Introduction
Gastric cancer (GC) is the second most common cause
of cancer-associated mortality in the world, and the incidence GC
is highest in East Asia, Eastern Europe and parts of Latin America
(1,2). However, the precise mechanisms
underlying gastric carcinogenesis are not yet completely understood
(3). Therefore, it is important to
identify the regulatory mechanisms and signaling pathways involved
in GC.
The achaete-scute homolog 2 (ASCL2) gene is a member
of the basic helix-loop-helix family of transcription factors,
which are downstream target of Wnt signaling and initiate
transcription by binding to the E-box (4). ASCL2 is involved in the determination
of neuronal precursors in the peripheral and central nervous
systems (5), and it is also a
cancer stem cell marker (6);
certain reports have revealed that ASCL2 promotes cell growth and
migration in colon cancer (7,8).
However, the function of ASCL2 in the metastasis of GC is poorly
understood.
MicroRNAs (miRNAs/miRs) are a class of endogenous
small non-coding RNAs with maximum length of 25 bp (8,9). At
present, >1,000 types of miRNA have been identified in the human
body (10). miRNAs are involved in
a number of physiological and pathological processes, including
cell proliferation, differentiation, apoptosis, migration,
invasion, organ development and tumor development (11,12).
miRNAs primarily affect the post-transcriptional level of gene
coding, and may inhibit or promote the expression of target genes.
In tumorigenesis, miRNAs may act as tumor suppressor genes or
carcinogenic genes (13). miR223
has been demonstrated to reverse epithelial-mesenchymal transition
(EMT), and to inhibit the migration and invasion of melanoma cells
by inducing the expression of E-cadherin protein (14). EMT refers to the process of
transition between epithelial cells and mesenchymal cells under
certain physiological or pathological conditions, which contributes
to the occurrence and development of tumors, particularly invasion
and metastasis (8). EMT has been
demonstrated to serve a critical role in gastric tumor migration
(15); therefore, it is important
to reveal the molecular mechanism underlying EMT, and its effect on
the invasion and metastasis of gastric tumors.
In the present clinical study, it was observed that
ASCL2 was upregulated in metastatic tumors compared with primary
tumors of patients. The present study investigated the effect of
ASCL2 on the invasion and migration of GC cells, and the underlying
mechanism.
Materials and methods
Sample collection
A total of 32 cases of gastric tumor and their
relevant metastases were obtained from patients, following the
provision of written informed consent. The patients were 41–91
years old and the male/female radio was 1.47. The samples were
collected between March 2009 and June 2014. The procedure was
approved by the ethical committee of Putuo Hospital Affiliated to
Shanghai University of Traditional Chinese Medicine (Shanghai,
China).
Cell culture
Human gastric carcinoma cell lines MKN-1 and SNU16
were obtained from the American Type Culture Collection (Manassas,
VA, USA) and were maintained in RPMI-1640 medium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) containing 10% fetal bovine serum
(FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C
and 5% CO2, with the medium changed every two days.
Cells were passaged at 80% confluence and seeded at 30% confluence
for maintenance of optimal proliferating conditions.
Immunohistochemistry
Immunohistochemical analysis was performed to
evaluate the ASCL2 expression in the adjacent tissues (3 cm next to
the tumor), primary gastric tumor or metastases. Following
deparaffinization and rehydration with ddH2O at room
temperature, the 10% formalin-fixed paraffin-embedded tumor tissue
sections (4 µm) were incubated with 3% hydrogen peroxide in
methanol to quench endogenous peroxidase activity. The sections
were blocked for 30 min with 1% bovine serum albumin (Sangon
Biotech Co., Ltd., Shanghai, China) and incubated with the primary
antibodies ASCL2 (ab116699; 1:100; Abcam, Cambridge, UK) at 4°C
overnight. The sections were washed with PBS and incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody
(ab205718; 1:100; Abcam) at room temperature for 1 h. The products
were visualized using a diaminobenzidine staining kit (Tiangen
Biotech Co., Ltd., Beijing, China) at room temperature for 2 min
and counterstained with hematoxylin at room temperature for 5 min.
The images were obtained by microscope (IX71; Olympus Corporation,
Tokyo, Japan).
ASCL2 overexpression assay
Lentivirus particles expressing ASCL2 were produced
by Shanghai GeneChem Co., Ltd (Shanghai, China). MKN-1 and SNU16
cells (5×104 each) were inoculated in 12-well plates and
incubated in complete medium with 1×108 TU/ml of
lentivirus. At 24 h following seeding, the medium was aspirated and
replaced with 1 ml fresh medium overnight. In order to obtain a
stable transfected cell line, the lentivirus-infected cells were
screened using culture medium containing 2 µg/ml puromycin, and the
culture medium was changed every three days.
Cell transfection
Hsa-miR-223 mimic (UGUCAGUUUGUCAAAUACCCC) and
negative control mimic (UCACAACCUCCUAGAAAGAGUAGA) were purchased
from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The cells at
60% confluence (determined by blood counting chamber) were seeded
in 6-well plates and were transfected with hsa-miR-223 or negative
control mimic at a final concentration of 100 nM using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Cells were collected 48 h
post-transfection for reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) analysis, western blotting or Transwell
assays.
Western blot analysis
Cell lysates or the homogenized tissues from tumor
xenografts were dissolved in RIPA lysis buffer (Beyotime Institute
of Biotechnology, Haimen, China), protein concentration was
determined by BCA method, and lysates were separated by 10%
SDS-PAGE and transferred to a nitrocellulose membrane, and GAPDH
was used as a control. Following blocking with 5% dried skimmed
milk for 2 h in room temperature, the membrane was probed with
specific primary antibodies ASCL2 (ab116699), Zeb-1 (ab203829),
E-cadherin (ab76055), Integrin (ab133557), Twist (ab50581),
Vimentin (ab8978), MMP-2 (ab92536), MMP-9 (ab73734) and GAPDH
(ab8245; all from Abcam and diluted at 1:1,000) overnight at 4°C,
followed by incubation with HRP-conjugated secondary IgG (H+L)
antibody (ab205718; 1:1,000; Abcam) at room temperature for 1 h.
The blots were processed with Immobilon™ western
chemiluminescent HRP substrate (EMD Millipore, Billerica, MA, USA)
and analyzed with a gel imaging analysis system and Image Lab
version 2.0.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Migration assay
MKN-1 and SNU16 cells and their transfectants, at
90–100% confluence in 6-well plates, were cultured overnight in
serum-free medium. The medium was replaced with PBS, and the
monolayers were wounded mechanically using a 10 µl Axygen pipette
tip (Axygen Scientific, Inc., Union City, CA, USA). Following
wounding, cells were rinsed twice with sterilized PBS and incubated
in RPMI-1640 medium containing 10% FBS at 37°C, under 5%
CO2, and digital images of the healing conditions were
obtained by microscope (IX71; Olympus Corporation, Tokyo, Japan) at
0 and 16 h.
Transwell assay
The invasive capacity of the two types of GC cells
overexpressing ASCL2 was evaluated by Transwell assay in a 24-well
plate. Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) was
diluted with 4°C pre-cooled serum-free medium to a final
concentration of 1 mg/ml. The upper chambers were coated with 100
µl diluted Matrigel. For the stably-transfected cells,
5×103/200 µl cells were seeded in the upper chambers and
fed with serum-free medium, and 500 µl medium with 20% FBS was
added to the lower chambers. The cells were allowed to invade the
Matrigel matrix for 8–24 h. The cells on the upper chamber were
scraped off with a cotton swab and the transmigrated cells were
fixed with 4% formaldehyde for 1 h at room temperature, washed with
PBS 3 times and stained with 0.5% crystal violet for 15 min,
followed by washing with PBS for 3 times, and then counted in 5
randomly selected microscopic fields (magnification, ×200). The
cell numbers were counted by microscope, and all of the experiments
were performed in triplicate.
RT-qPCR analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. First strand cDNA was synthesized using
PrimeScript™ RT enzyme mix I, oligo dT primers and
random hexamers (Takara Bio, Inc., Otsu, Japan). To determine the
fold changes in each gene and miR, qPCR was performed using the
first strand cDNA and the SYBR premix ExTaq™ Green II
(Takara Bio, Inc.). The primer sequences were as follows: ASCL2
forward, AACTTGAGCTGCTGGAGGGACA and reverse,
TCTTGGCCAGCATGGAAAACTC; β-actin forward, CTGGAACGGTGAAGGTGACA,
reverse, CGGCCACATTGTGAACTTTG; hsa-miR-223-3p mimic forward,
UGUCAGUUUGUCAAAUACCCCA and reverse, UGGGGUAUUUGACAAACUGACA;
hsa-miR-141-3p, Forward, TAACACTGTCTGGTAAAGATGG, Reverse,
CAGTGCGTGTCGTGGAGT; hsa-miR-137, Forward, TTATTGCTTAAGAATACGCGTAG,
Reverse, CAGTGCGTGTCGTGGAGT; miRNA mimic negative control forward,
UUUGUACUACACAAAAGUACUG and reverse, CAGUACUUUUGUGUAGUACAAA. The
Primers of the other miRNAs were purchased from Guangzhou RiboBio
Co., Ltd. Reaction and signal detection were measured using a qPCR
system (Bio-Rad Laboratories, Inc.). The obtained cDNA was
amplified using following procedure: 94°C (5 min), then 30 cycles
at 94°C (30 sec), 59°C (30 sec) and 72°C (30 sec). Expression
levels were calculated as the relative expression ratio using the
2−ΔΔCq method with β-actin as a reference. The qPCR was
performed in triplicate independently.
Prediction and detection of
EMT-associated protein binding to miR223
EMT-associated proteins binding to miR223 were
predicted using TargetScan (www.targetscan.org). A total of 412 transcripts were
predicted to be the targets of miR223; the cumulative weighted
context ++ score of zinc finger E-box-binding homeobox 1 (Zeb-1)
was 0.21, total context ++ score was −0.21, and the aggregate
probability of conserved targeting was 0.47. The present study
examined all the potential targets which may be involved in EMT,
migration and invasion by western blotting.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assays were performed using a ChIP assay kit
(Upstate Biotechnology, Inc., Lake Placid, NY, USA), according to
the manufacturer's protocol. Soluble chromatin was prepared from
cells overexpressing ASCL-2 and control cells. Chromatin was
immunoprecipitated with an antibody against ASCL2 (ab116699, Abcam,
UK), which was diluted at 1:100 at 4°C. The final DNA extracts were
amplified by qPCR using primer pairs in the human pre-miR223
promoter according to the protocol described above. Pre-miR223
primer sequences: Forward primer: 5′-CCACGCTCCGTGTATTTGAC-3′.
Reverse primer: 5′-CCGCACTTGGGGTATTTGAC-3′.
Luciferase reporter assay
Fragments of the pre-miR223 promoter 5′-flanking
sequence or the 3′ untranslated region (UTR) sequences of Zeb-1
were amplified using PCR and cloned into the luciferase reporter
vector pGL3-Basic (Promega Corporation, Madison, WI, USA). The day
prior to transfection, the cells were plated in 24-well plates at a
density of 5×104 cells/well. The pre-miR223
promoter-luciferase constructs or the 3′ UTR luciferase constructs
of Zeb-1 were transfected into the cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). To normalize for transfection efficiency, the
cells were simultaneously co-transfected with a pRLTK vector
expressing the Renilla luciferase enzyme (pRL; Promega
Corporation). The cells were harvested following 24 h, and the
luciferase activity was measured using the Dual Luciferase Reporter
Assay System (Promega Corporation) with a single sample
luminometer. In a similar way, the cells were transfected with the
pRL-TK vector. Pre-miR223 activity is presented as the percentage
of pGL3-control activity.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three different experiments. The Student's t-test was used to
analyze the comparisons between two groups, and analysis of
variance was used to analyze the comparisons between multiple
groups and followed by Newman-Keuls post hoc comparison test with
differences. The statistical significance of the results was
evaluated using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of ASCL2 is highest in
metastases, among adjacent normal tissues, primary gastric tumors
and gastric metastases
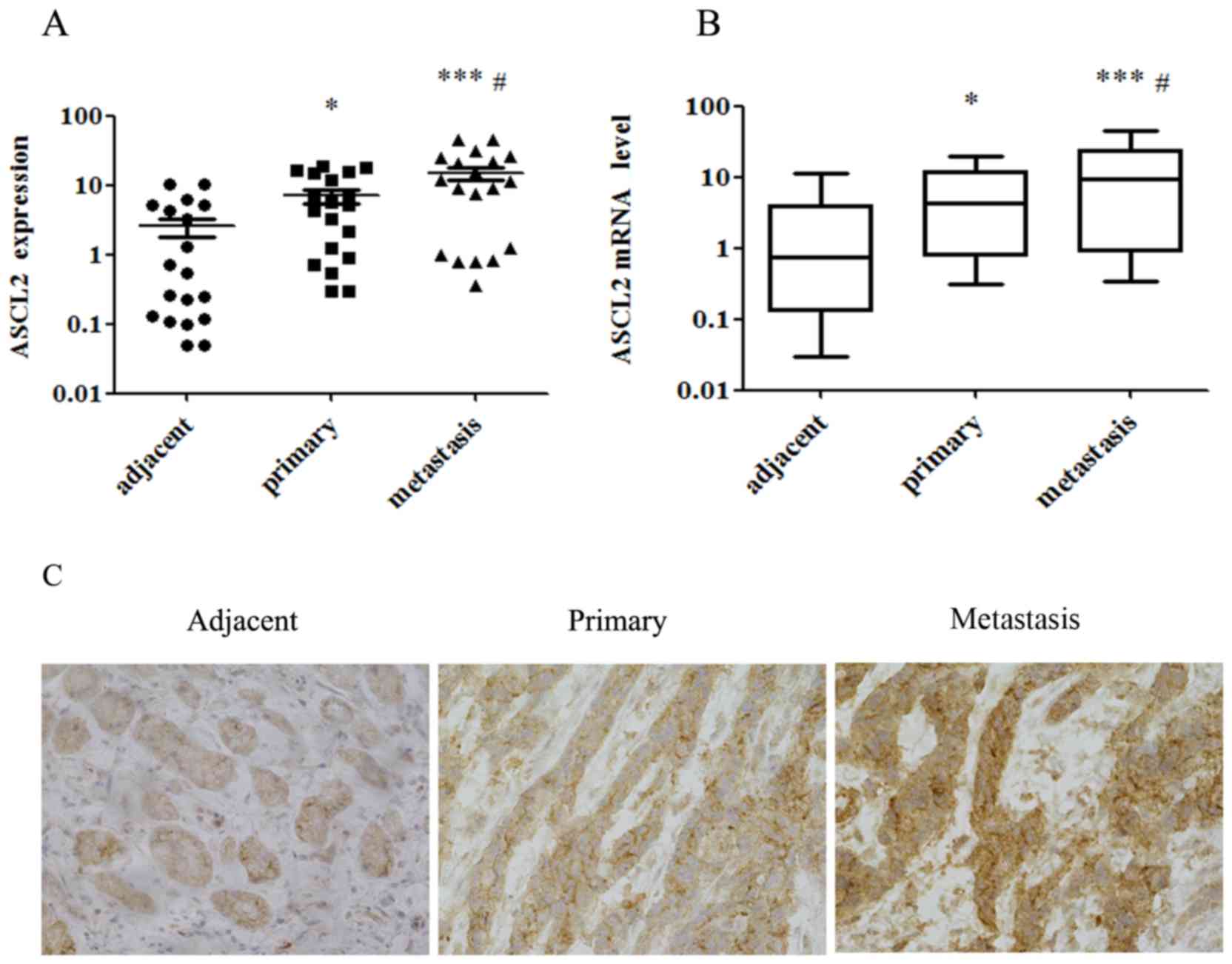
In order to study the expression level of ASCL2 in
different parts of GC, protein and RNA were extracted from 32 cases
of GC adjacent normal tissues, the primary gastric tumor and
metastatic cancer tissue, and the expression of ASCL2 was analyzed
using western blotting, qPCR and immunohistochemistry (Fig. 1). The expression of ASCL2 protein
in metastatic tissues was highest, and was at its lowest in normal
tissues (Fig. 1A and C), and the
mRNA level of ASCL2 in metastatic tissues of GC was significantly
higher compared with that in normal tissues (Fig. 1B), which was consistent with the
results of the western blotting and immunohistochemistry. It was
suggested that the high expression of ASCL2 may be associated with
the metastasis of GC cells in metastatic GC tissues.
ASCL2 expression contributes to cell
migration and invasion in MKN-1 and SNU16 cells
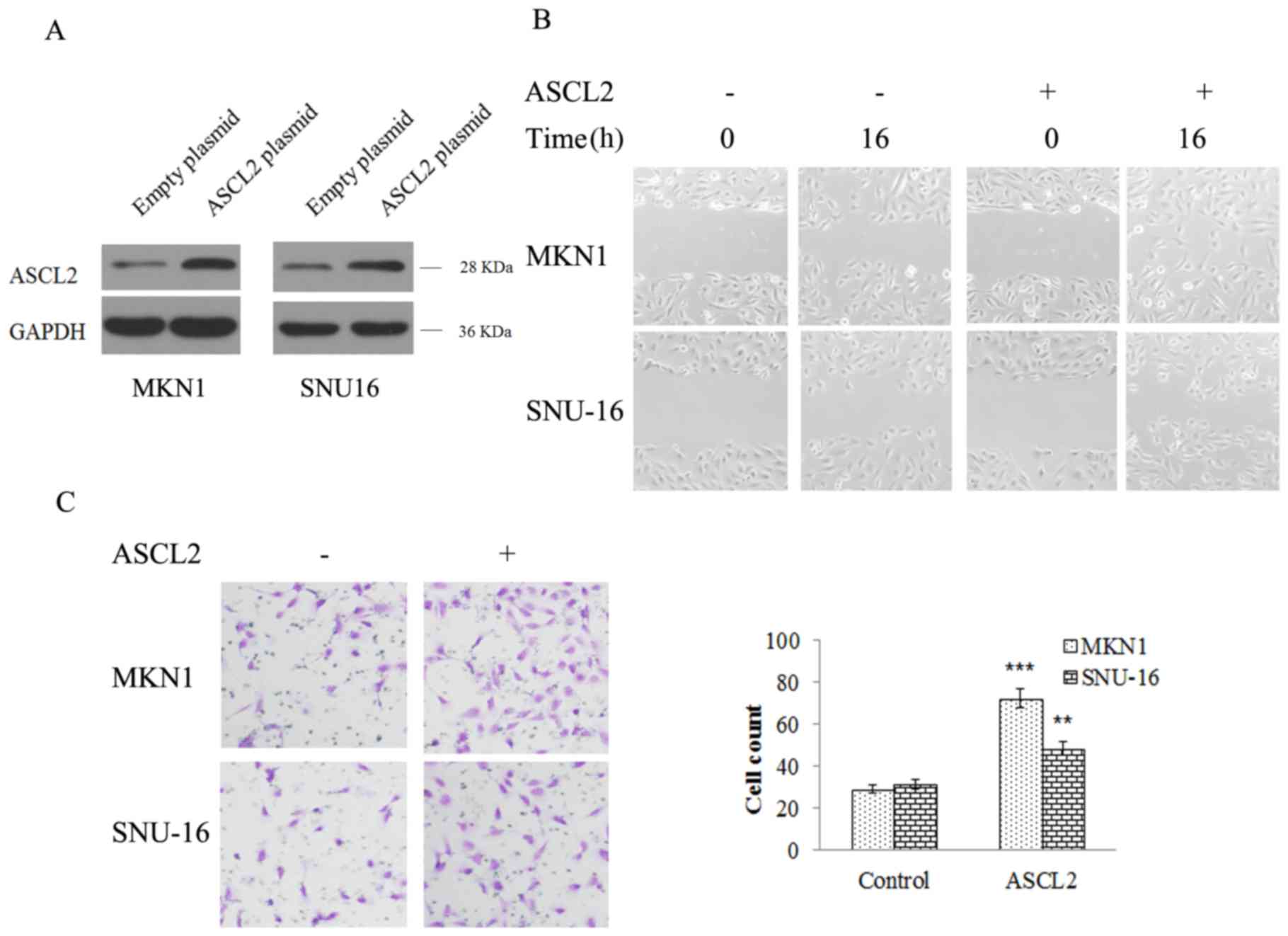
In order to further study the role of ASCL2 in the
metastasis of GC, the ASCL2 plasmid was transfected into MKN-1 and
SNU16 cells to construct ASCL2-overexpressing stably-transfected
cell lines (Fig. 2A). Transwell
experiments and wound healing assays were performed to study the
effect of ASCL2 on GC metastasis. From the wound healing assay, the
scratch width in ASCL2 overexpression and NC group cells was
detected at 16 h. The scratches in the NC group were significantly
wider compared with the ASCL2 overexpression group (Fig. 2B), which indicated that
overexpression of ASCL2 was able to increase cell migration and
invasion. In addition, a Transwell experiment was performed, the
results of which demonstrated that the number of MKN-1 and SNU16
cells in the ASCL2 overexpression group was significantly increased
compared with the NC group (Fig.
2C), indicating that the overexpression of ASCL-2 significantly
increased the invasive ability of MKN-1 and SNU16 cells. From the
above results, it was demonstrated that ASCL2 had a role in
promoting the migration and invasion of GC cells, although the
mechanism regulating the metastasis of GC via ASCL-2 remained
unclear.
ASCL2 promotes the expression of
EMT-inducing proteins
Since ASCL2 was able to promote the metastasis of GC
cells, the migration of cells was associated with the dynamic
alterations in adhesion between cytoskeletal proteins and the cell
matrix. In this process, EMT-inducing proteins served an important
role; therefore, it was hypothesized that ASCL2 may regulate
EMT-associated proteins during cell migration. To this end,
proteins were extracted from cells and western blotting was
performed. From the experimental results, it was observed that the
expression of E-cadherin in ASCL2-overexpressing cell lines
decreased, while Zeb-1, twist-related protein 1 (Twist), Integrin,
Vimentin, 72 kDa type IV collagenase (MMP-2) and matrix
metalloproteinase 9 (MMP-9) were upregulated in
ASCL2-overexpressing cell lines (Fig.
3), which indicated that ASCL2 upregulated EMT-inducing protein
expression and promoted cell migration. However, the mechanism
underlying the regulatory effect of ASCL2 on EMT-associated
proteins remained to be elucidated.
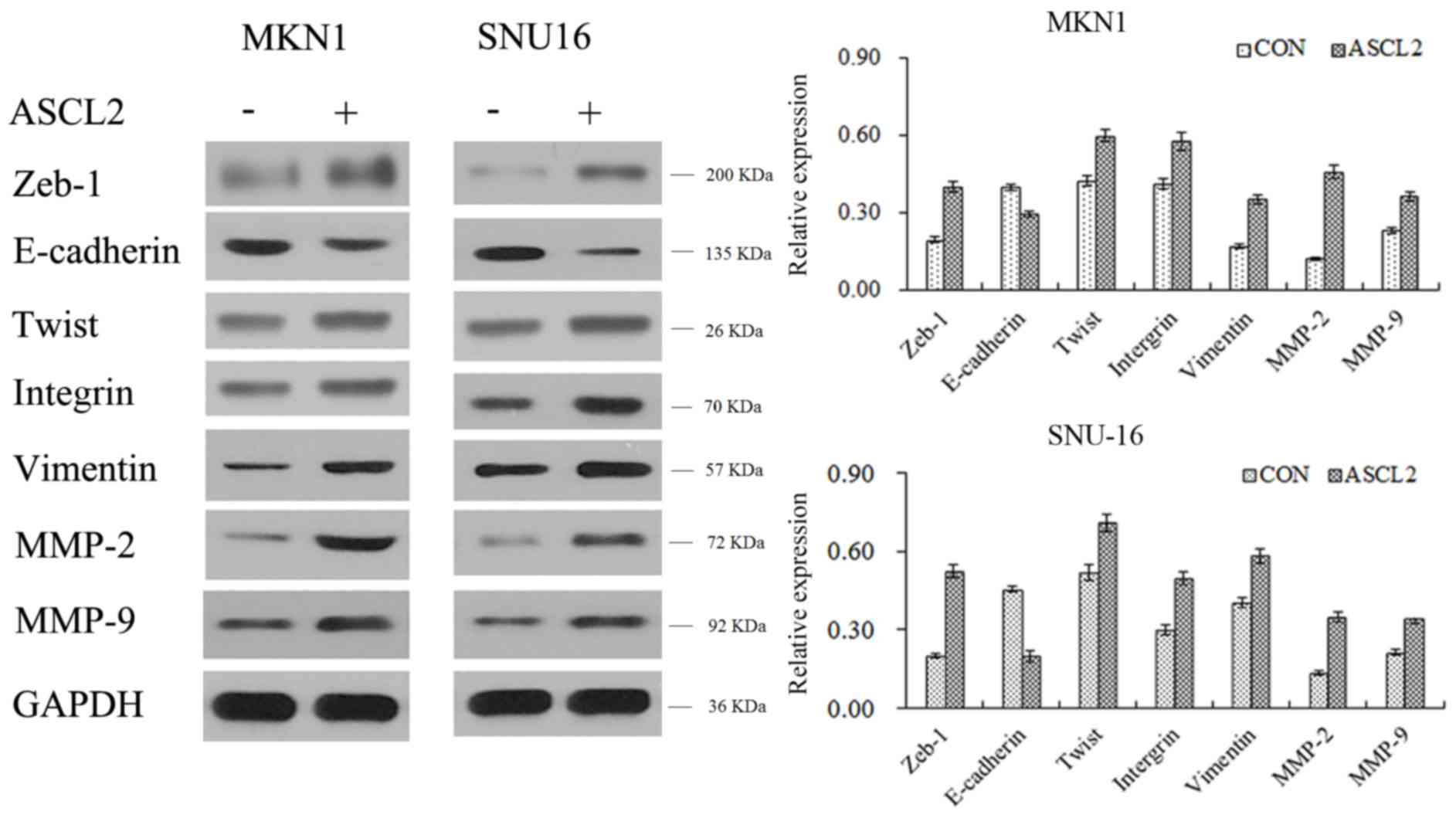 | Figure 3.ASCL2 promotes the expression of
epithelial-mesenchymal transition-inducing proteins. The expression
of the epithelium-associated protein E-cadherin, and the
mesenchyme-associated proteins Zeb-1, Twist, Integrin, Vimentin,
MMP-2 and MMP-9 were detected by western blot analysis. ASCL2,
achaete-scute homolog 2; Twist, twist-related protein 1; MMP-2, 72
kDa type IV collagenase; MMP-9, matrix metalloproteinase 9; Zeb-1,
zinc E-box-binding homeobox 1; CON, control. |
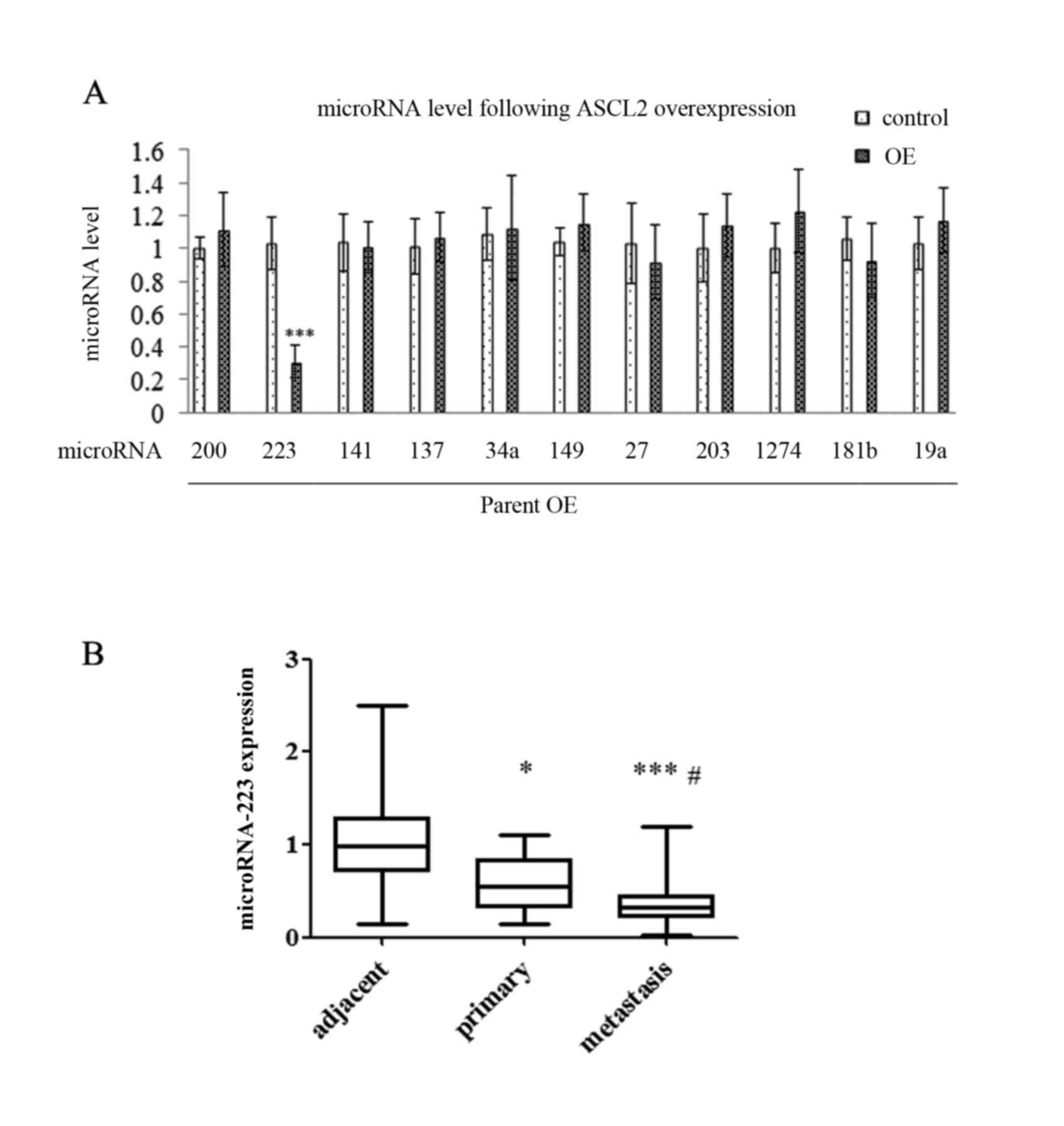
EMT-associated miRNAs alter following
ASCL2 overexpression in MKN-1 and SNU16 cells
The present study demonstrated that miRNAs are an
important switch in regulating gene expression and regulating
various life phenomena, including tumor cell proliferation,
differentiation, apoptosis and motility. It was hypothesized that
EMT-associated proteins may be mediated by EMT-associated miRNAs.
In order to study this hypothesis, RT-qPCR analysis was performed
on the ASCL2 overexpressing cell lines. The results illustrated a
significant reduction in miR223 out of a series of EMT-associated
miRNAs (Fig. 4A), which indicated
that ASCL2 regulated the expression of metastasis-associated
proteins by downregulating miR223. Furthermore, the expression
level of miRNA223 in the samples from patients was assessed by
RT-qPCR analysis and the data suggested that the level of miR223
was at its lowest in metastatic tissues and highest in normal
tissues (Fig. 4B).
Overexpressing miR223 attenuates the
EMT-promoting effect induced by ASCL2 expression
In order to study the role of miR223 in the
migration of GC cells, miR223 mimics and control mimics were
overexpressed in MNK1 and SNU16 cells (Fig. 5A), and a Transwell assay was
performed in which miR223 mimic was added to the ASCL2
overexpression group cells and the NC group cells, respectively
(Fig. 5B). The miR223 mimic was
able to reduce the number of penetrating cells in the ASCL2
overexpression and the NC groups (Fig.
5B), which indicated that ASCL2 may reduce the decreased cell
migration induced by miR223. EMT-associated protein binding to
miR223 was predicted using TargetScan (Fig. 5C). The results of the luciferase
reporter assay suggested that miR223 was able to bind to the 3′UTR
of Zeb-1 (Fig. 5D).
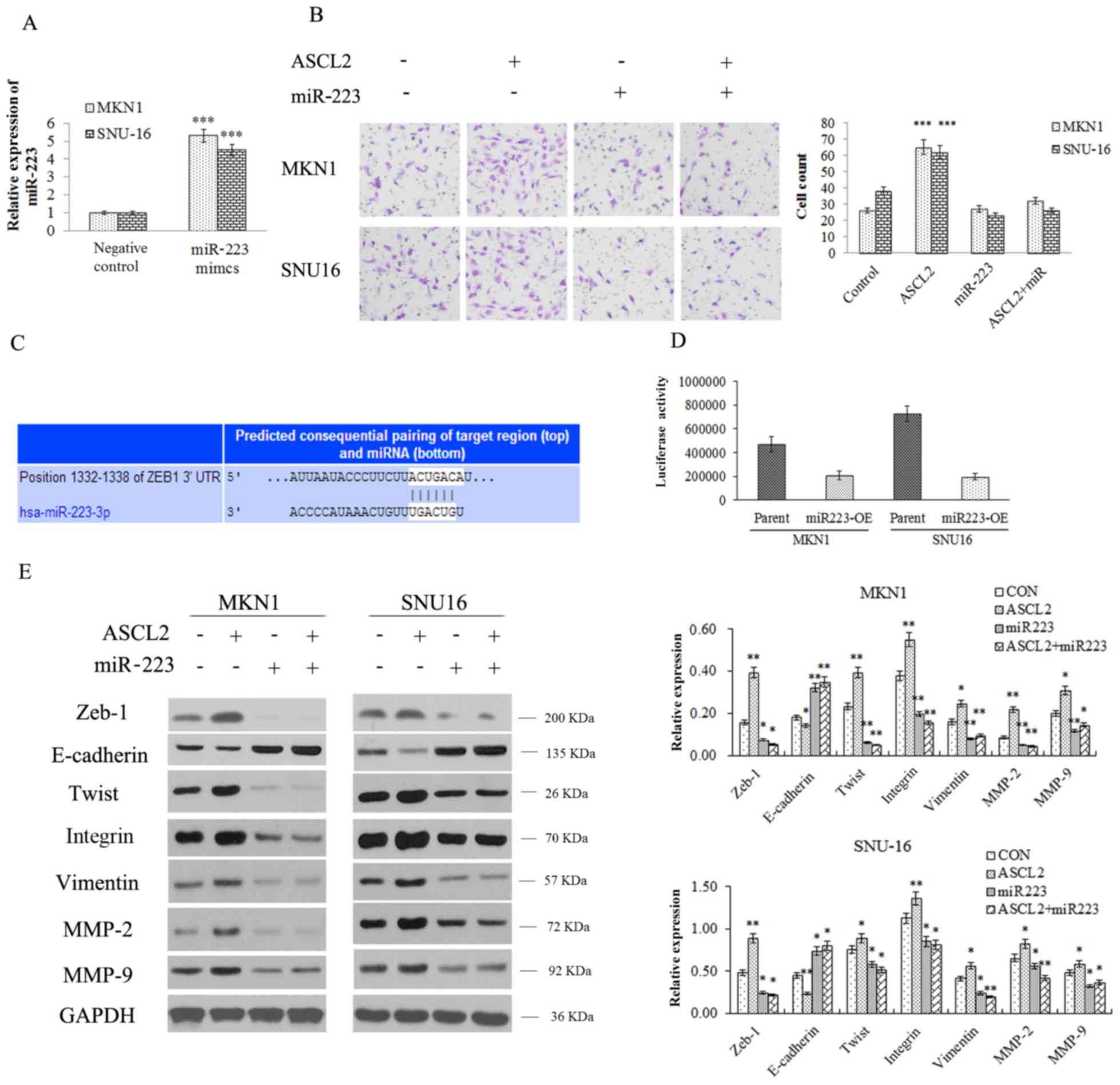 | Figure 5.Overexpressing miR223 attenuates the
EMT-promoting effect induced by ASCL2 expression. (A) The level of
miR223 was detected by quantitative polymerase chain reaction. (B)
The effect of miR223 on invasion in ASCL2-overexpressing MKN-1 and
SNU16 cells was evaluated by Transwell assay (magnification, ×200).
(C) EMT-associated proteins binding to miR223 were predicted using
TargetScan. (D) A luciferase reporter assay evaluated the binding
of miR223 to the 3′ UTR of Zeb-1. (E) The expression of the
epithelium-associated protein E-cadherin, and the
mesenchyme-associated proteins Zeb-1, Twist, Integrin, Vimentin,
MMP-2 and MMP-9 was detected by western blot analysis. *P<0.05,
**P<0.01, ***P<0.001 vs. respective control. miR, microRNA;
ASCL2, achaete-scute homolog 2; EMT, epithelial-mesenchymal
transition; UTR, untranslated region; OE, overexpression; Twist,
twist-related protein 1; MMP-2, 72 kDa type IV collagenase; MMP-9,
matrix metalloproteinase 9; Zeb-1, zinc E-box-binding homeobox 1;
CON, control. |
The effect of miR223 on EMT-associated proteins in
the NC and ASCL2 overexpression groups was additionally detected.
From the western blotting results, it was inferred that miR223
reduced EMT-associated protein expression in the two groups;
however, in the ASCL2 overexpression group, the reduction in the
expression of EMT-associated proteins (Zeb-1, Twist, Integrin,
Vimentin) and migration related proteins (MMP-2 and MMP-9) were
less marked compared with the NC group (Fig. 5E), indicating that ASCL2 may weaken
the biological function of miR223.
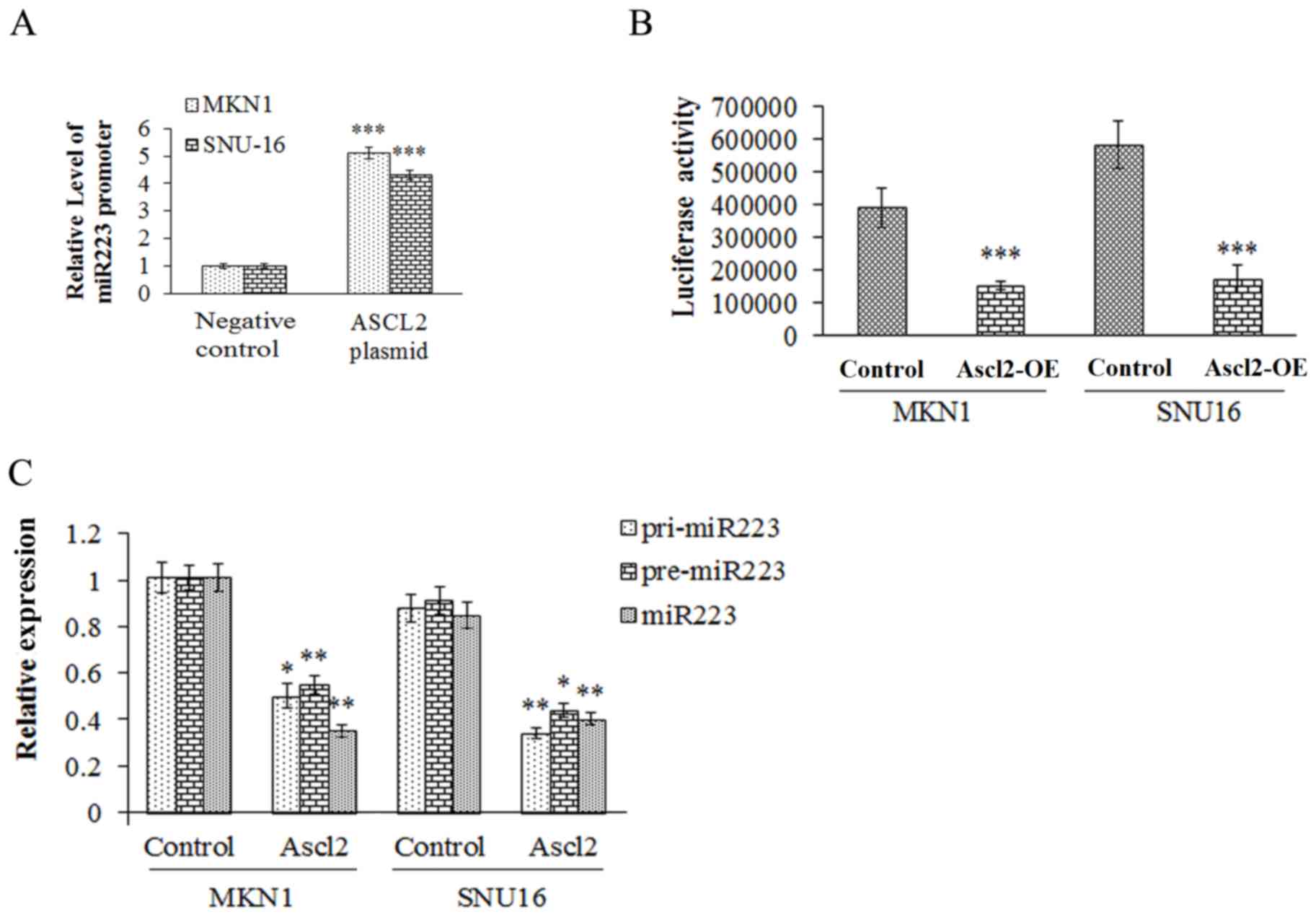
ASCL2 may interact with the promoter
of pre-miR223, and inhibit the maturation of miR223
In order to examine the role of ASCL2 in the
biological function of miR223, the interaction between ASCL2 and
miR223 was analyzed using ChIP and luciferase reporter assays. The
results demonstrated that ASCL2 interacted with pre-miR223
(Fig. 6A and B) to inhibit the
transformation of pre-miR223 to mature miR223 (Fig. 6C).
Discussion
GC is the most common malignant tumor of the
digestive tract. Due to the incidence of GC, its invasive and
metastatic characteristics, clear clinical symptoms and low cure
rate, GC ranks 4th among malignant tumors in the world (16,17).
At present, the pathogenesis of GC had not been completely
elucidated. The occurrence of GC originates from gastric epithelial
stem cells; normal gastric epithelial stem cells develop gene
mutations and thus promote the occurrence and development of
tumors. A small number of tumor stem cell-like cells have been
identified in GC tissues, which have infinite self-renewal,
multi-directional differentiation and strong proliferative
potential, and also promote the infiltration and recurrence of GC.
The mechanisms involved in the invasion and metastasis of GC cells
required further study. In the present study, the expression of the
ASCL2 gene was significantly increased in GC metastatic tissues, as
demonstrated by western blotting and qPCR analysis, which suggested
that high expression of ASCL2 may be associated with the metastasis
of GC cells.
ASCL2 is a basic/helical helix transcription factor
whose expression is confined to the LGr5-positive colonic basal
ganglion cells of the placenta, and small- and large-intestinal
crypts and crypt base columnar cells (1,7).
ASCL2 may interact with colorectal epithelial cells via the
homeobox protein CDX-2 (CDX2) proximal promoter to repress the
expression of CDX2 (18). It has
been reported that CDX2 gene overexpression may promote GC cellular
apoptosis. ASCL2 may promote colon cancer cell growth and
migration, and the upregulation of ASCL2 by promoter demethylation
may promote the growth of GC cells; the results of the present
study suggested that ASCL2 may be involved in the metastasis of GC
cells. In order to test this hypothesis, an ASCL2 gene
overexpression system was constructed in vitro, and the
effect of ASCL2 on the metastasis of GC cells was studied using
wound healing and Transwell assays. From the wound healing assay,
the migration of the ASCL2 overexpression and NC groups of MKN-1
and SNU16 cells were observed at 16 h. The scratches in the NC
group were significantly wider compared with those of the ASCL2
overexpression group; the scratches were partially-healed, although
the NC group exhibited clear scratches, which indicated that
overexpression of ASCL2 may increase cell migration ability. The
results of the Transwell assay demonstrated that the number of
MKN-1 and SNU16 cells in the NC group was significantly decreased
compared with the ASCL2 overexpression group, which indicated that
the overexpression of ASCL2 significantly increased the invasive
ability of MKN-1 and SNU16 cells. From the above results, it was
inferred that ASCL2 had promoted the migration and invasion of GC
cells, although the mechanism regulating the metastasis of GC via
ASCL2 remained unclear.
EMT is an important malignant biological behavior of
tumors. The invasion and migration of tumor cells may be enhanced
through EMT (19), and EMT serves
an important role in secondary metastasis and promotes primary
metastasis of gastric carcinoma, colon cancer, liver cancer, breast
cancer, ovarian cancer and lung cancer (20). The role of EMT in tumor invasion
and metastasis has become a research focus, and it was considered
to be the initial step in tumor invasion and metastasis (21). During EMT, epithelial cells lose
their epithelial characteristics, illustrated by the downregulation
of E-cadherin, while acquiring a mesenchymal phenotype,
characterized by the upregulation of mesenchymal proteins,
including vimentin and N-cadherin. In GC, the upregulation of ASCL2
may promote GC metastasis. EMT is an important promoter of tumor
metastasis; it was therefore hypothesized that ASCL2 may affect GC
cell metastasis by affecting the EMT process. In order to study the
association between ASCL2 and EMT, the alteration in EMT-associated
protein expression following overexpression of ASCL2 was examined
by western blotting. The results demonstrated that the expression
of E-cadherin in ASCL2-overexpressing cell lines decreased, while
Zeb-1, Twist, Integrin, Vimentin, MMP-2 and MMP-9 were upregulated
in ASCL2-overexpressing cell lines, indicating that ASCL2
upregulated EMT-inducing proteins and promoted cell migration.
However, the mechanism underlying the effect of ASCL2 on
EMT-associated proteins has seldom been studied.
A previous study confirmed that miRNAs are
associated with the occurrence, development, invasion and
metastasis of GC, and they have become some of the specific
molecular markers of GC (8). In
tumorigenesis, miRNAs act as tumor suppressor genes or carcinogenic
genes, and miR223 was able to reverse EMT, and inhibit the
migration and invasion of melanoma cells induced the expression of
E-cadherin protein. miR223 is located on the X chromosome (22). Previous studies have demonstrated
that miR223 is associated with tumor and inflammation-associated
signal transduction pathways (23). It has been reported that miR223 was
highly expressed in patients with acute myeloid leukemia (AML)
(22); further studies
demonstrated that miR223 served an important role in the regulation
of AML (24), although miR223 was
downregulated in chronic myelognous leukemia (CLL), and may be
associated with tumor burden and tumor invasion. In a previous
study, it was observed that ASCL2 may upregulate EMT-associated
protein expression to promote the metastasis of GC cells.
Therefore, the role of miR223 in GC cells, and the association
between ASCL2 and miR223, was the focus of the present study. It
was observed that miR223 expression was significantly reduced out
of a series of EMT-associated miRNAs. miR223 was able to decrease
the migratory ability and downregulate the expression of Zeb-1,
Twist, Integrin, Vimentin, MMP-2 and MMP-9 in GC cells; however, in
the ASCL2-overexpressing cell line, the decrease in the expression
of EMT-associated proteins was improved, thereby suggesting that
ASCL2 may affect the role of miRNAs. It was observed that ASCL2 was
able to bind to pre-miR223, which inhibited the maturation of
miR223 and weakened the biological function of miR223, thus
reducing the inhibition of GC cell metastasis.
The present study demonstrated that ASCL2 was able
to downregulate the expression level of miR223, contribute to EMT
and promote gastric tumor metastasis, indicating that ASCL2 may
serve as a therapeutic target in the treatment of GC. Future
studies are required to investigate the association between ASCL2
and other miRNAs, further enriching the molecular mechanism of GC
cell invasion and metastasis, and providing a novel effective
potential target for GC.
Acknowledgements
Not applicable.
Funding
The present study was funding by Innovation project
fund of the science and Technology Commission of Putuo (grant no.
2011PTKW003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QZ performed experimental design, specimen
collection and experimental techniques. JW was performed specimen
collection and experimental techniques. RZ performed experiment
assistance and writing. TC performed experimental design. CC and
DXF performed experimental techniques. YZ performed specimen
collection.
Ethics approval and consent to
participate
The procedure was approved by the ethical committee
of Putuo Hospital Affiliated to Shanghai University of Traditional
Chinese Medicine (Shanghai, China). All patients provided written
informed consent.
Consent for publication
The patient, or parent, guardian or next of kin
provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Kwon OH, Park JL, Baek SJ, Noh SM, Song
KS, Kim SY and Kim YS: Aberrant upregulation of ASCL2 by promoter
demethylation promotes the growth and resistance to 5-fluorouracil
of gastric cancer cells. Cancer Sci. 104:391–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Erratum: Estimates of the worldwide mortality from 25 cancers in
1990. Int J Cancer, 83, 18–29 (1999). Int J Cancer. 83:870–873.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuasa Y: Control of gut differentiation
and intestinal-type gastric carcinogenesis. Nat Rev Cancer.
3:592–600. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Massari ME and Murre C: Helix-loop-helix
proteins: Regulators of transcription in eucaryotic organisms. Mol
Cell Biol. 20:429–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyamoto T, Jinno Y, Sasaki T, Ikeda Y,
Masuzaki H, Niikawa N and Ishikawa M: Genomic cloning and
localization to chromosome 11p15.5 of the human achaete-scute
homolog 2 (ASCL2). Cytogenet Cell Genet. 73:312–314. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Flier LG, van Gijn ME, Hatzis P,
Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M,
Guryev V, Oving I, et al: Transcription factor achaete scute-like 2
controls intestinal stem cell fate. Cell. 136:903–912. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jubb AM, Chalasani S, Frantz GD, Smits R,
Grabsch HI, Kavi V, Maughan NJ, Hillan KJ, Quirke P and Koeppen H:
Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is
upregulated in intestinal neoplasia. Oncogene. 25:3445–3457. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu R, Yang Y, Tian Y, Bai J, Zhang X, Li
X, Peng Z, He Y, Chen L, Pan Q, et al: Ascl2 knockdown results in
tumor growth arrest by miRNA-302b-related inhibition of colon
cancer progenitor cells. PLoS One. 7:e321702012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs-the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gal H, Pandi G, Kanner AA, Ram Z,
Lithwick-Yanai G, Amariglio N, Rechavi G and Givol D: MIR-451 and
Imatinib mesylate inhibit tumor growth of Glioblastoma stem cells.
Biochem Biophys Res Commun. 376:86–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee NS, Kim JS, Cho WJ, Lee MR, Steiner R,
Gompers A, Ling D, Zhang J, Strom P, Behlke M, et al: miR-302b
maintains ‘stemness’ of human embryonal carcinoma cells by
post-transcriptional regulation of Cyclin D2 expression. Biochem
Biophys Res Commun. 377:434–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsujimoto H, Ono S, Ichikura T, Matsumoto
Y, Yamamoto J and Hase K: Roles of inflammatory cytokines in the
progression of gastric cancer: Friends or foes? Gastric Cancer.
13:212–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian Y, Pan Q, Shang Y, Zhu R, Ye J, Liu
Y, Zhong X, Li S, He Y, Chen L, et al: MicroRNA-200 (miR-200)
cluster regulation by achaete scute-like 2 (Ascl2): Impact on the
epithelial-mesenchymal transition in colon cancer cells. J Biol
Chem. 289:36101–36115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solt LA, Griffin PR and Burris TP: Ligand
regulation of retinoic acid receptor-related orphan receptors:
Implications for development of novel therapeutics. Curr Opin
Lipidol. 21:204–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Becker-Andre M, Andre E and DeLamarter JF:
Identification of nuclear receptor mRNAs by RT-PCR amplification of
conserved zinc-finger motif sequences. Biochem Biophys Res Commun.
194:1371–1379. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schuijers J, Junker JP, Mokry M, Hatzis P,
Koo BK, Sasselli V, Van der Flier LG, Cuppen E, van Oudenaarden A
and Clevers H: Ascl2 acts as an R-spondin/Wnt-responsive switch to
control stemness in intestinal crypts. Cell Stem Cell. 16:158–170.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carlberg C, Hooft van Huijsduijnen R,
Staple JK, DeLamarter JF and Becker-André M: RZRs, a new family of
retinoid-related orphan receptors that function as both monomers
and homodimers. Mol Endocrinol. 8:757–770. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jetten AM, Kurebayashi S and Ueda E: The
ROR nuclear orphan receptor subfamily: Critical regulators of
multiple biological processes. Prog Nucleic Acid Res Mol Biol.
69:205–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gawlas K and Stunnenberg HG: Differential
transcription of the orphan receptor RORbeta in nuclear extracts
derived from Neuro2A and HeLa cells. Nucleic Acids Res.
29:3424–3432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stamatopoulos B, Meuleman N, Haibe-Kains
B, Saussoy P, Van Den Neste E, Michaux L, Heimann P, Martiat P,
Bron D and Lagneaux L: microRNA-29c and microRNA-223
down-regulation has in vivo significance in chronic lymphocytic
leukemia and improves disease risk stratification. Blood.
113:5237–5245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mi S, Lu J, Sun M, Li Z, Zhang H, Neilly
MB, Wang Y, Qian Z, Jin J, Zhang Y, et al: MicroRNA expression
signatures accurately discriminate acute lymphoblastic leukemia
from acute myeloid leukemia. Proc Natl Acad Sci USA. 104:pp.
19971–19976. 2007; View Article : Google Scholar : PubMed/NCBI
|