Introduction
Prostate cancer (PCa) is the most commonly diagnosed
male cancer in Western countries and the second leading cause of
death in the US (1). Approximately
164,690 people are diagnosed with PCa, which cause approximately 9%
of cancer-related deaths among American men (1,2). PCa
is a heterogeneous and multi-focal cancer with highly variable
natural history, causing difficulty in predicting its initiation,
progression and prognosis. The combination of Gleason score, TNM
stage, lymph node status and serum prostate-specific antigen (PSA)
level has been commonly used to predict the prognosis of patients
with PCa (3,4). PSA testing is widely used for the
early clinical diagnosis of PCa, but it's true value remains
controversial because of its poor tumour specificity (3). According to statistics, PSA testing
may have led to false positives in 23–42% of all diagnosed cases;
furthermore, the patients receiving treatments, such as radical
prostatectomy or radiotherapy, must pay substantial costs and
subject themselves to serious side effects (5,6).
Approximately 25% of patients with PCa suffer from biochemical
recurrence (BCR) following radical prostatectomy (7). Therefore, identifying methods that
can be used to correctly distinguish indolent and aggressive
cancers and finding novel and effective biomarkers are critical to
improve the understanding of the biological progression of PCa and
to advance the diagnosis and prognosis of PCa in clinics.
Mitochondrial dysfunction is closely related with
cancer development (8–10). Cancer cells usually have
hyperexpression of mitochondrial genes, such as the leucine-rich
pentatricopeptide repeat containing (LRPPRC) gene that we have
reported previously (11,12). Mitochondrial genes play direct role
in regulating cancer proliferation, survival and therapeutic effect
(13,14).
Pentatricopeptide repeat domain protein 3 (PTCD3), a
mitochondrial ribosomal protein, is located on the human chromosome
2p11.2-p12 and encodes a polypeptide of 689 amino acids with an
estimated molecular weight of 79 kDa (15). PTCD3 is linked with mt-rRNA from
the small ribosomal subunit and plays a role in RNA binding and
mitochondrial gene expression. Some of its related pathways are
mitochondrial translation and organelle biogenesis and maintenance
(16). PTCD3 knockdown decreases
mitochondrial respiration and the activity of respiratory complexes
(17). Depleting PTCD3 in 143B
osteosarcoma cells generally affects mitochondrial protein
synthesis (16). Its prognostic
value in breast cancer and its ability to act as a therapeutic
target in lymphomas have been found in recent work (18,19).
However, few studies have been conducted to clarify the function of
PTCD3 in human cancer, and the role of PTCD3 in PCa remains
unknown. Therefore, we aimed to investigate PTCD3 expression in PCa
tissues and examine its clinical significance in this specific
malignancy.
Materials and methods
Patient tissue samples
For IHC analysis, a tissue microarray (TMA, n=78)
with detailed clinical information including 71 PCa tissues, 3
adjacent normal prostate tissue and 4 normal prostate tissue was
purchased from Xi'an Alenabio, Co., Ltd., (cat no: PR803c). All
patients whose prostate tissue samples were included in the TMA had
not received chemotherapy or radiotherapy before the surgery and
have not been diagnosed with any additional malignancies.
Meanwhile, we got TCGA dataset information at https://portal.gdc.cancer.gov/, and used Perl
programming language to merge the individual sample expression
files. Then we transformed the ENSemble ID into symbol ID, and
finally got the clinical data and mRNA sequence. The clinical
information of the TCGA public dataset including 498 PCa tissues
and 52 normal prostate tissues was used to investigate the
expression of PTCD3 at mRNA level and for survival analysis.
Detailed information on the clinical parameters of all patients in
this study is presented in Table
I.
 | Table I.Expression of PTCD3 and its
association with clinicopathologic features in prostate cancer. |
Table I.
Expression of PTCD3 and its
association with clinicopathologic features in prostate cancer.
|
|
| TMA | TCGA |
|---|
|
|
|
|
|
|---|
| Clinical
features | Case | Low, n (%) | High, n (%) | P-value | Case | x±s | P-value |
|---|
| Tissue |
|
Cancer | 71 | 31 (43.7) | 40 (56.3) | 1.000 | 498 | 911.22±222.35 | 0.011a |
|
Benign | 7 | 3 (42.9) | 4 (57.1) |
| 52 | 859.94±122.77 |
|
| Age, years |
|
<60 | 5 | 4 (80.0) | 1 (20.0) | 0.160 | 201 | 897.94±198.71 | 0.266 |
| ≥60 | 66 | 27 (40.9) | 39 (59.1) |
| 296 | 920.60±237.22 |
|
| Gleason score |
|
<7 | – | – | – | – | 44 | 866.50±105.94 |
<0.001b |
| =7 |
|
|
|
| 247 | 876.59±178.93 |
|
|
>7 | – | – | – |
| 206 | 962.83±272.90 |
|
| Serum PSA levels
(ng/ml) |
|
<10 | – | – | – | – | 8 | 1041.54±287.19 | 0.109 |
|
≥10 | – | – | – |
| 438 | 912.02±225.04 |
|
| Pathological
grade |
| ≤2 | 23 | 16 (69.6) | 7 (30.4) | 0.001b | – | – | – |
|
>2 | 44 | 12 (27.3) | 32 (72.7) |
| – | – |
|
| Clinical stage |
| I | 4 | 4 (100.0) | 0 (0.0) | 0.034a | – | – | – |
|
II–IV | 66 | 27 (40.9) | 39 (59.1) |
| – | – |
|
| Tumor invasion |
|
T1-T2 | 46 | 22 (47.8) | 24 (52.2) | 0.409 | 351 | 887.35±188.31 | 0.076 |
|
T3-T4 | 24 | 9 (37.5) | 15 (62.5) |
| 55 | 952.75±258.09 |
|
| Lymph node
metastasis |
| N0 | 58 | 25 (43.1) | 33 (56.9) | 0.662 | 344 | 909.60±212.73 | 0.384 |
| N1 | 12 | 6 (50.0) | 6 (50.0) |
| 80 | 933.45±251.57 |
|
| Distant
metastasis |
| M0 | 56 | 24 (42.9) | 32 (57.1) | 0.630 | 455 | 907.32±222.61 | 0.002b |
| M1 | 14 | 7 (50.0) | 7 (50.0) |
| 3 | 1307.89±492.13 |
|
| Survival time,
years |
|
<5 | – | – | – | – | 411 | 911.86±196.51 | 0.946 |
| ≥5 | – | – | – |
| 86 | 909.44±320.39 |
|
Mouse prostate tissue samples
PCa tissues from 11 month-old Prostate-specific PTEN
knockout (PTEN−/−) and 7 month-old TRAMP mice were
collected as reported previously (11,12)
and gifted to the present study by Dr. Fen Wang (Institute of
Biosciences and Technology, Texas A&M Health Science Center,
Houston, TX, USA). Normal prostate tissues were collected from
wild-type mice aged 5 or 11 months. PCa tissues from TRAMP model
mice which showed metastasis to the liver were collected at the
same age of 13 months.
Immunohistochemistry (IHC) analysis in
tissue microarray
The tissue microarray was deparaffinized with xylene
and rehydrated for further H&E or peroxidase (DAB)
immunohistochemistry staining employing a DAKO EnVision System
(Agilent Technologies, Inc., Santa Clara, CA, USA). Following a
brief proteolytic digestion and a peroxidase blocking of tissue
slides, the slides were incubated overnight with the primary
antibodies against PTCD3 (cat no: bs-4424R, at a dilution of 1:300)
at 4°C. After washing, slides were incubated with second antibody
labeled by HRP (rabbit). Finally, visualization was performed by 3,
30-diaminoben-zidine tetrahydrochloride (DAB) and counterstained by
hematoxylin. In each immunohistochemistry run, negative controls
were stained with isotype-matched control IgG. The final
immunoreactivity scores (IRS) of each case were calculated by
multiplying the two scores for the immunostaining intensity and
immunostaining percentage.
Immunohistochemistry staining in mouse
prostate tissue samples
Normal prostate tissues collected from wild-type
mice and PCa tissues collected from PTEN−/− mice or
TRAMP model mice were subjected to immunohistochemistry staining,
and the histological scores were assigned by 2 independent clinical
pathologists in a double-blinded manner. Prostate tissue samples
from TRAMP, PTEN−/− and wild-type mice were prepared for
immunohistochemistry staining as described for LRPPRC analysis
previously (12). Antibody against
PTCD3 (cat no: bs-4424R) was purchased from Beijing Biosynthesis
Biotechnology, Co., Ltd., (Beijing, China).
Cell culture
Two human PCa cell lines (DU145 and LNCaP) and one
benign prostate cell line (RWPE-1) were purchased from American
Type Culture Collection (Manassas, VA, USA). DU145 and LNCaP cell
lines were maintained in the 1640 Medium (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum, and RWPE-1 cells were cultured in DMEM Medium (HyClone; GE
Healthcare Life Sciences) supplemented with 10% fetal bovine serum.
The cells were cultured at 37°C in a humidified incubator with 5%
CO2.
Western blot analysis
Cells were collected and lysed in RIPA buffer
containing broad protease inhibitor cocktail (EASY packs; Roche
Diagnostics, Basel, Switzerland). The total protein concentration
in the supernatants collected after centrifugation was measured by
bicinchoninic acid (BCA). Equal amount of total protein (40 µg) was
loaded on and separated by SDS-PAGE (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and blotted with primary antibodies and
corresponding horse-radish peroxidase-conjugated secondary
antibodies. GAPDH was used as an internal loading control. The
primary antibodies anti-PTCD3 (cat. no: ab52099, dilution 1:1,000;
Hangzhou MultiSciences (Lianke) Biotech, Co., Ltd., Zhejiang,
China) and anti-GAPDH (cat. no: 5174, dilution 1:2,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) were used. Protein
bands were visualized with the GelDoc XR + chemiluminescent
detection system (Bio-Rad Laboratories, Inc.). Densitometric
analysis of the bands was performed using the ImageJ free software
(National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
Cells were digested and grown on glass coverslips
for 6 h. Then the cells were washed three times with PBS, fixed in
4% PFA for 20 min and permeabilized with 0.1% Triton X-100 in PBS
for 20 min. The cells were washed three times with PBS and blocked
with 2% BSA in PBS for 30 min to 1 h at room temperature. Cells
were incubated overnight at 4°C with anti-PTCD3 (cat. no: bs-4424R;
dilution 1:200) in 1% BSA in PBS followed by five-times washing
with PBS. Cells were incubated with DYLight649 goat anti-rabbit IgG
[H+L] secondary antibody (GAR6492; Hangzhou MultiSciences (Lianke)
Biotech, Co., Ltd.) at a 1:500 dilution in PBS at room temperature
for 1 h. After washing with PBS for five times, cells were
incubated with DAPI for 5 min followed by five-times washing with
PBS. Cells were then mounted with Fluoromount-G (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), and images were
captured using a confocal laser scanning microscope (LSM800)
equipped with a ×40 objective.
Statistical analysis
SPSS v.22.0 software (IBM Corp., Armonk, NY, USA)
was used for statistical analysis. Pearson's Chi-squared tests and
Fisher's exact test were used to analyze the association of PTCD3
protein expression with clinicopathological parameters. The
association between PTCD3 mRNA expression with patient's
clinicopathological was analyzed by Student's t-test (pairwise
comparison) and one-way analysis of variance plus least significant
difference post-hoc test (inter-group comparison). Kaplan-Meier
curve method was used for survival analysis, and the differences
were assessed by log-rank test. Further Univariate analysis
comparisons and multivariate survival analysis comparisons were
obtained by using Cox proportional hazards regression. The relative
risks of mortality were expressed as adjusted hazard ratios (HRs)
and their corresponding 95% confidence intervals (CIs). P<0.05
was considered to indicate a statistically significant
difference.
Results
The expression of PTCD3 protein is
increased in PCa and is associated with aggressive phenotypes
We first detected the expression of PTCD3 protein in
71 PCa tissues, 3 adjacent normal prostate tissues and 4 normal
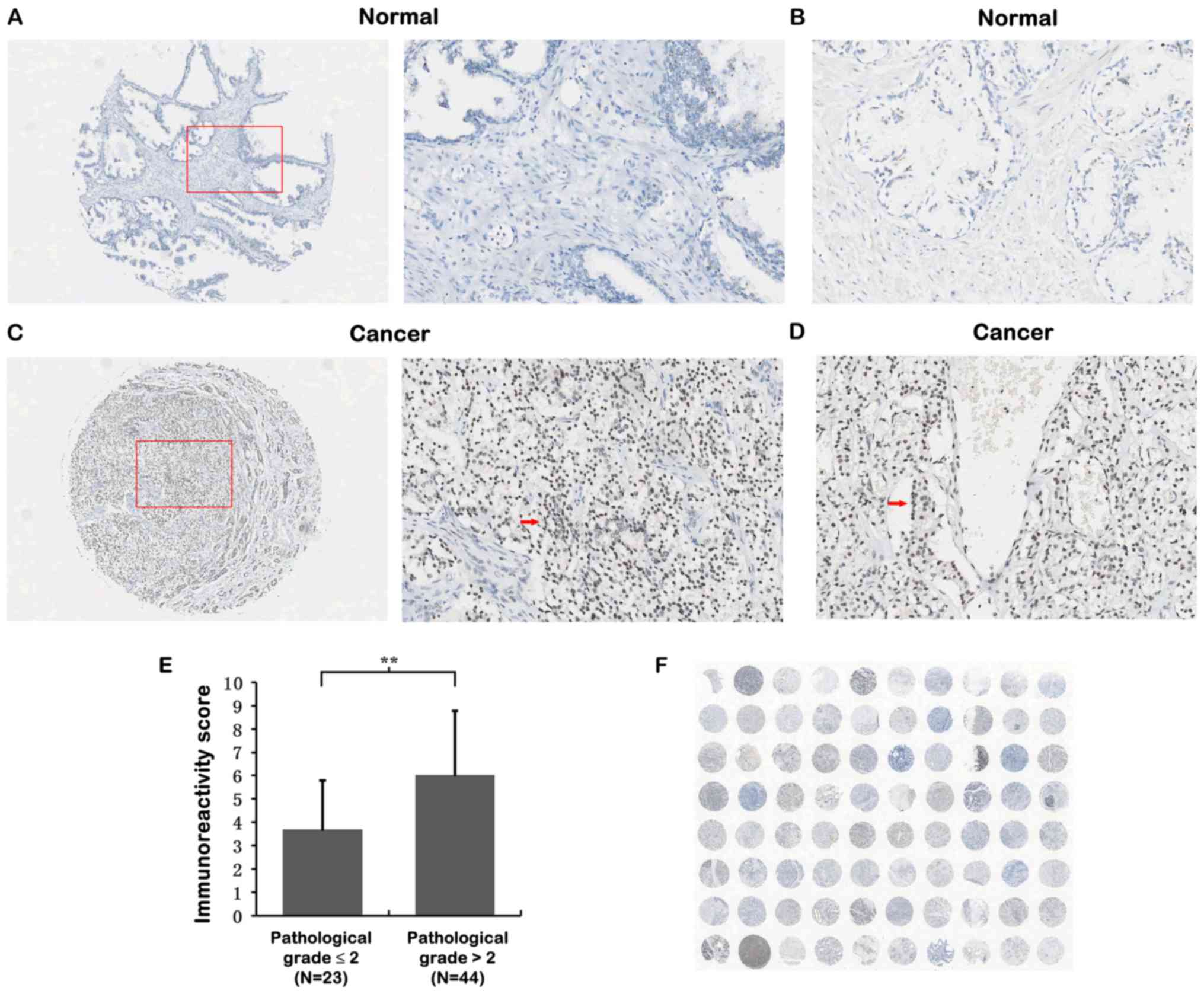
prostate tissues by IHC analysis (Table I). As shown in Fig. 1A-D, PTCD3 immunostainings appeared
strongly in PCa cancer cells in PCa tissues but weakly in normal
prostate tissues. Overexpression of PTCD3 protein was significantly
related to advanced PCa pathological grade (IRS 3.70±2.10 for
pathological grade ≤2 vs. 6.03±2.74 for pathological grade >2;
Fig. 1E) and higher clinical
stages of PCa tissues (Table I).
However, the levels of PTCD3 were not associated with age, distant
metastasis, lymph node metastasis and tumour invasion (Table I).
PTCD3 is overexpressed in PCa and is
correlated with gleason score and distant metastasis of PCa in TCGA
dataset
Publicly available data from the TCGA dataset, which
consists of 498 PCa tissues and 52 normal prostate tissue with
high-throughput sequencing data, were used to validate the
expression data of protein-coding genes (mRNA) (20). As shown in Table I, PTCD3 was overexpressed in the
PCa tissue samples (P=0.011) compared with that in benign prostate
tissues samples. Patients with PCa exhibiting high Gleason score
(P<0.001) and increased distant metastasis (P=0.002) showed high
PTCD3 mRNA expression levels. However, high PTCD3 expression was
not associated with age, serum PSA levels, tumour invasion, lymph
node metastasis, and survival time in the TCGA dataset
(P>0.05).
PTCD3 expression is elevated in the
tissue samples of prostate-specific PTEN−/− mouse model
and TRAMP model
To confirm the elevated PTCD3 expression in PCa
tissues and investigate the relationship between PTCD3 and tumour
progression, we further performed immunohistochemical (IHC)
analysis in the tissue samples of the prostate-specific
PTEN−/− mouse model and TRAMP model.
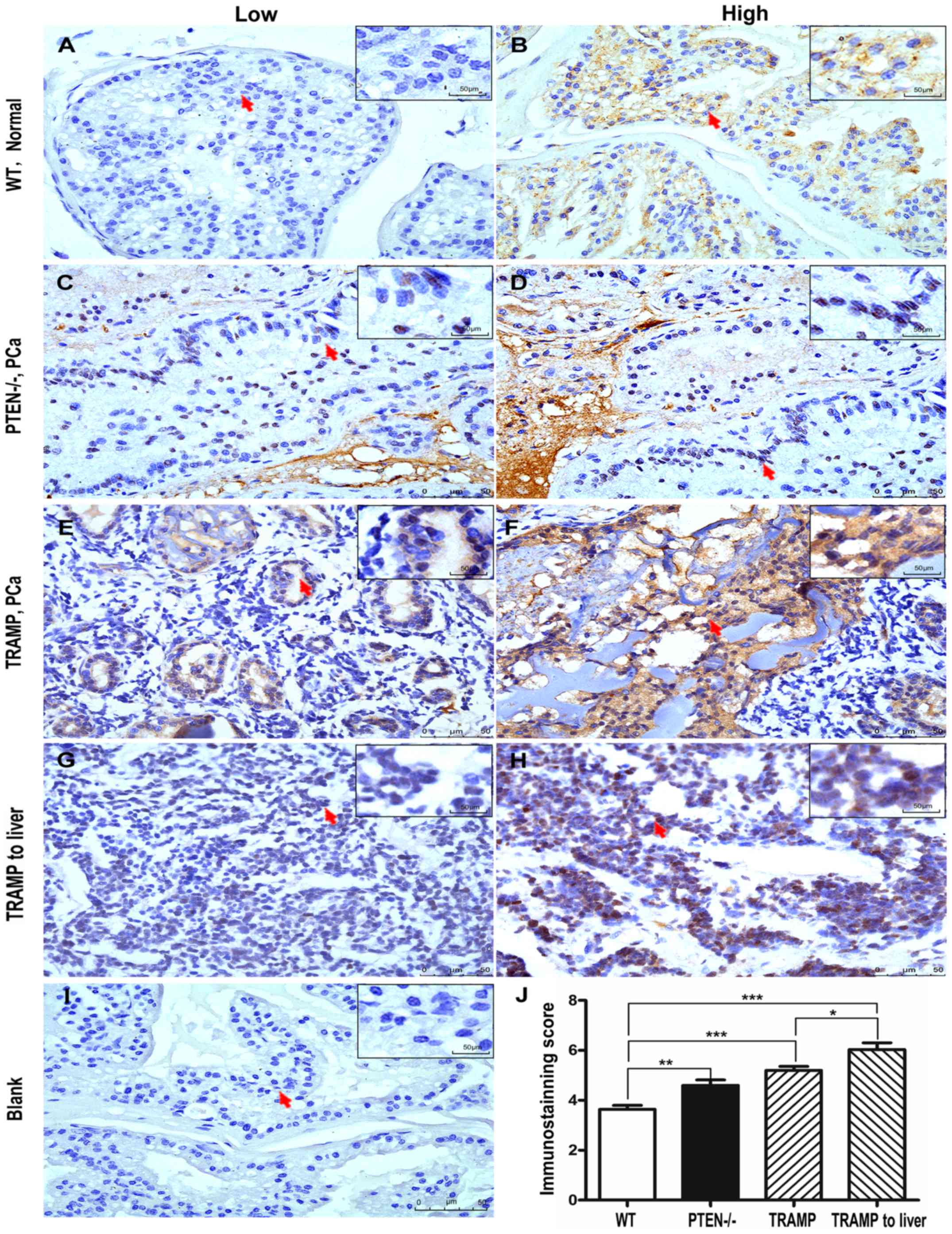
Similar to the results in human PCa tissues, PTCD3
levels were low in normal prostate tissues of WT mice but were
elevated in PCa samples from the PTEN knockout mice and TRAMP model
(Fig. 2). The PTCD3 levels between
the normal prostate tissues of WT mice and PCA in PTEN-deficient
mice or TRAMP model were significantly different (P<0.001;
Fig. 2J). We found that PTCD3
expression was significantly higher in PCa tissue from the
13-month-old TRAMP mouse model, which exhibited metastasis to the
liver, than in the other mouse samples. These data from mouse PCa
tissues were consistent with previous results in human PCa and
further confirmed that the levels of PTCD3 were correlated with the
aggressive progression of PCa.
Overexpression of PTCD3 is correlated
with Hormone-independence of human PCa
Given that the prostate is a hormone-regulated
organ, early-stage PCa is usually hormone dependent, whereas
late-stage PCa only becomes hormone independent after androgen
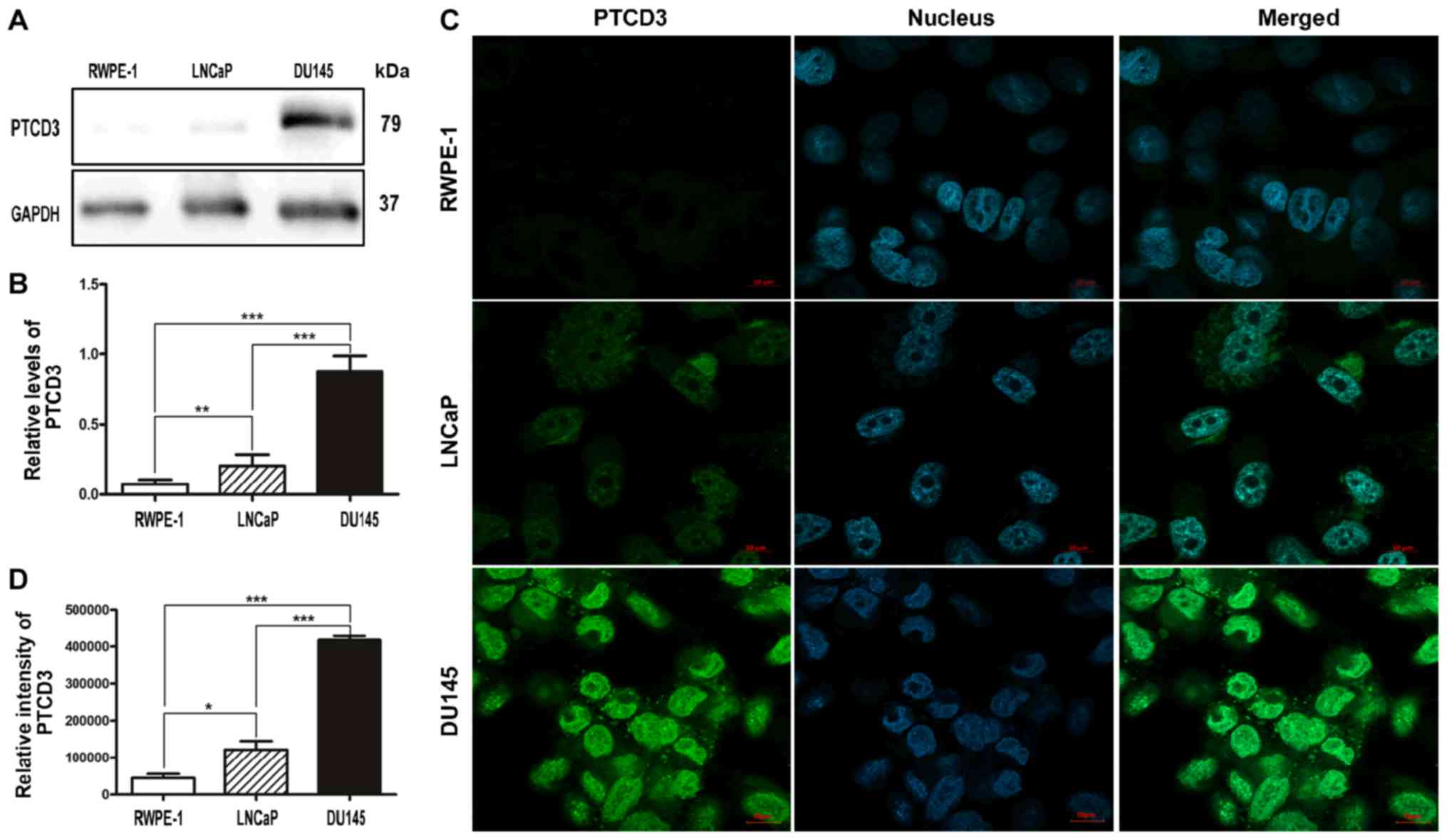
depletion treatment. We extracted proteins from two human PCa cell
lines (DU145 and LNCaP) and one benign prostate cell line (RWPE-1)
to determine the levels of PTCD3 in different stages of PCa. We
found that the levels of PTCD3 were lower in normal prostate cells
than in PCa cells, and the expression levels of PTCD3 in
hormone-dependent PCa cells (LnCaP) were lower than in
hormone-independent PCa cells (DU145) (Fig. 3A and B). This finding indicates
that the levels of PTCD3 were higher in hormone-independent PCa,
which is usually detected in patients with late-stage disease. To
confirm this result, we further performed immunofluorescence assays
in these three cell lines. We found that the expression levels of
PTCD3 were not detectable in RWPE-1 cell but were significantly
high in PCa cells including LNCaP and DU145 cells (Fig. 3C and D). Similar to the results of
Western blot analysis, the fluorescence intensities of PTCD3 in
DU145 cells were brighter than those in LNCaP and RWPE-1 cells
(Fig. 3C and D). The data from
normal prostate cells and PCa cells further confirmed that the
levels of PTCD3 were correlated with the hormone independence of
human PCa.
Overexpression of PTCD3 is associated
with poor prognosis of PCa in TCGA dataset
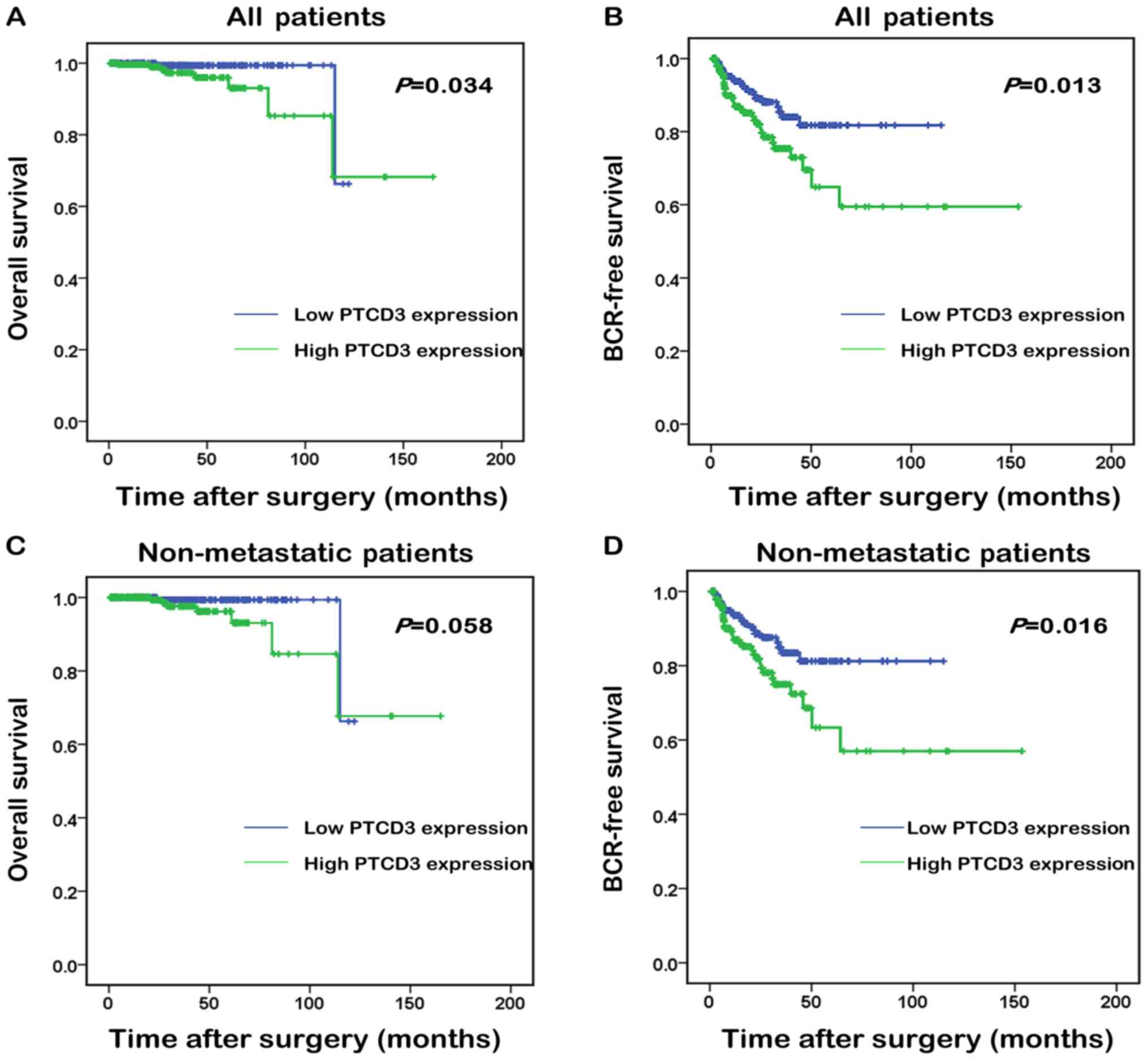
Overall survival and BCR-free survival are important
to PCa patients. Especially, BCR-free survival is authoritative to
PCa patients for its predictive function, which may affect the
follow-up treatments. Kaplan-Meier method was used to compare the
overall and BCR-free survival of patients with high and low PTCD3
expression to further evaluate the prognostic value of
overexpression of PTCD3 in patients with PCa. Median PTCD3 mRNA
expression in all PCa tissues was used as the cut-off point to
classify all cases into PTCD3 high (n=210, in TCGA dataset) and
PTCD3 low (n=215, in TCGA dataset) groups. Fig. 3 shows that all PCa patients with
high PTCD3 expression had shorter overall survival (Fig. 4A) than those with low PTCD3
expression (P=0.034). No statistically significant difference was
found between high PTCD3 and low PTCD3 groups in non-metastatic
patients (P=0.058; Fig. 4C). All
PCa patients and non-metastatic patients with high PTCD3 expression
showed shorter BCR-free survival (P=0.013, P=0.016; Fig. 4B and D) than those with low PTCD3
expression.
PTCD3 can serve as an independent
prognostic marker of PCa patients
We further utilized the Cox proportional hazard
model to assess whether or not PTCD3 is an independent prognostic
predictor for the survival of PCa patients documented in the TCGA
database. In Table II, the
univariate model analysis shows that the level of PTCD3 mRNA was a
significant prognostic factor for BCR-free survival in patients
with PCa with HR of 1.942, 95% CI of 1.141–3.306 and P=0.014.
Multivariate analysis using Cox proportional hazard model further
revealed that a high level of PTCD3 mRNA was a significant
independent prognostic marker for patients with PCa (HR of 1.956,
95% CI of 1.113–3.436 and P=0.020; Table II).
 | Table II.Prognostic value of PTCD3 for
BCR-free survival by Cox proportional hazards model. |
Table II.
Prognostic value of PTCD3 for
BCR-free survival by Cox proportional hazards model.
|
| BCR-free
survival |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Univariate
analysis |
|
|
| Gleason
score (<7 vs. =7 vs. >7) | 3.110
(1.841–5.255) |
<0.001c |
| Serum
PSA levels (<10 vs. ≥10) | 9.396
(3.978–22.196) |
<0.0001d |
| pN
stage (N0 vs. N1) | 1.879
(1.049–3.365) | 0.034a |
| PTCD3
expression (low vs. high) | 1.942
(1.141–3.306) | 0.014a |
| Multivariate
analysis |
|
|
| Gleason
score (<7 vs. =7 vs. >7) | 2.578
(1.378–4.825) | 0.003b |
| Serum
PSA levels (<10 vs. ≥10) | 6.579
(2.533–17.086) |
<0.001c |
| pN
stage (N0 vs. N1) | 1.659
(0.912–3.081) | 0.097 |
| PTCD3
expression (low vs. high) | 1.956
(1.113–3.436) |
0.020a |
Discussion
As a global, heterogeneous, and multifocal disease,
PCa continues to be a burden on the healthcare system (21). One in every six men can develop PCa
in his lifetime, and the incidence rate increases with age
(22). When PCa becomes metastatic
and invasive, it manifests an extremely unfavourable prognosis that
is commonly the primary cause of death. Thus, finding an effective
biomarker that can distinguish between indolent and aggressive PCa
and can predict the clinical outcome of patients with PCa may
benefit patient management and decrease morbidity.
PTCD3 is one of the mammalian mitochondrial
pentatricopeptide repeat (PPR) domain proteins (23) that play a critical role in
mitochondrial translation and organelle biogenesis and maintenance
(17,24). During carcinogenesis, the material
and energy in the cells are reshaped to support and help the
survival of cancer cells and to satisfy the requirements for the
synthesis of biological macromolecules and energy supplement in
cancer cells (25). Mitochondria
are an important stress receptor in cells and the central part of
all metabolic reactions (26) and
thus play a critical role in tumorigenesis. Our previous studies
showed that the mitochondrion-associated protein, LRPPRC, is one of
the mammalian mitochondrial PPR domain proteins that prevent the
degradation of the mitochondria; high LRPPRC levels can be used as
an independent biomarker for patients with late-stage PCa and poor
prognosis (11,12). Therefore, PTCD3 may have an
important function in PCa progression. D'Andrea et al
(19), recently identified that
the inhibition of the mitochondrial translation factor PTCD3 may be
detrimental to lymphomagenesis, and PTCD3 may act as a therapeutic
target in lymphomas (27).
However, the function of PTCD3 in human cancer was only
investigated in few studies, and the clinical significance of PTCD3
in the context of human PCa has not been reported yet.
In the present study, we found that PTCD3 played an
important role in PCa tumour progression by acting as an oncogene.
PTCD3 expression at both mRNA and protein levels was up-regulated
in the PCa tissues with advanced pathological or clinical stage.
High PTCD3 expression was dramatically associated with aggressive
tumour progression in patients with PCa, including high Gleason
score, short overall survival time, short BCR-free survival time
and high distant metastasis. The IHC results in mouse PCa tissues
also proved that elevated PTCD3 levels were positively correlated
with the aggressive progression of PCa. Furthermore, the results of
Western blot analysis and immunofluorescence assays performed in
normal prostate cells (RWPE-1) and PCa cells (DU145 and LNCaP)
showed that PTCD3 levels were lower in benign prostate cells than
in PCa cells. By contrast, the hormone-independent DU145 PCa cells
exhibited the strongest PTCD3 fluorescence intensity. These
findings indicated that PTCD3 overexpression was correlated with
the stages of human PCa. PTCD3 expression was identified as an
unfavourable prognostic factor of BCR-free survival in patients
with PCa. To our knowledge, this study is the first to investigate
the role of PTCD3 in the prognosis of PCa.
In conclusion, our data strongly suggested that
PTCD3 overexpression may play an important role in the
characteristics and aggressive behaviour of PCa, especially in
groups with advanced pathological or clinical stage and high
Gleason score. Additionally, PTCD3 upregulation is associated with
poor prognosis in patients with PCa. This protein may be a novel
prognostic biomarker for PCa and a potential therapeutic target for
patients with PCa. However, further study is necessary to gain a
full understanding of the underlying molecular mechanisms.
Acknowledgements
The authors would like to thank Dr. Fen Wang
(Institute of Biosciences and Technology, Texas A&M Health
Science Center, Houston, TX, USA) for providing mouse prostate
tissues samples.
Funding
The present study was supported by the Guangzhou
Municipal Science and Technology Project (grant no. 1563000448) and
National Natural Science Foundation of China (grant no.
81570189).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ, JW and YH designed the study and drafted the
manuscript. GJ, ZL and XL performed the experiments. YH, ZS and HW
analyzed and interpreted the data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Society AC: Cancer facts & figures
2016. American Cancer Society; Atlanta, GA: 2016
|
|
3
|
Center MM, Jemal A, Lortet-Tieulent J,
Ward E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blute ML Jr, Damaschke NA and Jarrard DF:
The epigenetics of prostate cancer diagnosis and prognosis: Update
on clinical applications. Curr Opin Urol. 25:83–88. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Draisma G, Etzioni R, Tsodikov A, Mariotto
A, Wever E, Gulati R, Feuer E and de Koning H: Lead time and
overdiagnosis in prostate-specific antigen screening: Importance of
methods and context. J Natl Cancer Inst. 101:374–383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
PSA-based screening for prostate cancer, .
Too many adverse effects. Prescrire Int. 21:215–217.
2012.PubMed/NCBI
|
|
7
|
Garg AD, Martin S, Golab J and Agostinis
P: Danger signalling during cancer cell death: Origins, plasticity
and regulation. Cell Death Differ. 21:26–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sehrawat A, Roy R, Pore SK, Hahm ER,
Samanta SK, Singh KB, Kim SH, Singh K and Singh SV: Mitochondrial
dysfunction in cancer chemoprevention by phytochemicals from
dietary and medicinal plants. Semin Cancer Biol. 47:147–153. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guerra F, Guaragnella N, Arbini AA, Bucci
C, Giannattasio S and Moro L: Mitochondrial dysfunction: A novel
potential driver of epithelial-to-mesenchymal transition in cancer.
Front Oncol. 7:2952017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ježek J, Cooper KF and Strich R: Reactive
oxygen species and mitochondrial dynamics: The yin and yang of
mitochondrial dysfunction and cancer progression. Antioxidants
(Basel). 7(pii): E132018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang X, Zhong W, Huang H, He H, Jiang F,
Chen Y, Yue F, Zou J, Li X, He Y, et al: Autophagy defects
suggested by low levels of autophagy activator MAP1S and high
levels of autophagy inhibitor LRPPRC predict poor prognosis of
prostate cancer patients. Mol Carcinog. 54:1194–1204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang X, Li X, Huang H, Jiang F, Lin Z, He
H, Chen Y, Yue F, Zou J, He Y, et al: Elevated levels of
mitochondrion-associated autophagy inhibitor LRPPRC are associated
with poor prognosis in patients with prostate cancer. Cancer.
120:1228–1236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Beckman KB, Caberto C, Kazma R,
Lum-Jones A, Haiman CA, Le Marchand L, Stram DO, Saxena R and Cheng
I: Association of genes, pathways, and haplogroups of the
mitochondrial genome with the risk of colorectal cancer: The
multiethnic cohort. PLoS One. 10:e01367962015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burch TC, Rhim JS and Nyalwidhe JO: Novel
altered mitochondrial genes in prostate cancer progression. Cancer
Res. 75:2015. View Article : Google Scholar
|
|
15
|
Genecards: Summaries for PTCD3 Gene.
https://www.genecards.org/cgi-bin/carddisp.pl?gene=PTCD3August
8–2018
|
|
16
|
Lightowlers RN and Chrzanowska-Lightowlers
ZM: Human pentatricopeptide proteins: Only a few and what do they
do? RNA Biol. 10:1433–1438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rackham O, Davies SM, Shearwood AM,
Hamilton KL, Whelan J and Filipovska A: Pentatricopeptide repeat
domain protein 1 lowers the levels of mitochondrial leucine tRNAs
in cells. Nucleic Acids Res. 37:5859–5867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madhavan S, Gusev Y, Singh S and Riggins
RB: ERRγ target genes are poor prognostic factors in
Tamoxifen-treated breast cancer. J Exp Clin Cancer Res. 34:452015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Andrea A, Gritti I, Nicoli P, Giorgio M,
Doni M, Conti A, Bianchi V, Casoli L, Sabò A, Mironov A, et al: The
mitochondrial translation machinery as a therapeutic target in
Myc-driven lymphomas. Oncotarget. 7:72415–72430. 2016.PubMed/NCBI
|
|
20
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esfahani M, Ataei N and Panjehpour M:
Biomarkers for evaluation of prostate cancer prognosis. Asian Pac J
Cancer Prev. 16:2601–2611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baade PD, Youlden DR and Krnjacki LJ:
International epidemiology of prostate cancer: Geographical
distribution and secular trends. Mol Nutr Food Res. 53:171–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manna S: An overview of pentatricopeptide
repeat proteins and their applications. Biochimie. 113:93–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baggio F, Bratic A, Mourier A, Kauppila
TE, Tain LS, Kukat C, Habermann B, Partridge L and Larsson NG:
Drosophila melanogaster LRPPRC2 is involved in coordination of
mitochondrial translation. Nucleic Acids Res. 42:13920–13938. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zong WX, Rabinowitz JD and White E:
Mitochondria and cancer. Mol Cell. 61:667–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oran AR, Adams CM, Zhang XY, Gennaro VJ,
Pfeiffer HK, Mellert HS, Seidel HE, Mascioli K, Kaplan J, Gaballa
MR, et al: Multi-focal control of mitochondrial gene expression by
oncogenic MYC provides potential therapeutic targets in cancer.
Oncotarget. 7:72395–72414. 2016. View Article : Google Scholar : PubMed/NCBI
|