability of trophoblasts.
Therefore, placental trophoblast cell
differentiation indeed involves an EMT-like process, which will be
the focus for our follow-up research. The present study is the
first to detect the effect of RBP4 on the invasion ability of
trophoblast cells and changes in MMP-related molecules, including
Snail, Slug, Vimentin, and E-Cadherin, which are directly related
to cell invasion ability. We plan to conduct additional studies in
the future to further elucidate these mechanisms.
Acknowledgements
The authors would like to thank the Experimental
Center of Capital Medical University, Beijing Chao-Yang Hospital
(Beijing, China) for their assistance and cooperation during the
present study.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81571455)
and Sino-RUS Cooperation Funds (grant no. 2015DFR31070).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and QW conceived and designed the experiments. HL
and TL performed the experiments. ZZ and GC analyzed the data. ZZ
and CL interpreted the data, and HL wrote the first draft of the
manuscript. ZZ and CL gave final approval of the results and
conclusions made in the manuscript. NZ analyzed the results and
developed the structure and arguments for the paper. HL, CL and ZZ
critically revised the paper for important intellectual content.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol was conducted in accordance
with the guidelines of the World Medical Association's Declaration
of Helsinki and was performed following approval from the Medical
Ethics Committee (11-S-59) of Beijing Chao-Yang Hospital, Capital
Medical University. All of the women enrolled in the present study
provided written informed consent prior to inclusion to the
study.
Patient consent for publication
All of the women enrolled in the present study
provided written informed consent prior to inclusion to the
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson UD, Olsson MG, Kristensen KH,
Åkerström B and Hansson SR: Review: Biochemical markers to predict
preeclampsia. Placenta. 33 Suppl:S42–S47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noris M, Perico N and Remuzzi G:
Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol.
1:98–114; quiz 120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naylor HM and Newcomer ME: The structure
of human retinol-binding protein (RBP) with its carrier protein
transthyretin reveals an interaction with the carboxy terminus of
RBP. Biochemistry. 38:2647–2653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goodman DS: Plasma retinol-binding
protein. Ann N Y Acad Sci. 348:378–390. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CH, Hsieh TJ, Lin KD, Lin HY, Lee MY,
Hung WW, Hsiao PJ and Shin SJ: Increased unbound retinol-binding
protein 4 concentration induces apoptosis through receptor-mediated
signaling. J Biol Chem. 287:9694–9707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu C, Zhang N, Yu H, Chen Y, Liang Y,
Deng H and Zhang Z: Proteomic analysis of human serum for finding
pathogenic factors and potential biomarkers in preeclampsia.
Placenta. 32:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Q, Liu C, Liu Y, Zhang N, Deng H and
Zhang Z: Serum markers of pre-eclampsia identified on proteomics. J
Obstet Gynaecol Res. 42:1111–1118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding J, Huang F, Wu G, Han T, Xu F, Weng
D, Wu C, Zhang X, Yao Y and Zhu X: MiR-519d-3p suppresses invasion
and migration of trophoblast cells via targeting MMP-2. PLoS One.
10:e01203212015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ball E, Bulmer JN, Ayis S, Lyall F and
Robson SC: Late sporadic miscarriage is associated with
abnormalities in spiral artery transformation and trophoblast
invasion. J Pathol. 208:535–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldman-Wohl D and Yagel S: Regulation of
trophoblast invasion: From normal implantation to pre-eclampsia.
Mol Cell Endocrinol. 187:233–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Solini A, Santini E, Madec S, Rossi C and
Muscelli E: Retinol-binding protein-4 in women with untreated
essential hypertension. Am J Hypertens. 22:1001–1006. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hendler I, Blackwell SC, Mehta SH, Whitty
JE, Russell E, Sorokin Y and Cotton DB: The levels of leptin,
adiponectin, and resistin in normal weight, overweight, and obese
pregnant women with and without preeclampsia. Am J Obstet Gynecol.
193:979–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JZ, Wong MH, Brennecke SP and Keogh
RJ: The effects of human chorionic gonadotrophin, progesterone and
oestradiol on trophoblast function. Mol Cell Endocrinol. 342:73–80.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jovanović M, Stefanoska I, Radojcić L and
Vićovac L: Interleukin-8 (CXCL8) stimulates trophoblast cell
migration and invasion by increasing levels of matrix
metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1.
Reproduction. 139:789–798. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karthikeyan VJ, Lane DA, Beevers DG, Lip
GY and Blann AD: Matrix metalloproteinases and their tissue
inhibitors in hypertension-related pregnancy complications. J Hum
Hypertens. 27:72–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duan C, Bauchat JR and Hsieh T:
Phosphatidylinositol 3-kinase is required for insulin-like growth
factor-I-induced vascular smooth muscle cell proliferation and
migration. Circ Res. 86:15–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang R, Leng H, Huang J, Du Y, Wang Y,
Zang W, Chen X and Zhao G: miR-337 regulates the proliferation and
invasion in pancreatic ductal adenocarcinoma by targeting HOXB7.
Diagn Pathol. 9:1712014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan C, Zhu Y, Zhang X, Chen X, Zheng W and
Yang J: Down-regulated aquaporin 5 inhibits proliferation and
migration of human epithelial ovarian cancer 3AO cells. J Ovarian
Res. 7:782014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Downward J: Mechanisms and consequences of
activation of protein kinase B/Akt. Curr Opin Cell Biol.
10:262–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kemi OJ, Ceci M, Wisloff U, Grimaldi S,
Gallo P, Smith GL, Condorelli G and Ellingsen O: Activation or
inactivation of cardiac Akt/mTOR signaling diverges physiological
from pathological hypertrophy. J Cell Physiol. 214:316–321. 2008.
View Article : Google Scholar : PubMed/NCBI
|