Introduction
Human parvovirus B19 (B19) and human bocavirus 1
(HBoV) are the only known pathogenic parvoviruses in human beings
(1,2). B19, discovered in 1975 by Cossart
during screening for hepatitis B virus (3), is highly infectious and causes
various pathological conditions including fifth disease in
children, persistent anemia in immunocompromised patients,
transient aplastic crises, hydrops fetalis in pregnant women,
arthropathy, and autoimmune diseases (4–7).
HBoV, discovered in 2005 by Allender et al (8), was first identified in the
respiratory nasopharyngeal aspirates of children with lower
respiratory tract infections. HBoV is known to be a significant
causative agent in acute respiratory tract infections, in which
wheezing is the most common symptom (9).
In the literature, intra-nuclear viral particles
typical of B19 were first documented in an electron microscopic
examination of fetal cardiac tissue (10); however, B19 is not regarded as a
cardiotropic virus (11). Notably,
a previous investigation identified B19 as a pathogenic agent in
cases of myocarditis in children and adolescents (12). More recently, an increasing body of
evidence has shown that B19 is strongly associated with
cardiovascular disorders. In the last decade, B19 has emerged as a
potential pathogenic agent in adult patients with inflammatory
heart disease (13). Indeed, the
B19 genome has been detected in endomyocardial biopsies of patients
with acute myocardial infarction (14). Another investigation found that the
persistence of B19 may be associated with progression of left
ventricular dysfunction (15). In
a study of 208 patients, dominantly higher prevalence of the B19
genome was found in endomyocardial biopsies of inpatients with
inflammatory cardiomyopathy or myocarditis compared with controls
(16). These findings strongly
indicate a connection between B19 and heart disorders.
Phospholipase A2 (PLA2)-like activity of B19-VP1
unique region (VP1u) has been identified (17) and associated with its infectivity
and the pathogenesis of many disorders (18–21).
Recently, B19-VP1u has been associated with cardiac disorders
(22,23). Abnormal ultrastructural changes in
the myocardia and elevated levels of myocardial functional enzymes,
including aspartate aminotransferase (AST), lactate dehydrogenase
(LDH), creatine kinase (CK), creatine kinase isoenzyme (CK-MB) and
alpha-hydroxybutyric acid dehydrogenase (alpha-HBDH), have been
detected in mice receiving recombinant B19-VP1u proteins (22). Similarly, dilated cardiomyopathy
has been observed in BALB/c mice immunized with VP1u; an
observation clinically relevant to B19-associated cardiac damage
(24). Although a recent
investigation indicated that HBoV-VP1u also has a PLA2 motif, and
could exhibit sPLA2 activity (25), little is known about the role of
HBoV-VP1u in cardiac injury. Therefore, this study compared
B19-VP1u and HBoV-VP1u with respect to their potential roles in
inducing injury in H9c2 cardiomyocytes.
Materials and methods
Plasmids
Plasmid pEGFP-C1 was purchased from CLONTECH
(Clontech Laboratories, Inc., Mountainview, CA, USA). The B19-VP1u
and B19-VP1uD175A genes described in our previous study (26) were ligated into the pEGFP-C1
expression vector, which are known as pET32a-B19-VP1u and
pET32a-B19-VP1uD175A. A 387-bp DNA fragment encompassing
nucleotides 3056–3442 of the Taiwan HBoV strain (TW125_07: GeneBank
accession no. EU984241.1) provided by Centers for Disease Control,
Taipei, Taiwan (27) was amplified
by the polymerase chain reaction (PCR) using the following primers,
including 5′-GCAGATCTATGCCTCCAATTAAG-3′ (forward primer) and
5′-GCGTCGACTGAGGTTCCTGG-3′ (reverse primer). The HBoV-VP1u and
HBoV-VP1uV12A, the mutant form of HBo-VP1u without sPLA2 activity
(25,28), were constructed into pEGFP-C1,
which are known as pET32a-HBoV-VP1u and pET32a-HBoV-VP1uV12A. All
constructants were verified by DNA sequencing analysis forwardly
and reversely.
Secreted sPLA2 catalytic activity
The secreted sPLA2 activity was detected as cribbed
elsewhere (27). The recombinant
protein samples, including B19-VP1u, B19-VP1uD175A, HBoV-VP1u and
HBoV-VP1uV12A, were assayed for sPLA2 activity by using a
colorimetric assay (cat. no. 765001, sPLA2 Activity kit; Cayman
Chemical), in accordance with the manufacturer's instructions, with
dynamic colorimetric measurements at the optical density of 414 nm
determined every minute for 10 min. Results are revealed as
micromoles per minute per milliliter.
Cell culture, transfection and stable
clones
H9c2 cardiac myoblast cells were purchased from ATCC
and cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a 5% CO2
incubator. A total of 1×106 cells were grown to 70–80%
confluence in a 100 mm2 dish before transfection. The
transfection reaction was performed using Lipofectamine reagent
with PLUS reagent (Invitrogen; Thermo Fisher Scientific, Inc.) with
2 µg of the plasmids, pEGFP, pEGFP-B19-VP1u, pEGFP-B19-VP1uD175A (a
mutant of the B19-VP1u region), pEGFP-HBoV-VP1u and
pEGFP-HBoV-VP1uV12A (a mutant of HBoV-VP1u region), respectively.
To determine the transfection efficiency, pCMV-SPORT-β-gal plasmid
(0.5 µg/transfection, Invitrogen; Thermo Fisher Scientific, Inc.)
and co-transfected with the constructs mentioned above,
respectively. After X-gal staining (29), cells were fixed with chilled
methanol and the extent of β-gal expression was measured by
determining the ratio of the X-gal-stained area to the area of each
observation field under a light microscope. No significant
variation was observed among all groups (data not shown). The cells
were then cultured in serum-free DMEM for 12 h at 37°C in a 5%
CO2 incubator and subsequently in DMEM with 10% FBS. The
stable clones were obtained by G418 selection at a concentration of
600 mg/ml (Promega Corporation, Madison, WI, USA) in DMEM
containing 10% FBS for 8 weeks. The expression levels of EGFP and
the EGFP-B19-VP1u, EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and
EGFP-HBoV-VP1uV12A fusion proteins were examined by using a Zeiss
Axioplan-2 epifluorescence microscope (Carl Zeiss AG, Oberkochen,
Germany) and by western blot analysis.
Fluorescence microscopy
Expression of recombinant EGFP, EGFP-B19-VP1u,
EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A in H9c2
cells was observed with a Zeiss Axioplan-2 fluorescence microscope
(Carl Zeiss AG). Transfected H9c2 cells were fixed by 4%
paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked
with 1% BSA in phosphate-buffered saline (PBS) for 10 min. The cell
nuclei were stained with DAPI (blue). Excitation filters/emission
filters were set at 480/535 and 358/460 nm for green fluorescent
protein (GFP) and DAPI, respectively. Digital images of the cells
were recorded by using a spot camera system.
Reverse transcription-semi
quantitative (RT-sq)PCR
All procedures were carried out in a designated PCR
clean area. RNA was extracted from infected cells using Trizol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
isolated from the cells transfected with EGFP, EGFP-B19-VP1u,
EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A,
respectively. The RNA samples were suspended in diethyl
pyrocarbonate (DEPC)-treated water, quantified, and then stored at
−80°C until use. RNA concentration and purity were determined by a
spectrophotometer by calculating the ratio of optical density at
wavelengths of 260 and 280 nm. The first-strand cDNA for PCR was
synthesized from total RNA (2 µg) using the ImProm-II Reverse
Transcription System (Promega Corporation). The cDNAs encoding
monocyte chemoattractant protein 2 [MCP2, also known as chemokine
ligand 8 (CCL-8)], IFN-gamma-inducible protein 10 [IP-10, also
known as CSC motif chemokine 10 (CXCL10)] and GAPDH were amplified
by using a multiplex PCR kit (cat. no. MP-70070; Maxim Biotech,
Inc., Rockville, MD, USA). The intensity of MPC2, IP-10 and GAPDH
were then quantified using densitometric apparatus (Alpha-Imager
2200; ProteinSimple, San Jose, CA, USA).
Protein extraction
The cells were centrifuged at 800 g for 5 min and
washed twice with ice-cold PBS twice. The cell pellets were then
suspended in 600 µl of PRO-PREP™ buffer (iNtRON Biotech,
Gyeonggi-do, Korea) and chilled on ice for 1 h. The supernatant
containing protein extracts were then collected by centrifugation
at 17,982 g for 5 min at 4°C. Protein concentration of the samples
was determined by a modified Bradford assay using a
spectrophotometer (Hitachi U3000; Hitachi, Ltd., Tokyo, Japan) at
595 nm with BSA as the standard.
Immunoblotting
Protein samples were separated by 10 or 12% SDS-PAGE
and electrophoretically transferred to nitrocellulose membranes (GE
Healthcare, Chicago, IL, USA). After blocking with 5% non-fat dry
milk in PBS, antibodies against GFP (cat. no. 460092; Invitrogen;
Thermo Fisher Scientific, Inc.), TNF-α (cat. no. sc-8301), IL-6
(cat. no. sc-1265), IL-1β (cat. no. sc-7884), NF-κB p65 (cat. no.
sc-109) (1:500-1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and β-actin (1:5,000; MAB1501, Chemicon; EMD Millipore,
Billerica, MA, USA) were diluted in PBS with 2.5% BSA and incubated
with the membranes for 1.5 h with gentle agitation at room
temperature. The membranes were then incubated with horseradish
peroxidase (HRP)-conjugated secondary antibody (cat. nos. sc-2004
or sc-2005; Santa Cruz Biotechnology, Inc.). Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore) and a
chemiluminescence imaging analyzer (GE ImageQuant TL 8.1; GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA) were used to detect
the antigen-antibody complexes. The blotting results were then
quantified using densitometric apparatus (Alpha-Imager 2200;
ProteinSimple).
Statistical analysis
All of the statistical analyses were performed using
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA) by one-way analysis of variance (One-way ANOVA) followed by
Tukey's multiple-comparisons test. Data were represented as mean ±
SEM and verified at least three independent experiments. P<0.05
was considered to indicate a statistically significant difference.
The significant differences were stressed with symbols as shown in
figures.
Results
Expression of recombinant B19-VP1u and
HBoV-VP1u
To detect the expression of the recombinant EGFP,
EGFP-B19-VP1u, EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and
EGFP-HBoV-VP1uV12A proteins in H9c2 cells, an epifluorescence
microscope and western blot analysis were employed. The upper panel
of Fig. 1A shows photographs of
H9c2 cells that were transfected with pEGFP, pEGFP-B19-VP1u,
pEGFP-B19-VP1uD175A, pEGFP-HBoV-VP1u and pEGFP-HBoV-VP1uV12A. The
expressions of EGFP, EGFP-B19-VP1u, EGFP-B19-VP1uD175A,
EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A was observed as indicated by
the arrow in the figure. The nuclei of H9c2 cells were stained with
DAPI. The expression of the EGFP, EGFP-B19-VP1u,
EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A
recombinant proteins was further verified using antibodies against
EGFP (Fig. 1B).
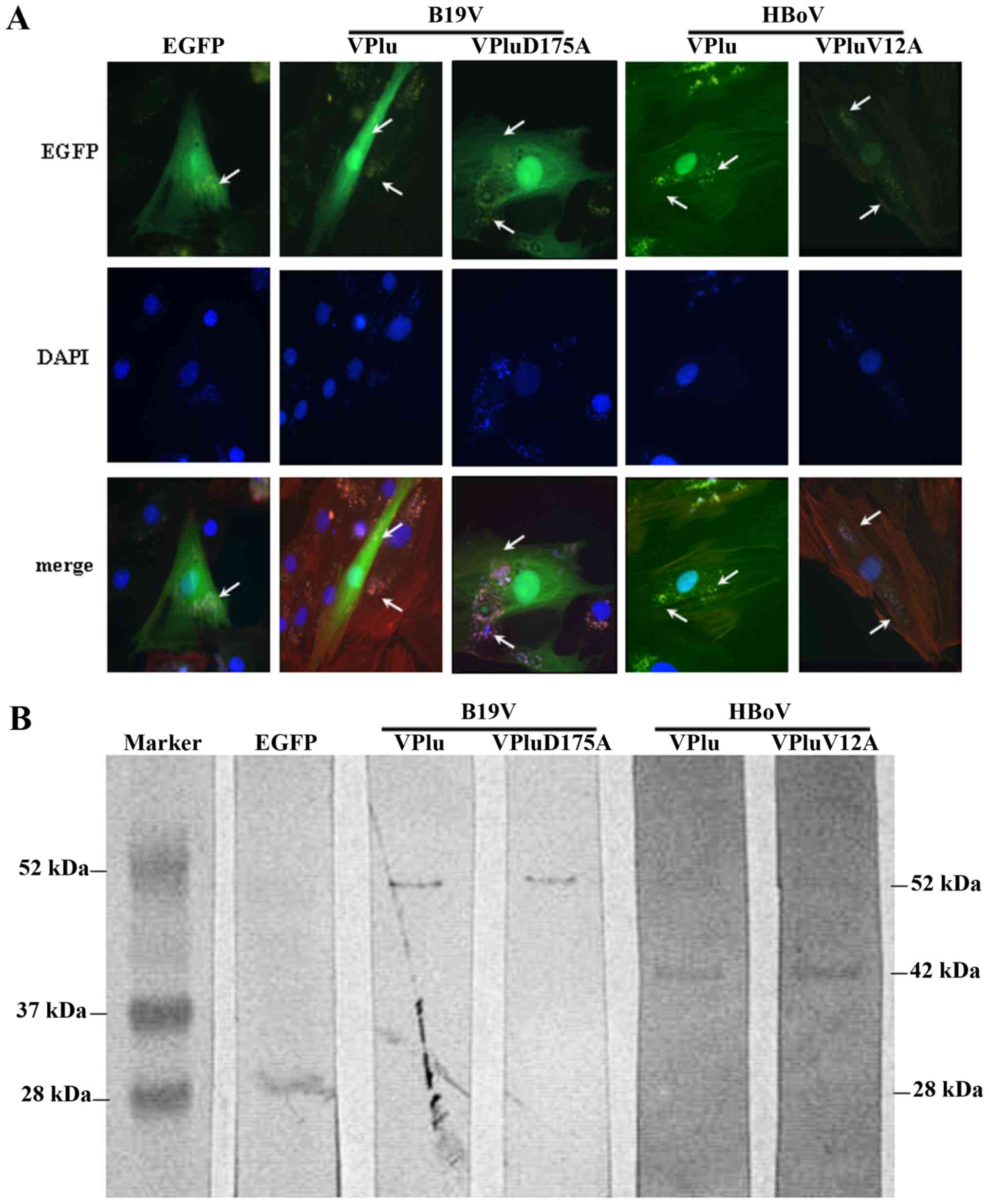 | Figure 1.Expression of EGFP, EGFP-B19-VP1u,
EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A in H9c2
cells. (A) Representative images of H9c2 cells transfected with
pEGFP, pEGFP-B19-VP1u, pEGFP-B19-VP1uD175A, pEGFP-HBoV-VP1u or
pEGFP-HBoV-VP1uV12A, observed under a fluorescence microscope. EGFP
expression is indicated with white arrows (magnification, ×200).
(B) Expression of EGFP, EGFP-B19-VP1u, EGFP-B19-VP1uD175A,
EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A recombinant proteins was
detected using antibodies against EGFP. Similar results were
observed in triplicate experiments. |
Secreted sPLA2 activity of recombinant
B19-VP1u and HBoV-VP1u
To determine the sPLA2 catalytic activity of the
purified recombinant proteins, an sPLA2 activity assay was
performed. Table I summarizes the
sPLA2 activities in B19-VP1u, B19-VP1uD175A, HBoV-VP1u and
HBoV-VP1uV12A recombinant proteins. As a positive control, bvPLA2
exhibited an sPLA2 activity of 0.368±0.009 µmol/min/ml, whereas
B19-VP1uD175A and HBoV-VP1uV12A exhibited no detectable sPLA2
activities. Accordingly, sPLA2 activities were detected for
B19-VP1u (400 ng) and HBoV-VP1u (400 ng) at the values of
0.098±0.012 and 0.046±0.007 µmol/min/ml, respectively (Table I).
 | Table I.Determination of sPLA2 activity. |
Table I.
Determination of sPLA2 activity.
| Proteins | sPLA2 activity
(mmol/min/ml) |
|---|
| bvPLA2 (10 ng) | 0.368±0.009 |
| B19-VP1u (400
ng) | 0.098±0.012 |
| B19-VP1uD175A (400
ng) | ND |
| HBoV-VP1u (400
ng) | 0.046±0.007 |
| HBoV-VP1uV12A (400
ng) | ND |
Effects of recombinant B19-VP1u and
HBoV-VP1u on chemokine expression in H9c2 cells
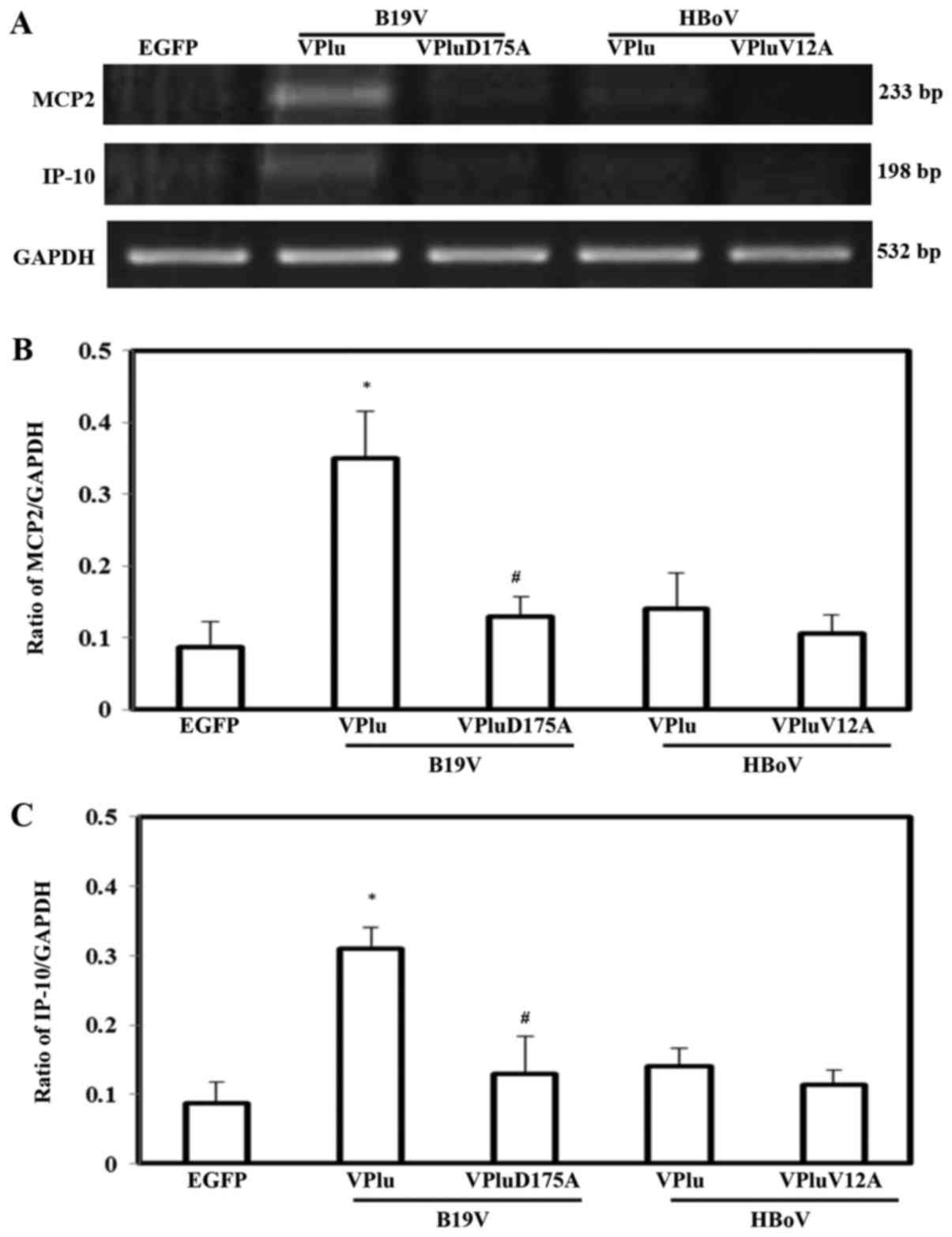
To study the effects of EGFP, EGFP-B19-VP1u,
EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A on
chemokine expression, the mRNA expressions of MCP2 and IP-10 were
measured (Fig. 2). Significantly
higher levels of MCP2 and IP-10 mRNA were detected in H9c2 cells
transfected with pEGFP-B19-VP1u, but not in those cells transfected
with pEGFP-HBoV-VP1u, pEGFP-B19-VP1uD175A or pEGFP-HBoV-VP1uV12A,
relative to expression in cells transfected with pEGFP. Quantified
results of MCP2 and IP-10 mRNA levels based on GAPDH level are
shown in Fig. 2B and C.
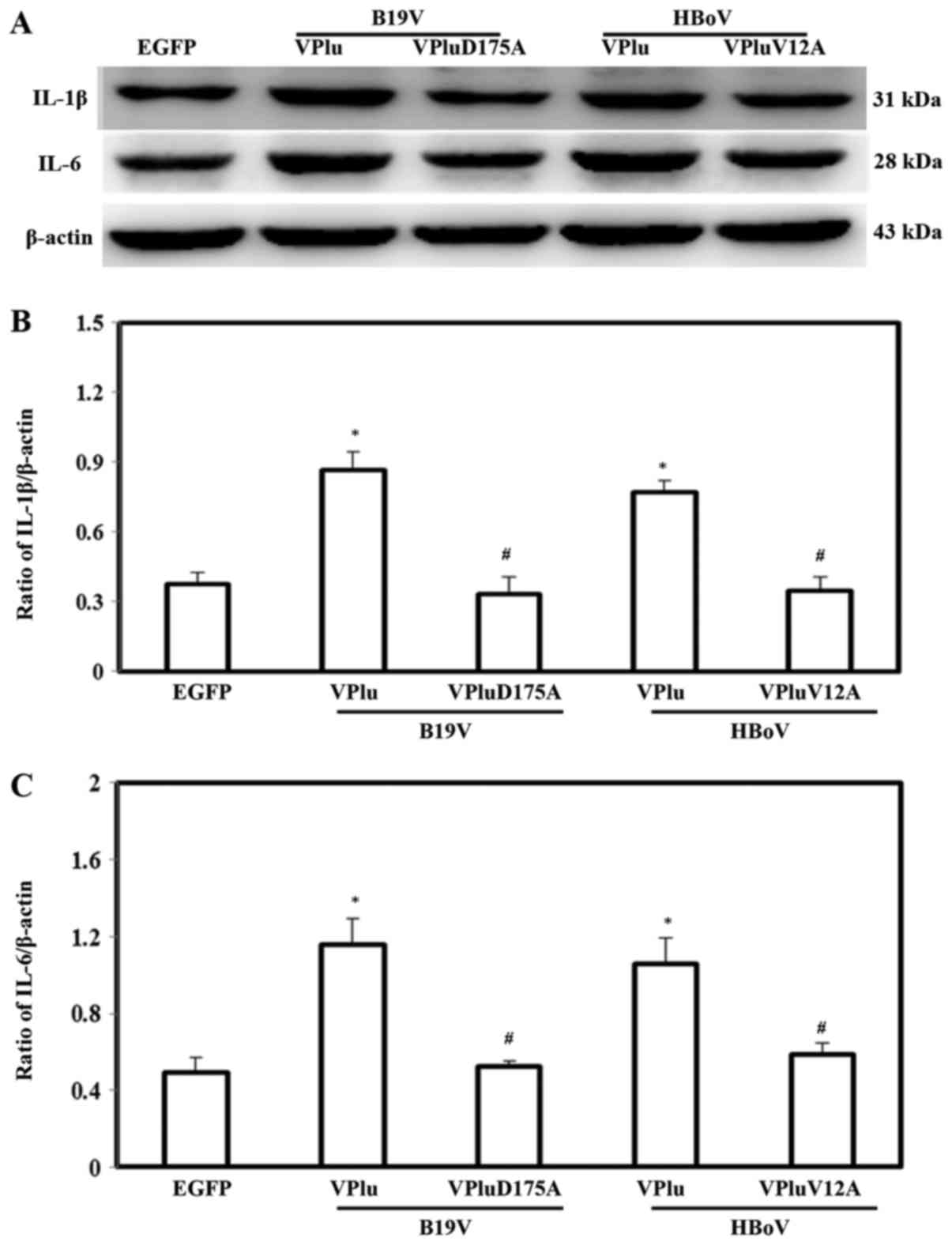
Effects of recombinant B19-VP1u and
HBoV-VP1u on inflammatory cytokines expression in H9c2 cells
To examine the effects of EGFP-B19-VP1u,
EGFP-B19-VP1uD175A, EGFP-HBoV-VP1u and EGFP-HBoV-VP1uV12A on the
expressions of proinflammatory cytokines, IL-1β, IL-6 and TNF-α
levels were detected by western blotting. Significantly higher
protein expression of IL-1β and IL-6 was detected in H9c2 cells
transfected with pEGFP-B19-VP1u and pEGFP-HBoV-VP1u, respectively,
compared with in those cells transfected with pEGFP. Conversely, no
significant difference in the expression of either IL-1β or IL-6
was detected in H9c2 cells transfected with pEGFP-B19-VP1uD175A and
pEGFP-HBoV-VP1uV12A, respectively, from that in cells transfected
with pEGFP. The lower panel in Fig.
3 shows quantitative results concerning the expression levels
of IL-1β and IL-6 normalized to β-actin. Notably, significantly
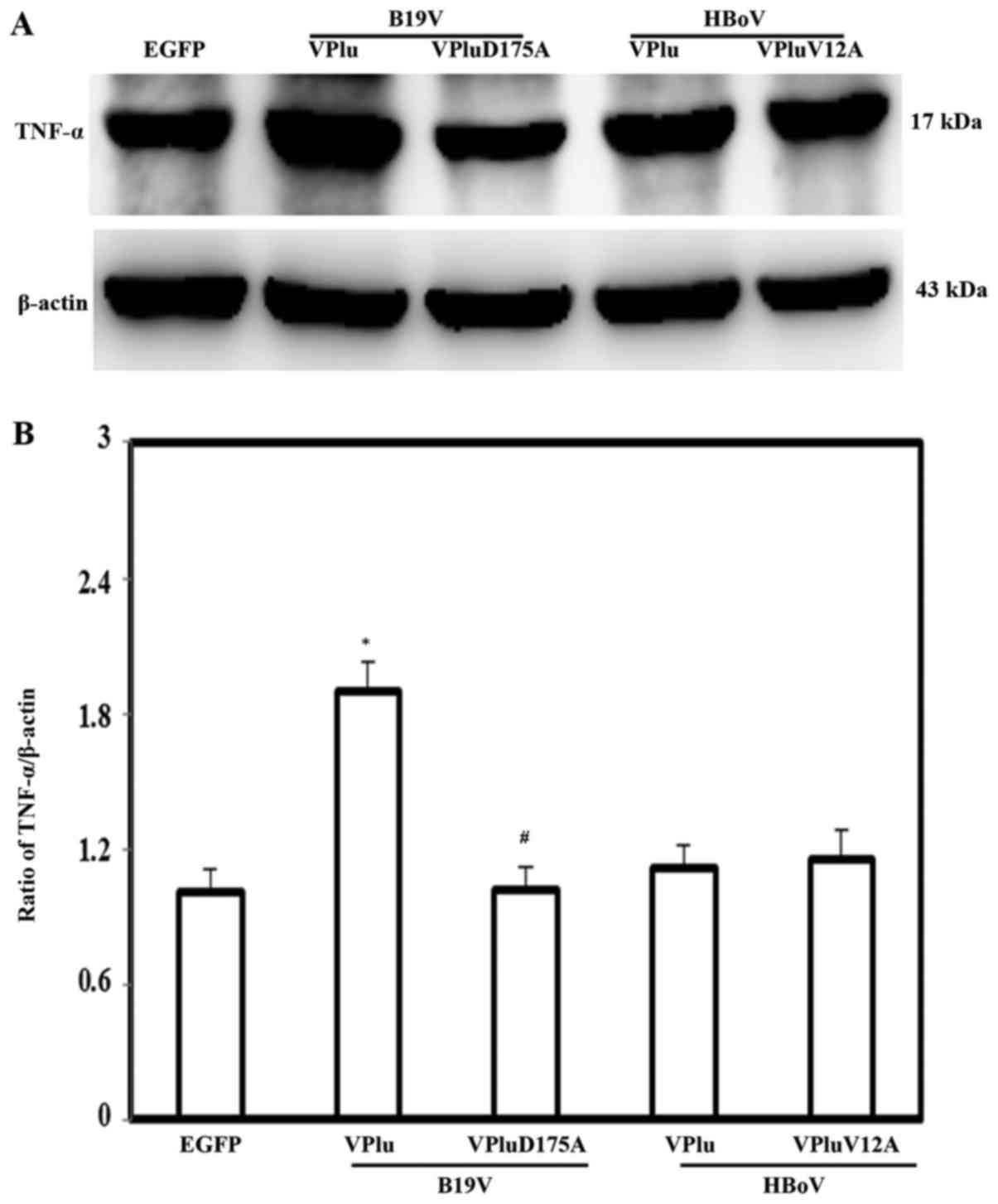
higher expression of TNF-α was observed only in H9c2 cells
transfected with pEGFP-B19-VP1u, and not in those cells transfected
with pEGFP-HBoV-VP1u, pEGFP-B19-VP1uD175A or pEGFP-HBoV-VP1uV12A,
relative to that in cells transfected with pEGFP. The lower panel
in Fig. 4 shows quantitative
results concerning TNF-α levels normalized to β-actin. To identify
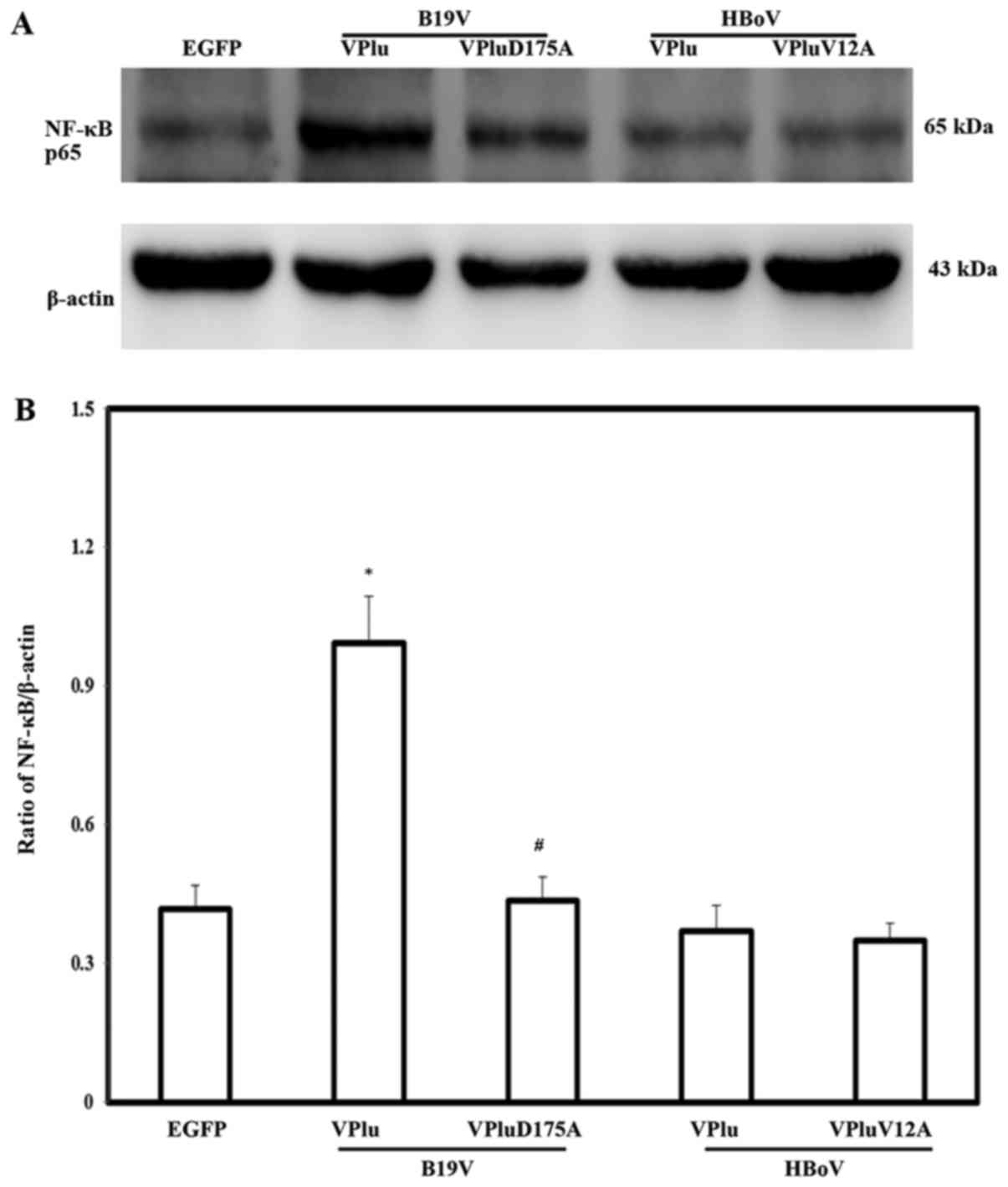
the potential signaling involved in B19-VP1u-mediated cytokine
induction, the expression of NF-κB was detected. Significantly
higher expression of NF-κB protein was detected in H9c2 cells
transfected with pEGFP-B19-VP1u, compared with in those cells
transfected with pEGFP. No significant difference in the expression
of NF-κB protein was detected between H9c2 cells that were
transfected with pEGFP-HBoV-VP1u, pEGFP-B19-VP1uD175A or
pEGFP-HBoV-VP1uV12A, and those cells transfected with pEGFP. The
lower panel in Fig. 5 shows
quantitative results concerning NF-κB levels normalized to
β-actin.
Discussion
B19 and HBoV are the only established pathogenic
parvoviruses in the literature, and are responsible for many
diseases in human beings and thought to circulate globally.
Although both B19-VP1u and HBoV-VP1u exhibit sPLA2-like activity
(27), only B19-VP1u has been
associated with cardiac diseases (22,23).
The present investigation, to the best of our knowledge, is the
first to evaluate the involvement of HBoV-VP1u in inducing cardiac
inflammatory cytokines, and the difference between B19-VP1u and
HBoV-VP1u with respect to induced cytokine profiles. B19-VP1u was
found herein to induce significant expression of inflammatory
chemokines and cytokines, including MCP2, IP-10, IL-1β, IL-6 and
TNF-α, in H9c2 cells, whereas HBoV-VP1u only induced significant
expression of IL-1β and IL-6 in the H9c2 cells. These findings
demonstrate for the first time the effect of HBoV-VP1u on cardiac
inflammation and the different cytokine profiles induced by
B19-VP1u and HBoV-VP1u in H9c2 cells.
The acute response to heart injury involves the
production of inflammatory cytokines; however, sustained and
long-term inflammation is a primary cause of further damage that
can manifest as cardiac hypertrophy and chronic heart failure
(30). Both experimental and
clinical studies have shown that increased levels of inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β and IL-6, are important in the pathogenesis of chronic
heart injuries, contributing to cardiac remodeling by influencing
hypertrophy, fibrosis and apoptosis (31). Although all of these cytokines are
involved in cardiac disorders, various results indicate that the
healthy heart does not express TNF, whereas the failing heart
generates substantial levels (32), suggesting a role of TNF-α in more
severe cardiac disorder. Therefore, various chemokines, such as
CXCL10 (IP-10) and CCL8 (MCP2), may also be associated with various
heart disorders, including atherosclerosis, ischemia of the
myocardium and cardiac fibrosis (33–35).
Over recent decades, mounting evidence has indicated a pathogenic
role of B19 in various cardiac disorders, including myocarditis,
acute myocardial infarction, left ventricular dysfunction,
inflammatory cardiomyopathy and heart failure (10,12–16).
In this study, B19-VP1u induced significant expression of MCP2,
IP-10, TNF-α, IL-1β and IL-6 in H9c2 cells and HBoV-VP1u induced
significant expression of IL-1β and IL-6. Therefore, although
B19-VP1u and HBoV-VP1u may differ in their induction of
proinflammatory factors in H9c2 cells, the above results imply that
both B19-VP1u and HBoV-VP1u are involved in cardiac disorders.
However, further investigations must be performed to verify the
precise role of HBoV-VP1u in cardiac injuries.
B19 infection has been strongly associated with
sPLA2 activity, mediated by its VP1u region. Meanwhile, a previous
study reported that VP1u in HBoV exhibited sPLA2-like enzymatic
activity (25). The sPLA2-like
motif of the VP1-unique (VP1u) region of B19 and HBoV has been
demonstrated to be critical to B19 neutralization and infectivity
(2,17–19,27,36).
However, the role of sPLA2-like activity in the potential
pathogenic function of HBoV in cardiac injury remains unclear. In
this study, different profiles of inflammatory indicators were
induced in H9c2 cells by B19-VP1u and HBoV-VP1u, respectively.
Notably, no induction of the inflammatory indicators was observed
in H9c2 cells that were transfected with the mutant forms of
B19-VP1u and HBoV-VP1u. These findings demonstrate, to the best of
our knowledge for the first time, the critical role of the sPLA2
activity of B19-VP1u and HBoV-VP1u in inducing cardiac inflammatory
cytokines.
Interestingly, the chemokine MCP-2 and IP-10 were
not significantly induced in H9c2 cells that were transfected with
pEGFP-HBoV-VP1u, indicating the possibility that the VP1-u region
of HBoV has a different specificity and only mildly affects the
induction of chemokines in cardiac cells. However, further work is
required to clarify the precise mechanism of HBo-VP1u in cardiac
cells. Overall, B19-VP1u probably has a more prominent role than
HBoV-VP1u in the inflammatory responses that are associated with
cardiac injury.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Chung Shan
Medical University and Chi-Mei Medical Center cooperative project
(grant no. CMCSMU10502). Consumptive materials were partially
supported by the National Science Council (grant nos.
NSC99-2320-B-040-007-MY3 and NSC101-2314-B-040-008). The funders
had no role in study design, data collection and analysis, decision
to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HPH was involved in the study conception and design,
drafting and revising of the manuscript, and analysis of data. CCC
was involved in drafting of the manuscript, study conception and
design, and performing experiments. DWL and YFS performed
experiments. TCH and BST was involved in the study conception and
design, drafting and revising of the manuscript, analysis of data
and study supervision.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berns KI and Parrish CR:
ParvoviridaeFields Virology. Knipe DM, Howley PM, Cohen JI, Griffin
DE, Lamb RA, Martin MA, Racaniello VR and Roizman B: 6th.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 1768–1791.
2013
|
|
2
|
Qiu J, Söderlund-Venermo M and Young NS:
Human parvoviruses. Clin Microbiol Rev. 30:43–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cossart YE, Field AM, Cant B and Widdows
D: Parvovirus-like particles in human sera. Lancet. 1:72–73. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown KE and Young NS: Parvovirus B19 in
human disease. Annu Rev Med. 48:59–67. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heegaard ED and Brown KE: Human parvovirus
B19. Clin Microbiol Rev. 15:485–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Young NS and Brown KE: Parvovirus B19. N
Engl J Med. 350:586–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Page C, François C, Goëb V and Duverlie G:
Human parvovirus B19 and autoimmune diseases. Review of the
literature and pathophysiological hypotheses. J Clin Virol.
72:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allander T, Tammi MT, Eriksson M, Bjerkner
A, Tiveljung-Lindell A and Andersson B: Cloning of a human
parvovirus by molecular screening of respiratory tract samples.
Proc Natl Acad Sci USA. 102:pp. 12891–12896. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jartti T, Hedman K, Jartti L, Ruuskanen O,
Allander T and Söderlund-Venermo M: Human bocavirus-the first 5
years. Rev Med Virol. 22:46–64. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naides SJ and Weiner CP: Antenatal
diagnosis and palliative treatment of non-immune hydrops fetalis
secondary to fetal parvovirus B19 infection. Prenat Diagn.
9:105–114. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feldman AM and McNamara D: Myocarditis. N
Engl J Med. 343:1388–1398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murry CE, Jerome KR and Reichenbach DD:
Fatal parvovirus myocarditis in a 5-year-old girl. Hum Pathol.
32:342–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamparter S, Schoppet M, Pankuweit S and
Maisch B: Acute parvovirus B19 infection associated with
myocarditis in an immunocompetent adult. Hum Pathol. 34:725–728.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kühl U, Pauschinger M, Bock T, Klingel K,
Schwimmbeck CP, Seeberg B, Krautwurm L, Poller W, Schultheiss HP
and Kandolf R: Parvovirus B19 infection mimicking acute myocardial
infarction. Circulation. 108:945–950. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pankuweit S, Moll R, Baandrup U, Portig I,
Hufnagel G and Maisch B: Prevalence of the parvovirus B19 genome in
endomyocardial biopsy specimens. Hum Pathol. 34:497–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pankuweit S, Lamparter S, Schoppet M and
Maisch B: Parvovirus B19 genome in endomyocardial biopsy specimen.
Circulation. 109:e1792004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dorsch S, Liebisch G, Kaufmann B, von
Landenberg P, Hoffmann JH, Drobnik W and Modrow S: The VP1 unique
region of parvovirus B19 and its constituent phospholipase A2-like
activity. J Virol. 76:2014–2018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Filippone C, Zhi N, Wong S, Lu J, Kajigaya
S, Gallinella G, Kakkola L, Söderlund-Venermo M, Young NS and Brown
KE: VP1u phospholipase activity is critical for infectivity of
full-length parvovirus B19 genomic clones. Virology. 374:444–452.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leisi R, Ruprecht N, Kempf C and Ros C:
Parvovirus B19 uptake is a highly selective process controlled by
VP1u, a novel determinant of viral tropism. J Virol.
87:13161–13167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu TC, Tsai CC, Chiu CC, Hsu JD and Tzang
BS: Exacerbating effects of human parvovirus B19 NS1 on liver
fibrosis in NZB/W F1 mice. PLoS One. 8:e683932013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai CC, Chiu CC, Hsu JD, Hsu HS, Tzang BS
and Hsu TC: Human parvovirus B19 NS1 protein aggravates liver
injury in NZB/W F1 mice. PLoS One. 8:e597242013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nie X, Zhang G, Xu D, Sun X, Li Z, Li X,
Zhang X, He F and Li Y: The VP1-unique region of parvovirus B19
induces myocardial injury in mice. Scand J Infect Dis. 42:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tzang BS, Lin TM, Tsai CC, Hsu JD, Yang LC
and Hsu TC: Increased cardiac injury in NZB/W F1 mice received
antibody against human parvovirus B19 VP1 unique region protein.
Mol Immunol. 48:1518–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bogomolovas J, Šimoliūnas E, Rinkūnaitė I,
Smalinskaitė L, Podkopajev A, Bironaitė D, Weis CA, Marx A,
Bukelskienė V, Gretz N, et al: A novel murine model of parvovirus
associated dilated cardiomyopathy induced by immunization with
VP1-unique region of parvovirus B19. Biomed Res Int.
2016:16271842016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu XW, Liu WP, Qi ZY, Duan ZJ, Zheng LS,
Kuang ZZ, Zhang WJ and Hou YD: Phospholipase A2-like activity of
human bocavirus VP1 unique region. Biochem Biophys Res Commun.
365:158–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tzang BS, Tsay GJ, Lee YJ, Li C, Sun YS
and Hsu TC: The association of VP1 unique region protein in acute
parvovirus B19 infection and anti-phospholipid antibody production.
Clin Chim Acta. 378:59–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiu CC, Shi YF, Yang JJ, Hsiao YC, Tzang
BS and Hsu TC: Effects of human Parvovirus B19 and Bocavirus VP1
unique region on tight junction of human airway epithelial A549
cells. PLoS One. 9:e1079702014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zádori Z, Szelei J, Lacoste MC, Li Y,
Gariépy S, Raymond P, Allaire M, Nabi IR and Tijssen P: A viral
phospholipase A2 is required for parvovirus infectivity. Dev Cell.
1:291–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonnerot C, Rocancourt D, Briand P,
Grimber G and Nicolas JF: A beta-galactosidase hybrid protein
targeted to nuclei as a marker for developmental studies. Proc Natl
Acad Sci USA. 84:pp. 6795–6799. 1987; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Valaperti A: Drugs targeting the canonical
NF-κB pathway to treat viral and autoimmune myocarditis. Curr Pharm
Des. 22:440–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gullestad L, Ueland T, Vinge LE, Finsen A,
Yndestad A and Aukrust P: Inflammatory cytokines in heart failure:
Mediators and markers. Cardiology. 122:23–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feldman AM, Combes A, Wagner D, Kadakomi
T, Kubota T, Li YY and McTiernan C: The role of tumor necrosis
factor in the pathophysiology of heart failure. J Am Coll Cardiol.
35:537–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Frangogiannis NG: Chemokines in the
ischemic myocardium: From inflammation to fibrosis. Inflamm Res.
53:585–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Braunersreuther V, Mach F and Steffens S:
The specific role of chemokines in atherosclerosis. Thromb Haemost.
97:714–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ardigo D, Assimes TL, Fortmann SP, Go AS,
Hlatky M, Hytopoulos E, Iribarren C, Tsao PS, Tabibiazar R and
Quertermous T; ADVANCE Investigators, : Circulating chemokines
accurately identify individuals with clinically significant
atherosclerotic heart disease. Physiol Genomics. 31:402–409. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ros C, Gerber M and Kempf C:
Conformational changes in the VP1-unique region of native human
parvovirus B19 lead to exposure of internal sequences that play a
role in virus neutralization and infectivity. J Virol.
80:12017–12024. 2006. View Article : Google Scholar : PubMed/NCBI
|