Introduction
Biliary atresia (BA), a unique disease of infancy,
is caused by fibro-inflammatory destruction of extra- or
intra-hepatic bile ducts. BA leads to cholestasis and progressive
liver fibrosis and cirrhosis, as well as end-stage liver disease in
some infants, who then require a liver transplantation (1,2).
Worldwide, the incidence of BA ranges from 1 in 5,000 to 1 in
20,000 live births, which is responsible for nearly one-third of
all neonatal cholestasis cases and more than 90% of obstructive
cholestatic cases (3). If
untreated, the prognosis is extremely poor, with death from liver
cirrhosis occurring within 2 years (4). Although a type of surgery knows as
the Kasai procedure has markedly improved the prognosis of BA, life
quality of the majority of patients remains relatively poor, and
long-term survival depends on a liver transplant (5).
Over the past decade, mesenchymal stem cells (MSCs)
have been extensively investigated as potential therapeutic options
for the treatment of various degenerative diseases and immune
disorders (6,7). Focus on MSCs lies in their potential
for self-renewal, ability to differentiate into multiple cell
lineages, and immunoregulatory properties, as well as their ability
to secrete several types of anti-fibrotic molecules (8), such as hepatocyte growth factor
(9). Furthermore, unlike embryonic
stem cells, the use of MSCs does not give rise to ethical issues,
and they have a safer profile in terms of carcinogenesis. To date,
the effects of bone marrow-derived MSCs (BMMSCs) on liver fibrosis
induced by BA remain largely unknown. In the present study, we
investigated the anti-fibrotic potential of BMMSCs in a murine
model of BA induced by RRV administration.
Materials and methods
Ethics statement
The present study was reviewed and approved by the
Ethics Committee of the Jiangxi Provincial Children's Hospital
(Jiangxi, China) and all of the experiments were carried out in
accordance with relevant guidelines and regulations. All procedures
adhered to the Chinese Veterinary Medical Association Guide.
Animals
Pregnant BALB/c mice aged 10 to 12 weeks and
4-week-old healthy BALB/c nonpregnant mice were purchased from SLAC
Inc. (Shanghai, China). The animals were housed in groups in a
specific pathogen-free facility under artificial lighting
conditions (12:12 h), with food and water available ad libitum.
Murine BA model induced by RRV
A RRV strain (MMU 18006) was purchased from the
American Type Culture Collection (Manassas, VA, USA), and amplified
in MA104 cells. The experimental model of BA was established as
described in previous study (10).
Briefly, neonatal mice were injected with 20 µl of
1.2×105 pfu/ml of RRV or supernatant of MA104 cell
culture medium (controls) intraperitoneally within 24 h of birth.
Infected mice that died within the first 2 days or that were not
fed by their mothers were excluded from further analysis. All mice
were weighed every day and observed the signs of cholestasis
(icterus of non-fur-covered skin, color and quality of stools, and
bilirubin in urine) until the day of sacrifice. Liver samples were
collected for histological analysis and western blot analysis.
BMMSC isolation, purification, and
characterization
BMMSCs were obtained from 4-week-old BALB/c mice.
BMMSC isolation and purification protocols were performed in
accordance with method described previously (11). To exclude hematopoietic stem cells
and leucocytes, a magnetic bead-based mouse cell depletion kit
(Miltenyi Biotec, Bergisch Gladbach, Germany) containing anti-CD45,
anti-CD11b, anti-CD5, anti-Gr1 (Ly-6/C), and anti-TER 119
monoclonal antibodies was used. The expression profiles of BMMSC
surface markers were determined by flow cytometry. BMMSC plasticity
(i.e., differentiation into chondrogenic, adipogenic, and
osteogenic lineages) was assessed using a commercial kit (StemPro;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), following the
manufacturer's instructions. The purified cells were kept in
standard culture media until the day of transplant.
BMMSC transplantation
Infected mice received an intraperitoneal injection
of 1×106 BMMSCs in a single dose 7 days post-challenge.
All the animals included in the experimental groups were sacrificed
via broking the neck 14 days post-inoculation, and livers were
harvested for further studies.
Collection of blood and tissue
samples
After 14 days of the inoculation, mice were
intraperitoneally injected 1% pentobarbital sodium at 80 mg/kg,
then blood samples were withdrawn from the ventral main vein of the
mice and allowed to clot. Serum was separated by centrifugation at
2,000 rpm for 10 min at 4°C and used for the assessment of liver
function. Afterwards, the animals were killed by cervical
dislocation. The livers were dissected out and portions placed in
10% neutral buffered formalin (pH 7.4) for subsequent
histopathological examination. The remaining tissues were kept deep
frozen at −80°C.
Measurements of clinical biochemistry
parameters
Murine blood samples were collected after the
experiments and analyzed by the hospital's clinical laboratory
using a Hitachi Pre-Analytical Process Automation System with a
7600 Clinical Analyzer (Hitachi, Ltd., Tokyo, Japan). The
parameters analyzed were alanine aminotransferase (ALT), aspartate
aminotransferase (AST), total bilirubin (TBIL), and direct
bilirubin (DBIL).
Western blot analysis
Frozen liver samples were lysed with RIPA III lysis
buffer (Sangon Biotech, Shanghai, China). After determination of
the protein concentration with a BCA kit (Thermo Fisher Scientific,
Inc.), equal amount of proteins from different samples were
subjected to 10% SDS-PAGE gels following electrophoretic transfer
to nitrocellulose membranes. The membranes were then blocked with
5% nonfat milk and incubated with the primary antibodies against
laminin (LN), transforming growth factor-β (TGF-β), tumor necrosis
factor-α (TNF-α), collagen IV (all Abcam, Cambridge, MA, USA,
α-smooth muscle actin (α-SMA; Cell Signaling Technology, Inc.,
Danvers, MA, USA), α-1 type 1 collagen (COL1A1) (Abcam), or β-actin
(Cell Signaling Technology, Inc.). After incubation with
corresponding horseradish peroxidase-coupled secondary antibody
(Beyotime Institute of Biotechnology, Haimen, China), the membrane
was developed using an enhanced chemiluminescence system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Densitometric analysis was
performed using ImageJ software (National Institutes of Health,
Bethesda, MD, USA), using β-actin as an endogenous control.
Measurement of antioxidant enzyme
activities
Liver tissues were homogenized in saline using a
Teflon homogenizer (Glas Col, Vernon Hills, CA, USA). The sample
was then centrifuged at 3,000 rpm for 15 min at 4°C, and the
resultant supernatant was collected. The homogenate was then used
for assessments of oxidative stress markers. Antioxidant enzyme
activities were measured using an SOD, GSH, and MDA activity
detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), following the manufacturer's instructions.
Histology and
immunohistochemistry
Liver specimens were fixed in 10% neutral-buffered
formalin and embedded in paraffin. Then, the embedded tissues were
cut into 5 µm-thick serial sections. Next, the sections were
deparaffinized in xylene, hydrated through graded ethanol, and
stained with hematoxylin and eosin (H&E) or Masson's trichrome
for histopathological analysis. For immunohistochemistry, a
three-step immunohistochemical protocol was used, including
sectioning, dewaxing, and hydrating the tissues. After
deparaffinized with xylene and rehydrated with graded ethanol,
paraffin sections of tissues were antigen repaired with Tris-EDTA,
followed by incubation with 3% H2O2 at room
temperature for 10 min. Then, the slides were blocked with 5% goat
serum (diluted in PBS) for 1 h at room temperature and incubated
overnight at 4°C with primary antibody against collagen IV, LN,
α-SMA, COL1A1, TGF-β or TNF-α at the indicated dilutions, followed
by incubation with secondary antibody and rinsing with PBS. Cell
nuclei were counterstained with Harris hematoxylin solution.
Statistical analysis
Statistical analysis was performed using SPSS 23.0
software (IBM Corp., Armonk, NY, USA). Data were expressed as the
mean ± standard deviation. One-way analysis of variance followed by
Tukey's post-hoc test were used to compare the means of multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Liver enzymatic function and bilirubin
metabolism recovered after BMMSC treatment
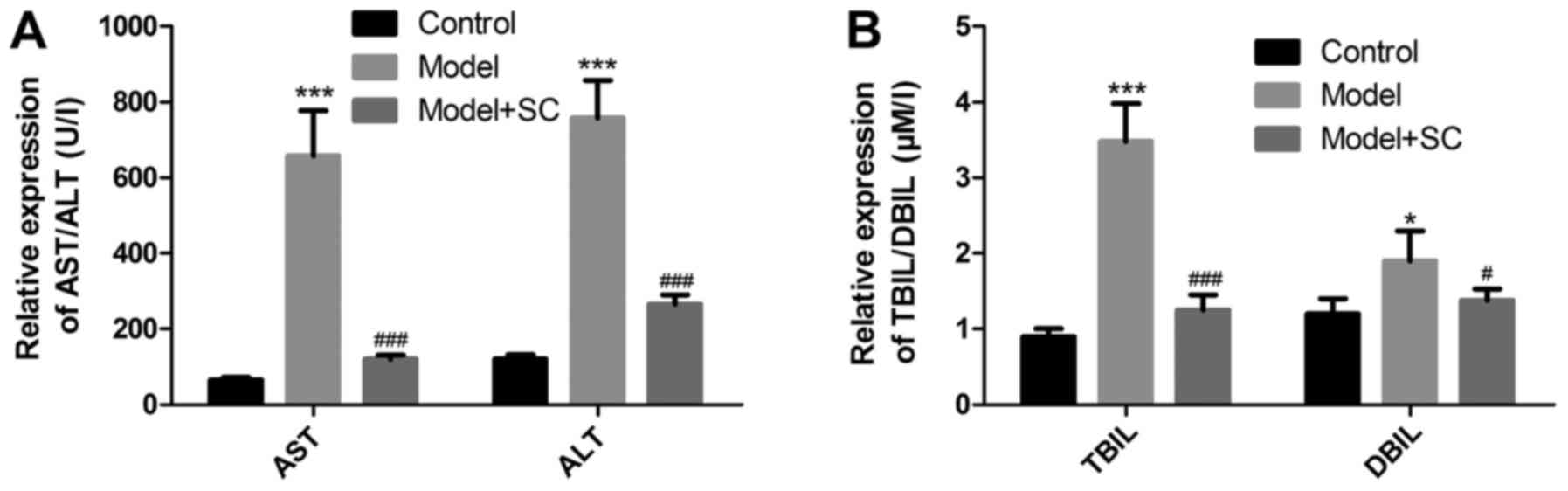
Liver enzyme levels such as AST, ALT, and the levels
of bilirubin metabolism TBIL and DBIL were upregulated in the
RRV-induced BA murine model as compared with the control group
(Fig. 1). Following BMMSC
transplantation, liver enzyme and bilirubin metabolism levels
markedly decreased in the RRV-induced BA murine model (Fig. 1), indicating that the BMMSCs
greatly improved liver function.
Effect of BMMSC treatment on liver
oxidative stress
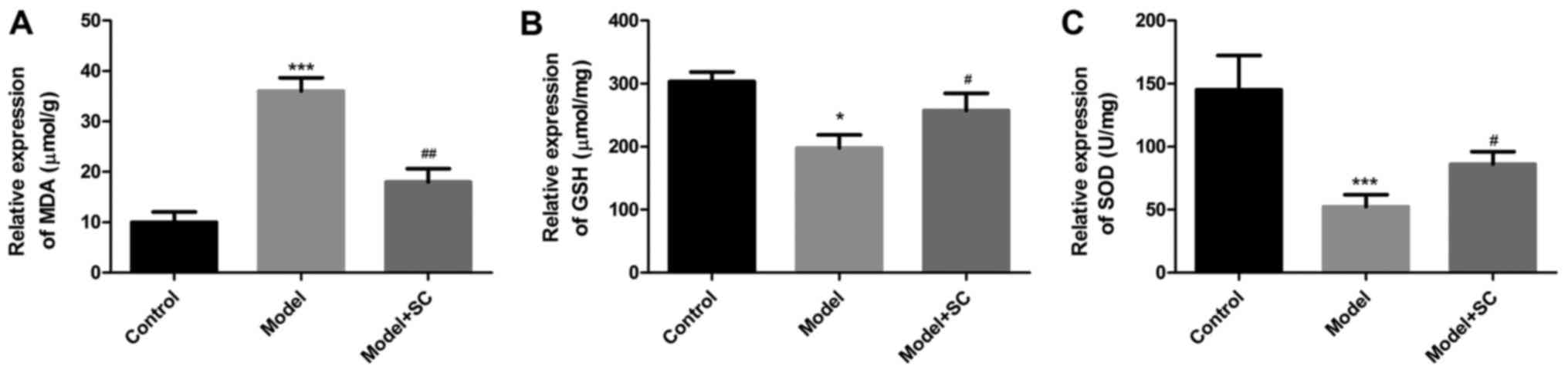
As shown in Fig. 2,
MDA levels significantly increased and GSH and SOD levels
significantly decreased in the BA model as compared with those of
the control group. BMMSC treatment reversed these effects, with
clearly reduced MDA levels and increased GSH and SOD levels as
compared with those in the nontreated RRV-induced BA group. These
data indicated that BMMSC treatment dramatically inhibited liver
oxidative stress in RRV-induced BA mice.
BMMSC treatment reduced the expression
of proteins associated with liver fibrosis
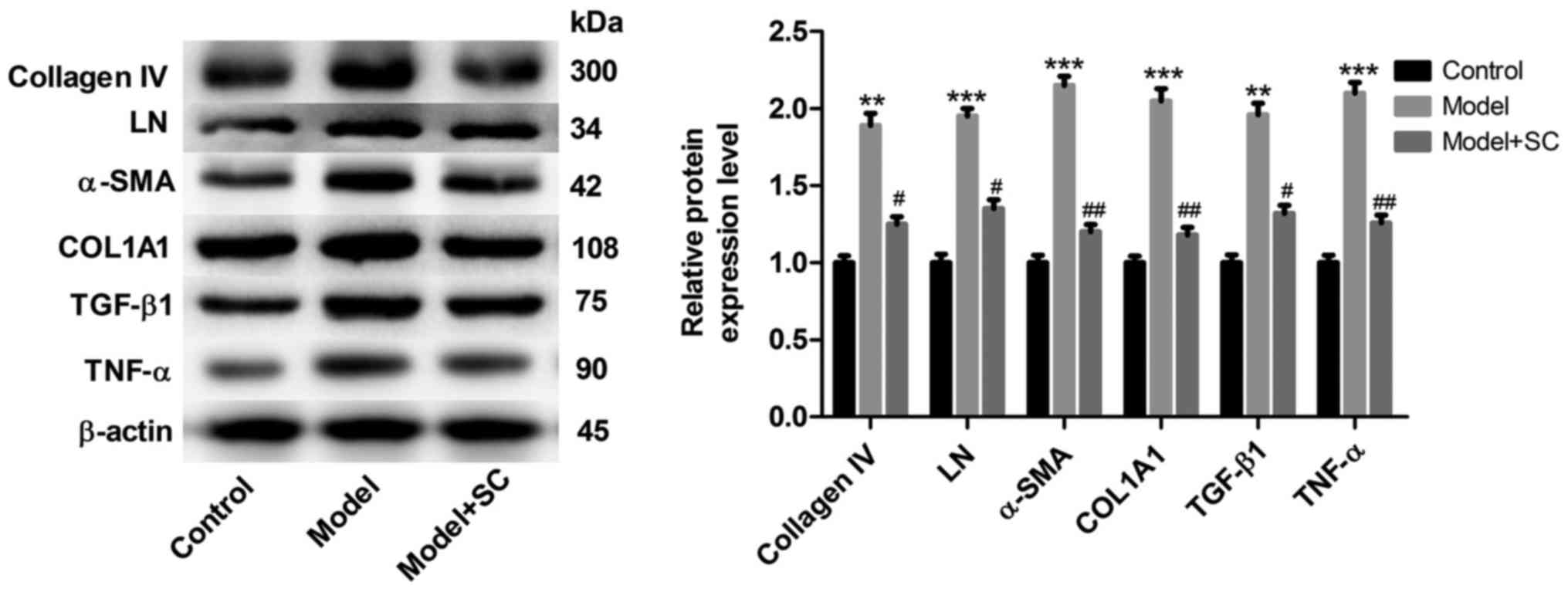
Expression levels of collagen IV, LN, α-SMA, COL1A1,
TGF-β1 and TNF-α in liver tissues increased significantly in the BA
group when compared with those in the control group (Fig. 3). And BMMSCs treatment
significantly decreased the protein expression of collagen IV, LN,
α-SMA, COL1A1, TGF-β1, and TNF-α levels (Fig. 3). Moreover, results from
immunohistochemical staining of collagen IV, LN, α-SMA, COL1A1,
TGF-β1, and TNF-α proteins in liver tissues from the different
groups (control group, BA group, and BA + BMMSC treatment group)
were consistent with those assessed by western blotting (Fig. 4). Taken together, these results
indicated that BMMSCs administration appeared to relieve liver
fibrosis caused by BA.
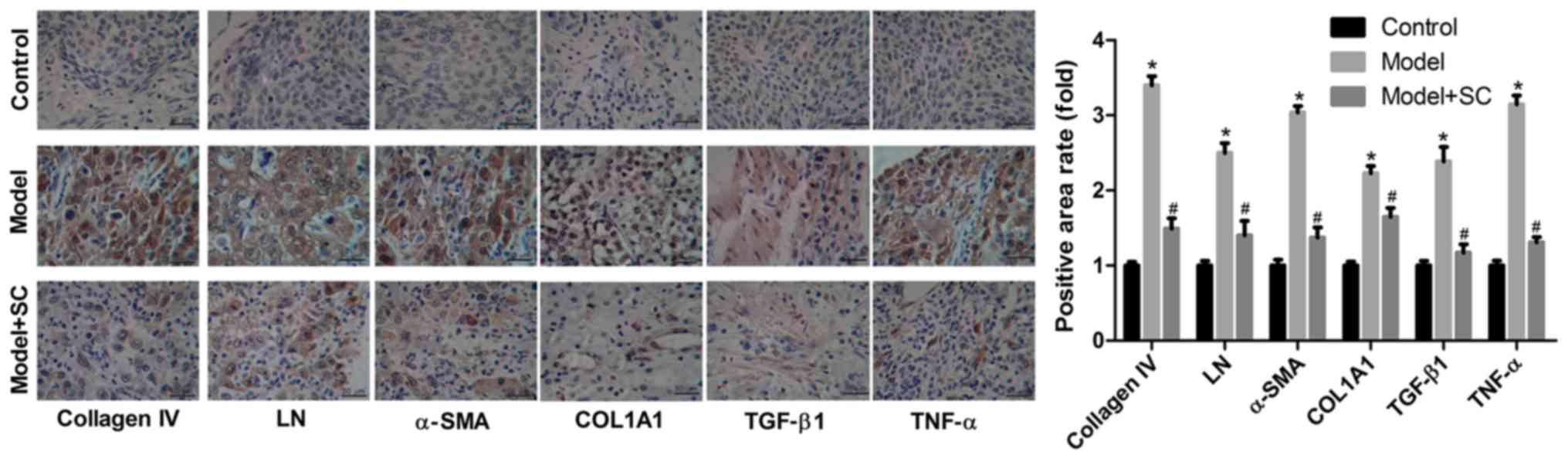 | Figure 4.Immunohistochemistry analysis of the
expression of proteins associated with liver fibrosis. Protein
expression in liver tissues from the different groups: Control, BA,
model and BA + BMMSC (model + SC). Magnification, ×100; scale
bars=50 µm. *P<0.05 vs. control group; #P<0.05 vs.
BA model group. LN, laminin; TGF-β, transforming growth factor-β;
TNF-α, tumor necrosis factor-α; COL1A1, collagen 1 type α1; α-SMA,
α-smooth muscle actin; BMMSC/SC, bone marrow-derived mesenchymal
stem cells; BA, biliary atresia. |
The effect of the BMMSC treatment on
histology
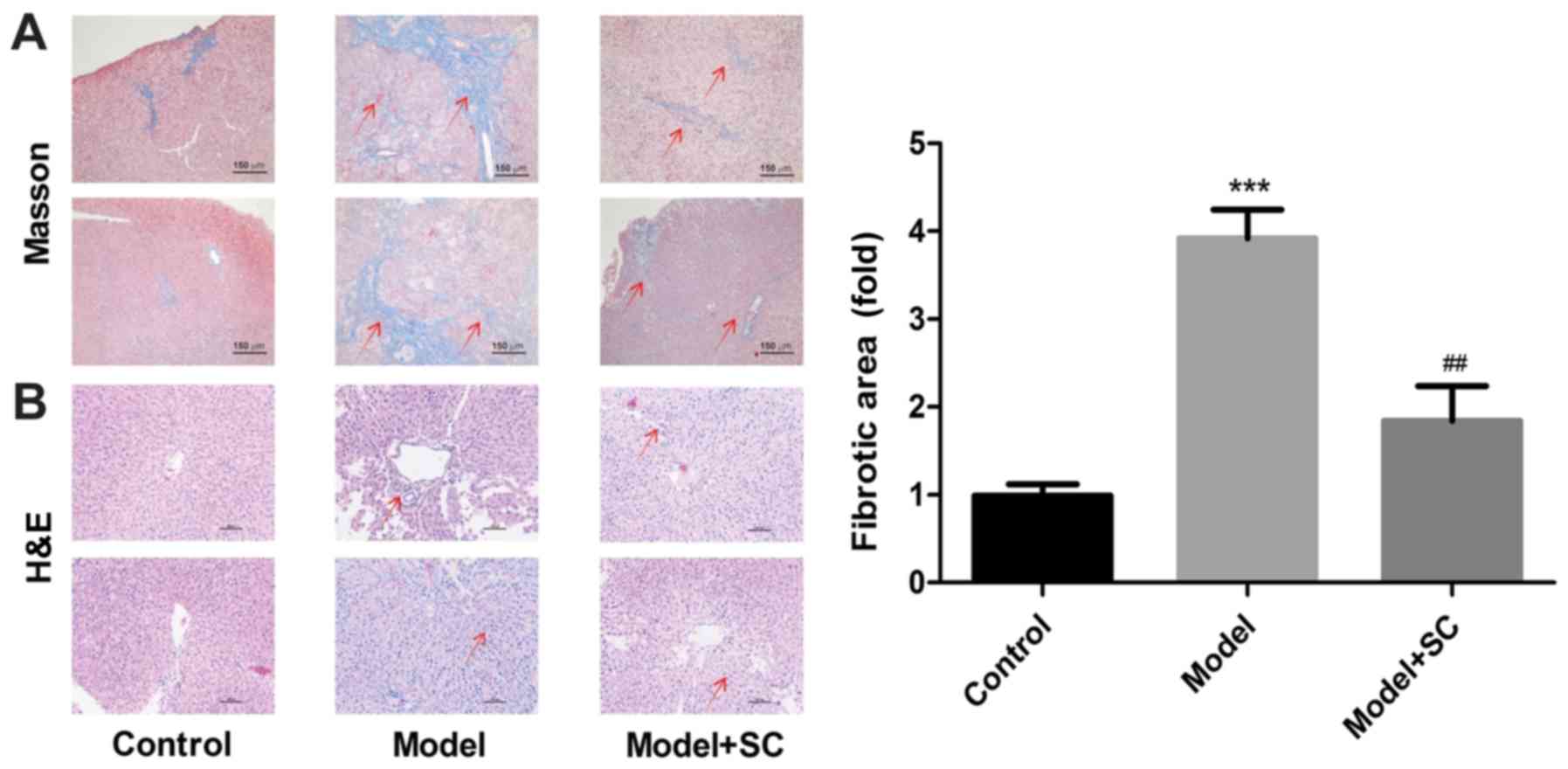
Liver sections of the control group displayed normal
histological features, whereas liver sections in the BA group
showed massive diffuse and progressive histological alterations,
with massive mononuclear cellular infiltration and marked fibrosis
(Fig. 5A and B). In the
BMMSC-treated group, most of the histological alterations observed
in the BA group disappeared, and cell histology was more or less
similar to that observed in the control group (Fig. 5A and B). These findings suggested
that BMMSC treatment alleviated liver fibrosis in a murine model of
RRV-induced BA.
Discussion
BA is a devastating disease of newborns that occurs
within the first few months after birth. The disease is
characterized by liver inflammation, fibrosis, and obstruction of
the bile duct (12). Although the
etiology of BA is largely unclear, human and mouse data have
implicated that a perinatal viral infection is a possible
pathogenic mechanism (13). In the
murine model of BA, RRV infection of newborn BALB/c mice results in
inflammatory cholangiopathy, with a pathological phenotype similar
to that seen in human BA (14,15).
BA mice develop bilirubinuria and jaundice, with pale-colored
stools and growth retardation, culminating in death. Based on this,
in the present study, we constructed a murine model of BA by RRV
administration and explored the antifibrotic roles of BMMSCs in BA
mice.
The present study demonstrated that BMMSCs treatment
significantly restored liver enzymatic function and bilirubin
metabolism and inhibited oxidative stress and alleviated liver
fibrosis of the RRV-induced murine model of BA. Our results were
consistent with those of a previous study (16). Our study points to a potentially
novel approach to ameliorate the fibrotic response of BA
patients.
Besides, we showed that the administration of RRV
led to a parallel increase of MDA, an oxidative stress marker, with
a subsequent decrease of antioxidant markers (GSH and SOD). Active
oxygen species play essential roles in host defense mechanisms
against disease. However, excessive production of active oxygen
species caused by chronic inflammation may lead to tissue injury
and disease progression (17). In
many BA patients, Kasai's hepatic portoenterostomy fails to resolve
upregulation of oxidative stress, even in cases of mild liver
dysfunction without jaundice (18). The aforementioned finding suggests
that antioxidant therapy may be necessary for the treatment of BA
patients. And our study demonstrated that BMMSCs transplantation
significantly rescued the decrease of GSH and SOD and increase of
MDA induced by RRV-administration, indicating the important value
of BMMSCs in the treatment of BA. LN, an extracellular matrix
component, is a major non-collagenous glycoprotein synthesized by
hepatic stellate cells (HSCs) and deposited in the basement
membrane of the liver (19).
Previous study revealed that elevated levels of LN were correlated
with the degree of perisinusoidal fibrosis (20). In the present study, the liver
content of LN was significantly increased in the RRV-induced BA
model, whereas it was significantly decreased after BMMSC
treatment. In addition to LN, collagen, another extracellular
matrix protein, plays an important role in fibrosis (21). Accordingly, we determined the
effect of BMMSC therapy on the collagen content of liver tissue.
Both collagen IV and COL1A1 were highly expressed in liver tissues
of the model group, and the BMMSC treatment markedly reduced their
expression. As demonstrated in previous research, activated HSCs
are a major source of matrix proteins in diseased livers (22). Accordingly, we evaluated the
formation of α-SMA, an indicator of HSC activation (22), by immunohistochemical staining and
western blotting of liver samples. The results showed that α-SMA
expression was elevated in the BA group, whereas it was clearly
reduced in the BA BMMSC-treated group.
MSCs exert inhibitory effects on monocytes,
dendritic cells, macrophages, and natural killer (NK) cells, which
are part of the innate immune system. Besides, MSCs inhibit the
maturation of monocytes into dendritic cells, which play a role in
antigen presentation to naive T-cells and repress the secretion of
TNF-α, INF-γ, and interleukin-12 by dendritic cells and increase
their secretion of interleukin-10, thereby reducing their
pro-inflammatory potential (23).
In contrast to MSCs, HSC activation in response to injury results
in the secretion of TGF-β1, which has potent fibrogenic effects in
both autocrine and paracrine patterns (24). However, BMMSCs could suppress the
activity of HSCs and reduce the secretion of specific cytokines,
such as TGF-β1 and interleukin-6 (24). In accordance with this finding, our
study revealed that treatment with BMMSCs significantly inhibited
inflammatory reactions in the liver by decreasing the secretion of
pro-inflammatory factors, such as TNF-α and TGF-β1.
Overall, the results of the present study point to
the potential beneficial role of BMMSCs in the inhibition of
fibrosis progression induced by RRV in mice.
Acknowledgements
Not applicable.
Funding
The present was supported by the National Natural
Science Foundation of China (grant nos. 81460118, 81760115 and
81660092), Education Department Scientific Research Foundation
(grant no. GJJ14769) and Health Development Planning Commission
Science Foundation of Jiangxi Province (grant no. 2012A135).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TX and SZ conceived and designed the study. JL, YC,
JX, HH and ZL performed the experiments. LY and JH performed the
histological examination of the liver. ZL and YX analyzed and
interpreted the data. JL and YC wrote the paper. ZL, YX, LY and JH
reviewed and edited the manuscript. All authors read and approved
the manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of the Jiangxi Provincial Children's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Hartley JL, Davenport M and Kelly DA:
Biliary atresia. Lancet. 374:1704–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mieli-Vergani G and Vergani D: Biliary
atresia. Semin Immunopathol. 31:371–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sira MM, Taha M and Sira AM: Common
misdiagnoses of biliary atresia. Eur J Gastroenterol Hepatol.
26:1300–1305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
You Z, Wen J, Cheng L, Ye H and Li B:
Screening of targeted genes in extrahepatic bile ducts of mice with
experimental biliary atresia. Mol Med Rep. 12:4326–4331. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zagory JA, Nguyen MV and Wang KS: Recent
advances in the pathogenesis and management of biliary atresia.
Curr Opin Pediatr. 27:389–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Wu Q, Wang Y, Li L, Bu H and Bao J:
Senescence of mesenchymal stem cells (Review). Int J Mol Med.
39:775–782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Zhang H, Huang B, Miao G, Yan X,
Gao G, Luo Y, Chen H, Chen W and Yang L: Mesenchymal stem cells
reverse highfat dietinduced nonalcoholic fatty liver disease
through suppression of CD4+ T lymphocytes in mice. Mol Med Rep.
17:3769–3774. 2018.PubMed/NCBI
|
|
8
|
Sioud M, Mobergslien A, Boudabous A and
Floisand Y: Mesenchymal stem cell-mediated T cell suppression
occurs through secreted galectins. Int J Oncol. 38:385–390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berardis S, Lombard C, Evraerts J, El
Taghdouini A, Rosseels V, Sancho-Bru P, Lozano JJ, van Grunsven L,
Sokal E and Najimi M: Gene expression profiling and secretome
analysis differentiate adult-derived human liver stem/progenitor
cells and human hepatic stellate cells. PLoS One. 9:e861372014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang R, Lin Z, Lui VCH, Wong KKY, Tam
PKH, Lee P, Lok CN, Lamb JR, Chen Y and Xia H: Silver nanoparticle
treatment ameliorates biliary atresia syndrome in rhesus rotavirus
inoculated mice. Nanomedicine. 13:1041–1050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohanty SK, Donnelly B, Dupree P, Lobeck
I, Mowery S, Meller J, McNeal M and Tiao G: A point mutation in the
rhesus rotavirus VP4 protein generated through a rotavirus reverse
genetics system attenuates biliary atresia in the murine model. J
Virol. 91:e005102017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bezerra JA: Potential etiologies of
biliary atresia. Pediatr Transplantat. 9:646–651. 2005. View Article : Google Scholar
|
|
14
|
Petersen C, Grasshoff S and Luciano L:
Diverse morphology of biliary atresia in an animal model. J
Hepatol. 28:603–607. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riepenhoff-Talty M, Schaekel K, Clark HF,
Mueller W, Uhnoo I, Rossi T, Fisher J and Ogra PL: Group A
rotaviruses produce extrahepatic biliary obstruction in orally
inoculated newborn mice. Pediatr Research. 33:394–399. 1993.
View Article : Google Scholar
|
|
16
|
Elmahdy NA, Sokar SS, Salem ML, Sarhan NI
and Abou-Elela SH: Anti-fibrotic potential of human umbilical cord
mononuclear cells and mouse bone marrow cells in CCl4- induced
liver fibrosis in mice. Biomed Pharmacother. 89:1378–1386. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cadenas E: Biochemistry of oxygen
toxicity. Annu Rev Biochem. 58:79–110. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asakawa T, Tanaka Y, Asagiri K, Kobayashi
H, Tanikawa K and Yagi M: Oxidative stress profile in the
post-operative patients with biliary atresia. Pediatr Surg Int.
25:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang N, Zhang Y, Liu Z, Fu T, Liang Q and
Ai X: Correlation analysis between four serum biomarkers of liver
fibrosis and liver function in infants with cholestasis. Biomed
Rep. 5:107–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baranova A, Lal P, Birerdinc A and
Younossi ZM: Non-invasive markers for hepatic fibrosis. BMC
Gastroenterol. 11:912011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Komers R, Carew R, Winbanks CE, Xu
B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K,
Gregorevic P, et al: Suppression of microRNA-29 expression by
TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc
Nephrol. 23:252–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Williams EJ, Benyon RC, Trim N, Hadwin R,
Grove BH, Arthur MJ, Unemori EN and Iredale JP: Relaxin inhibits
effective collagen deposition by cultured hepatic stellate cells
and decreases rat liver fibrosis in vivo. Gut. 49:577–583. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y,
Yu XD and Mao N: Human mesenchymal stem cells inhibit
differentiation and function of monocyte-derived dendritic cells.
Blood. 105:4120–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jang YO, Jun BG, Baik SK, Kim MY and Kwon
SO: Inhibition of hepatic stellate cells by bone marrow-derived
mesenchymal stem cells in hepatic fibrosis. Clin Mol Hepatol.
21:141–149. 2015. View Article : Google Scholar : PubMed/NCBI
|