Introduction
The rising incidence of osteoarthritis in developed
countries has caused numerous socio-economic problems (1–3). For
this reason, more effective treatments are needed. One approach to
treating the damaged cartilage in osteoarthritis is matrix-induced
autologous chondrocyte implantation (MACI), a process that requires
millions of cells (4). However, an
important drawback of MACI is that chondrocytes propagated in an
in vitro culture tend to lose their primary function and
phenotype in a process known as dedifferentiation. Thus, during
MACI, type I collagen production increases relative to type II
collagen, which is uncommon in hyaline cartilage chondrocytes
(5). To overcome this drawback,
several studies have differentiated multipotent and pluripotent
stem cell populations into chondrocyte-like cells. Multipotent stem
cells, such as mesenchymal stem cells (MSCs), can be easily
obtained from numerous different sources in the body, including fat
and bone marrow. However, the low concentration of MSCs in the
general cell population requires propagation in an in vitro
culture. In addition, MSCs may not be feasible for the treatment of
degenerative diseases because both the number of these cells and
their proliferative capacity decrease with age (6–8). For
this reason, other cell sources, such as pluripotent stem cells
which have unlimited proliferative and self-renewal ability would
seem to be a better option for therapeutic purposes (9,10).
However, the use of pluripotent stem cells, especially human
embryonic stem cells (hESCs), is controversial and may raise
ethical issues. These objections can be overcome by using induced
pluripotent stem cells (iPSCs), although such cells have several
limitations, including safety issues related to their tumorigenic
potential and the unknown efficiency of differentiation into
chondrocytes. Additionally, some studies suggest that there is an
important difference between iPSCs and hESCs at the molecular level
(11). Other disadvantages of
iPSCs are the high cost of culture and the low reprogramming
efficiency (11,12).
Various chondrogenic differentiation protocols have
been described in recent years, including high density mass,
micromass (13), monolayer culture
(14), and embryoid bodies (EB)
formation which is probably the most common protocol (15,16).
The EB-based protocol takes advantage of the natural ability of
pluripotent stem cells to form three germ layers. EBs can be
derived through a variety of methods, including suspension culture,
hanging-drops, or size-defined wells (17,18).
However, the heterogeneous size of EBs affects their
microenvironment because oxygen levels, growth factors, and
nutrient concentration all vary depending on the EB depth in the
culture. Moreover, changes in these factors could affect the
spontaneous differentiation process (19). Besides the physical properties, the
number of cells used in EB-formation also influences signalling
pathways. Apart from size, one of the potential regulators of EB
differentiation is the non-canonical WNT pathway, which directly
impacts the differentiation of cells into specific germ layers.
Hwang et al (20) showed
that WNT5a (a regulator of vasculogenesis) was elevated in 150 µm
diameter hydrogel wells used to form size-defined EBs, whereas EBs
formed in 450 µm wells presented increased expression of WNT11 (a
regulator of cardiomyogenic cells). Nevertheless, at present, the
influence of the size of EBs derived from human pluripotent cells
on chondrogenic fate remains poorly understood due to a lack of
data.
In this context, the aim of this study was to
demonstrate how the number of cells used for EB formation could
improve differentiation protocols used to create chondrocyte-like
cells for regenerative purposes and/or for the study of
chondrogenesis. In addition, we sought to determine the effect of
cell colony size and culture time on the spontaneous
differentiation of hESCs and further chondrogenic
differentiation.
Materials and methods
HESC culture
The BG01V hESC line (American Type Culture
Collection, Manassas, VA; USA) was cultured in mitomycin-C-treated
mouse embryonic fibroblasts (MEFs; passage 3; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) seeded on a culture dish previously
coated with Matrigel™ (Corning Incorporated, Corning, NY, USA). The
hESCs were cultured in DMEM/F12 (Merck Millipore, Germany)
supplemented with 20% KnockOut™ Serum Replacement (KnockOut™ SR,
Thermo Fisher Scientific Inc., MA, USA), 1% penicillin-streptomycin
(Sigma-Aldrich; Merck KGaA), 1 mM non-essential amino acid (NEAA;
Sigma-Aldrich; Merck KGaA), 0.2 mM 2-mercaptoethanol
(Sigma-Aldrich; Merck KGaA), and 10 ng/ml basic fibroblast growth
factor (bFGF) (Sigma-Aldrich; Merck KGaA). Cells were cultured at
37°C in a humidified atmosphere containing 5% CO2 and
95% air.
Embryoid body formation
The hESC colonies were dissociated enzymatically
with 0.1% EDTA/Trypsin (Sigma-Aldrich; Merck KGaA). Then the cells
were counted and seeded onto 96-well plate dedicated for suspension
cell culture (inertGrade™; BRAND GMBH + CO KG, Wertheim, Germany)
in varying cell numbers (500, 1,000, 1,500, and 2,000 cells per
well) (Fig. 1) with the addition
of 10 µM ROCK-inhibitor Y-27632 (Sigma-Aldrich; Merck KGaA). Next,
cells were left for spontaneous aggregation for 15 days. The medium
(hESC medium without bFGF) was changed every second day.
Measurement of EB size and
homogeneity
The diameter of the EBs was measured with a tool
provided by the manufacturer of the IS-OptaView programme
(Opta-Tech, Warszawa, Poland) which was designed for use with the
inverted-microscope MF-100F (Opta-Tech). EB size was measured on
the 5th, 10th and 15th day of culture (n=25 for each variant and
day). Homogeneity of the formed EBs (n=2,300) was also
evaluated.
Chondrogenic differentiation of
EBs
EBs (n=20) were transferred onto 6-well plates
coated with Matrigel™ (Corning Incorporated). After 24 h, the
medium was replaced with a chondrogenic medium (ChM) (day 0)
composed of DMEM/F12 (Sigma-Aldrich; Merck KGaA), 2% KnockOut™ SR
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), 1 mM sodium
pyruvate (Biowest, Nuaillé, France), 10−7 M
dexamethasone, 50 µM ascorbic acid, 50 µM L-proline, 1%
penicillin-streptomycin (all provided by Sigma-Aldrich; Merck
KGaA), 1% ITS+ premix (Corning Incorporated), and 10 ng/ml TGF-β3
(ImmunoTools, Friesoythe, Germany). The EBs were cultured for 21
days and the medium was changed every second day.
Total RNA isolation, cDNA synthesis
and reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
To isolate the total RNA, 25 EBs from suspension
culture or EBs after chondrogenic differentiation were collected
and suspended in TRI Reagent (Sigma-Aldrich; Merck KGaA). The
procedure was performed according to the manufacturer's
instructions. Then 1 µg of total RNA was used for cDNA synthesis
with iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's specifications.
Next, the gene expression profile was evaluated by RT-qPCR by
applying FAM-labelled probes (Roche Applied Science, Penzberg,
Germany). The thermocycling conditions were as follows:
Preincubation for 10 min at 95°C, followed by the amplification
step (denaturation at 95°C for 10 sec, annealing at 60°C for 30 sec
and extension at 72°C for 1 sec), and then cooling at 40°C for 30
sec. Primers and specific probes were designed using the online
Roche Universal ProbeLibrary software. All primers used in this
study are listed in Table I. Gene
expression was estimated by the 2−ΔΔCq method (21). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as the reference gene. The data were
normalized to the expression level of the control cell populations
of HC-402-05a (Human Chondrocytes; Cell Applications Inc., San
Diego, CA, USA) or the BG01V cell line.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Target mRNA | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) | Reference
sequence |
|---|
| ACAN |
CGTGAATCAGAATCAACTGCTG |
GTGTCCCTCTGTCTCCTTGC |
NM_013227.3 |
| ACTA2 (SMA) |
CTGTTCCAGCCATCCTTCAT |
TCATGATGCTGTTGTAGGTGGT |
NM_001141945.1 |
| BRACHYURY (T) |
GATGATCGTGACCAAGAACG |
CTTCCAGCGGTGGTTGTC |
NM_003181.2 |
| CD44 |
AGCCTACTGCAAATCCAAACA |
GAAGCTCTGAGAATTACTCTGCTG |
NM_000610.3 |
| CDH2 |
ATCCGACGAATGGATGAAAG |
CTGTGGGGTCATTGTCAGC |
NM_001792.3 |
| COL1A2 |
GGCAGTGATGGAAGTGTGG |
CCAACAGCTCCAATTTCACC |
NM_000089.3 |
| COL2A1 |
TTCTGGAGACCAAGGTGCTT |
TTCCATTAGCACCATCTTTGC |
NM_001844.4 |
| FGFR3 |
AGAGGCCCACCTTCAAGC |
CGACAGGTCCAGGTACTCGT |
NM_000142.4 |
| FOXA2 |
CGCCCTACTCGTACATCTCG |
AGCGTCAGCATCTTGTTGG |
NM_021784.4 |
| MIXL1 |
AAGCGCACGTCTTTCAGC |
GCCTGTTCTGGAACCATACCT |
NM_031944.1 |
| NANOG |
ATGCCTCACACGGAGACTGT |
AAGTGGGTTGTTTGCCTTTG |
NM_024865.2 |
| NCAM1 |
CGACCATCCACCTCAAAGTC |
CGGAGGCTTCACAGGTAAGA |
NM_000615.6 |
| PAX6 |
GGCACACACACATTAACACACTT |
GGTGTGTGAGAGCAATTCTCAG |
NM_000280.3 |
| SOX2 |
TGCCTCTTTAAGACTAGGACTGAGA |
GCCGCCGATGATTGTTATTA |
NM_003106.3 |
| SOX9 |
CTCGCCACACTCCTCCTC |
CGCTTCAGGTCAGCCTTG |
NM_000346.3 |
| TNC |
GCTCAAAGCAGCCACTCATT |
CCCATATCTGGAACCTCCTCT |
NM_002160.3 |
| VIM |
GGGACCTCTACGAGGAGGAG |
CTTTGTCGTTGGTTAGCTGGT |
NM_003380.3 |
Alcian blue staining
Alcian blue staining was performed to confirm the
deposition of proteoglycans (a marker of cartilage formation).
Firstly, EBs (n=6) were transferred into a 12-well plate coated
with Matrigel™. After 21 days of differentiation, cells were fixed
with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 20 min.
After three washes in phosphate buffered saline (PBS; Biowest),
cells were incubated with 1% Alcian blue (Sigma-Aldrich; Merck
KGaA) with 0.1 N HCl (POCH S.A., Gliwice, Poland) (pH=1.0). Next,
stained cells were rinsed in 0.1 N HCl and washed twice in PBS.
Then, pictures were taken under 40× magnification using a tool
provided by the manufacturer of the IS-OptaView programme
(Opta-Tech) which was designed for use with the inverted-microscope
MF-100F (Opta-Tech). All steps were performed at room temperature.
The HC-402-05a and the BG01V cell lines were used as controls.
Indirect immunofluorescence
Indirect immunofluorescence staining was performed
to confirm the presence of chondrogenic marker expression and the
absence of pluripotent cells after differentiation. Cells were
washed with phosphate-buffered saline (Biowest) and then fixed with
cooled methanol for 20 min at −20°C. Next, the cells were blocked
with PBS containing 1% bovine serum albumin (BSA; Biowest) and 0.5%
Tween (POCH, Poland). Cells were then incubated for 1 h at 37°C
with primary antibodies diluted in PBS containing 1% BSA at
optimised dilution (Table II).
After the washes, a secondary antibody anti-rabbit conjugated with
Alexa Fluor 488 or anti-mouse IgG conjugated with Alexa Fluor 488
(in all cases, diluted 1:500; Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA) were added and incubated for 1 h at
37°C. After these washes, the nuclei were stained with
4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) at 1:10,000
dilution (Sigma-Aldrich; Merck KGaA). Images were taken using the
IS-Opta-View application from the fluorescence module of the
MW-100F inverted microscope (Opta-Tech). A hESC cell line and
HC-402-05a were used as controls. To indicate the specific binding
of primary antibodies, the control reaction with 1% BSA was
performed (data not shown).
 | Table II.List of primary and secondary
antibodies used in the present study. |
Table II.
List of primary and secondary
antibodies used in the present study.
| A, Primary
antibodies |
|
| Antibody | Host/isotype | Supplier (cat.
no.) | Dilution |
|---|
| NANOG | Mouse/IgG1 | BD Pharmingen; BD
Biosciences, San Jose, CA, USA (560482) | 1:50 |
| SOX9 | Rabbit/IgG | Abcam, Cambridge,
UK (ab71762) | 1:50 |
| Type II
collagen | Rabbit/IgG | Abcam
(ab34712) | 1:100 |
| Chondroitin
sulphate | Mouse/IgM | Abcam
(ab11570) | 1:100 |
|
| B, Secondary
antibodies |
|
|
Antibody |
Host/isotype | Supplier (cat.
no.) |
Dilution |
|
| Anti-mouse IgG
conjugated with Alexa Fluor 488 | Donkey/IgG | Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA
(715-545-150) | 1:500 |
| Anti-rabbit IgG
conjugated with Alexa Fluor 488 | Donkey/IgG | Jackson
ImmunoResearch laboratories, Inc., (715-546-152) | 1:500 |
| Anti-mouse IgM
conjugated with Alexa Fluor 488 | Donkey/IgG | Jackson
ImmunoResearch laboratories, Inc., (715-545-140) | 1:500 |
Statistical analysis
All experiments were performed in triplicate and the
data are presented as the mean ± standard deviation of three
replicates. The results were compared using two-way analysis of
variance with a Holm-Sidak post-hoc test, and unpaired tailed
Student t-test (GraphPad Prism version 6.01; GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Measurement of EB diameter and
efficacy of EB formation
To assess the influence of EB size on chondrogenic
differentiation, hESC cells (500, 1,000, 1,500 and 2,000 per well)
were seeded and cultured for 15 days. During suspension culture,
the EBs were assessed by microscope to determine the size
(diameter) and homogeneity (formation of single, round sphere)
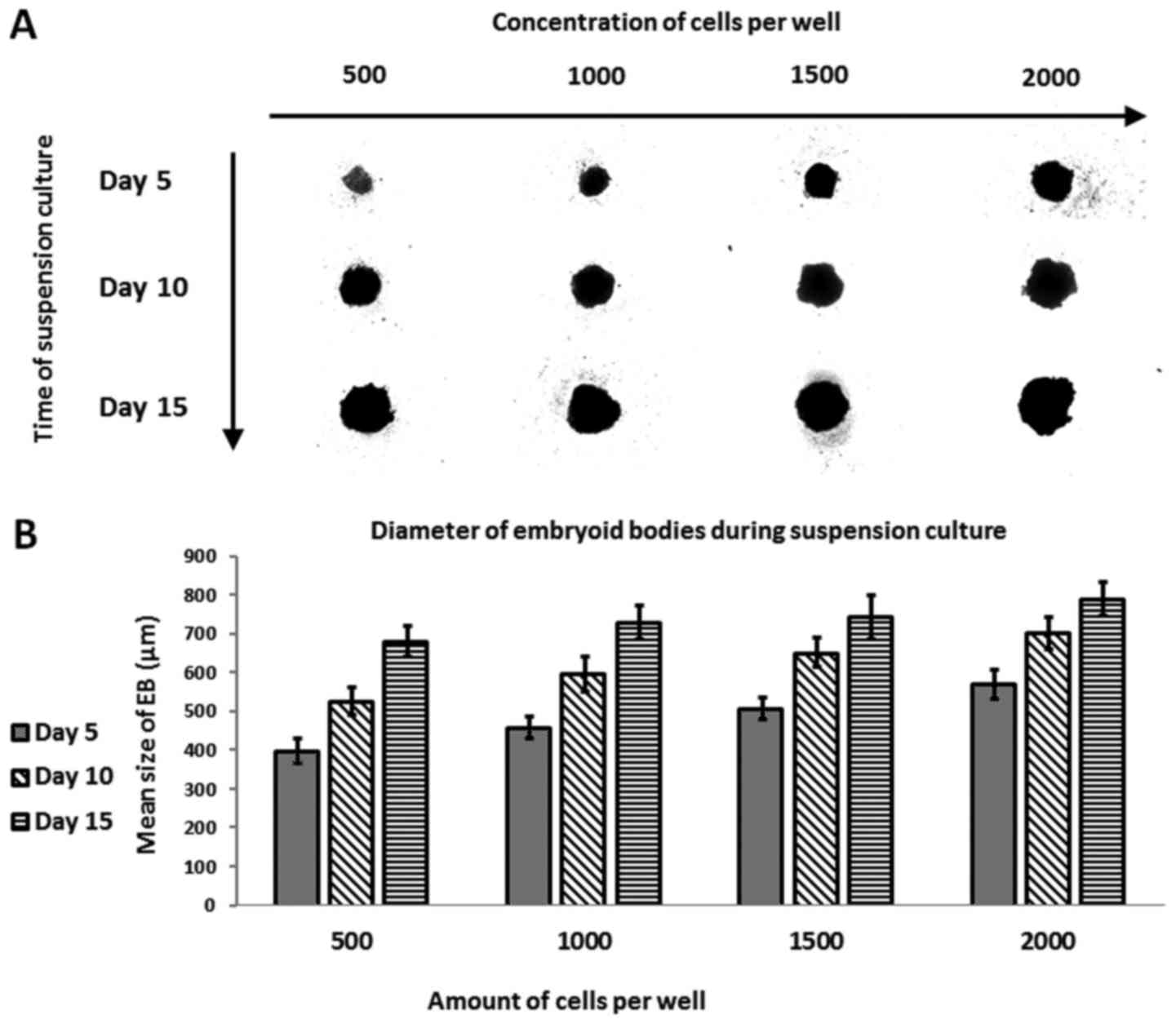
(Fig. 1A and B). Most (78.1%) of
the EBs were homogenous (Table
III). EBs formed in wells seeded with 500 cells measured a mean
of 397.92±31.91 µm in diameter at day 5, 526.06±36.46 µm at day 10,
and 680.33±38.46 µm at the final cell count (day 15, the end of the
suspension culture). The corresponding values for wells seeded with
1,000 cells were as follows: 457.85±28.46, 596.17±44.5, and
728.1±42.94 µm on days 5, 10 and 15, respectively. In wells with
1,500 cells, the values on days 5, 10 and 15 were: 508±28.31,
651.31±38.49, and 743.04±56.96 µm, respectively. The largest EBs
were formed in wells seeded with 2,000 cells, as follows:
568.91±36.02, 702.36±41.27, and 789.06±42.30 µm. These changes in
EB diameters indicate increased cell proliferation in the smaller
EBs formed in 500-cell wells vs. those with 2,000 cells.
 | Table III.Efficiency of homogeneous formation
of spherical EBs in BRAND inertGRADE™ 96-well plates. |
Table III.
Efficiency of homogeneous formation
of spherical EBs in BRAND inertGRADE™ 96-well plates.
| Feature of EBs per
well (n=2,300) | Percentage (%) |
|---|
| Homogeneous (single
spherical EB) | 78.09 |
| Heterogeneous
(double-triple-spherical, elongated EBs) | 21.91 |
The number of cells used for EB
formation affects the spontaneous differentiation of these cells
into three germ layers
Gene expression was estimated on days 5, 10 and 15
of the EB suspension culture procedure. The following markers were
evaluated to assess spontaneous differentiation (Fig. 2A-H): pluripotency (NANOG, SRY [sex
determining region Y]-box 2 (SOX2)], primitive streak mesoendoderm
[MIXL, Brachyury (T)], ectoderm (VIM, PAX6), endoderm [forkhead box
protein A2 (FOXA2)], and mesenchyme [α-smooth muscle actin
(α-SMA)]. Expression of the pluripotency markers NANOG (Fig. 2A) and SOX2 (Fig. 2B) decreased in all well variants
compared to controls. EBs expressing favourable pluripotent markers
were not observed in any of the variants. Expression of the
mesodermal marker Brachyury (Fig.
2C) was most significant on day 15 in the 500 and 1,000 cell
wells, indicating the pro-mesodermal potential of those EBs
compared to controls and the other variants (e.g., 1,500 and 2,000
cell wells) at other time points. Surprisingly, the mesodermal
marker MIXL1 (Fig. 2D) was more
highly expressed in EBs formed in 500 and 1,000 cell wells at day
15 vs. controls and other variants. No differences in expression of
the mesenchymal marker SMA (Fig.
2E) were observed in EBs compared to hESCs. During suspension
culture, SMA expression increased in all cell colony variants, with
the highest expression observed at day 10 in EBs cultured in 2,000
cell cultures compared to the other culture variants. Compared to
controls, expression of the endoderm marker gene FOXA2 (Fig. 2F) was lower on days 5 and 10 but
higher on day 15. Expression of this marker increased throughout
the suspension culture period in all EB populations. A significant
increase was observed at day 10 in the 2,000-cell wells and at day
15 in the 1,000-cell wells. Compared to BG01V, expression of the
ectoderm marker VIM (Fig. 2G)
decreased notably in all variants on days 5, 10, and 15. The only
exceptions were in the 500 and 1,000 cell EB: On day 15, VIM
expression in EBs was similar to the control. Similarly, the 500
and 1,000 cell variants presented the highest expression of VIM
compared to the 1,500 and 2,000 cell cultures assessed earlier in
the suspension culture. PAX6 (Fig.
2H) expression increased over time, achieving levels comparable
to controls at days 10 and 15. PAX6 expression was highest in the
500 and 1,000 cell variants on day 10, and in the 500 cell variant
on day 15 compared to the other groups.
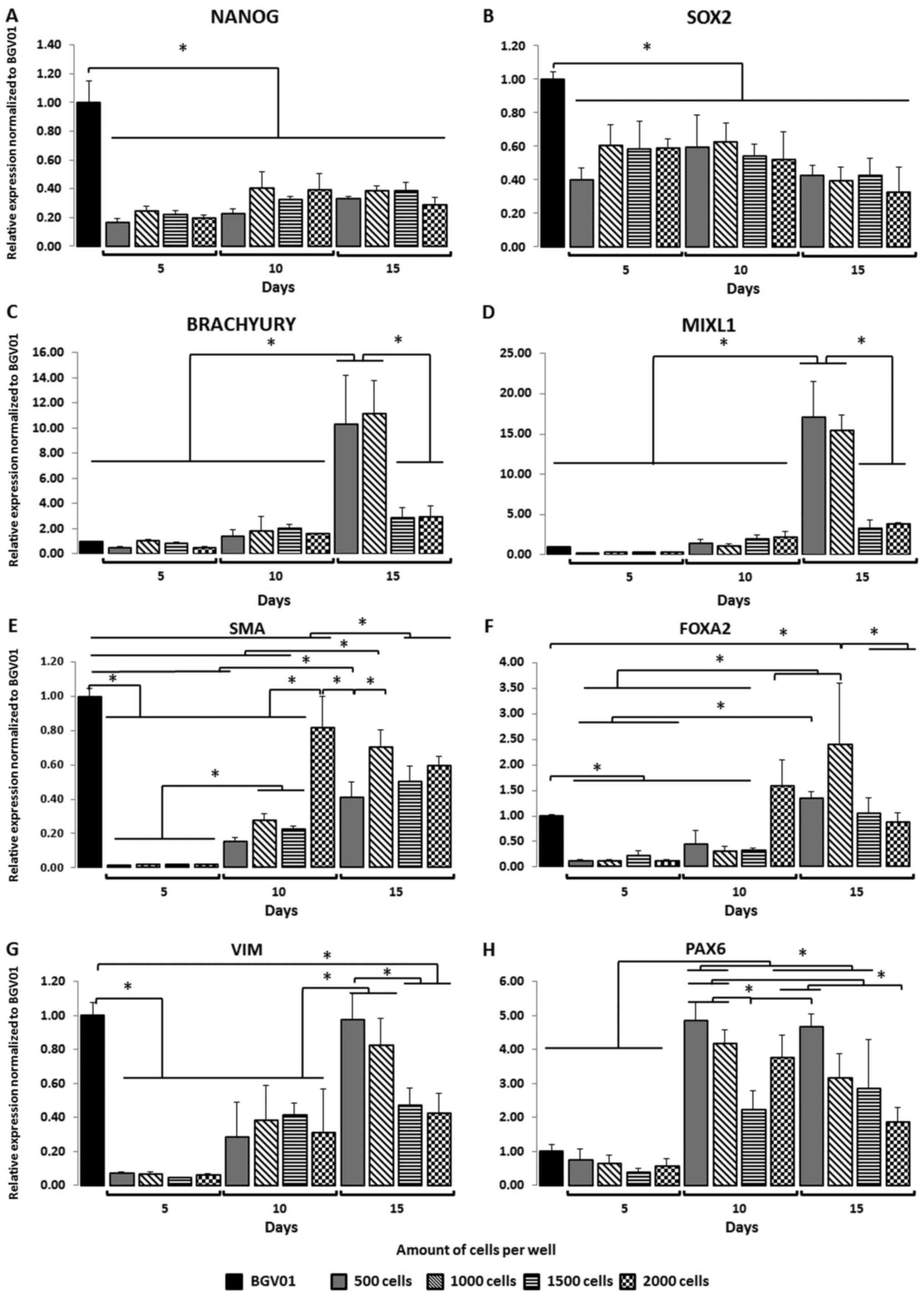 | Figure 2.Size of EBs and time (days) in the
suspension culture affects the expression of germ layer specific
genes. During suspension cell culture, the pluripotency markers,
(A) NANOG and (B) SOX2, decreased among all studied variants. Gene
expression analysis indicated that the mesoderm, (C) Brachyury and
(D) MIXL1, germ layer was favoured in EBs formed from 500 and 1,000
cell wells when compared with the 1,500 and 2,000 cell wells at the
end of suspension culture (day 15). The mesenchymal marker (E) SMA
and endoderm marker (F) FOXA2 did not predominantly express in the
studied variants. Gene expression analysis indicated that the
ectoderm germ layer, (G) VIM and (H) PAX6, was favoured in EBs
formed from 500 and 1,000 cell wells when compared with the 1,500
and 2,000 cell wells at the end of the suspension culture (day 15).
The y-axis represents the relative expression of analysed genes,
normalized to the BG01V cell line. Data are presented as the mean ±
standard deviation. *P<0.05, as indicated. EBs, embryoid bodies;
SOX2, sex determining region Y-box 2; MIXL1, mix paired-like
homeobox; α-SMA, α-smooth muscle actin; FOXA2, forkhead box protein
A2; VIM, vimentin; PAX6, paired box 6. |
The influence of EB size on
chondrogenic differentiation
Based on the gene expression of EBs evaluated during
suspension culture, EBs formed in the larger (2,000 cell) colonies
presented lower levels of mesodermal expression whereas the EBs
with the greatest mesodermal expression were formed in the smaller
(500 cell) wells. EBs were collected on days 5 and 15 to test the
hypothesis that prolonged time in suspension culture would improve
differentiation into chondrocytes due to the increasing expression
of mesodermal markers over time. To perform this test, we used a
serum-free chondrogenic medium supplemented with TGF-β3. After
differentiation, gene expression analysis was performed to identify
genes associated with pluripotency (NANOG and SOX2); mesoderm (T);
chondrocytes [SOX9, FGFR3, CD44, type I collagen (COL1A2), COL2A1
and ACAN], and mesenchymal condensation [Neuronal Cell Adhesion
Molecule (NCAM), N-cadherin (CDH2) and tenascin-C (TNC)] (Figs. 3 and 4).
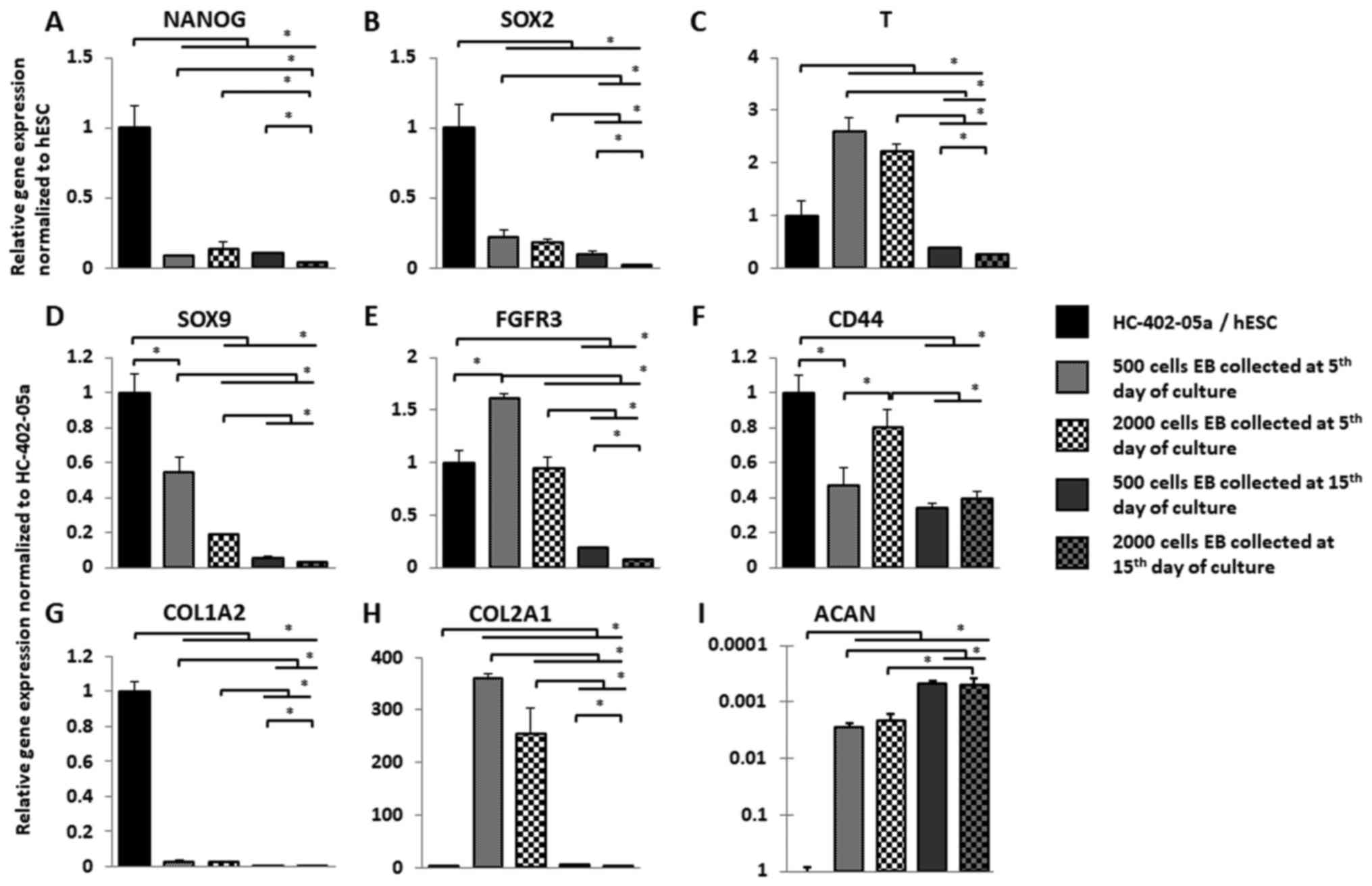 | Figure 3.Smaller EBs exhibit more
prochondrogenic outcomes. Following collection of EBs from
suspension culture, the 3 week chondrogenic differentiation
protocol was performed and gene expression was analysed. Expression
of (A) NANOG and (B) SOX2 decreased in all studied variants.
Expression of (C) T, (D) SOX9 and (E) FGFR3 was significantly
higher in EBs formed in 500-cell wells when compared with
2,000-cell wells. (F) CD44 was significantly expressed in
differentiated 2,000-cell wells EB collected on the fifth day of
suspension culture. (G) The low level of COL1A2 expression was
observed in all studied variants following chondrogenic
differentiation. (H) Expression of COL2A1 was significantly higher
in EBs formed in 500-cell wells when compared with 2,000-cell
wells. (I) ACAN expression increased only in EBs collected on day
5. Overall, 15 days of suspension culture resulted in a lower
expression of analysed genes in chondrogenically differentiated EBs
formed from 500- and 2,000-cell wells. The y-axis represents the
relative expression of analysed genes, normalized to the BG01V or
HC-402-05a cell line. Data are presented as the mean ± standard
deviation. *P<0,05, as indicated. EBs, embryoid bodies; hESC,
human embryonic stem cells; SOX, sex determining region Y-box; T,
brachyury gene; FGFR3, fibroblast growth factor receptor 3; CD44,
cluster of differentiation 44; COL, collagen; ACAN, aggrecan. |
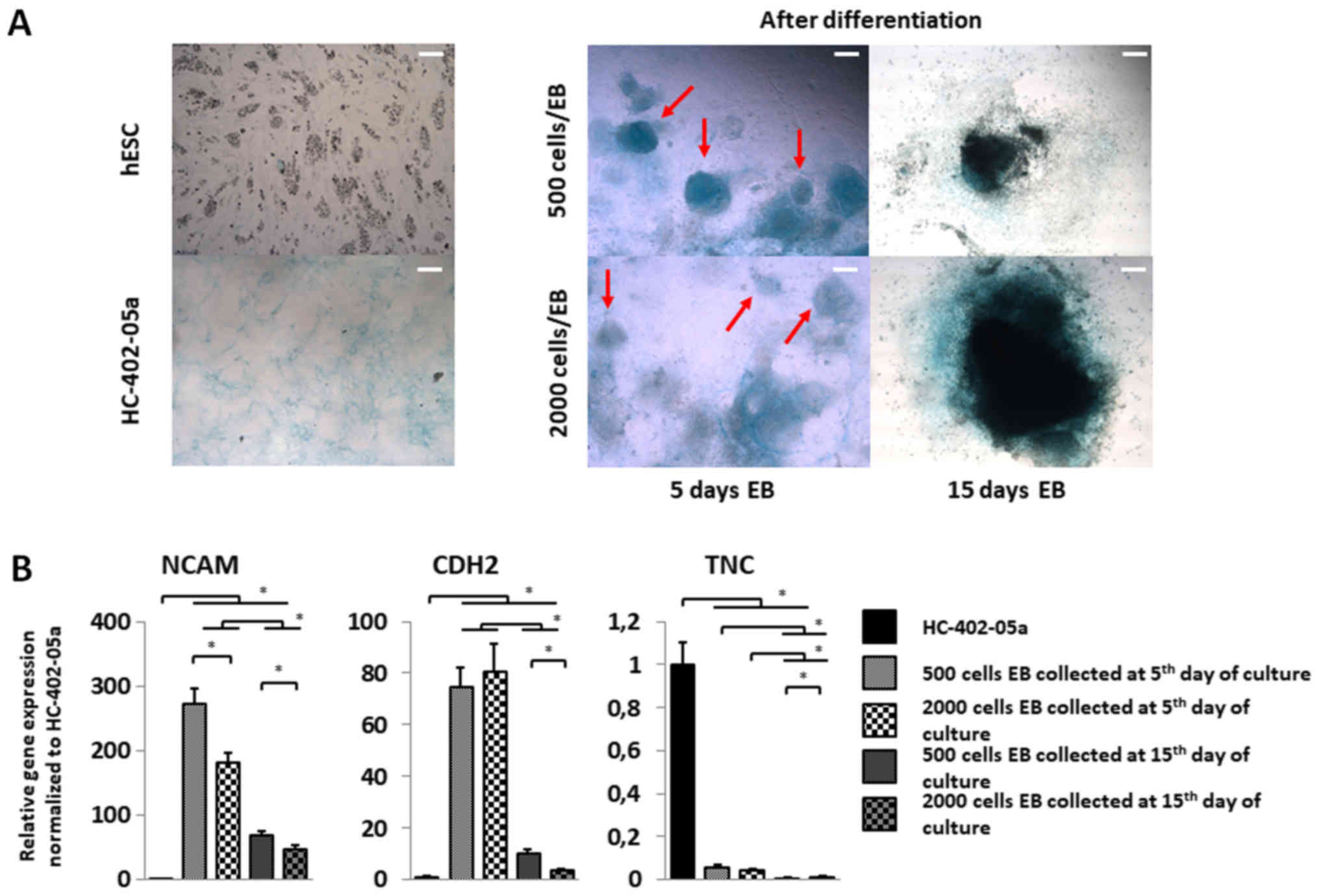 | Figure 4.Cells differentiated from smaller EBs
exhibit increased expression of genes associated with mesenchymal
condensation and a more condensed cartilage nodule structure. (A)
EBs harvested on day 5 of the suspension culture for chondrogenic
differentiation presented more condensed cartilage-like nodules
(red arrows). On day 15, the presence of intensively stained areas
indicated a dense extracellular matrix in those structures. Scale
bars, 200 µm. (B) To confirm mesenchymal condensation, reverse
transcription-quantitative polymerase chain reaction of TNC, CDH2
and NCAM was performed. Mesenchymal condensation progressed more
quickly in cells differentiated in 500-cell wells collected on day
5 of suspension culture. The y-axis represents the relative
expression of analysed genes, normalized to the HC-402-05a cell
line. Data are presented as the mean ± standard deviation.
*P<0,05, as indicated. EBs, embryoid bodies; hESC, human
embryonic stem cells; TNC, tenascin C; CDH2, cadherin 2; NCAM,
neural cell adhesion molecule. |
Compared to hESC cells, NANOG and SOX2 expression
decreased significantly in the differentiated EBs (Fig. 3A and B). Among the various cell
colony variants, EBs differentiated in 2,000-cell wells collected
on day 15 presented the lowest expression of pluripotency markers;
by contrast, expression of T (Fig.
3C) in differentiated EB cells was higher on day 5 compared to
controls and compared to EBs collected on day 15. No significant
differences in gene expression levels between EBs formed in 500 and
2,000 cell colonies were observed on day 5. SOX9 expression
(Fig. 3D) decreased in all
differentiated EBs relative to SOX9 expression in the HC-402-05a
cell line. SOX9 expression was highest on day 5 in EBs formed in
500 cell cultures. FGFR3 (Fig. 3E)
expression increased in EBs formed in 500 cell wells compared to
controls, but not on day 5 in 2,000 cell-well cultures.
Interestingly, FGFR3 expression was lower in differentiated EBs on
day 15 vs. EBs collected on day 5. CD44 (Fig. 3F) expression was higher in EBs
compared to controls, with the highest expression on day 5 in EBs
formed in 2,000 cell cultures. CD44 expression in EBs formed in 500
and 2,000 cell cultures on day 5 and in EBs formed on day 15 from
500 cell colonies were similar, perhaps due to the dense matrix
produced during 15 days of suspension culture.
After the differentiation process was complete, we
analysed the genes related to extracellular matrix production. All
differentiated EBs presented decreased expression of COL1A2
compared to controls (Fig. 3G).
However, EBs formed in 500 and 2,000 cell colonies and collected at
day 5 expressed type I collagen more highly than EBs collected on
day 15. COL2A1 is a marker of successful chondrogenic
differentiation (Fig. 3H). The
highest expression of COL2A1 was observed in EBs formed in 500 cell
cultures harvested on day 5. On days 5 and 15, EBs differentiated
in 500 cell cultures expressed COL2A1 more highly than EBs
harvested from 2,000 cell wells. ACAN (Fig. 3I) expression a marker of
chondrocytes associated with the production of proteoglycans was
much lower in the differentiated EB cells compared to HC-402-05a.
However, EBs collected on day 5 expressed ACAN more highly than
those harvested on day 15. No significant changes in ACAN
expression were observed among the various EB culture sizes
collected on the same days.
The influence of EB size on cartilage
nodule formation
Chondrocytes produce a specific extracellular matrix
(ECM) consisting mostly of proteoglycans and collagens.
Glycosaminoglycan (GAG) deposition was marked by alcian blue
staining. Blue staining and more condensed cartilage-like nodules
were more evident in EBs formed from 500 cell colonies compared to
the 2,000 cell cultures (Fig. 4A).
In EBs harvested on day 15, cartilage nodule condensation was
difficult to assess because the cells had less motility due to the
dense ECM formed during suspension culture. To explain the
different phenotypes of these nodules and to confirm the influence
of EB size on the mesenchymal condensation process, we estimated
the expression of genes (i.e., NCAM, TNC and CDH2) associated with
the condensation process (Fig.
4B). NCAM expression was higher in all of the cell colony
variants vs. controls (Fig. 4B).
The greatest in NCAM expression was observed on day 5 in EBs formed
from 500 cell cultures. CDH2 expression was elevated in all cell
culture variants compared to the HC-402-05a cell line (Fig. 4B). CDH2 expression did not differ
significantly between EBs in 500 and 2,000 cell cultures collected
on day 5, although CDH2 levels were much higher compared to EBs
harvested on day 15. CDH2 expression was higher on day 15 in EBs
formed from 500 vs. 2,000 cell colonies. By contrast, TNC
expression decreased in all studied populations compared to control
cells (Fig. 4B). No significant
differences were observed between TNC expression on day 5 in EBs
from the 500 and 2,000 cell colonies. TNC expression was slightly
higher in EBs collected on day 5 vs. day 15. Those results indicate
the presence of pre-chondrocytes and the formation of cartilage
nodules in differentiated EBs harvested on days 5 and 15 of
suspension culture.
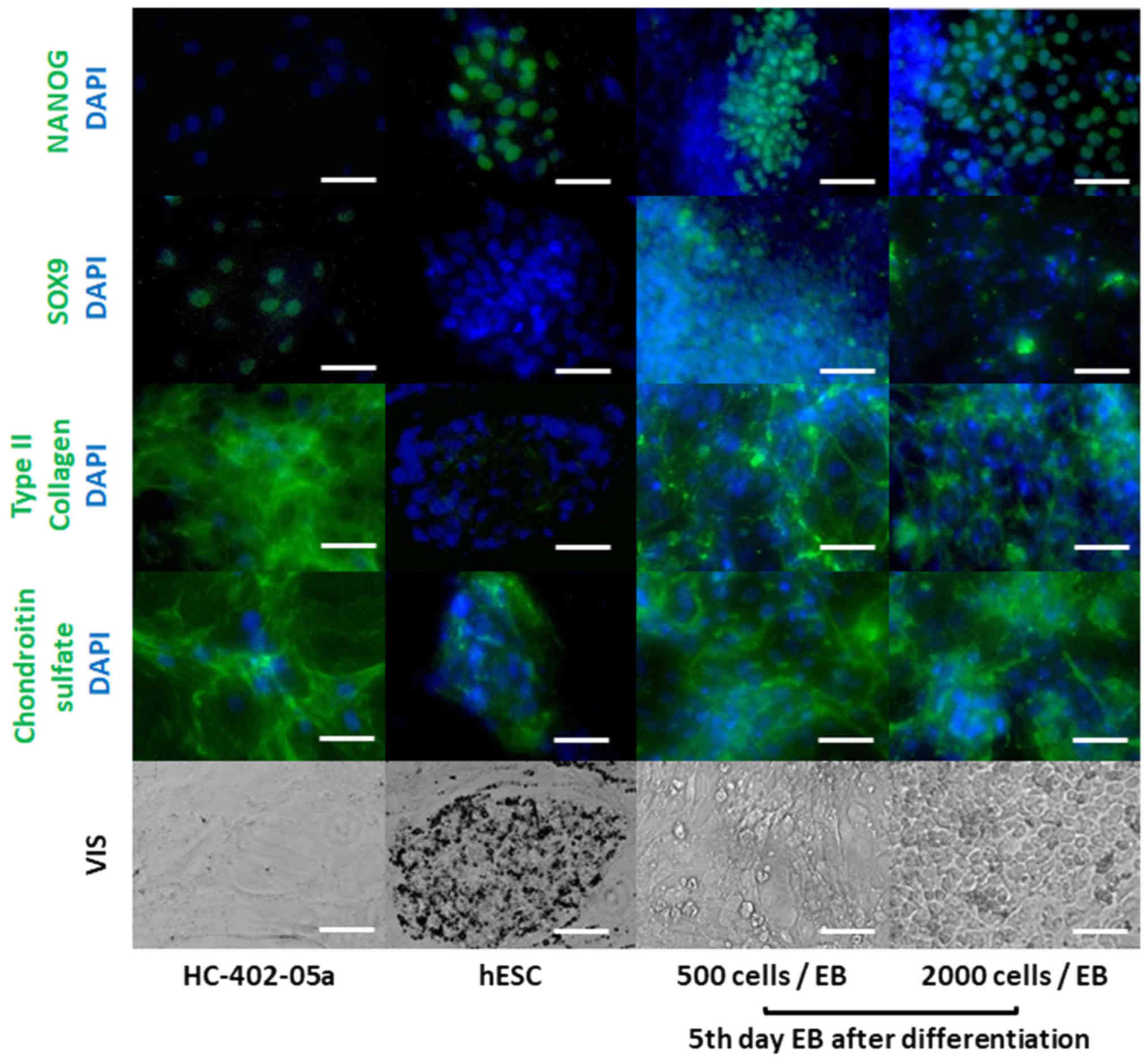
Chondrocyte-like and pluripotent cells
are observed in differentiated EBs
Indirect immunofluorescence staining was performed
for SOX9, type II collagen, chondroitin sulphate, and NANOG to
confirm the presence of proteins related to chondrocyte-like cells
and the absence of pluripotent stem cells in differentiated EBs
(Fig. 5). Staining showed that 5
day EBs formed from 500 and 2,000 cell cultures and harvested after
3 weeks of the differentiation process the number of cells
expressing NANOG was lower than hESCs. Staining also confirmed SOX9
expression in the differentiated cells. ECM compounds
characteristic of chondrocytes (such as type II collagen) were
observed in the differentiated EBs. Moreover, we observed an
expansive distribution pattern of chondroitin sulphate in these EBs
in contrast to the consistent distribution observed in hESCs. Due
to decreased cell motility and the high concentration of ECM
deposition in large EBs after 15 days of suspension culture, we
were unable to evaluate microscopically the specificity of
immunofluorescence staining after 3 weeks differentiation process.
Immunofluorescence staining was unable to determine the most
prochondrogenic of the various differentiated EB populations.
Overall, these results indicate the presence of chondrocyte-like
cells and the differentiated cells were also heterogeneous.
Discussion
In our previous work we indicated that the EBs based
differentiation protocol into chondrocyte-like cells is good, but
not perfect (15,22). For that purpose, we wanted to see
if elongation of suspension culture would influence the
chondrogenesis process. That is why the main aim of the present
study was to evaluate the influence the number of cells in the
culture on spontaneous and chondrogenic differentiation of EBs. The
EB formation process was carried out in low-adhesion round
bottom-well plates specifically designed for suspension culture in
order to closely control the number of cells seeded in the wells
and thus to obtain more homogenous EBs. After differentiation, most
(approximately 78%) of the EBs were homogenous in size and
spherical. Although this is a relatively good outcome, published
reports indicate lithographic well-design techniques can yield much
higher rates of homogenous EBs (23–25).
We also established the influence of varying cell quantities used
for EB formation on spontaneous differentiation into three germ
layers. Because the mesodermal germ layer is a chondrocyte
precursor, we focused on the EBs that displayed the highest
expression of mesoderm markers. We found that EBs formed in 500 and
1,000 cell cultures for 15 days expressed the mesodermal markers T
and MIXL1 more highly than in other variants. Ng et al
(26) showed that expression of
the mesoderm germ layer markers in three hESC lines was higher in
EBs formed in 500 and 1,000 cells. There is a strong correlation
between EBs with a strong expression of mesoderm germ layer markers
and the cell culture microenvironment. The global gene expression
of EBs propagated in a standard suspension culture plate indicates
a stronger upregulation of cardiac and mesoderm-related genes
compared to ectoderm and endoderm genes, a phenomenon that can be
explained by the diffusion of endogenous molecules secreted by EBs
into the local microenvironment (27).
The results described above have prompted further
research into the effect of the number of cells and the duration of
suspension culture on chondrogenic differentiation. Based on the
expression of Brachury and MIXL1, we decided to verify our
hypothesis by differentiation of 500 cell wells EBs collected after
15 days in suspension culture. Additionally, we choose the 500 cell
wells EBs after 5 days in suspension culture. This variant was
chosen based on other studies revelations, where various
progenitors derived from mesodermal germ layer where obtained more
efficiently from younger and smaller EBs (26,28–30).
Due to low expression of mesodermal markers as a variant with less
prochondrogenic potential we choose 2,000 cell wells after 5th and
15th day of suspension culture. In our study, we estimated the
expression of genes related to pluripotency and chondrogenesis
after 21 days of chondrogenic differentiation. Unfortunately, the
differentiated cells still expressed pluripotency markers (i.e.,
NANOG and SOX2). This is a common problem in all currently
available differentiation techniques, and the continued expression
of pluripotency markers even after completion of the
differentiation process has been associated with an increased risk
of teratomas (31). However, the
use of oleic acid synthesis inhibitors, which is crucial for
maintaining stemness and viability of pluripotent stem cells, could
increase the clinical safety of differentiated cells. In our study,
the differentiated EBs collected after 5 days in the suspension
culture showed significant upregulation of T and FGFR3 compared to
larger EBs and those collected after 15 days. SOX9 expression
decreased compared to controls (articular chondrocyte cell line)
but increased compared to EBs obtained from the other cell culture
variants. Those results suggest that the chondrocyte-like cells
obtained in this study were at the early stages of chondrogenesis.
Chondrogenesis was confirmed by the upregulation of FGFR3 and
COL2A1 in smaller EBs, which was correlated with the parallel
upregulation of T. This correlation is related to early limb
formation, where T expression enhances recruitment of mesoderm
cells towards chondroprogenitors via the FGFR3 pathway. Our
findings with regards to the expression of molecules associated
with ECM suggest chondrocytes in early stages of differentiation In
addition, the expression of COL2A1, CD44, COL1A2 and ACAN decreased
compared to controls, indicating an immature chondrocyte phenotype
(14,32). Another reason to explain why the
obtained chondrocytes were premature is the behaviour of
chondrocytes in native tissue, where ECM proteins are better
expressed in three dimensional environments rather than in
monolayer cultures. Other explanation for the premature
chondrocytes includes culture conditions. We established the
culture conditions in normoxia, which could also have negatively
influenced the efficiency of chondrogenic differentiation given
that hypoxic conditions are known to be more prochondrogenic.
Similarly, the size of the EBs can also influence chondrogenic
differentiation, as one study showed in EBs derived from mouse
embryonic stem cells (28).
We also used alcian blue staining to evaluate the
effect of EB mass on the deposition of proteoglycans, estimating
the expression of genes related to mesenchymal condensation. In
differentiated EBs, we observed blue stained areas, indicating the
presence of deposited proteoglycans. EBs cultured for a longer time
presented intensively stained areas, which could be interpreted as
indicating more chondrogenic properties. Previous studies of the
ECM composition of EBs have shown that the matrix is composed of
laminins, type IV collagen, versican, fibronectin, hyaluronian,
and/or type I collagen, which could explain the large,
positively-stained areas of ECM in older EBs after differentiation
(33,34). As we expected, those findings could
affect expression of genes related to the mesenchymal condensation
process. Based on our results, we can make the following
conclusions: first, the immaturity of the obtained chondrocytes was
due to low TNC expression (35);
second, the mesenchymal condensation process was more advanced in
younger cells obtained from smaller (500 cell) cultures, which
could be explained by higher NCAM levels. The relation between CDH2
and NCAM during chondrogenesis could explain the lack of clear
differences in CDH2 expression between EBs formed from 500 and
2,000 cell cultures and collected on day 5. Studies of the role of
CDH2 and NCAM during in vitro chondrogenesis in chick limb
buds have shown that CDH2 expression decreases during the
finalization of chondrocyte maturation with a parallel increase in
NCAM expression (36). The low
expression of CDH2 in older differentiated EBs could explain the
low cell motility, which was due to dense ECM observed in the
intensively stained areas.
Immunofluorescence staining of the differentiated
cells confirms the presence of NANOG, SOX9, type II collagen, and
positively-stained chondroitin sulphate cells. Importantly, the
pattern of chondroitin sulphate signalling in differentiated cells
was similar to that observed in the chondrocyte cell line. Our
results indicate the limitations of this approach: We obtained a
mixed population of differentiated and undifferentiated cells,
which is the main disadvantage of this technique. As mentioned
previously, we did not passage or sort the cells during the
experiments in order to achieve a broad overview of the influence
of EB size on the chondrogenic process. This explains why we
observed an increased accumulation of ECM in the EBs harvested on
day 15, which did not allow us to estimate the influence of EB size
and culture time on protein levels by immunofluorescence
staining.
In this study, we have shown that several factors
the duration of suspension culture, EB size, and particularly the
number of plated cells used to form the EBs can significantly
affect spontaneous differentiation and EB heterogeneity. In
particular, we found that differentiated EBs formed from 500 cell
cultures and collected on the 5th day of suspension culture
presented better prochondrogenic properties. We determined that the
decreased cellular mass of pluripotent stem cells used to form EBs
positively affects chondrogenic differentiation and mesenchymal
condensation. Differentiation of hESCs into chondrocytes with
intermediate EBs generates chondrocyte-like cells at early stages
of chondrogenesis. Therefore, additional steps are needed to obtain
chondrocyte-like cells at more advanced stages of chondrogenesis, a
finding that suggests that existing protocols will need to be
modified.
Acknowledgements
The authors would like to thank Mr. Bradley Londres
for his invaluable assistance in editing the final text.
Funding
The present study was funded by the National Science
Centre allocated on the basis of (grant no.
2012/07/E/NZ3/01819).
Availability of data and material
All data generated or analysed during this study are
included in this published article.
Authors' contributions
WMS and TT were responsible for experimental design.
KK and KJ performed the experiments. WMS, MSL, KK, MR and KJ
analysed and interpreted the results. All authors were responsible
for the writing and preparation of the manuscript and figures.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Windt TS, Vonk LA, Brittberg M and
Saris DBF: Treatment and prevention of (Early) osteoarthritis using
articular cartilage repair-fact or fiction? A systematic review.
Cartilage. 4 3 Suppl:5S–12S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Wei X, Zhou J and Wei L: The
age-related changes in cartilage and osteoarthritis. Biomed Res
Int. 2013:9165302013.PubMed/NCBI
|
|
3
|
Madry H, Luyten FP and Facchini A:
Biological aspects of early osteoarthritis. Knee Surg Sports
Traumatol Arthrosc. 20:407–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teixeira Moreira LS, Leijten JC, Sobral J,
Jin R, van Apeldoorn AA, Feijen J, van Blitterswijk C, Dijkstra PJ
and Karperien M: High throughput generated micro-aggregates of
chondrocytes stimulate cartilage formation in vitro and in vivo.
Eur Cell Mater. 23:387–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caron MM, Emans PJ, Coolsen MM, Voss L,
Surtel DA, Cremers A, van Rhijn LW and Welting TJ:
Redifferentiation of dedifferentiated human articular chondrocytes:
Comparison of 2D and 3D cultures. Osteoarthritis Cartilage.
20:1170–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carroll SH and Ravid K: Differentiation of
mesenchymal stem cells to osteoblasts and chondrocytes: A focus on
adenosine receptors. Expert Rev Mol Med. 15:e12013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solchaga LA, Penick KJ and Welter JF:
Chondrogenic differentiation of bone marrow-derived mesenchymal
stem cells: Tips and tricksMethods in molecular biology. 698.
Vemuri M, Chase LG and Rao MS: Humana Press; Totowa, NJ: pp.
253–278. 2011, View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanawa M, Igarashi A, Ronald VS, Higashi
Y, Kurihara H, Sugiyama M, Saskianti T, Pan H and Kato Y:
Age-dependent decrease in the chondrogenic potential of human bone
marrow mesenchymal stromal cells expanded with fibroblast growth
factor-2. Cytotherapy. 15:1062–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ko JY, Kim KI, Park S and Im GI: In vitro
chondrogenesis and in vivo repair of osteochondral defect with
human induced pluripotent stem cells. Biomaterials. 35:3571–3581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsumaki N, Okada M and Yamashita A: iPS
cell technologies and cartilage regeneration. Bone. 70:48–54. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robinton DA and Daley GQ: The promise of
induced pluripotent stem cells in research and therapy. Nature.
481:295–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beers J, Linask KL, Chen JA, Siniscalchi
LI, Lin Y, Zheng W, Rao M and Chen G: A cost-effective and
efficient reprogramming platform for large-scale production of
integration-free human induced pluripotent stem cells in chemically
defined culture. Sci Rep. 5:113192015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toh WS, Guo XM, Choo AB, Lu K, Lee EH and
Cao T: Differentiation and enrichment of expandable chondrogenic
cells from human embryonic stem cells in vitro. J Cell Mol Med.
13:3570–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oldershaw RA, Baxter MA, Lowe ET, Bates N,
Grady LM, Soncin F, Brison DR, Hardingham TE and Kimber SJ:
Directed differentiation of human embryonic stem cells toward
chondrocytes. Nat Biotechnol. 28:1187–1194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suchorska WM, Augustyniak E, Richter M and
Trzeciak T: Comparison of four protocols to generate
chondrocyte-like cells from human induced pluripotent stem cells
(hiPSCs). Stem Cell Rev. 13:299–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahmoudifar N and Doran PM: Chondrogenesis
and cartilage tissue engineering: The longer road to technology
development. Trends Biotechnol. 30:166–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon SH, Ju J, Park SJ, Bae D, Chung HM
and Lee SH: Optimizing human embryonic stem cells differentiation
efficiency by screening size-tunable homogenous embryoid bodies.
Biomaterials. 35:5987–5997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Preda MB, Burlacu A and Simionescu M:
Defined-size embryoid bodies formed in the presence of serum
replacement increases the efficiency of the cardiac differentiation
of mouse embryonic stem cells. Tissue Cell. 45:54–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Winkle AP, Gates ID and Kallos MS:
Mass transfer limitations in embryoid bodies during human embryonic
stem cell differentiation. Cells Tissues Organs. 196:34–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang YS, Chung BG, Ortmann D, Hattori N,
Moeller HC and Khademhosseini A: Microwell-mediated control of
embryoid body size regulates embryonic stem cell fate via
differential expression of WNT5a and WNT11. Proc Natl Acad Sci USA.
106:16978–16983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suchorska WM, Lach MS, Richter M,
Kaczmarczyk J and Trzeciak T: Bioimaging: An useful tool to monitor
differentiation of human embryonic stem cells into chondrocytes.
Ann Biomed Eng. 44:1845–1859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dias AD, Unser AM, Xie Y, Chrisey DB and
Corr DT: Generating size-controlled embryoid bodies using laser
direct-write. Biofabrication. 6:0250072014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu F, Sridharan B, Wang S, Gurkan UA,
Syverud B and Demirci U: Embryonic stem cell bioprinting for
uniform and controlled size embryoid body formation.
Biomicrofluidics. 5:222072011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pettinato G, Wen X and Zhang N: Formation
of well-defined embryoid bodies from dissociated human induced
pluripotent stem cells using microfabricated cell-repellent
microwell arrays. Sci Rep. 4:74022014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ng ES, Davis RP, Azzola L, Stanley EG and
Elefanty AG: Forced aggregation of defined numbers of human
embryonic stem cells into embryoid bodies fosters robust,
reproducible hematopoietic differentiation. Blood. 106:1601–1603.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giobbe GG, Zagallo M, Riello M, Serena E,
Masi G, Barzon L, Di Camillo B and Elvassore N: Confined 3D
microenvironment regulates early differentiation in human
pluripotent stem cells. Biotechnol Bioeng. 109:3119–3132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Messana JM, Hwang NS, Coburn J, Elisseeff
JH and Zhang Z: Size of the embryoid body influences chondrogenesis
of mouse embryonic stem cells. J Tissue Eng Regen Med. 2:499–506.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cha JM, Bae H, Sadr N, Manoucheri S,
Edalat F, Kim K, Kim SB, Kwon IK, Hwang YS and Khademhosseini A:
Embryoid body size-mediated differential endodermal and mesodermal
differentiation using polyethylene glycol (PEG) Microwell Array.
Mac Res. 23:245–255. 2015. View Article : Google Scholar
|
|
30
|
Lee EJ, Lee HN, Kang HJ, Kim KH, Hur J,
Cho HJ, Lee J, Chung HM, Cho J, Cho MY, et al: Novel embryoid
body-based method to derive mesenchymal stem cells from human
embryonic stem cells. Tissue Eng Part A. 16:705–715. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng A, Kapacee Z, Peng J, Lu S, Lucas
RJ, Hardingham TE and Kimber SJ: Cartilage repair using human
embryonic stem cell-derived chondroprogenitors. Stem Cells Transl
Med. 3:1287–1294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang SL, Harnish E, Leeuw T, Dietz U,
Batchelder E, Wright PS, Peppard J, August P, Volle-Challier C,
Bono F, et al: Compound screening platform using human induced
pluripotent stem cells to identify small molecules that promote
chondrogenesis. Protein Cell. 3:934–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goh SK, Olsen P and Banerjee I:
Extracellular matrix aggregates from differentiating embryoid
bodies as a scaffold to support ESC proliferation and
differentiation. PLoS One. 8:e618562013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shukla S, Nair R, Rolle MW, Braun KR, Chan
CK, Johnson PY, Wight TN and McDevitt TC: Synthesis and
organization of hyaluronan and versican by embryonic stem cells
undergoing embryoid body differentiation. J Histochem Cytochem.
58:345–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Okamura N, Hasegawa M, Nakoshi Y, Iino T,
Sudo A, Imanaka-Yoshida K, Yoshida T and Uchida A: Deficiency of
tenascin-C delays articular cartilage repair in mice.
Osteoarthritis Cartilage. 18:839–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tavella S, Raffo P, Tacchetti C, Cancedda
R and Castagnola P: N-CAM and N-cadherin expression during in vitro
chondrogenesis. Exp Cell Res. 215:354–362. 1994. View Article : Google Scholar : PubMed/NCBI
|