Introduction
Acute pancreatitis (AP) is an inflammatory disease
of the pancreas, which is characteristically sterile and results in
acinar cell necrosis. In its most severe form, AP is associated
with multi-organ failure and mortality (1,2). The
overproduction of various cytokines and non-cytokine inflammatory
mediators may account for AP systemic manifestations, such as tumor
necrosis factor (TNF)-α (3).
Inflammatory mediator, TNF-α was upregulated in the serum of
patients with severe AP and was an effective discriminator of AP
severity (4).
Toll-like receptor (TLR) signaling pathways exert
essential proinflammatory activities in AP (5). TLRs form a major group of
damage-associated molecular pattern receptors. Among the TLRs, TLR9
is the only receptor for detecting DNA (self and non-self). Hence,
DNA released from damaged cells triggers inflammation via TLR9 in
immune cells (6). TLR9 is
expressed in the rat pancreas and is involved in cerulein-induced
pancreatitis (7). CpG
oligodeoxynucleotide (CpG-ODN) is a short (~20 bp), single-stranded
synthetic DNA fragment containing the immunostimulatory CpG motif,
a potent TLR9 agonist, which activates dendritic cells (DCs) and B
cells to produce type I interferons and inflammatory cytokines
(8). Bacterial and viral genomic
DNA containing the CpG motif (CpG-DNA) and its analog,
oligodeoxynucleotide-containing CpG motif (CpG-ODNs) are powerful
activators of the innate immune system (8).
To the best of our knowledge, the effect of
CpG-ODN1826 on sodium taurocholate-induced pancreas damage in rats
at different points in time has yet to be reported. In the present
study, the aim was to investigate the temporal differences in the
expression levels of TLR9 and early-stage inflammatory cytokines
(TNF-α) in rats subjected to AP, and to determine the role of TLR9
in AP from edema to necrosis. In the present study, AP and CpG + AP
animal models were established in Sprague Dawley (SD) rats over
time periods of 0 to 12 h. Pathological changes were observed by
hematoxylin and eosin (H&E) staining and TLR9 protein
expression levels in the pancreas were detected by western
blotting. Serum TNF-α protein levels were detected by enzyme linked
immunosorbent assay (ELISA). In addition, AMS was assessed using an
amylase activity kit. The present study may provide the optimal
therapeutic time points and methods to prevent the progression from
edema to cell death during AP.
Materials and methods
Animals and treatments
A total of 104 adult male SD rats (weight at the
start of the experiments, 220±20 g) provided by Kunming Medical
University (Kunming, China) animal center were employed in the
current study. Rats were housed in a temperature of 25±1.0°C and a
humidity of ~50%, under a 12-h light/dark cycle, with food and
water provided ad libitum. Experiments were conducted
according to the guidelines for the care and use of experimental
animals established by the Ministry of Science and Technology of
the People's Republic of China (approval no. 2006-398), and was
approved by the Laboratory Animal Management Committee of Kunming
Medical University. All efforts were made to minimize animal
stress/distress. SD rats were randomly divided into eight groups as
presented in Table I.
 | Table I.Grouping and treatment of
Sprague-Dawley rats. |
Table I.
Grouping and treatment of
Sprague-Dawley rats.
| Group | H&E
staining/H&E score | Western
blotting/ELISA/serum amylase tests |
|---|
| Control | n=5 | n=8 |
| AP 3 h | n=5 | n=8 |
| AP 6 h | n=5 | n=8 |
| AP 12 h | n=5 | n=8 |
| CpG | n=5 | n=8 |
| CpG + AP 3 h | n=5 | n=8 |
| CpG + AP 6 h | n=5 | n=8 |
| CpG + AP 12 h | n=5 | n=8 |
Animal models
Prior to the experiment, the rats were fasted for 24
h with free access to water. They were anesthetized by
intraperitoneal injection (0.5 ml/100 g) with 7% chloral hydrate.
According to Yu et al (9),
the ventral midline was incised for entry into the abdominal cavity
to expose the general bile duct and the pancreatic duct. The
biliopancreatic duct was occluded with a vascular clip for entry to
the distal duodenum. The animals in the experimental group were
infused slowly (3 ml/h) with 5% sodium taurocholate (0.1 ml/100 g;
Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) using four pediatric
scalp acupuncture needles at the proximal end. The control group
was infused with the equivalent quantity of normal saline. To
prevent large quantities of drug reflux, the needle was not removed
for 10 min after the injection. After modeling, the rat skin was
disinfected and the peritoneum was closed using a continuous
single-layer suture, while the rest was closed using a full-layer
simple-interval suture. Subsequent to closing the abdomen, the rats
were injected with 5 ml saline in the inside position of the lower
limb muscle to restore the water lost during surgery. In addition,
in order to investigate the role of TLR9, CpG-OND1826 (3′-tcc atg
acg ttc ctg acg tt-′5) was synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China) and injected into the enterocoelia (1 µg/µl; 200
µl) prior to establishing the AP model.
Tissue preparation
For all experiments, animals were deeply
anesthetized with 7% chloral hydrate (0.5 ml/100 g body weight).
Blood samples (1.5 ml) were collected via abdominal aorta puncture
under anesthesia. All blood samples were centrifuged at 100 × g for
5 min at 37°C and the supernatant fluid (serum) was collected,
aliquoted and stored at −20°C for the ELISA and AMS tests. The
pancreas was removed and divided into two segments. One segment was
stored at −80°C for western blot analysis. For subsequent
pathological examination and immunohistochemistry, the other part
was fixed with 10% paraformaldehyde at room temperature for 4
h.
H&E staining
The pancreas samples were fixed in formalin and
embedded in paraffin routinely, as described by Han et al
(10). The paraffin blocks were
sliced into continuous 5-µm sections. The sections were dewaxed
with xylene, and washed with ethanol and water. The sections were
stained with hematoxylin (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) at room temperature for 5 min,
differentiated, washed, stained with eosin (Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 2 min,
dehydrated with graded ethanol and cleared with xylene. Then, the
sections were mounted onto slides and observed under an Olympus
BH-2 microscope (Olympus Corporation, Tokyo, Japan); images were
captured using the image acquisition system of the microscope. The
total surface of the slides was scored by two pathologists, blinded
to the experimental conditions and with expertise in pancreatic
pathology, for edema, acinar necrosis, hemorrhage and fat necrosis,
inflammation and perivascular infiltrate, according to Schmidt
et al (11), to determine
the severity of injury.
Western blot analysis
In order to determine TLR9 protein expression
levels, pancreatic tissues were lysed and homogenized in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) including protease inhibitor cocktail
(Roche Diagnostics GmbH, Mannheim, Germany). Equal amounts (100 µg)
of extracted protein samples were resolved by 15% SDS-PAGE, using
electrophoresis buffer (24.8 mM Tris, 192 mM glycine and 0.1% SDS)
at 60 V for 30 min and 100 V for 1.5 h. The precipitated proteins
were separated and transferred to polyvinylidene difluoride
membranes at 350 mA for 4 h, using transfer buffer (24.8 mM Tris,
192 mM glycine and 10% methanol). After blocking with 5% nonfat
milk in TBS containing 3% Tween-20 for 1 h at room temperature, the
membranes were incubated with rabbit polyclonal anti-TLR 9 antibody
(cat. no. ab37154; dilution 1:2,500; Abcam, Cambridge, MA, USA) or
rabbit polyclonal anti-GAPDH antibody (cat. no. ab37168; dilution
1:5,000; Abcam) in TBS overnight at 4°C. The membranes were rinsed
4 times in TBST prior to incubation at 37°C for 1.5 h with
anti-rabbit secondary antibody (cat. no. SAB3700840; dilution
1:5,000; Sigma-Aldrich; Merck KGaA). Subsequently, the membranes
were rinsed in TBST 4 times and protein bands were visualized by
enhanced chemiluminescence using SuperSignal West Pico
Chemiluminescent substrate (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Blots were semi-quantified by densitometry using
Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunohistochemistry (IHC)
According to the method of Sholl et al
(12), TLR9 IHC was performed on 8
µm-thick formalin-fixed paraffin-embedded tissue sections. Slides
were baked at 37°C in a constant temperature drying oven,
deparaffinized in xylene, dehydrated through graded alcohols, and
subjected to antigen retrieval with 0.01 M citrate buffer (pH 6.0)
in a microwave oven at 98°C for 15 min. All further steps were
performed at room temperature in a hydrated chamber. Slides were
pretreated with 3% H2O2 (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) for 15 min to quench endogenous
peroxidase activity, and washed in 50 mM Tris-Cl (pH 7.4). Slides
were blocked using 5% normal goat serum (Gibco; Thermo Fisher
Scientific, Inc.), and subsequently incubated with rabbit anti-TLR
9 antibody (dilution, 1:300) overnight at 4°C. Slides were washed
with TBS and incubated at 37°C with the ImmPRESS™ Reagent
anti-rabbit immunoglobulin (Ig) G secondary antibody (dilution,
1:250; cat. no. MP-7401; Vector Laboratories, Inc., Burlingame, CA,
USA) for 2 h. The slides were rinsed with TBS five times, each for
5 min. Finally, the sections were visualized by 0.05% DAB staining
(Beyotime Institute of Biotechnology). A negative control was
created by replacing the primary antibody with 2% goat serum to
ascertain the specificity of antibody staining. Immunoreactive
products were observed and photographed under a light microscope
(Leica Microsystems GmbH, Wetzlar, Germany) coupled with a
computer-assisted video camera.
Detection of serum inflammatory
cytokines by ELISA
Anti-TNF-α (cat. no. 70-EK3822/2; Multisciences
Lianke Biotech, Co., Ltd., Hangzhou China) IgG autoantibody titers
were measured using commercially available ELISA kits according to
the manufacturer's instructions. Serum samples were diluted to 1:20
in assay diluent. Diluted serum samples were added to 96-well
plates. Subsequent to rinsing off any unbound substances,
peroxidase-conjugated goat polyclonal anti-rat IgG antibodies
included in the kit were added to the wells. Development was
performed using a microplate reader set to 450 nm to reflect the
level of anti-TNF-α autoantibodies bound in the initial step for
each sample.
ELISA quantification
To compare results from different plates, test
sample ODs were adjusted relative to the positive and negative
control samples supplied in each kit. The mean OD of duplicate
wells was calculated. The index value of each tested serum was
defined by the following formula: Index=(OD of tested serum-OD of
negative control)/(OD of positive control-OD of negative
control)×100. A cutoff ELISA value of 9 U/ml was used (where ≥9.0
U/ml represents a positive result) according to the manufacturer's
instructions.
Pancreatic amylase test
Measurement of serum or ascites pancreatic amylase
was performed according to the instructions of the amylase test kit
(cat. no. TE0203-200T; Beijing Leagene Biotechnology Co., Ltd.,
Beijing China). Samples were diluted 1:20 in normal saline. The
diluted samples (3 µl) were added to the wells with 37.5 µl AMS
assay buffer, mixed, and incubated in 37°C for 7.5 min.
Subsequently, 37.5 µl KI working solution and 225 µl distilled
water were added. For the blank control, 3 µl distilled water was
used instead of the samples. Development was performed using a
microplate reader set to 660 nm. The blank control was set as 0,
and the OD was read for the samples and the blank control. The
following calculation was performed: Sample AMS activity
(U/dl)=[(blank control-sample)/(blank control ×80)×20].
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 independent experiments. The statistical significance of the
differences between groups was assessed using independent samples
Student's t-test for pair-wise comparisons or one-way analysis of
variance followed by a post hoc lest significant difference or
Games-Howell test for multiple comparisons. Statistical analysis
was performed using SPSS software version 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Pathological examination
The pancreas samples in the control rats were normal
(Fig. 1A). In the AP 3 h group,
the tissue samples exhibited a mild edema and red blood cells
(RBCs) were observed (Fig. 1B). In
the AP 6 h group, certain pancreas samples exhibited edema.
Furthermore, certain cells were degenerated and a greater number of
RBCs were observed when compared with that in the AP 3 h group
(Fig. 1C). In the AP 12 h group,
the pancreas samples exhibited hemorrhages, pancreatic cell
degeneration and necrosis (Fig.
1D). Subsequent to the CpG-OND1826 injection, in the AP 0 h
(CpG) group, the tissue samples were normal (Fig. 1E). However, the morphological
structure of the tissue samples was worse than that of the tissue
samples from the CpG-OND1826-treated animals at 3 h (Fig. 1F), 6 h (Fig. 1G) and 12 h (Fig. 1H).
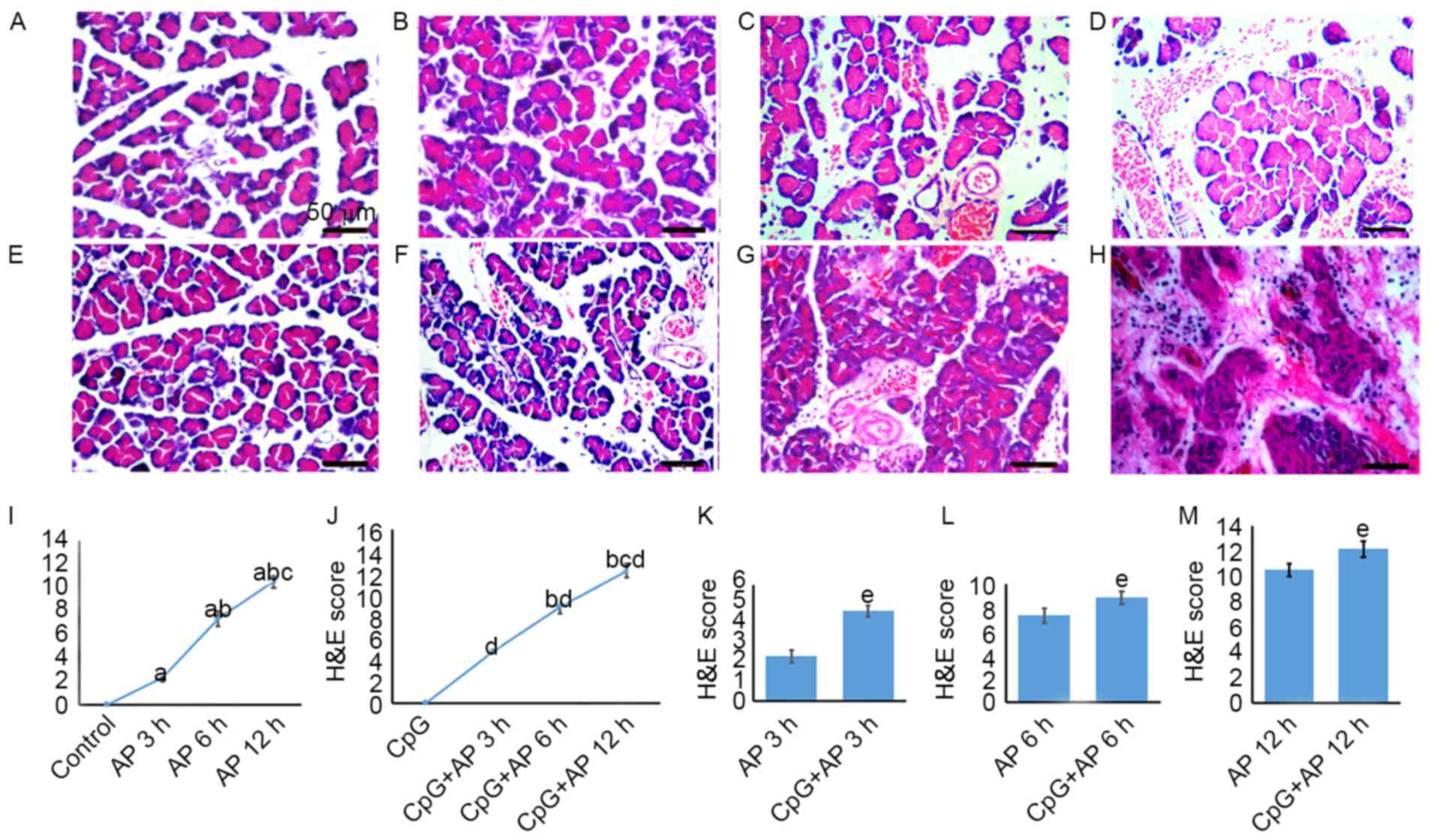 | Figure 1.H&E staining and scoring. H&E
staining of the (A) control group, (B) AP 3 h group, (C) AP 6 h
group, (D) AP 12 h group, (E) CpG group, (F) CpG + AP 3 h group,
(G) CpG + AP 6 h group and the (H) CpG + AP 12 h group. Scale bar,
50 µm. H&E scores of the (I) control, and AP 3, 6 and 12 h
groups; (J) CpG, and CpG + AP 3, 6 and 12 h groups; (K) AP 3 h and
CpG +AP 3 h groups; (L) AP 6 h and CpG +AP 6 h groups; (M) AP 12 h
and CpG + AP 12 h groups. aP<0.05 vs. control group;
bP<0.05 vs. 3 h group; cP<0.05 vs. 6 h
group; dP<0.05 vs. CpG group; eP<0.05
vs. AP group. H&E, hematoxylin and eosin; AP, acute
pancreatitis. |
The H&E score was higher in the AP 3 h, 6 h and
12 h groups when compared with that in the control group (Fig. 1I; all P<0.05). Furthermore, the
score was greater in the AP 6 h (P<0.05) and 12 h (P<0.05)
groups compared with that in the AP 3 h group, and it increased
further at 12 h (P<0.05; Fig.
1I). Additionally, the H&E score was greater in the CpG +
AP 3, 6 and 12 h groups (P<0.01 for all) compared with that in
the CpG group (Fig. 1J). In
addition, the score was increased in the CpG + AP 3 (Fig. 1K), 6 (Fig. 1L) and 12 h (Fig. 1M) groups when compared with that in
the CpG-OND1826 untreated groups (P<0.05 for all). However, no
significant difference was identified between the CpG and control
groups (P>0.05).
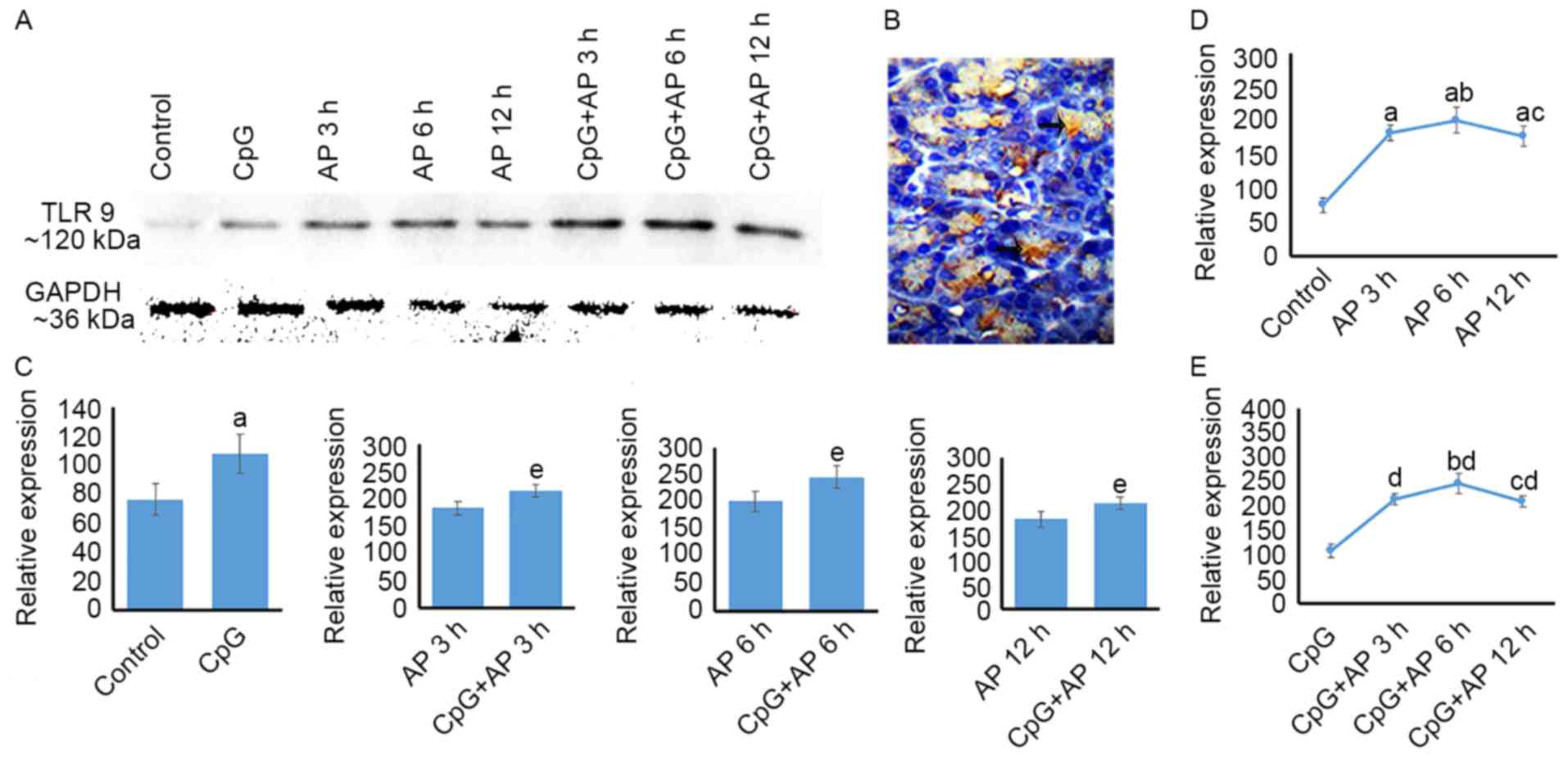
Temporal differences in TLR 9 protein
expression in the AP and CpG + AP groups
The levels of TLR 9 protein expression are presented
in Fig. 2. TLR9 protein was
expressed in pancreatic cells (Fig.
2B). The levels of TLR 9 protein expression were upregulated in
the AP 3, 6 and 12 h groups when compared with those in the control
group (Fig. 2A and D; P<0.05).
Additionally, TLR9 expression levels were upregulated in the AP 6 h
group compared with those in the AP 3 and 12 h groups (P<0.05;
Fig. 2A and D). However, no
difference in TLR9 expression levels was identified between the AP
6 and 12 h groups (P>0.05; Fig. 2A
and D). Following CpG-OND injection, the expression level of
TLR9 was upregulated when compared with that in the AP 0 (control),
3, 6 and 12 h groups (P<0.05 for all; Fig. 2A and C). The levels of TLR9 protein
expression were upregulated in the CpG + AP 3, 6 and 12 h groups
(P<0.01 for all) compared with that in the CpG group (Fig. 2A and E). Furthermore, TLR9
expression levels in CpG + AP 6 h group was higher than that in the
CpG + AP 3 and 12 h (P<0.05; Fig.
2A and E).
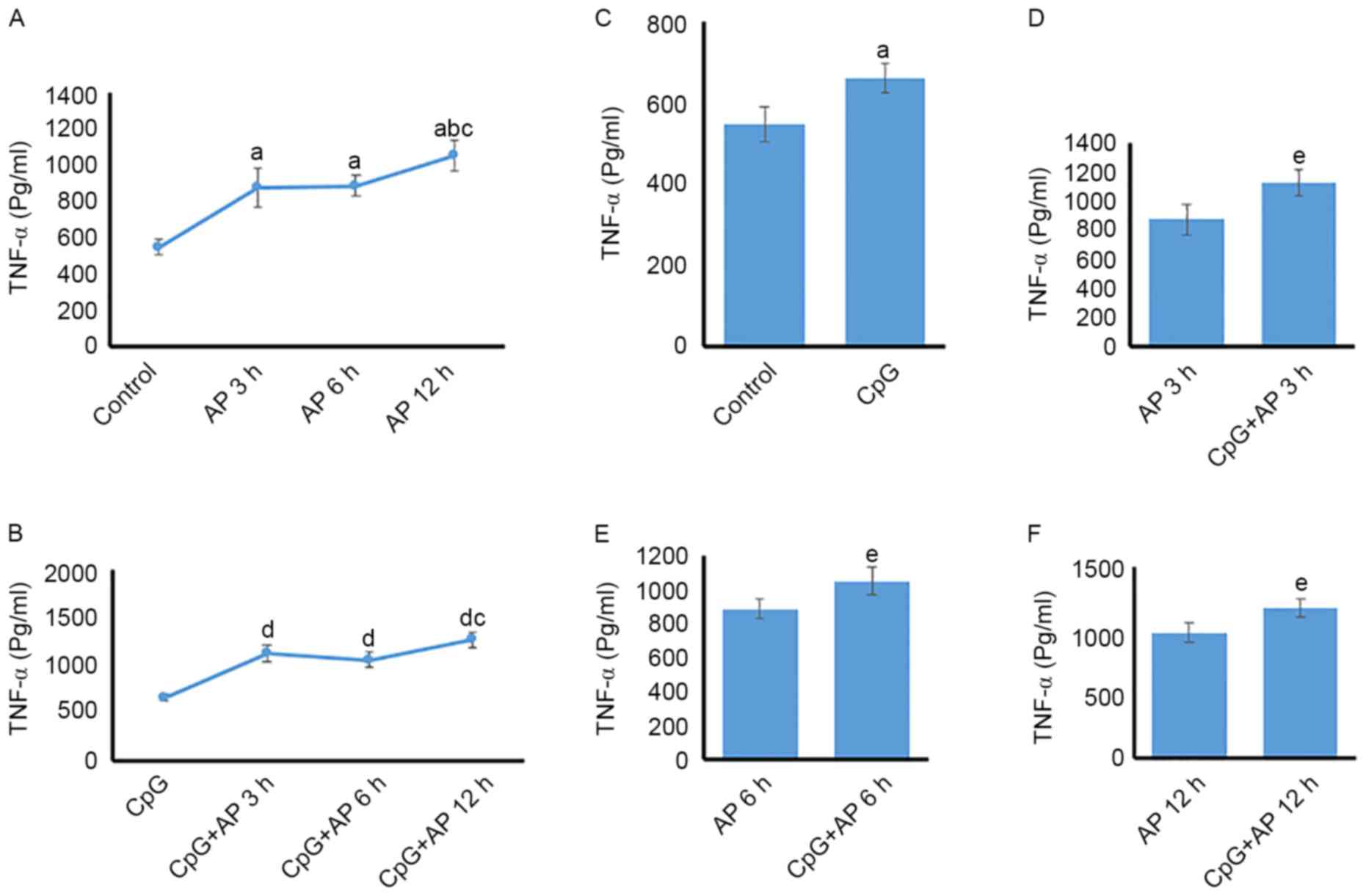
Temporal differences in serum TNF-α
protein expression levels
TNF-α protein expression was upregulated in the AP
3, 6, and 12 h groups (P<0.05 for all) compared with that in the
control group (Fig. 3A).
Additionally, the level of TNF-α protein expression was higher in
the 12 h group than in the AP 3 and 6 h groups (P<0.05; Fig. 3A). However, no significant
difference was identified between the AP 3 and 6 h groups
(P>0.05; Fig. 3A). TNF-α
expression levels in the CpG + AP 3, 6, 12 h group were increased
when compared with those in the CpG group (P<0.05 for all;
Fig. 3B). However, no significant
difference was identified between the CpG + AP 3 and 6 h
(P>0.05; Fig. 3B). In addition,
CpG-OND1826 administration caused upregulation of TNF-α protein
expression when compared with that in the AP 0 (control; Fig. 3C), 3 (Fig. 3D), 6 (Fig. 3E), and 12 h groups (Fig. 3F; P<0.05 for all).
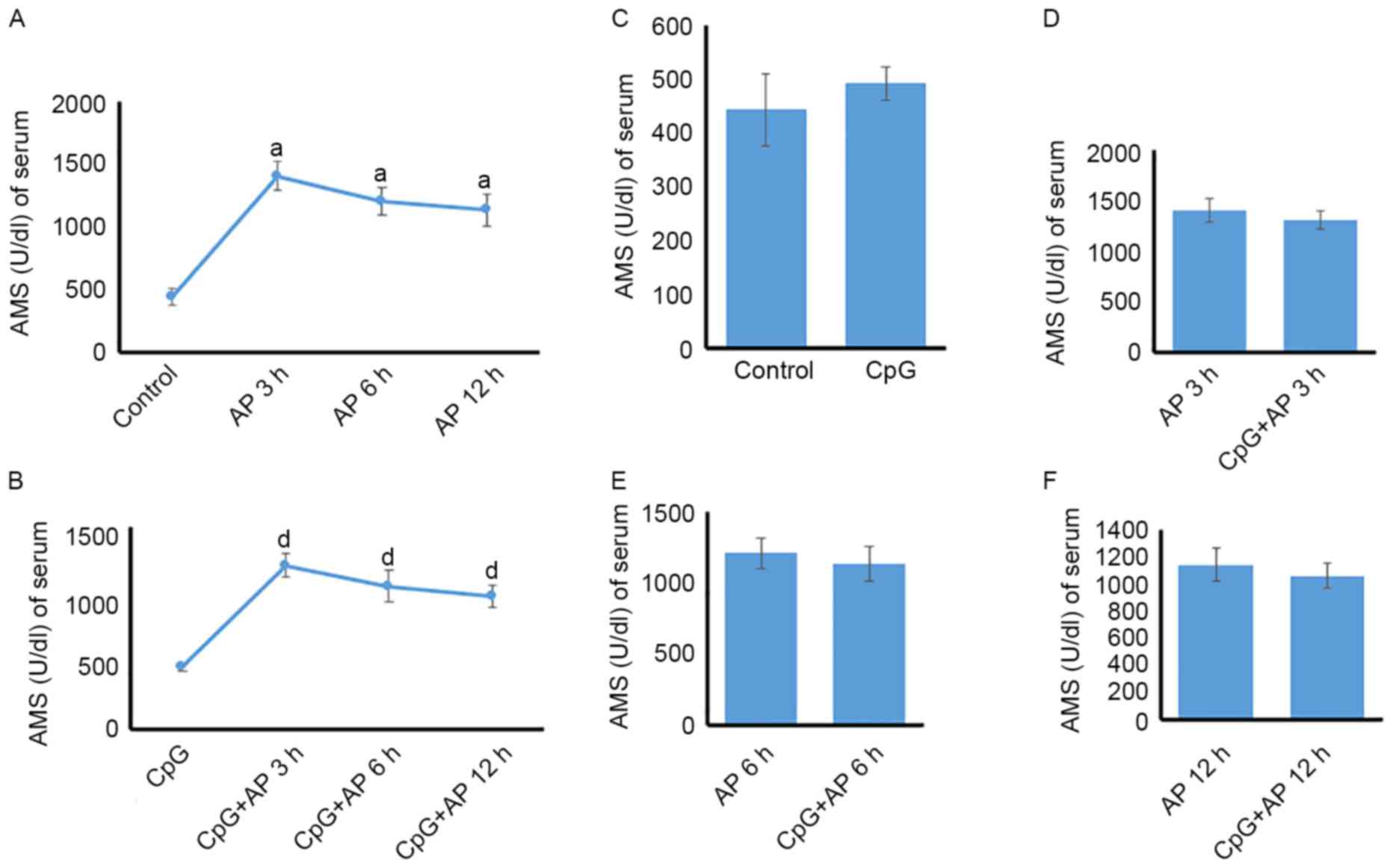
Temporal differences in serum AMS
AMS was upregulated in the AP 3, 6, and 12 h groups
(P<0.01 for all) compared with that in the control group
(Fig. 4A). However, no significant
difference was identified among the AP 3, 6 and 12 h groups
(P>0.05; Fig. 4A). Following
treatment with CpG, the tendency of AMS was the same as that in the
AP groups (Fig. 4B). However, no
significant change was observed between the AP and CpG + AP groups
at 0, 3, 6, and 12 h (Fig.
4C-F).
Discussion
Significance of pathological, TLR9,
TNF-α and serum AMS changes at different time points in AP
rats
Pathological examination indicated that the severity
of pancreatic tissue damage following AP increased over time.
Additionally, TLR9 protein expression was upregulated subsequent to
AP. TLR9 is a key determinant of the innate immune response in
infectious and sterile injury (13). Previously, Hoque et al
(2) demonstrated that host genomic
DNA is markedly elevated in the blood very early in the course of
experimental AP, which is consistent with a role for TLR9 in
sensing initial pancreatic injury. In addition, serum DNA was
demonstrated to be significantly elevated in patients with severe
AP (SAP) (2). Furthermore, TLR9
was higher in 6 h than that in 3 h. Zeng et al (7) also demonstrated that TLR9 was
expressed in the pancreas of AP-induced rats and control animals.
Furthermore, previous studies indicated that TLR9 is expressed by
pancreatic ductal and endothelial cells (2), and resident immune cells
(predominantly macrophages) (14).
In the AP 3 h group in the present study, tissue samples presented
a mild edema and RBCs were observed. In the AP 6 h group, certain
pancreas samples also presented edema. Furthermore, certain cells
were degenerated and more RBCs were observed when compared with
that in the AP 3 h group. Xiang et al (15) indicated that chronic stress
increases TLR9 expression levels in peritoneal macrophages and
chronic stress promotes cell apoptosis via TLR9. When necrosis
occurred in a large area of the pancreatic tissue, TLR9 expression
levels were significantly decreased at 12 h compared with those at
3 and 6 h, but were greater than those in the control group. Thus,
these data indicate that TLR9 may be important in AP.
In addition, serum TNF-α protein levels and AMS were
persistently increased from 3 until 12 h in rats subjected to AP.
Expression levels of TNF-α protein at AP 12 h were higher than
those in AP 3 and 6 h. In addition, Wang et al (16) demonstrated that serum AMS
activities and TNF-α expression levels significantly increased in
the SAP group, when compared with the control group. Their data was
consistent with the present results. In addition, previous studies
indicated that higher expression levels of TNF-α were detected in
mild AP and SAP mice at varying time points post induction
(14,17,18).
Furthermore, during the pathogenic process of SAP, aberrant
activation of inflammatory cells and excessive production of
inflammatory mediators, such as TNF-α and IL-6 causes pancreatic
necrosis and systemic inflammatory response syndrome, as well as
multiple organ dysfunction syndrome (17). In addition, the decrease in serum
TNF-α expression levels in AP rats inhibits the inflammatory
response to AP and exerts a significant protective effect on the
pancreatic tissue and function in AP rats (14). Gomes et al (19) demonstrated that TLR9 is required
for mitogen-activated protein kinase/nuclear factor-κB activation,
followed by IL-12 and TNF-α production. Thus, the serum TNF-α
expression level may be associated with TLR9 activity in AP rats at
3 and 6 h. At 12 h, the serum TNF-α level was high; however, TLR9
protein expression was downregulated. To investigate the functions
of TLR9, CpG-OND 1826 was employed.
Function of CpG-OND 1826 at different
time points in AP rats
Krieg (20)
demonstrated CpG-ODN to be a double-edged sword, as it may improve
the host immune function; however, it may lead to autoimmune
diseases. Thus, the function of CpG-ODN is complicated, and in
order to investigate its function at different time points, AP
model rats were used. The present data showed that TLR9 expression
levels increased following CpG-OND administration. Furthermore,
pancreatic injury was more serious in the CpG + AP groups and the
expression level of TNF-α was upregulated compared with that in the
AP groups. Akira (21)
demonstrated that TLR stimulation induces the innate immune
response through the increased secretion of proinflammatory
cytokines, such as TNF-α, IL-1β and IL-6. However, Lee et al
(14) indicated that simultaneous
stimulation and restimulation of TLR7 and TLR9 with specific
agonists induced tolerance in mouse macrophages, thereby reducing
the production of proinflammatory cytokines, TNF-α and IL-6, when
compared with individual stimulation. In addition, no significant
difference was identified between the AP and CpG + AP groups in
ASM, and data indicated that there was no statistical correlation
between the magnitude of the hyperamylasemia disease and the final
outcome (22).
In conclusion, these data indicate that CpG-ODN1826
aggravates sodium taurocholate-induced AP in rats, which is
accompanied by an increase of TLR9 and TNF-α expression levels from
3 to 12 h following treatment. Therefore, the present results
suggested that the downregulation of TLR9 and/or TNF-α protein
levels may offer protection against pancreatic injury following
AP.
Acknowledgements
The present study was supported by the Yunnan
Provincial Science and Technology Department Foundation of China
(grant no. 2015FB024).
References
|
1
|
Kim MJ, Bae GS, Jo IJ, Choi SB, Kim DG,
Shin JY, Lee SK, Kim MJ, Shin S, Song HJ and Park SJ: Loganin
protects against pancreatitis by inhibiting NF-κB activation. Eur J
Pharmacol. 765:541–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoque R, Malik AF, Gorelick F and Mehal
WZ: Sterile inflammatory response in acute pancreatitis. Pancreas.
41:353–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weng TI, Wu HY, Chen BL, Jhuang JY, Huang
KH, Chiang CK and Liu SH: C/EBP homologous protein deficiency
aggravates acute pancreatitis and associated lung injury. World J
Gastroenterol. 19:7097–7105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Wu Y, Zhang S, Zhang J, Ji F, Bo W,
Guo X and Li Z: Baicalein protect pancreatic injury in rats with
severe acute pancreatitis by inhibiting pro-inflammatory cytokines
expression. Biochem Biophys Res Commun. 466:664–669. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding JL, Zhou ZG, Zhou XY, Zhou B, Wang L,
Wang R, Zhan L, Sun XF and Li Y: Attenuation of acute pancreatitis
by peroxisome proliferator-activated receptor-α in rats: The effect
on toll-like receptor signaling pathways. Pancreas. 42:114–122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shintani Y, Drexler HC, Kioka H,
Terracciano CM, Coppen SR, Imamura H, Akao M, Nakai J, Wheeler AP,
Higo S, et al: Toll-like receptor 9 protects non-immune cells from
stress by modulating mitochondrial ATP synthesis through the
inhibition of SERCA2. EMBO Rep. 15:438–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng YJ, Song JM, Li Y, Wang R, Zhou B,
Zhou ZG, Liu HY and Xu B: Toll-like receptor 9 is expressed in rat
pancreas and is involved in cerulein-induced pancreatitis.
Pancreas. 36:212–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma C, Spies NP, Gong T, Jones CX and Chu
WM: Involvement of DNA-PKcs in the type I IFN response to CpG-ODNs
in conventional dendritic cells in TLR9-dependent or -independent
manners. PloS One. 10:e01213712015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu C, Huang L, Li X, Zhu H, Li Z and Yu X:
Spatial and temporal differences of HMGB1 expression in the
pancreas of rats with acute pancreatitis. Int J Clin Exp Pathol.
8:6928–6935. 2015.PubMed/NCBI
|
|
10
|
Han J, Xu X, Zhang B, Chen B and Hang W:
Expression of ATF4 and RUNX2 in periodontal tissue of pressure side
during orthodontic tooth movement in rat. Int J Clin Exp Med.
8:934–938. 2015.PubMed/NCBI
|
|
11
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sholl LM, Sun H, Butaney M, Zhang C, Lee
C, Jänne PA and Rodig SJ: ROS1 immunohistochemistry for detection
of ROS1-rearranged lung adenocarcinomas. Am J Surg Pathol.
37:1441–1449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoque R, Farooq A, Malik A, Trawick BN,
Berberich DW, McClurg JP, Galen KP and Mehal W: A novel
small-molecule enantiomeric analogue of traditional (−)-morphinans
has specific TLR9 antagonist properties and reduces sterile
inflammation-induced organ damage. J Immunol. 190:4297–4304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HJ, Kim KC, Han JA, Choi SS and Jung
YJ: The early induction of suppressor of cytokine signaling 1 and
the downregulation of toll-like receptors 7 and 9 induce tolerance
in costimulated macrophages. Mol Cells. 38:26–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang Y, Yan H, Zhou J, Zhang Q, Hanley G,
Caudle Y, LeSage G, Zhang X and Yin D: The role of toll-like
receptor 9 in chronic stress-induced apoptosis in macrophage. PloS
One. 10:e01234472015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang MM, Zhang T, Yang LH, Liu LW, Chen
XC, Zhou MT and Chen BC: Sedum sarmentosun bunge extraction
ameliorated severe acute pancreatitis-induced lung injury: An
experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi.
35:228–233. 2015.(In Chinese). PubMed/NCBI
|
|
17
|
Zhang X, Feng G, Weng W, Liang J, Lin N,
Cai Y, Xu R, Zhou N, Yuan M, Yuan W and Xia X: Protective effects
of baicalin and octreotide on intestinal mucosa of rats with severe
acute pancreatitis. Turk J Gastroenterol. 20:108–115.
2009.PubMed/NCBI
|
|
18
|
Xue QM, Pan H, Huang L and Li N: Effects
of acupuncture at ST25 on inflammatory mediators and nuclear factor
κB activation in a rat model of severe acute pancreatitis. Acupunct
Med. 33:299–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomes MT, Campos PC, Pereira Gde S,
Bartholomeu DC, Splitter G and Oliveira SC: TLR9 is required for
MAPK/NF-κB activation but does not cooperate with TLR2 or TLR6 to
induce host resistance to Brucella abortus. J Leukoc Biol.
99:771–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krieg AM: Therapeutic potential of
toll-like receptor 9 activation. Nat Rev Drug Discov. 5:471–484.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akira S: Innate immunity to pathogens:
Diversity in receptors for microbial recognition. Immunol Rev.
227:5–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vissers RJ, Abu-Laban RB and McHugh DF:
Amylase and lipase in the emergency department evaluation of acute
pancreatitis. J Emerg Med. 17:1027–1037. 1999. View Article : Google Scholar : PubMed/NCBI
|