Introduction
Prostate cancer (PCa) is the most common malignant
tumor in the male genitourinary system, and the incidence of cancer
is fifth in the world (1). It is
well known that the incidence of PCa in China ranks one hundred
seventieth in the country, which has been rising from 2008 to 2012
(2,3), speculating that the number of people
with PCa will continue to increase in the future (2).
MicroRNAs (miRNAs) are a class of non-coding small
RNAs that regulate gene expression in a post-transcriptional manner
(4,5). Recent studies have demonstrated that
miRNA regulation serves a role in health and disease (6,7).
Abnormal expression of miRNAs may lead to initiation and
development of tumors (8–10). Previous studies have also indicated
that a number of miRNAs may be delivered to human cells by food
intake, therefore, miRNAs are relevant to human health (11,12).
It has also been demonstrated that incidence, development,
treatment, prognosis and recurrence of PCa are associated with
abnormal expression of certain miRNAs (13–15).
The authors of the present study hypothesized that miRNAs may aid
in our understanding of pathogenesis and the basis for molecular
diagnosis of PCa, and have attempted to evaluate the prognosis and
to suggest novel treatment methods for PCa.
Microbubble ultrasound contrast agent is a safe,
novel, stable and efficient gene transfer vector (13–15).
In this technique, the gene of interest is contained in a
microbubble, and when the microbubble breaks itis released.
Microbubble destruction induced by vibration increases the
permeability of local cells and produces an irreversible sound
hole, which may promote entry of a gene into the nucleus and
increase its expression and transfection efficiency (16). Furthermore, microbubbles transport
genes or drugs efficiently to avoid degradation by blood
endonucleases and other lytic enzymes (17,18).
By using this method of ultrasound-targeted microbubble destruction
(UTMD), genes and drugs may reach target tissues or organs through
the blood circulatory system. The goal of UTMD is to reduce the
extent of adverse systemic responses (19). Research and clinical studies of
UTMD primarily focus on cancer, anti-tumor therapies, thrombosis,
thrombolytic therapy, inflammation, drug delivery and gene
therapies (20,21). It has been widely demonstrated that
microbubble-based techniques may improve gene transfection
efficiency (reviewed in 20) and are considered as a novel approach
to cancer treatment (18).
Based on the potential role of miR-205 in the
molecular mechanism underlying PCa development (22), the aim of the present study was to
investigate the transfection efficiency and safety of UTMD-mediated
transfection of miR-205 to PC-3 cells. Furthermore, the present
study attempted to investigate the role served by miRNAs in the
development of PCa and the feasibility of UTMD-mediated gene
therapy.
Materials and methods
Cell culture
RWPE-1 normal prostate cells and PCa cell lines
VCaP, LNCaP, PC-3, and DU145 were purchased from American Type
Culture Collection (Manassas, VA, USA). All cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 2 mM L-glutamine in an atmosphere of 5%
CO2 at 37°C.
Cell treatment
For cisplatin treatment, DU145 and PC-3 cells were
seeded (1×105 cells/well) in six-well plates, and
treated with 0, 1, 2, 4, 6, 10 and 15 µg/ml cisplatin (Beijing
Solarbio Science and Technology Co., Ltd., Beijing, China) at room
temperature for 48 h. For miRNA transfection, the miR-205 mimics
and miRNA negative controls (miR-NC) were purchased from Shanghai
Gene Chem Co., Ltd. (Shanghai, China). PC-3 and DU145 cells were
seeded (1×105 cells/well) in six-well plates and
transfected with miR-205 mimics (target sequence:
5′-GATTTCAGTGGAGTGAAGTTCAGGAGGCAT-3′, C=1.6 µg/µl) and miR-NC
(target sequence: 5′-CCAGTATTAACTGTGCTGCTGA-3′, C=1.3 µg/µl) using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h according to the manufacturer's
protocol. Subsequently, cells were harvested for further
experiments.
miRNA-microbubble preparation and transfection.
Microbubbles were obtained by sonication of an aqueous dispersion
comprising 1,2-distearoyl-3-trimethylammoniumpropane (0.4 mg/ml;
Avanti Polar Lipids Inc., Alabaster, AL, USA) with perfluoropropane
gas, polyethyleneglycol-2000 stearate (1 mg/ml; Avanti Polar Lipids
Inc.), and distearoylphosphatidylcholine (2 mg/ml, DSPCa; Avanti
Polar Lipids Inc.) (23).
Microbubbles were examined by an inverted microscope (Guangmi,
GMSP-5, Shanghai, China; www.shgmyq.com/). miR-205 and miR-NC were separately
added into the microbubbles, and incubated at 37°C for 30 min. PC-3
cells were transfected with a mixture of miR-205/miR-NC and
microbubbles using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc) for 48 h according to
the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. First-strand cDNA was reverse
transcribed from the total RNA using the RevertAid First Strand
cDNA synthesis kit (Thermo Fisher Scientific, Inc.). The
temperature and time of the reaction were 85°C for 5 min, 4°C for 5
min. The qPCR assay was performed using SYBR-Green PCR Master Mix
kit (Takara Biotechnology Co., Ltd., Dalian, China) and an ABI 7500
real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used for the
PCR: Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for
5 sec, 60°C for 34 sec. Primers for target genes and U6 (the
internal loading control) were designed using the Primer Premier
software version 5.0 (Premier Biosoft International, Palo Alto, CA,
USA) and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The following primer sequences were used for PCR: miR-205
forwad, 5′-TGGGCTGAGTCCCTCT-3′ and reverse,
5′-GAGGGACGGGTGATGGGCAGATTGG-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′
(reverse). Expression levels were normalized to U6, and relative
expression values were calculated using the 2−∆∆Cq
method (24).
Western blot analysis
Total protein was extracted from cells using
ProteoPrep Total Extraction Sample kit (Sigma-Aldrich; Merck KGaA).
Cell lysates were collected following centrifugation at 12,000 × g
at 4°C for 20 min. Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to detect protein concentrations. Each
protein sample (30 µg) was separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad
Laboratories, Inc.). The PVDF membranes were treated with the
following primary antibodies: Caspase 9 (1:1,000; cat. no. ab25758;
Abcam, Cambridge, UK), cleaved-caspase 9 (1:1,000; cat. no. ab2324;
Abcam), cytochrome c (cyto c; 1:1,000; cat. no. ab28146; Abcam),
epithelial (E)-cadherin (1:1,000; cat. no. ab133597; Abcam), matrix
metalloproteinase 9 (MMP-9; 1:1,000; cat no. ab73734; Abcam);
phosphorylated (p)-extracellular signal-regulated kinase (ERK)1/2
(1:1,000; cat. no. 9101; New England BioLabs, Inc., Ipswich, MA,
USA), ERK1/2 (1:1,000; cat. no. ab17942; Abcam), β-actin (1:1,000;
cat. no. ab8226; Abcam) overnight at 4°C. The following day, the
membranes were incubated with a horseradish conjugated-conjugated
secondary antibody (Donkey anti-rabbit IgG H&L, 1:7,000, cat
no. ab98488; Goat anti-mouse IgG H&L, 1:8,000, cat no.
ab150117; Rabbit anti-mouse IgG H&L, 1:8,000, cat no. ab175743;
Abcam) for 1 h at room temperature. Expression was visualized using
the Enhanced Chemiluminescence Detection kit (EMD Millipore,
Billerica, MA, USA).
Cell viability
Cell viability was measured using the Cell Counting
Kit (CCK)-8 assay (Beyotime Institute of Biotechnology Co., Ltd.,
Shanghai, China). Cells (1×104 cells/well) were seeded
into a 96-well plate and cultured at 37°C in a 5% CO2
incubator for 48 h. CCK-8 solution (10 µl) was added into each well
and incubated at 37°C for 4 h. The absorbance was measured at a
wavelength of 450 nm with a microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Flow cytometry
After PC-3 cells (1×106 cells/ml) were
treated with: i) 1X PBS (blank); ii) cisplatin (2 µg/ml); iii)
cisplatin (2 µg/ml) + scrambled-miRNA (miR-NC; 100 µg) + UTMD also
referred as UTMD-mediated miR-NC group; iv) cisplatin (2 µg/ml) +
miR-205 (100 µg) + UTMD; v) cisplatin (2 µg/ml) + miR-205 (100 µg),
also referred as miR-205 group; and vi) cisplatin (2 µg/ml)+UTMD.
Cells were digested with 0.25% EDTA-trypsin (Weike; Shanghai,
China; www.weike21.com/), and dispersed. Cell
suspension was centrifuged in 500 × g at 37°C for 5 min, and
collected. Subsequently, cells were washed with 1X PBS, and
re-suspended using 1X binding buffer and double stained with the
Annexin V-FITC/PI Staining kit (BD Biosciences, Franklin Lakes, NJ,
USA). Finally, apoptotic cells were detected by flow cytometry
{Taomsun, TMS-2050 [FlowJo 10; Version: 10.2 64 (Bit), Suzhou,
Jiangsu, China}. Apoptotic rates of treated PC-3 cells were
quantified by graphPad prism 7 software.
Terminal deoxynucleotidyl transferase-mediated dUTP
nick-end labeling (TUNEL) and 4′,6-diamidino-2-phenylindole
(DAPI) staining. Cell apoptosis was detected using the In-Situ
Cell Death Detection kit (R&D Systems, Inc., Minneapolis, MN,
USA), according to the manufacturer's protocol. Cells
(1×105 cells/well) were seeded in 24-well plates, and
treated with: i) 1X PBS (blank); ii) cisplatin (2 µg/ml); iii)
cisplatin (2 µg/ml) + scrambled-miRNA (miR-NC; 100 µg) + UTMD, also
referred as UTMD-mediated miR-NC group; iv) cisplatin (2 µg/ml) +
miR-205 (100 µg)+UTMD; v) cisplatin (2 µg/ml) + miR-205 (100 µg),
also referred as miR-205 group; and vi) cisplatin (2 µg/ml) + UTMD.
Subsequently, cells were fixed in 4% paraformaldehyde at 4°C for 30
min, permeabilized in 0.1% Triton X-100, and treated with 50 µl
fluorescein-12-dUTP or 60 µl DAPI at room temperature for 30 min.
The fluorescence of cells in the middle of each well was detected
by fluorescence microscope (Zeiss Axiovert 100 M; Zeiss GmbH, Jena,
Germany).
Wound-healing assay
The wound-healing assay was used to determine the
effects of UTMD-mediated miR-205 delivery on cell migration.
Treated cells were seeded in the six-well plates (1×106
cells/well) and cultured in RPMI-1640 medium for 12 h at 37°C in a
5% CO2 incubator. A 100 µl pipette tip was used to
create a straight scratch and the images captured were used as the
baseline. Subsequently, cells were treated as mentioned earlier.
Finally, cells were washed three times with 1X PBS to remove the
suspended cells, and new images were captured.
Invasion assay. Cell invasion assay was performed
using Transwell chambers. Matrigel inserts were placed in the upper
compartment and incubated for 30 min in an incubator at 5%
CO2 and 37°C. A 200 µl solution with serum-free medium
containing treated cells (5×105 cells/well) was seeded
in the upper compartment. The lower compartment was filled with 600
µl medium supplemented with 10% FBS. Following 24 h of incubation,
the migratory cells were fixed with methanol and stained with
crystal violet at room temperature for 25 min. Cell numbers were
counted in five representative fields and images were captured
under a fluorescence microscope.
Statistical analysis
All results are presented as the mean ± standard
deviation of three independent experiments. Statistical analysis
was performed using SPSS software (version 13.0; SPSS, Inc.,
Chicago, IL, USA). The differences between groups were assesses by
one-way analysis of variance followed by Dunnett's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
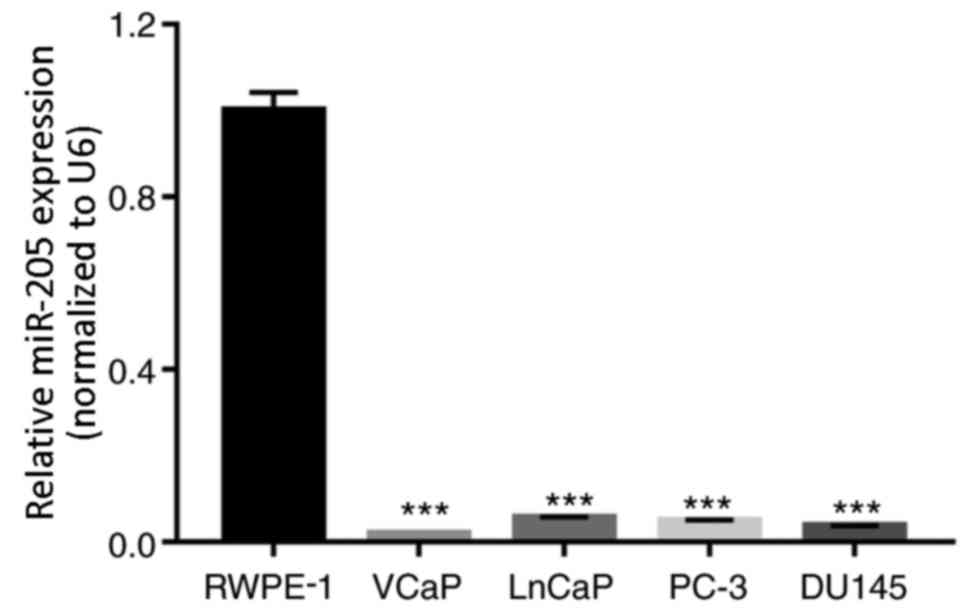
miR-205 expression is decreased in
human PCa cells
RT-qPCR assays were performed to detect the
expression level of miR-205 in the normal prostate cell line RWPE-1
and in the PCa cell lines VCaP, LnCaP, PC-3 and DU145. The results
indicated that the expression levels of miR-205 were significantly
lower in PCa cells compared with RWPE-1 cells (P<0.001; Fig. 1).
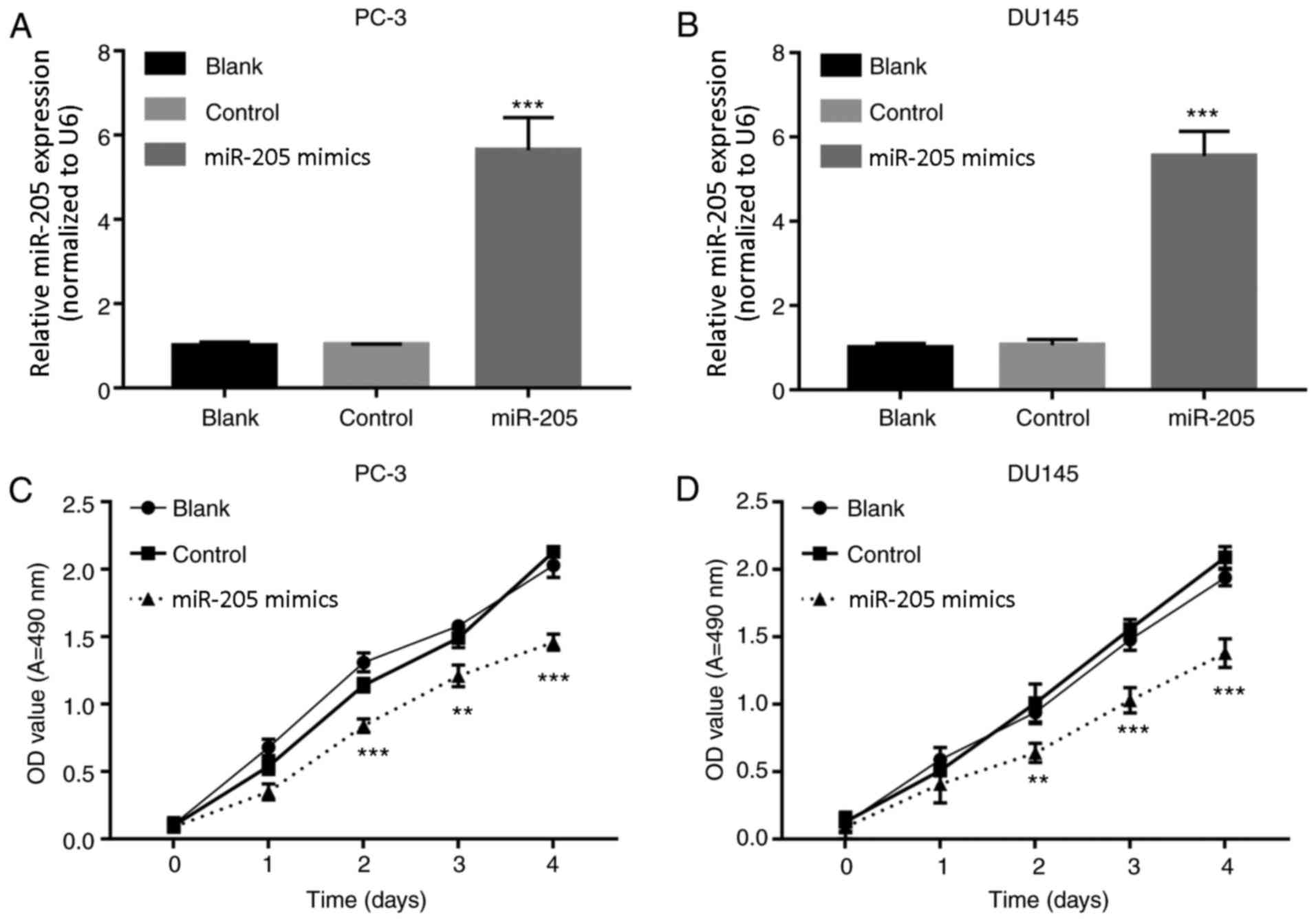
miR-205 inhibits prostate cancer cell
proliferation
The effects of miR-205 overexpression on PCa cells
were detected. PC-3 and DU145 cells were treated with PBS (Blank),
miRNA-NC (Control) and miR-205 mimics (miR-205). RT-qPCR results
demonstrated that miR-205 expression was significantly increased in
the miR-205 mimics treated groups compared with the respective
control groups (P<0.001; Fig. 2A
and B). In addition, miR-205 mimics-treated PC-3 and DU145
cells exhibited a significant reduction in proliferation compared
with the respective control groups at 48 h (Fig. 2C and D).
UTMD-mediated miR-205 transfection
inhibits cisplatin-modulated cell proliferation
Cationic microbubble technology is an effective
method for miRNA delivery (25).
As presented in Fig. 3A, the
microbubbles were imaged using an inverted microscope. It is proven
that cisplatin has an anti-PCa effect (26). In this experiment, to screen for
the optimum cisplatin concentration to treat DU145 and PC-3 cells,
CCK-8 assay was performed to detect viability of DU145 and PC-3
cells treated with 0, 1, 2, 4, 6, 10 and 15 µg/ml cisplatin. The
results demonstrated that cisplatin markedly decreased the
viability of DU145 and PC-3 cells in a dose dependent manner,
compared with the untreated control group (Fig. 3B). Additionally, cisplatin
inhibited the viability of PC-3 cells more than that of DU145
cells. The IC50 of cisplatin was 2 µg/ml in PC-3 cells. Therefore,
subsequent experiments were performed using 2 µg/ml cisplatin in
PC-3 cells.
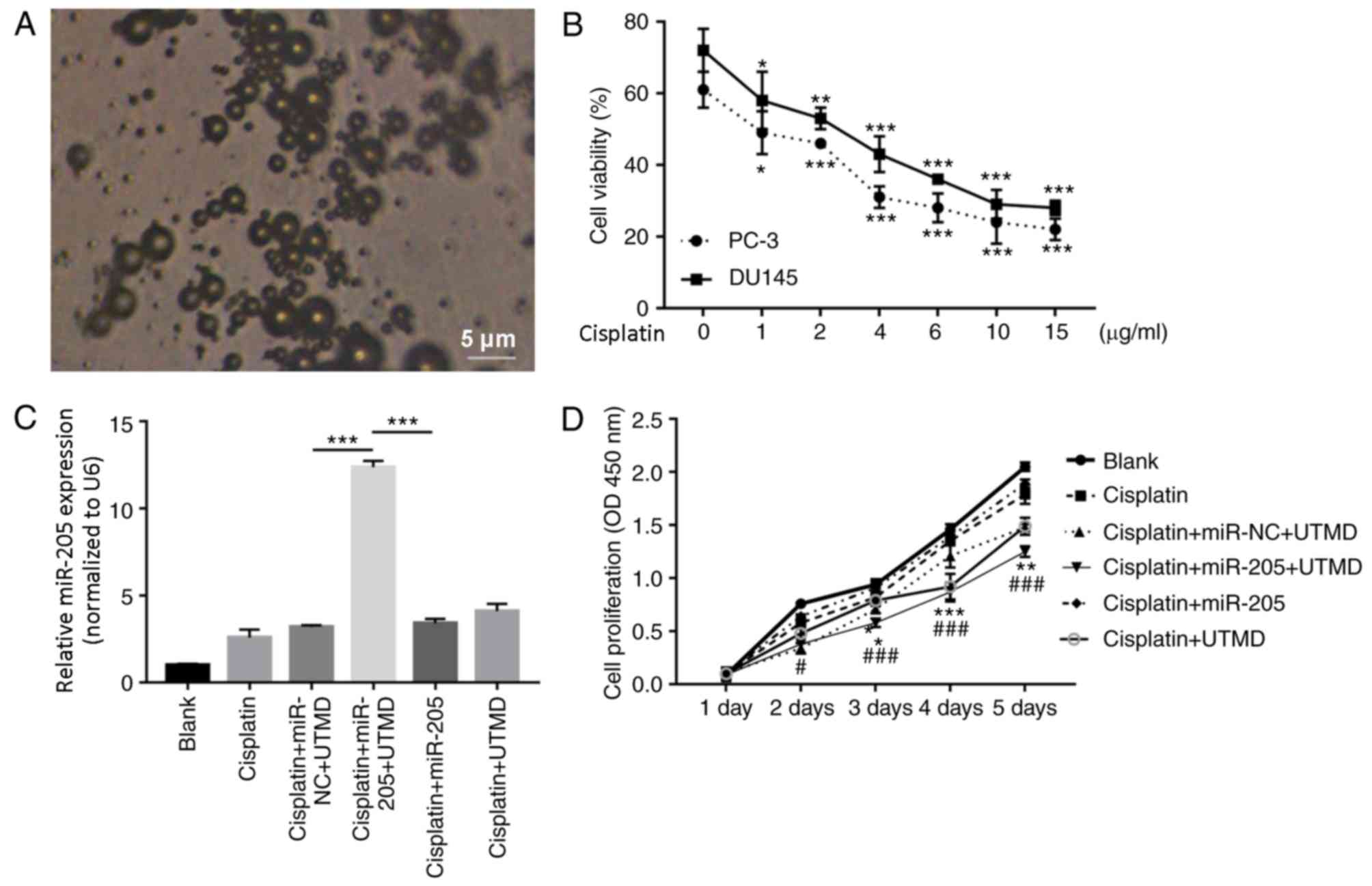 | Figure 3.UTMD-mediated miR-205 transfection
inhibits cell proliferation modulated by cisplatin. (A)
Microbubbles were examined by an inverted microscope. Scale bar, 5
µm; magnification, ×100. (B) Cell viability was detected by Cell
Counting kit-8 assay in DU145 and PC-3 cells treated with 0, 1, 2,
4, 6, 10, 15 µg/ml cisplatin for 48 h. *P<0.05, **P<0.01 and
***P<0.001 vs. the respective untreated control group at 48 h.
(C) Relative expression level of miR-205 determined by reverse
transcription-quantitative polymerase chain reaction assay in PC-3
cells. ***P<0.001. (D) PC-3 cell proliferation. Data are
presented as the mean ± standard deviation; *P<0.05, **P<0.01
and ***P<0.001 vs. the cisplatin + miR-NC + UTMD group;
#P<0.05 and ###P<0.001 vs. the cisplatin+miR-205 group. miR,
microRNA; NC, negative control; OD, optical density; UTMD,
ultrasound-targeted microbubble destruction. |
To investigate whether UTMD-mediated miR-205 serves
a role in PCa, PC-3 cells were treated with: i) 1X PBS (blank); ii)
cisplatin (2 µg/ml); iii) cisplatin (2 µg/ml) + scrambled-miRNA
(miR-NC; 100 µg) + UTMD; iv) cisplatin (2 µg/ml) + miR-205 (100
µg)+UTMD, also referred as UTMD-mediated miR-NC group; v) cisplatin
(2 µg/ml) + miR-205 (100 µg), also referred as miR-205 group; and
vi) cisplatin (6 µg/ml) + UTMD. RT-qPCR results indicated that
UTMD-mediated miR-205 transfection significantly increased miR-205
expression compared with the UTMD-mediated miR-NC group and the
miR-205 group (P<0.001; Fig.
3C). CCK-8 results indicated that UTMD-mediated miR-205
transfection significantly inhibited the proliferation of PC-3
cells compared with the UTMD-mediated miR-NC group and the miR-205
group (Fig. 3D).
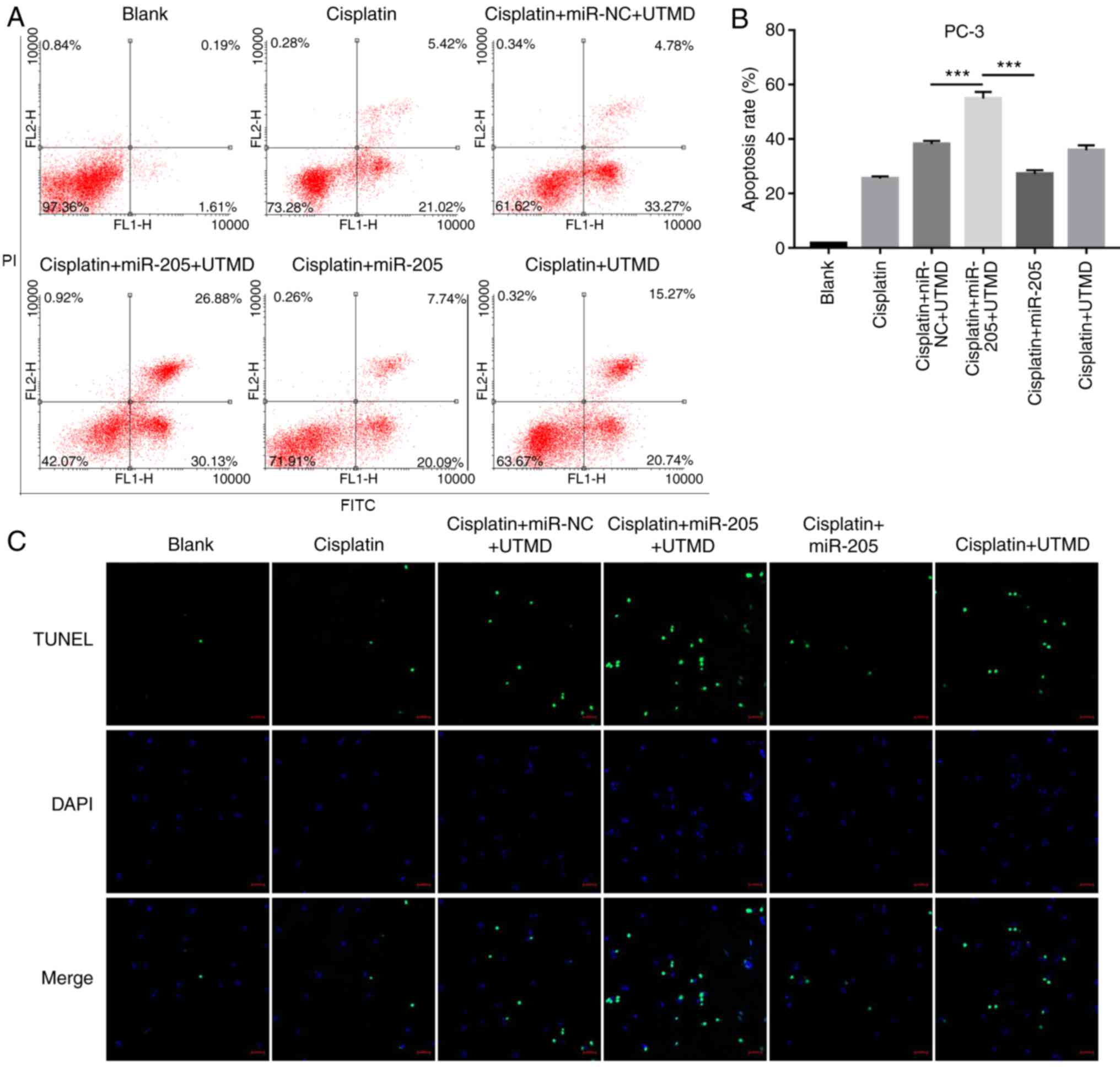
UTMD-mediated miR-205 delivery
promotes cisplatin-modulated apoptosis
Flow cytometric analysis results demonstrated that
UTMD-mediated miR-205 transfection significantly increased
apoptotic rates in PC-3 cells compared with the UTMD-mediated
miR-NC transfected group and the miR-205 group (Fig. 4A and B). In addition, the results
of the TUNEL assay indicated that about 30 apoptotic cells were
stained by fluorescence in UTMD-mediated miR-205 transfection
group, which was higher compared with the UTMD-mediated miR-NC
transfection group and with the miR-205 group (Fig. 4C).
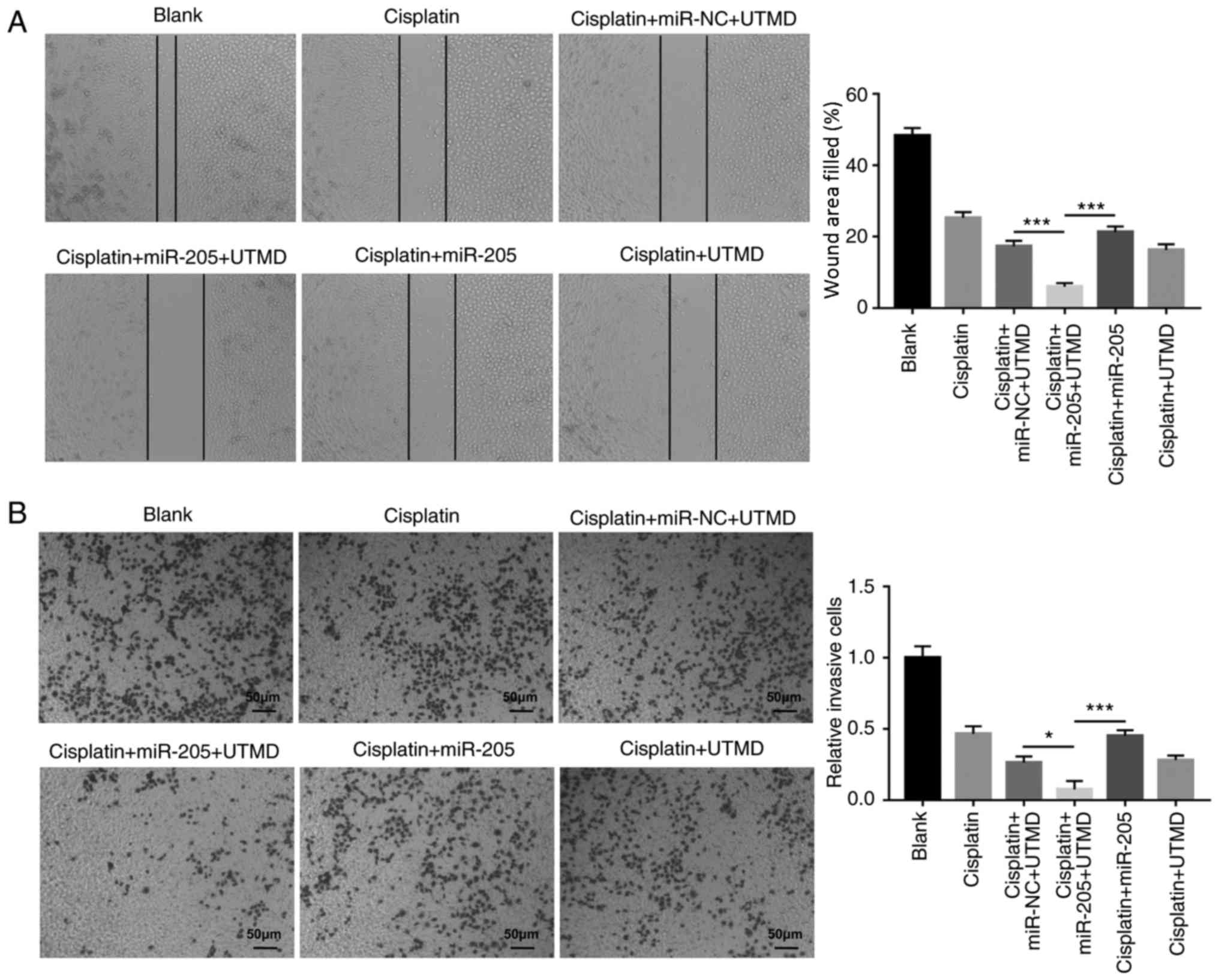
UTMD-mediated miR-205 transfection inhibits PC-3
cell migration and invasion modulated by cisplatin. In order to
further investigate the biological significance of UTMD-mediated
miR-205 transfection in PCa cells, wound-healing and Matrigel
invasion assays were performed. The results demonstrated that
UTMD-mediated miR-205 delivery decreasedPC-3 cell migration and
invasion in cells co-treated with cisplatin compared with the
UTMD-mediated miR-NC transfection group and with the miR-205 group
(Fig. 5A and B).
UTMD-mediated miR-205 transfection
increases expression of caspase-9, cleaved-caspase 9, cytoc and
E-cadherin, and decreases expression of MMP-9 and p-ERK
To investigate the potential mechanism of
UTMD-mediated miR-205 delivery on the inhibition of apoptosis and
invasion of PC-3 cells modulated by cisplatin, the protein
expression levels of apoptosis-associated genes (including
caspase-9, cleaved-caspase 9 and cytoc), E-cadherin, MMP-9, ERK and
p-ERK were evaluated using western blot analysis. The results
demonstrated that in comparison with the UTMD-mediated miR-NC
transfection group and with the miR-205 group, UTMD-mediated
miR-205 transfection notably upregulated the expression of
caspase-9, cleaved-caspase 9 and cytoc, which suggested that
miR-205 may promote cell apoptosis. UTMD-mediated miR-205
transfection also resulted in increased protein expression levels
of the epithelial marker E-cadherin and in decreased expression of
MMP-9, which suggested that miR-205 may inhibit
epithelial-mesenchymal transition (EMT). Furthermore, results
demonstrated that UTMD-mediated miR-205 delivery markedly decreased
p-ERK expression, which suggested that miR-205 may downregulate the
ERK signaling pathway (Fig.
6).
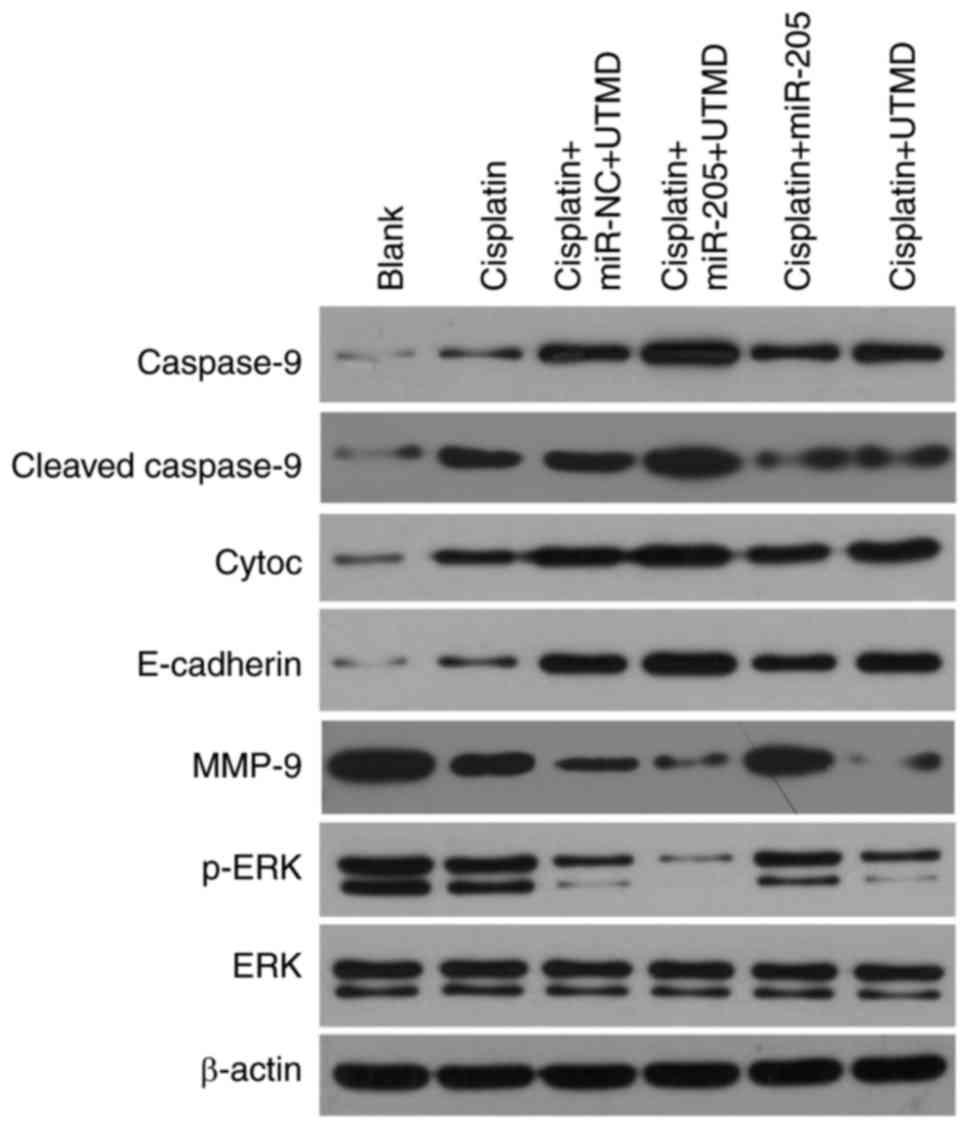 | Figure 6.UTMD-mediated miR-205 transfection
increases the expression ofcaspase-9, cleaved-caspase 9, cyto c and
E-cadherin, and decreases the expression of MMP-9 and p-ERK, as
demonstrated using western blot analysis. Cyto c, cytochrome c;
E-cadherin, epithelial-cadherin; ERK, extracellular
signal-regulated kinase; miR, microRNA; MMP-9, matrix
metalloproteinase-9; NC, negative control; p, phosphorylated; UTMD,
ultrasound-targeted microbubble destruction. |
Discussion
miRNAs serve roles in proliferation,
differentiation, cell cycle, apoptosis, migration and invasion
(27,28). A number of miRNAs have been
previously identified that may serve roles as novel markers for
diagnosis of multifarious tumors (29). miRNAs, acting as oncogenes or tumor
suppressor genes, may become novel therapies against cancer
(30). miR-205 is a highly
conserved miRNA that was identified based on the conserved sequence
of mouse and Takifugu rubripes (31), and was subsequently identified in
human and zebrafish (32,33). Previous studies have indicated that
miR-205 serves roles in the development of various tumors,
including colorectal cancer, PCa, adeno carcinoma, endometrial
cancer, non-small cell lung cancer and nasopharyngeal carcinoma
(34). In the present study,
miR-205 was down-regulated in PCa cells and inhibited PCa cell
proliferation.
UTMD is a novel, safe, non-invasive technology
(35). Compared with viral vector
transfection technology, the combined use of UTMD and non-viral
vectors is a safer and more effective method of increasing the gene
and drug transfection efficiencies (36–38).
UTMD involves the attachment of genes to microbubbles that may
subsequently be injected and circulated through blood vessels and
then destroyed by ultrasound insonation at the target site
(39). Microbubble destruction
leads to increased capillary permeability, generating holes in the
cell membrane releasing the ‘payload’, which is subsequently
incorporated intracellularly (40).
Previous studies have demonstrated that miRNAs can
enhance cisplatin anti-PCa effects (41–43).
The present study evaluated UTMD-mediated miR-205 delivery in PCa
cells to determine whether this delivery system facilitated gene
delivery in PCa cells, with the aim to investigate alterations in
cell proliferation, apoptosis, migration and invasion, and to
elucidate the regulatory functions of miR-205 in PCa. The results
of the present study demonstrated that UTMD-mediated miR-205
transfection inhibited PCa cell proliferation, migration and
invasion, and promoted apoptosis modulated by cisplatin. In
addition, the results demonstrated that UTMD-mediated miR-205
delivery upregulated the protein expression levels of
apoptosis-associated genes caspase-9, cleaved-caspase 9 and
cytoc.
UTMD is a novel tool for organ-specific gene
delivery through the progress of sonoporation allowing for
efficient macromolecule transfer into cells (44). In the present study, UTMD-mediated
miR-205 significantly inhibited PCa cell proliferation, migration
and invasion, and induced apoptosis, compared with miR-205. The
results suggested that UTMD-mediated delivery of miRNA is a
potential platform for PCa therapy.
The mitogen-activated protein kinase (MAPK) pathway
is an information dissemination and aggregation pathway that
mediates nuclear reactions caused by an extracellular signal. MAPK
is composed of three main pathways, including ERK, JNK and p38
(45). ERK1 and ERK2 mediate
extracellular signals into the nucleus through a signal
transduction cascade, activating a series of effect or molecules in
the nucleus, which regulate biological activities, including cell
proliferation and apoptosis (46).
A previous study demonstrated that receptor tyrosine-protein kinase
ErbB2 inhibits miR-205 transcription through the Ras/Raf/dual
specificity mitogen-activated protein kinase MEK/ERK pathway in
breast cancer (47). miR-205 is
involved in osteogenic differentiation of bone mesenchymal stem
cells via the DNA-binding protein SATB2/Runt-related transcription
factor 2 and ERK/MAPK pathways (48). miR-205 is also reported to
downregulate p-MAPK levels in breast cancer (49). Results from the present study
demonstrated that UTMD-mediated miR-205 transfection led to reduced
p-ERK expression, which suggested that miR-205 may regulate ERK
signaling pathway.
EMT has been hypothesized to be associated with
tumor invasion and metastasis, and it is regulated by multiple
biological molecules and signaling pathways (50). miRNAs are a class of non-coding
RNAs, which can negatively regulate mRNA expression of target genes
(51). One previous study
demonstrated that certain miRNAs (miRNA-9, miRNA23b and
miRNA-17-92) suppress the invasion and metastasis of cancer by
regulating EMT-related factors (E-cadherin and MMP-9) (52). Up-regulation of E-cadherin
increases intercellular adhesion (53) and it has been demonstrated that
drugs significantly decrease the metastasis of lung cancer via
down-regulation of MMP9 (54). A
number of studies have demonstrated that ERK1/2 serves arole in the
process of EMT in a number of tumors (55–57).
Another previous study demonstrated that miR-205 regulates invasion
and migration of laryngeal squamous cell carcinoma by AKT-mediated
EMT (58). In the present study,
UTMD-mediated miR-205 transfection upregulated the expression of
E-cadherin and downregulated MMP-9 expression, suggesting that
miR-205 may inhibit EMT in PC-3 cells. Therefore, UTMD-mediated
miR-205 delivery is a potential method of PCa treatment.
In conclusion, the present study used UTMD to
successfully transfect PCa cells withmiR-205 mimics plasmid; the
results demonstrated that cell proliferation, migration and
invasion were suppressed, and apoptosis was increased, which may
aid in future efforts of miRNA inhibition in vivo.
Furthermore, the present study demonstrated that UTMD-mediated
miR-205 delivery increased the expression levels of E-cadherin and
decreased the expression of MMP-9 and p-ERK, which suggested that
the ERK signaling pathway may serve a role in the development and
progression of PCa. UTMD-mediated miR-205 delivery may be a novel
molecular targeted therapy for the treatment of PCa.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ren SC, Chen R and Sun YH: Prostate cancer
research in China. Asian J Androl. 15:350–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye D and Zhu Y: Epidemiology of prostate
cancer in China: An overview and clinical implication. Zhonghua Wai
Ke Za Zhi. 53:249–252. 2015.(In Chinese). PubMed/NCBI
|
|
4
|
Ibrahim SA, Hassan H and Götte M: MicroRNA
regulation of proteoglycan function in cancer. FEBS J.
281:5009–5022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janga SC and Vallabhaneni S: MicroRNAs as
post-transcriptional machines and their interplay with cellular
networks. Adv Exp Med Biol. 722:59–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guedes J, Cardoso AL and de Lima Pedroso
MC: Involvement of microRNA in microglia-mediated immune response.
Clin Dev Immunol. 2013:1868722013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
8
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McGuire A, Brown JA and Kerin MJ:
Metastatic breast cancer: The potential of miRNA for diagnosis and
treatment monitoring. Cancer Metastasis Rev. 34:145–155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang
Y, Li J, Bian Z, Liang X, Cai X, et al: Exogenous plant MIR168a
specifically targets mammalian LDLRAP1: Evidence of cross-kingdom
regulation by microRNA. Cell Res. 22:107–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gismondi A, Di Marco G and Canini A:
Detection of plant microRNAs in honey. PLoS One. 12:e01729812017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gandellini P, Folini M and Zaffaroni N:
Emerging role of microRNAs in prostate cancer: Implications for
personalized medicine. Discov Med. 9:212–218. 2010.PubMed/NCBI
|
|
14
|
Leite KR, Morais DR, Florez MG, Reis ST,
Iscaife A, Viana N, Moura CM, Silva IA, Katz BS, Pontes J Jr, et
al: The role of microRNAs 371 and 34a in androgen receptor control
influencing prostate cancer behavior. Urol Oncol. 33(267): e15–22.
2015.
|
|
15
|
Sun X, Liu Z, Yang Z, Xiao L, Wang F, He
Y, Su P, Wang J and Jing B: Association of microRNA-126 expression
with clinicopathological features and the risk of biochemical
recurrence in prostate cancer patients undergoing radical
prostatectomy. Diagn Pathol. 8:2082013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tinkov S, Bekeredjian R, Winter G and
Coester C: Microbubbles as ultrasound triggered drug carriers. J
Pharm Sci. 98:1935–1961. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanguino A, Lopez-Berestein G and Sood AK:
Strategies for in vivo siRNA delivery in cancer. Mini Rev Med Chem.
8:248–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ibsen S, Schutt CE and Esener S:
Microbubble-mediated ultrasound therapy: A review of its potential
in cancer treatment. Drug Des Devel Ther. 7:375–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayer CR, Geis NA, Katus HA and
Bekeredjian R: Ultrasound targeted microbubble destruction for drug
and gene delivery. Expert Opin Drug Deliv. 5:1121–1138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kiessling F, Fokong S, Koczera P, Lederle
W and Lammers T: Ultrasound microbubbles for molecular diagnosis,
therapy, and theranostics. J Nucl Med. 53:345–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Miyoshi H and Nakamura M:
Encapsulated ultrasound microbubbles: Therapeutic application in
drug/gene delivery. J Control Release. 114:89–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer-the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leong-Poi H, Kuliszewski MA, Lekas M,
Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ and
Lindner JR: Therapeutic arteriogenesis by ultrasound-mediated
VEGF165 plasmid gene delivery to chronically ischemic skeletal
muscle. Circ Res. 101:295–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang D, Gao YH, Tan KB, Zuo ZX, Yang WX,
Hua X, Li PJ, Zhang Y and Wang G: Inhibition of hepatic fibrosis
with artificial microRNA using ultrasound and cationic
liposome-bearing microbubbles. Gene Ther. 20:1140–1148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kubota H, Fukuta K, Yamada K, Hirose M,
Naruyama H, Yanai Y, Yamada Y, Watase H, Kawai N, Tozawa K and
Yasui T: Feasibility of metronomic chemotherapy with
tegafur-uracil, cisplatin, and dexamethasone for
docetaxel-refractory prostate cancer. J Rural Med. 12:112–119.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tricoli JV and Jacobson JW: MicroRNA:
Potential for cancer detection, diagnosis, and prognosis. Cancer
Res. 67:4553–4555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wienholds E, Kloosterman WP, Miska E,
Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen
S and Plasterk RH: MicroRNA expression in zebrafish embryonic
development. Science. 309:310–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao Y, Wu S, Zhao R and Deng Q: MiR-205
promotes proliferation, migration and invasion of nasopharyngeal
carcinoma cells by activation of AKT signalling. J Int Med Res.
44:231–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Sun Z, Ren P, Lee RJ, Xiang G, Lv
Q, Han W, Wang J, Ge S and Xie M: Ultrasound-targeted microbubble
destruction (UTMD) assisted delivery of shRNA against PHD2 into
H9C2 cells. PLoS One. 10:e01346292015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen H and Hwang JH: Ultrasound-targeted
microbubble destruction for chemotherapeutic drug delivery to solid
tumors. J Ther Ultrasound. 1:102013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen ZY, Lin Y, Yang F, Jiang L and Ge Sp:
Gene therapy for cardiovascular disease mediated by ultrasound and
microbubbles. Cardiovasc Ultrasound. 11:112013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wan C, Li F and Li H: Gene therapy for
ocular diseases meditated by ultrasound and microbubbles (Review).
Mol Med Rep. 12:4803–4814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma J, Du LF, Chen M, Wang HH, Xing LX,
Jing LF and Li YH: Drug-loaded nano-microcapsules delivery system
mediated by ultrasound-targeted microbubble destruction: A
promising therapy method. Biomed Rep. 1:506–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nande R, Howard CM and Claudio PP:
Ultrasound-mediated oncolytic virus delivery and uptake for
increased therapeutic efficacy: State of art. Oncolytic Virother.
4:193–205. 2015.PubMed/NCBI
|
|
41
|
Liu F, Wang J, Fu Q, Zhang X, Wang Y, Liu
J, Huang J and Lv X: VEGF-activated miR-144 regulates autophagic
survival of prostate cancer cells against Cisplatin. Tumour Biol.
Nov 13–2015.(Epub ahead of print).
|
|
42
|
Pennati M, Lopergolo A, Profumo V, De
Cesare M, Sbarra S, Valdagni R, Zaffaroni N, Gandellini P and
Folini M: miR-205 impairs the autophagic flux and enhances
cisplatin cytotoxicity in castration-resistant prostate cancer
cells. Biochem Pharmacol. 87:579–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou P, Ma L, Zhou J, Jiang M, Rao E, Zhao
Y and Guo F: miR-17-92 plays an oncogenic role and conveys
chemo-resistance to cisplatin in human prostate cancer cells. Int J
Oncol. 48:1737–1748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng X, Ji P and Hu J: Sonoporation using
microbubbles promotes lipofectamine-mediated siRNA transduction to
rat retina. Bosn J Basic Med Sci. 11:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hasegawa T, Adachi R, Iwakata H, Takeno T,
Sato K and Sakamaki T: ErbB2 signaling epigenetically suppresses
microRNA-205 transcription via Ras/Raf/MEK/ERK pathway in breast
cancer. FEBS Open Bio. 7:1154–1165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu N, Feng C, Jiang Y, Miao Q and Liu H:
Regulative effect of Mir-205 on osteogenic differentiation of bone
mesenchymal stem cells (BMSCs): Possible Role of SATB2/Runx2 and
ERK/MAPK Pathway. Int J Mol Sci. 16:10491–10506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iorio MV, Casalini P, Piovan C, Di Leva G,
Merlo A, Triulzi T, Menard S, Croce CM and Tagliabue E:
microRNA-205 regulates HER3 in human breast cancer. Cancer Res.
69:2195–2200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schwarzenbach H: The clinical relevance of
circulating, exosomal miRNAs as biomarkers for cancer. Expert Rev
Mol Diagn. 15:1159–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin CW, Kao SH and Yang PC: The miRNAs and
epithelial-mesenchymal transition in cancers. Curr Pharm Des.
20:5309–5318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pieters T and van Roy F: Role of cell-cell
adhesion complexes in embryonic stem cell biology. J Cell Sci.
127:2603–2613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li L, Wang S, Yang X, Long S, Xiao S, Wu W
and Hann SS: Traditional Chinese medicine, Fuzheng KangAi
decoction, inhibits metastasis of lung cancer cells through the
STAT3/MMP9 pathway. Mol Med Rep. 16:2461–2468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ha GH, Park JS and Breuer EK: TACC3
promotes epithelial-mesenchymal transition (EMT) through the
activation of PI3K/Akt and ERK signaling pathways. Cancer Lett.
332:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pan H, Jiang T, Cheng N, Wang Q, Ren S, Li
X, Zhao C, Zhang L, Cai W and Zhou C: Long non-coding RNA BC087858
induces non-T790M mutation acquired resistance to EGFR-TKIs by
activating PI3K/AKT and MEK/ERK pathways and EMT in non-small-cell
lung cancer. Oncotarget. 7:49948–49960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang H, Sun JD, Yan LJ and Zhao XP:
PDGF-D/PDGFRβ promotes tongue squamous carcinoma cell (TSCC)
progression via activating p38/AKT/ERK/EMT signal pathway. Biochem
Biophys Res Commun. 478:845–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang B, Lv K, Chen W, Zhao J, Luo J, Wu J,
Li Z, Qin H, Wong TS, Yang W, et al: miR-375 and miR-205 regulate
the invasion and migration of laryngeal squamous cell carcinoma
synergistically via AKT-Mediated EMT. Biomed Res Int.
2016:96527892016. View Article : Google Scholar : PubMed/NCBI
|