Introduction
Atherosclerosis may lead to ischemia of the heart,
brain or extremities and may further lead to infarction, which is
the primary cause of mortality in the United States, Europe and
much of Asia (1). The stability of
plaques in coronary arteries is of great importance, as the rupture
of plaques may lead to fatal complications such as myocardial
infarction.
Purinergic 2X7 receptor (P2X7R) is a ligand-gated
cation channel that is expressed by most immune cells such as
macrophages, monocytes and lymphocytes (2). P2X7R is a member of the purinergic
receptor family that is involved in the production and activation
of the inflammatory cytokine interleukin (IL)-1β and modulates the
inflammatory response (3). A
previous study demonstrated that P2X7R was highly expressed in
endothelial cells and macrophages that infiltrate atherosclerotic
plaques of human carotid arteries (4); in addition, P2X7R serves a crucial
role in the development of atherosclerosis by regulating the
activation of the NACHT, LRR and PYD domains-containing protein 3
(NLRP3) inflammasome (5).
Matrix metalloproteinase (MMP)-9 is a 92 kDa protein
that belongs to a family of zinc- and calcium-dependent proteases
(6). MMP-9 is considered to have
various pathological functions. A number of studies have
demonstrated the key role of MMP-9 in atherosclerosis was in the
rupture of plaques through the degradation of the extracellular
matrix (7,8). Extracellular matrix metalloproteinase
inducer (EMMPRIN; also known as CD147 or basigin) is a highly
glycosylated transmembrane protein that was first described in
tumor cells (9). Previous studies
have demonstrated that EMMPRIN, as an upregulator of local MMP-9
expression (10), was involved in
numerous physiological and pathological processes, including tumor
invasion (9) and atherosclerosis
(11). It has also been indicated
that during differentiation from monocytes into macrophages, the
expression of EMMPRIN and MMP-9 is significantly increased
(12), thereby accelerating the
transition of stable plaques into unstable plaques through
atherogenic cells (13).
Consequently, downregulation of EMMPRIN and MMP-9 expression may
ameliorate the development of atherosclerosis. Notably, several
studies have revealed that P2X7R regulates the expression of MMP-9
and that P2X7R is involved in fibrosis progression in the lungs
(14) and liver (15). Upon ATP stimulation, P2X7R in human
peripheral blood mononuclear cells was reported to mediate MMP-9
activities by rapidly increasing the release of MMP-9 and
decreasing the release of tissue inhibitor of metalloproteinases 1
(TIMP-1) (16). However, whether
P2X7R expressed in phorbol 12-myristate 13-acetate (PMA)-induced
THP-1 cells is able to regulate EMMPRIN and MMP-9 expression
remains unexplored.
AMPK is a cellular energy sensor that acts as a
kinase to maintain various processes of energy homoeostasis, such
as fatty acid oxidation, protein synthesis and glucose uptake
(17–19). Our previous study demonstrated that
the inhibition of AMPKα with compound C (a specific AMPK inhibitor)
reduced MMP-9 and EMMPRIN expression levels in PMA-induced THP-1
cell differentiation, which suggested that activation of the AMPKα
pathway may be involved in the regulation of EMMPRIN and MMP-9
expression in PMA-induced macrophages (20). Moreover, a number of previous
studies have reported that MAPK signaling pathways are special
regulators for EMMPRIN and MMP-9 (21,22).
On the basis of these results, the present study hypothesized that
AMPKα and MAPK signaling pathways may regulate the levels of
EMMPRIN and MMP-9 expression. However, whether P2X7R regulate the
activation of AMPKα and MAPK signaling pathways is still
unknown.
Therefore, the present study aimed at exploring the
role of P2X7R in mediating the expression of EMMPRIN and MMP-9 in
PMA-induced THP-1 cells and to further reveal its mechanisms.
Materials and methods
Cell culture and treatment
The human monocytic cell line THP-1 was obtained
from American Type Culture Collection (Manassas, VA, USA) and
maintained at a density of 106 cells/ml as the control
group in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 10 mM
4-(2-hydroxyethyl)-1piperazineethanesulphonic acid (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and 1% penicillin/streptomycin
solution at 37°C in a 5% CO2 incubator. Cells were
cultured in six-well at a density of 106 cells/ml for 48
h at 37°C in the presence of 100 nM PMA (20), which allowed them to differentiate
into adherent macrophages. Cells were pretreated with 100 µM
A-438079 (Selleck Chemicals, Houston, TX, USA) for 1 h at 37°C
(23), and then stimulated with
100 nM PMA for another 48 h at 37°C, which was added directly to
the medium.
Protein isolation and western blot
analysis
Following treatment, cells were washed with cold PBS
(pH 7.4) and cell pellets were lysed for 30 min with a lysis buffer
(0.5% Nonidet P-40; 50 mmol/l Tris-HCl, pH 7.5; 1 mmol/l EDTA; 1
mmol/l EGTA and 150 mmol/l NaCl; containing 10% glycerol; 50 mmol/l
sodium fluoride; 10 mmol/l sodium pyrophosphate; 1 mmol/l sodium
orthovanadate; 80 µmol/l β-glycerophosphate; 1 mmol/l
phenylmethylsulfonyl fluoride; 10 µg/ml aprotinin; 100 µg/ml
soybean trypsin inhibitor and 10 µg/ml leupeptin), followed by
centrifugation at 4°C for 10 min at 12,000 × g. Protein
concentrations were measured by BCA protein assay (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The protein extracts
were denatured and the solubilized proteins (20 µg) subjected to
electrophoresis by 10% SDS-PAGE. Proteins were subsequently
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with TBS
containing 0.05% Tween-20 (TBST) and 5% skimmed milk for 1 h at
room temperature, followed by probing with primary antibodies
against GAPDH (cat. no. 5174), P2X7R (cat. no. 13809), matrix
metalloproteinase (MMP)-9 (cat. no. 13667), 5′-AMP-activated
protein kinase (AMPK)α (cat. no. 5832), phosphorylated (p)-AMPKα
(cat. no. 50081), p38 (cat. no. 8690), p-p38 (cat. no. 9215), c-Jun
N-terminal kinase (JNK) (cat. no. 9252), p-JNK (cat. no. 9255) (all
1:1,000 in TBST; Cell Signaling Technology, Inc., Danvers, MA,
USA), EMMPRIN (1:1,000 in TBST; cat. no. ab666, Abcam, Cambridge,
MA, USA), extracellular signal-regulated kinase (ERK)1/2 (cat. no.
sc81457) or p-ERK1/2 (cat. no. sc81492) (both 1:300 in TBST; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
Following primary antibody incubations, the membranes were
incubated with goat anti-rabbit or goat anti-mouse secondary
antibody (1:1,000; cat. no. A0239 or cat. no. A0216, respectively,
Beyotime Institute of Technology, Haimen, China) for 1 h. Protein
bands were visualized by Enhanced Chemiluminescence Detection
Reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
results were analyzed with Quantity One software 4.62 (Bio-Rad
Laboratories, Inc.) and data were normalized based on GAPDH.
RNA isolation, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA from 106 cells treated with
indicated conditions was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized using the Reverse
Transcription Reagent, according to the manufacturer's protocol
(cat. no. N8080234, Thermo Fisher Scientific, Inc.) and RT-qPCR was
performed with the SYBR Premix Ex Taq kit (cat. no. DRR041; Takara
Biotechnology Co., Ltd., Dalian, China) according to the following
PCR conditions: initial denaturation at 95°C for 30 sec followed by
50 cycles of amplification at 95°C for 5 sec and 60°C for 34 sec.
The amplified fluorescent signal was detected by the ABI-7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primer sequences used in the study were as
follows: MMP-9 (NCBI accession no. NM_004994.2), forward
5′-TGACGCCGCTCACCTTCACT-3′, reverse 5′-CGCGCCATCTGCGTTTCCAA-3′;
EMMPRIN (NCBI accession no. NM_001728.3), forward
5′-TTGGAGGTTGTAGGACCGGCGA-3′, reverse 5′-TGGGACCCTGCCCTTCAAACCA-3′;
and GAPDH (NCBI accession no. NM_001256799.2), forward
5′-CCGCATCTTCTTTTGCGTCGCC-3′, reverse 5′-TCTCAGCCTTGACGGTGCCA-3′.
The expression compared was using the 2−ΔΔCq method
(24). All results were normalized
with GAPDH.
Gelatin zymography
Cells (1×106 cells/well) in the
logarithmic phase were seeded in 6-well plates and incubated in
serum-free medium with or without 100 µM A-438079 for 1 h at 37°C
in a 5% CO2 incubator, followed by incubation with 100
nM PMA for an additional 48 h at 37°C. Culture supernatants were
collected and 20 µl aliquots were loaded onto a 10% polyacrylamide
gel containing 1 mg/ml gelatin. Following electrophoresis, gels
were washed twice with 2.5% Triton X-100 (37°C; 30 min each) and
incubated at 37°C for 18 h in developing buffer comprising 10 mM
Tris base, 40 mM Tris-HCl, 200 mM NaCl, 10 mM CaCl2,
0.02% Brij-35. Gels were subsequently stained with 0.5% (w/v)
Coomassie Brilliant Blue R-250 for 2 h at room temperature,
followed by destaining with a solution containing 50% methanol, 10%
glacial acetic acid and 40% water. MMP-9-digested regions were
visualized as light bands against a dark background. An image of
each gel was captured by an Odyssey Imaging System and analyzed by
Image Studio 5.2.5 (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Statistical analyses were performed using SPSS v18
software (SPSS Inc., Chicago, IL, USA). Three or more groups were
compared using one-way analysis of variance (ANOVA) with
Student-Newman-Keuls and Dunnett methods as post-hoc analysis if
the result of ANOVA was significant. Data were presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed at least three times.
Results
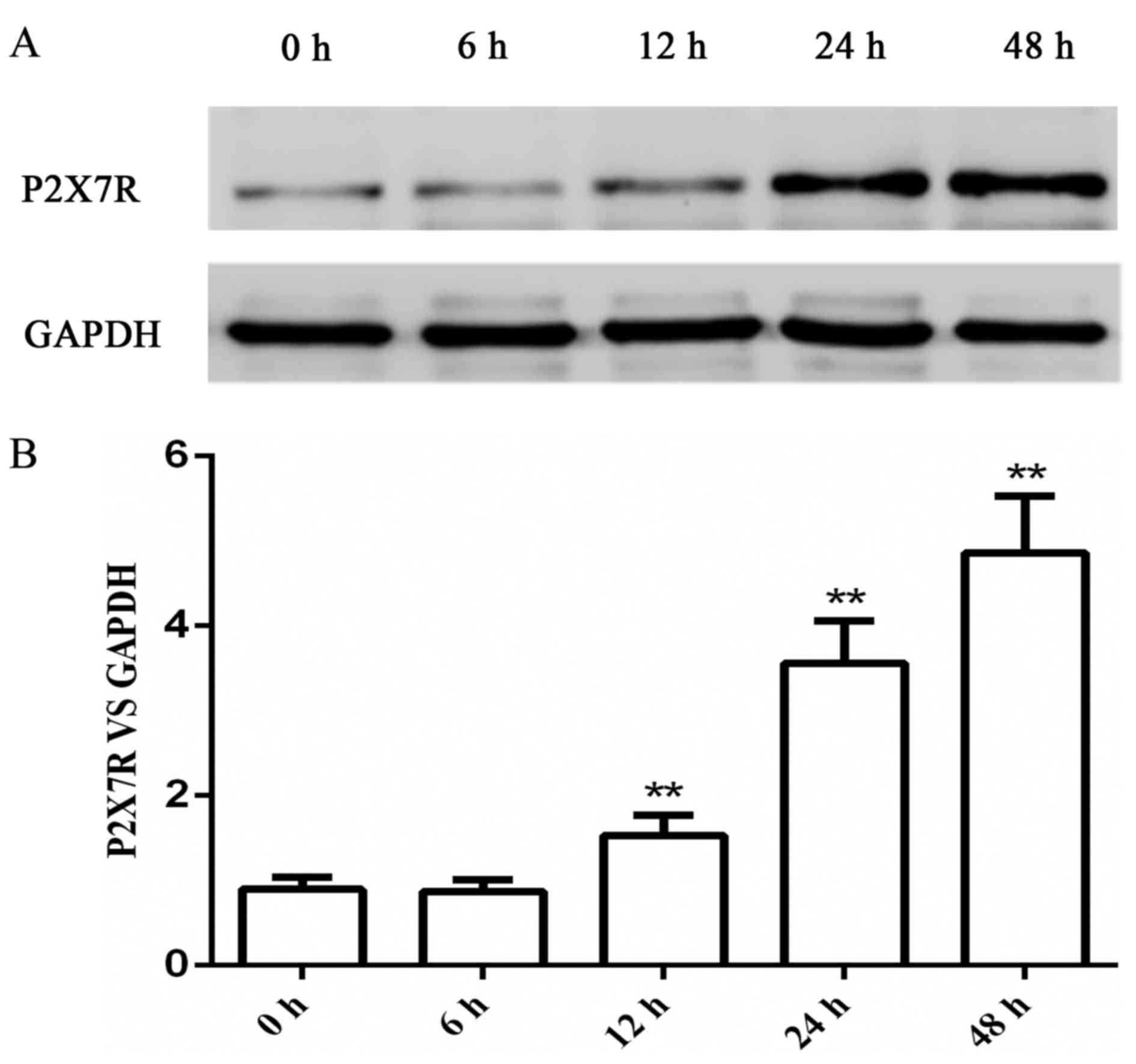
PMA treatment stimulated the
expression of P2X7R in a time-dependent manner
The use of PMA to induce THP-1 cells to
differentiate into macrophages is a classical cell model that is
widely used to explore the inflammatory function of macrophages
in vitro (25,26). THP-1 cells were treated with 100 nM
PMA for different incubation periods ranging between 6 and 48 h.
The protein expression level of P2X7R was measured by western blot
analysis, which indicated that the level of PMA-stimulated P2X7R
expression was in a time-dependent manner (Fig. 1). Therefore, the cells were treated
with 100 nM PMA for 48 h in the subsequent experiments.
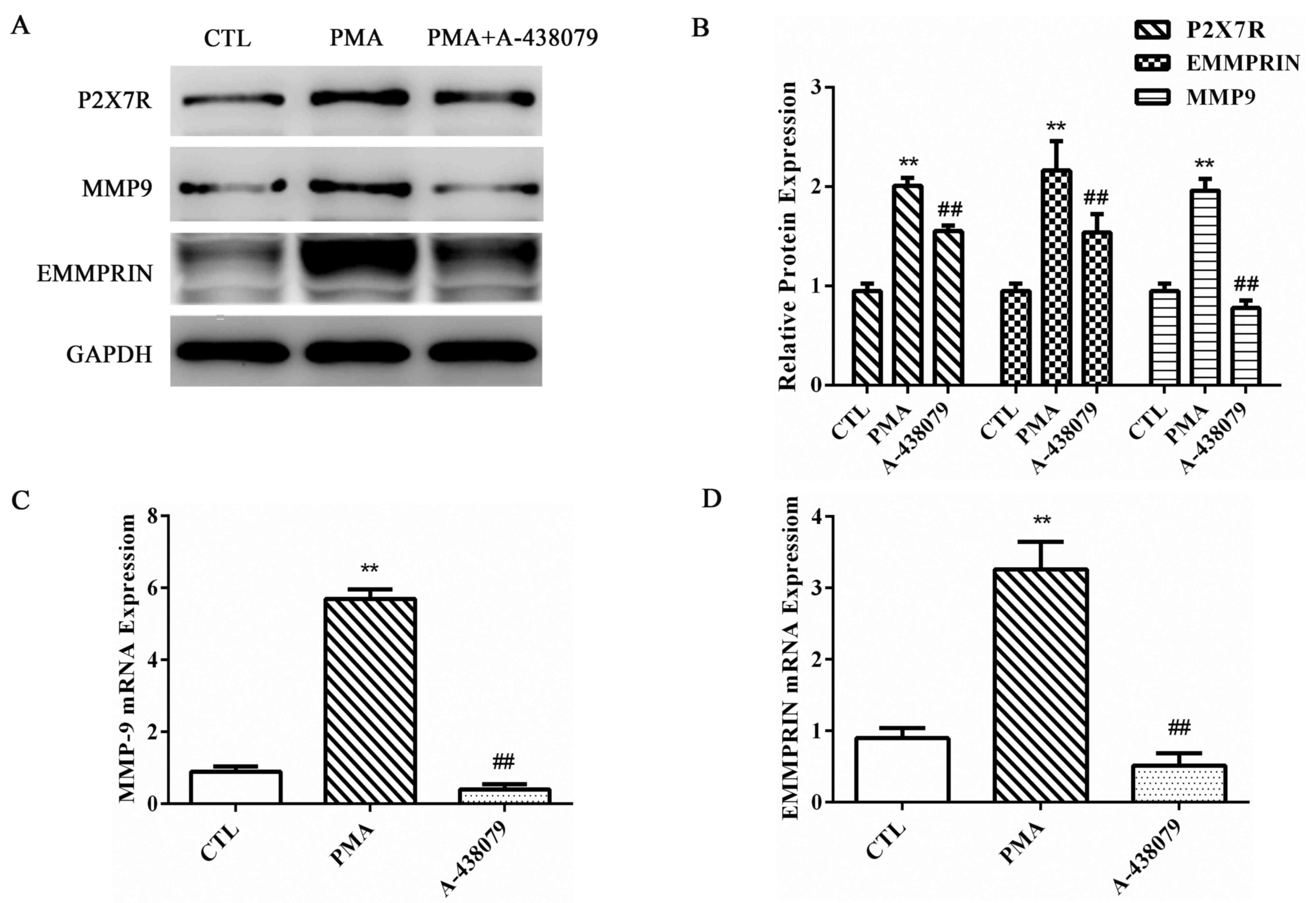
Inhibition of P2X7R reduces MMP-9 and
EMMPRIN expression and MMP-9 activity in PMA-induced
macrophages
THP-1 cells were pretreated with A-438079, a
specific antagonist of P2X7R, for 1 h, followed by incubation with
100 nM PMA for 48 h to determine the effects of P2X7R inhibition on
MMP-9 and EMMPRIN expression in PMA-induced macrophages. MMP-9 and
EMMPRIN protein (Fig. 2A and B)
and mRNA (Fig. 2C and D,
respectively) expression levels were significantly increased in the
PMA-induced macrophages, and the suppression of P2X7R expression by
A-438079 treatment significantly inhibited the PMA-upregulated
expression level of MMP-9 (Fig.
2). EMMPRIN, which is the most well-characterized and major
cell surface regulator of MMP-9 (27), exhibited a similar reduction in
expression as MMP-9 following suppression of P2X7R expression
(Fig. 2). These results indicated
that P2X7R inhibition affected the expression of EMMPRIN and MMP-9
in PMA-induced macrophages at both the protein and mRNA levels.
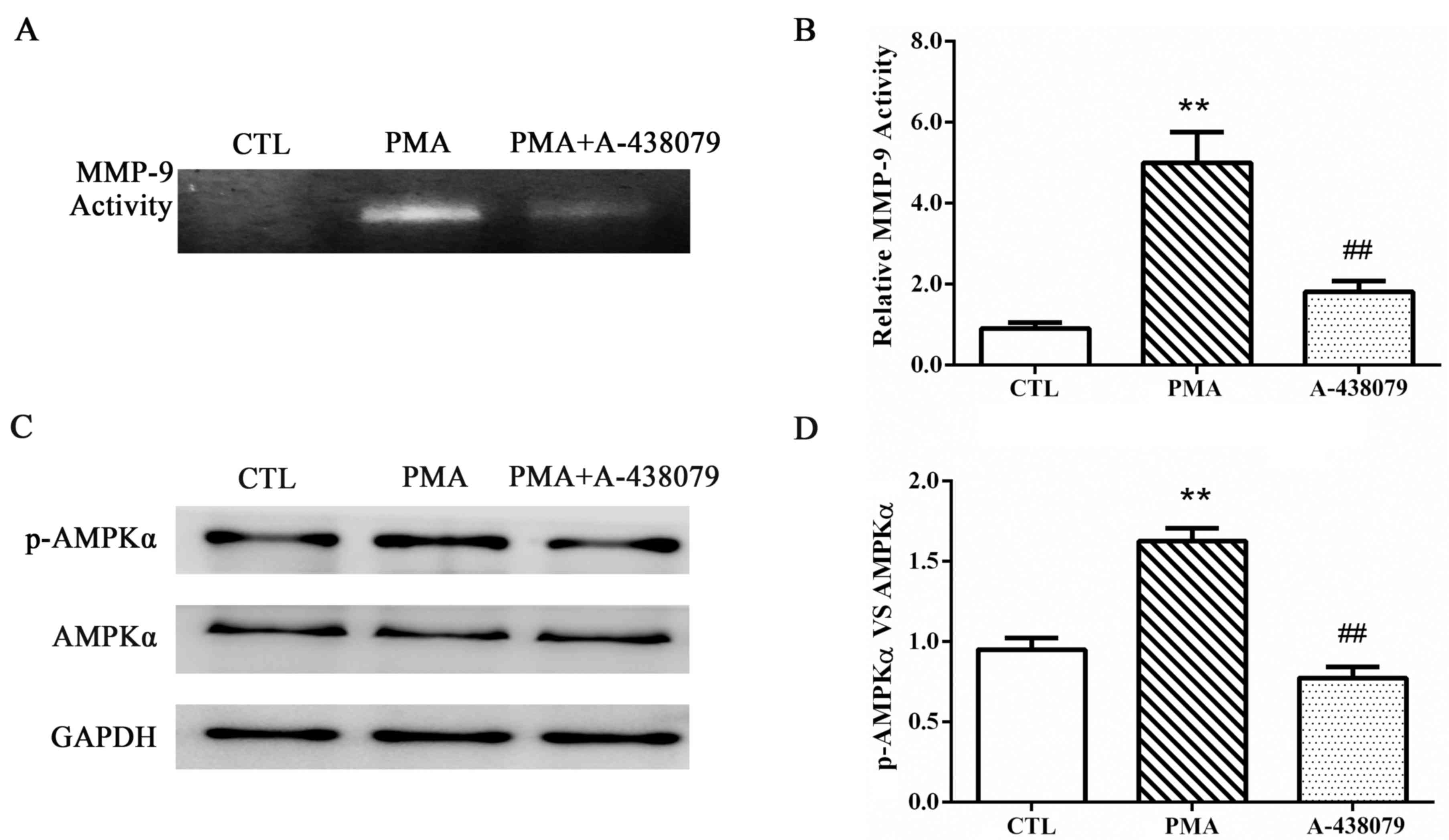
The effects of P2X7R on the enzymatic activities of
MMP-9 were examined by gelatin zymography. As previously reported
(20), following staining with
Coomassie Blue, an unstained transparent band was observed at ~92
kDa; this band represented the theoretical size of the gelatin
digested by MMP-9. In the THP-1-derived macrophages, MMP-9 activity
was significantly increased in cells treated with PMA, compared to
untreated control cells (Fig. 3A and
B), and A-438079-inhibited expression of P2X7R significantly
reduced MMP-9 activity.
P2X7R mediates AMPK activation induced
by PMA
The potential mechanism associated with P2X7R
regulation of MMP-9 and EMMPRIN expression in PMA-induced
macrophages was determined by examining the potential involvement
of the AMPK pathway. Cells were pretreated with A-438079 for 1 h
and induced with PMA for another 48 h. The protein expression
levels of p-AMPKα and total AMPKα were examined by western blot
analysis (Fig. 3C and D). PMA
treatment induced the activation of AMPKα in THP-1 cells, and the
phosphorylation of AMPKα was significantly reduced by A-438079
co-treatment. This result suggested that the inhibition of P2X7R
inhibited AMPKα activation.
P2X7R inhibition suppresses
mitogen-activated protein kinase (MAPK) pathway in PMA-induced
THP-1 cells
Previous studies indicated that PMA treatment
promoted the expression of EMMPRIN and MMP-9 by activating the MAPK
signaling pathway (20).
Therefore, whether P2X7R regulated the expression of EMMPRIN and
MMP-9 through the MAPK pathway was examined. To verify this
hypothesis, THP-1 cells were pretreated with A-438079 for 1 h prior
to incubation with PMA for 48 h. The inhibition of P2X7R by
A-438079 co-treatment significantly decreased the PMA-induced
phosphorylation of ERK1/2, p38 MAPK and JNK (Fig. 4A-D). These results suggested that
the MAPK pathway may be involved in the regulation of EMMPRIN and
MMP-9 expression by P2X7R in PMA-induced THP-1 cells.
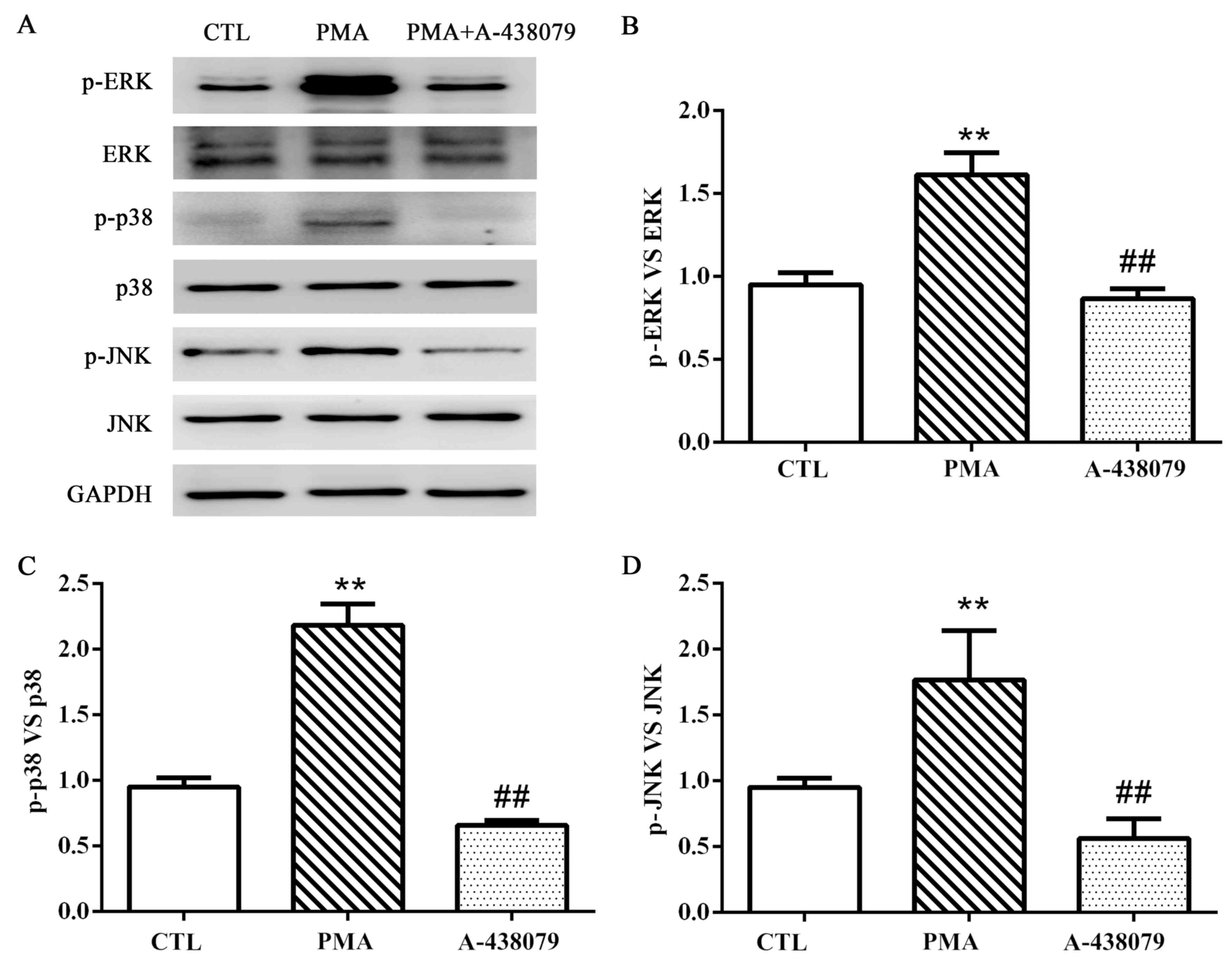 | Figure 4.P2X7R regulates the phosphorylation of
ERK1/2, p38 and JNK. (A) Protein expression levels of ERK, p-ERK,
p38, p-p38, JNK, p-JNK and GAPDH were examined by western blot
analysis. (B-D) Protein quantification was carried out by
densitometric analysis. Proteins were normalized to the internal
control GAPDH. Data are expressed as the mean ± standard deviation;
n=3; **P<0.01 vs. CTL group; ##P<0.01 vs. PMA
group. CTL, untreated control group; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; p,
phosphorylated; P2X7R, purinergic 2X7 receptor; PMA, phorbol
12-myristate 13-acetate. |
Discussion
Regulation of plaque stability is vital to patients
with atherosclerosis, particularly in cases of thrombosis and fatal
complications. Previous studies have suggested that the involvement
of P2X7R in atherosclerotic regulation was through different
targets, such as the NLRP3 inflammasome (4,28).
However, despite the general proinflammatory effects of P2X7R, the
mechanism by which it mediates atheromatous progression has been
poorly investigated. Additionally, the elevated expression levels
of EMMPRIN and MMP-9 have been correlated with advanced
atherosclerotic lesions, followed by plaque rupture and myocardial
infarction (13,29). The present study demonstrated that
P2X7R inhibition by A-438079 significantly downregulated the
expression of EMMPRIN and MMP-9 at the protein and mRNA levels,
probably by suppressing the AMPK and MAPK pathways in PMA-induced
THP-1 cells. Therefore, P2X7R may be a potential therapeutic target
for ameliorating the development of atherosclerotic plaques.
P2X7R is considered to be only activated in
circumstances in which the local concentration of ATP increases,
such as infection and injury, or in tumor microenvironments
(30). However, currently unknown
allosteric modulators may serve a role in P2X7R activity in
vivo by decreasing its Km for ATP so that P2X7R may be
activated even at low nucleotide concentrations (2). Similar to this hypothesis, our
previous research demonstrated that the expression of P2X7R is
highly elevated when stimulated by PMA in monocytes-derived
macrophages (31). Therefore, the
present study this cell model was used to explore the underlying
biological mechanism. Although there is no explicit association
between PMA and ATP, it was speculated that the stimulation of PMA
may influence the extracellular concentration of ATP, but this
needs to be investigated further. The present results suggested the
possibility of P2X7R serving a role in the differentiation of THP-1
cells from monocytes to macrophages, but this needs to be studied
further.
Furthermore, the regulation between P2X7R and MMP-9
in different physiologic and pathologic processes has also been
reported. For example, P2X7 receptor activated by ATP stimulation
in human peripheral blood mononuclear cells was revealed to rapidly
increase MMP-9 release and thus enhanced extracellular MMP-9
activity (16). P2X7R was also
demonstrated to be involved in the regulation of the blood-brain
barrier by mediating MMP-9 activities and the degradation of the
extracellular matrix (32,33). The present results revealed that
the inhibition of P2X7R expression may significantly inhibit the
PMA-induced upregulation of MMP-9 expression and activity. In
addition, EMMPRIN, as the major cell surface regulator of MMP-9,
was also regulated by P2X7R in PMA-induced THP-1 cells.
To determine the molecular mechanisms by which P2X7R
may regulate the expression of EMMPRIN and MMP-9 in differentiated
macrophages, the level of phosphorylated AMPKα was investigated. A
previous study revealed that the activation of AMPKα may be induced
by PMA treatment (20). In
addition, another study reported that P2X7R was able to mediate the
activation of AMPK during autophagy induced by LL-37 in macrophages
(34). On the basis of these
results, the present study hypothesized that P2X7R may regulate the
activation of AMPKα to adjust the levels of EMMPRIN and MMP-9
expression in PMA-induced macrophages. As expected, the inhibition
of P2X7R expression in the present study led to the reduced
activation of the AMPKα pathway, and the downregulation of EMMPRIN
and MMP-9 expression. Consequently, AMPKα activation may be
necessary for P2X7R to regulate the expression of MMP-9 and EMMPRIN
in PMA-induced macrophages. Notably, our previous data indicated
that compound C also suppressed the phosphorylation of MAPK
signaling, including the ERK, JNK and p38 pathways in PMA-induced
macrophages (20). Therefore, the
activation of the AMPK pathway is the upstream of MAPK in THP-1
cells stimulated with PMA.
Furthermore, P2X7R serves an essential role in the
regulation of MAPK pathways during physiological and pathological
processes, such as sympathoexcitatory response in myocardial
infarction (35) and the
differentiation of bone marrow stem cells into osteoblasts
(36–38). In the present study, the activation
of the MAPK signaling pathway was examined in PMA-induced THP-1
cells and it was revealed that the inhibition of P2X7R
significantly decreased the phosphorylation of ERK1/2, p38 MAPK and
JNK in these macrophages. These results indicated that MAPK
pathways may serve an essential role in the regulation of EMMPRIN
and MMP-9 expression by P2X7R.
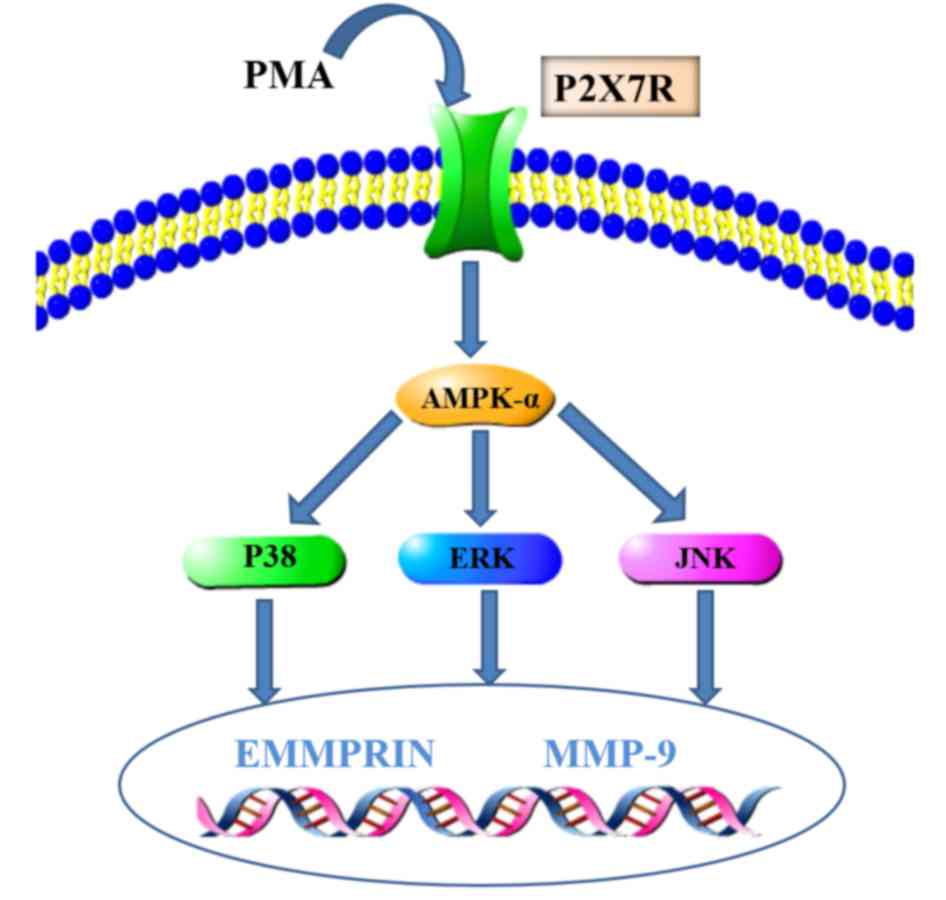
In conclusion, P2X7R expression was significantly
increased in the PMA-induced macrophages, and the inhibition of
P2X7R expression was followed by the downregulation of EMMPRIN and
MMP-9 expression, which probably occurred through the suppression
of AMPK and MAPK signaling pathway activation. AMPK, as an upstream
activator of MAPK signaling, may be involved in the regulation of
EMMPRIN and MMP-9 expression in PMA-induced macrophages (20). Therefore, the present study
suggested that P2X7R may regulate EMMPRIN and MMP-9 expression
through AMPK/MAPK signaling in PMA-induced macrophages and the
schematic model is illustrated in Fig.
5. These data provided novel insights into the regulatory
mechanisms of EMMPRIN and MMP-9 and suggested that P2X7R may be a
potential strategy for combating plaque ruptures.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural
Science Foundation of China (grant no. 81670227), The Traditional
Chinese Medicine Administration of Zhejiang Province (grant no.
2016ZA137) and The Wenzhou Science & Technology Bureau (grant
nos. Y20150036 and Y20150035).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL, ZH and WH conceived and designed the study. LL,
SH, ZW, ZZ and JH performed the experiments. ZZ and ZH analyzed and
integrated the results. LL wrote the paper. ZZ, JH and ZH reviewed
and edited the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Ross R: ATHEROSCLEROSIS-An inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartlett R, Stokes L and Sluyter R: The
P2X7 receptor channel: Recent developments and the use of P2X7
antagonists in models of disease. Pharmacol Rev. 66:638–675. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baroja-Mazo A and Pelegrin P: Modulating
P2X7 receptor signaling during rheumatoid arthritis: New
therapeutic approaches for bisphosphonates. J Osteoporo.
2012:4082422012. View Article : Google Scholar
|
|
4
|
Piscopiello M, Sessa M, Anzalone N,
Castellano R, Maisano F, Ferrero E, Chiesa R, Alfieri O, Comi G,
Ferrero ME and Foglieni C: P2X7 receptor is expressed in human
vessels and might play a role in atherosclerosis. Int J Cardiol.
168:2863–2866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng K, Liu L, Wei D, Lv Y, Wang G, Xiong
W, Wang X, Altaf A, Wang L, He D, et al: P2X7R is involved in the
progression of atherosclerosis by promoting NLRP3 inflammasome
activation. Int J Mol Med. 35:1179–1188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu S, Koren E, Chan Y, Koscec M, Sheehy
A, Kolodgie F, Virmani R and Feder D: Effects of everolimus on
macrophage-derived foam cell behavior. Cardiovasc Revasc Med.
15:269–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moustardas P, Kadoglou NP, Katsimpoulas M,
Kapelouzou A, Kostomitsopoulos N, Karayannacos PE, Kostakis A and
Liapis CD: The complementary effects of atorvastatin and exercise
treatment on the composition and stability of the atherosclerotic
plaques in ApoE knockout mice. PloS One. 9:e1082402014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Biswas C, Zhang Y, DeCastro R, Guo H,
Nakamura T, Kataoka H and Nabeshima K: The human tumor cell-derived
collagenase stimulatory factor (renamed EMMPRIN) is a member of the
immunoglobulin superfamily. Cancer Res. 55:434–439. 1995.PubMed/NCBI
|
|
10
|
Yoon YW, Kwon HM, Hwang KC, Choi EY, Hong
BK, Kim D, Kim HS, Cho SH, Song KS and Sangiorgi G: Upstream
regulation of matrix metalloproteinase by EMMPRIN; extracellular
matrix metalloproteinase inducer in advanced atherosclerotic
plaque. Atherosclerosis. 180:37–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Jin R, Zhu X, Yan J and Li G:
Function of CD147 in atherosclerosis and atherothrombosis. J
Cardiovasc Transl Res. 8:59–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Major TC, Liang L, Lu X, Rosebury W and
Bocan TM: Extracellular matrix metalloproteinase inducer (EMMPRIN)
is induced upon monocyte differentiation and is expressed in human
atheroma. Arterioscler Thromb Vasc Biol. 22:1200–1207. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joghetaei N, Stein A, Byrne RA, Schulz C,
King L, May AE and Schmidt R: The extracellular matrix
metalloproteinase inducer (EMMPRIN, CD147)-a potential novel target
in atherothrombosis prevention? Thromb Res. 131:474–480. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riteau N, Gasse P, Fauconnier L, Gombault
A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VFJ,
Marchand-Adam S, Crestani B, et al: Extracellular ATP Is a danger
signal activating P2X7receptor in lung inflammation and fibrosis.
Am J Respir Crit Care Med. 182:774–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang C, Yu W, Cui H, Wang Y, Zhang L, Han
F and Huang T: P2X7 blockade attenuates mouse liver fibrosis. Mol
Med Rep. 9:57–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu BJ and Wiley JS: Rapid ATP-induced
release of matrix metalloproteinase 9 is mediated by the P2X7
receptor. Blood. 107:4946–4953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Habets DD, Coumans WA, Voshol PJ, den Boer
MA, Febbraio M, Bonen A, Glatz JF and Luiken JJ: AMPK-mediated
increase in myocardial long-chain fatty acid uptake critically
depends on sarcolemmal CD36. Biochem Biophys Res Commun.
355:204–210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Jia Q, Xiao J, Jiao H and Lin H:
Glucocorticoids retard skeletal muscle development and myoblast
protein synthesis through a mechanistic target of rapamycin
(mTOR)-signaling pathway in broilers (gallus gallus domesticus).
Stress. 18:686–698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwanaka N, Egawa T, Satoubu N, Karaike K,
Ma X, Masuda S and Hayashi T: Leucine modulates contraction- and
insulin-stimulated glucose transport and upstream signaling events
in rat skeletal muscle. J Appl Physiol. 108:274–282. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao J, Han Z, Tian L, Chen K, Fan Y, Ye B,
Huang W, Wang C and Huang Z: Curcumin inhibits EMMPRIN and MMP-9
expression through AMPK-MAPK and PKC signaling in PMA induced
macrophages. J Transl Mad. 12:2662014. View Article : Google Scholar
|
|
21
|
Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung
DI, Hahn JH, Kim YM, Park SH and Lee H: A splice variant of CD99
increases motility and MMP-9 expression of human breast cancer
cells through the AKT-, ERK-, and JNK-dependent AP-1 activation
signaling pathways. J Biol Chem. 281:34833–34847. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SJ, Kim CE, Yun MR, Seo KW, Park HM,
Yun JW, Shin HK, Bae SS and Kim CD: 4-Hydroxynonenal enhances MMP-9
production in murine macrophages via 5-lipoxygenase-mediated
activation of ERK and p38 MAPK. Toxicol Appl Pharmacol.
242:191–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo J, Lee S, Wu D, Yeh J, Ellamushi H,
Wheeler AP, Warnes G, Zhang Y and Bo X: P2X7 purinoceptors
contribute to the death of Schwann cells transplanted into the
spinal cord. Cell Death Dis. 4:e8292013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Auwerx J: The human leukemia cell line,
THP-1: A multifacetted model for the study of monocyte-macrophage
differentiation. Experientia. 47:22–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin Z: The use of THP-1 cells as a model
for mimicking the function and regulation of monocytes and
macrophages in the vasculature. Atherosclerosis. 221:2–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang QM, Wang H, Li YF, Xie ZY, Ma Y, Yan
JJ, Gao YF, Wang ZM and Wang LS: Inhibition of EMMPRIN and MMP-9
expression by epigallocatechin-3-gallate through 67-kda laminin
receptor in PMA-induced macrophages. Cell Physiol Biochem.
39:2308–2319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erlinge D and Burnstock G: P2 receptors in
cardiovascular regulation and disease. Purinergic Signal. 4:1–20.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Newby AC: Metalloproteinase expression in
monocytes and macrophages and its relationship to atherosclerotic
plaque instability. Arterioscler Thromb Vasc Biol. 28:2108–2114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lenertz LY, Gavala ML, Zhu Y and Bertics
PJ: Transcriptional control mechanisms associated with the
nucleotide receptor P2X7, a critical regulator of immunologic,
osteogenic, and neurologic functions. Immunol Res. 50:22–38. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong F, Ye B, Cao J, Cai X, Lin L, Huang
S, Huang W and Huang Z: Curcumin represses NLRP3 inflammasome
activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced
macrophages. Front Pharmacol. 7:3692016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang F, Zhao K, Zhang X, Zhang J and Xu B:
ATP induces disruption of tight junction proteins via il-1
Beta-dependent MMP-9 activation of human blood-brain barrier in
vitro. Neural Plast. 2016:89285302016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rubio-Araiz A, Perez-Hernandez M, Urrutia
A, Porcu F, Borcel E, Gutierrez-Lopez MD, O'Shea E and Colado MI:
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) disrupts
blood-brain barrier integrity through a mechanism involving P2X7
receptors. Int J Neuropsychopharmacol. 17:1243–1255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rekha RS, Muvva Rao SS, Wan M, Raqib R,
Bergman P, Brighenti S, Gudmundsson GH and Agerberth B:
Phenylbutyrate induces ll-37-dependent autophagy and intracellular
killing of mycobacterium tuberculosis in human macrophages.
Autophagy. 11:1688–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Q, Xu H, Hao L, Ma G, Sun J, Song X,
Ding F and Wang N: P2X7 receptor regulates sympathoexcitatory
response in myocardial infarction rats via NF-kappaB and MAPK
pathways. Am J Transl Res. 9:4954–4962. 2017.PubMed/NCBI
|
|
36
|
Okumura H, Shiba D, Kubo T and Yokoyama T:
P2X7 receptor as sensitive flow sensor for ERK activation in
osteoblasts. Biochem Biophys Res Commun. 372:486–490. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li W, Li G, Zhang Y, Wei S, Song M, Wang
W, Yuan X, Wu H and Yang Y: Role of P2 × 7 receptor in the
differentiation of bone marrow stromal cells into osteoblasts and
adipocytes. Exp Cell Res. 339:367–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sathanoori R, Sward K, Olde B and Erlinge
D: The ATP receptors P2X7 and P2X4 modulate high glucose and
palmitate-induced inflammatory responses in endothelial cells. PLoS
One. 10:e01251112015. View Article : Google Scholar : PubMed/NCBI
|