Introduction
Allergic rhinitis (AR) is one of the common types of
rhinitis; previous epidemiological studies revealed an average
morbidity rate of 10–20% with an increasing annual tendency
(1,2). Over the past few years, AR morbidity
and severity have gradually increased in developed countries, and
AR has become a serious problem that is damaging public health. AR
may have a strong effect on the lives of people; apart from the
influence on the daily lives and careers, AR may induce some
complications, including nasosinusitis, bronchial asthma and
Eustachian tube dysfunction; however, the development of treatments
for AR has gained increasing attention. The pathogenesis of AR and
the pathogenic factors involved are diverse and complex, but are
not well understood. Current treatments for AR maintain the stage
of symptom remission; however, complete healing is desired.
Previous studies have demonstrated that allergic
diseases are not only localized but also may be systemic without
the full elucidation of pathogenesis (3,4).
Eosinophils (EOS) have remained the investigative focus in the
pathogenesis of allergic diseases, and tissue invasion by numerous
EOS is an important characteristic of this type of disease
(5). Eotaxins are members of the
C-C chemokine family that are able to induce the migration of EOS
into inflammatory tissues by combining with C-C chemokine receptor
3 (CCR3) expressed in EOS (6).
Different from most chemokines that function by binding to
receptors, Eotaxin signals are specifically transduced via CCR3
(6). As a transmembrane G
protein-coupled receptor, CCR3 is one of the main chemokine
receptors, and CCR3 controls the recruitment of EOS into local
inflammatory tissues (6). It was
reported that Eotaxin also served an important role within in
situ hematopoiesis of EOS in the inflammatory tissues by means
of CCR3 (7).
CCR3 is a specific receptor for Eotaxin; therefore,
CCR3 deficiency may lead to reduced binding of Eotaxin and
subsequent inhibition of phosphatidylinositol-3-kinase (PI3K)
signaling pathway activation and the inactivation of its subunit
PI3Kγ (8). Thus, various
downstream signaling responses may be affected. The activation and
migration of EOS and associated progenitor cells in the marrow
might be suppressed and, consequently, the amount of mature EOS in
the nasal mucosa may decrease, which may reduce degranulation and
reduce AR morbidity rates (9). The
morbidity of AR involving stimulation by allergens may activate the
binding of Eotaxin to CCR3 and induce the key downstream PI3 K
signaling pathway (8). This
process may trigger the activation and migration of EOS in the
marrow to the peripheral tissues and nasal mucosa (8). CCR3 knockout in the marrow cells may
inhibit the activation and migration of EOS and subsequently
prevent AR by inhibiting PI3K signaling (8,9).
These previous results revealed that gene therapy may be used to
regulate EOS and CCR3, which may provide novel insights into the
treatment of AR.
The present study established an AR model mouse and
examined the effects of CCR3 gene knockout on
inflammation-associated damage and EOS numbers in nasal mucosa,
peripheral blood, and nasal washings. In addition, alterations in
the expression levels of EOS peroxidase (EPO), EOS cationic protein
(ECP), major basic protein (MBP), interferon-γ (IFN-γ), interleukin
(IL)-4, IL-10, and immunoglobulin E (IgE) were investigated by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and ELISA.
Materials and methods
Materials
Aluminum hydroxide was purchased from Damao Chemical
Reagent Factory (Tianjin, China). The hematoxylin and eosin
(H&E) staining kit was obtained from Boster Biological
Technology (cat. no. AR11800-1; Pleasanton, CA, USA). The Leukocyte
Separation Medium kit for the separation of mouse peripheral blood
was obtained from Yanjin Biological (WBC1092, Shanghai, China).
Mouse interferon-γ (IFN-γ; cat. no. SEA033Hu), interleukin (IL)-4
(cat. no. SEA077Mu), IL-10 (cat. no. SEA056Mu), and IgE (cat. no.
SEA545Mu) ELISA kits were from Cloud-Clone Corp. (Wuhan, China).
Ovalbumin (OVA; cat. no. P0003) was purchased from Beijing Solarbio
Science & Technology Co. Ltd., (Beijing, China). Rapid Wright's
Staining kit was obtained from Nanjing KeyGen Biotech Co., Ltd.
(cat. no. KGA225; Jiangsu, China). TRIzol Reagent (cat. no. CW0580)
and HiFi Script first strand cDNA synthesis kit (cat. no. CW2569)
were obtained from CW Biotech (Beijing, China).
Animals
A total of 20 specific pathogen free (SPF) normal
BALB/c mice (10 male and 10 female; age, 6–8 weeks; weight, 20–28
g) were obtained from Experimental Animal Center of Medical college
of Nanchang University (Jiangxi, China). A total of 20 SPF
CCR3−/−BALB/c mice (10 male and 10 female; age, 6–8
weeks; weight, 20–28 g) were purchased from the Jackson Laboratory
(Strain J005440; Bar Harbor, ME, USA). The mice were raised with
free access to water and food in a sterile environment of 18–29°C,
40–70% relative humidity and a 12/12 h light/dark cycle. The
CCR3−/− BALB/c mice were identified by gene analysis,
including DNA extraction, PCR amplification, and agarose gel
electrophoresis according to the instructions provided by Jackson
Laboratory (Bar Harbor, ME, USA). B6 was the genomic DNA
(wild-type), which served as negative control. Target fragments of
wild-type and mutant were 120 and 280 bp, respectively. PCR
template was not directly used for electrophoretic detection. It
meant that template control was not set. The study protocol was
reviewed and approved by The Institutional Animal Care and Use
Committee, Nanchang University, [Jiangxi, China; approval number
YanLinShen (2013) No. 16] and was conducted in accordance with the
guidelines established by the Chinese Council of Animal Care
(10).
AR model mouse establishment
A total of 20 SPF normal BALB/c mice and 20 SPF
CCR3−/− BALB/c mice were randomly divided into four
groups (n=10/group): i) Normal CCR3+/+ control (CG); ii)
CCR3+/+ AR model (AR); iii) CCR3−/−CG; and
iv) CCR3−/−AR. Each mouse in the AR and
CCR3−/−AR groups was intraperitoneally injected with a
mixture of 10 µg OVA and 4 mg aluminum hydroxide twice per day, on
day 1 and day 15 to induce sensitization. Subsequently, between
days 21 and 27, 1 mg/ml OVA was dripped into the noses of these
mice twice per day for provocation. An equal volume of normal
saline was similarly administrated to the nasal cavities of mice in
the CG and CCR3−/−CG groups. Nasal washings were
collected. At 24 h after the final administration of the OVA drip,
all mice were anesthetized with 0.6% sodium pentobarbital (70
mg/kg) and euthanized. The upper palates of mice were dissected and
the bilateral nasal septum mucosae were collected. The mucosae were
fixed in 4% paraformaldehyde buffer at room temperature for 24 h
for subsequent experiments. In addition, the chests of mice were
opened and blood was extracted from the ventriculus dexter using
injection syringes and into plain tubes, which were stored at 4°C
for future analyses.
H&E staining
Fixed nasal mucosae were dehydrated in a gradient of
70, 80 and 90% ethanol, embedded in paraffin and sectioned (4 µm).
Sections were deparaffinized with dimethylbenzene at room
temperature for 10 min, rehydrated in graded ethanol at room
temperature for 5 min and stained with H&E at room temperature
for 15 sec, according to the manufacturer's protocols. The sections
were mounted with neutral resins and characterized under a
conventional light microscope (XDZ-103; Lingcheng Biotech,
Shanghai, China). EOS morphology was observed and EOS numbers were
counted.
Wright's staining
Separation medium (5 ml, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to a 15 ml centrifuge tube. Blood (15
ml) was carefully sampled with a suction pipette and added to the
surface of the separation medium. The mixture was centrifuged at
400–500 × g and room temperature for 20–30 min. The ring-like milky
layer of cells (one or two layers) was carefully collected with a
pipette. Following the addition of 10 ml cleaning solution, the
cells were mixed. The mixture was centrifuged at 250 × g and room
temperature for 10 min. The cell pellet was washed three times with
cleaning solution to obtain the leukocytes. Nasal washings were
centrifuged at 250 × g and room temperature for 5 min. The pellet
was resuspended in 0.3 ml phosphate buffer solution. Finally, blood
leukocyte smears and nasal washings smears were prepared by
smearing the suspension onto slides.
Following natural drying at room temperature for 30
min, smears were fixed in 4% paraformaldehyde at room temperature
for 15 min. Solution A from the Wright's staining kit was applied
to start staining for 1 min; solution B was added at double the
volume of solution A, and the slides were gently agitated to mix
the solutions. Staining was maintained at room temperature for 10
min. The staining solution was carefully washed away from one side
of the slide with purified water; the surrounding water-drop was
blotted with filter paper. Following natural drying at room
temperature for 30 min, the slides were analyzed under a microscope
(XDZ-103; Lingcheng Biotech). A total of 6 fields were examined per
slide. EOS morphology was observed and EOS numbers were
counted.
RT-qPCR
The blood was centrifuged at 2,000 × g and room
temperature for 20–30 min to obtain the serum. Total RNA was
extracted from the serum (200 µl) and nasal washings (200 µl) using
TRIzol (Life Technologies; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Total RNA concentration
and purity of the samples were determined using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA was reverse
transcribed to cDNA with the HiFi Script First Strand cDNA
synthesis kit, according to the manufacturer's protocol. Target
genes, including CCR3, EPO, ECP, and MBP were detected using a CFX
Connect PCR machine (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
using primers synthesized by Sangon Biotech Co., Ltd., (Shanghai,
China); primer sequences are provided in Table I, and GAPDH served as an internal
control. The PCR system (25 µl) contained 9.5 µl RNase free ddH20,
1 µl cDNA, 1 µl forward primer, 1 µl reverse primer, and 12.5 µl
2×ULtraSYBR Mixture (CW0957; Beijing CWBIO, Beijing, China). The
PCR thermocycling conditions were as follows: Pre-denaturation for
3 min at 95°C; followed by 40 cycles of denaturation for 10 sec at
95°C, annealing for 30 sec at 50°C (GAPDH, CCR3, and ECP) or 55°C
(EPO and MBP), elongation for 30 sec at 72°C. Amplification
products (5 µl) were loaded onto a 1% agarose gel for
electrophoresis, stained with ethidium bromide, and images were
captured with a ChemiDoc XRS Gel Imaging System (Bio-Rad
Laboratories, Inc.). The relative expression was calculated by the
2−ΔΔCq method (11).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer (5′→3′) |
|---|
| GAPDH | F:
GCAAGTTCAACGGCACAG |
|
| R:
CGCCAGTAGACTCCACGAC |
| CCR3 | F:
TGCTGAGATGTCCCAATA |
|
| R:
GCCAGGTCCAGATGTTTA |
| MBP | F:
ACCCACTATGGCTCCCTG |
|
| R:
CAATCCTCTTCCCTTTCCTT |
| ECP | F:
AGGCGAAGCGTGAGTTGC |
|
| R:
GATCGGGAATGTTGGTGC |
| EPO | F:
CGGGCGAAGACAAACAAAGG |
|
| R:
GGGCGGTAGAAGCCAAAGATC |
ELISA
Serum and nasal washing supernatant were used to
determine the levels of IFN-γ (pg/ml), IL-4 (pg/ml), IL-10 (pg/ml),
and IgE (ng/ml) expression by ELISA, following the manufacturer's
protocols. Expression levels were determined using a PT-3502 G
microplate reader (Potenov Tech, Beijing, China).
Statistical analysis
Each experiment was replicated three times. All data
were expressed as the mean ± standard deviation. Statistical
analysis was performed by using one-way analysis of variance
followed by a Tukey's post hoc test with SPSS software 11.5 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Mice identification
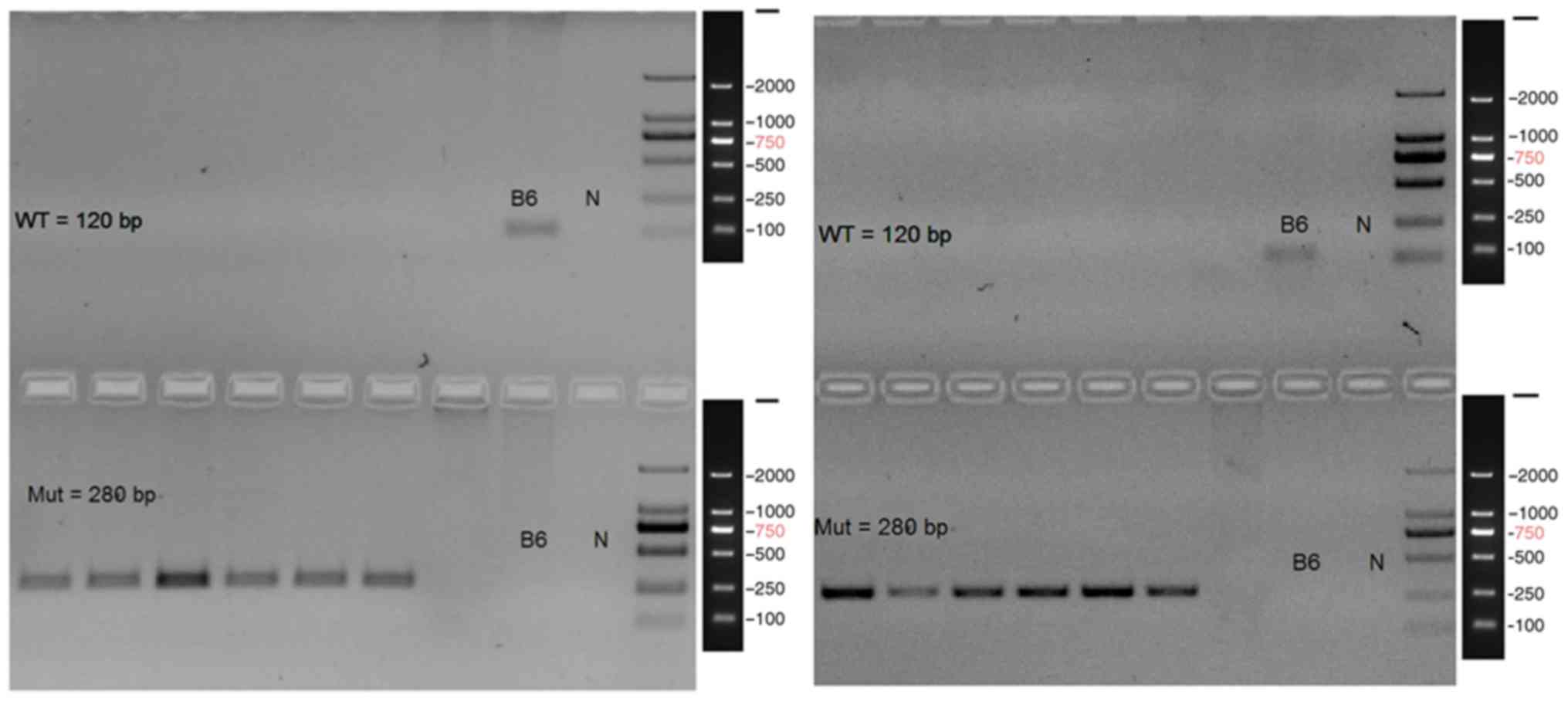
The homozygous genotypes of the CCR3 gene knockout
mice that were used in the following were confirmed by PCR analysis
(Fig. 1). The left gel shows the
PCR identification result of Nos. 1–6 CCR3 gene knockout mice. The
right gel shows the PCR identification result of nos. 7–12 CCR3
gene knockout mice. Bands at 120 and 280 bp represent the wild-type
and mutant, respectively. There were no bands in the location of
120 bp in the left six lanes, but in the location of 280 bp mutant
gene, notable bands were observed. Bands at 280 bp showed that the
CCR3 gene was completely knocked out. This suggested that these
mice were homozygotes with CCR3 gene knockout.
Histological examination of nasal
mucosae by H&E staining
Nasal mucosae were examined by H&E staining. In
the CG and CCR3−/−CG groups (Fig. 2A and B, respectively), the nasal
mucosae were intact with explicit structure and without notable
invasion of inflammatory cells. However, in the AR group (Fig. 2C), detachment of epithelial cells
with irregular arrangement, structural disorder of mucosal layer
and invasion of numerous inflammatory cells, including EOS, were
observed. Moreover, local swelling of nasal mucosae was observed.
Notably, in the CCR3−/−AR group (Fig. 2D), the damage of mucosal epithelial
cells and the detachment of epidermal cells were markedly
alleviated as compared with the AR group; however, slight edema in
epithelial cells and mild invasion of a few inflammatory cells were
observed.
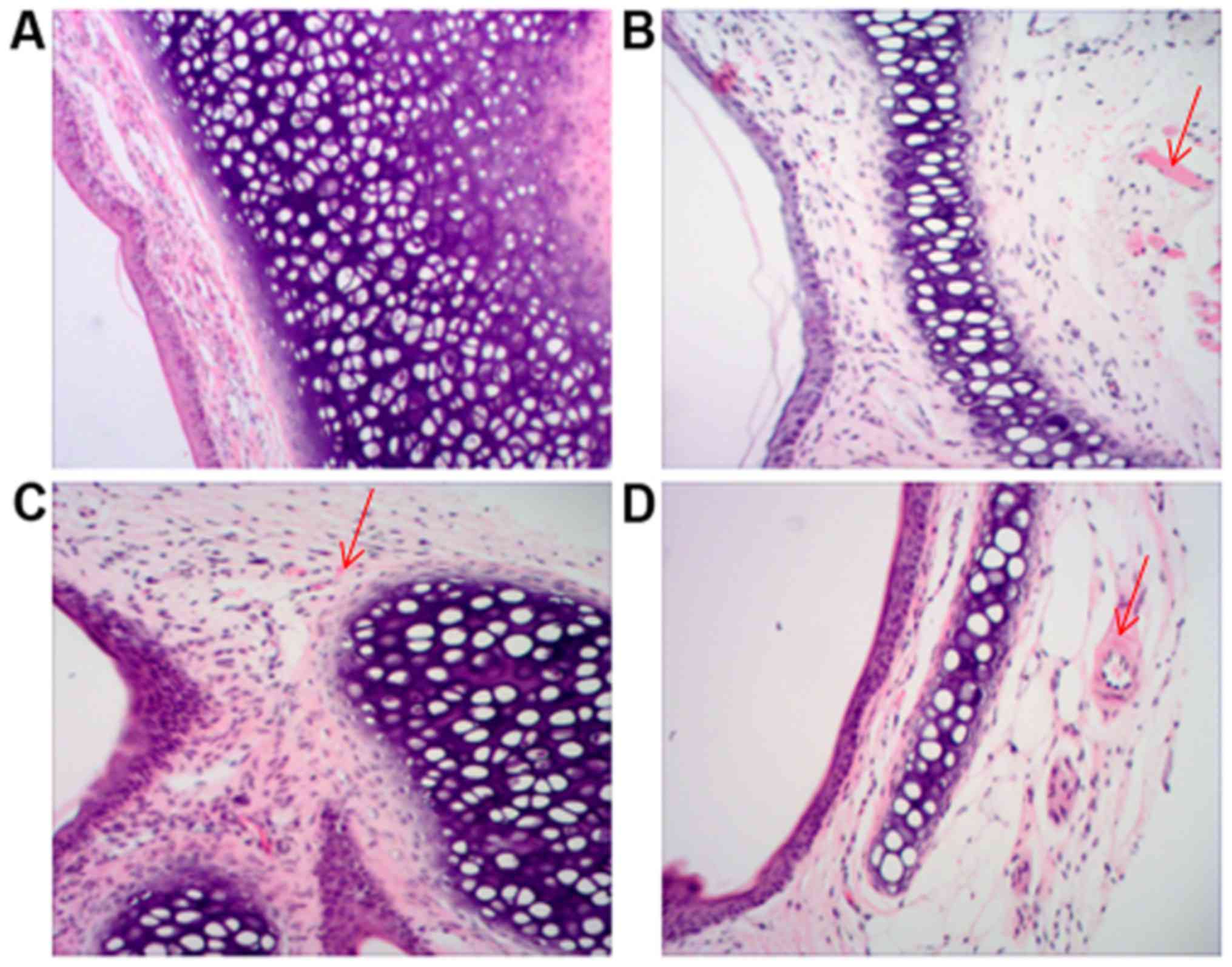 | Figure 2.Histological examination of nasal
mucosae. Sections of nasal mucosae from mice in the (A) CG, (B)
CCR3−/−CG, (C) AR and (D) CCR3−/−AR groups
were examined by hematoxylin and eosin staining; Detachment of
epithelial cells with irregular arrangement, structural disorder of
the mucosal layer and invasion of numerous inflammatory cells,
including EOS, are indicated by arrows (magnification, ×100). AR,
allergic rhinitis model; CG, normal control; CCR3, C-C chemokine
receptor 3; EOS, eosinophil. |
In addition, total EOS numbers in the nasal mucosae
of the various groups were ranked in the following order, from
highest to lowest (Table II):
AR>CCR3−/−AR>CG>CCR3−/−CG. Compared
with the CG group, the average EOS numbers were significantly
increased in the AR group (P<0.05), and the average EOS numbers
were also significantly higher in the CCR3−/−AR group
compared with the average EOS numbers in the CCR3−/−CG
group (P<0.05). Although the average EOS numbers in the
CCR3−/−CG group were lower compared with those in the CG
group, no significant difference was identified. Compared with the
AR group, EOS numbers in the CCR3−/−AR group were
significantly reduced (P<0.05), which suggested that EOS in
these mice had reduced invasive ability. These data indicated that
CCR3 gene deficiency may have suppressed the invasion of
inflammatory cells and relieved the nasal mucosae damage in AR
model mice.
 | Table II.EOS numbers in the nasal mucosae of
mice in the CG, AR, CCR3−/−CG, and CCR3−/−AR
groups. |
Table II.
EOS numbers in the nasal mucosae of
mice in the CG, AR, CCR3−/−CG, and CCR3−/−AR
groups.
| Groupa | EOS numbers
(×106) |
|---|
| CG | 1.21±0.13 |
| AR |
18.23±1.21b |
|
CCR3−/−CG | 1.19±0.20 |
|
CCR3−/−AR |
8.32±1.27b,c |
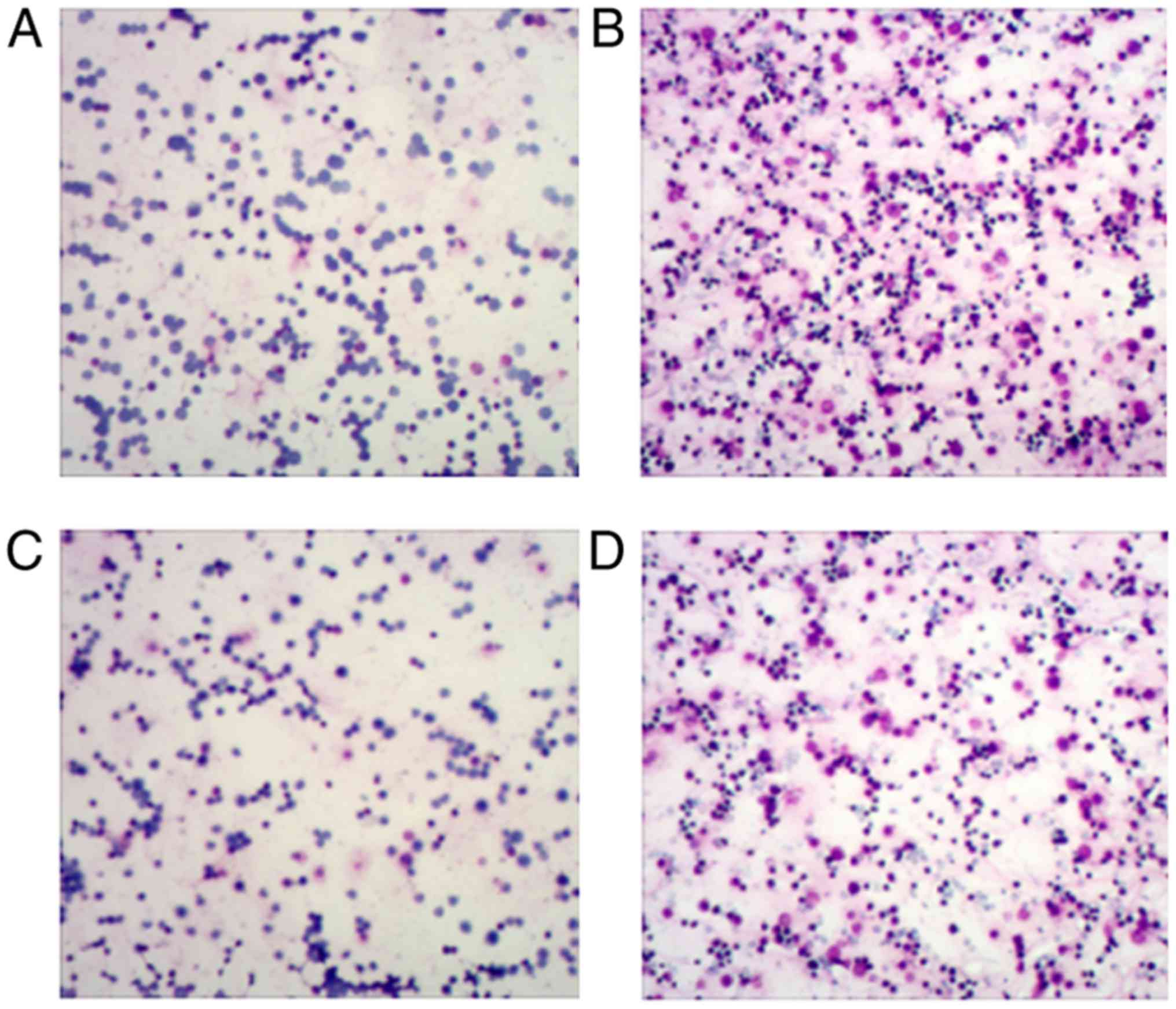
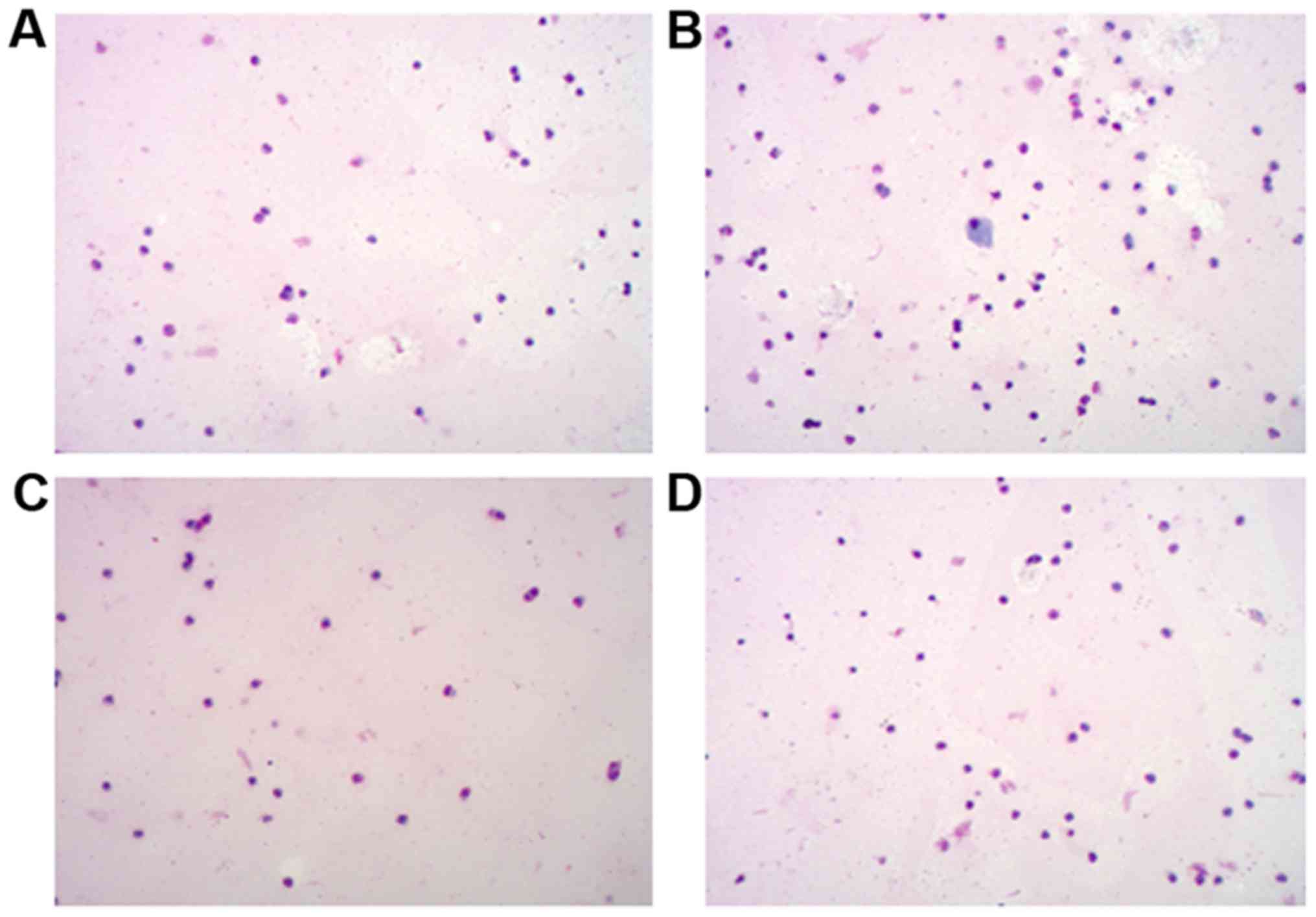
Examination of blood smear and nasal
washing smears by Wright's staining
Blood and nasal washing smears from mice in each
group were examined by Wright's staining. Similar results were
identified between the blood (Fig.
3; Table III) and nasal
washings (Fig. 4; Table IV) samples. Compared with the CG
group, the AR group had sharply increased EOS numbers (P<0.05),
and the EOS numbers were also remarkably higher in the
CCR3−/−AR group compared with the EOS numbers in the
CCR3−/−CG group (P<0.05). Compared with the AR group,
EOS numbers in the peripheral blood smear and the nasal washing
smears were notably reduced in the CCR3−/−AR group
(P<0.05). Although average EOS numbers in the peripheral blood
smear and nasal washing smears were decreased by 17.4 and 9.2%,
respectively, in the CCR3−/−CG group compared with in
the CG group, there was no significant difference. It was suggested
that CCR3 deficiency may be capable of weakening the invasion of
inflammatory cells in AR model mice.
 | Table III.EOS numbers in the peripheral blood
smears from mice in the CG, AR, CCR3−/−CG, and
CCR3−/−AR groups. |
Table III.
EOS numbers in the peripheral blood
smears from mice in the CG, AR, CCR3−/−CG, and
CCR3−/−AR groups.
| Groupa | EOS numbers
(×106) |
|---|
| CG | 0.23±0.15 |
| AR |
2.51±0.21b |
|
CCR3−/−CG | 0.19±0.10 |
|
CCR3−/−AR |
1.22±0.24b,c |
 | Table IV.EOS numbers in the nasal washings
smear in the CG, AR, CCR3−/−CG and CCR3−/−AR
groups. |
Table IV.
EOS numbers in the nasal washings
smear in the CG, AR, CCR3−/−CG and CCR3−/−AR
groups.
| Groupa | EOS numbers
(×106) |
|---|
| CG | 1.31±0.14 |
| AR |
2.83±0.71b |
|
CCR3−/−CG | 1.19±0.20 |
|
CCR3−/−AR |
2.12±0.57b,c |
mRNA expression levels of CCR3, EPO,
ECP, and MBP in the peripheral serum and nasal washings
mRNA expression levels of CCR3, EPO, ECP, and MBP
were determined by RT-qPCR on the peripheral blood and nasal
washings of mice following various treatments, and similar trends
in mRNA expression levels were observed (Figs. 5 and 6, respectively). For example, CCR3 mRNA
was not detected in the CCR3−/−CG and
CCR3−/−AR groups, whereas expression levels in the AR
group were significantly higher compared with CCR3 mRNA expression
in the CG group (P<0.05). The mRNA expression levels EPO, ECP,
and MBP exhibited similar trends among the different groups;
compared with the CG group, mRNA expression levels were
significantly increased in the AR group (P<0.05), and the
expression levels were also significantly higher in the
CCR3−/−AR group compared with expression levels in the
CCR3−/−CG group (P<0.05). In addition, the
CCR3−/−AR group exhibited significantly lower EPO, ECP,
and MBP mRNA expression levels compared with the respective
expression levels in the AR group (P<0.05); however, no
significant differences were identified between the CG and
CCR3−/−CG groups.
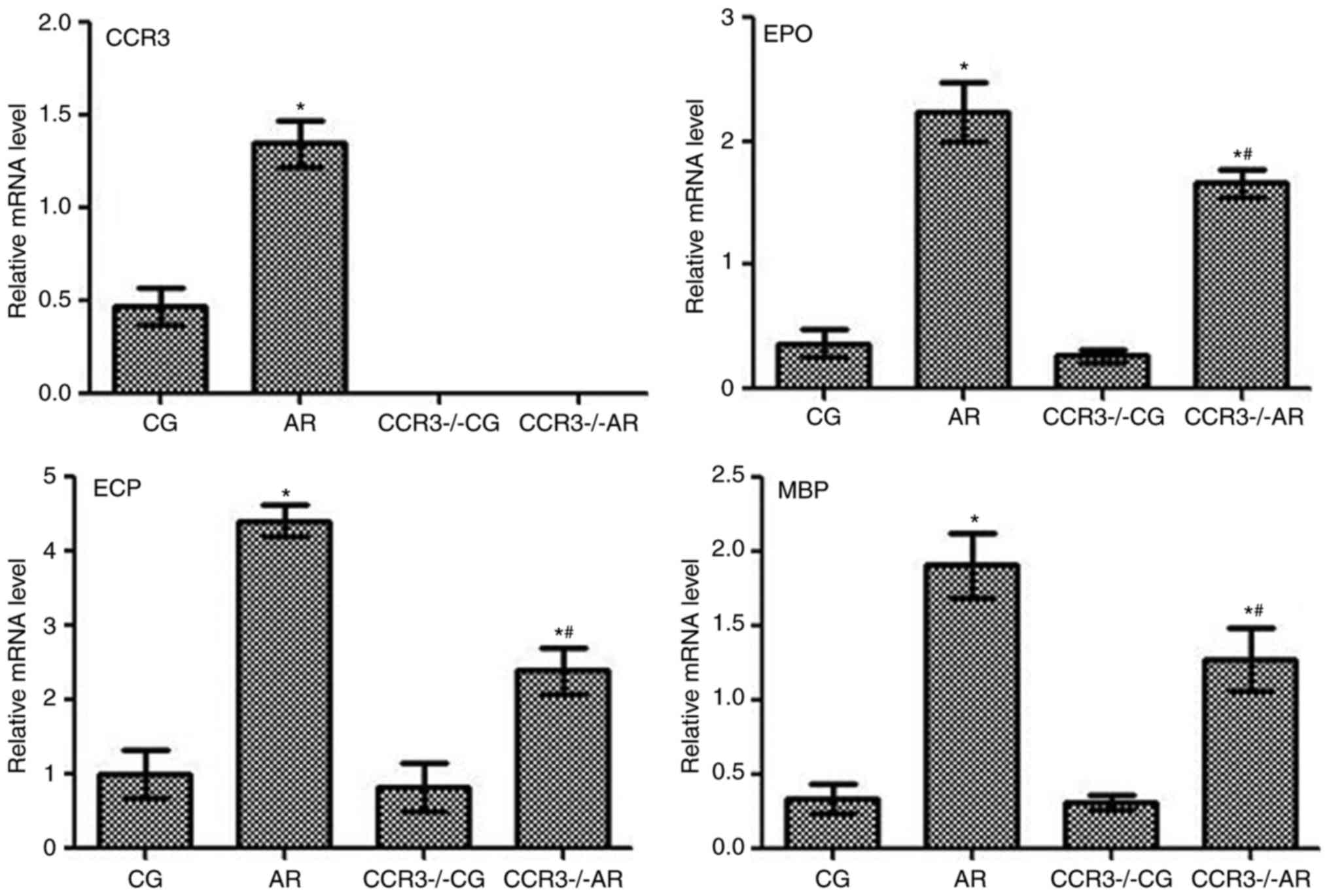 | Figure 5.mRNA expression levels of CCR3, EPO,
ECP, and MBP in the peripheral serum of mice in various groups were
determined by reverse transcription-quantitative polymerase chain
reaction. *P<0.05, AR group vs. the CG group and the
CCR3−/−AR group vs. the CCR3−/−CG group.
#P<0.05, CCR3−/−AR group vs. the AR group.
AR, allergic rhinitis model; CCR3, C-C chemokine receptor-3; CG,
normal control; CCR3−/−CG, CCR3 knockout control;
CCR3−/−AR, AR model with CCR3 knockout; ECP, eosinophil
cationic protein; EPO, eosinophil peroxidase; MBP, major basic
protein. |
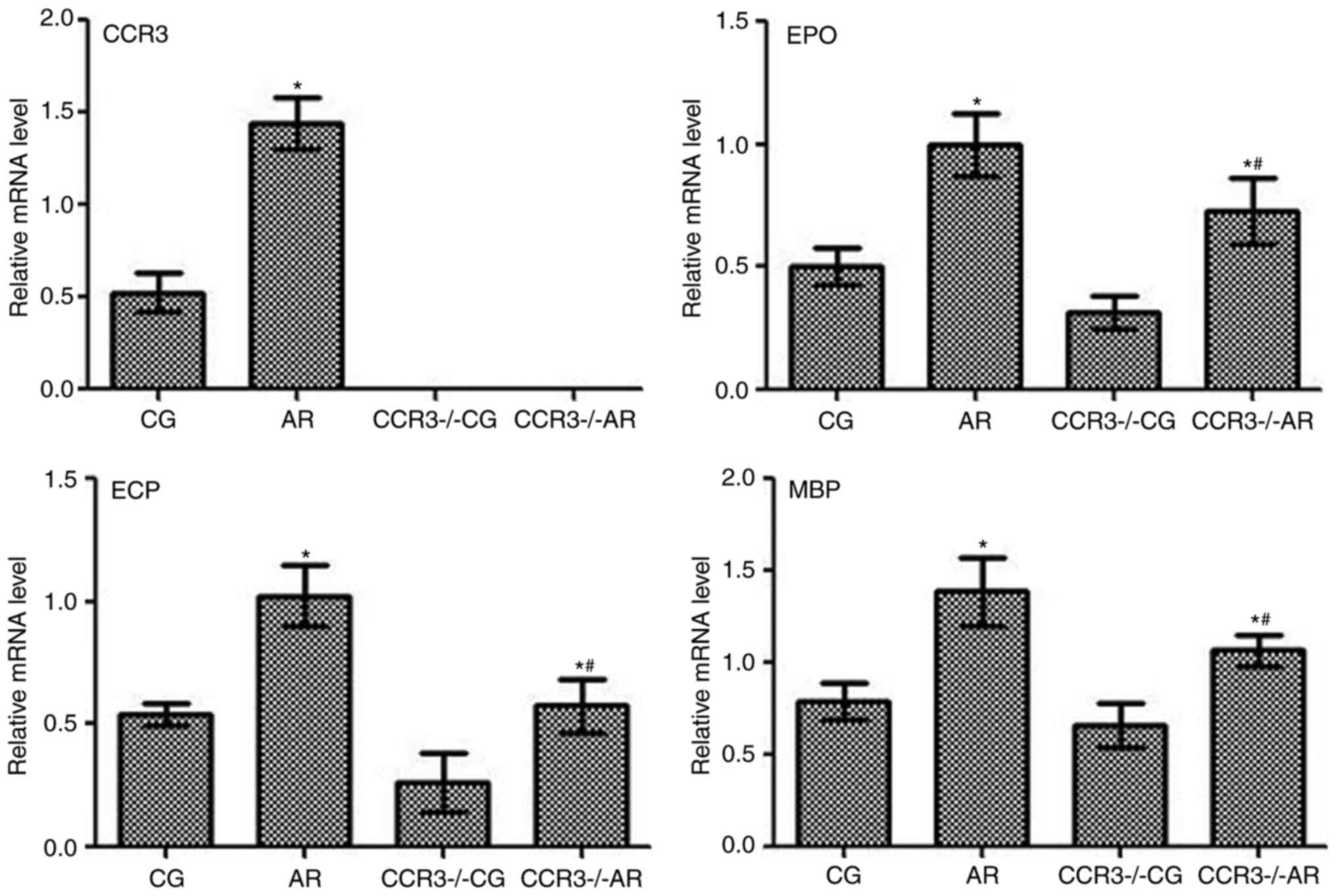 | Figure 6.mRNA expression levels of CCR3, EPO,
ECP, and MBP in the nasal washings of mice in various groups were
determined by reverse transcription-quantitative polymerase chain
reaction. *P<0.05, AR group vs. the CG group and the
CCR3−/−AR group vs. the CCR3−/−CG group.
#P<0.05, CCR3−/−AR group vs. the AR group.
AR, allergic rhinitis model; CCR3, C-C chemokine receptor-3; CG,
normal control; CCR3−/−CG, CCR3 knockout control;
CCR3−/−AR, AR model with CCR3 knockout; ECP, eosinophil
cationic protein; EPO, eosinophil peroxidase; MBP, major basic
protein. |
Expression levels of IL-4, IgE, IFN-γ,
and IL-10, in the peripheral serum and nasal washings
The levels of IL-4, IgE, IFN-γ, and IL-10 in the
peripheral blood and nasal washings were evaluated by ELISA
(Figs. 7 and 8). IL-4, IgE, IFN-γ, and IL-10 expression
levels between the CG and CCR3−/−CG groups were similar.
The expression levels of IL-4 and IgE were significantly increased
in mice in the AR group compared with CG group mice (P<0.05);
similar results were observed between the CCR3−/−AR
group compared with the CCR3−/−CG group (P<0.05).
IL-4 and IgE expression levels in the peripheral blood were
significantly decreased in the CCR3−/−AR mice compared
with the AR group mice (P<0.05); however, no significant
difference was identified in the nasal washings between these two
groups (P>0.05).
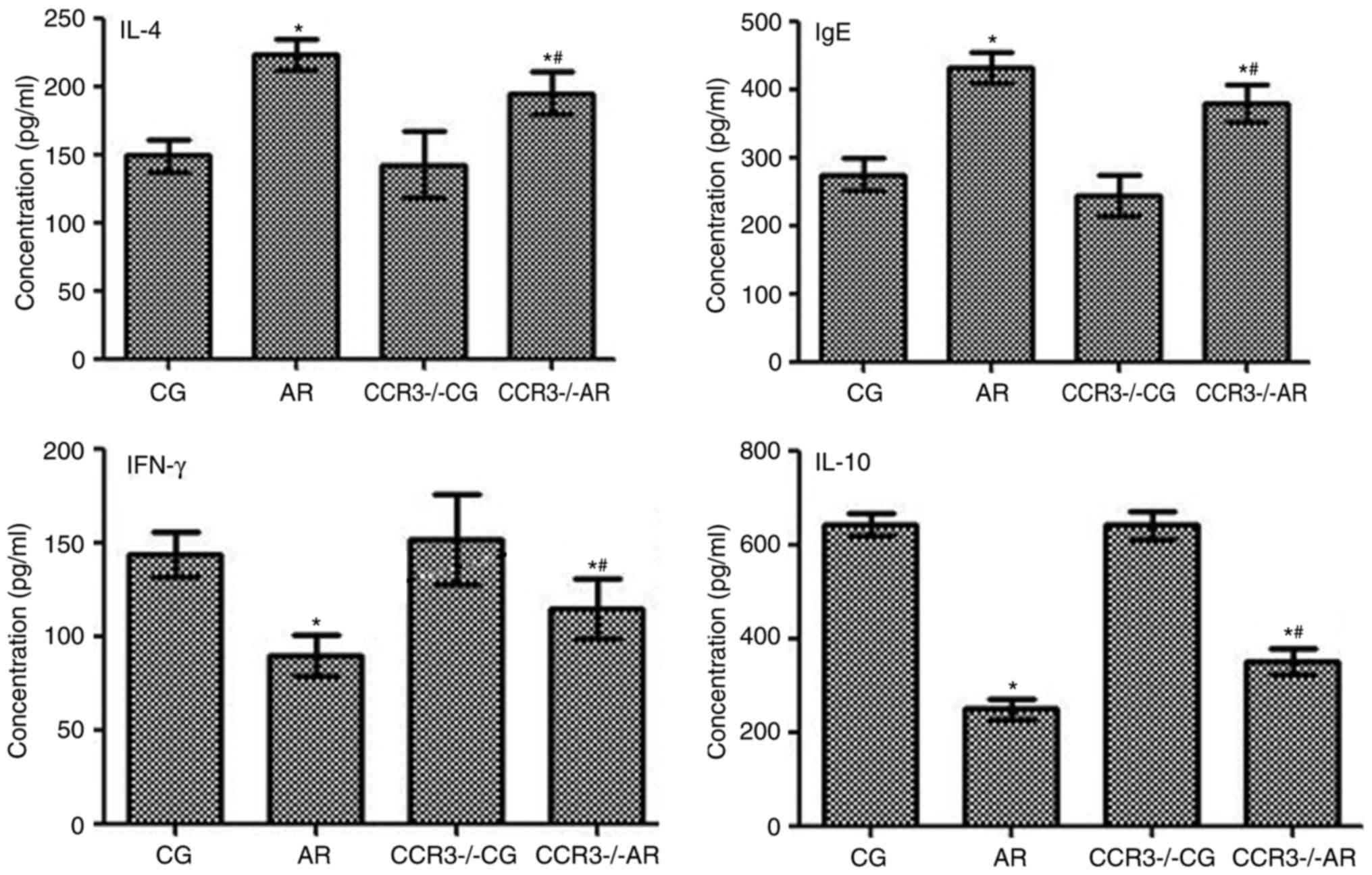 | Figure 7.Expression levels of IFN-γ, IL-4,
IL-10, and IgE in the peripheral serum of mice were evaluated by
ELISA. *P<0.05, AR vs. CG and CCR3−/−AR vs.
CCR3−/−CG; #P<0.05, CCR3−/−AR
vs. AR. AR, allergic rhinitis model; CCR3, C-C chemokine
receptor-3; CCR3−/−AR, AR model with CCR3 knockout;
CCR3−/−CG, CCR3 knockout control; CG, normal control;
IFN-γ, interferon-γ; IgE, immunoglobulin E; IL-10,
interleukin-10. |
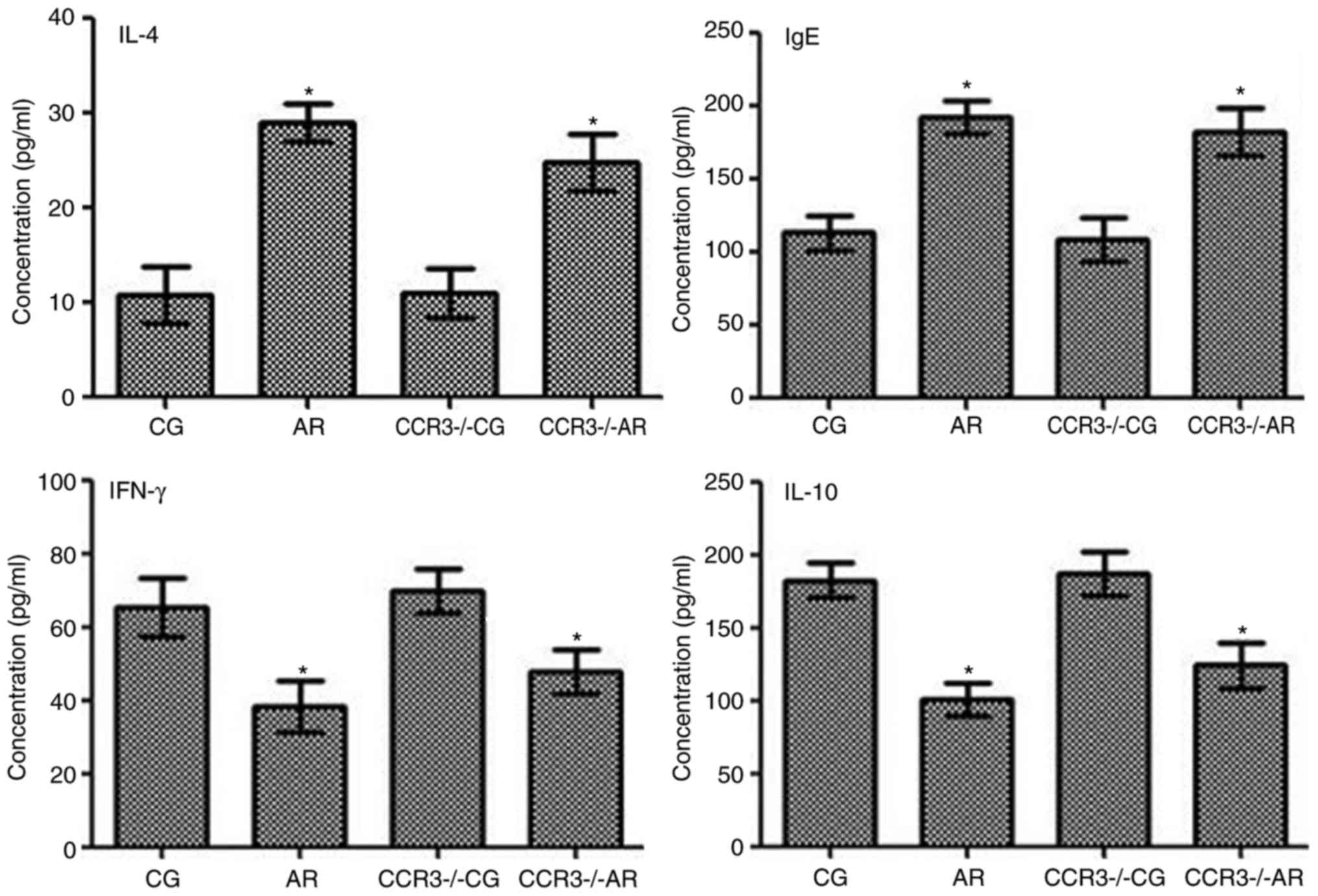 | Figure 8.Expression levels of IFN-γ, IL-4,
IL-10, and IgE in the nasal washings of mice in various groups were
evaluated by ELISA. *P<0.05, AR vs. CG and CCR3−/−AR
vs. CCR3−/−CG. AR, allergic rhinitis model; CCR3, C-C
chemokine receptor-3; CCR3−/−AR, AR model with CCR3
knockout; CCR3−/−CG, CCR3 knockout control; CG, normal
control; IFN-γ, interferon-γ; IgE, immunoglobulin E; IL-10,
interleukin-10. |
Conversely, IFN-γ and IL-10 expression levels in the
peripheral blood and the nasal washings were significantly reduced
in the AR group compared with the CG group, as well as in the
CCR3−/−AR group compared with the CCR3−/−CG
(both P<0.05). In the peripheral blood, IFN-γ and IL-10
expression levels in the CCR3−/−AR group were
significantly upregulated compared with levels in the AR group
(P<0.05); however, in the nasal washings, no significant
differences were identified for the IFN-γ and IL-10 expression
levels between the AR and the CCR3−/−AR groups
(P>0.05).
Discussion
The main effector cells of AR are EOS, which are a
type of leukocyte. EOS are featured with 2–3 segments of nucleus
and are full of acidophil granules in the cytoplast. The matured
EOS contains primary and secondary granules. The secondary granules
contain numerous proteins, including ECP, MBP, EPO, and EOS-derived
neurotoxin (12). The life cycle
of EOS is divided into three stages: In the marrow, peripheral
blood, and tissues. EOS mainly exist in the epithelial tissues of
hollow organs, including the intestinal epithelium and respiratory
epithelium. In the present study, the peripheral blood and the
nasal mucosae of AR model mice were revealed to contain numerous
EOS.
In the AR mice, local swelling of nasal mucosae was
observed, which may have been caused by histamine- and
kinin-induced angiotelectasis and the subsequent enhancement of
vascular wall permeability by stimulation of the receptors located
on sensory nerves and blood vessel (13). Histamine is also known to augment
glandular secretion and plasma effusion (13). Plasma effusion may have resulted in
the nasal mucosal edema and swelling (14). Retention of a large number of
exudates within connective tissues may oppress the superficial
blood vessels, which ultimately led to pallor of the mucosae
(14). The aforementioned
pathological alterations occurred repeatedly, generating mucosal
epithelial hyperplasia and mucosae thickening in consequence.
Additionally, under the influence of the aforementioned factors,
parasympathetic nerves may release acetylcholine, which may have
maintained high levels of glandular secretion and induced the
presence of abundant watery rhinorrhea (15). Mastocytes and basophils may release
inflammatory mediators to aggravate the inflammatory reaction;
however, they may secrete EOS chemotactic factors (15). These processes induced the
aggregation of numerous EOS around the glands and blood vessels,
and some EOS may enter into the secretions (15). Additionally, numerous EOS locally
invaded into tissues, which is an important pathological feature of
AR (16). These data agreed with
the present study results, which demonstrated that EOS numbers in
the nasal mucosae, peripheral blood, and nasal washings of AR model
mice were significantly higher compared with the CG mice.
Basic pathological alterations of AR include
angiotelectasis, enhancement of vascular wall permeability, augment
of glandular secretion, and invasion of EOS (17). Following nasal provocation, the
expression of numerous factors, including histamine, kinins,
leukotriene B4, and EOS-derived factors (such as ECP, MBP, EPO, and
EOS-derived neurotoxin) were upregulated in nasal secretions,
(18,19). Similarly, the present study results
demonstrated that EOS invasion and mRNA expression levels of EPO,
ECP, and MBP were significantly increased in the peripheral blood
and nasal washings of AR model mice.
The chemokine receptor CCR3 is an ~350
amino-acid-long transmembrane protein on the surface of immature
eosinophil progenitor cells and mature EOS that exhibits homology
with other C-C chemokine receptors (20). The combination of CCR3 and Eotaxin
[also known as C-C motif chemokine 11 (CCL11)] may activate the G
protein-dependent intracellular signaling pathways and further
facilitate the release of EOS progenitor cells and mature EOS from
the marrow into the AR affected tissues (20), which may serve an important role in
the invasion of EOS into target sites (21,22).
The binding of CCR3 and ligands was previously reported to amplify
downstream signaling cascade effects (23). Its mechanism was as follows: The
binding of the Gα subunit and guanosine triphosphate activated the
downstream factors, including phospholipase C, phosphatidylinositol
and calcium ion via dissociation of Gβγ dimer subunit (23). In addition, its signal may mediate
inflammatory reaction cascades and induce the degranulation of
inflammatory cells by activating the mitogen activated protein
kinase pathway (23). The ligands
of CCR3 include Rantes (also known as CCL5), Eotaxin, Eotaxin-2
(also known as CCL24), Eotaxin-3 (also known as CCL26), monocyte
chemotactic protein 2 (MCP-2; also known as CCL8), MCP-3 (also
known as CCL7), and MCP-5 (also known as CCL12). The affinity of
CCR3 to different chemokines varies; CCL11, CCL24, and CCL26
transduce signals only via CCR3, but not other receptors. CCR3
serves a crucial role in the accumulation of EOS in inflammatory
tissues, which indicates that CCR3 is closely associated with
numerous type-I allergic diseases, including AR (24).
In the present study, EOS numbers were significantly
reduced in CCR3−/− mice bearing AR. Expression levels of
the secondary EOS granule proteins MBP, ECP, and EOP were not
significantly different between the CG and CCR3−/−CG
mice; however, compared with the AR group, the levels of MBP, ECP,
and EOP were significantly reduced in the mice of
CCR3−/−AR group. This may have resulted from the
deficiency of CCR3; as the CCR3−/− mice did not produce
CCR3, there was no CCR3 to bind to the ligands. CCR3 is a gene
related to EOS. In AR mice, EOS numbers were elevated, but after
CCR3 knockout, the EOS numbers were reduced. Therefore, there was a
difference between CCR3 knockout and non-knockout in the AR model.
However, there was no increase in the EOS numbers in the CG mice,
so there was no significant relationship between CCR3 knockout and
non-knockout in the CG mice. Consequently, the downstream signaling
cascade effects of amplification may not be induced, which
downregulated the expression of the secondary granules of EOS.
Therefore, CCR3 knockout relieved the inflammatory reaction in the
genesis and development of AR by downregulating the expression of
EPO, ECP, and MBP in the present study. These findings were in
accord with a previous study (25).
IL-10 is an anti-inflammatory cytokine, and its
major effect cells are antigen-presenting cells and T lymphocytes
(26). During the different stages
of the immune response process, IL-10 reverses the
hyper-responsiveness of the body to allergens, which leads to
immune tolerance (27).
Furthermore, IL-10 exhibits other various immunomodulatory effects,
including immunosuppression, immune anergy, and anti-inflammation.
The major roles of the immunosuppressive effects of IL-10 are to
suppress the secretion of cytokines from certain cells, including
monocytes and natural killer cells, to reduce the generation of IgE
and to protect the body from allergen-induced airway inflammation
(28). During the inhibition of
inflammatory response, inflammatory mediators derived from
dendritic cells and macrophages may be partially inhibited by
IL-10. In addition, previous studies using AR model mice revealed
that IL-10 may partially alleviate asthmatic airway inflammation
(29–32). In the present study, AR model mice
(with and without CCR3 deficiency) expressed lower levels of IL-10
compared with control mice. Notably, CCR3−/−AR mice
exhibited a significant increase in IL-10 expression levels in the
serum compared with CCR3+/+AR mice, which suggested that
CCR3 knockout was capable of relieving the inflammatory reaction in
the genesis and development of AR by upregulating IL-10 expression.
CCR3 knockout may upregulate IL-10 expression by influencing the
expression of TH2 cytokines and Eotaxin (33).
IL-4 is a differentiation factor and growth factor
of B cells, and it may serve an important role in the responses of
B cells to antigenic stimulation (34). Wherein, the responses include the
proliferation of B cells and antibody secretion from B cells
(34). The allergen of
pathological specimens collected from patients with AR from
symptomatic to asymptomatic status was exposed in vitro
(35). mRNA level of IL-4 was
identified to be markedly increased and same class-switch of B
cells may produce IgE, but in non-AR, the phenomenon of same
class-switch from B cells to IgE did not exist (35). Furthermore, IL-4 is able to induce
the class-switch of B cells from producing other immune globulins
to producing IgE (35). It is
noted in particular that IL-4 promotes a strong transition of IgG
to IgE (36). The silencing of the
IL-4 may significantly suppress the occurrence of T helper (Th)-2
cell response (37). The
synergistic effects of IL-4 and IL-3 restrain the secretion of
IFN-γ, which is significant in the genesis of hypersensitivity
diseases such as AR. Conversely, IL-4 is able to raise the
expression of vascular cell adhesion molecule-1 and substance P,
leading to the upregulation of major histocompatibility complex
expression and the subsequent strengthening of immune response,
followed by the final aggravation of EOS accumulation and invasion
(37). IgE was produced by B cells
in the lymphatic tissues of respiratory tract and intestinal tract
(38). When the body is exposed to
an allergen, the antigen will bind to IgE on the surface of
basophilic granulocytes, mastocytes, and vascular endothelial cells
in tissues, leading to the release of numerous active factors,
including leukotriene, prostaglandin, endothelin, thromboxane A2,
and histamine and the subsequent attack of type-I allergic
responses. This is in accordance with the results of the present
study. It was identified that the expression of IL-4 and IgE was
upregulated in AR mice and CCR3 knockout was able to alleviate the
inflammation response in the genesis and development of AR by
downregulating the expression of IL-4 and IgE. CCR3 knockout may
downregulate IL-4 expression by influencing the expression of TH2
cytokines and Eotaxin as well (39). Furthermore, IL-4 affected B
lymphocytes, thereby downregulating the IgE expression (39).
IFN is a type of active protein mainly produced by
the monocytes and lymphocytes (40). IFN is able to enhance the activity
of T lymphocyte, NK cells, and macrophages, thereby elevating the
immunomodulatory effects of these cells and the antiviral
capability of the bodies (40).
There are three types of common IFN, and IFN-γ is an example of a
Type II IFN. The immunomodulatory effect is the major biological
activity of IFN-γ (40). In
addition, IFN-γ exhibits antiviral and anti-proliferative
activities to a certain extent (40). As a Th1-associated cytokine, IFN-γ
was reported to affect the microenvironment of CD+ T
cell differentiation, induce the shift of Thl/Th2 balance and cause
the transformation CD+ T to Th1 type, which accordingly
interdicts the differentiation of Th2 cytokines and the production
of IL-4 (41,42). Additionally, IFN-γ may reduce the
production of Th2 type cytokines by inhibiting the generation of
Th2 cell-specific transcription factor and Th2 type cells (42). IFN-γ and IL-4 are important
regulatory factors for synthesizing IgE (42). A previous report revealed that
intranasal inhalation of IFN-γ may effectively inhibit the
synthesis of IL-4 and IL-5 in AR model rats, thus reducing the
level of OVA-specific IgE (43).
In the present study, IFN-γ expression levels were significantly
reduced in the AR model mice (AR and CCR3−/−AR) compared
with the control mice (CG and CCR3−/−CG). However, in
the CCR−/−AR mice, IFN-γ expression levels were
significantly increased following compared with CCR+/+AR
model mice, which indicated that the CCR3 knockout may alleviate
the inflammatory response in the genesis and development of AR by
upregulating IFN-γ expression.
In conclusion, CCR3 gene knockout was demonstrated
to reduce the number of invading EOS and the inflammatory response
in the AR mice by downregulating the expression of EPO, ECP, MBP,
IL-4, and IgE and upregulating the expression of IL-10 and IFN-γ.
Regulators and modulators of CCR3, eosinophil granule proteins, and
immune factors may potentially be developed as drugs to treat AR in
clinic. These results may serve as guidelines for the design and
development of promising drugs and strategies to treat allergic
diseases such as AR. It should be noted that the results of the
present study may require further validation in larger animals with
a large-scale investigation in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360160), Talent
Team Project of Jiangxi Province (grant no. 20161BCB24010), and
Leading Talent Training Program of Ganpo Talent 555 Project.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, KL, JW and YL designed the study and wrote the
manuscript. HP, QP, SW and YJ collected and analyzed the data. All
authors performed the experiments.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by The
Institutional Animal Care and Use Committee, Nanchang University,
[Jiangxi, China; approval number YanLinShen (2013) No. 16] and was
conducted in accordance with the guidelines established by the
Chinese Council of Animal Care (10).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Casale TB and Dykewicz MS: Clinical
implications of the allergic rhinitis-asthma link. Am J Med Sci.
327:127–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brozek JL, Bousquet J, Baena-Cagnani CE,
Bonini S, Canonica GW, Casale TB, van Wijk RG, Ohta K, Zuberbier T,
Schünemann HJ, et al: Allergic Rhinitis and its Impact on Asthma
(ARIA) guidelines: 2010 revision. J Allergy Clin Immunol.
126:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo S and Kemphues KJ: Par-1, a gene
required for establishing polarity in C. elegans embryos, encodes a
putative Ser/Thr kinase that is asymmetrically distributed. Cell.
81:611–620. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnes NC, Sharma R, Lettis S and
Calverley PM: Blood eosinophils as a marker of response to inhaled
corticosteroids in COPD. Eur Respir J. 47:1374–1382. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu XH, Liao B, Xu Y, Liu K, Huang Y,
Huang QL and Liu YH: Downregulation of mouse CCR3 by lentiviral
shRNA inhibits proliferation and induces apoptosis of mouse
eosinophils. Mol Med Rep. 15:696–702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCaffrey AP, Meuse L, Pham TT, Conklin
DS, Hannon GJ and Kay MA: RNA interference in adult mice. Nature.
418:38–39. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shamri R, Young KM and Weller PF: PI3K,
ERK, p38 MAPK and integrins regulate CCR3-mediated secretion of
mouse and human eosinophil-associated RNases. Allergy. 68:880–889.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saito Y, Takeda M, Nishikawa J, Konno Y,
Tamaki M, Itoga M, Kobayashi Y, Moritoki Y, Ito W, Chihara J and
Ueki S: The effect of pharmacological PI3Kγ inhibitor on
eotaxin-induced human eosinophil functions. Pulm Pharmacol Ther.
27:164–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun DM, Yue BF, Sun RZ, Wang TQ, Pang WY,
Kong Q, Zhu DS, Li N and Qin C: Laboratory animal-Guideline of
welfare ethical review (Draft for approval). Laboratory Animal
Welfare and Ethics Committee CALAS Beijing. 252017.
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stone KD, Prussin C and Metcalfe DD: IgE,
mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 125
2 Suppl 2:S73–S80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
González DA, Barbieri van Haaster MM,
Quinteros Villarruel E, Brandt M, Benítez MB, Stranieri GM and
Orman B: Histamine stimulates secretion of extracellular vesicles
with nucleotidase activity in rat submandibular gland. Arch Oral
Biol. 85:201–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Irani YD, Scotney PD, Klebe S, Mortimer
LA, Nash AD and Williams KA: An anti-VEGF-B antibody fragment
induces regression of pre-existing blood vessels in the rat cornea.
Invest Ophthalmol Vis Sci. 58:3404–3413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shu XL and Zhou D: AR model of wind speed
time series and its rapid implementation. Spatial Struc. 2003.(In
Chinese).
|
|
16
|
Noda A: A test of the adaptive market
hypothesis using a time-varying AR model in Japan. Financ Res Lett.
17:66–71. 2016. View Article : Google Scholar
|
|
17
|
Dong B, Ma J, Ye C, Bao Y, Yang A and Xiu
H: Establishment of rat model of proliferative diabetic
retinopathy. J Third Military Med Univ. 34:1218–1222, (In
Chinese).
|
|
18
|
Naclerio RM, Meier HL, Kagey-Sobotka A,
Adkinson NF Jr, Meyers DA, Norman PS and Lichtenstein LM: Mediator
release after nasal airway challenge with allergen. Am Rev Respir
Dis. 128:597–602. 1983.PubMed/NCBI
|
|
19
|
Rådinger M and Lötvall J: Eosinophil
progenitors in allergy and asthma-do they matter? Pharmacol Ther.
121:174–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian M, Chen L, Ma L, Wang D, Shao B, Wu
J, Wu H and Jin Y: Expression and prognostic significance of
CCL11/CCR3 in glioblastoma. Oncotarget. 7:32617–32627. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ponath PD, Qin S, Post TW, Wang J, Wu L,
Gerard NP, Newman W, Gerard C and Mackay CR: Molecular cloning and
characterization of a human eotaxin receptor expressed selectively
on eosinophils. J Exp Med. 183:2437–2448. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sallusto F, Mackay CR and Lanzavecchia A:
Selective expression of the eotaxin receptor CCR3 by human T helper
2 cell. Science. 277:2005–2007. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyagaki T, Sugaya M, Murakami T, Asano Y,
Tada Y, Kadono T, Okochi H, Tamaki K and Sato S: CCL11-CCR3
interactions promote survival of anaplastic large cell lymphoma
cells via ERK1/2 activation. Cancer Res. 71:2056–2065. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahrens R, Waddell A, Seidu L, Blanchard C,
Carey R, Forbes E, Lampinen M, Wilson T, Cohen E, Stringer K, et
al: Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1
mediates eosinophil recruitment and function in pediatric
ulcerative colitis. J Immunol. 181:7390–7399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu XH, Liao B, Liu K and Liu YH: Effect
of RNA interference therapy on the mice eosinophils CCR3 gene and
granule protein in the murine model of allergic rhinitis. Asian Pac
J Trop Med. 7:226–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pylaeva E, Potekhina A, Provatorov S,
Ruleva N, Masenko VP, Noeva E, Krasnikova TL and Arefieva TI:
Diminished frequency of CD4+IL10+ T-lymphocytes predicts coronary
atherosclerosis progression. Atherosclerosis. 241:e922015.
View Article : Google Scholar
|
|
27
|
Zhou J and Yang X: Interleukin-10 in
bronchial asthma. Foreign Med Sci (Section of Pediatr). 32:31–33.
2005.(In Chinese).
|
|
28
|
Ning J, Zhu J and Zou Y: Relationship
between interleukin-10/interferon γ and bronchial asthma in
children: Reports of 29 cases. New Chin Med. 37:643–644. 2006.(In
Chinese).
|
|
29
|
Fu CL, Ye YL, Lee YL and Chiang BL:
Effects of overexpression of IL-10, IL-12, TGF-beta and IL-4 on
allergen induced change in bronchial responsiveness. Respir Res.
7:722006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tao W, Wen F, Yao H and Sun Y: Influence
of Erythropoietin and inflammatory cytokines on pathogenesis of
cerebral palsy. Chin J Rehabil Theor Pract. 14:62–64. 2008.(In
Chinese).
|
|
31
|
Doi Y, Kiyohara Y, Kubo M, Ninomiya T,
Wakugawa Y, Yonemoto K, Iwase M and Iida M: Elevated C-reactive
protein is a predictor of the development of diabetes in a general
Japanese population: the Hisayama Study. Diabetes Care.
28:2497–2500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu M and Wang Z: Treatment status of
anti-inflammatory immunotherapy for bronchial asthma. Pract Pharm
Clin Remedies. 13:454–456. 2010.(In Chinese).
|
|
33
|
Jiang P, Lin Q and Zhao A: Selective
induction of CCR3, CCR5 and CXCR3 expression by IL-4 and IL-10
influences the spontaneous resorption abortion rate in the
CBA/JxDBA/2 mouse model. Prog Obstetri Gynecol. 56:560–565.
2009.
|
|
34
|
Li GY, Wang JJ, Hu XY and Chen LC: Effect
of jianpi tongqiao pill to serum total IgE IL-4 content of rat
model of AR. Chin Arch Trad Chin Med. 2011.(In Chinese).
|
|
35
|
Fiset PO, Cameron L and Hamid Q: Local
isotype switching to IgE in airway mucosa. J Allergy Clin Immunol.
116:233–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sudowe S, Arps V, Vogel T and Kölsch E:
The role of interleukin-4 in the regulation of sequential isotype
switch from immunoglobulin G1 to immunoglobulin E antibody
production. Scand J Immunol. 51:461–471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu G, Zhang R, Wen W, Yan Z, Yu S and Sun
W: An investigation on the effects of dexamethasone on IgE, IL-4
and IL-17 levels in the serum of rats with experimental allergic
rhinitis. Chin Otorhinolar J Integr Med. 14:69–72. 2006.(In
Chinese).
|
|
38
|
Zhang YN, Song J, Wang H, Wang H, Zeng M,
Zhai GT, Ma J, Li ZY, Liao B, Wang BF, et al: Nasal
IL-4(+)CXCR5(+)CD4(+) T follicular helper cell counts correlate
with local IgE production in eosinophilic nasal polyps. J Allergy
Clin Immunol. 137:462–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sung YY, Kim SH, Kim DS, Lee JE and Kim
HK: Illicium verum extract and trans-anethole attenuate
ovalbumin-induced airway inflammation via enhancement of Foxp3+
regulatory T cells and inhibition of Th2 cytokines in mice.
Mediators Inflamm. 2017:75068082017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sakai S, Kauffman KD, Sallin MA, Sharpe
AH, Young HA, Ganusov VV and Barber DL: CD4 T cell-derived IFN-γ
plays a minimal role in control of pulmonary mycobacterium
tuberculosis infection and must be actively repressed by PD-1 to
prevent lethal disease. PLoS Pathog. 12:e10056672016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayashi N, Yoshimoto T, Izuhara K, Matsui
K, Tanaka T and Nakanishi K: T Helper 1 cells stimulated with
ovalbumin and IL-18 induce airway hyperresponsiveness and lung
fibrosis by IFN-γ and IL-13 production. Proc Natl Acad Sci USA.
104:pp. 14765–14770. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kuwahara M, Ise W, Ochi M, Suzuki J,
Kometani K, Maruyama S, Izumoto M, Matsumoto A, Takemori N,
Takemori A, et al: Bach2-Batf interactions control Th2-type immune
response by regulating the IL-4 amplification loop. Nat Commun.
7:125962016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kotowicz K, Dixon GL, Klein NJ, Peters MJ
and Callard RE: Biological function of CD40 on human endothelial
cells: Costimulation with CD40 ligand and interleukin-4 selectively
induces expression of vascular cell adhesion molecule-1 and
P-selectin resulting in preferential adhesion of lymphocytes.
Immunology. 100:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|