Introduction
Ovarian cancer is the most fatal gynecological
cancer and the fifth most common cause of cancer mortality in women
(1–3). Epigenetic and genetic factors can
influence the genesis and development of ovarian cancer. Notably,
activation of the canonical signal transducer and activator of
transcription 3 (STAT3) signaling pathway has a crucial role in
inducing tumorigenesis, progression, invasion and metastasis
(4–8). Phosphorylated STAT3 translocates to
nucleus where it activates its target genes, including B-cell
lymphoma 2 (Bcl-2), cyclin D1 and survivin. These genes regulate
cell proliferation and apoptosis, thereby mediating cancer
initiation, invasion, and progression (4–11). A
combination of surgical operation and chemotherapy is currently the
most popular therapeutic strategy for patients with ovarian cancer
(1,2). The most common chemotherapy regimens
for treatment of ovarian cancer employ a combination of carboplatin
and paclitaxel. Although up to 80% of patients respond well to
these chemotherapy drugs initially, a majority of patients suffer
from recurrence and metastasis due to chemoresistance. The
discovery and identification of novel drugs and therapeutic targets
in ovarian cancer are required, and botanical products are
attractive candidates.
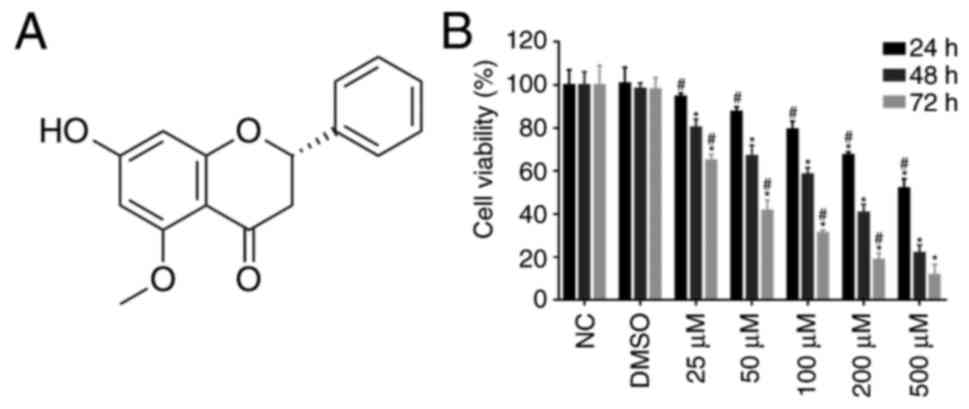
Alpinetin (7-hydroxy-5-methoxyflavanone;
C16H14O4; Fig. 1A) is a natural flavonoid primarily
derived from the roots or seeds of Alpinia katsumadai Hayata
that is known to exhibit antibacterial, anti-inflammatory and other
important therapeutic properties (12–18).
In previous studies, alpinetin was reported to inhibit
proliferation of human tumor cells, including lung cancer and
gastroenteric cancer cells (12–16,19),
indicating the potential anticancer properties of this compound.
However, the effects of alpinetin on ovarian cancer cells and its
underlying mechanisms of action remain largely unknown.
In the present study, the effects of alpinetin on
SKOV3 ovarian cancer cells were investigated. The results
demonstrated that alpinetin inhibited SKOV3 cell proliferation, led
to arrest in the G1/S transition phase and altered expression
levels of apoptosis-related protein. In addition, alpinetin
suppressed the invasion ability of SKOV3 cells by inhibiting cell
migration as well as altering protein expression levels of matrix
metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs). Alpinetin also suppressed colony and
spheroid formation by SKOV3 cells. It was hypothesized that STAT3
signaling pathway may mediate the anti-ovarian cancer effects of
alpinetin.
Materials and methods
Chemicals and reagents
Alpinetin (>98% high-performance liquid
chromatography purity) was purchased from Chengdu Must
Biotechnology Co., Ltd. (Chengdu, China). Alpinetin was dissolved
in dimethylsulfoxide (DMSO) to a concentration of 200 mM before
use. RPMI-1640 medium, PBS, trypsin/EDTA solution and fetal bovine
serum (FBS) were purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Cell Counting kit-8 (CCK)-8, bicinchoninic
acid (BCA) protein assay kit, Cell Apoptosis Detection kit and Cell
Cycle Analysis kit were purchased from Beyotime Institute of
Biotechnology (Haimen, China). Antibodies against α-tubulin
(ab52866; 1:2,000), cyclin D1 (ab134175; 1:10,000), surviving
(ab469; 1:2,000), Bcl-2 (ab59348; 1:1,000), Bcl-2-associated X
protein (Bax; ab32503; 1:2,000), TIMP-1 (ab61224; 1:500), TIMP-2
(ab180630; 1:500), MMP-2 (ab92536; 1:1,000) and MMP-9 (ab137867;
1:1,000) were obtained from Abcam (Cambridge, UK). Antibodies
against cyclin-dependent kinase (CDK)4 (11026-1-AP; 1:500), CDK6
(14052-1-AP; 1:500) and c-myc (10828-1-AP; 1:500) were purchased
from Wuhan Sanying Biotechnology (Wuhan, China). Collagen solution
(04902, 3 mg/ml) was obtained from STEMCELL Technologies, Inc.
(Vancouver, BC, Canada) Antibodies against STAT3 (4904P; 1:1,000),
phosphorylated (p)-STAT3 (9145P; 1:1,000), cleaved caspase-3
(9664s; 1:1,000) and cleaved poly (ADP-ribose) polymerase (PARP,
5625S, 1:1,000) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Fluorescent-labeled secondary antibodies
(ABIN2169623/ABIN2169616, 1:10,000) were purchased from LI-COR
Biosciences (Lincoln, NE, USA). CellTiter-Glo® 3D Cell
Viability Assay kit (G9683) was purchased from Promega Corporation
(Madison, WI, USA).
Cell culture
Human SKOV3 ovarian cancer cell line was obtained
from EK-Bioscience (Shanghai, China). SKOV3 cells were cultured in
RPMI-1640 supplemented with 10% FBS, penicillin (100 IU) and
streptomycin (100 µg/ml) at 37°C in a humidified atmosphere
containing 5% CO2.
Cell viability
SKOV3 cells were seeded into 96-well plates (5,000
cells/well) and allowed to adhere overnight before being treated
with different concentrations of alpinetin. DMSO was used as
vehicle control and RPMI-1640 with 10% FBS was used as negative
control. The maximum concentration of DMSO is 0.25%. Subsequently,
cells were cultured for 24, 48 and 72 h, and analyzed using the
CCK-8 assay according to manufacturer's instructions. Absorbance
was measured at 450 nm using a PerkinElmer Victor3 1420 Multilabel
Counter (PerkinElmer, Inc., Waltham, MA, USA). Cell viability (%)
was calculated using the following formula: Cell viability=(average
absorbance of samples/average absorbance of controls) ×100. All
tests were repeated three times.
Apoptosis assay
SKOV3 cells were seeded into 6-well plates
(2×105 cells/well). Following treatment with different
concentrations of alpinetin for 48 h, SKOV3 cells were stained with
Annexin V and propidium iodide (PI) using an Apoptosis Detection
kit (BB-4101, BestBio, Inc., Shanghai, China) according to the
manufacturer's instructions. Briefly, cells were digested from
plates, washed twice with cold PBS and resuspended in an Annexin
V-fluorescein isothiocyanate (FITC) binding buffer. Annexin V-FITC
was added to the cells and mixed gently, and allowed to incubate
for 15 min at 4°C in the dark. PI staining solution was then added
and incubated for 5 min at 4°C in the dark. Lastly, cells were kept
on ice in the dark and immediately analyzed using a FACSCalibur
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Cell
apoptosis was quantified FlowJo.7.6.1 software (FlowJo LLC,
Ashland, OR, USA).
Cell cycle analysis
SKOV3 cells were seeded into 6-well plates
(2×105 cells/well). After culturing in RPMI-1640 without
FBS overnight for synchronization, SKOV3 cells were treated with
different concentrations of alpinetin for an additional 48 h before
cells were harvested and fixed in pre-cooled 70% ethanol at 4°C
overnight. Fixed cells were washed with PBS and then stained with
PI for 30 min at 4°C in the dark. Stained cells were acquired using
a FACSCalibur flow cytometer (BD Biosciences) and cell cycle was
analyzed using FlowJo.7.6.1 software (FlowJo LLC).
Western blot analysis
Following treatment with different concentrations of
alpinetin for 48 h, SKOV3 cells were digested from plates and
resuspended in ice-cold cell lysis buffer (Beyotime Biotech Co.,
Ltd.) containing complete™ mini protease inhibitor
cocktail tablet (Roche Diagnostics, Basel, Switzerland). Protein
concentration was determined using a BCA kit according to the
manufacturer's instructions. Proteins (30 µg/lane) were separated
using 10% SDS-PAGE and transferred onto a polyvinylidene fluoride
membrane, then blocked with 5% skimmed milk. Membranes were
incubated with primary antibodies overnight at 4°C. Subsequently,
membranes were incubated with secondary antibodies (IRDye700 and
IRDye800; goat anti-mouse/rabbit; 1:10,000; LI-COR Biosciences) for
1 h at 37°C and visualized using the Odyssey infrared imaging
system (LI-COR Biosciences). The gray value of protein bands were
semi-quantified using Image Studio Lite 5.2.5 (LI-COR Biosciences);
the protein levels are presented as a ratio of the average gray
value of each protein to the average gray value of α-tubulin.
Wound healing assay
SKOV3 cells were seeded in 12-well plates
(1×105 cells/ml) and allowed to adhere overnight. A
‘wound’ was introduced by scratching the wells using a sharp tip
and cells were further incubated for 24 h with 100 µM alpinetin
under serum-free conditions. The gap was then imaged under a
microscope (CKX31; Olympus Corporation, Tokyo, Japan) and
quantified using Image-Pro Plus 5.1 (Media Cybernetics, Inc.,
Rockville, MD, USA) before and after treatment.
Colony formation assay
SKOV3 cells were seeded into a 96-well plate (200
cells/well) and allowed to adhere overnight. Subsequently, cells
were treated with different concentrations of alpinetin for 7 days.
The cell culture medium was removed and staining solution (0.05%
crystal violet, 1% formaldehyde and 1% methanol) was added into
plate for 20 min. The staining solution was removed, the plate was
washed under running water and colonies were counted using an
inverted microscope.
3D spheroid assay and
CellTiter-Glo® 3D cell viability assay
SKOV3 cells were seeded at 5,000 cells/well in
ultra-low attachment 96-well plates (Corning Incorporated, Corning,
NY, USA) together with collagen (5 µl/well). Cells were cultured
for 24 h in 37°C and spheroid formation was captured using a
microscope (IX83; Olympus Corporation). Subsequently, alpinetin was
added to wells and DMSO was used as vehicle control. Following 7
days of culture, the spheroids were imaged under a microscope
(IX83; Olympus Corporation) before 100 µl CellTiter-Glo®
3D reagent was added and allowed to incubate at room temperature in
the dark. Luminescence was measured using a Multilabel Counter
(PerkinElmer, Inc.).
Statistical analysis
Data are presented as the means ± standard
deviation. The data were subjected to one-way analysis of variance.
Differences between two groups were determined using Dunnett's
test, and multiple means were compared using Tukey's test. All
analyses were conducted using SPSS version 22.0.0.1 (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Alpinetin inhibits proliferation of
the SKOV3 ovarian cancer cell line
SKOV3 cells were treated with increasing
concentrations of alpinetin for 24, 48 and 72 h. The CCK-8 assay
was used to investigate whether alpinetin inhibits proliferation of
ovarian cancer cells. As shown in Fig.
1B, alpinetin treatment significantly decreased the viability
of SKOV3 cells in a dose-and time-dependent manner.
Alpinetin induces cell apoptosis and
alters expression levels of apoptosis-associated proteins in SKOV3
cells
Due to the effect of alpinetin on proliferation of
SKOV3 cells, it was of interest to perform a cell apoptosis assay
to validate the anticancer effect of alpinetin. The results
demonstrated that alpinetin induced apoptosis of SKOV3 cells in a
dose-dependent manner (Fig. 2A).
In addition, treatment of SKOV3 cells with increasing
concentrations of alpinetin significantly downregulated the protein
expression levels of Bcl-2, whereas upregulated the protein
expression levels of Bax as well as cleaved caspase-3 and PARP
(Fig. 2B).
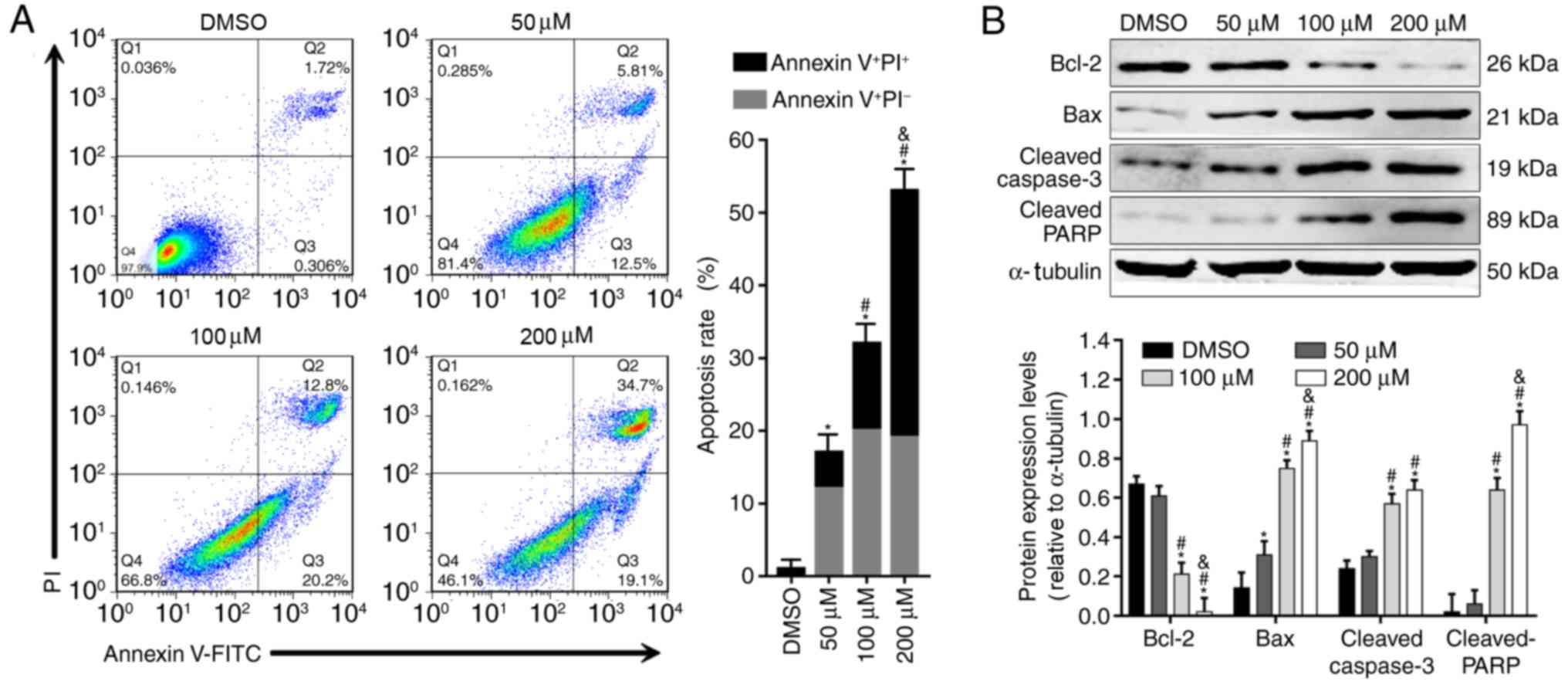 | Figure 2.Analysis of apoptosis in
alpinetin-treated SKOV3 cells. (A) Apoptosis rates of SKOV3 cells
following treatment with increasing concentrations of alpinetin
(50, 100 and 200 µM) for 48 h. Q1: FITC−PI+;
Q2: FITC+PI+; Q3:
FITC−PI−; Q4: FITC+PI−.
(B) Western blot analysis of apoptosis-related protein expression
levels in SKOV3 cells following treatment with increasing
concentrations of alpinetin (50, 100 and 200 µM) for 48 h. Each
experiment was performed three times and representative data are
shown. *P<0.05 vs. DMSO(vehicle control), #P<0.05
vs. 50 µM, &P<0.05 vs. 100 µM. Bax,
Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; DMSO,
dimethylsulfoxide; FITC, fluorescein isothiocyanate; PARP, poly
(ADP-ribose) polymerase; PI, propidium iodide. |
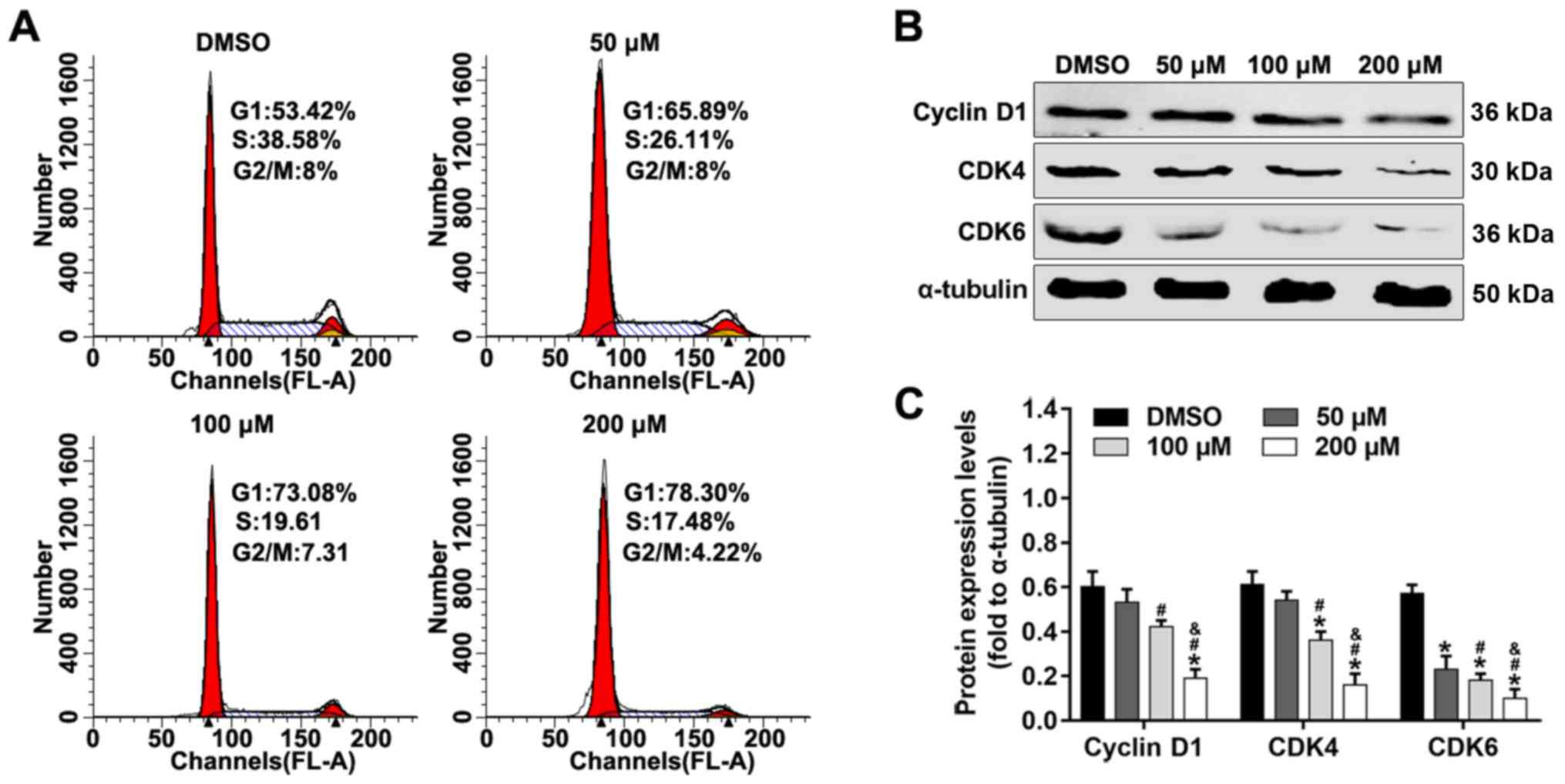
Alpinetin inhibits G1/S transition and
alters expression levels of cell cycle-related proteins
In order to investigate the mechanism underlying
alpinetin-mediated inhibition of proliferation of SKOV3 cells, cell
cycle was analyzed by flow cytometry and apoptosis-related proteins
were detected using western blotting. As shown in Fig. 3A, the number of cells in the
G1 phase increased whereas the number cells in the S
phase were decreased following alpinetin treatment compared with
the DMSO control group. In addition, treatment of SKOV3 cells with
increasing concentrations of alpinetin significantly downregulated
the protein expression levels of cyclin D1, CDK4 and CDK6 (Fig. 3B and C).
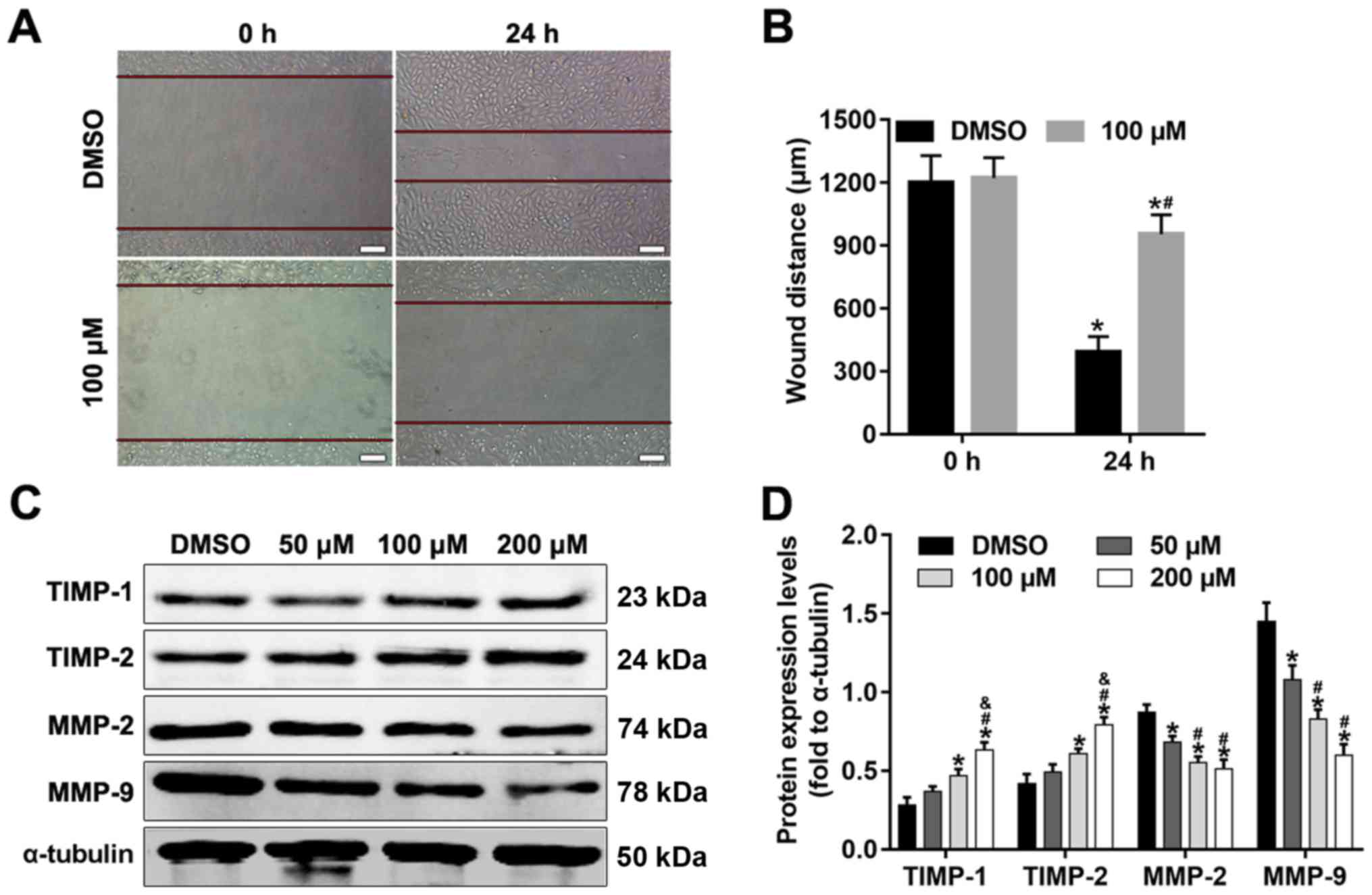
Alpinetin inhibits SKOV3 migration and
alters the protein expression levels of TIMPs and MMPs
Migration is one of the key traits of malignant
cancer cells. MMPs are relevant for cancer as they are a family of
migration promoting factors, whilst TIMPs are inhibitors of MMPs.
In the present study, the effects of alpinetin on the migration of
SKOV3 cells were evaluated. As shown in Fig. 4A and B, the wound distance was
significantly higher in the alpinetin group compared with in the
DMSO control group. The results indicated that alpinetin decreased
the migratory capacity of SKOV3 cells. In addition, MMP-2 and MMP-9
protein expression levels were significantly decreased in
alpinetin-treated cells compared with in control cells (Fig. 4C and D). Conversely, TIMP-1 and
TIMP-2 expression levels were increased in the alpinetin-treated
groups (Fig. 4C and D).
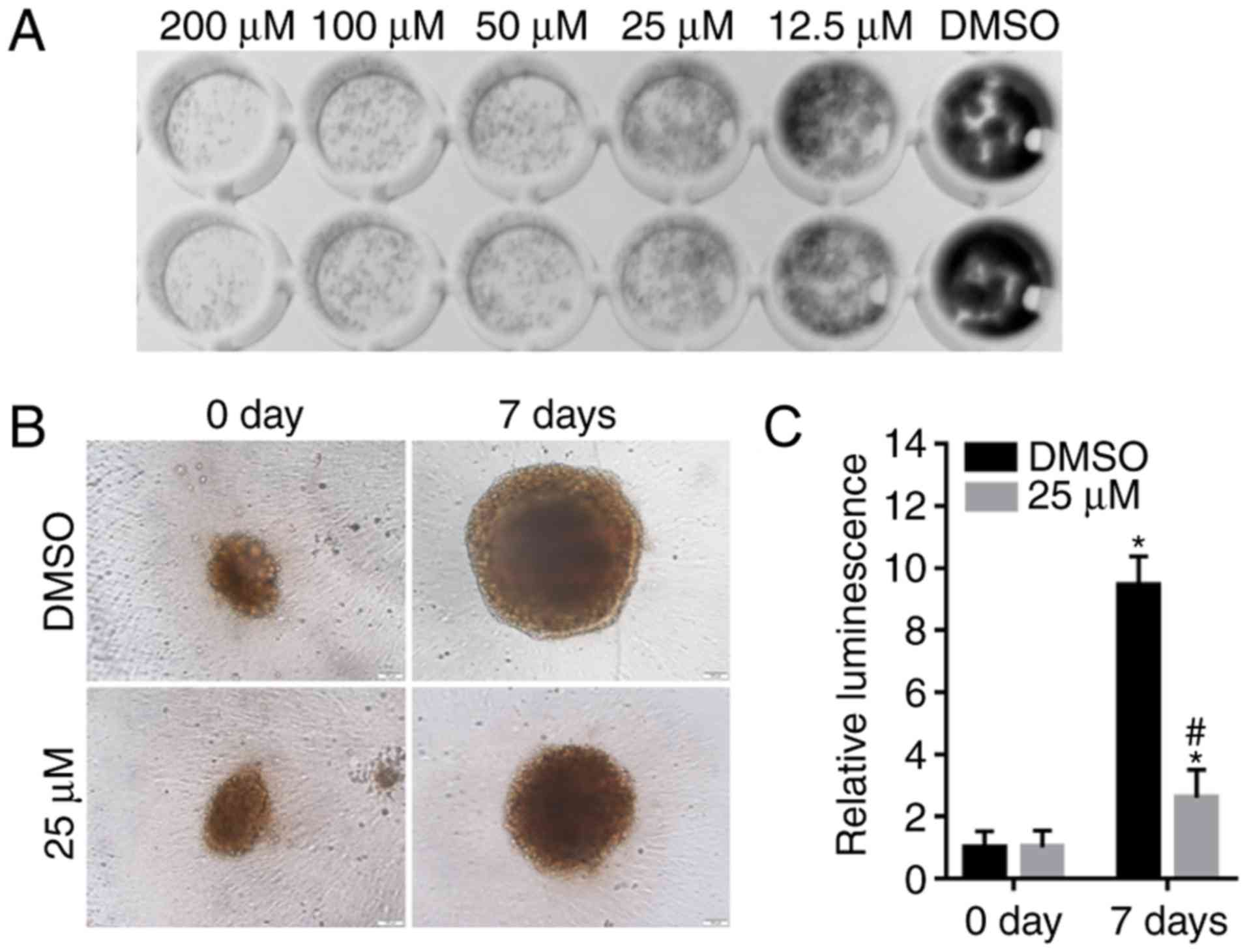
Alpinetin suppresses colony and
spheroid formation of SKOV3 cells
The colony formation ability of SKOV3 cells
following alpinetin treatment was also evaluated in the present
study and the results demonstrated that treatment with alpinetin
significantly suppressed colony formation (Fig. 5A). In addition, the anticancer
effects of alpinetin on SKOV3 cells were evaluated in a 3D tumor
spheroid culture. Alpinetin decreased the diameter of spheroids
compared with the DMSO control group (Fig. 5B) and the cell viability of SKOV3
spheroids was also significantly decreased (Fig. 5C).
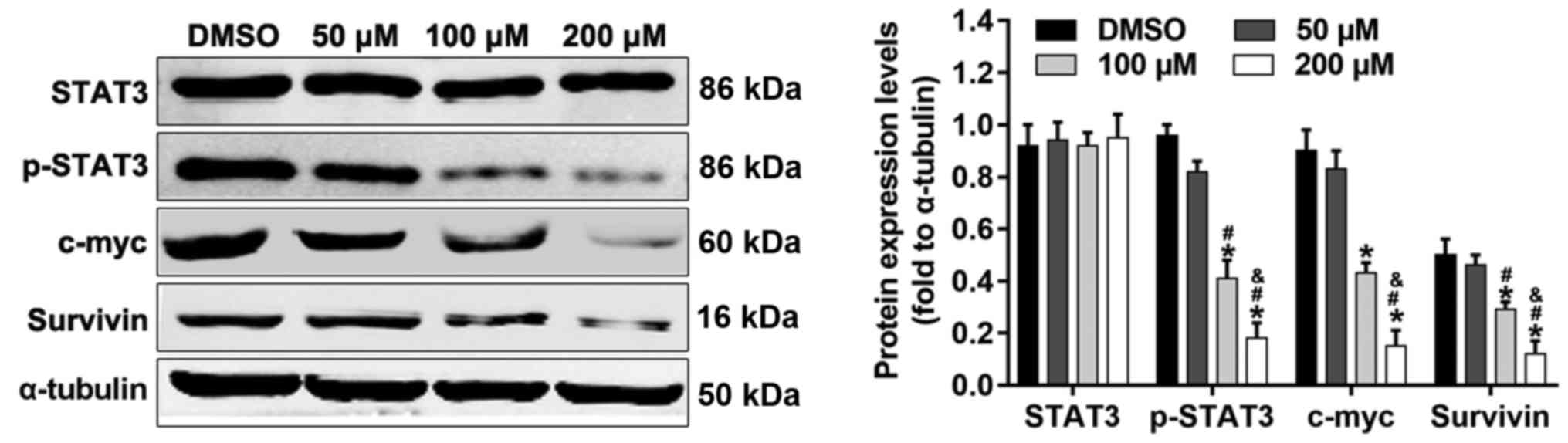
STAT3 signaling is involved in the
anticancer effects of alpinetin
STAT3 signaling serves an important role in cell
proliferation and survival in ovarian cancer. Cyclin D1, Bcl-2 and
survivin are STAT3-regulated gene products that are associated with
cell proliferation or apoptosis. In order to ascertain whether
STAT3 signaling is involved in the anticancer effects of alpinetin
on SKOV3 cells, the protein expression levels of STAT3, p-STAT3,
c-myc and survivin were detected by western blotting after
treatment of cells with different concentrations of alpinetin. The
results indicated that alpinetin significantly decreased the
phosphorylation of STAT3, and the protein expression levels of
c-myc and survivin compared with the DMSO control group (Fig. 6).
Discussion
Several studies have indicated that alpinetin can
inhibit proliferation and induce apoptosis in different cancer cell
lines, with numerous signaling pathways, including Notch and
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein
kinase B (Akt) signaling pathway thought to be involved its
anticancer activity (12,15). However, the effects of alpinetin on
ovarian cancer remained poorly understood. The aim of the present
study was to investigate the anticancer efficacy of alpinetin and
its underlying mechanism of action in ovarian cancer. Alpinetin, a
major bioactive component from the roots or seeds of Alpinia
katsumadai Hayata, may target SKOV3 ovarian cancer cells in
vitro by suppressing proliferation and migration as well as
inhibiting the STAT3 signaling pathway.
The results demonstrated that alpinetin inhibited
proliferation and induced cell apoptosis of SKOV3 ovarian cancer
cells in a dose- and time-dependent manner. Bcl-2 family members
are important mediators of cell apoptosis through activating
caspase. Caspase-3 is a crucial member of the caspase family of
proteases, whereby cleaved caspase-3 can cleave PARP and induce
apoptosis. The cleaving of caspase-3 and PARP has an important role
during cell apoptosis. In the present study, alpinetin
significantly decreased the expression levels of anti-apoptotic
protein Bax and increased the expression levels of pro-apoptotic
proteins Bcl-2, cleaved caspase-3 and PARP in SKOV3 cells.
Similarly, Wu et al (12)
reported that alpinetin induces cell apoptosis, upregulates the
protein expression levels of Bax and cytochrome c, and
downregulates the protein expression levels of Bcl-2, X-linked
inhibitor of apoptosis protein and Bcl-extra large (XL) in A549
lung cancer cells. In addition, cells were arrested in the
G1 phase by alpinetin. Cyclin-D1, CDK4 and CDK6 are
crucial for the transition from G1 to S phase of the
cell cycle and their protein expression levels were significantly
decreased in SKOV3 cells following alpinetin treatment. Therefore,
it may be hypothesized that alpinetin-mediated downregulation of
cyclin-D1, CDK4 and CDK6 protein expression leads to G1
cell cycle phase arrest. These results indicated that alpinetin
inhibited cell proliferation, induced cell apoptosis through
G1 phase arrest and altered expression of
apoptosis-related proteins.
The migratory ability of SKOV3 cells was also
detected after alpinetin treatment. The results revealed that
alpinetin significantly decreased the migratory capacity of SKOV3
cells. MMPs and TIMPs are the crucial factors for tumor invasion
(7,20), whereby MMPs can promote cancer
invasion and TIMPs act as inhibitors of MMPs. In the present study,
MMP-2 and MMP-9 expression levels were significantly decreased in
alpinetin-treated cells compared with in DMSO control cells.
Conversely, TIMP-1 and TIMP-2 expression levels were increased in
the alpinetin-treated groups. Similarly, Park et al
(21) demonstrated that Alpinia
katsumadai suppresses cell migration by inhibiting
transglutaminase 2, MMP-2 and MMP-9 expression in HT-1080 cells.
Therefore, it can be hypothesized that alpinetin suppresses the
migration of ovarian cancer cells via downregulation of MMPs and
upregulation of its inhibitors.
The effects of alpinetin on SKOV3 colony and
spheroid formation following treatment with alpinetin was also
evaluated. The results demonstrated that treatment with alpinetin
significantly suppressed the colony formation capacity of SKOV3
ovarian cancer cells and significantly decreased the diameter as
well as cell viability of SKOV3 spheroids compared with the DMSO
control group. These results further confirm the anticancer effects
of alpinetin on ovarian cancer.
Numerous signaling pathways, including STAT3,
PI3K/Akt, β-catenin and Notch signaling pathways can influence
tumorigenesis, progression, invasion and metastasis of ovarian
cancer (5,12,15,22).
Activation of the canonical STAT3 signaling pathway serves a vital
role in ovarian cancer. Activated p-STAT3 translocates to nucleus
where it activates its downstream target genes, including cyclin-D1
and c-myc, which promote cell cycle progression, as well as
survivin, Bcl-2, Bcl-XL and myeloid cell leukemia 1, which suppress
apoptosis (5,6,8–11).
STAT3 also regulates the expression of MMP-9 and vascular
endothelial growth factor, both of which are associated with
invasion and angiogenesis (5,6,8–11).
In the present study, alpinetin significantly altered the protein
expression levels of cyclin-D1, c-myc, survivin, Bcl-2, Bax,
TIMP-1, TIMP-2, MMP-2, MMP-9 as well as cleaved caspase-3 and PARP
in SKOV3 cells. Therefore, it may be hypothesized that alpinetin
exerts its anticancer effects on SKOV3 cells through STAT3
signaling. Subsequently, activation of STAT3 signaling in SKOV3
cells after alpinetin treatment was investigated. The results
demonstrated that alpinetin inhibited activation of STAT3 signaling
in a dose-dependent manner. Previous studies using A549 lung cancer
cells and glioma stem cells revealed that the anticancer effects of
alpinetin involved inhibition of PI3K/AKT and Notch signaling
(12,15). These results implied that
inhibition of ovarian cancer cell proliferation and migration by
alpinetin resulted from downregulation of STAT3 signaling, and
other signaling pathways may also be involved.
In conclusion, alpinetin represents a novel
potential anti-ovarian cancer agent, which has the ability to
suppress proliferation, promote cell cycle arrest and inhibit
invasion via STAT3 signaling in vitro; however, in
vivo studies are required to confirm these results. Overall,
alpinetin may be a promising compound for treatment of ovarian
cancer.
Acknowledgements
The authors would like to thank all of the
colleagues in Department of Gynecology, Women's Hospital, Zhejiang
University, for their technical assistance and helpful
suggestions.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ was involved in project development, data
collection, data analysis and manuscript writing. XG, JS and DH
conducted data collection and data analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Bax
|
Bcl-2-associated X protein
|
|
Bcl
|
B-cell lymphoma
|
|
CDK
|
cyclin-dependent kinase
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
MMP
|
matrix metalloproteinase
|
|
PARP
|
poly (ADP-ribose) polymerase
|
|
VEGF
|
vascular endothelial growth factor
|
|
PI3K/Akt
|
phosphatidylinositol-4, 5-bisphosphate
3-kinase/protein kinase B
|
|
STAT
|
signal transducer and activator of
transcription
|
|
TIMP
|
tissue inhibitor of
metalloproteinase
|
References
|
1
|
Chien J and Poole EM: Ovarian cancer
prevention, screening, and early detection: Report from the 11th
biennial ovarian cancer research symposium. Int J Gynecol Cancer.
27 9S Suppl 5:S20–S22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi JY, Lee S, Yun SM, Suh DH, Kim K, No
JH, Jeong EH and Kim YB: Active hexose correlated compound (AHCC)
inhibits the proliferation of ovarian cancer cells by suppressing
signal transducer and activator of transcription 3 (STAT3)
activation. Nutr Cancer. 70:109–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li C, Hong L, Liu C, Min J, Hu M and Guo
W: Astragalus polysaccharides increase the sensitivity of SKOV3
cells to cisplatin. Arch Gynecol Obstet. 297:381–386. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen SH, Murphy DA, Lassoued W, Thurston
G, Feldman MD and Lee WM: Activated STAT3 is a mediator and
biomarker of VEGF endothelial activation. Cancer Biol Ther.
7:1994–2003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai L, Zhang G, Tong X, You Q, An Y, Wang
Y, Guo L, Wang T, Zhu D and Zheng J: Growth inhibition of human
ovarian cancer cells by blocking STAT3 activation with small
interfering RNA. Eur J Obstet Gynecol Reprod Biol. 148:73–80. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar J, Fang H, McCulloch DR, Crowley T
and Ward AC: Leptin receptor signaling via Janus kinase 2/Signal
transducer and activator of transcription 3 impacts on ovarian
cancer cell phenotypes. Oncotarget. 8:93530–93540. 2017.PubMed/NCBI
|
|
7
|
Jia Z, Jia Y, Guo FJ, Chen J, Zhang XW and
Cui MH: Phosphorylation of STAT3 at Tyr705 regulates MMP-9
production in epithelial ovarian cancer. PLoS One. 12:e1836222017.
View Article : Google Scholar
|
|
8
|
Hirano T, Ishihara K and Hibi M: Roles of
STAT3 in mediating the cell growth, differentiation and survival
signals relayed through the IL-6 family of cytokine receptors.
Oncogene. 19:2548–2556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landen CN, Lin YG, Armaiz Pena GN, Das PD,
Arevalo JM, Kamat AA, Han LY, Jennings NB, Spannuth WA, Thaker PH,
et al: Neuroendocrine modulation of signal transducer and activator
of transcription-3 in ovarian cancer. Cancer Res. 67:10389–10396.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
11
|
Harada D, Takigawa N and Kiura K: The role
of STAT3 in non-small cell lung cancer. Cancers (Basel). 6:708–722.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu L, Yang W, Zhang SN and Lu JB:
Alpinetin inhibits lung cancer progression and elevates
sensitization drug-resistant lung cancer cells to cis-diammined
dichloridoplatium. Drug Des Devel Ther. 9:6119–6127.
2015.PubMed/NCBI
|
|
13
|
Wang Z, Lu W, Li Y and Tang B: Alpinetin
promotes Bax translocation, induces apoptosis through the
mitochondrial pathway and arrests human gastric cancer cells at the
G2/M phase. Mol Med Rep. 7:915–920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang B, Du J, Wang J, Tan G, Gao Z, Wang Z
and Wang L: Alpinetin suppresses proliferation of human hepatoma
cells by the activation of MKK7 and elevates sensitization to
cis-diammined dichloridoplatium. Oncol Rep. 27:1090–1096. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Yan Z, Liu X, Che S, Wang C and
Yao W: Alpinetin targets glioma stem cells by suppressing Notch
pathway. Tumor Biol. 37:9243–9248. 2016. View Article : Google Scholar
|
|
16
|
Du J, Tang B, Wang J, Sui H, Jin X, Wang L
and Wang Z: Antiproliferative effect of alpinetin in BxPC-3
pancreatic cancer cells. Int J Mol Med. 29:607–612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajmala Shireen P, Abdul Mujeeb VM and
Muraleedharan K: Theoretical insights on flavanones as antioxidants
and UV filters: A TDDFT and NLMO study. J Photochem Photobiol B.
170:286–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huo M, Chen N, Chi G, Yuan X, Guan S, Li
H, Zhong W, Guo W, Soromou LW, Gao R, et al: Traditional medicine
alpinetin inhibits the inflammatory response in Raw 264.7 cells and
mouse models. Int Immunopharmacol. 12:241–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malami I, Abdul AB, Abdullah R, Kassim NK,
Rosli R, Yeap SK, Waziri P, Etti IC and Bello MB: Correction: Crude
extracts, flavokawain B and alpinetin compounds from the rhizome of
Alpinia mutica induce cell death via UCK2 enzyme inhibition and in
turn reduce 18S rRNA biosynthesis in HT-29 cells. PLoS One.
12:e1702332017. View Article : Google Scholar
|
|
20
|
Sun X, Lin L, Chen Y, Liu T, Liu R, Wang
Z, Mou K, Xu J, Li B and Song H: Nitidine chloride inhibits ovarian
cancer cell migration and invasion by suppressing MMP-2/9
production via the ERK signaling pathway. Mol Med Rep.
13:3161–3168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park MK, Jo SH, Lee HJ, Kang JH, Kim YR,
Kim HJ, Lee EJ, Koh JY, Ahn KO, Jung KC, et al: Novel suppressive
effects of cardamonin on the activity and expression of
transglutaminase-2 lead to blocking the migration and invasion of
cancer cells. Life Sci. 92:154–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang JM, Min J, Li BS, Hong SS, Liu C, Hu
M, Li Y, Yang J and Hong L: Therapeutic effects of punicalagin
against ovarian carcinoma cells in association with β-catenin
signaling inhibition. Int J Gynecol Cancer. 26:1557–1563. 2016.
View Article : Google Scholar : PubMed/NCBI
|