Introduction
Ischemia/reperfusion (I/R) injury is an important
factor in determining the resistance of the beneficial effects of
revascularization on neurons in the treatment of ischemic stroke
(1,2). Minimizing the severe outcomes of I/R
is of clinical importance due to the effects associated with
hemorrhagic transformation and disruption of the blood-brain
barrier (3). A large body of
evidence has demonstrated that apoptosis, with an increase in the
apoptosis regulator Bcl-2 (Bcl-2)-associated-X (Bax)/Bcl-2 ratio
and caspase-3, functions as an important part of the pathogenesis
of cerebral I/R injury (4,5). The advantages of inhibiting apoptosis
have been demonstrated; however, the precise mechanisms underlying
these dynamic regulations in cerebral I/R injury require further
investigation.
The src homology 2 (SH2) B adaptor protein 1 (SH2B1)
belongs to the SH2 domain-containing adaptor protein family, and is
a crucial metabolic mediator in the regulation of energy, insulin
resistance and glucose homeostasis (6). Additionally, upon stimulation, SH2B1
exerts a series of pathophysiological functions in cellular
proliferation, apoptosis, myogenesis, cardiac hypertrophy and
myocardial infarction that are dependent on binding to particular
protein tyrosine kinases (7–10),
including both cytoplasmic tyrosine kinases, such as Janus kinases
(JAK)1–3 and receptor tyrosine kinases against nerve growth factor,
insulin and platelet-derived growth factor. Among such kinases, the
activation of JAK2, as the first reported binding target of SH2B1,
may be potentiated by SH2B1 to induce downstream effectors,
including the signal transducer and activator of transcription 3
(STAT3) (7,11); however, the fundamental details of
SH2B1 and the JAK2/STAT3 signaling pathway and corresponding
regulation during cerebral I/R injury have not been determined.
The activity of the JAK2/STAT3 signaling pathway
appears to exhibit different consequences in response to a variety
of pathological stresses, in particular, anti-apoptotic properties
against I/R injury (12–14). Previous studies have indicated the
importance of SH2B1 in promoting cardiac hypertrophy via the
activation of the JAK2/STAT3 signaling pathway (7,11),
which is supported by reports of pharmacological inhibition of JAK2
that negates cardiac aberration in SH2B1 transgenic mice (7,11).
However, further investigation into whether the anti-apoptotic
effects mediated by the JAK2/STAT3 signaling pathway via SH2B1
serves a role in neuroprotection in cerebral I/R injury is
required. The present study demonstrated that SH2B1 overexpression
attenuated neuronal apoptosis during 6 h of oxygen-glucose
deprivation (OGD) followed by 24 h of reoxygenation (OGD/R) injury,
which may be associated with the activation of the JAK2/STAT3
signaling pathway.
Materials and methods
PC12 cell culture
PC12 cells were obtained from the American Type Cell
Culture Collection (ATCC; Manassas, VA, USA) and were maintained in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
antibiotics (100 U/ml penicillin and streptomycin) in a humidified
incubator at 37°C with an atmosphere of 5% CO2 and 95%
air (15). Cells were supplied
with fresh medium three times per week and were passaged at a 1:3
ratio twice per week.
Adenoviral vector establishment
Adenoviral vectors expressing SH2B1 (Ad-SH2B1) or
green fluorescent protein (Ad-GFP) were synthesized and provided by
Shanghai GeneChem, Inc. (Shanghai, China). Sequences were obtained
from https://blast.ncbi.nlm.nih.gov/Blast.cgi (Accession
no. 89817). In brief, the entire coding region of target gene SH2B1
was cloned into shuttle vector GV135 (Shanghai GeneChem, Inc.). The
co-transfection of 293T cells (ATCC) with a SH2B1 recombinant
shuttle vector and an auxiliary packaging plasmid (Microbix
Biosystems Inc., Toronto, ON, Canada) was performed using an AdMax
Adenovirus Packaging system (AdMax Local, Los Angeles, CA, USA)
according to the manufacturer's protocol (16). By applying Cre/loxP enzymes, the
recombinant adenovirus Ad-SH2B1 and the Ad-GFP negative control
were obtained. Subsequently, adenoviruses were amplified, purified
by an Adeno-X™ Virus Purification kit (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer's protocols, and
routinely titrated to 1×1010 PFU/ml.
Adenoviral transduction and
experimental design
For viral transfection, PC12 cells (1×105
cells per 24-well plate) were incubated with Ad-SH2B1 or Ad-GFP for
4 h at 37°C, prior to glucose deprivation as described below, at
different multiplicities of infections as calculated via: Viral
titer × viral volume/cell number (0, 10, 20, 40 and 80 MOI), and
finally, 20 MOI was selected for the following experiments due to
~95% transfection efficiency with no significant effects on cell
viability as determined by flow cytometry described below. After 4
h of incubation at 37°C, medium was removed, and PC12 cells were
further incubated at 37°C with complete medium (DMEM+FBS) for 48 h
prior to OGD/R treatment (17).
Each experiment was repeated in triplicate.
To determine the association between SH2B1 and
OGD/R-induced neuronal impairment, PC12 cells were randomly
allocated to four groups: i) Control group (untransfected, no
OGD/R); ii) OGD/R group; iii) Ad-SH2B1 transfection plus OGD/R
(Ad-SH2B1 + OGD/R); and iv) Ad-GFP transfection plus OGD/R (Ad-GFP
+ OGD/R). To further detect the underlying mechanisms of the
anti-OGD/R effects conferred by SH2B1 in PC12 cells, an additional
experiment was conducted in which Ad-SH2B1 or Ad-GFP was transduced
into PC12 cells as aforementioned in the absence or presence of a
JAK2 inhibitor (AG490, 10 µM, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). These cells were then subjected to OGD/R treatment as
described (17). The inhibitor was
added into the medium 30 min at 37°C prior to the OGD procedure
(17).
OGD/R establishment
To mimic the I/R procedure in vitro, oxygen
deprivation was induced in an anaerobic chamber containing 95%
N2 and 5% CO2 at 37°C, and glucose
deprivation was concurrently induced by maintaining cells in a
glucose-free Hanks' Balanced Salt Solution (Invitrogen; Thermo
Fisher Scientific, Inc.) for 6 h at 37°C. Subsequently, PC12 cells
from the OGD/R-treated groups were removed from the anoxia
incubator and cultured under normal conditions as aforementioned to
induce reoxygenation for an additional 24 h (17,18).
Evaluation of cell injury
Cell injury was assessed by measuring the content of
lactate dehydrogenase (LDH) in the culture medium using an LDH
diagnostic kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer's protocol (17). LDH activity was then calculated
using a microplate spectrophotometer (Shimadzu Corporation, Kyoto,
Japan) at a wavelength of 440 nm. Alternations in absorbance were
expressed as concentration units per liter (17).
Cell viability assay
Cell viability was measured using a Cell Counting
kit-8 (CCK-8) assay according to the manufacturer's protocol
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) (15). Briefly, PC12 cells from all four
groups were seeded into 96-well plates (1×104
cells/well) and were washed with PBS.CCK-8 solution (10 µl) was
then added to each well for an additional 4 h at 37°C. The
absorbance at 450 nm was determined with a microplate reader
(Biotek Instruments, Inc., Winoski, VT, USA). Cell viability was
expressed as a percentage of the control group cells.
Flow cytometric analysis of cell
apoptosis
The apoptotic rate was detected by flow cytometry
with an apoptosis detection kit (BD Pharmingen; BD Biosciences)
(15). Briefly, following the
corresponding interferences, cells were digested by 0.25% trypsin,
collected from 24-well culture plates, washed twice with PBS and
centrifuged at 20,000 × g at 4°C for 5 min. Then, PC12 cells from
all four groups were incubated with 5 µl of 7-aminoactinomycin D
(7-AAD) dye at room temperature for 15 min. Subsequently, 1 µl
Annexin V-allophycocyanin (APC) was added into the cell suspension,
and maintained for 15 min in the dark. Finally, cells were analyzed
by fluorescence-activated cell sorting via a flow cytometric
analysis (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
CytExpert 1.0 software (Beckman Coulter, Inc., Brea, CA, USA).
Cells that stained positively for Annexin V-APC or 7-AAD were
considered as apoptotic cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect mRNA expression as
previously described (15,17,18).
Briefly, total RNA from all four PC12 cells group was extracted and
purified with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and reverse-transcribed into cDNA with a commercial
PrimeScript™ II 1st strand cDNA synthesis kit (Takara Bio, Inc.,
Otsu, Japan) according to the manufacturer's protocol. RT-qPCR was
performed with an ABI Prism 7500 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in the presence of
primers and SYBR® Green Supermix (Bio-Rad Laboratories,
Inc.). The following thermocycling conditions were used for the
PCR: Initial denaturation at 50°C for 2 min; 40 cycles of
denaturation at 95°C for 30 sec; annealing at 56°C for 30 sec; and
extension at 72°C for 30 sec. The mRNA expression levels of
targeted genes were normalized to the reference gene β-actin, and
calculated using the comparative quantification method
(2−ΔΔCq) (19). The
primer sequences used in the present study are listed as follows:
SH2B1, forward 5′-CCCGTCTTAGCATTCCCTGT-3′ and reverse,
5′-GGCTTAGGCACTCTTGGATGT-3′; β-actin, forward
5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′.
Western blot analysis
Western blotting was performed to measure protein
expression as previously described (15,17,18).
In brief, PC12 cells of all four groups were lysed with ice-cold
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Jiangsu, China), supplemented with 1 mmol/l PMFS, 1
µg/ml leupeptin and 1 µg/ml pepstatin as the protease inhibitors
(Beyotime Institute of Biotechnology). Following centrifugation at
20,000 × g at 4°C for 5 min, total extracted protein was collected
from the supernatant, and the protein concentration was measured
with a commercial Bicinchoninic Acid protein assay kit (Beyotime
Institute of Biotechnology). A total of 50 µg protein lysate was
loaded in each lane and separated by 8–12% SDS-PAGE. Targeted
proteins were then transferred onto nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA) via an electroblotting technique.
Non-specific bindings were blocked with 5% non-fat milk dissolved
in tris-buffered saline with 0.1% Tween-20 (TBST) for 2 h at room
temperature. Following three washes with TBST, membranes were
incubated with primary antibodies overnight at 4°C against SH2B1
(1:800; sc-136065; Santa Cruz Biotechnolgy, Inc., CA, USA), Bax
(1:500; ab32503; Abcam, Cambridge, UK), Bcl-2 (1:600; cat. no.
2876S; Cell Signaling Technology, Inc., Danvers, MA, USA), cleaved
caspase-3 (1:400; cat. no. 9661L; Cell Signaling Technology, Inc.),
cleaved caspase-9 (1:500; cat. no. 9507S; Cell Signaling
Technology, Inc.), total (t)-JAK2 (1:500; cat. no. 3230S; Cell
Signaling Technology, Inc.), phosphorylated (p)-JAK2 (1:800; cat.
no. 3776S; Cell Signaling Technology, Inc.), t-STAT3 (1:800
dilution; 4904P; Cell Signaling Technology, Inc.) and p-STAT3
(1:600; cat. no. 9134S; Cell Signaling Technology, Inc.). GAPDH
(1:1,000; ab181602; Abcam) was utilized as an internal reference
control. Subsequently, the membranes washed three times with TBST
and were incubated with a horseradish peroxidase-conjugated rabbit
anti-rat IgG secondary antibody (1:1,000; BA1058; Wuhan Boster
Biological Technology Ltd., Wuhan, China). The protein bands were
visualized by an enhanced chemiluminescence reagent (Thermo Fisher
Scientific, Inc.), and the signal intensities were calculated by
BandScan 5.0 software (Glyko, Inc., Novato, CA, USA).
Statistical analysis
Data were presented as the mean ± standard
deviation. All statistical analyses were performed using standard
SPSS software (version 19.0, IBC Corp., Armonk, NY, USA). A
Student's t-test was utilized for between-group comparisons.
Multiple comparisons were conducted with one-way analysis of
variance, followed by a Student-Newman-Keuls-q-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
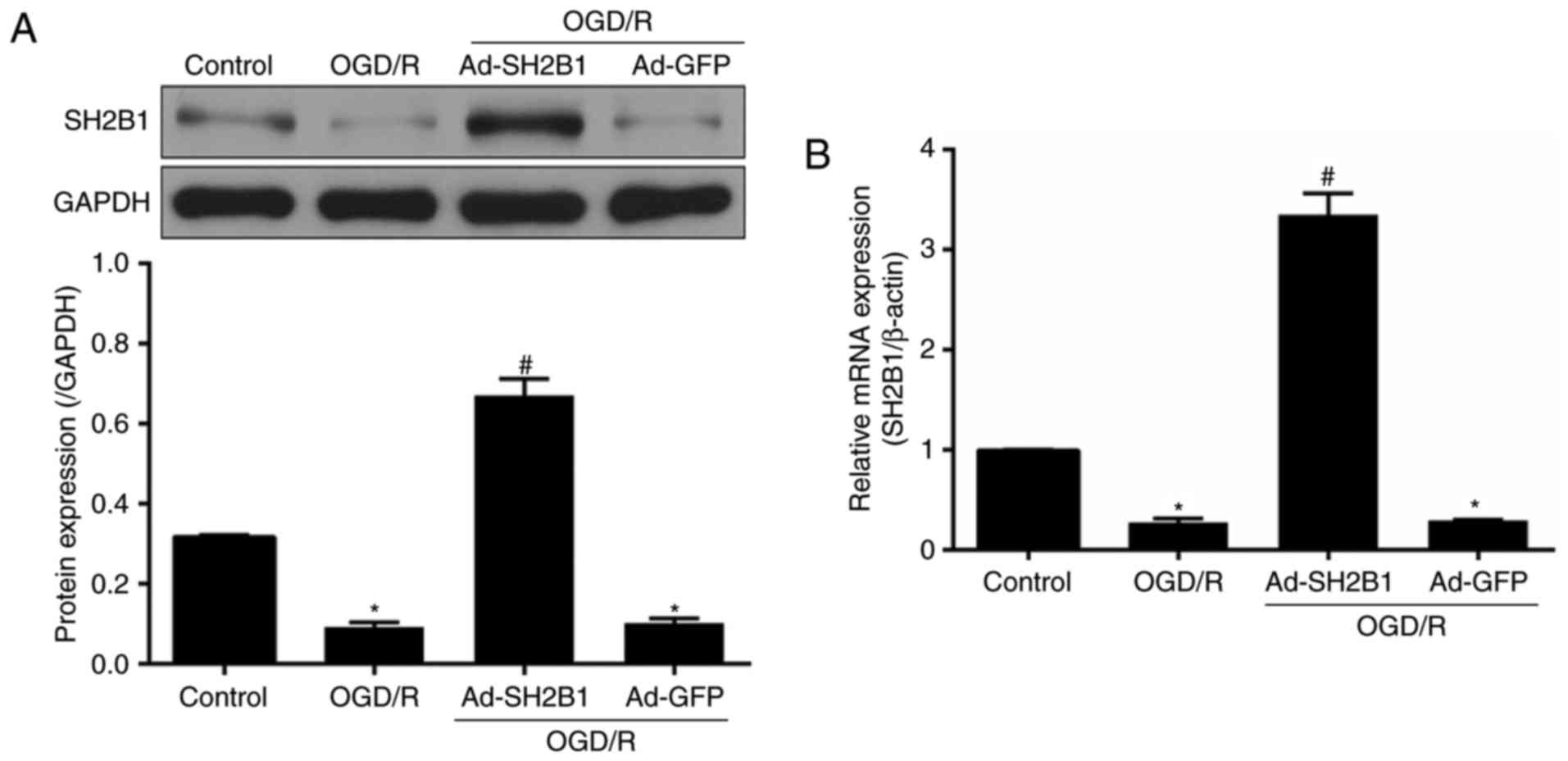
OGD/R is associated with the
downregulation of SH2B1 expression
To investigate the association between SH2B1 and
cerebral I/R injury, mRNA and protein expression levels of SH2B1
were detected in PC12 cells following OGD/R. Compared with the
control group, SH2B1 expression at both the mRNA and protein levels
was significantly suppressed in OGD/R-treated untransfected PC12
cells (P<0.05; Fig. 1).
Ad-SH2B1 transfection significantly increased SH2B1 expression
(Ad-SH2B1 + OGD/R group vs. OGD/R group; P<0.05), indicating
efficient transduction of the adenovirus. In addition, SH2B1
expression was not significant altered between the Ad-GFP + OGD/R
and OGD/R groups (P>0.05).
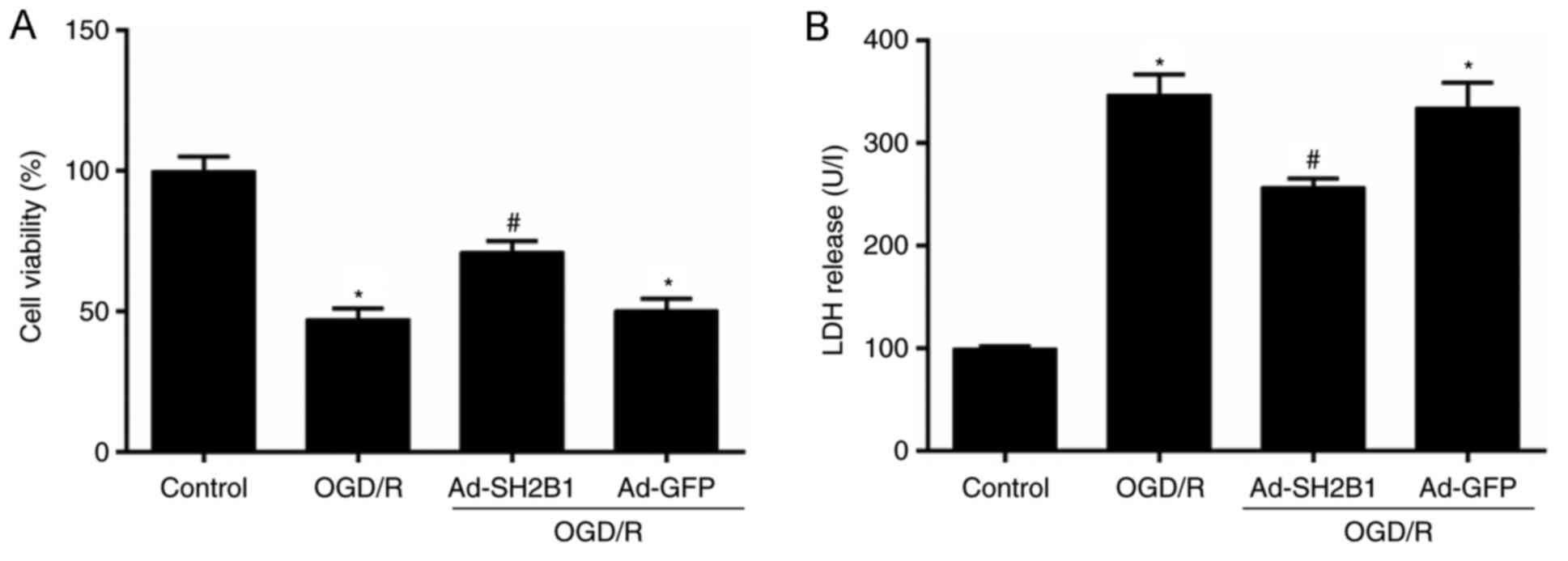
Upregulation of SH2B1 increases cell
viability and reduces LDH release
To determine whether neuronal SH2B1 upregulation
alleviated OGD/R-induced injury, cell viability and LDH (necrotic
biomarker) release in PC12 cells were measured by CCK-8 and ELISA,
respectively. Compared with the control group, OGD/R treatment
induced neuronal cell death as observed by the decrease in cell
viability (Fig. 2A); however, LDH
activity was increased (P<0.05; Fig. 2B). Conversely, these effects were
significantly reversed by SH2B1 upregulation under the OGD/R
condition (Ad-SH2B1 + OGD/R group vs. OGD/R group; P<0.05). The
aforementioned parameters demonstrated no significant differences
between the OGD/R and Ad-GFP + OGD/R groups (P>0.05). These data
indicated that SH2B1 may serve neuroprotective roles in cerebral
I/R injury.
Upregulation of SH2B1 reduces
OGD/R-induced apoptosis
To investigate the potential mechanisms of the
neuroprotective effects of SH2B1 in OGD/R, the apoptotic rate and
apoptotic mediators, including Bax, Bcl-2 and cleaved caspase-9/3
were detected by flow cytometry and western blotting, respectively.
In line with the flow cytometry results, the expression levels of
Bax and cleaved caspase-9/3 revealed similar pattern of
alternations. As presented in Fig.
3, OGD/R-induced untransfected PC12 cells demonstrated an
aggravated apoptotic rate, and upregulated Bax and cleaved
caspase-9/3 expression levels; however, Bcl-2 anti-apoptotic
effector expression levels were lower compared with in the control
group (P<0.05). When OGD/R-inducted cells were pre-treated with
Ad-SH2B1, the aforementioned parameters were significantly inversed
(Ad-SH2B1 + OGD/R group vs. OGD/R group, P<0.05). Additionally,
no significant differences were observed between the Ad-GFP + OGD/R
and OGD/R groups (P>0.05). These results suggested that the
effects of SH2B1 on OGD/R-induced PC12 cells may be associated with
the deactivation of the apoptotic cascade.
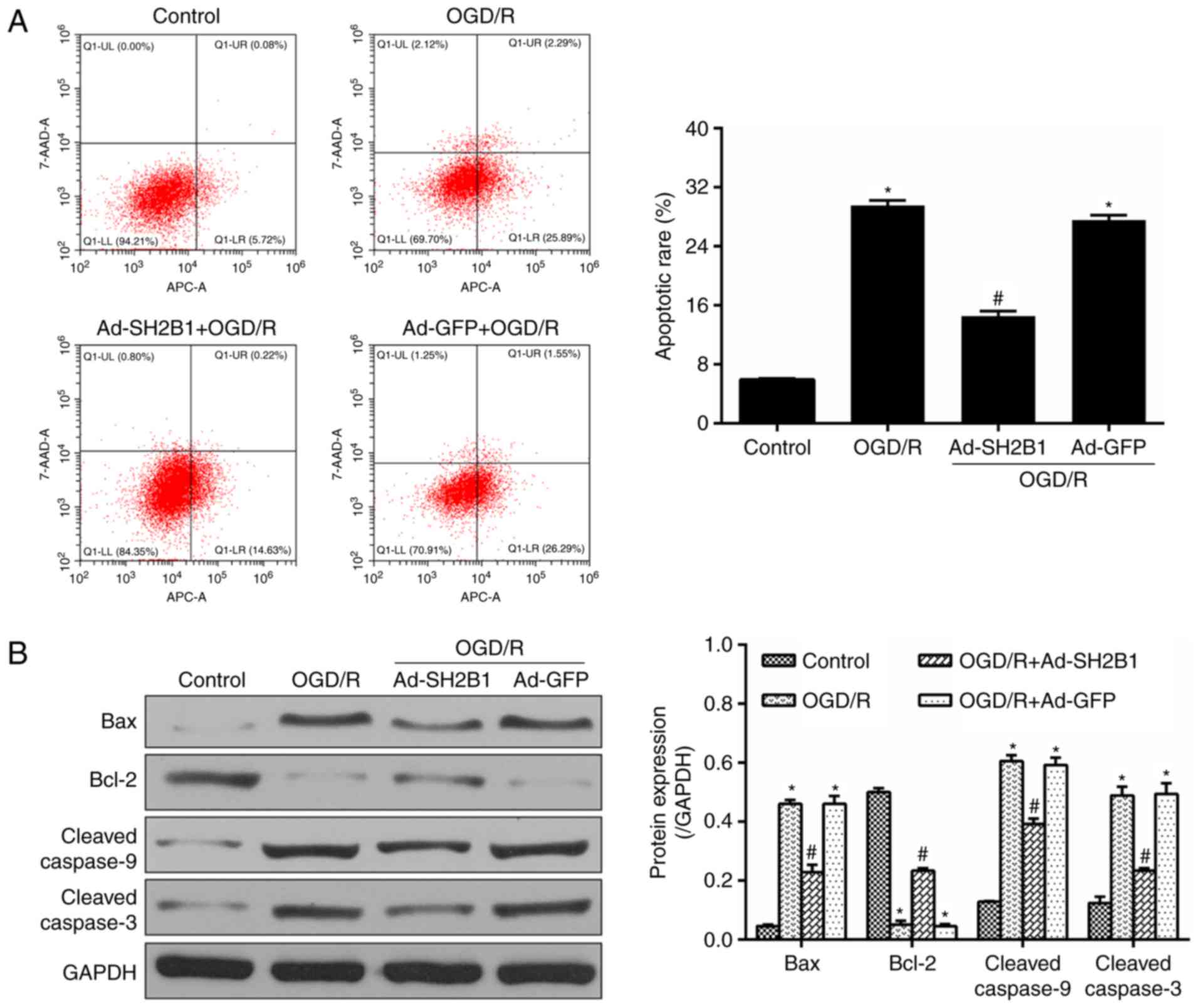 | Figure 3.Upregulation of SH2B1 abolishes
OGD/R-induced apoptosis. (A) Flow cytometry analysis and
quantitative data for apoptotic rates in PC12 cells following
OgD/R. (B) Representative protein bands and quantified evaluation
of apoptosis-associated mediators including Bax, Bcl-2 and cleaved
caspase-9/3. Data are presented as the mean ± standard deviation.
n=3 per group. *P<0.05 vs. control group; #P<0.05
vs. OGD/R group. 7-AAD, 7-aminoactinomycin D; Ad, adenovirus
vector; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated-X; GFP,
green fluorescent protein; OGD/R, oxygen-glucose deprivation and
reoxygenation; SH2B1, src homology 2 B adaptor protein 1; Ad-SH2B1
+ OGD/R, Ad-SH2B1 transfection plus OGD/R; Ad-GFP + OGD/R, Ad-GFP
transfection plus OGD/R. |
Upregulation of SH2B1 activates the
JAK2/STAT3 signaling pathway in OGD/R injury
The JAK2/STAT3 signaling pathway was extensively
investigated as a major anti-apoptotic mediator in OGD/R injury
(12,13). To investigate whether the
JAK2-STAT3 axis was involved in the favorable effects of SH2B1 on
OGD/R, the expression levels of JAK2 and STAT3 in PC12 cells were
analyzed. As presented in Fig. 4,
following OGD/R induction, protein expression levels of p-JAK2 and
p-STAT3 were significantly reduced (OGD/R group vs. control group,
P<0.05); however, Ad-SH2B1 delivery into OGD/R-treated PC12
cells significantly increased p-JAK2 and p-STAT3 expression levels
compared with in the OGD/R group (P<0.05). Ad-GFP transfection
resulted in no significant alterations in the expression levels of
p-JAK2 and p-STAT3 (Ad-GFP + OGD/R group vs. OGD/R group,
P>0.05). In addition, t-JAK2 and t-STAT3 revealed no significant
differences in expression levels among the four groups
(P>0.05).
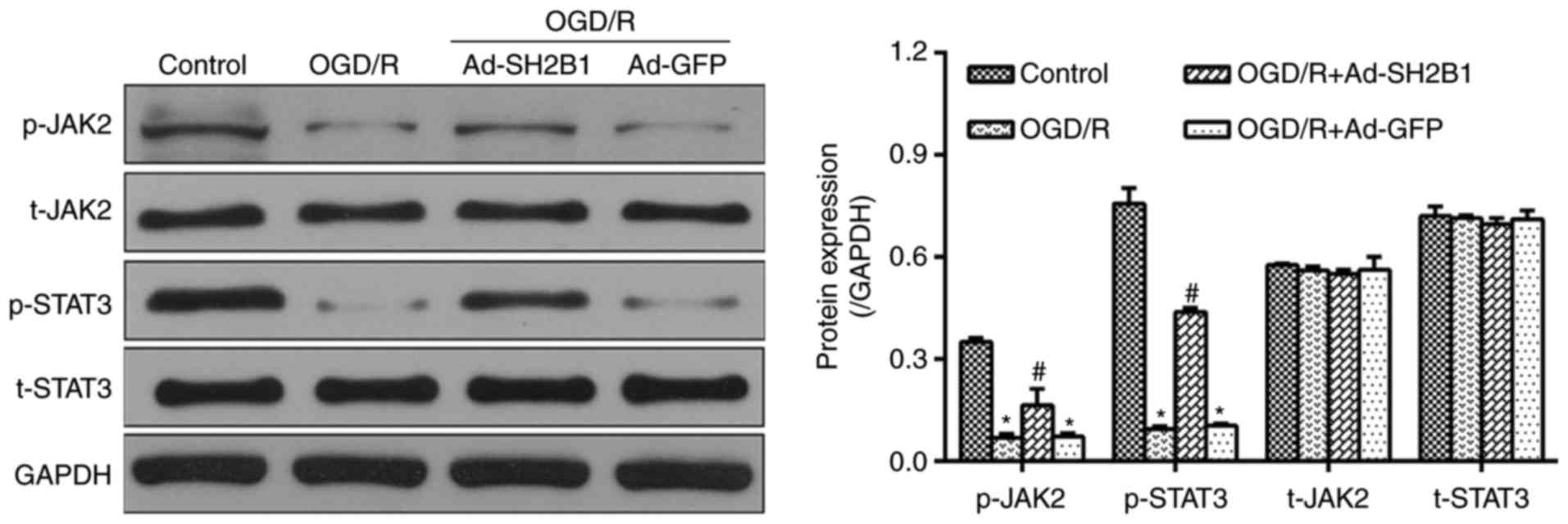 | Figure 4.Upregulation of SH2B1 activates the
JAK2/STAT3 signaling pathway in OGD/R injury. Representative
western blotting bands for p-JAK2, JAK2, p-STAT3 and STAT3 were
observed by western blot analysis. The histogram demonstrates the
statistical measurements for the relative intensities normalized to
GADPH. Data are presented as the mean ± standard deviation of three
independent experiments. *P<0.05 vs. control;
#P<0.05 vs. OGD/R. Ad, adenovirus vector; GFP, green
fluorescent protein; JAK2, Janus kinase 2; OGD/R, oxygen-glucose
deprivation and reoxygenation; p, phosphorylated; SH2B1, src
homology 2 B adaptor protein 1; STAT3, signal transducer and
activator of transcription 3; t, total. |
Inhibitory roles of SH2B1 in OGD/R are
dependent on the JAK2/STAT3 signaling pathway
To further examine whether the inhibitor effects of
SH2B1 on OGD/R injury were dependent upon the activation of the
JAK2/STAT3 signaling pathway, virus-transfected and OGD/R-treated
PC12 cells were exposed to the JAK2 inhibitor AG490. As presented
in Fig. 5, the expression levels
of p-JAK2/STAT3 (Fig. 5A), cell
viability and LDH activity (Fig.
5B), as well as the apoptotic rate (Fig. 5C) were significantly different
between the Ad-GFP and Ad-SH2B1 groups under the OGD/R + PBS
condition; however, significantly differences were also observed
between the Ad-SH2B1 + OGD/R + PBS group compared with the presence
of AG490. Treatment with AG490, however, abrogated the effects of
SH2B1 overexpression on p-JAK2/STAT3 expression levels, viability
and cell death relative to OGD/R + PBS + Ad-SH2B1-treated PC12
cells (P<0.05). Overall, these data demonstrated that the
protective effects of SH2B1 against OGD/R may be largely dependent
upon the activation of the JAK2/STAT3 signaling pathway and the
suppression of apoptosis.
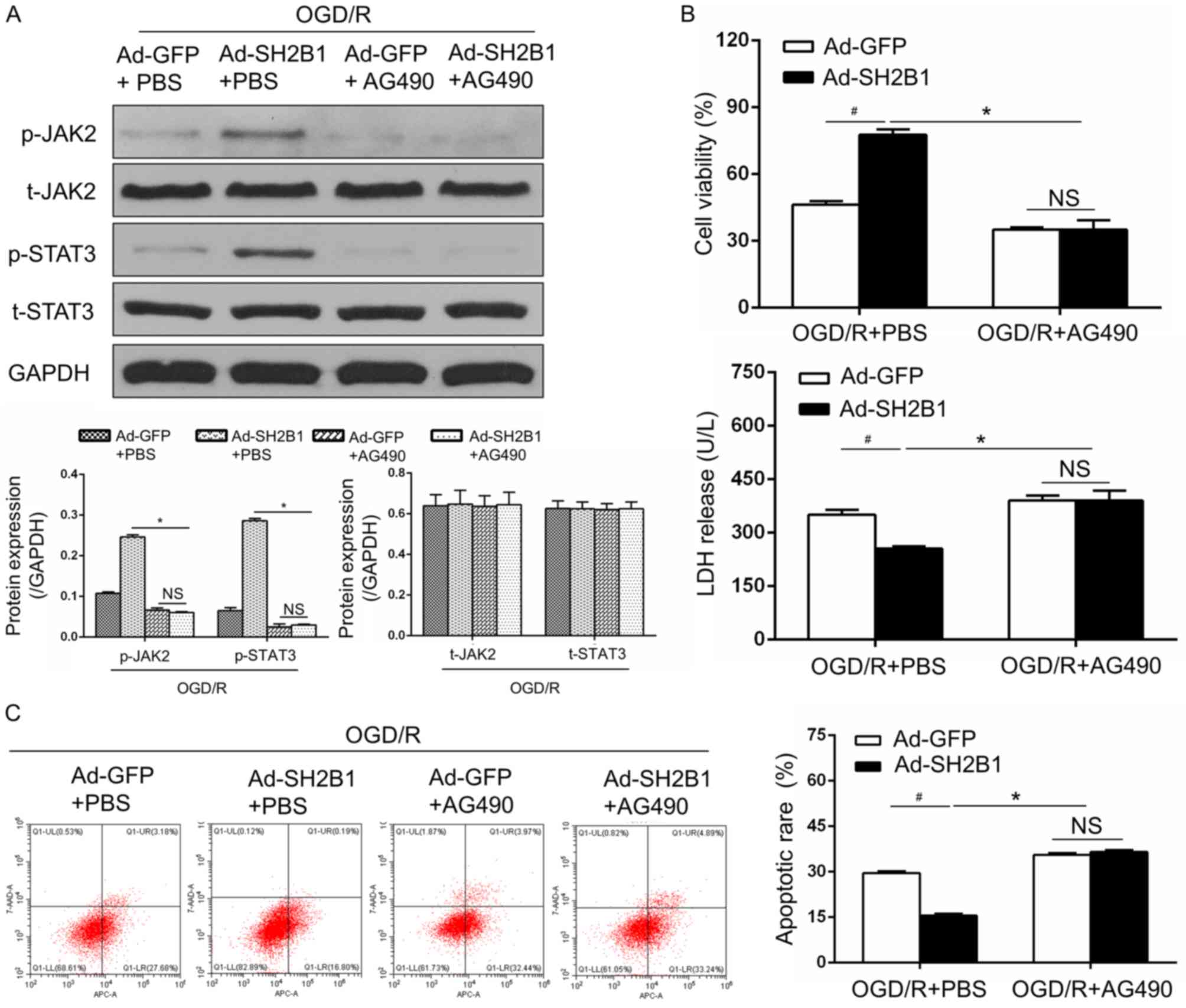 | Figure 5.Inhibitory roles of SH2B1 against
OGD/R are dependent on the JAK2/STAT3 signaling pathway. (A)
Protein expression levels of JAK2/STAT3 signaling
pathway-associated proteins were measured by western blot analysis.
(B) Cell Counting Kit-8 assay was used to evaluate cell viability,
and LDH release into the culture medium was detected by ELISA. (C)
Flow cytometry images and quantification of apoptosis in each
group. Data from three independent experiments are presented as the
mean ± standard deviation. #P<0.05 vs. OGD/R + Ad-GFP
+ PBS group. *P<0.05 vs. OGD/R + Ad-SH2B1 + PBS group. 7-AAD,
7-aminoactinomycin D; Ad, adenovirus vector; APC, allophycocyanin;
GFP, green fluorescent protein; JAK2, Janus kinase 2; AG490, JAK2
inhibitor; NS, no significance; OGD/R, oxygen-glucose deprivation
and reoxygenation; p, phosphorylated; SH2B1, src homology 2 B
adaptor protein 1; STAT3, signal transducer and activator of
transcription 3; t, total. |
Discussion
The present study provided evidence that selective
overexpression of SH2B1 may exhibit anti-I/R effects, as it
attenuated neuronal apoptosis in vitro. This conclusion was
upheld with a series of novel experiments: Ad-SH2B1 transfection
followed by OGD/R resulted in SH2B1 overexpression in neural-like
PC12 cells; SH2B1 overexpression prior to the OGD/R procedure
significantly enhanced cell viability but repressed LDH release,
which accompanied by a reduction in the expression of
apoptosis-associated cascade. SH2B1 overexpression activated the
JAK2/STAT3 survival pathways, while inhibition of the JAK2/STAT3
axis reversed neuron-protective roles of SH2B1 during OGD/R. To the
best of the authors knowledge, the present study is the first to
report the association between the anti-I/R effects of SH2B1 on
apoptosis and the JAK2/STAT3 signaling pathway.
Timely and successful revascularization serves as
the cornerstone for the treatment of ischemic stroke (1,2).
Additionally, ischemic brain tissue is susceptible to secondary
reperfusion injury that minimizes benefits of blood
re-establishment itself (1,2).
Considerable advances in the investigation of the molecular
mechanisms of cerebral I/R injury have been made; however,
efficient treatment methods to abolish the detrimental outcomes or
cerebral I/R are required (1,2).
Numerous studies have verified that the activities of the apoptotic
cascade, which induces particular mediators, including Bax, Bcl-2
and cleaved caspase-3, serve as the central etiology in the
pathogenesis of cerebral I/R injury (4,12).
Therefore, more extensive studies focusing on the interventions of
prolonged apoptosis suggest that the repression of apoptosis may be
a promising way to reduce the severity of I/R injuries (4,12);
however, at present, there are limitations in reducing
I/R-associated impairments (1–4).
Consistently, the results of the present study revealed that PC12
cells subjected to SH2B1 overexpression and OGD/R were less
vulnerable to apoptosis, as manifested by reduced expression levels
of pro-apoptotic markers, including Bax and cleaved caspase-3, and
an increase in the anti-apoptotic marker Bcl-2. In parallel, the
apoptotic index and LDH release (necrotic marker) were reduced, and
cell viability was improved following Ad-SH2B1 transduction. Thus,
the findings of the present study provide compelling insights into
the beneficial effects exerted by SH2B1 on neuronal cells in I/R
injury.
As the pivotal cell survival signaling pathway in
cerebral I/R prevention, the JAK2/STAT3 signaling pathway emerges
as a powerful effector that resists cell death and apoptosis
(12–14). Induction of JAK2 and STAT3 are
primarily correlated with its protein expression and
phosphorylation activities. Therefore, regulating the JAK2/STAT3
signaling pathway may facilitate the understanding for the
management of cerebral I/R (12,13);
however, whether a JAK2/STAT3-mediated mechanism is involved in the
anti-apoptotic effects of SH2B1 remain to be determined in cerebral
I/R. In the present study, p-JAK2 and p-STAT3 expression levels
were profoundly increased following SH2B1 overexpression under the
OGD/R condition. Furthermore, abolishment of the JAK2/STAT3
signaling pathway by pharmacological inhibitor, AG490, contributed
to apoptosis and OGD/R injury in the presence or absence of SH2B1
overexpression in the present study. These data suggested that the
beneficial neurological effects of SH2B1 against I/R may be
particularly dependent on the JAK2/STAT3 signaling pathway and
associated apoptosis-inhibiting mechanisms.
SH2B1, known as an adapter protein, exerts its
versatile biological potencies primarily by binding to kinases,
including JAK2 (6–9). Emerging data revealed that SH2B1
shares many critical roles in regulating the migration, apoptosis
and proliferation of cancer cells (20). At present, the involvement of SH2B1
in cardiovascular insults have also drawn significant attention in
cardiac hypertrophy and myocardial infarction (7,10).
Recent reports from Wu et al (7) and others (10) have systematically demonstrated that
SH2B1 directly binds to JAK2 in cardiomyocytes, thus aggravating
hypertrophy in a JAK2/STAT3-dependent manner. Providing that the
JAK2/STAT3 axis contributes outstanding functions in the
attenuation of cerebral I/R injury (12–14),
it appears to be reasonable to propose that SH2B1 may participate
in cerebral I/R by activating the JAK2/STAT3 signaling pathway.
Additionally, the activation of the SH2B1-JAK2-STAT3 axis
exclusively alleviated OGD/R injury in PC12 cells as suggested by
the data of the present study. Furthermore, compared with the
diverse roles of SH2B1 in cerebral I/R and cardiac hypertrophy,
SH2B1 may confer pleiotropic roles, potentially in a
context-specific manner; however, further investigations are
required.
SH2B1 couples upstream stimulators of tyrosine
kinases with downstream effectors by creating multi-protein
complexes, thereby regulating the catalytic actions of bound
enzymes that exhibit a stimulus-specific pattern (7–10).
For instance, SH2B1 overexpression appears to be sufficient to
induce the formation of the SH2B1/JAK2 complex thereby elevating
protein kinase B (AKT) activities in pancreatic b-cells in
vivo and in vitro in diabetic models (9). Furthermore, the dual functionalities
of SH2B1 constituting the aggravation or inhibition of pathological
events have been verified recently; Blandino-Rosano et al
(21) identified that upregulation
of the SH2B1/JAK2 complex is responsible for a positive feedback
mechanism in inducing pancreatic β-cell survival. Another study
confirmed that SH2B1 enhances insulin and leptin signaling by
activating phosphoinositide 3-kinase-AKT/mitogen-activated kinases
and JAK2, respectively (22). Chen
et al (20) indicated that
re-establishment of SH2B1 reverses the impeding roles of
microRNA-326-3p on proliferation and metastasis potentially via the
activation of the JAK2/Ras-related C3 botulinum toxin substrate 1
signaling pathway. Furthermore, evidence of the positive
associations between SH2B1 and cardiac remodeling have also
demonstrated these similarities (7,11).
Conversely, other investigations have affirmed that global deletion
of SH2B1 leads to severe obesity and glucose intolerance, while
restoration of SH2B1 may correct metabolic disorders in mice
(8). In support of these findings,
the present study directly rendered the notion that SH2B1
overexpression via adenoviral vectors was effective in protecting
PC12 cells against OGD/R injury. Therefore, the aforementioned
discrepancies may harbor deeper insights and increased complexity
of the SH2B1-associated mechanism involved in variety of
pathological conditions. A recent study revealed another epigenetic
mechanism of SH2B1 on progressing myogenesis by erasing histone H3
lysine 9 (H3K9) trimethylation (me3) and inducing H3K4me3 on the
promoters/enhancers of corresponding genes (23). Providing the association between
SH2B1 and epigenetic modification (microRNA or histone methylation)
and their participations in I/R injury, further investigation is
required to improve understanding of the potential regulatory
networks associated with SH2B1 during the pathogenesis of cerebral
I/R injury.
In conclusion, the present study revealed that SH2B1
is an intrinsic positive mediator for I/R-induced neuronal
apoptosis and that the SH2B1-JAK2-STAT3 axis may be considered as a
compelling therapeutic target for the prevention of cerebral I/R.
Notably, further studies are required to examine whether SHB21
directly affects other signaling pathways against cerebral I/R
injury apart from the JAK2/STAT3 pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81671238).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY and LZ made substantial contributions to the
conception and design of the present study. YS and NW performed the
experiments including cell culture, adenoviral transfection, OGD/R
establishment, apoptotic detection and western blotting assay. QS,
ZC and YW performed data interpretation and statistical analysis,
and were involved in drafting the manuscript or revising it
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SH2B1
|
src homology 2 B adaptor protein 1
|
|
OGD/R
|
oxygen-glucose deprivation and
reoxygenation
|
|
I/R
|
ischemia/reperfusion
|
|
JAK2
|
Janus kinase 2
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Jian Z, Ding S, Deng H, Wang J, Yi W, Wang
L, Zhu S, Gu L and Xiong X: Probenecid protects against
oxygen-glucose deprivation injury in primary astrocytes by
regulating inflammasome activity. Brain Res. 1643:123–129. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu G, Zhu L, Yuan X, Chen H, Xiong R,
Zhang S, Cheng H, Shen Y, An H, Li T, et al: Britanin ameliorates
cerebral ischemia-reperfusion injury by inducing the Nrf2
protective pathway. Antioxid Redox Signal. 27:754–768. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Park JH, Maharjan S, Park JA,
Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, et al: Sac-1004,
a vascular leakage blocker, reduces cerebral ischemia-reperfusion
injury by suppressing blood-brain barrier disruption and
inflammation. J Neuroinflammation. 14:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Min HM, Wang Y, Ren DY, Cheng X, Li J,
Jiang XQ, Min LQ and Bao CF: Protective effect of 2-deoxy-D-glucose
on the brain tissue in rat cerebral ischemia-reperfusion models by
inhibiting Caspase-apoptotic pathway. Histol Histopathol. 32:57–67.
2017.PubMed/NCBI
|
|
5
|
Nakka VP, Gusain A and Raghubir R:
Endoplasmic reticulum stress plays critical role in brain damage
after cerebral ischemia/reperfusion in rats. Neurotox Res.
17:189–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rui L: SH2B1 regulation of energy balance,
body weight, and glucose metabolism. World J Diabetes. 5:511–526.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu G, Liu Y, Huang H, Tang Y, Liu W, Mei
Y, Wan N, Liu X and Huang C: SH2B1 is critical for the regulation
of cardiac remodelling in response to pressure overload. Cardiovasc
Res. 107:203–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren D, Zhou Y, Morris D, Li M, Li Z and
Rui L: Neuronal SH2B1 is essential for controlling energy and
glucose homeostasis. J Clin Invest. 117:397–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Morris DL, Jiang L, Liu Y and Rui
L: SH2B1 in β-cells regulates glucose metabolism by promoting
β-cell survival and islet expansion. Diabetes. 63:585–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prudente S, Morini E, Larmon J, Andreozzi
F, Di Pietro N, Nigro A, Gervino EV, Mannino GC, Bacci S, Hauser
TH, et al: The SH2B1 obesity locus is associated with myocardial
infarction in diabetic patients and with NO synthase activity in
endothelial cells. Atherosclerosis. 219:667–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikeda Y, Takimoto E and Komuro I: SH2B1: A
new player in the regulation of cardiac hypertrophic response in
failing hearts. Cardiovasc Res. 107:197–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Zhang X, Zhang J, Kang N, Zhang N,
Wang H, Xue J, Yu J, Yang Y, Cui H, et al: Diosmin protects against
cerebral ischemia/reperfusion injury through activating JAK2/STAT3
signal pathway in mice. Neuroscience. 268:318–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shyu WC, Lin SZ, Chiang MF, Chen DC, Su
CY, Wang HJ, Liu RS, Tsai CH and Li H: Secretoneurin promotes
neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway
in murine models of stroke. J Clin Invest. 118:133–148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Li H and Li M: Curcumin protects
against cerebral ischemia-reperfusion injury by activating
JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med.
8:14985–14991. 2015.PubMed/NCBI
|
|
15
|
Zhang JF, Zhang L, Shi LL, Zhao ZH, Xu H,
Liang F, Li HB, Zhao Y, Xu X, Yang K and Tian YF: Parthenolide
attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β
pathway in PC12 cells. Biomed Pharmacother. 89:1159–1165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HB and Yang J, Ding JW, Chen LH, Li
S, Liu XW, Yang CJ, Fan ZX and Yang J: RNAi-mediated
down-regulation of CD47 protects against
ischemia/reperfusion-induced myocardial damage via activation of
eNOS in a rat model. Cell Physiol Biochem. 40:1163–1174. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao T, Feng JZ, Xu GH, Fu J, Li XG and Qin
XY: Minocycline promotes neurite outgrowth of PC12 cells exposed to
oxygen-glucose deprivation and reoxygenation through regulation of
MLCP/MLC signaling pathways. Cell Mol Neurobiol. 37:417–426. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Zhu X, Chen M, Ge Q, Shen Y and Pan
S: Resveratrol protects PC12 cells against OGD/R-induced apoptosis
via the mitochondrial-mediated signaling pathway. Acta Biochim
Biophys Sin (Shanghai). 48:342–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen W, Wang J, Liu S, Wang S, Cheng Y,
Zhou W, Duan C and Zhang C: MicroRNA-361-3p suppresses tumor cell
proliferation and metastasis by directly targeting SH2B1 in NSCLC.
J Exp Clin Cancer Res. 35:762016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blandino-Rosano M, Scheys JO,
Jimenez-Palomares M, Barbaresso R, Bender AS, Yanagiya A, Liu M,
Rui L, Sonenberg N and Bernal-Mizrachi E: 4E-BP2/SH2B1/IRS2 are
part of a novel feedback loop that controls β-cell mass. Diabetes.
65:2235–2248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen Y, Xia Y, Meng S, Lim NK, Wang W and
Huang F: SH2B1 is involved in the accumulation of amyloid-β42 in
alzheimer's disease. J Alzheimers Dis. 55:835–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen KW, Chang YJ, Yeh CM, Lian YL, Chan
MW, Kao CF and Chen L: SH2B1 modulates chromatin state and MyoD
occupancy to enhance expressions of myogenic genes. Biochim Biophys
Acta. 1860:270–281. 2017. View Article : Google Scholar : PubMed/NCBI
|