Introduction
Prolactinoma is the most common hormone-secreting
pituitary tumor, with an estimated prevalence of 10 per million
adults (1). Prolactinoma may
induce gonadal and sexual dysfunction related to hyperprolactinemia
in addition to other symptoms related to tumor expansion (2). The clinical presentation of
prolactinoma in females is usually more obvious compared with
males, particularly owing to the manifestation of classical
amenorrhea-galactorrhea syndrome (3). Dopamine agonists are commonly used to
treat prolactinoma, as it normalizes serum prolactin (PRL) levels
and reduces tumor size (4). For
patients with drug resistant tumors, those that are pregnant or
have malignant prolactinoma, transsphenoidal adenoma resection is
considered a second-line therapy (5). However, the prognosis of patients
undergoing surgery, particularly for those with invasive
macroprolactinomas, is still poor (6). Therefore, novel therapies are
required for the treatment of dopamine agonist-resistant
prolactinomas.
Estrogen is an important hormone that serves a key
role in regulation of physiological processes and cell growth
(7). Because estrogen stimulates
the proliferation of pituitary lactotrophs and promotes the
synthesis and secretion of PRL, estrogen receptors (ERs) may also
be involved in the progression of prolactinomas (8). ERα has been implicated in
prolactinoma proliferation and PRL secretion by regulating various
growth factors, including pituitary tumor transforming gene, basic
fibroblast growth factor and transforming growth factor β1 (TGFβ1)
(9). Therefore, ER inactivation
may reduce PRL hypersecretion and control lactotroph adenomatous
growth (10). Notably, a
significant correlation between ERα and PRL, as well as tumor
volume and TGFβ1 expression, was observed in patients with
prolactinoma (11). In addition,
increased activity of estrogen-responsive genes was previously
demonstrated to promote estrogen-regulated tumor proliferation
and/or PRL secretion in patients with prolactinoma (12). Although ERs are considered a
potential therapeutic target in prolactinoma, the regulatory
mechanisms of ERs in prolactinoma are not yet fully understood.
The inositol-requiring enzyme 1 (IRE1)/X-box binding
protein 1 (XBP1) signaling pathway is a conserved unfolded protein
response pathway that is involved in endoplasmic reticulum stress
(13). Under normal circumstances
IRE1 binds to glucose-regulated protein, 78 kDa (GRP78) in the
endoplasmic reticulum, whereas endoplasmic reticulum stress may
inhibit this interaction. IRE1 activation facilitates the recovery
of endoplasmic reticulum stress by removing an intron from the XBP1
mRNA. This results in a frame-shift in the XBP1 coding sequence,
which leads to translation of the active XBP1 isoform (S) (14). The isoform encoded by the unspliced
mRNA, XBP1(U), which is constitutively expressed, and thought to
function as a negative feedback regulator of XBP1(S), which shuts
off transcription of target genes during the recovery phase of ER
stress (13). Previous studies
have also demonstrated that the IRE1/XBP1 signaling pathway serves
an important role in tumor progression. In the present study, the
ER antagonist fulvestrant was used to treat the GH3 prolactinoma
cell line, and cellular proliferation, apoptosis and preprolactin
(PPL) and PRL secretion levels were monitored over a period of
time. In addition, the protein expression levels of IRE1, XBP1 and
GRP78 in fulvestrant-treated GH3 cells were measured. The results
demonstrated that fulvestrant may have an inhibitory effect on GH3
prolactinoma cells by targeting the IRE1/XBP1 signaling
pathway.
Materials and methods
Cell culture and treatment
GH3 prolactinoma cell line was purchased from the
cell culture center of Sun Yat-Sen University (Guangzhou, China).
Cells were cultured in Dulbecco's modified Eagle's medium (BD
Biosciences, Franklin Lakes, NJ, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified atmosphere containing 5%
CO2. The medium was replaced every 48 h and cells in the
logarithmic growth phase were used in all subsequent treatments:
According to preliminary results (data not shown) GH3 cells
(1×105 cells/ml) were seeded in 96-well plates and
treated with 10−6 mol/l fulvestrant (ICI-182,780, Abmole
bioscience, Hongkong, China; www.abmole.cn/search?q=fulvestrant). Cells were
harvested at 2, 4, 8, 12 and 24 h post-treatment. Untreated cells
(0 h) were used as controls.
Cell growth and MTT viability
assay
Cell growth was observed using an Olympus CKX41
inverted microscope (Olympus Corporation, Tokyo, Japan). Cell
viability was quantified by MTT assay (Shanghai Jiang Lai
Biotechnology Co., Ltd., China), according to the manufacturer's
protocol. Briefly, 100 µg/ml MTT solution was added to each well.
Following 4 h of incubation, the supernatant was removed and 150 µl
dimethylsulphoxide was added to dissolve the formazan crystals.
Optical density was measured at 490 nm using an ultraviolet
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Hoechst 33258 staining
Following fulvestrant treatment, morphological
changes of the nuclei were observed over time (0–24 h) by Hoechst
33258 staining (Beyotime Institute of Biotechnology, Shanghai,
China). Briefly, cells were washed with PBS three times and fixed
in 4% paraformaldehyde at room temperature (25°C) for 20 min.
Subsequently, cells were permeabilized by 0.5% Triton X-100 for 15
min, followed by staining with Hoechst 33258 for 10 min in the
dark. Samples were washed with PBS three times prior to mounting,
and images were captured under an Olympus CX21 fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Cell apoptosis assay
Following fulvestrant treatment, GH3 cell apoptosis
was quantified by staining with 5 µl Annexin V-fluorescein
isothiocyanate and 2.5 µl propidium iodide (Miltenyi Biotec Inc.,
CA, USA). Following incubation on ice for 10 min in the dark, 400
µl 1X Annexin V binding buffer was added. Stained cells were
detected using an Attune™ NxT version 2.5 flow cytometer
(Thermo Fisher Scientific, Inc.; Attune NxT software version 2.5).
Apoptosis rate was calculated as percentage of cells in Q2 which
corresponds to early stage apoptosis.
PPL and PRL assays
Following treatment with fulvestrant, GH3 cells were
seeded into 96-well plates (1×105 cells/well) and
cultured for 24 h at 37°C in a humidified atmosphere containing 5%
CO2. The PPL and PRL concentrations in the cell culture
supernatants of GH3 cells from different treatment groups were
detected using rat PRL (rPRL; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), according to the manufacturer's protocol. PRL and
PRL concentrations were calculated using a standard curve of known
concentration.
Western blot analysis
Total protein was extracted from GH3 cells in the
different treatment groups using RIPA cell lysis buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following
centrifugation at 10,000 × g for 15 min at 4°C, 40 µg proteins were
separated by 12% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked with 5% skim milk for 1 h at 4°C and incubated with
primary antibodies against IRE1 (cat. no. ab48187; 1:1,000), XBP1
(cat. no. ab37152; 1:500), GRP78 (cat. no. ab21685; 1:500) and
β-actin (cat. no. ab8227; 1:1,000; all purchased from Abcam,
Cambridge, UK) overnight at 4°C. Subsequently, membranes were
washed with Tris-buffered saline containing 0.05% Tween-20 (TBST)
three times and incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibodies (cat. no. A0208; 1:5,000;
Beyotime Institute of Biotechnology, Haimen, China) for 1 h at
25°C. Membranes were washed a final time with TBST and the protein
bands were visualized using the Enhanced Chemiluminescence Reagent
(Beyotime Institute of Biotechnology). Protein expression levels
were quantified using AlphaView software 3.2 CD in a FluorChem M
Ultraviolet Gel Imaging System (ProteinSimple, Santa Clara, CA,
USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS version
17.0 (SPSS, Inc., Chicago, IL, USA). Comparisons between different
groups were determined by one-way analysis of variance followed by
the Least Significant Difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Fulvestrant inhibits GH3 cell
viability
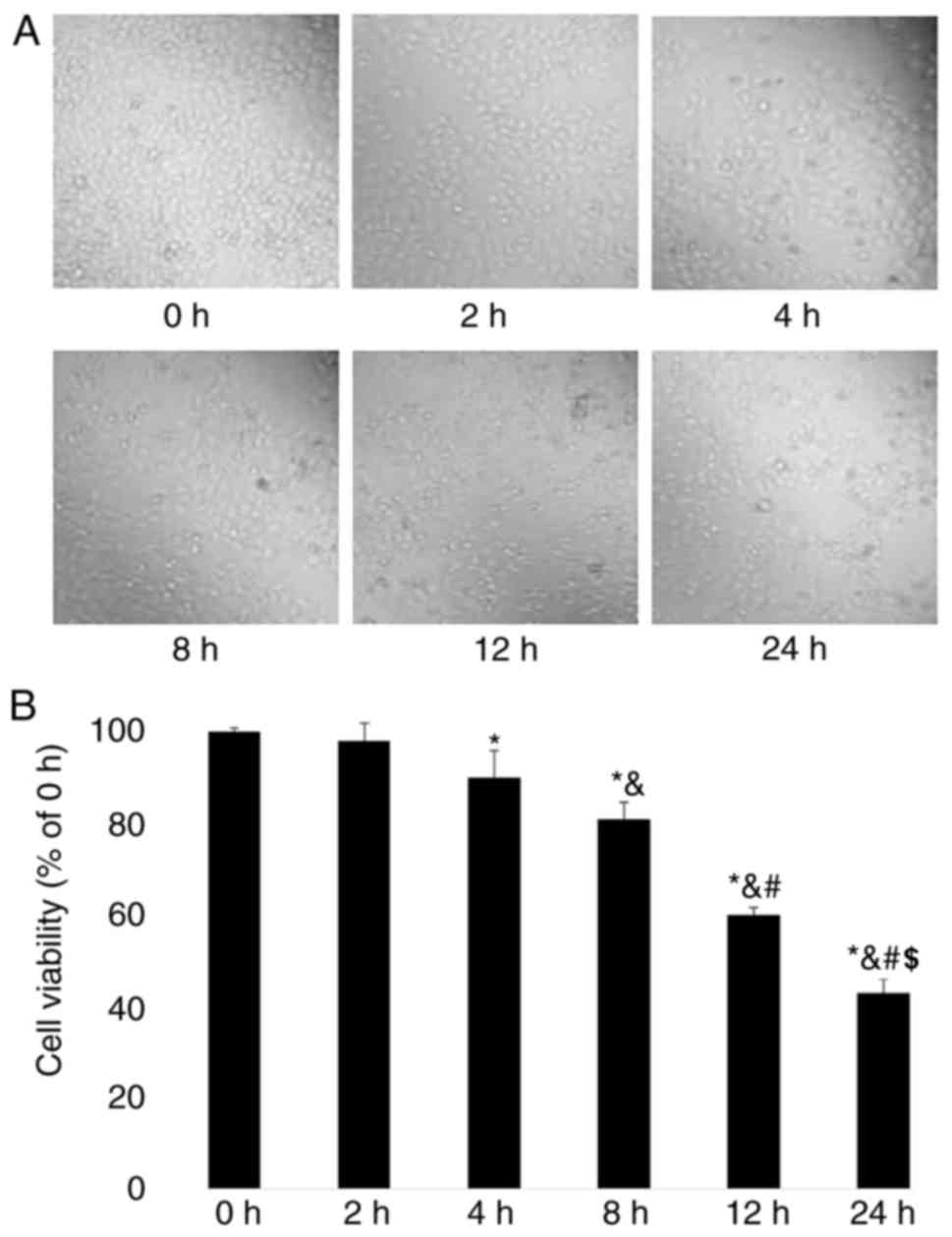
The effects of fulvestrant on GH3 cell viability
were evaluated. Microscopic observation revealed that the cell
density of GH3 cells was reduced after 4 h of fulvestrant treatment
(Fig. 1A); following 24 h of
treatment, the density of GH3 cells was gradually reduced in time.
The viability of GH3 cells was quantified using MTT assay, which
demonstrated that cell viability was significantly reduced in a
time-dependent manner between 8 and 24 h following fulvestrant
treatment (P<0.05; Fig.
1B).
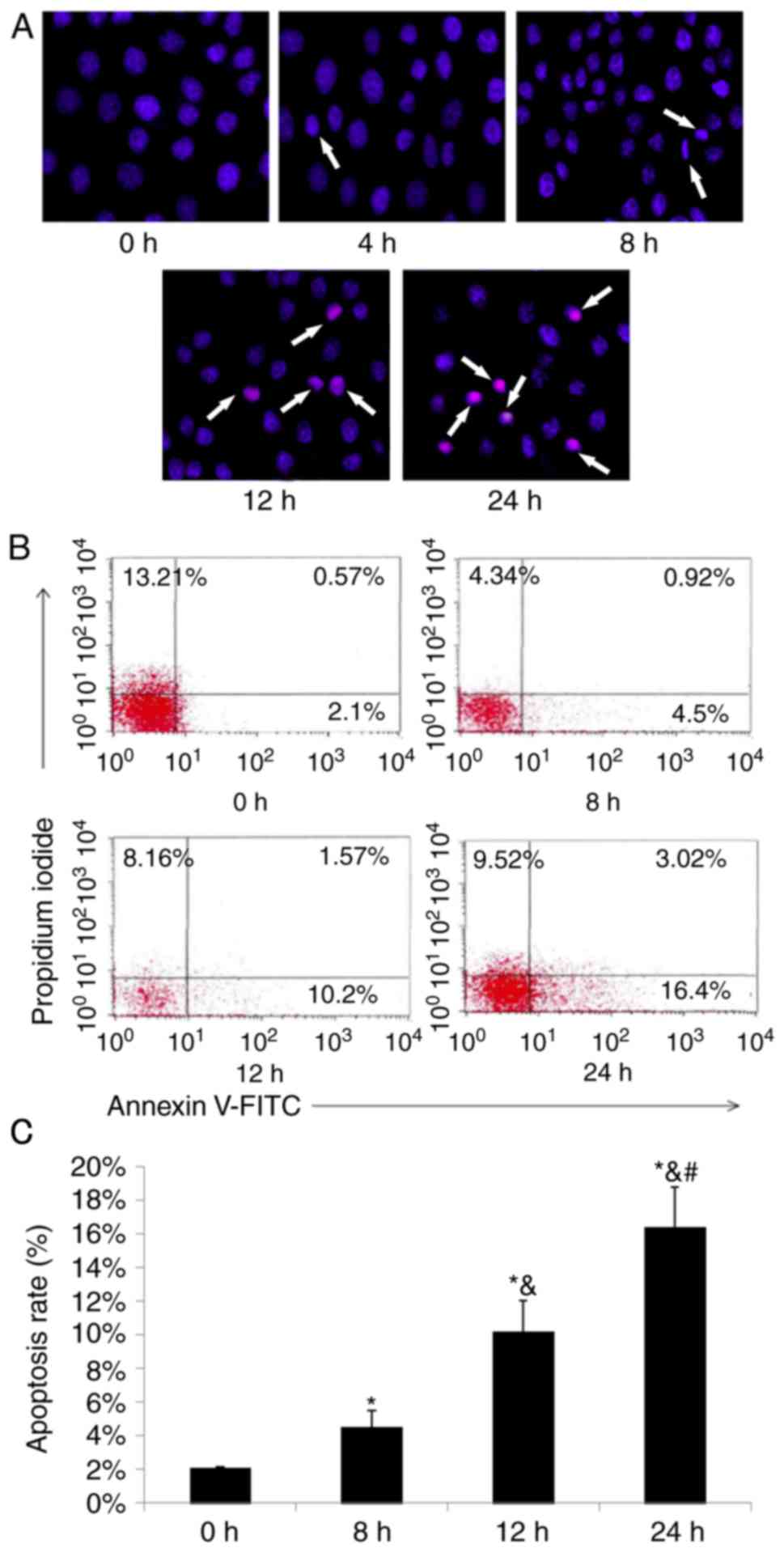
Fulvestrant promotes apoptosis of GH3
cells
As cell viability was significantly reduced
following fulvestrant treatment, cell apoptosis was analyzed. Prior
to treatment with fulvestrant, GH3 cells exhibited regular and full
nuclei, uniformly colored chromatin, as determined by microscopic
observation (Fig. 2A 0 h).
However, obvious shrinkage and fragmentation of the nuclei were
observed in GH3 cells at 8 h following fulvestrant treatment. As
treatment time increased, the apoptotic morphology of GH3 cells
became more apparent (Fig. 2A).
Subsequently, the apoptotic rates of GH3 cells were determined by
flow cytometry, which revealed that the apoptotic rates of GH3
cells treated with fulvestrant significantly increased in a
time-dependent manner (Fig. 2B and
C). At 8, 12 and 24 h of fulvestrant treatment, the early
apoptotic rates of GH3 cells were 4.5, 10.2 and 16.4%,
respectively.
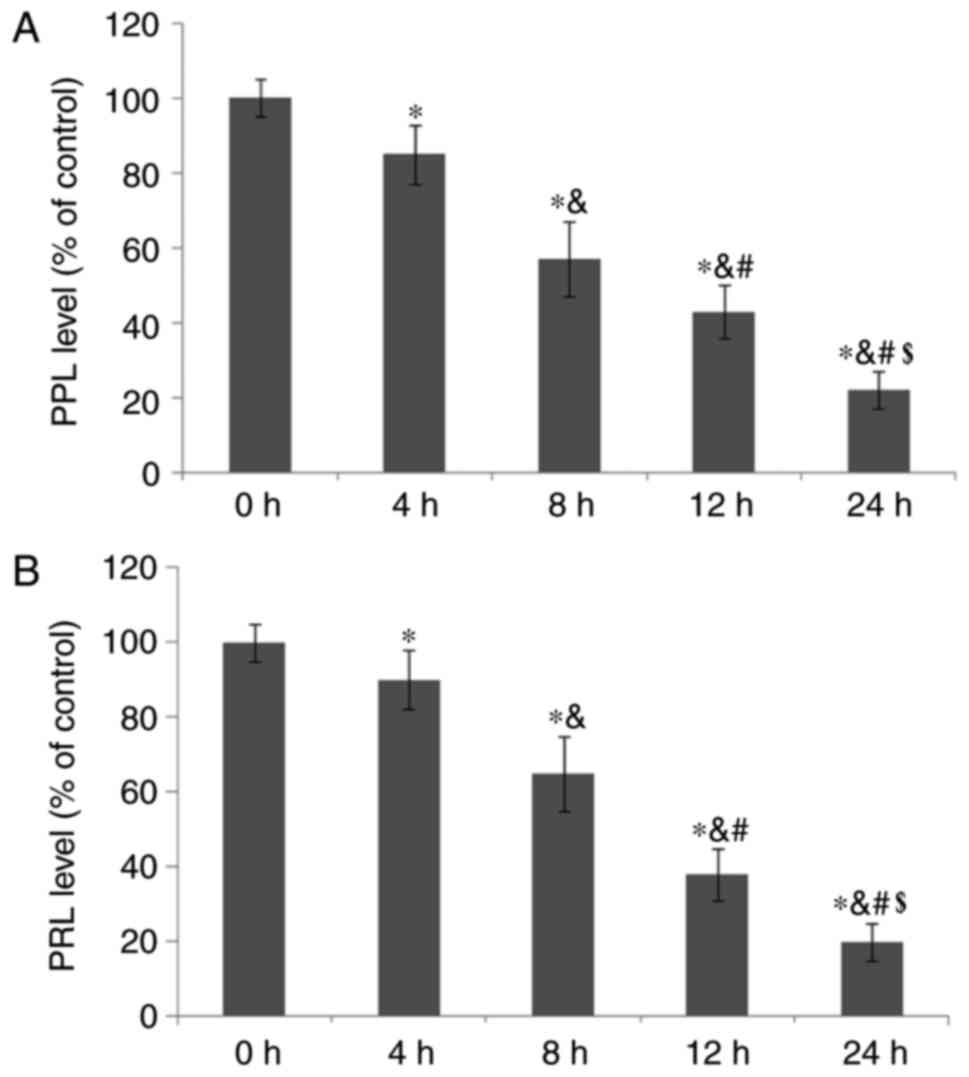
Fulvestrant reduces PPL and PRL
secretion from GH3 cells
The effects of fulvestrant treatment on PPL and PRL
secretion from GH3 cells were also evaluated. The results
demonstrated that PPL and PRL secretion levels were significantly
reduced in a time-dependent manner following treatment with
fulvestrant (P<0.05; Fig. 3A and
B, respectively).
Fulvestrant reduces IRE1, XBP1 and
GRP78 expression levels in GH3 cells
To examine the potential mechanisms of action of
fulvestrant on GH3 cells, the protein expressions levels of IRE1,
XBP1 and GRP78 were detected following treatment (Fig. 4A); the expression levels of IRE1,
XBP1 and GRP78 were significantly reduced by fulvestrant in a
time-dependent manner (Fig.
4B).
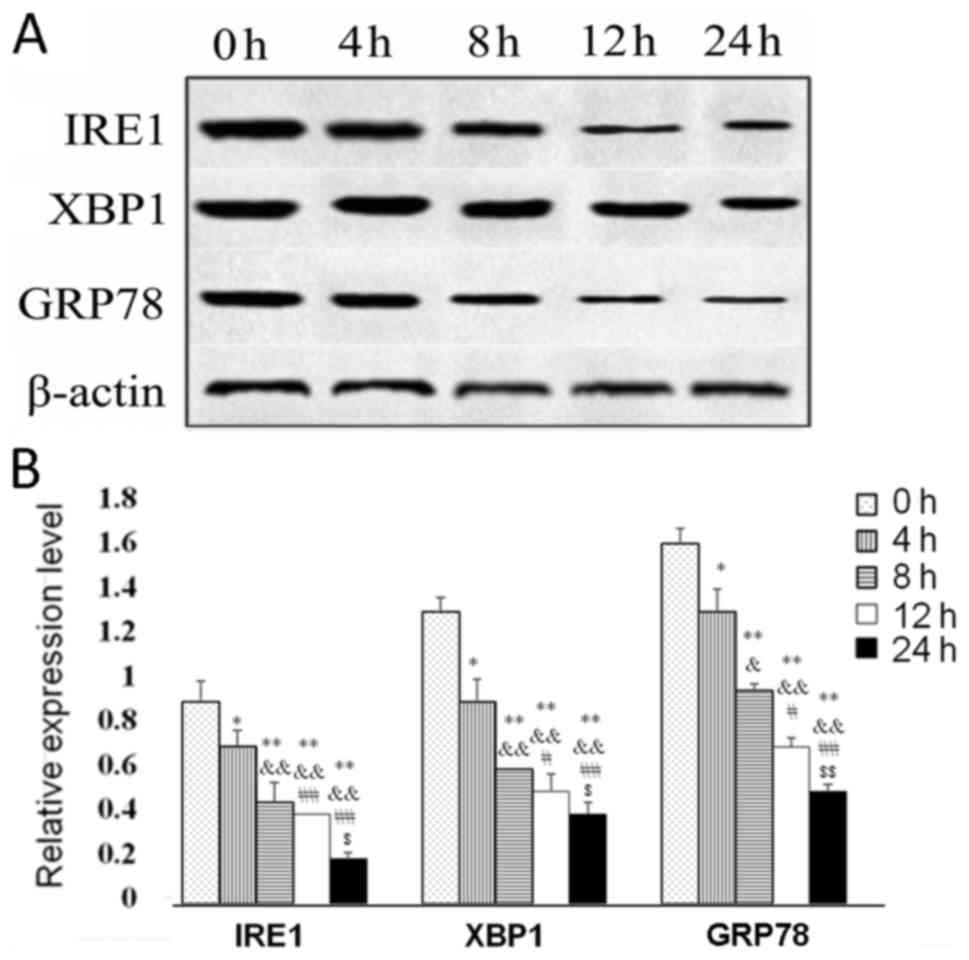 | Figure 4.Protein expression levels of IRE1,
XBP1 and GRP78 in GH3 cells treated with fulvestrant for 0, 2, 4,
8, 12 and 24 h. (A) Representative western blot images of IRE1, XBP
and GRP78 protein expression levels; β-actin was used as a loading
control. (B) Quantification of protein expression level from (A).
*P<0.05 vs. 0 h; &P<0.05 vs. 4 h;
#P<0.05 vs. 8 h; $P<0.05 vs. 12 h.
**P<0.01 vs. 0 h; &&P<0.01 vs. 4 h;
##P<0.01 vs. 8 h; $$P<0.01 vs. 12 h.
GRP78, glucose-regulated protein, 78 kDa; IRE1, inositol-requiring
enzyme 1; XBP1, X-box binding protein 1. |
Discussion
Prolactinoma is a common type of hormone-secreting
tumor in the pituitary gland (15). As significant correlations have
been identified between ERs and the occurrence, development and
invasion of prolactinomas, ERs may be an effective therapeutic
target for prolactinoma (11).
Fulvestrant is an ERα antagonist that exhibits great potential for
the treatment of cancer (16). It
has been reported previously that fulvestrant inhibits tumor
proliferation and PRL secretion by blocking ERα in a F344 rat model
of prolactinoma (17). In
addition, it was identified that fulvestrant significantly inhibits
cell proliferation and PRL secretion in an MMQ prolactinoma cell
line by inhibiting ERα (18). In
the present study, fulvestrant treatment reduced the viability of
GH3 cells and increased the apoptotic rate of GH3 cells. In
addition, PPL and PRL secretion by GH3 cells were significantly
reduced following fulvestrant treatment. These results suggested
that fulvestrant may exhibit antitumor effects on prolactinoma.
Binding of estrogen to the ER may induce prolactinoma by promoting
pituitary lactotroph proliferation and PRL secretion (17). ERα activation has been demonstrated
to promote prolactinoma cell proliferation by regulating the
transcription of various target genes, including PRL, B-cell
CLL/lymphoma 2 (Bcl-2), vascular endothelial growth factor and
matrix metalloproteinase 9 (19).
However, fulvestrant blocks the interaction between estrogen and
ER, thereby inhibiting proliferation of prolactinoma cells. It was
hypothesized that fulvestrant may be used for the effective
treatment of prolactinoma in the future.
The IRE1/XBP1 pathway is a conserved unfolded
protein response pathway that is involved in endoplasmic reticulum
stress and is considered to be an important pathway in tumor
progression (13,20,21).
It has been reported that the IRE1α/XBP1 pathway promotes cell
proliferation and progression of melanoma by activating interleukin
6/signal transducer and activator of transcription 3 signaling
(22). The IRE1α/XBP1 signaling
pathway is also involved in the development of multiple myeloma and
is closely associated with the effects of treatment and prognosis
(23). IRE1, XBP1 and GRP78 are
three key components in the IRE1/XBP1 pathway, and their abnormal
expression is commonly associated with tumor progression. For
example, ERβ-induced downregulation of IRE1α and XBP1 was reported
to be associated with decreased survival of breast cancer cells
(24), whereas increased
expression of IRE1α was demonstrated to promote cell proliferation
and invasion of colorectal carcinoma cells (25). GRP78 is a binding target of IRE1 in
the endoplasmic reticulum, and the GRP78-specific monoclonal
antibody MAb159 has been identified to inhibit tumor growth and
metastasis by regulating the phosphoinositide 3-kinase pathway
(26). In the present study,
significantly decreased expression levels of IRE1, XBP1 and GRP78
were observed in GH3 cells treated with fulvestrant, which occurred
in a time-dependent manner. These findings are consistent with
previous studies, and they further demonstrated that the inhibitory
effects of fulvestrant on GH3 cells were associated with the
IRE1/XBP1 signaling pathway. As previously described, upregulation
of wild-type ERβ1 or treatment with ERβ agonists enhances apoptosis
of breast cancer cells in the presence of pharmacological inducers
of endoplasmic reticulum stress. However, targeting Bcl-2 to the
endoplasmic reticulum of ERβ1-expressing cells prevents apoptosis
induced by endoplasmic reticulum stress (27). Therefore, the present study
hypothesized that fulvestrant promotes endoplasmic reticulum
stress-regulated apoptosis of GH3 cells by regulating the IRE1/XBP1
signaling pathway.
In conclusion, fulvestrant inhibited proliferation
and promoted apoptosis of GH3 cells by downregulating the IRE1/XBP1
signaling pathway. However, the present study was limited to in
vitro experiments. Further studies on the anti-prolactinoma
effects of fulvestrant in an in vivo model are required to
better understand its regulatory mechanisms.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and YW designed the study. CW, MB, XW, CT, DZ and
LC performed the cell experiments. CW, GL and LX analyzed and
interpreted the data. JS performed the morphological examination.
CW drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gillam MP, Molitch ME, Lombardi G and
Colao A: Advances in the treatment of prolactinomas. Endocr Rev.
27:485–534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu J, Zheng X, Zhang W and Yang H: Current
drug withdrawal strategy in prolactinoma patients treated with
cabergoline: A systematic review and meta-analysis. Pituitary.
18:745–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colao A, Sarno AD, Cappabianca P, Briganti
F, Pivonello R, Somma CD, Faggiano A, Biondi B and Lombardi G:
Gender differences in the prevalence, clinical features and
response to cabergoline in hyperprolactinemia. Eur J Endocrinol.
148:325–331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beran RG: ‘Prolactinoma: Are dopamine
agonists still first choice?’. Intern Med J. 41:7572011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casanueva FF, Molitch ME, Schlechte JA,
Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A,
Fahlbusch R, et al: Guidelines of the pituitary society for the
diagnosis and management of prolactinomas. Clin Endocrinol (Oxf).
65:265–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klibanski A: Clinical practice.
Prolactinomas. N Engl J Med. 362:1219–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jameera Begam A, Jubie S and Nanjan MJ:
Estrogen receptor agonists/antagonists in breast cancer therapy: A
critical review. Bioorg Chem. 71:257–274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorski J, Wendell D, Gregg D and Chun TY:
Estrogens and the genetic control of tumor growth. Prog Clin Biol
Res. 396:233–243. 1997.PubMed/NCBI
|
|
9
|
Lv H, Li C, Gui S, Sun M, Li D and Zhang
Y: Effects of estrogen receptor antagonist on biological behavior
and expression of growth factors in the prolactinoma MMQ cell line.
J Neurooncol. 102:237–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kansra S, Yamagata S, Sneade L, Foster L
and Ben-Jonathan N: Differential effects of estrogen receptor
antagonists on pituitary lactotroph proliferation and prolactin
release. Mol Cell Endocrinol. 239:27–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv H, Li C, Gui S and Zhang Y: Expression
of estrogen receptor α and growth factors in human prolactinoma and
its correlation with clinical features and gender. J Endocrinol
Invest. 35:174–180. 2012.PubMed/NCBI
|
|
12
|
Chaidarun SS, Swearingen B and Alexander
JM: Differential expression of estrogen receptor-beta (ER beta) in
human pituitary tumors: Functional interactions with ERα and a
tumor-specific splice variant. J Clin Endocrinol Metab.
83:3308–3315. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hay MP, Jiang D, Kozak M, Niwa M and Koong
AC: Inhibition of the IRE1α-XBP1 pathway: A new approach to
targeting the tumour microenvironment. Journal. 2015.
|
|
14
|
Chen Y and Brandizzi F: IRE1: ER stress
sensor and cell fate executor. Trends Cell Biol. 23:547–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rigante M, Massimi L, Parrilla C, Galli J,
Caldarelli M, Di Rocco C and Paludetti G: Endoscopic
transsphenoidal approach versus microscopic approach in children.
Int J Pediatr Otorhinolaryngol. 75:1132–1136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagykalnai T, Landherr L, Laczo I and Piko
B: Fulvestrant (Faslodex ®)for hormone sensitive breast
cancer. A review. Magy Onkol. 59:251–257. 2015.(In Hungarian).
|
|
17
|
Cao L, Gao H, Gui S, Bai G, Lu R, Wang F
and Zhang Y: Effects of the estrogen receptor antagonist
fulvestrant on F344 rat prolactinoma models. J Neurooncol.
116:523–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leng L and Zhang Y: Effects of an estrogen
receptor antagonist on proliferation, prolactin secretion and
growth factor expression in the MMQ pituitary prolactinoma cell
line. J Clin Neurosci. 18:1694–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leng L and Zhang Y: Effects of an estrogen
receptor antagonist on proliferation, prolactin secretion and
growth factor expression in the MMQ pituitary prolactinoma cell
line. J Clin Neurosci. 18:1694–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koong AC, Chauhan V and Romero-Ramirez L:
Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol Ther.
5:756–759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Henkel A and Green RM: The unfolded
protein response in fatty liver disease. Semin Liver Dis.
33:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen C and Zhang X: IRE1α-XBP1 pathway
promotes melanoma progression by regulating IL-6/STAT3 signaling. J
Transl Med. 15:422017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Li Q, She T, Li H, Yue Y, Gao S,
Yan T, Liu S, Ma J and Wang Y: IRE1α-XBP1 signaling pathway, a
potential therapeutic target in multiple myeloma. Leuk Res.
49:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rajapaksa G, Nikolos F, Bado I, Clarke R,
Gustafsson JA and Thomas C: ERβ decreases breast cancer cell
survival by regulating the IRE1/XBP-1 pathway. Oncogene.
34:4130–4141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin C, Jin Z, Chen NZ, Lu M, Liu CB, Hu WL
and Zheng CG: Activation of IRE1α-XBP1 pathway induces cell
proliferation and invasion in colorectal carcinoma. Biochem Biophys
Res Commun. 470:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra
SK, Krasnoperov V, Dong D, Liu S, Li D, et al: Monoclonal antibody
against cell surface GRP78 as a novel agent in suppressing PI3K/AKT
signaling, tumor growth, and metastasis. Clin Cancer Res.
19:6802–6811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rajapaksa G, Nikolos F, Bado I, Clarke R,
Gustafsson JÅ and Thomas C: ERβ decreases breast cancer cell
survival by regulating the IRE1/XBP-1 pathway. Oncogene.
34:4130–4141. 2015. View Article : Google Scholar : PubMed/NCBI
|