Introduction
Of all gynecological diseases, breast cancer has the
highest incidence worldwide (1).
There are a number of causes of breast cancer, including aberrant
estrogen receptor signaling (2),
genetic and environmental factors (3–5) and
inflammation (6). However, breast
cancer is not as invasive as other gynecological tumors, including
cervical and ovarian cancers. There are two types of breast cancer
cell lines (MDA-MB-231 and MCF-7) that are frequently used as
models of breast cancer progression and which exhibit varying
metastatic potential (7). Multiple
different signaling pathways control the metastatic potential of
these cells, including those involved in growth and apoptosis
(8,9), hypoxia-associated gene expression
(10), proteasomal activation and
serine/threonine-protein kinase mTOR (mTOR) (11), protein Wnt5a (12), phosphatidylinositol 3-kinase
(13), nuclear factor-κB (NF-κB)
(14) and nuclear factor erythroid
2-related factor 2 signaling (15). These pathways may also contribute
to resistance against first-line endocrine therapy (16).
Toll-like receptors (TLRs) are ancient microbial
pattern recognition receptors that are highly conserved from
Drosophila to humans. TLR4 activates myeloid differentiation
factor 88 (MyD88) upon receiving tumor antigen information and
promotes the resting state of NF-κB nuclear translocation, finally
activating gene transcription (17). By contrast, TLR4 may also allow
tumor cells to escape host immune surveillance through the MyD88
signaling pathway. Li et al (18) identified that high expression
levels of TLR4 and MyD88 were associated with poor overall survival
rates in patients with epithelial ovarian cancer (EOC). Inhibition
of TLR4/MyD88 signaling may therefore be a useful tool in promoting
DNA repair and maintaining immune responses following ultraviolet
radiation-induced damage, which contributes to the development of
nonmelanoma skin cancer (19).
High levels of MyD88 are also associated with reduced survival
rates of patients with EOC (20).
Atractylenolide-I, a novel TLR4-antagonist, inhibits lymphocyte
antigen 96 (MD-2)-mediated TLR4/MyD88 signaling, making it a
potential therapy for patients with EOC (21). Finally, targeting the
cyclooxygenase 2/prostaglandin E2 and TLR/MyD88 signaling pathways
in gastric cancer cells suppresses inflammation and maintains
stemness (22).
High mobility group box 1 (HMGBl), an endogenous
ligand for TLR4, has attracted much attention in recent years.
HMGB1 is an abundant non-histone nuclear transcription factor and
is involved in the growth and metastasis of prostate (23), colorectal (24), gastric (25), liver (26) and lung (27) tumors. TLR4 acts as a transmembrane
receptor that is able to activate MyD88-dependent signaling in
response to the binding of HMGB1. HMGB1-mediated TLR4/MyD88
signaling has been implicated in the invasion and metastasis of a
number of different cancer cell types (18,19).
However, the role of TLR4/MyD88 in human breast cancer progression
has not been well characterized.
A previous study identified that the mRNA expression
levels of TLR4 and MYD88 were significantly higher in breast cancer
cells compared with fibroadenoma cells and adjacent normal tissues;
high protein expression levels of TLR4 and MyD88 were also
associated with poor clinical prognosis (28). The current study aimed to examine
the mechanisms underlying cancer cell invasion mediated by TLR4 and
MyD88. MDA-MB-231 and MCF-7 represent human breast cell lines with
varying metastatic and invasive potential. Generally, MCF-7 cells
are non-invasive, while MDA-MB-231 cells are highly invasive
(29) and used to examine the
mechanisms of breast cancer metastasis (30). The present study used these two
cellular models of invasion to examine the association between
TLR4, MyD88 and HMGB1 expression levels and metastatic
potential.
Materials and methods
Cell culture
MCF-7 and MDA-MB-231 cells were purchased from the
cell bank of the Chinese Academy of Sciences (Shanghai, China).
MDA-Kb2 cells were purchased from Shanghai Composite Biology Co.,
Ltd (http://www.xiangbio.com/; Shanghai,
China). Normal human breast tissues were donated by the First
Affiliated Hospital of Fujian Medical University (Fujian, China).
Additional instruments and reagents used are in Table I.
 | Table I.Expression of TLR4, MyD88 and HMGB1
in breast cancer and cancer-adjacent tissues. |
Table I.
Expression of TLR4, MyD88 and HMGB1
in breast cancer and cancer-adjacent tissues.
|
|
| TLR4 |
|
| MyD88 |
|
| HMGB1 |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Group | No. of
specimens | − | + | χ2 | P-value | − | + | χ2 | P-value | − | + | χ2 | P-value |
|---|
| Breast cancer | 100 | 54 | 46 | 88.72 | <0.001 | 59 | 41 | 86.65 | <0.001 | 24 | 76 | 67.68 | <0.001 |
| Cancer-adjacent
tissues | 20 | 16 | 4 |
|
| 15 | 5 |
|
| 12 | 8 |
|
|
MDA-MB-231 and MDA-Kb2 cells were cultured in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.) and MCF-7 cells were cultured in DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 0.01 mg/ml
bovine insulin and 10% FBS. All cells were cultured in a humidified
incubator with 5% CO2 at 37°C.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde at room
temperature for 30 min, permeabilized with 0.05% Triton X-100 and
blocked with 1% bovine serum albumin (Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature. The cells were
subsequently washed using 1X Tween and PBS (PBST) solution
(Beyotime Institute of Biotechnology, Haimen, China) and incubated
with anti-HMGB1 (cat. no. ab18256; 1:50; Abcam, Cambridge, UK),
anti-TLR4 (cat. no. ab22048; 1:50; Abcam) and anti-MyD88 (cat. no.
9284; 1:50; Cell Signaling Technology, Inc., Danvers, MA, USA)
monoclonal antibodies for 1 h at room temperature and washed five
times with 1X PBST, followed by incubation with Alexa Fluor 555
labeled donkey anti-mouse immunoglobulins (1:100; cat. no. A0460;
Beyotime Institute of Biotechnology, Shanghai, China) for 1 h at
room temperature. The cells were treated with DAPI (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 15 min at room temperature in
dark to stain nuclei. Fluorescence images (×400) were captured
using an Olympus confocal scanning microscope (Olympus Corporation,
Tokyo, Japan). The relative fluorescence intensity was calculated
with ImageJ densitometry software (version 1.6, National Institutes
of Health, Bethesda, MD, USA).
Immunohistochemistry
A total of 100 tissue samples were collected from
patients with breast cancer of different stages from the specimen
repository of the Department of Breast and Thyroid Surgery, The
First Affiliated Hospital of Fujian Medical University. These
samples were initially obtained between January 2012 and December
2013, and were documented for age, family cancer history, tumor
size, histological grade, tumor stage and axillary lymph node
metastasis. A total of 20 cancer-adjacent tissues were used as a
control.
All patients were women aged 35–70 years, with a
median age of 44 years, and all gave written informed consent.
Ethical approval was obtained from the ethics committee of The
First Affiliated Hospital of Fujian Medical University [(2014)106].
None of the patients received neoadjuvant chemotherapy or
radiotherapy prior to biopsy, although each patient received
individualized adjuvant chemotherapy. The tumors were staged per
the American Joint Cancer Committee Guidelines (31) and included 21 cases of Stage I
disease, 38 cases of Stage II disease and four cases of Stage III
disease. Histological grading was referred to as the standard of
diagnosis and treatment (19).
Cancer and control tissues were used for HMGB1, TLR4
and Myd88 protein detection. Antibodies used for
immunohistochemical staining included anti-HMGB1 (cat. no. ab18256;
1:50; Abcam), anti-TLR4 (cat. no. ab22048; 1:50; Abcam) and
anti-MyD88 (cat. no. 9284; 1:50; Cell Signaling Technology, Inc.).
Briefly, tissue slides were deparaffinized in xylene and rehydrated
with an ethanol gradient, consisting of 5-minute washes using
absolute 95, 80 and 70% ethanol. Then the sections were subjected
to antigen retrieval by boiling in 0.01 mol/l sodium citrate buffer
(pH 6.0) in a microwave oven for 10 min. Following blocking of
endogenous peroxidase activity with 0.3% hydrogen peroxide and
blocking nonspecific protein binding with 1.5% normal goat serum
(Thermo Fisher Scientific, Inc.) at room temperature for 1 h the
sections were incubated overnight with primary antibodies at 4°C in
a humidified chamber. The sections were subsequently incubated with
biotinylated goat anti-mouse IgG (cat. no. ab64255; Abcam) for 30
min at 37°C and proteins were detected with 3,3′-diaminobenzidine.
To evaluate the presence or absence of lung metastasis, the lung
tissues were serially cut into 5-µm slices and every 10th section
was stained with hematoxylin for 10 min, rinsed with running water,
differentiated with hydrochloric acid and then stained with eosin
for 5 min at room temperature The number of metastases in the lungs
was calculated by two independent pathologists.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA (2 mg)
was reverse transcribed to cDNA with PrimeScript™ 1st Strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China) and
RT-PCR was carried out with TaKaRa Ex Taq® (Takara
Biotechnology Co., Ltd.). RT-PCR was performed according to the
following conditions: Pre-denaturation at 95°C for 5 min, followed
by 35 cycles of 95°C for 1 min, 60°C for 45 sec and 72°C for 45
sec, and a final extension at 72°C for 10 min. The primers for
RT-PCR were as follows: TLR4 forward (F),
5′-AATGGATCAAGGACCAGAGG-3′ and reverse (R),
5′-CAGCCAGCAAGAAGCATCAG-3′; MYD88 F, 5′-CGCCGGATGGTGGTGGTTGT-3′ and
R, 5′-TGTAGTCGCAGACAGTGATGAACC-3′; HMGB1 F,
5′-AATACGAAAAGGATATTGCT-3′ and R, 5′-GCGCTAGAACCAACTTAT-3′; and
GAPDH F, 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′ and R,
5′-CGTCATCCCTGCTTGCTGATCCACATCTGC-3′. The relative mRNA expression
of target genes was normalized to GAPDH with the method of
2−ΔΔCq (32).
Western blot analysis
Whole cell lysates were prepared using
radioimmunoprecipitation assay buffer [1% Triton X-100, 150 mmol/l
NaCl, 1 mmol/l EGTA, 50 mmol/l Tris-HCl, 0.1% sodium dodecyl
sulfate (SDS), 1% sodium deoxycholate and phenylmethylsuphonyl
fluoride; Cell Signaling Technology, Inc.] and western blotting was
performed as previously described (33). The concentration of protein was
determined by bicinchoninic acid kit (Sigma-Aldrich, Merck KGaA).
The same amount of proteins (30 µg) were separated by 12.5%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
Following being blocked with 5% non-fat milk at room temperature
for 1 h, the membranes were treated with anti-TLR4 (cat. no.
ab22048; 1:100; Abcam) and anti-MyD88 (cat. no. 9284; 1:100; Cell
Signaling Technology, Inc.). Antibodies at 4°C overnight. The next
morning, the membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse secondary antibody (Santa Cruz
Biotechnology, Inc.; cat. no. sc-2031; 1:10,000) for 2 h at room
temperature. After being washed, bands were developed with enhanced
chemiluminescence kit (Sigma-Aldrich, Merck KGaA) and captured with
Gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The relative expression of target proteins was calculated with
Quantity One Software V4.2 (Bio-Rad Laboratories, Inc.) normalized
to GAPDH.
Statistical analysis
Data derived from at least three separate and
independent experiments were expressed as the mean ± standard
deviation. Statistical differences of different groups were
performed with SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
χ2 tests and the Fisher exact probability method were
used to compare the differences between expression levels of HGBM1,
TLR4 and MyD88 in each group, and the association between their
expression levels and the clinicopathological features of breast
cancer. A one-way analysis of variance with Dunnett's post-hoc
analysis was used for comparisons between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of TLR4, MYD88 and
HMGB1 vary according to metastatic potential in breast cancer
cells
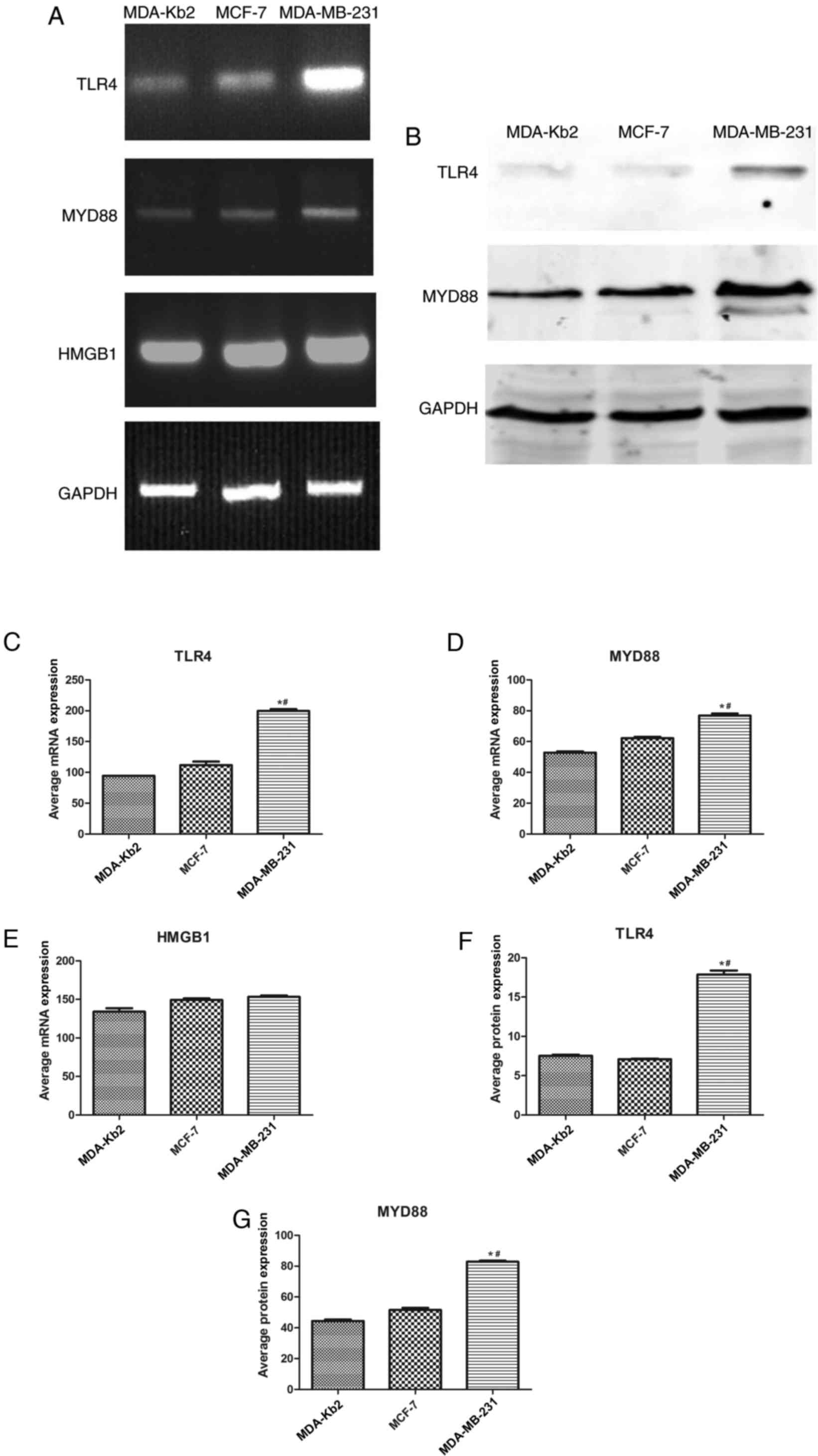
It was identified that TLR4, MYD88 and HMGB1 were
expressed to a marked extent in MDA-MB-231, MCF-7 and MDA-Kb2
cells. The respective expression levels of TLR4 and MYD88 in
MDA-MB-231 cells were 10.43 and 2.09 times higher compared with
those in MCF-7 cells (P<0.05; Fig.
1). No significant differences were observed between the
expression levels of either TLR4 or MYD88 in MCF-7 and MDA-Kb2
cells. TLR4 protein expression in MDA-MB-231 cells was 2.6 times
higher compared with MCF-7 cells and 2.4 times higher compared with
MDA-Kb2 cells. The protein expression levels of MYD88 in MDA-MB-231
cells were 1.6 times higher compared with MCF-7 cells and 1.8 times
higher compared with MDA-Kb2 cells (P<0.05; Fig. 1). HMGB1 was also expressed in these
three cell lines (Fig. 1).
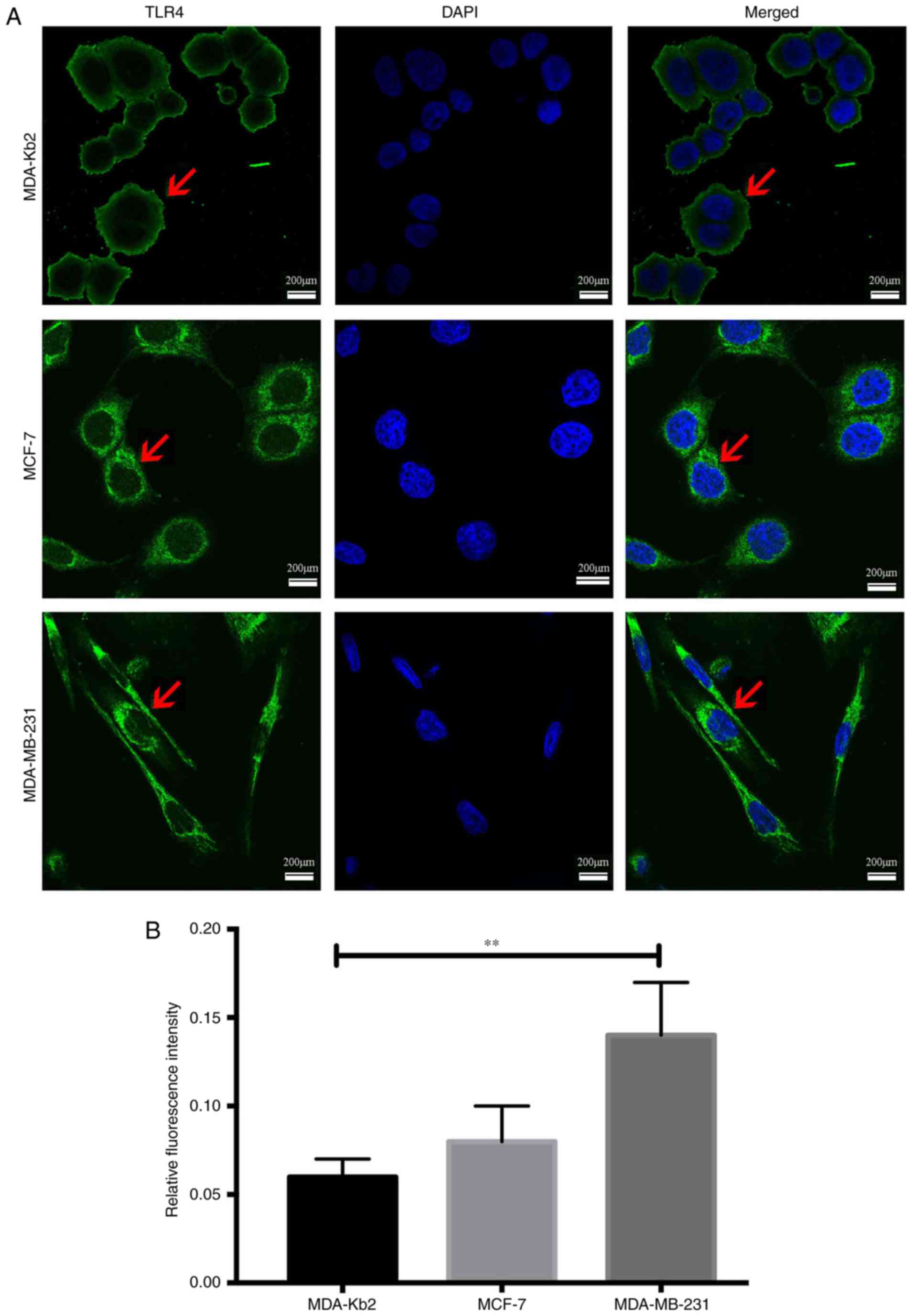
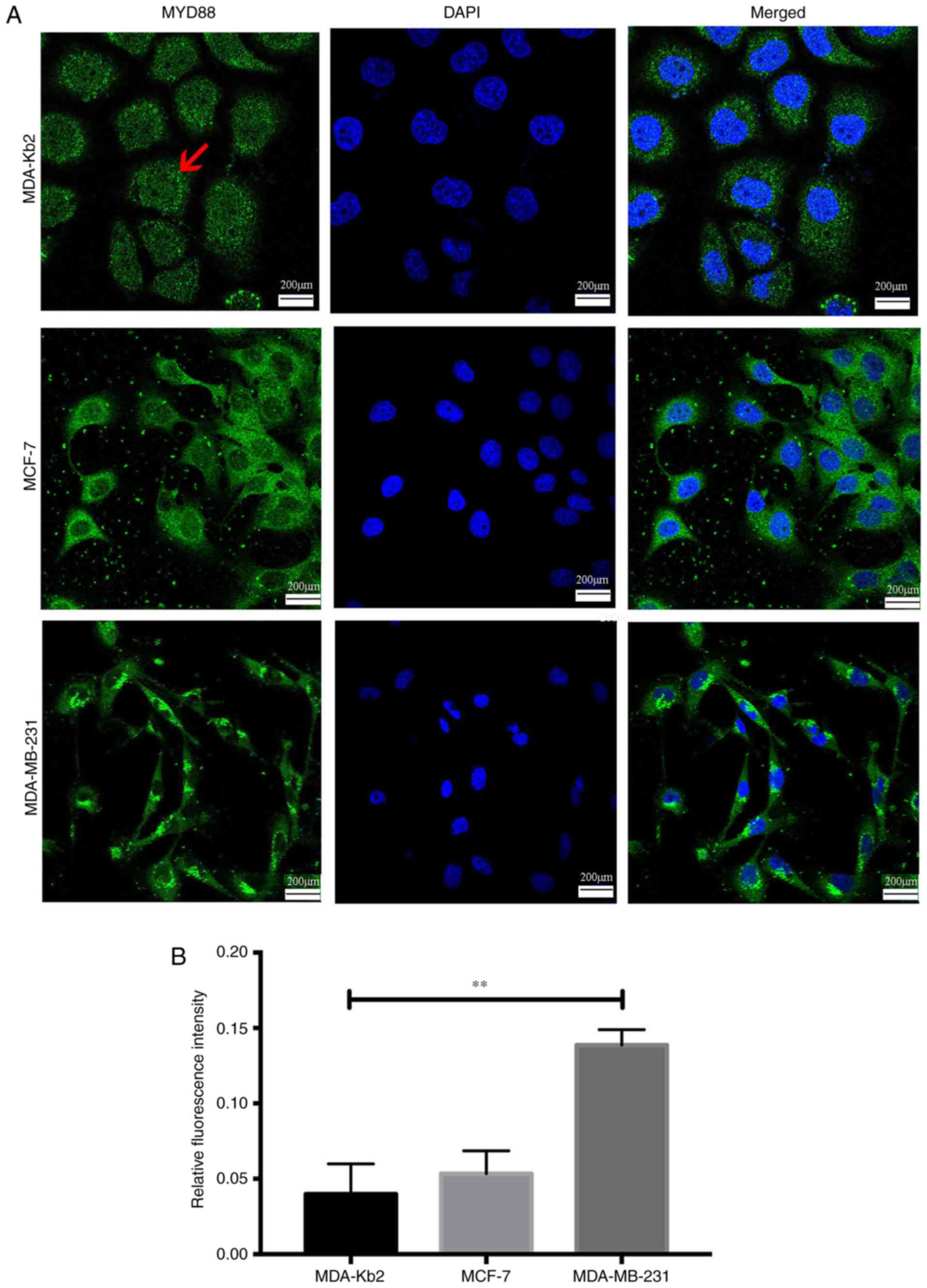
The average fluorescence intensity of TLR4 protein
expression in MDA-MB-231 cells was 0.14 and 0.08 in MCF-7 cells
(P<0.01; Fig. 2). There were no
significant differences between the intensities in MCF-7 and
MDA-Kb2 cells (P>0.05). MyD88 protein was primarily expressed in
the cytoplasm of all three cell types. The average fluorescence
intensity of MyD88 in MDA-MB-231 and MCF-7 cells was 0.136 and
0.05, respectively (P<0.01; Fig.
3), while there was no significant difference between the
levels in MCF-7 and MDA-Kb2 cells (P>0.05). These results
suggested that an association may exist between TLR4 and MyD88 and
the invasive potential of breast cancer cells.
Expression of TLR4 and MyD88
correlates with the metastatic potential of tumors from patients
with breast cancer
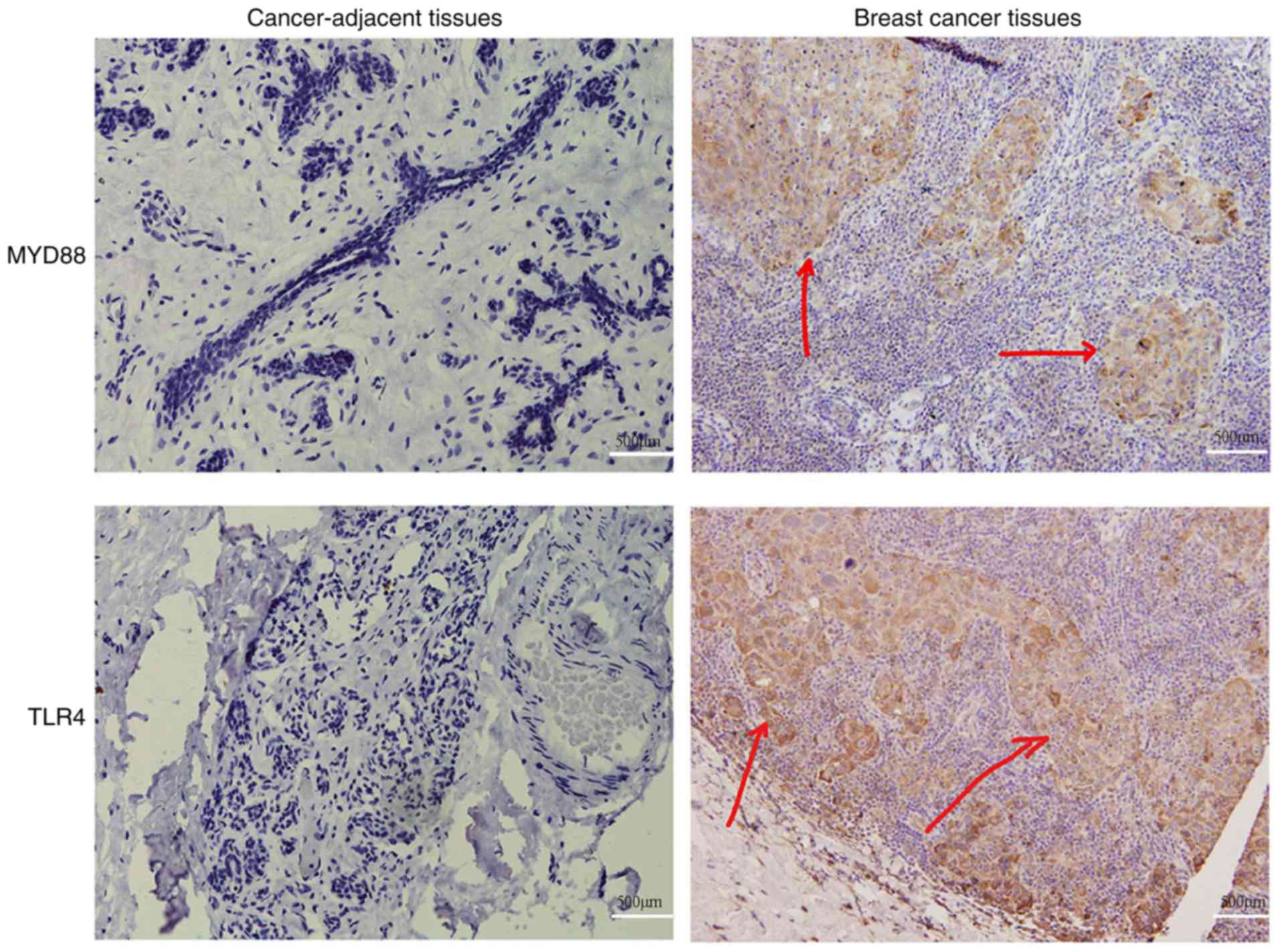
To examine the association between TLR4/MyD88
signaling and breast cancer progression, breast tumors were
collected from patients and used to detect the protein expression
levels of TLR4 and MyD88. Randomly selected cancer-adjacent tissues
were used as controls. It was identified that TLR4 was primarily
localized to the plasma membrane as fine brown granules (Fig. 4). Expression of TLR4 was identified
in 46% (46/100 cases) of breast tumors and 20% (4/20 cases) of
cancer-adjacent tissues (P<0.001; Table I). MyD88 was primarily localized to
the cytoplasm as brownish yellow granules (Fig. 4). Expression of MyD88 was
identified in 41% (41/100 cases) of breast tumors and 25% (5/20
cases) of cancer-adjacent tissues (P<0.001; Table I). These results were consistent
with the expression patterns of TLR4 and MyD88 in vitro.
TLR4, MyD88 and HMGB1 protein
expression is associated with poor prognosis in patients with
breast cancer
To determine whether there was a correlation between
the expression levels of TLR4, MyD88 and HMGB1 and breast cancer
invasion, clinical and pathological data were collected from the
100 cases of breast cancer used in the expression analysis. It was
identified that the expression rates of TLR4 in patients with or
without axillary lymph node metastasis were 68.8 and 55%
(P<0.05), respectively. TLR4 expression rates for samples from
patients with Stage I/II or Stage III disease were 33.8 and 71.9%
(P<0.001; Table II),
respectively. The expression rates of MyD88 in samples with or
without axillary lymph nodes metastasis were 59 and 25.6%
(P<0.05), respectively. MyD88 expression rates in samples from
patients with Stage I/II or Stage III disease were 44.1 and 65.6%,
respectively (P<0.05; Table
III). The expression rates of HMGB1 in samples with or without
axillary lymph node metastasis were 63.9 and 41% (P<0.05),
respectively. For samples from patients with Stage I/II or Stage
III disease, the rates were 41.2 and 68.7%, respectively
(P<0.05; Table IV).
 | Table II.TLR4 expression and the corresponding
breast cancer clinical pathological features. |
Table II.
TLR4 expression and the corresponding
breast cancer clinical pathological features.
|
|
| TLR4 |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | No. | − | + | χ2 | P-value |
|---|
| Axillary lymph node
metastasis |
|
Yes | 61 | 19 | 42 | 12.45 | 0.002 |
| No | 39 | 27 | 12 |
|
|
| Histological
grade |
|
Yes | 68 | 45 | 23 | 8.155 | 0.006 |
| No | 32 | 9 | 23 |
|
|
 | Table III.MyD88 expression and the
corresponding breast cancer clinical pathological features. |
Table III.
MyD88 expression and the
corresponding breast cancer clinical pathological features.
|
|
| MyD88 |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | No. | − | + | χ2 | P-value |
|---|
| Axillary lymph node
metastasis |
|
Yes | 61 | 25 | 36 | 6.094 | 0.015 |
| No | 39 | 29 | 10 |
|
|
| Histological
grade |
|
Yes | 68 | 38 | 30 | 5.366 | 0.039 |
| No | 32 | 11 | 21 |
|
|
 | Table IV.HMGB1 expression and the
corresponding breast cancer clinical pathological features. |
Table IV.
HMGB1 expression and the
corresponding breast cancer clinical pathological features.
|
|
| HMGB1 |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | No. | − | + | χ2 | P-value |
|---|
| Axillary lymph node
metastasis |
|
Yes | 61 | 22 | 39 | 6.994 | 0.017 |
| No | 39 | 23 | 16 |
|
|
| Histological
grade |
|
Yes | 68 | 40 | 28 | 5.587 | 0.028 |
| No | 32 | 10 | 22 |
|
|
Discussion
Breast cancer is one of the most common cancer types
in women and is the second most common among all cancer types
worldwide. Targeted medicine has become more widespread, as it
improves the diagnosis, prognosis and treatment of patients with
breast cancer (31). More
effective molecular targets of carcinogenesis and metastasis in
breast cancer are being identified every year, including erb-b2
receptor tyrosine kinase 2 (34),
X-C motif chemokine receptor 1 (35) and the mTOR effectors ribosomal
protein S6 kinases B1 and 2, and eukaryotic translation initiation
factor 4E-binding protein 1 (36).
TLR4/MyD88 signaling occurs primarily during
inflammation (37), and activation
of the TLR4 complex may control the pathophysiology of a number of
human diseases, including cardiovascular disorders, diabetes,
metabolic syndrome, autoimmune disorders, neuroinflammatory
disorders, autism and chronic fatigue syndrome (38). Repressing TLR4/MyD88 signaling
decreases cell viability, activates apoptosis and increases the
levels of inflammatory factors following Bacillus Calmette-Guerin
infection in macrophages (39).
Furthermore, ochratoxin DefiA induces immune-associated toxicity
via reactive oxygen species-mediated TLR4/MyD88 signaling in
porcine alveolar macrophages (40), and curcumin (41) and irisin (42) exert their biological functions by
inhibiting TLR4/MyD88/NF-κB signaling. The tumor-associated
inflammatory microenvironment may serve a pivotal role in the
progression and prognosis of a number of cancer types, including
ovarian, rectal and prostate cancer (43). Methicillin-resistant
Staphylococcus aureus infection may enhance non-small cell
lung cancer metastasis by upregulating TLR4 signaling (44), and polysaccharopeptide exerts
immunomodulatory effects through TLR4-TIRAP/MAL-MyD88 signaling in
peripheral blood mononuclear cells from patients with breast cancer
(45).
Cellular invasion is a common characteristic of
malignant tumors. Tumor invasiveness is frequently accompanied by
the overexpression and activation of oncogenes, or the loss of
tumor suppressors. The estrogen receptor-positive human breast
cancer cell line MCF-7, which has a low metastatic potential, is
the most common cellular model of breast cancer. By contrast,
MDA-MB-231, which is estrogen receptor-negative, has a high rate of
invasion and spontaneous metastasis. The results of the present
study demonstrated that HMGB1, TLR4 and MyD88 were expressed in
MCF-7, MDA-MB-231 and MDA-Kb2 cells. It was identified that the
protein and mRNA expression levels of TLR4 and MyD88 were
significantly higher in MDA-MB-231 cells compared with MCF-7 cells.
These results demonstrated a positive association between
TLR4/MyD88 expression and invasive potential, which is consistent
with the results of other studies performed on colorectal and
ovarian cancer (18,46). However, the levels of HMGB1, the
ligand of TLR4, demonstrated no statistical differences between
cell lines, suggesting that the expression of the TLR4 receptor,
and not its ligand, is the key regulatory factor that determines
invasiveness.
To further examine the association between the
TLR4/MyD88 pathway and breast cancer progression, the expression
levels of TLR4 and MyD88 in breast tumors from patients with
various stages of disease were assessed. The results demonstrated
that the expression levels of TLR4 and MyD88 were significantly
increased in breast tumors compared with normal breast tissue.
These levels were positively correlated with axillary lymph node
metastasis and histological grade. This observation confirmed the
association between the TLR4/MyD88 pathway and breast cancer and
may provide a novel potential biomarker and therapeutic target to
aid in the prognosis and treatment of patients with this type of
cancer.
In conclusion, the expression levels of TLR4/MyD88
were positively correlated with the metastatic potential of breast
cancer cells and tumors. The expression levels of TLR4/MyD88 may be
used as a biomarker to evaluate the prognosis and guide the
treatment of patients with breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the Key Clinical
Specialty Discipline Construction Program of Fujian, P.R.C.
Professor Development Fund (grant no. JS14021) and the Natural
Science Foundation of Fujian Province (grant no. 2015J01386).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KLW managed the study and the materials, and
constructed the target breast cancer cell lines; HHZ was
responsible for the choice of experimental materials and performed
the IHC experiments; YJF examined the mRNA and protein levels of
TLR; YZZ determined the localization of TLR4 in cells; LJK detected
the expression of HMGB1, TLR4 and MyD88; LC assisted cell culture
and data sorting; FZ performed MCF-7 and MDA-MB-231 cell culture
and data sorting; LFY constructed and identified TLR-siRNA plasmid
and transfected cells; and XJC supervised the project and
determined the breast cancer clinical pathological features.
Ethics approval and consent to
participate
Ethical approval was obtained from the ethics
committee of The First Affiliated Hospital of Fujian Medical
University [Fujian, China; (2014)106]. All patients gave written
informed consent
Patient consent for publication
All patients were given written informed consent.
Ethical approval was obtained from the ethics committee of The
First Affiliated Hospital of Fujian Medical University
[(2014)106].
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dall GV and Britt KL: Estrogen effects on
the mammary gland in early and late life and breast cancer risk.
Front Oncol. 7:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou PT, Li B, Ji J, Wang MM and Gao CF: A
systematic review and meta-analysis of the association between OGG1
Ser326Cys polymorphism and cancers. Med Oncol. 32:4722015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YL, Zhang HM, Pan HM, Bao YH, Xue J,
Wang TC, Dong XC, Li XL and Bao HG: The relationship between
apolipoprotein E gene ε2/ε3/ε4 polymorphism and breast cancer risk:
A systematic review and meta-analysis. Onco Targets Ther.
9:1241–1249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falzon C, Radel R, Cantor A and
d'Arripe-Longueville F: Understanding narrative effects in physical
activity promotion: The influence of breast cancer survivor
testimony on exercise beliefs, self-efficacy, and intention in
breast cancer patients. Support Care Cancer. 23:761–768. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrow RJ, Etemadi N, Yeo B and Ernst M:
Challenging a misnomer? The role of inflammatory pathways in
inflammatory breast cancer. Mediators Inflamm. 2017:47548272017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang A, Zhao H, Cai J and Jiang WG:
Possible effect of muscle-relaxant anaesthetics on invasion,
adhesion and migration of breast cancer cells. Anticancer Res.
36:1259–1265. 2016.PubMed/NCBI
|
|
8
|
Chen JC, Chang NW, Chung JG and Chen KC:
Saikosaponin-A induces apoptotic mechanism in human breast
MDA-MB-231 and MCF-7 cancer cells. Am J Chin Med. 31:363–377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang S, Zhou Q and Yang X: Caspase-3
status is a determinant of the differential responses to genistein
between MDA-MB-231 and MCF-7 breast cancer cells. Biochim Biophys
Acta. 1773:903–911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El Guerrab A, Cayre A, Kwiatkowski F,
Privat M, Rossignol JM, Rossignol F, Penault-Llorca F and Bignon
YJ: Quantification of hypoxia-related gene expression as a
potential approach for clinical outcome prediction in breast
cancer. PLoS One. 12:e01759602017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noh WC, Kim YH, Kim MS, Koh JS, Kim HA,
Moon NM and Paik NS: Activation of the mTOR signaling pathway in
breast cancer and its correlation with the clinicopathologic
variables. Breast Cancer Res Treat. 110:477–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Kipps TJ and Zhang S: Wnt5a
signaling in normal and cancer stem cells. Stem Cells Int.
2017:52952862017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boyault S, Drouet Y, Navarro C, Bachelot
T, Lasset C, Treilleux I, Tabone E, Puisieux A and Wang Q:
Mutational characterization of individual breast tumors: TP53 and
PI3K pathway genes are frequently and distinctively mutated in
different subtypes. Breast Cancer Res Treat. 132:29–39. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mandal A, Bhatia D and Bishayee A:
Anti-inflammatory mechanism involved in pomegranate-mediated
prevention of breast cancer: The role of NF-κB and Nrf2 signaling
pathways. Nutrients. 9:E4362017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orlowski RZ and Dees EC: The role of the
ubiquitination-proteasome pathway in breast cancer: Applying drugs
that affect the ubiquitin-proteasome pathway to the therapy of
breast cancer. Breast Cancer Res. 5:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castrellon AB: Novel strategies to improve
the endocrine therapy of breast cancer. Oncol Rev. 11:3232017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kadowaki N, Ho S, Antonenko S, Malefyt RW,
Kastelein RA, Bazan F and Liu YJ: Subsets of human dendritic cell
precursors express different toll-like receptors and respond to
different microbial antigens. J Exp Med. 194:863–869. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Block MS, Vierkant RA, Fogarty ZC,
Winham SJ, Visscher DW, Kalli KR, Wang C and Goode EL: The
inflammatory microenvironment in epithelial ovarian cancer: A role
for TLR4 and MyD88 and related proteins. Tumour Biol.
37:13279–13286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harberts E, Zhou H, Fishelevich R, Liu J
and Gaspari AA: Ultraviolet radiation signaling through TLR4/MyD88
constrains DNA repair and plays a role in cutaneous
immunosuppression. J Immunol. 194:3127–3135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
d'Adhemar CJ, Spillane CD, Gallagher MF,
Bates M, Costello KM, Barry-O'Crowley J, Haley K, Kernan N, Murphy
C, Smyth PC, et al: The MyD88+ phenotype is an adverse prognostic
factor in epithelial ovarian cancer. PLoS One. 9:e1008162014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang JM, Zhang GN, Shi Y, Zha X, Zhu Y,
Wang MM, Lin Q, Wang W, Lu HY, Ma SQ, et al: Atractylenolide-I
sensitizes human ovarian cancer cells to paclitaxel by blocking
activation of TLR4/MyD88-dependent pathway. Sci Rep. 4:38402014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Echizen K, Hirose O, Maeda Y and Oshima M:
Inflammation in gastric cancer: Interplay of the
COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.
Cancer Sci. 107:391–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gnanasekar M, Kalyanasundaram R, Zheng G,
Chen A, Bosland MC and Kajdacsy-Balla A: HMGB1: A promising
therapeutic target for prostate cancer. Prostate Cancer.
2013:1571032013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Zhang Z, Zhang Y, Chen X, Guo S,
Lei Y, Xu Y, Ji C, Bi Z and Wang K: HMGB1-mediated autophagy
modulates sensitivity of colorectal cancer cells to oxaliplatin via
MEK/ERK signaling pathway. Cancer Biol Ther. 16:511–517. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang QY, Wu LQ, Zhang T, Han YF and Lin
X: Autophagy-mediated HMGB1 release promotes gastric cancer cell
survival via RAGE activation of extracellular signal-regulated
kinases 1/2. Oncol Rep. 33:1630–1638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong YD, Cui L, Peng CH, Cheng DF, Han BS
and Huang F: Expression and clinical significance of HMGB1 in human
liver cancer: Knockdown inhibits tumor growth and metastasis in
vitro and in vivo. Oncol Rep. 29:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng A, Tu Z and Yin B: The effect of
HMGB1 on the clinicopathological and prognostic features of
non-small cell lung cancer. Oncotarget. 7:20507–20519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Zhao F, Zhang H, Zhu Y, Wu K and
Tan G: Significance of TLR4/MyD88 expression in breast cancer. Int
J Clin Exp Pathol. 8:7034–7039. 2015.PubMed/NCBI
|
|
29
|
Gkretsi V, Stylianou A, Louca M and
Stylianopoulos T: Identification of Ras suppressor-1 (RSU-1) as a
potential breast cancer metastasis biomarker using a
three-dimensional in vitro approach. Oncotarget. 8:27364–27379.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun N, Xu HN, Luo Q and Li LZ: Potential
indexing of the invasiveness of breast cancer cells by
mitochondrial redox ratios. Adv Exp Med Biol. 923:121–127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Denkert C, Liedtke C, Tutt A and von
Minckwitz G: Molecular alterations in triple-negative breast
cancer-the road to new treatment strategies. Lancet. 389:2430–2442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurien BT and Scofield RH: Western
blotting: An introduction. Methods Mol Biol. 1312:17–30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Milanezi F, Leitão D, Ricardo S, Augusto I
and Schmitt F: Evaluation of HER2 in breast cancer: Reality and
expectations. Expert Opin Med Diagn. 3:607–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang XL, Qi LG, Lin FJ and Ou ZL: The role
of the chemokine receptor XCR1 in breast cancer cells. Breast
Cancer (Dove Med Press). 9:227–236. 2017.PubMed/NCBI
|
|
36
|
Karlsson E, Pérez-Tenorio G, Amin R,
Bostner J, Skoog L, Fornander T, Sgroi DC, Nordenskjöld B, Hallbeck
AL and Stål O: The mTOR effectors 4EBP1 and S6K2 are frequently
coexpressed, and associated with a poor prognosis and endocrine
resistance in breast cancer: A retrospective study including
patients from the randomised Stockholm tamoxifen trials. Breast
Cancer Res. 15:R962013. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Totsuka N, Kim YG, Kanemaru K, Niizuma K,
Umemoto E, Nagai K, Tahara-Hanaoka S, Nakahasi-Oda C, Honda S,
Miyasaka M, et al: Toll-like receptor 4 and MAIR-II/CLM-4/LMIR2
immunoreceptor regulate VLA-4-mediated inflammatory monocyte
migration. Nat Commun. 5:47102014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lucas K and Maes M: Role of the Toll Like
receptor (TLR) radical cycle in chronic inflammation: Possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xue X, Qiu Y and Yang HL: Immunoregulatory
role of MicroRNA-21 in macrophages in response to bacillus
calmette-guerin infection involves modulation of the TLR4/MyD88
signaling pathway. Cell Physiol Biochem. 42:91–102. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu H, Hao S, Gan F, Wang H, Xu J, Liu D
and Huang K: In vitro immune toxicity of ochratoxin A in porcine
alveolar macrophages: A role for the ROS-relative TLR4/MyD88
signaling pathway. Chem Biol Interact. 272:107–116. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahimifard M, Maqbool F, Moeini-Nodeh S,
Niaz K, Abdollahi M, Braidy N, Nabavi SM and Nabavi SF: Targeting
the TLR4 signaling pathway by polyphenols: A novel therapeutic
strategy for neuroinflammation. Ageing Res Rev. 36:11–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mazur-Bialy AI, Pocheć E and Zarawski M:
Anti-inflammatory properties of irisin, mediator of physical
activity, are connected with TLR4/MyD88 signaling pathway
activation. Int J Mol Sci. 18:E7012017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
An J, Li Z, Dong Y, Ren J and Guo K:
Methicillin-resistant staphylococcus aureus infection exacerbates
NSCLC cell metastasis by up-regulating TLR4/MyD88 pathway. Cell Mol
Biol (Noisy-le-grand). 62:1–7. 2016.PubMed/NCBI
|
|
45
|
Wang J, Dong B, Tan Y, Yu S and Bao YX: A
study on the immunomodulation of polysaccharopeptide through the
TLR4-TIRAP/MAL-MyD88 signaling pathway in PBMCs from breast cancer
patients. Immunopharmacol Immunotoxicol. 35:497–504. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aviello G, Corr SC, Johnston DG, O'Neill
LA and Fallon PG: MyD88 adaptor-like (Mal) regulates intestinal
homeostasis and colitis-associated colorectal cancer in mice. Am J
Physiol Gastrointest Liver Physiol. 306:G769–G778. 2014. View Article : Google Scholar : PubMed/NCBI
|