Introduction
Lung cancer is one of the most common malignant
tumours worldwide, with an increasing incidence each year; its
morbidity has increased >10-fold in male and female patients in
the last five decades (1).
Non-small cell lung cancer (NSCLC) represents >80% of lung
cancer cases. A total of 40–50% of patients with NSCLC are at an
advanced stage and are treated with radiotherapy and chemotherapy,
although these therapies lack sensitivity (2).
With the discovery of a series of tumour-targeting
genes, a number of targeted gene drugs against NSCLC, including
epidermal growth factor receptor-tyrosine kinase inhibitor
(EGFR-TKI), are being developed. In recent years, EGFR, a
transmembrane receptor glycoprotein, has been an important target
of gene therapy against NSCLC (2,3). In
tumours, including NSCLC, thyroid cancer and colorectal cancer,
EGFR signalling pathways are frequently abnormally activated or
highly expressed, which indicates the correlation between tumour
progression and EGFR-associated gene expression. The abnormal
expression of any protein in the downstream transduction pathways
of EGFR promotes the erroneous transduction of proliferation
signals to the cell nucleus, thus inducing unusual hyperplasia of
cells as a consequence (4–6). EGFR, which was reported to be
abnormally activated and overexpressed, is the initial point of
these pathways, and therefore becomes an important effector target
for drug therapy against NSCLC (7). Competitively combining with the ATP
binding site of tyrosine kinase domain to ATP, EGFR-TKI is able to
inhibit the phosphorylation and activation of EGFR tyrosine kinase,
and delay the EGFR signal transduction system, to restrain cell
proliferation and accelerate apoptosis, thus achieving the purpose
of tumour inhibition (7,8). For over a decade, EGFR-TKIs,
including gefitinib and erlotinib, have been clinically identified
to have a specific curative effect on patients with an EGFR gene
mutation (9,10). However, like other
chemotherapeutics, the problem of drug resistance has gradually
emerged with the promotion of clinical applications (11). Recently, immunotherapy has become
the most revolutionary treatment in patients with NSCLC (12). Wang et al (13) demonstrated that inactivity of the
EGFR/mitogen-activated protein kinase pathway was associated with
the reversal of PD-L1-mediated immune evasion in NSCLC in an in
vivo study. Therefore, research into effective targets of EGFR
and the internal mechanisms involved in the immune evasion of NSCLC
is of significance.
The B7 family is the principal co-stimulatory
molecule family in T-lymphocyte activation, and includes B7-1,
B7-2, B7-H1, B7-H2, H7-H3 and B7-4 (14–16).
Inamura et al (17)
reported a significant association between high B7-H3 expression
with wild-type EGFR and smoking in patients, indicating the
potential effectiveness of an anti-B7-H3 therapy for EGFR wild-type
or smoking-associated lung cancer. In 2013, Zhu et al
(18) identified a novel
co-stimulatory pathway regulating human T-cell responses, the
B7-H5/CD28 homologue (CD28H) pathway. A recent study in pancreatic
cancer indicated the loss of B7-H5, one of the co-stimulatory
molecules in the B7 molecule family, which may contribute to immune
evasion (19). To the best of our
knowledge, there has been no direct research into the association
between and mechanism of B7-H5 and EGFR. The present study aimed to
determine the possible association between EGFR and B7-H5 in the
immune evasion of NSCLC, and attempted to investigate the
associated pathway.
Materials and methods
Tissues and cells
A total of 42 patients with NSCLC at The First
People's Hospital of Huzhou (Huzhou, China) were included in the
present study. All cancer tissues specimens were obtained from
surgical tumour resections, and their adjacent normal lung tissue
specimens were obtained simultaneously as the negative control. The
normal and cancer tissues represented matched pairs from each
patient. Basic clinical and pathological data for these patients
was collected with their written informed consent. The study was
approved by the ethics committee of The First People's Hospital of
Huzhou. The cell lines BEAS-2B, A549, NCI-H1299, NCI-H1755 and 95D
were all obtained from Shanghai Yansheng Industrial Co. Ltd.
(Shanghai, China). Cells were maintained in Dulbecco's modified
Eagle medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing with 10% fetal bovine serum, and 1%
streptomycin/penicillin (Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2.
Grouping
NCI-H1299 cells were divided into four groups:
Control group, mock group, silencing (si)EGFR group and EGFR-TKI
group. Cells in the mock group were infected with a blank vector.
Cells in the siEGFR group were infected with the recombinant
plasmids of siEGFR. In the EGFR-TKI group, cells were treated with
gefitinib as a positive control (Cardinal Health, Inc., Dublin, OH,
USA).
Cell infection and treatment
Recombinant plasmids of siEGFR and siB7-H5 were
purchased from Shanghai Quanyang Biotechnology Co., Ltd. (Shanghai,
China) and the sequences were as follows: siEGFR antisense
3′-UUCCGCAUUCCUCGUCUAUUU-5′ and sense 5′-GGCGUAAGGAGCAGAUAAAUU-3′;
siB7-H5 antisense 3′-UUCGUCGCACAAUUCACAAAU-5′ and sense
5′-GCAGCGUGUUAAGUGUUUAUU-3′; and a negative siRNA control antisense
3′-TTAAGAGGCUUGCACAGUGCA-5′ and sense 5′-UUCUCCGAACGUGUCACGUTT-3′.
Cells in the logarithmic growth phase were seeded into a 6-well
plate at a density of 2×106 to culture for 24 h.
Recombinant plasmids were transfected into cells, according to the
manufacturer's protocol of the Invitrogen Lipofectamine®
LTX (Thermo Fisher Scientific, Inc., Waltham, MA, USA). A total of
2 µg pIRES2-ZsGreen1-vector or pIRES2-ZsGreen1-ARHGAP18 (Sangon
Biotech Co., Ltd., Shanghai, China), 5 µl Lipofectamine®
LTX (Thermo Fisher Scientific, Inc.) and 250 µl Opti-Minimum
Essential Medium (Shanghai Haoran Biological Technology Co., Ltd.,
Shanghai, China) was prepared, mixed and incubated at room
temperature for 25 min. Subsequently, 500 µl mixture was added into
the 6-well plate with RPMI 1640 medium (Thermo Fisher Scientific,
Inc.). Finally, following a 48-h culture period, transfected cells
were harvested for the following experiments.
Cell Counting Kit-8 (CCK-8) assay
Cell viability in each group was detected with a
CCK-8 Κit (Beyotime Institute of Biotechnology, Haimen, China).
Cells at a density of 3×104 cells/ml were grouped and
seeded into 96-well plates at 100 µl per well, and incubated at
37°C in a 5% CO2 incubator for 4 h. Following the
addition of 10 µl CCK reagent to each well, cells were placed into
the 5% CO2 incubator at 37°C for 1–4 h. The optical
density (OD) value of each group was observed at 450 nm using a
spectrophotometer (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany).
Flow cytometry (FCM)
Cells in the logarithmic phase were collected and
seeded into 6-well plates at a density of 1×105 cells
per well. Cells were digested with EDTA-free trypsin (Beyotime
Institute of Biotechnology), stained with Annexin V-fluorescein
isothiocyanate and propidium iodide (Shanghai BestBio Science Co.,
Ltd., Shanghai, China), and incubated in the dark at 4°C for 15
min. The cell cycle distribution and apoptosis rate of each group
was detected using an EPICS XL-MCL flow cytometer (Beckman Coulter,
Inc., Brea, CA, USA) with an excitation wavelength of 488 nm and an
emission wavelength of 530 nm. Data was analysed using FCS Express
version 3.0 (De Novo Software, Glendale, CA, USA).
ELISA analysis
The transforming growth factor (TGF)-β and
interleukin (IL)-10 content in cells were measured using a TGF-β1
ELISA kit (Abcam, Cambridge, UK; cat. no. ab100647) and an IL-10
ELISA kit (Abcam; cat. no. ab100549). Detection was performed
according to the manufacturer's protocol. OD values were read at
420 nm using a spectrophotometer (Sigma-Aldrich; Merck KGaA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Cells were seeded into 6-well plates at a density of
2×106 cells/well. Total RNA was extracted using TRIzol
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The concentration of the extracted RNA was assessed using
a UV spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA was
synthesized using reverse transcriptase, a poly (A) polymerase and
tagged oligo (dT) primers in a 20 µl reaction volume for RT-qPCR
(miScript; Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's protocol. SYBR Green PCR Master Mix (Takara Bio,
Inc., Otsu, Japan) was used to quantitate the expression levels of
target genes. The amplification assay was performed on an Applied
Biosystems PCR system (ABI 7500; Thermo Fisher Scientific, Inc.).
PCR was performed by activating the DNA polymerase at 95°C for 5
min, followed by 40 cycles of two-step PCR (95°C for 10 sec and
60°C for 30 sec) and a final extension at 75°C for 10 min then
holding at 4°C. GAPDH was applied as the internal control to
monitor the efficiency of qPCR. All primers in the study were
designed by Sangon Biotech Co., Ltd. The specific primer sequences
for each gene are listed as follows: Forward
5′GGTGAGTGGCTTGTCTGGAA3′ and reverse 5′CCTTACGCCCTTCACTGTGT3′ for
EGFR (product, 152 bp); forward 5′GAACTGGATGCCCTTGTGGT3′ and
reverse 5′TGCTGTCTGTGCCTTCATGT3′ for B7-H5 (product, 123 bp);
forward 5′CCAGGCGGTGATGTGGATCT3′ and reverse
5′GTGCTGGTAGCCATGAGGGT3′ for Survivin (product, 139 bp); forward
5′GCCAGCAAACTGGTGCTCAA3′ and reverse 5′CCAACCACCCTGGTCTTGGA3′ for
apoptosis regulator Bax (Bax; product, 126 bp); forward
5′CACTGGCCAGGGTCAGAGTT3′ and reverse 5′TGGCCATAGACCCTGTCAGC3′ for
apoptosis regulator Bcl-2 (Bcl-2; product, 85 bp); forward
5′GGGCTACCATGCCAACTTCT3′ and reverse 5′GACACAGAGATCCGCAGTCC3′ for
TGF-β (product, 384 bp); forward 5′GGCTTGGGGCTTCCTAACTG3′ and
reverse 5′GGGAATCCCTCCGAGACACT3′ for IL-10 (product, 99 bp);
forward 5′GATCCCCAGGGCTCAAACAT3′ and reverse
5′GAAAAGGCGCAGTTTACGCT3′ for cyclooxygenase-2 (COX-2; product, 126
bp) and forward 5′AATGGGCAGCCGTTAGGAAA3′ and reverse
5′GCGCCCAATACGACCAAATC3′ for GAPDH (product, 168 bp). Each reaction
was run in triplicate. The 2−ΔΔCq method was used for
quantification (20).
Western blotting
Cells were seeded in 6-well plates at a density of
2×106 cells/well, and grouped. Cells were harvested and
washed twice with PBS, and lysed in ice-cold
radioimmunoprecipitation assay buffer (Whiga Technology Co., Ltd.,
Guangzhou, China) with a freshly mixed 0.01% protease inhibitor
(phenylmethylsulfonyl fluoride; BeijingBioLab Science and
Technology Co., Ltd., Beijing, China), followed by incubation for
30 min on ice. Cell lysates were centrifuged at 10,000 × g for 5
min at 4°C, and the supernatants containing 20–30 µg protein were
collected, and protein concentration was determined using a BCA
protein assay kit (Thermo Fisher Scientific, Inc.). Samples were
run on a 10% SDS-PAGE gel, and electrophoretically transferred to a
nitrocellulose membrane (Merck KGaA). To block the nonspecific
proteins, 5% fat-free milk was incubated with the membrane for 2 h
at room temperature. Membranes were incubated with the following
primary specific antibodies at 4°C for 6 h and then at room
temperature for 4 h: Anti-EGFR antibody (1:1,000; Abcam; cat. no.
ab52894), anti-B7-H5 antibody (1:1,000; Abcam; cat. no. ab201565),
anti-Survivin antibody (1:5,000; Abcam; cat. no. ab76424), anti-Bax
antibody (1:1,000; Abcam; cat. no. ab32503), anti-Bcl-2 antibody
(1:1,000; Abcam; cat. no. ab692), anti-TGF-β antibody (1:1,000;
Abcam; cat. no. ab92486), anti-vascular endothelial growth factor
(VEGF) receptor 2 antibody (1:1,000; Abcam; cat. no. ab10972),
anti-IL-10 antibody (1:800; Abcam; cat. no. ab34843), anti-COX-2
antibody (1:1,000; Abcam; cat. no. ab15191), anti-tyrosine-protein
kinase JAK2 (JAK2; phosphorylated Y1007) antibody (1:1,000; Abcam;
cat. no. ab195055), anti-JAK2 antibody (1:1,000; Abcam; cat. no.
ab39636), anti-signal transducer and activator of transcription 3
(STAT3; phosphorylated Y705) antibody (1:2,000; Abcam; cat. no.
ab76315), anti-STAT3 antibody (1:1,000; Abcam; cat. no. ab68153),
and anti-GAPDH antibody (1:2,000; Abcam; cat. no. ab8245). The
horseradish peroxidase secondary antibodies were goat anti-mouse
immunoglobulin (Ig)G heavy and light chains (H&L; 1:2,000;
Abcam; cat. no. ab6789), goat anti-rabbit IgG H&L (1:2,000;
Abcam; cat. no. ab6721), and donkey anti-goat IgG H&L (1:2,000;
Abcam; cat. no. ab6885). The membranes were then incubated at room
temperature for 1 h. Blots were visualized via enhanced
chemiluminescence (ECL; Thermo Fisher Scientific, Inc.). An ECL
system (Amersham; GE Healthcare, Chicago, IL, USA) was used to
detect the bands. The density of the blots was read with the
Quantity One software version 2.4 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Data is expressed as the mean ± standard deviation.
Prism GraphPad version 6.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) was used to analyze the data. Differences among
groups were evaluated via one-way analysis of variance followed by
Tukey's multiple comparisons test. The χ2 test was used
for the analysis of the significance of EGFR and
clinicopathological characteristics. P<0.05 was considered to
indicate a statistically significant difference.
Results
Negative correlation between EGFR and
B7-H5 expression in lung cancer tissues and cell lines
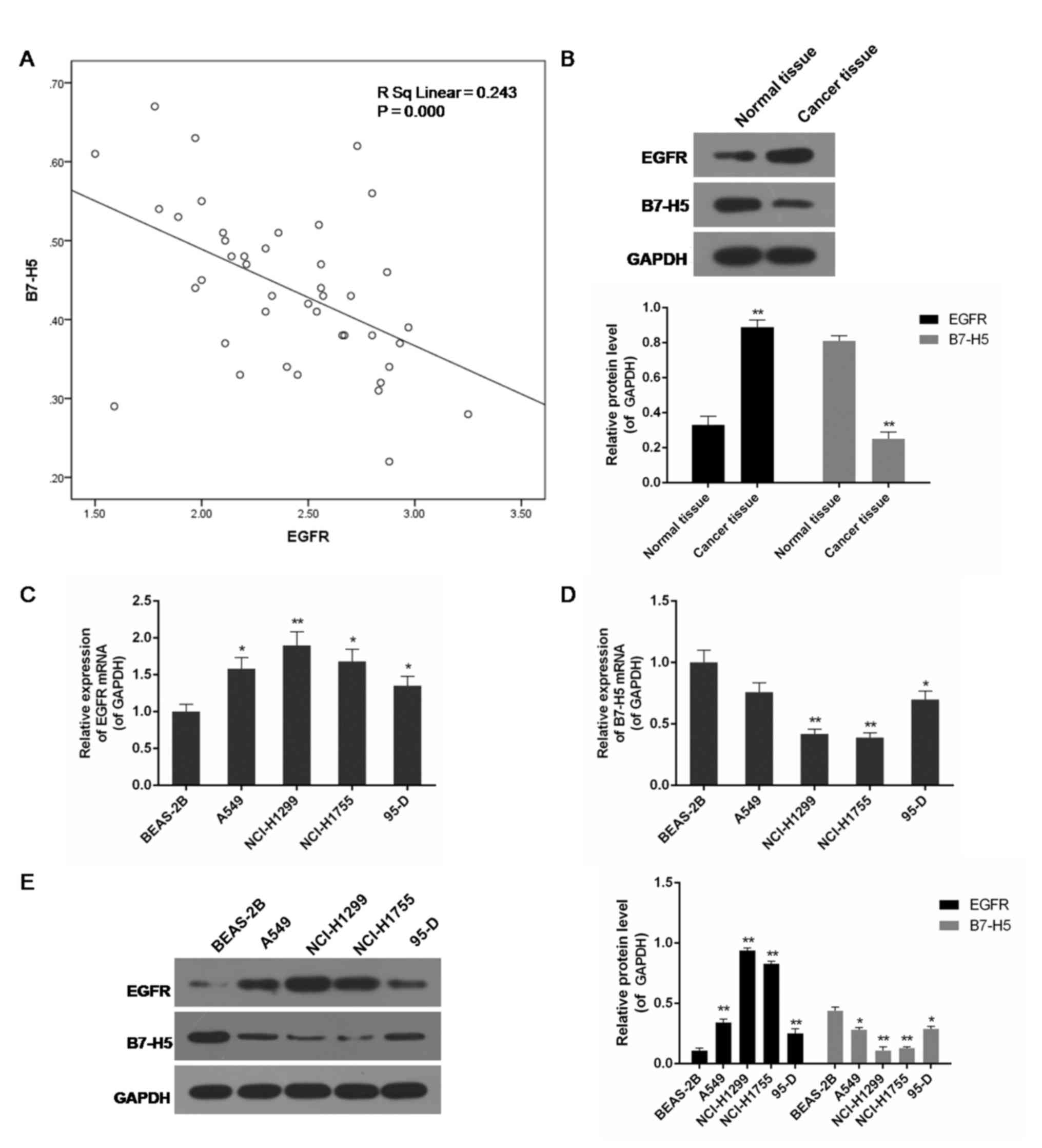
The present study included a total of 42 patients
from our hospital. The expression of EGFR and B7-H5 in
surgically-resected NSCLC specimens and adjacent normal cancer
tissues was assessed. Compared with normal tissues, the level of
EGFR in cancer specimens was increased, while the expression of
B7-H5 was decreased, and the correlation analysis indicated a
negative association between them (P<0.01; Fig. 1A and B). EGFR mRNA and B7-H5 mRNA
were observed to be negatively correlated. Examination of the
correlation between EGFR expression and clinical pathological
features demonstrated a positive association between increased EGFR
expression levels and tumour-node-metastasis staging (Table I). A negative association between
EGFR and B7-H5 mRNA and protein expression levels was observed in
lung cell lines. In the lung cancer cell lines A549, NCI-H1299,
NCI-H1755 and 95-D, particularly NCI-H1299 and NCI-H1755, EGFR
expression was significantly increased, whereas B7-H5 was
significantly inhibited compared with the normal lung cell line
BEAS-2B (P<0.05 or P<0.01; Fig.
1C-E).
 | Table I.Association between EGFR and clinical
data of patients with non-small cell lung cancer. |
Table I.
Association between EGFR and clinical
data of patients with non-small cell lung cancer.
| Cancer staging | Sex,
male/female | Age, <59/≥59
years | EGFR expression,
lower/higher |
|---|
| TNM |
| I | 5/2 | 4/3 | 5/2 |
| II | 8/3 | 5/6 | 4/7 |
|
III | 15/9 | 9/15 | 5/19 |
| P-values | 0.802 | 0.639 | 0.043a |
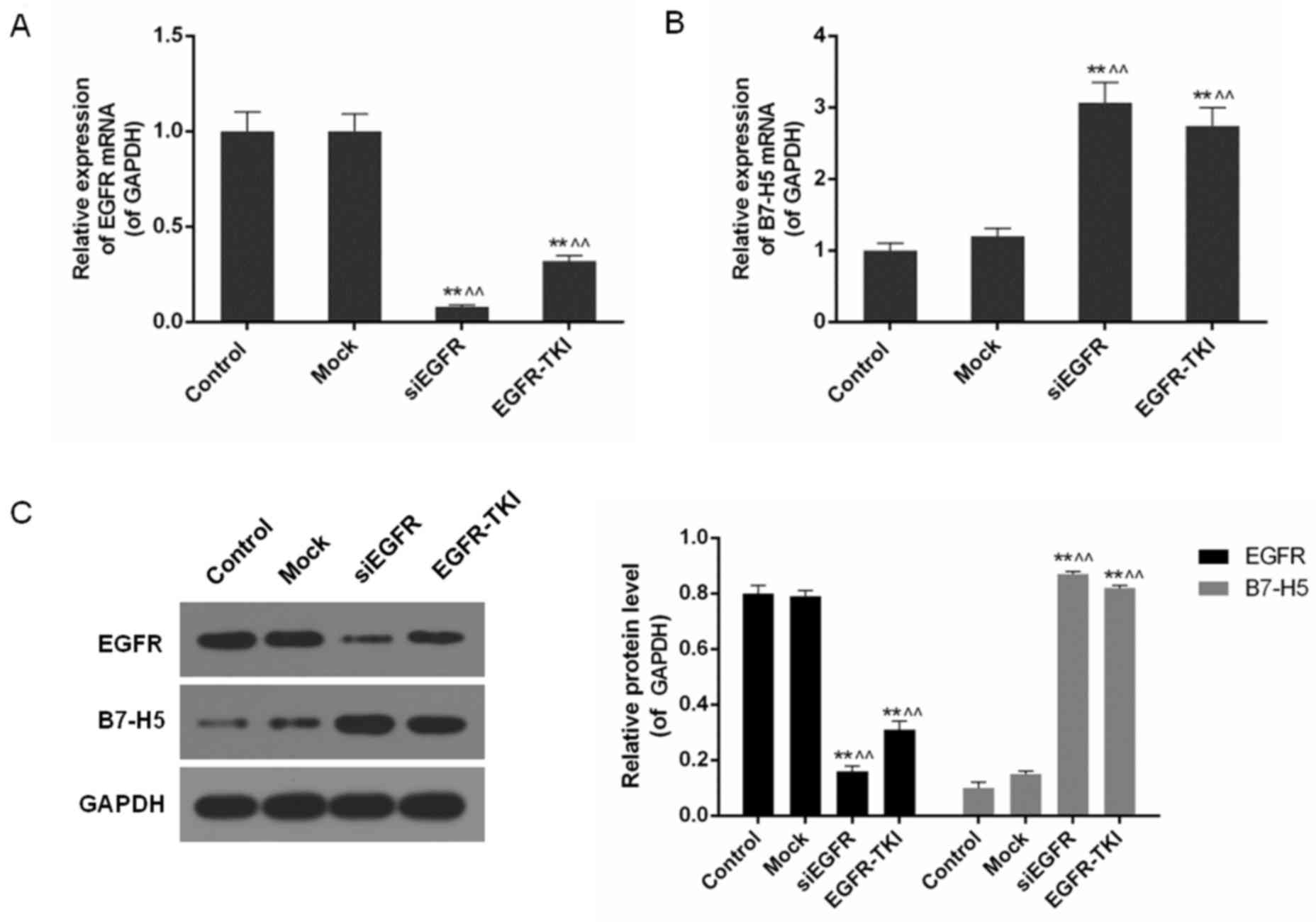
Low expression of EGFR increases B7-H5
levels in siEGFR- or EGFR-TKI-treated NCI-H1299 cells
It was detected that EGFR gene silencing and
EGFR-TKI significantly inhibited EGFR mRNA and protein expression
in NCI-H1299 cells, particularly in the siEGFR group (P<0.01;
Fig. 2). In contrast to the low
levels of EGFR expression, B7-H5 gene products were significantly
increased (P<0.01; Fig. 2B and
C). B7-H5 protein levels in the siEGFR and EGFR-TIKI groups
were >4 times higher compared with the control and mock groups,
where EGFR was highly expressed (P<0.01; Fig. 2C).
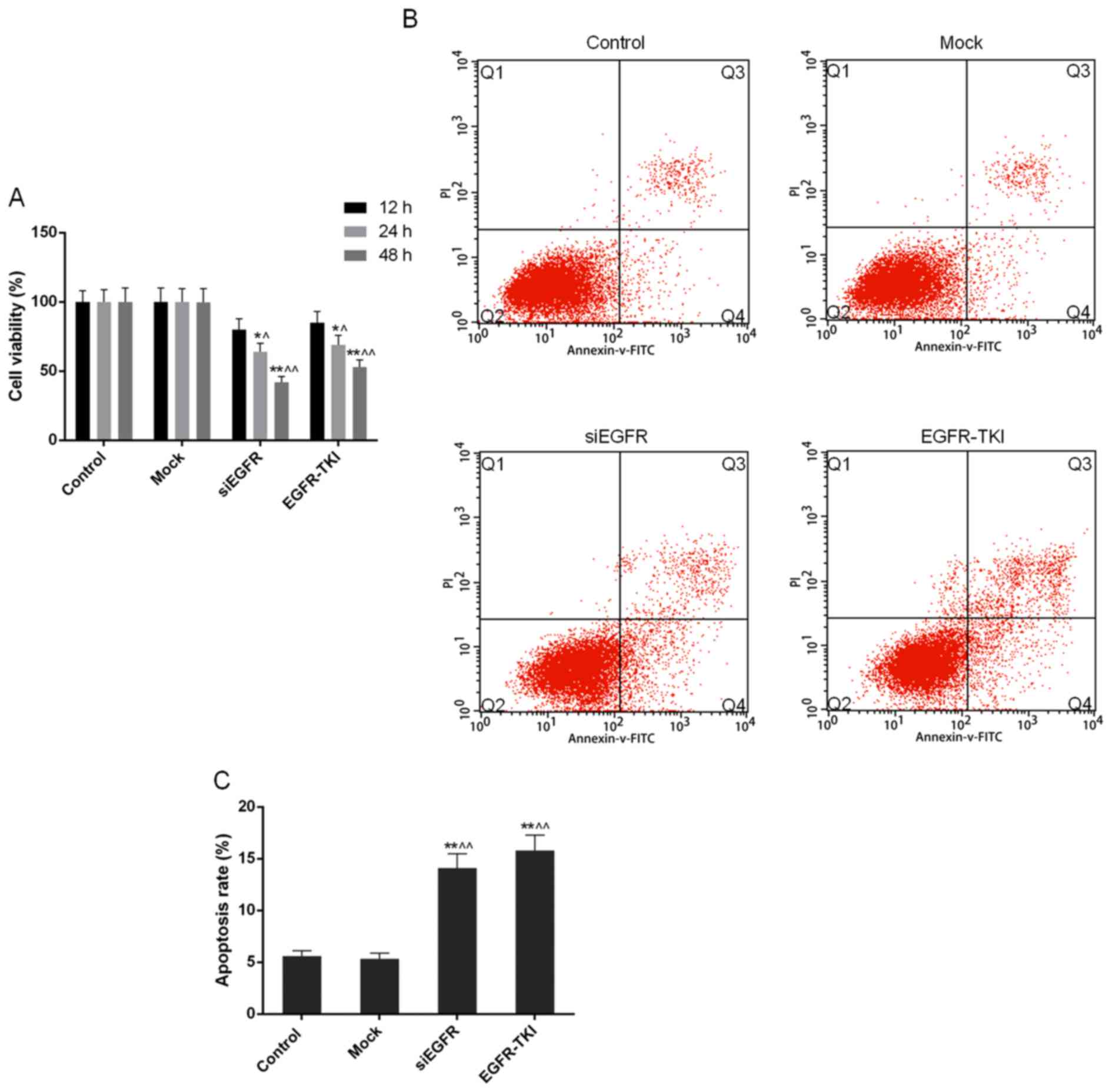
Cell viability is reduced when EGFR
expression is inhibited in NCI-H1299 cells
Compared with the control and mock groups, it was
observed through the CCK-8 assay that the inhibition of EGFR
expression in siEGFR- and EGFR-TKI-treated cells was able to
markedly decrease the cell viability and proliferation of NCI-H1299
cells; this effect occurred in a time-dependent manner. Cell
viability in the siEGFR and EGFR-TKI groups, following incubation
for 24 and 48 h, was significantly different compared with that in
the control and mock groups (P<0.05 or P<0.01; Fig. 3A).
Apoptosis rate of NCI-H1299 cells
increases when EGFR expression is inhibited
In the present study, the FCM results demonstrated
that the apoptosis rate of NCI-H1299 cells was significantly
increased to 14.09±1.38% in the siEGFR group and to 15.79±1.50% in
the EGFR-TKI group, from ~5% in the control and mock groups
(P<0.01; Fig. 3B and C).
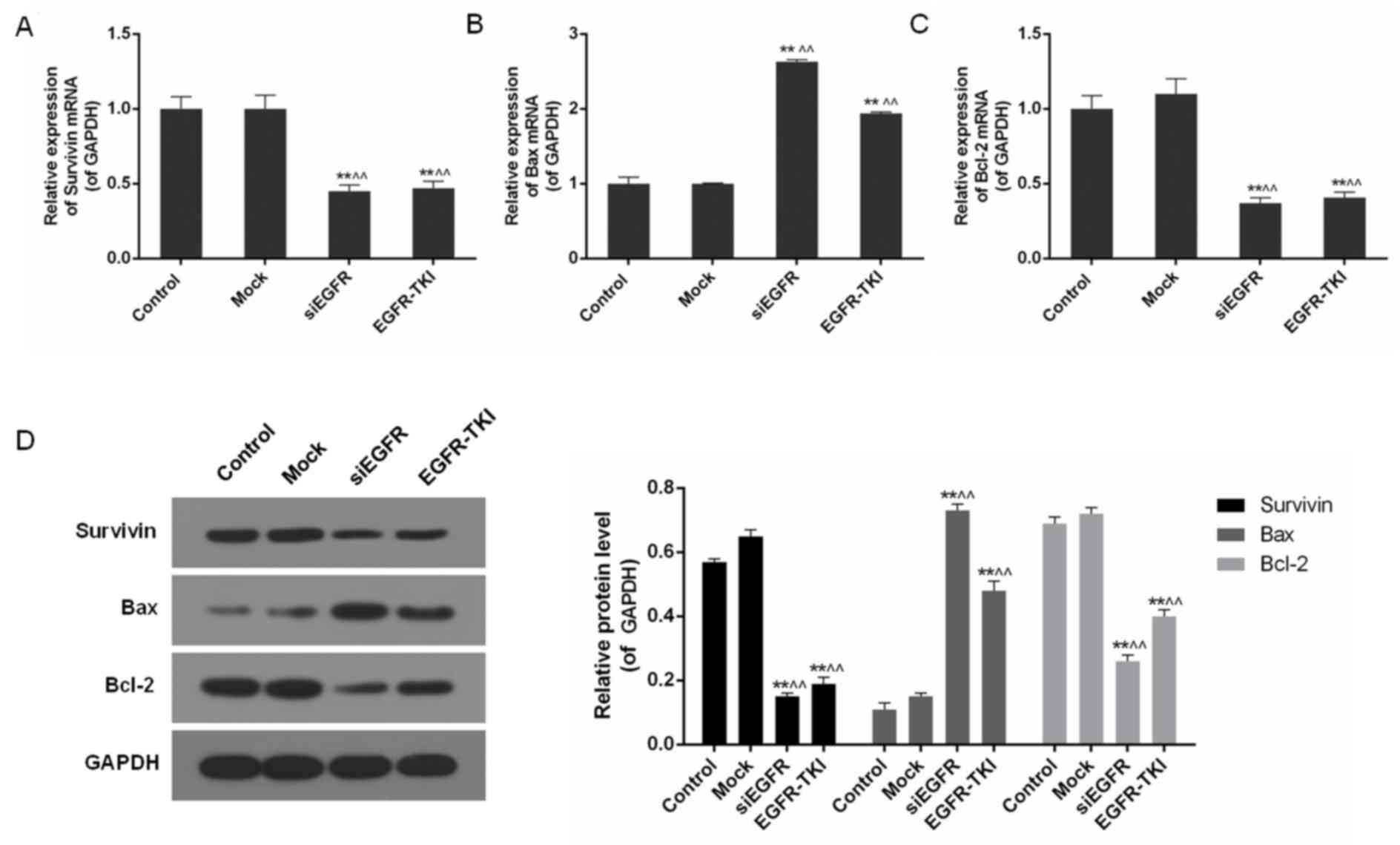
Inhibition of EGFR expression in
NCI-H1299 cells downregulates Survivin and Bcl-2
In addition to the inhibition of EGFR, mRNA and
protein levels of apoptosis-associated genes including Survivin,
Bax and Bcl-2, were differentially-expressed in the siEGFR and
EGFR-TKI groups, compared with the control and mock groups. In
EGFR-inhibited NCI-H1299 cells, expression of the anti-apoptotic
genes Survivin and Bcl-2 was significantly downregulated, whereas
expression of the pro-apoptotic gene Bax was significantly
increased, particularly in the siEGFR group (P<0.01; Fig. 4).
Inhibition of EGFR expression in
NCI-H1299 cells downregulates TGF-β, VEGF, IL-10 and COX-2
Inhibition of EGFR expression in the siEGFR and
EGFR-TKI groups was observed to dramatically reduce the expression
of immune cell cytokines, including TGF-β, VEGF, IL-10 and COX-2.
The ELISA detected a significant decrease in TGF-β and IL-10 in
EGFR-inhibited cells (P<0.01; Fig.
5A and B). It was additionally observed via qPCR and western
blotting that the mRNA and protein expression of TGF-β, VEGF, IL-10
and COX-2 was significantly downregulated compared with the control
and mock groups (P<0.01; Fig.
5C-G).
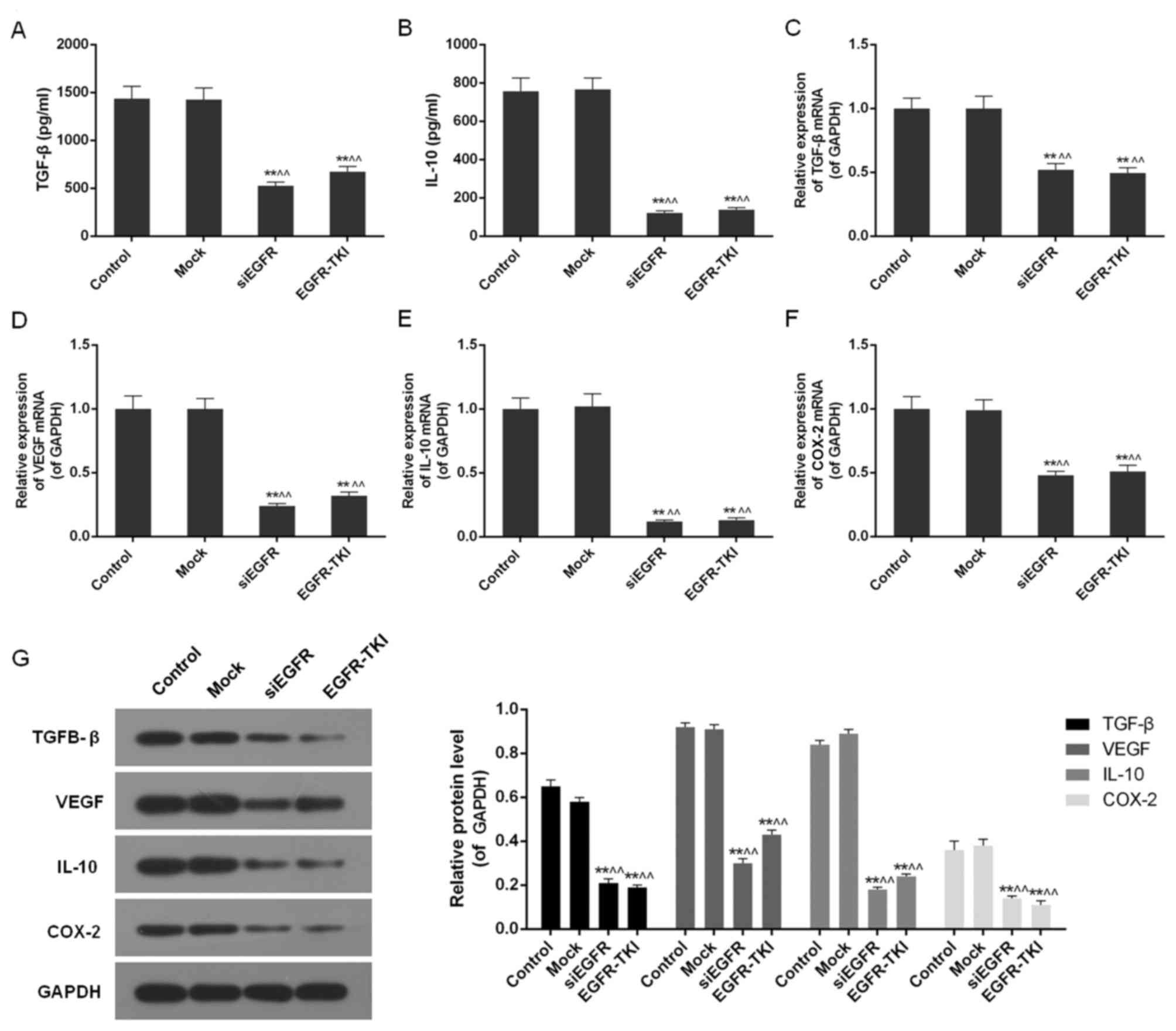 | Figure 5.TGF-β, VEGF, IL-10 and COX-2 content
in the control, mock, siEGFR and EGFR-TKI groups. (A) The TGF-β
content was decreased in EGFR-inhibited NCI-H1299 cells. (B) The
IL-10 content was decreased in EGFR-inhibited NCI-H1299 cells. (C)
The expression of TGF-β mRNA was downregulated in EGFR-inhibited
NCI-H1299 cells. (D) The expression of VEGF mRNA was downregulated
in EGFR-inhibited NCI-H1299 cells. (E) The expression of IL-10 mRNA
was downregulated in EGFR-inhibited NCI-H1299 cells. (F) The
expression of COX-2 mRNA was downregulated in EGFR-inhibited
NCI-H1299 cells. (G) Inhibition of EGFR expression decreased the
protein expression levels of TGF-β, VEGF, IL-10 and COX-2. Data are
presented as the mean ± standard deviation; n=3. **P<0.01 vs.
control group; ^^P<0.01 vs. mock group. EGFR,
epidermal growth factor receptor; si, small interfering; TKI,
tyrosine kinase inhibitor; TGF-β, transforming growth factor-β;
IL-10, interleukin-10; VEGF, vascular endothelial growth factor;
COX-2, cyclooxygenase-2. |
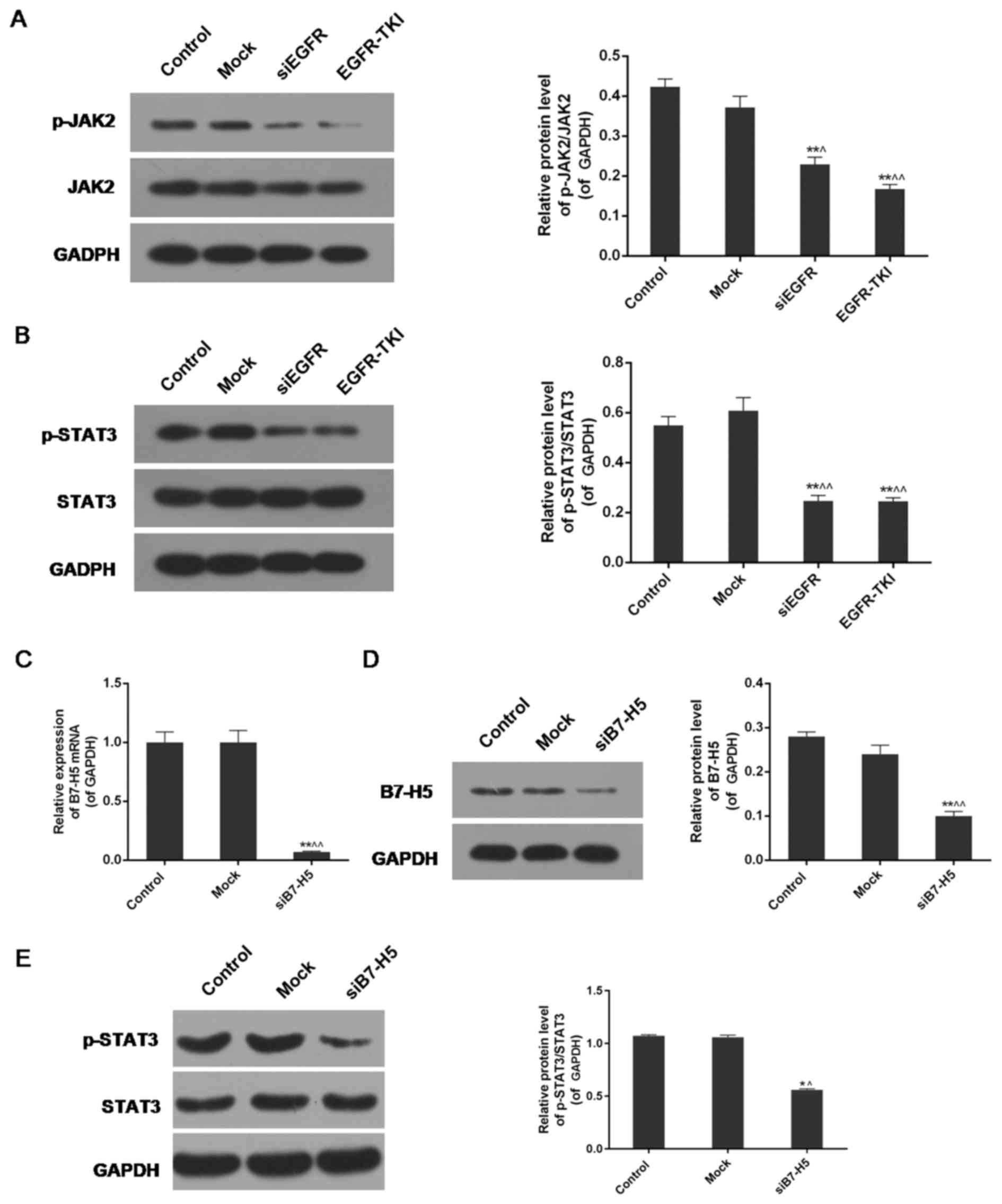
Inhibition of EGFR expression in
NCI-H1299 cells inhibits the phosphorylation of JAK2 and STAT3
The expression levels of phosphorylated (p-)JAK2,
JAK2, p-STAT3 and STAT3 were analysed via western blotting. The
results indicated a significant decrease in the protein expression
levels of p-JAK2 and p-STAT3 in the siEGFR and EGFR-TKI groups
compared with the control and mock groups. For JAK2 and STAT3,
there were no notable differences in their expression among the
four groups. The expression levels of p-JAK2/JAK2 and p-STAT3/STAT3
were decreased in EGFR-inhibited cells compared with normal
NCI-H1299 cells (P<0.01; Fig. 6A
and B).
B7-H5 silencing partially enhances the
STAT3 signalling pathway under EGFR-TKI conditions
B7-H5 gene silencing significantly downregulated the
expression of B7-H5 in NCI-H1299 cells, at the mRNA and protein
levels (P<0.01; (Fig. 6C and
D). The results of the western blotting revealed that weak
expression of B7-H5 under treatment with EGFR-TKI resulted in
reduced phosphorylation of STAT3. The p-STAT3/STAT3 ratio of
protein expression was decreased from 1.07±0.01 and 1.06±0.02 in
the control and mock groups, respectively, to 0.56±0.01 in the
siB7-H5 group (P<0.01; Fig.
6E). The decrease was less marked compared with the EGFR-TKI
group in Fig. 6B.
Discussion
In the present study, a negative correlation between
EGFR and B7-H5 expression was demonstrated in lung cancer tissues,
in addition to a number of lung cancer cell lines, including A549,
NCI-H1299, NCI-H1755 and 95-D. In lung cancer, B7-H5 was weakly
expressed, whereas EGFR expression was abundant; by contrast, high
expression of B7-H5 and low expression of EGFR was detected in
normal cells. In the present study, the inhibition of EGFR
expression by EGFR gene silencing and EGFR-TKI decreased the
viability of NCI-H1299 cells in a time-dependent manner;
furthermore, the apoptosis rate was increased ~2-fold compared with
normal NCI-H1299 cells, thus implying the inhibiting influence of
EGFR downregulation on tumour cells.
The anti-tumour action mechanism of the body
primarily involves cell-mediated immunity and humoral immunity.
Cell-mediated immunity is the principal mechanism of anti-tumour
action; however, during tumorigenesis, tumour cells are able to
evade monitoring and attack the immune system to continue mitosis
and growth (in a variety of ways). Effective activation and
functional mediation of T lymphocytes requires the synergistic
effect of dual signal control. The first signal is the complex
formed by an antigen peptide and the major histocompatibility
complex, which is identified by a T cell receptor, and the second
signal is the receptor combined with a B7-family antigen-presenting
cell or other co-stimulatory molecules. In the absence of the
second signal, although the first signal exists, the T lymphocyte
may not effectively proliferate to modulate the immune response,
resulting in tumour immune evasion (21,22).
A recent study in pancreatic cancer indicated that the loss of
B7-H5 may contribute to immune evasion (19). Zhu et al (18) reported that B7-H5 was
constitutively expressed in macrophages and may be induced on
dendritic cells, and additionally demonstrated that the B7-H5/CD28H
interaction co-stimulated human T cell growth and cytokine
production. It has been confirmed that alterations in tumour
immunity result from alterations in gene activity and gene products
in tumour cells. Tumorigenesis and metastasis are closely
associated with the immunity of the human body and the internal
environment of tumour cells (23,24).
Recent studies have indicated that there may exist cells that have
different functions against normal human tissues surrounding the
microenvironment of tumour cells (25–27).
Without an effective anti-tumour immune response, these cells may
induce tumorigenesis and metastasis via inhibition of the immune
response of local tumours. Immune cells in the tumour
microenvironment are immunological effector cells that serve an
important role in tumour recognition and immune defence to attack
tumour cells (28–30). However, it was demonstrated that
tumour cells are able to secrete a number of inhibitors, modify
antigens on the cell surface, or negatively regulate gene
expression so as to escape immune cell recognition and destruction.
This may eventually promote the formation, development and
metastasis of tumour cells (31).
In the present study, a marked downregulation of the expression of
the anti-apoptotic proteins Survivin and Bcl-2, and an upregulation
of the pro-apoptotic protein Bax, was detected in EGFR-inhibited
groups. The decreasing levels of Bcl-2/Bax promoted apoptosis, and
resulted in increased apoptosis rates in the siEGFR and EGFR-TKI
groups compared with normal cells.
A number of lines of evidence have indicated that
numerous cellular factors, including TGF-β, VEGF, IL-10 and COX-2,
are involved in immunosuppression (32–34).
As a multifunctional cellular factor, TGF-β has an inhibitory
effect on the immune system, and the immunosuppression activated by
TGF-β in the microenvironment of tumours is considered to be one of
the most important elements for cancer cells to evade immune
surveillance. TGF-β inhibits the multiplication of T lymphocytes by
suppressing the secretion of IL-2, which is one of the key factors
in stimulating the proliferation and differentiation of T
lymphocytes (35). IL-10 is
primarily secreted by Th2 cells, mononuclear P macrophages, B
lymphocytes, and keratinocytes under normal conditions; however, an
unusual increase in its expression occurs during tumorigenesis. In
the body of a cancer patient, the Th2 cell subset, the primary
source of IL-10, increases abnormally. Furthermore, T regulatory
cells, another source of IL-10, secrete high levels of IL-10 and
low levels of IL-2; additionally, tumours themselves may generate
IL-10, leading to a rise in the expression of IL-10 (36). Previous studies demonstrated that
VEGF has a strong inhibitory effect on the differentiation of
hematopoietic stem cells (CD34+) to dendritic cells, so
as to further influence the proliferation of specific cytotoxic T
lymphocytes, thus protecting tumours from the monitoring of the
immune system (37,38). The membrane-conjugated protein
COX-2 is able to induce apoptosis in immune cells by activating
caspase-3, inhibit the antitumor activity of T lymphocytes and NK
cells by inducing the production of prostaglandin E2, inhibit the
generation of tumour necrosis factor and restrain antibody
synthesis by B lymphocytes, consequently reducing the ability of
the body to combat tumours while promoting tumour angiopoeisis
(39). In the present study,
inhibition of EGFR expression and high expression of B7-H5
significantly decreased expression of the four previously mentioned
cellular factors, including TGF-β, VEGF, IL-10 and COX-2 in
NCI-H1299 cells. Weak expression of these four factors suppresses
tumorigenesis and development, and decreases the capability of a
cell to escape immune surveillance.
STAT is a regulatory factor of signal transduction.
STAT3 is the key signalling cascade compound in the STAT family,
which is involved in the response of various cytokines, including
the JAK/STAT signalling pathway. STAT3 is phosphorylated by JAK at
a tyrosine residue, and subsequently transferred to the cell
nucleus to activate its target gene. The JAK/STAT signalling
pathway is associated with cell proliferation, differentiation and
apoptosis; however, continuous activation of the pathway leads to
abnormal cell proliferation and malignant transformation (40,41).
p-STAT3, the activated form of STAT3, may act on specific DNA
binding sites in the cell nucleus to directly or indirectly
upregulate the expression of anti-apoptotic genes, including Bcl-2,
Bcl-2-like protein 1 and cyclin D1/D2 to regulate the proliferation
and apoptosis of cells (42,43).
To date, high expression of activated p-STAT3 has been detected in
a number of types of cancer, including gastric cancer, colon
cancer, prostate cancer and breast cancer (44–46).
Abnormal activation of the JAK/STAT signalling pathway may induce
growth disorders, and promote malignant cell transformation and
tumorigenesis (47). Compared with
normal tissues and cells, the activation of STAT components,
particularly STAT3, exists in the majority of human cancer tissues
and cells to mediate cell proliferation, apoptosis, differentiation
and other physiological activities (46). The present study demonstrated that
in NCI-H1299 cells, the JAK2/STAT3 signalling pathway is activated,
and inhibiting the expression of EGFR markedly reduced the
phosphorylation of JAK2 and STAT3 to restrain its activation. The
results indicated the important association between EGFR expression
levels and activation of the JAK2/STAT3 signalling pathway. Due to
the negative correlation between EGFR and B7-H5, the expression of
B7-H5 was markedly increased while that of EGFR was inhibited,
highlighting the possible role of B7-H5 in EGFR-regulated tumour
immune escape. However, when EGFR and B7-H5 were inhibited, STAT3
activation remained suppressed, which may have resulted from the
fact that the effects of EGFR on regulating the JAK2/STAT3 pathway
are attributed to complex factors and mechanisms, and B7-H5 is only
one of the influential elements. Apart from the significant
association between EGFR and certain members of the B7 family,
including B7-H5 and B7-H3, Zhang et al (48) reported that the expression of
programmed death-ligand 1 may be regulated by EGFR activation in
esophageal squamous cell carcinoma, and revealed the important role
of EGFR-mediated immune escape. Furthermore, in triple-negative
breast cancer cells, Lim et al (49) recently illustrated that EGFR
signaling promoted tumor progression and immune evasion by
elevating the level of aerobic glycolysis. The complete mechanism
involved in EGFR-targeted therapy for immune escape requires
further study.
The results of the present study demonstrated a
notable negative correlation between EGFR and B7-H5 expression
levels, in lung cancer tissues and cancer cell lines. Inhibiting
the expression of EGFR by gene silencing and EGFR inhibition
significantly reduced the cell viability and increased the
apoptosis of NCI-H1299 cells, by upregulating the expression of
Survivin and Bcl-2. The protein expression levels of TGF-β, VEGF,
IL-10 and COX-2 were decreased by weak activation of the JAK2/STAT3
signalling pathway. EGFR may be involved in immune evasion,
possibly through regulation of B7-H5 expression in NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Nature Fund (grant no. 2017C33178).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZD and GZ conceived and designed the study. WX
analyzed and interpreted the patient data regarding the use of
cancer tissues and cell lines. LZ performed the cell experiments.
This report has been approved by all authors.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First People's Hospital of Huzhou.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nand M, Maiti P, Pant R, Kumari M, Chandra
S and Pande V: Virtual screening of natural compounds as inhibitors
of EGFR 696–1022 T790M associated with non-small cell lung cancer.
Bioinformation. 12:311–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Remon J, Besse B and Soria JC: Successes
and failures: What did we learn from recent first-line treatment
immunotherapy trials in non-small cell lung cancer? BMC Med.
15:552017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirsch FR, Varella-Garcia M, Bunn PA Jr,
Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C and Franklin WA:
Epidermal growth factor receptor in non-small-cell lung carcinomas:
Correlation between gene copy number and protein expression and
impact on prognosis. J Clin Oncol. 21:3798–3807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki S, Dobashi Y, Sakurai H, Nishikawa
K, Hanawa M and Ooi A: Protein overexpression and gene
amplification of epidermal growth factor receptor in nonsmall cell
lung carcinomas. An immunohistochemical and fluorescence in situ
hybridization study. Cancer. 103:1265–1273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woodburn JR: The epidermal growth factor
receptor and its inhibition in cancer therapy. Pharmacol Ther.
82:241–250. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wakeling AE, Guy SP, Woodburn JR, Ashton
SE, Curry BJ, Barker AJ and Gibson KH: ZD1839 (Iressa): An orally
active inhibitor of epidermal growth factor signaling with
potential for cancer therapy. Cancer Res. 62:5749–5754.
2002.PubMed/NCBI
|
|
9
|
Inoue A, Suzuki T, Fukuhara T, Maemondo M,
Kimura Y, Morikawa N, Watanabe H, Saijo Y and Nukiwa T: Prospective
phase II study of gefitinib for chemotherapy-naive patients with
advanced non-small-cell lung cancer with epidermal growth factor
receptor gene mutations. J Clin Oncol. 24:3340–3346. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sonobe M, Manabe T, Wada H and Tanaka F:
Mutations in the epidermal growth factor receptor gene are linked
to smoking-independent, lung adenocarcinoma. Br J Cancer.
93:355–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Costa DB, Schumer ST, Tenen DG and
Kobayashi S: Differential responses to erlotinib in epidermal
growth factor receptor (EGFR)-mutated lung cancers with acquired
resistance to gefitinib carrying the L747S or T790M secondary
mutations. J Clin Oncol. 26:1184–1186. 2008. View Article : Google Scholar
|
|
12
|
Rolfo C, Caglevic C, Santarpia M, Araujo
A, Giovannetti E, Gallardo CD, Pauwels P and Mahave M:
Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv
Exp Med Biol. 995:97–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Jia Y, Zhao S, Zhang X, Wang X,
Han X, Wang Y, Ma M, Shi J and Liu L: BIN1 reverses PD-L1-mediated
immune escape by inactivating the c-MYC and EGFR/MAPK signaling
pathways in non-small cell lung cancer. Oncogene. 36:6235–6243.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lenschow DJ, Walunas TL and Bluestone JA:
CD28/B7 system of T cell costimulation. Annu Rev Immunol.
14:233–258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong H and Chen L: B7-H1 pathway and its
role in the evasion of tumor immunity. J Mol Med (Berl).
81:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kryczek I, Wei S, Zhu G, Myers L, Mottram
P, Cheng P, Chen L, Coukos G and Zou W: Relationship between B7-H4,
regulatory T cells, and patient outcome in human ovarian carcinoma.
Cancer Res. 67:8900–8905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inamura K, Yokouchi Y, Kobayashi M,
Sakakibara R, Ninomiya H, Subat S, Nagano H, Nomura K, Okumura S,
Shibutani T and Ishikawa Y: Tumor B7-H3 (CD276) expression and
smoking history in relation to lung adenocarcinoma prognosis. Lung
Cancer. 103:44–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Y, Yao S, Iliopoulou BP, Han X,
Augustine MM, Xu H, Phennicie RT, Flies SJ, Broadwater M and Ruff
Wetal: B7-H5 costimulates human T cells via CD28H. Nat Commun.
4:20432013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Byers JT, Paniccia A, Kaplan J, Koenig M,
Kahn N, Wilson L, Chen L, Schulick RD, Edil BH and Zhu Y:
Expression of the Novel Costimulatory Molecule B7-H5 in Pancreatic
Cancer. Ann Surg Oncol. 22 Suppl 3:S1574–S1579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woodland DL and Kohlmeier JE: Migration,
maintenance and recall of memory T cells in peripheral tissues. Nat
Rev Immunol. 9:153–161. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katayama A, Ogino T, Bandoh N, Nonaka S
and Harabuchi Y: Expression of CXCR4 and its down-regulation by
IFN-gamma in head and neck squamous cell carcinoma. Clin Cancer
Res. 11:2937–2946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghebeh H, Mohammed S, Al-Omair A, Qattan
A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah
A, et al: The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is
expressed in breast cancer patients with infiltrating ductal
carcinoma: Correlation with important high-risk prognostic factors.
Neoplasia. 8:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:pp. 3360–3365. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y,
Chen Y, Shi Q, Yu G and Zhang X: PD-L1 expression analysis in
gastric carcinoma tissue and blocking of tumor-associated PD-L1
signaling by two functional monoclonal antibodies. Tissue Antigens.
69:19–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghebeh H, Barhoush E, Tulbah A, Elkum N,
Al-Tweigeri T and Dermime S: FOXP3+ Tregs and B7-H1+/PD-1+ T
lymphocytes co-infiltrate the tumor tissues of high-risk breast
cancer patients: Implication for immunotherapy. BMC cancer.
8:572008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pettit SJ, Ali S, O'Flaherty E, Griffiths
TR, Neal DE and Kirby JA: Bladder cancer immunogenicity: Expression
of CD80 and CD86 is insufficient to allow primary CD4+ T cell
activation in vitro. Clin Exp Immunol. 116:48–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim S, Buchlis G, Fridlender ZG, Sun J,
Kapoor V, Cheng G, Haas A, Cheung HK, Zhang X and Corbley Metal:
Systemic blockade of transforming growth factor-beta signaling
augments the efficacy of immunogene therapy. Cancer Res.
68:10247–10256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaspar NJ, Li L, Kapoun AM, Medicherla S,
Reddy M, Li G, O'Young G, Quon D, Henson M and Damm D Letal:
Inhibition of transforming growth factor beta signaling reduces
pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol.
72:152–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erkanli S, Bolat F, Kayaselcuk F, Demirhan
B and Kuscu E: COX-2 and survivin are overexpressed and positively
correlated in endometrial carcinoma. Gynecol Oncol. 104:320–325.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li MO, Sanjabi S and Flavell RA:
Transforming growth factor-beta controls development, homeostasis,
and tolerance of T cells by regulatory T cell-dependent and
-independent mechanisms. Immunity. 25:455–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoechst B, Ormandy LA, Ballmaier M, Lehner
F, Kruger C, Manns MP, Greten TF and Korangy F: A new population of
myeloid-derived suppressor cells in hepatocellular carcinoma
patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology.
135:234–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kopfstein L, Veikkola T, Djonov VG,
Baeriswyl V, Schomber T, Strittmatter K, Stacker SA, Achen MG,
Alitalo K and Christofori G: Distinct roles of vascular endothelial
growth factor-D in lymphangiogenesis and metastasis. Am J Pathol.
170:1348–1361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kamstock D, Elmslie R, Thamm D and Dow S:
Evaluation of a xenogeneic VEGF vaccine in dogs with soft tissue
sarcoma. Cancer Immunol Immunother. 56:1299–1309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu XH, Kirschenbaum A, Yu K, Yao S and
Levine AC: Cyclooxygenase-2 suppresses hypoxia-induced apoptosis
via a combination of direct and indirect inhibition of p53 activity
in a human prostate cancer cell line. J Biol Chem. 280:3817–3823.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bahrenberg G, Behrmann I, Barthel A,
Hekerman P, Heinrich PC, Joost HG and Becker W: Identification of
the critical sequence elements in the cytoplasmic domain of leptin
receptor isoforms required for Janus kinase/signal transducer and
activator of transcription activation by receptor heterodimers. Mol
Endocrinol. 16:859–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bjorbaek C, Uotani S, da Silva B and Flier
JS: Divergent signaling capacities of the long and short isoforms
of the leptin receptor. J Biol Chem. 272:32686–32695. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T and Uenoyama
Yetal: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Du XL, Yang H, Liu SG, Luo ML, Hao JJ,
Zhang Y, Lin DC, Xu X, Cai Y, Zhan QM and Wang MR: Calreticulin
promotes cell motility and enhances resistance to anoikis through
STAT3-CTTN-Akt pathway in esophageal squamous cell carcinoma.
Oncogene. 28:3714–3722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Zhang J, Wang L, Wei H and Tian
Z: Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human
lung cancer in xenograft mice. BMC Cancer. 7:1492007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang WT, Yang SF, Wu CC, Chen WT, Huang
YC, Su YC and Chai CY: Expression of signal transducer and
activator of transcription 3 and suppressor of cytokine signaling 3
in urothelial carcinoma. Kaohsiung J Med Sci. 25:640–646. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yakata Y, Nakayama T, Yoshizaki A, Kusaba
T, Inoue K and Sekine I: Expression of p-STAT3 in human gastric
carcinoma: Significant correlation in tumour invasion and
prognosis. Int J Oncol. 30:437–442. 2007.PubMed/NCBI
|
|
47
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: Clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang W, Pang Q, Yan C, Wang Q, Yang J, Yu
S, Liu X, Yuan Z, Wang P and Xiao Z: Induction of PD-L1 expression
by epidermal growth factor receptor-mediated signaling in
esophageal squamous cell carcinoma. Onco Targets Ther. 10:763–771.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lim SO, Li CW, Xia W, Lee HH, Chang SS,
Shen J, Hsu JL, Raftery D, Djukovic D and Gu Hetal: EGFR signaling
enhances aerobic glycolysis in triple-negative breast cancer cells
to promote tumor growth and immune escape. Cancer Res.
76:1284–1296. 2016. View Article : Google Scholar : PubMed/NCBI
|