Introduction
Osteoporosis is a systemic bone disease
characterized by low bone mass, damaged bone microstructure,
increased fragility and susceptibility to fractures (1). Osteoporosis may be divided into
primary osteoporosis and secondary osteoporosis. The former is
subdivided into postmenopausal, elderly and idiopathic
osteoporosis, and the latter includes osteoporosis caused by any
disease or drug that affects the physiological function of bones,
such as long-term and high-dose intake of glucocorticoids (2). Generally speaking, postmenopausal
osteoporosis occurs in women during menopause, elderly osteoporosis
affects people aged >70, and idiopathic osteoporosis mainly
occurs in teenagers, although its pathogenesis remains to be
elucidated (3). It has been
previously reported that there are tens of millions of female and
male osteoporosis patients in the United States, and billions of
Chinese people suffer from low bone mass (3). Minor trauma may lead to fractures,
teratogenesis, disability or death and other serious adverse
consequences in osteoporosis patients, and therefore, osteoporosis
has become one of primary factors affecting the quality of life of
the elderly (4).
Under the action of the Wnt signaling pathway,
mesenchymal stem cells differentiate into osteoblasts (5). The classic Wnt/β-catenin signal
pathway in osteoblasts also regulates the formation of osteoclasts
(6). Osteoblasts promote the
expression of two factors required in the formation of osteoclasts,
macrophage colony-stimulating factor and receptor activator for
nuclear factor-κB ligand (RANKL). Osteoblasts also secrete and
express osteoprotegerin (OPG), which is the decoy receptor of
RANKL, and binding to osteoprotegerin may inhibit the interaction
of RANK/RANKL, to inhibit the formation of osteoclasts (7). In osteoclast progenitor cells, when
RANKL activates its receptor, osteoclasts will be stimulated to
produce reactive oxygen species (7,8).
Therefore, RANKL and OGP are key molecules bridging bone formation
and bone resorption in bone remodeling.
Phosphoinositol 3-kinase (PI3K)/protein kinase B
(AKT) is one of the most important signaling pathways that regulate
cell proliferation, differentiation, survival, migration and
metabolism (9). A previous study
demonstrated that many signaling molecules involved in ossification
selectively activate genes associated with the PI3K/AKT signaling
pathway, and disturb the dynamic balance between bone formation and
bone resorption during bone remodeling via the regulation of
osteoblast and osteoclasts; therefore, this signaling pathway has a
very important role in the incidence and development of
osteoporosis (10).
Celastrus orbiculatus Thunb. belongs to the
Celastrus genus of the Celastraceae family, and its root,
stem, fruit and leaves may be used as medicine. It has been
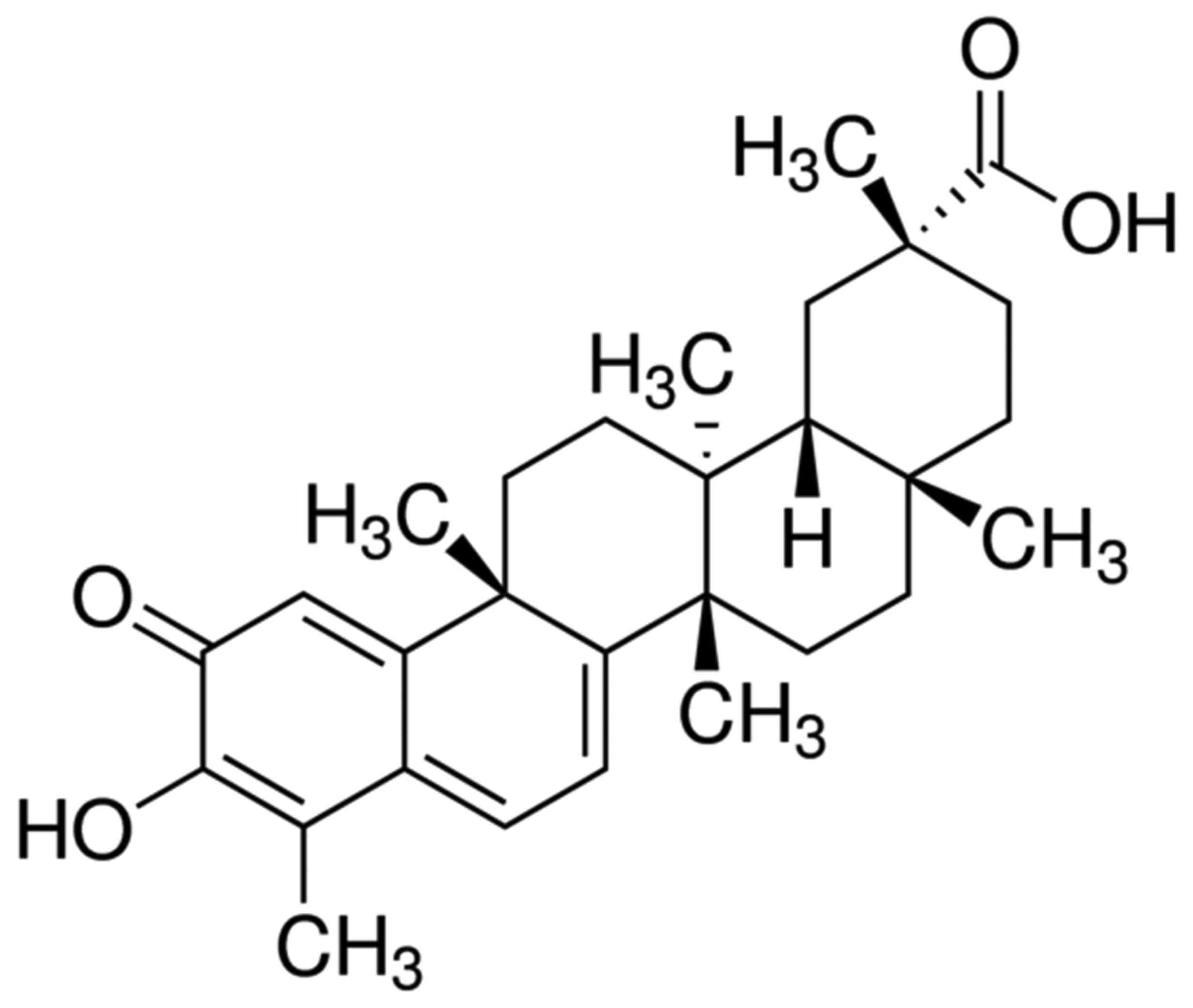
demonstrated that Celastrol (Fig.
1) has anti-oxidative and anti-inflammatory properties,
inhibits atherosclerosis by lipoprotein oxidative modification and
prevents against inflammation (11). Previous studies have revealed that
Celastrol has anti-inflammatory, anti-bacterial, anti-viral,
anti-fertility and insect-resistance functions, and has been used
for the treatment of rheumatism, rheumatoid arthritis, blood
diseases, skin diseases and as an agricultural insecticide
(11,12). In the present study, bioinformatics
analysis was used to investigate the effects of Celastrol on
glucocorticoid-induced osteoporosis (GIOP) and the potential
underlying molecular mechanisms.
Materials and methods
Animal treatment
Male C57BL/6J mice (8-weeks old, 20–22 g, n=30) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd (Beijing, China). All mice were provided with food and
water ad libitum, and were housed at a temperature of
22–23°C, a humidity of 55–60% and a 12/12 h light/dark cycle. The
mice were randomly divided into three groups: i) Vehicle group
(n=10); ii) GIOP model group (n=10); and iii) Celastrol treatment
group (n=10). Mice were injected intramuscularly with 5 mg/kg body
weight dexamethasone three times a week for 12 weeks. Mice in the
Celastrol treatment group were injected with a daily dose of 1
mg/kg Celastrol (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
12 weeks. The study was approved by the Ethics Committee of the
Department of Minimally Invasive Spine Surgery, The 309th Hospital
of the People's Liberation Army (Beijing, China). Following
treatment with Celastrol, body weight was determined. Urine calcium
(cat. no. C004-2), creatinine (cat. no. A032) and tartrate
resistant acid phosphatase-5b (TRACP-5b; cat. no. A058) were
quantified using ELISA kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Qiagen, Inc., Valencia, CA, USA). cDNA was synthesized using a
High-Capacity cDNA Reverse Transcription kit (cat. no. 4368813;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
RT-qPCR instrument (model ABI 7300) was used to analyze
aleurain-like protease (ALP), triiodothyronine receptor auxiliary
protein (TRAP), cathepsin K, osteocalcin, bone morphogenetic
protein 2 (BMP-2), type I collagen, runt-related transcription
factor 2 (Runx-2) mRNA expression levels using a SYBR
Green-containing PCR kit (Shanghai GenePharma, Co., Ltd., Shanghai,
China). The primer sequences used for qPCR were as follows: ALP
forward, 5′-CCAGGGCGTACGGAGGCCATT-3′ and reverse,
5′-GACCAAATTACGGCGTAGCCTC-3′; TRAP forward,
5′-AGCATAAGGGTCCAAGTCCAA-3′ and reverse,
5′-TACCAAAAGCGGCGTAGTTA-3′; cathepsin K forward,
5′-AGGCGGAGGTCGATGCCCCG-3′ and reverse,
5′-CACGATGATGTCACCCTCGATGT-3′; osteocalcin forward,
5′-ATGAGAGCCCTCACACTCCT-3′ and reverse, 5′-CTTGGACACAAAGGCTGCAC-3′;
BMP-2 forward, 5′-CAGCTTCCACCATGAAGAAT-3′, and reverse,
5′-CCAACCTGGTGTCCAAAAGT-3′; type I collagen forward,
5′-CCTGGATGCCATCAAAGTCT-3′, and reverse,
5′-ACTGCAACTGGAATCCATCG-3′; Runx-2 forward,
5′-CTCCCTGAACTCTGCACCAA-3′, and reverse,
5′-GTTCTGAAGCACCTGAAATGCG-3′; and GAPDH forward,
5′-ACAGGGGAGGTGATAGCATT-3′ and reverse,
5′-GACCAAAAGCCTTCATACATCTC-3′. The PCR conditions were as follows:
Initial denaturation for 10 min at 95°C; followed by 40 cycles of
denaturation for 30 sec at 95°C, annealing for 30 sec at 60°C and a
final extension for 30 sec at 72°C. The method for quantification
used was the 2−∆∆Cq method (13).
Western blot analysis
Total proteins were extracted from tissue samples
using radioimmunoprecipitation assay (Thermo Fisher Scientific,
Inc.) and the protein concentration was quantified in triplicate
using the Pierce™ bicinchoninic acid protein assay kit
(Thermo Fisher Scientific, Inc.). A total of 25 µg was subjected to
8–12% SDS-PAGE and directly transferred to nitrocellulose membranes
(Sigma-Aldrich; Merck KGaA). Membranes were blocked with 5% non-fat
milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h at
37°C and then hybridized with the following primary antibodies:
Anti-Wnt (cat. no. ab32249; 1:500; Abcam, Cambridge, UK),
anti-β-catenin (cat. no. ab16051; 1:500; Abcam), PI3K (cat. no.
sc-7174; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
phosphorylated (p)-AKT (cat. no. sc-7985-R; 1:500; Santa Cruz
Biotechnology, Inc.), p-glycogen synthase kinase-3 (GSK-3; cat. no.
sc-81497; 1:500; Santa Cruz Biotechnology, Inc.), prostaglandin E2
(PGE-2; cat. no. ab96189; 1:500; Abcam), caspase-3 (cat. no.
sc-98785; 1:500; Santa Cruz Biotechnology, Inc.) and GAPDH (cat.
no. sc-25778; 1:5,000; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The membranes were incubated with anti-rabbit
horseradish peroxidase-conjugated secondary antibodies (cat. no.
7074; 1:5,000; Cell Signaling Technology, Inc., Danvers, MA, USA)
at the room temperature in the dark for 2 h. The blots were
developed using enhanced chemiluminescence plus kits (GE
Healthcare, Chicago, IL, USA), and densitometric analysis was
performed using Image_Lab_3.0 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All experimental data are presented as the mean ±
standard error of the mean. Comparison between groups was performed
using a one-way analysis of variance followed by Tukey's Honest
Significant Difference post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
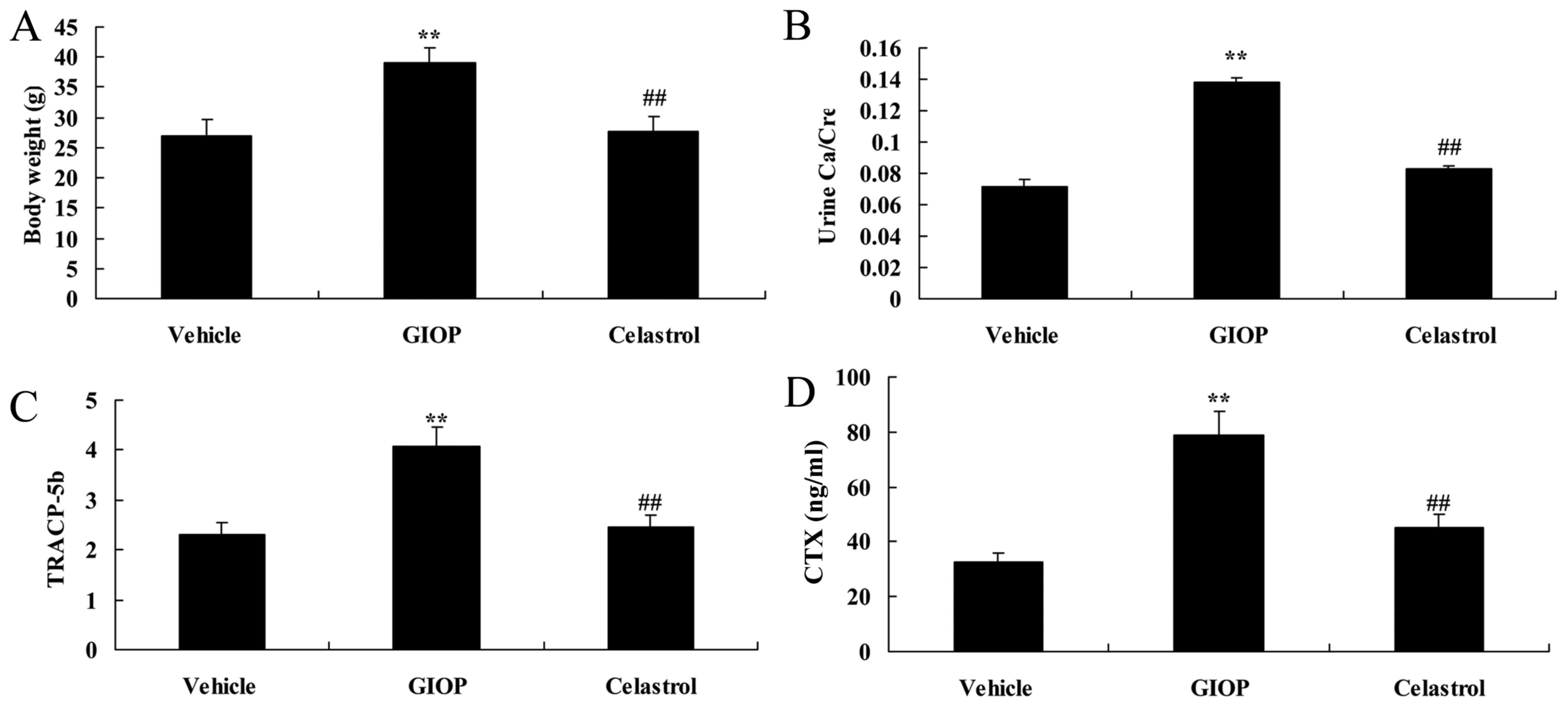
Celastrol reduces body weight, urine
Ca/Cre, TRACP-5b, C-terminal telopeptide of type I collagen
(CTX)
Body weight, urine Ca/Cre, TRACP-5b and CTX were
increased in GIOP mice when compared with the vehicle group
(Fig. 2). Celastrol treatment
inhibited these factors when compared with the GIOP group (Fig. 2).
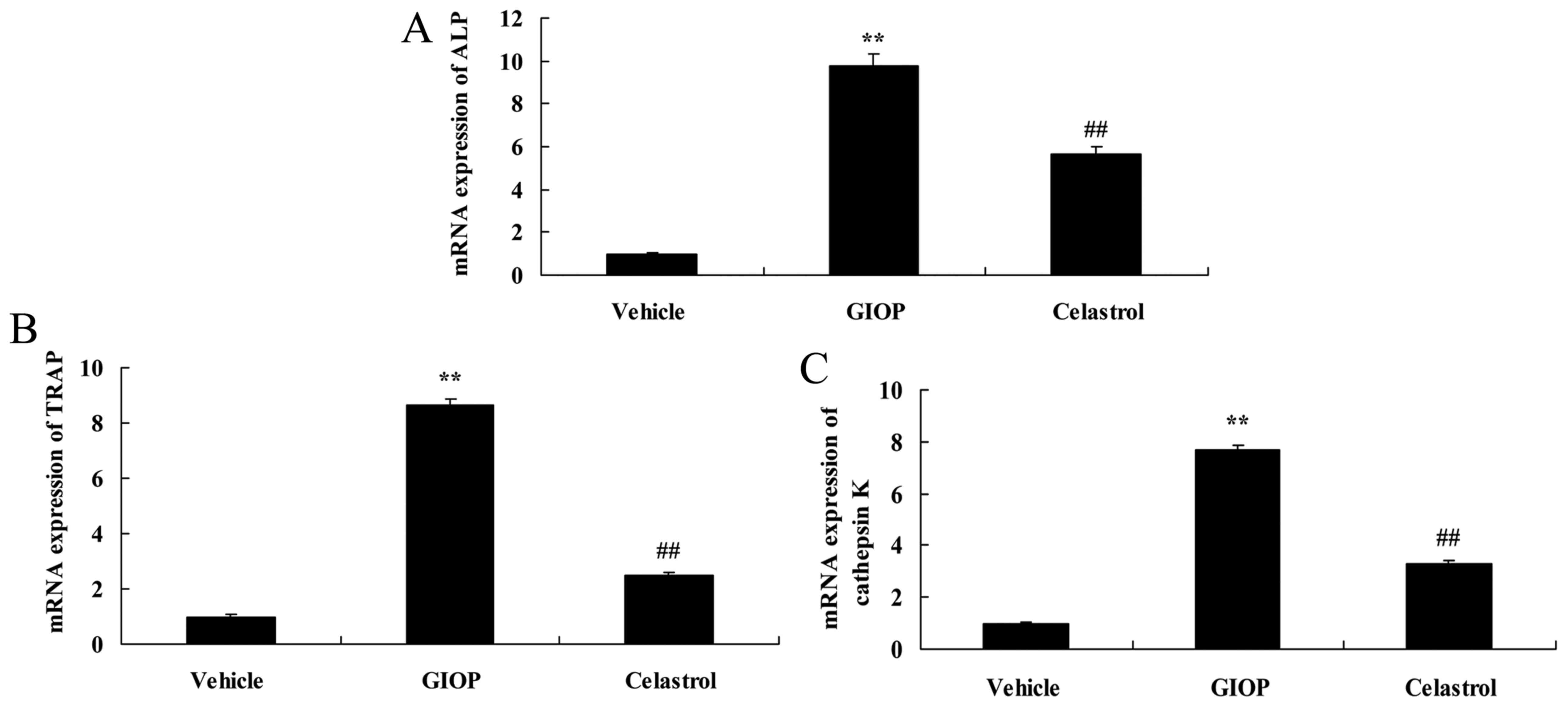
Celastrol reduces ALP, TRAP and
cathepsin K mRNA expression levels
The present study quantified ALP, TRAP and cathepsin
K mRNA expression levels in GIOP mice following Celastrol
treatment. Fig. 3 demonstrated
that ALP, TRAP and cathepsin K mRNA expression levels in GIOP mice
were higher compared with the vehicle group. Treatment with
Celastrol significantly reduced ALP, TRAP and cathepsin K mRNA
expression levels compared with the GIOP group (Fig. 3).
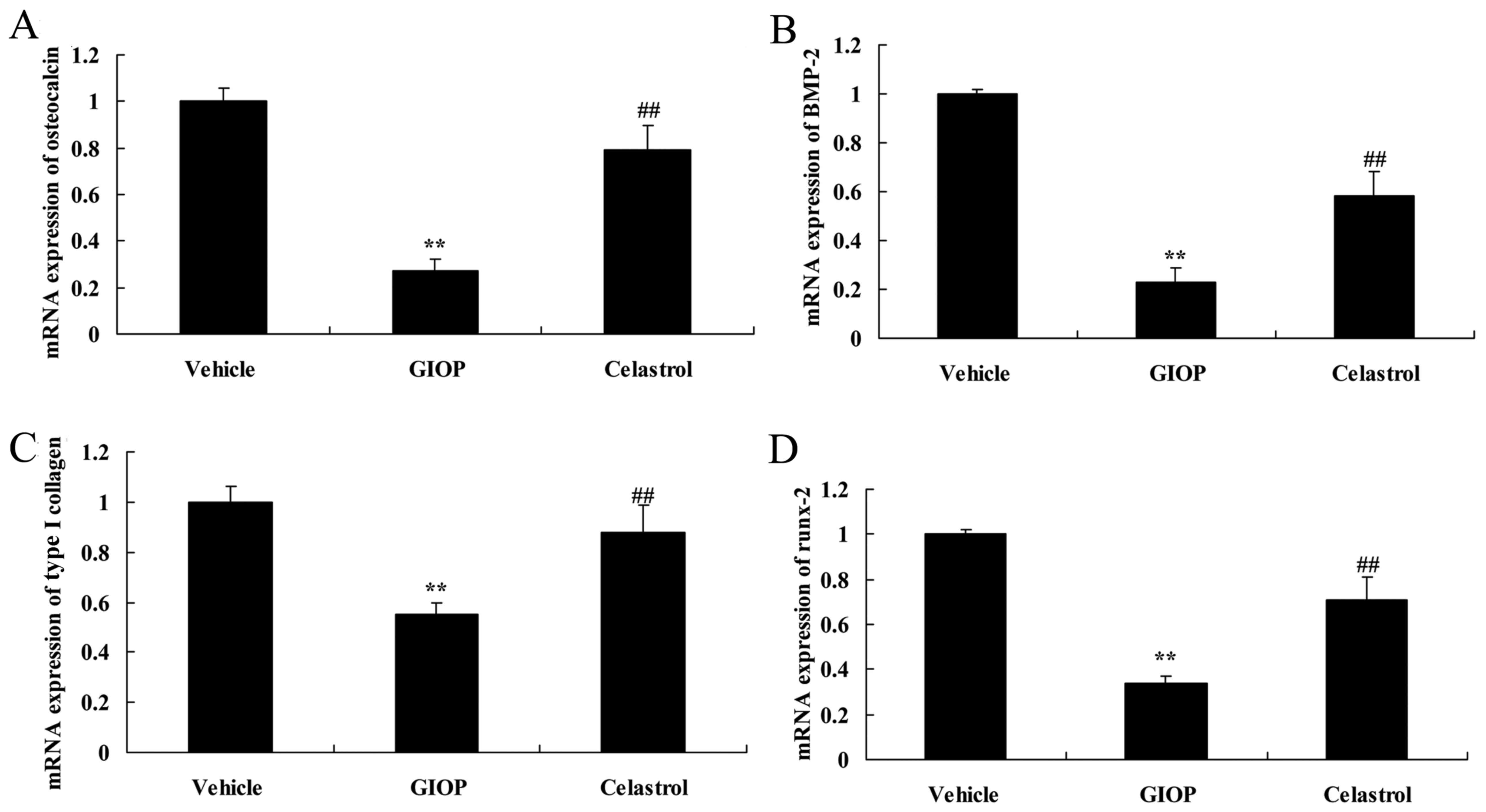
Celastrol inhibits osteocalcin, BMP-2,
type I collagen, Runx-2 mRNA expression levels
Osteocalcin, BMP-2, type I collagen, Runx-2 mRNA
expression levels were determined in GIOP mice after Celastrol
treatment. There was significant inhibition of osteocalcin, BMP-2,
type I collagen, runx-2 mRNA expression in GIOP mice, compared with
the vehicle group, which was significantly reversed in the group
which received Celastrol treatment compared with the GIOP group
(Fig. 4).
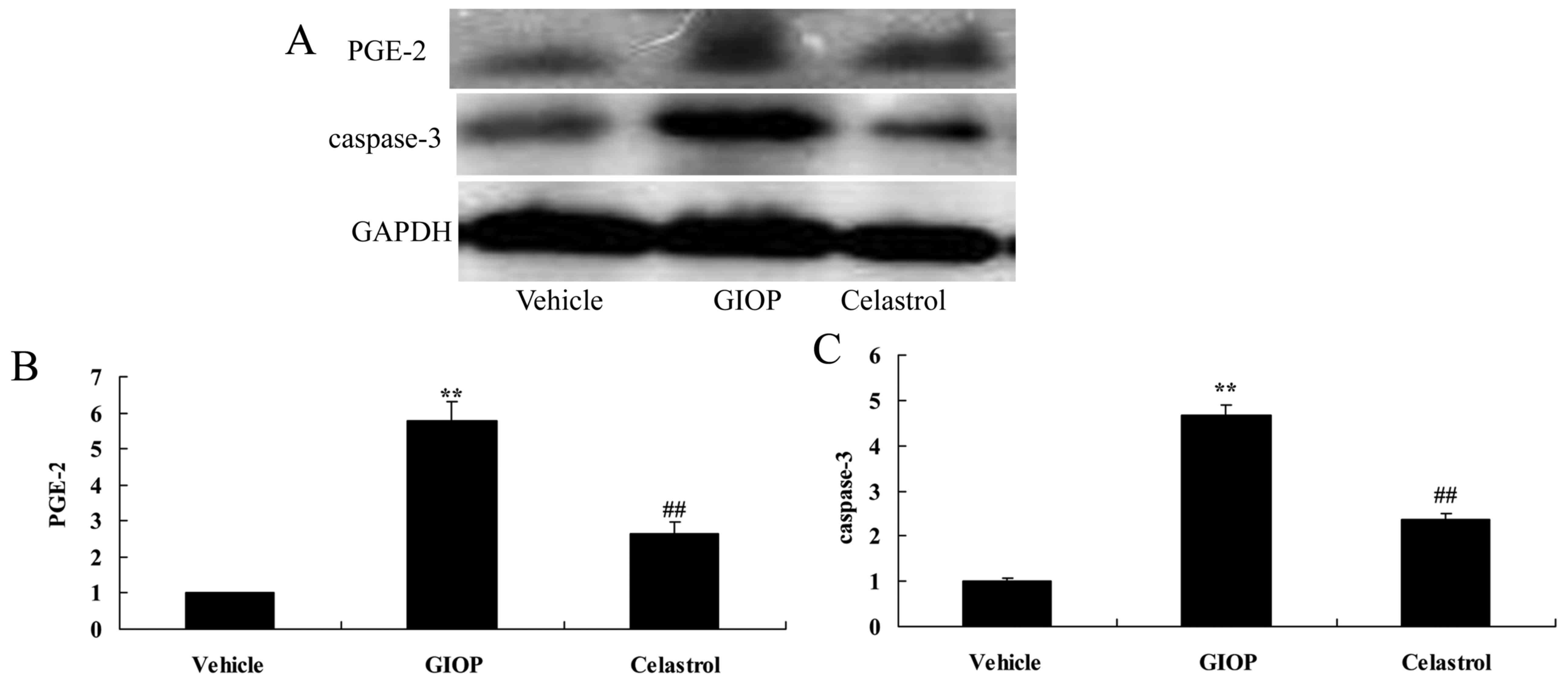
Celastrol reduces PGE-2 and caspase-3
protein expression levels
The mechanism of Celastrol on PGE-2 and caspase-3
protein expression was investigated using western blot analysis.
PGE-2 and caspase-3 protein expression levels in GIOP mice were
higher compared with the vehicle group (Fig. 5). Treatment with Celastrol
significantly reduced PGE-2 and caspase-3 protein expression levels
when compared with the GIOP group (Fig. 5).
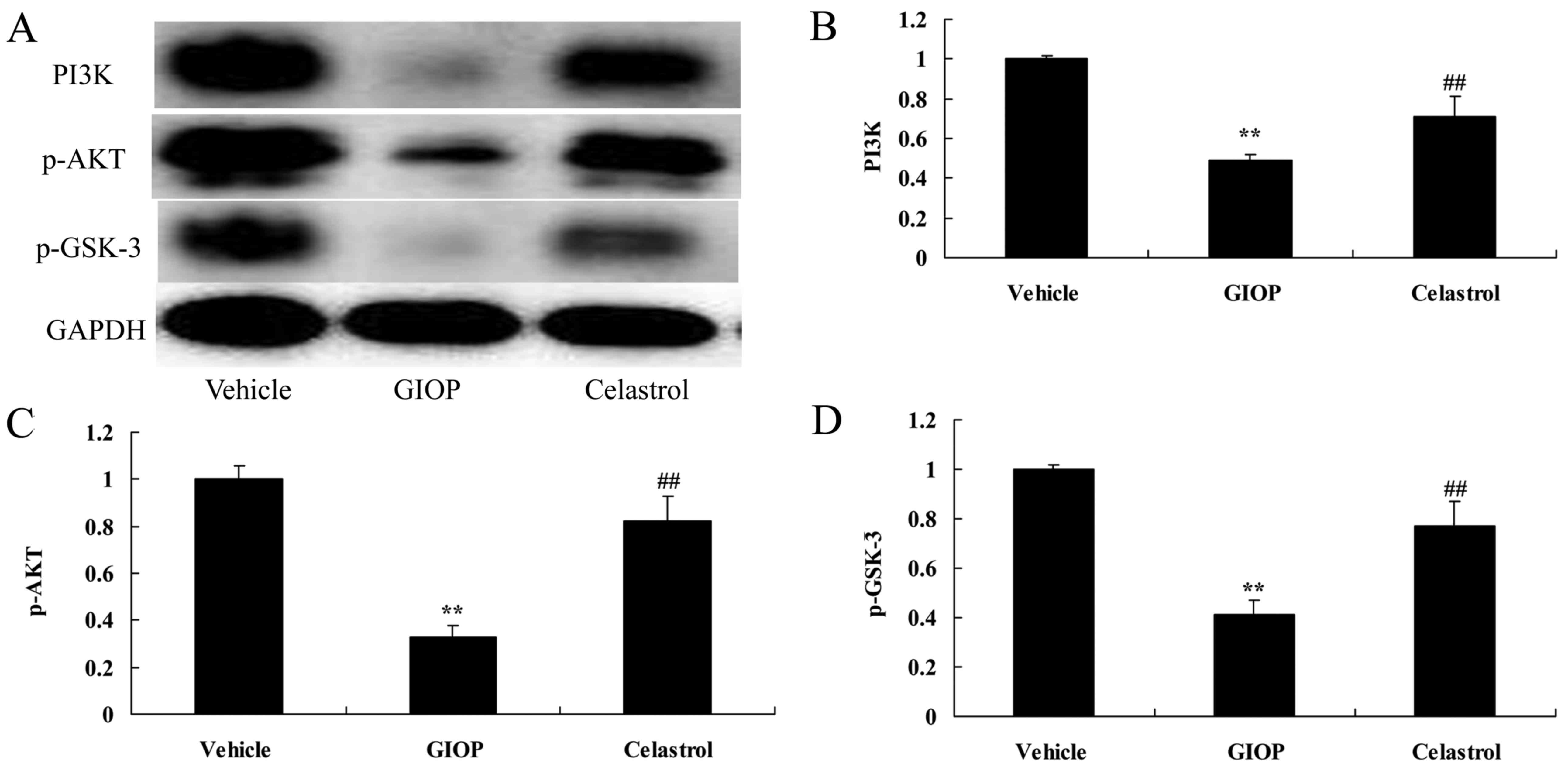
Celastrol increases PI3K, p-AKT and
p-GSK-3 protein expression levels
To test the anti-apoptotic mechanism of Celastrol on
osteoporosis, PI3K, p-AKT and p-GSK-3 protein expression levels
were measured using western blot analysis. The results of western
blot analysis showed that PI3K, p-AKT and p-GSK-3 protein
expressions were significantly suppressed in GIOP mice compared
with the vehicle group (Fig. 6).
Celastrol treatment significantly increased PI3K, p-AKT and p-GSK-3
protein expression levels when compared with the GIOP group
(Fig. 6).
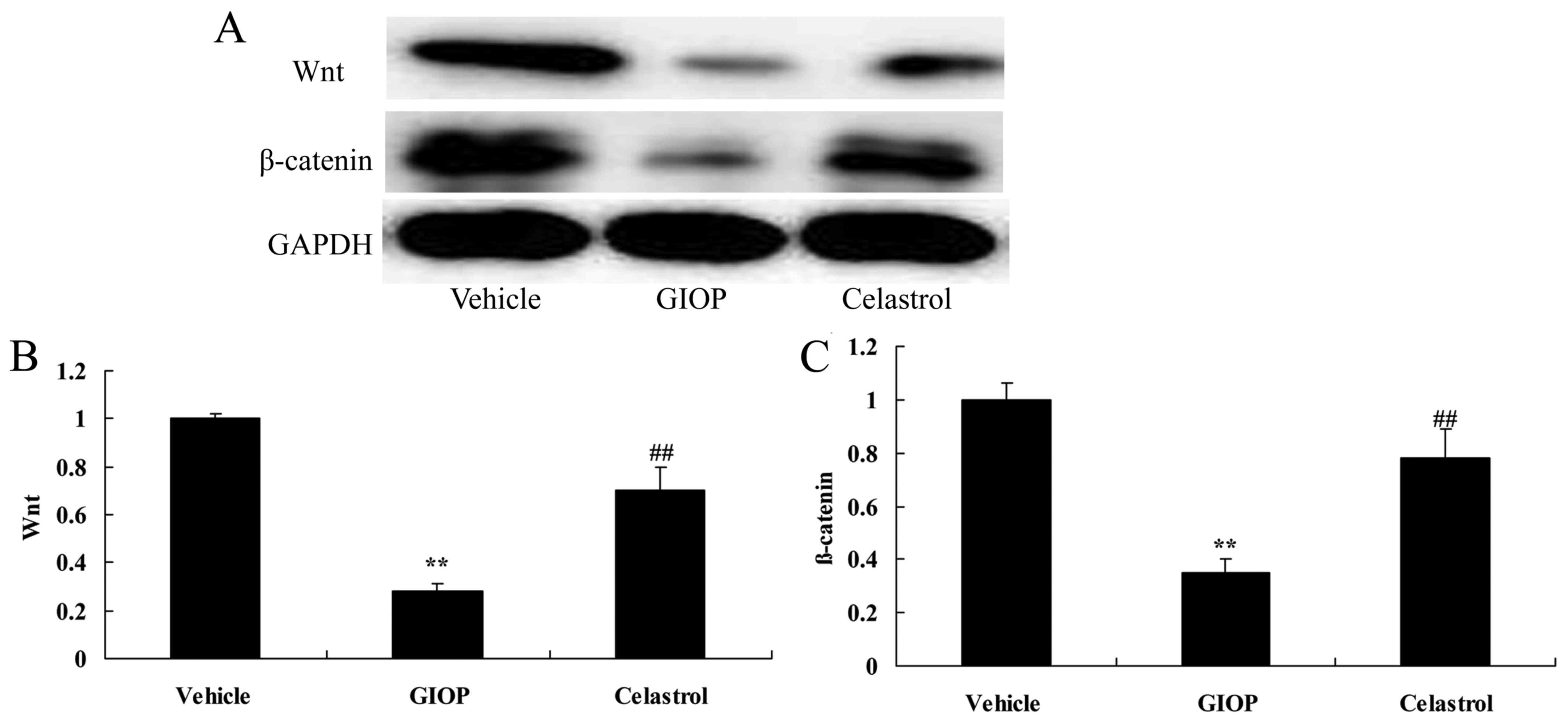
Celastrol increases Wnt and β-catenin
protein expression levels
The underlying molecular mechanism by which
Celastrol regulates osteoporosis was subsequently investigated. It
was determined that Wnt and β-catenin protein expression levels
were significantly inhibited in GIOP mice compared with the vehicle
group (Fig. 7). Treatment with
Celastrol significantly increased Wnt and β-catenin protein
expression levels in GIOP mice (Fig.
7).
Discussion
Osteoporosis is a systemic bone disease
characterized by low bone mass, damaged bone microstructure,
increased fragility and susceptibility to fracture (14). Minor trauma in osteoporosis
patients may lead to development of fractures, disability or death;
therefore, osteoporosis has become one of the primary factors
affecting the quality of life of the elderly (15). Estrogen deficiency-induced bone
loss is believed to be the primary cause of elderly osteoporosis
(15). To the best of our
knowledge the present study may provide the first evidence in
examining whether Celastrol inhibits body weight, osteoporosis and
PGE-2 and caspase-3 protein expression levels in GIOP mice.
Under normal circumstances, the PI3K/AKT signaling
pathway selectively affects the physiological function of
osteoblasts and osteoclasts and is activated by oxidative stress.
The PI3K/AKT pathway acts on specific target genes, such as
forkhead transcription factor (FOXO) and GSK-3β, to reduce the
oxidative damage of osteoblasts and osteoclasts (10). Previous studies have revealed that
insulin, insulin growth factor and other growth factors activate
the PI3K/AKT signaling pathway, selectively regulating Wnt, FOXO,
BMP and RANKL, and other signaling pathways, affecting the
formation and differentiation of osteoblasts and osteoclasts and
their functions, to regulate bone mass and bone strength (9,16).
Therefore, as the center regulating the function of osteoblasts and
osteoclasts, the PI3K/AKT signaling pathway has an important role
in maintaining the dynamic equilibrium of bone tissues under normal
physiological stimulation and pathological conditions and PI3K/AKT
may be a target for the treatment of osteoporosis (16). Shrivastava et al (17) demonstrated that Celastrol induced
apoptosis in breast cancer via the PI3K/AKT pathway. The present
study revealed that Celastrol significantly induced PI3K, p-AKT and
p-GSK-3 protein expression levels in GIOP mice. This data suggested
that Celastrol may have a significant effect on the suppression of
bone cell apoptosis in GIOP mice via the PI3K/AKT pathway.
The Wnt signaling pathway regulates the growth,
development, illness, aging and mortality (18). In the Wnt signaling pathway,
activation of Wnt leads to the phosphorylation of the signaling
molecule β-catenin and its accumulation in the nucleus, which
interacts with T cytokine/lymphoid enhancement factor to mediate
Wnt-induced gene transcription, and guide the differentiation of
bone marrow mesenchymal stem cells into osteoblasts (6,19).
In bones, the Wnt/β-catenin pathway is vital for osteogenic
differentiation and β-catenin may bind to nuclear transcription
factors after entering the nucleus, to regulate a variety of
proteins associated with osteogenic differentiation (5). BMP-2, a subtype of the transforming
growth factor-β superfamily, has an important role in bone
formation and bone metabolic balance in adults (20). C2C12 cells may differentiate from
muscle cells into osteoblasts under the continuous stimulation of
BMP-2 (21). A previous study has
revealed that β-catenin is vital to osteogenic differentiation and
is downstream of the Wnt/β-catenin pathway and regulates osteogenic
differentiation via the Wnt autocrine loop (22). The present study demonstrated that
treatment with Celastrol significantly promoted Wnt and β-catenin
protein expression levels in GIOP mice. Lin et al (12) previously reported that Celastrol
ameliorates ulcerative colitis-associated colorectal cancer through
β-catenin expression. The findings of the present study are
consistent with previous finding (12) regarding the role of Celastrol as an
effective activator of the Wnt/β-catenin pathway in GIOP mice and
has a protective effect.
In conclusion, Celastrol treatment reduced body
weight, prevented osteoporosis and inhibited PGE-2 and caspase-3
protein expression levels in GIOP mice via the PI3K/AKT and Wnt
signaling pathways. The present study in conjunction with
previously published findings, suggested that Celastrol may be a
potential therapeutic drug against osteoporosis in the clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL designed the study; JX, QL, YW, JL, LG and GW
performed the experiments; JX and XL analyzed the data; XL wrote
the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Department of Minimally Invasive Spine Surgery, The 309th
Hospital of the People's Liberation Army (Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reginster JY, Kaufman JM, Goemaere S,
Devogelaer JP, Benhamou CL, Felsenberg D, Diaz-Curiel M, Brandi ML,
Badurski J, Wark J, et al: Maintenance of antifracture efficacy
over 10 years with strontium ranelate in postmenopausal
osteoporosis. Osteoporos Int. 23:1115–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen GZ, Xu YX, Zhang JW, Liu SH and Guo
ZY: Effect of acupoint catgut-embedding on the quality of life,
reproductive endocrine and bone metabolism of postmenopausal women.
Chin J Integr Med. 16:498–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McColm J, Hu L, Womack T, Tang CC and
Chiang AY: Single- and multiple-dose randomized studies of
blosozumab, a monoclonal antibody against sclerostin, in healthy
postmenopausal women. J Bone Miner Res. 29:935–943. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tee SI, Yosipovitch G, Chan YC, Chua SH,
Koh ET, Chan YH, Tan SS, Tsou IY and Tan SH: Prevention of
glucocorticoid-induced osteoporosis in immunobullous diseases with
alendronate: A randomized, double-blind, placebo-controlled study.
Arch Dermatol. 148:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang F, Wang Y, Zhao Y, Zhan Q, Yu P, Wang
J and Xue C: Sialoglycoprotein isolated from eggs of carassius
auratus ameliorates osteoporosis: An effect associated with
regulation of the Wnt/β-catenin pathway in rodents. J Agric Food
Chem. 64:2875–2882. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karner CM and Long F: Wnt signaling and
cellular metabolism in osteoblasts. Cell Mol Life Sci.
74:1649–1657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanzaki H, Shinohara F, Itohiya K,
Yamaguchi Y, Katsumata Y, Matsuzawa M, Fukaya S, Miyamoto Y, Wada S
and Nakamura Y: RANKL induces Bach1 nuclear import and attenuates
Nrf2-mediated antioxidant enzymes, thereby augmenting intracellular
reactive oxygen species signaling and osteoclastogenesis in mice.
FASEB J. 31:781–792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu DR, Lee JN, Oh GS, Kim HJ, Kim MS and
Lee SH: The inhibitory effect of beta-lapachone on RANKL-induced
osteoclastogenesis. Biochem Biophys Res Commun. 482:1073–1079.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xi JC, Zang HY, Guo LX, Xue HB, Liu XD,
Bai YB and Ma YZ: The PI3K/AKT cell signaling pathway is involved
in regulation of osteoporosis. J Recept Signal Transduct Res.
35:640–645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li XJ, Zhu Z, Han SL and Zhang ZL:
Bergapten exerts inhibitory effects on diabetes-related
osteoporosis via the regulation of the PI3K/AKT, JNK/MAPK and NF-κB
signaling pathways in osteoprotegerin knockout mice. Int J Mol Med.
38:1661–1672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan Y, Cui ZJ, Sun B, Han LP, Li CJ and
Chen LM: Celastrol attenuates oxidative stress in the skeletal
muscle of diabetic rats by regulating the AMPK-PGC1alpha-SIRT3
signaling pathway. Int J Mol Med. 37:1229–1238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin L, Sun Y, Wang D, Zheng S, Zhang J and
Zheng C: Celastrol ameliorates ulcerative colitis-related
colorectal cancer in mice via suppressing inflammatory responses
and epithelial-mesenchymal transition. Front Pharmacol. 6:3202016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koh JM, Chung DJ, Chung YS, Kang MI, Kim
IJ, Min YK, Oh HJ, Park IH, Lee YS, Kravitz B, et al: Assessment of
Denosumab in Korean postmenopausal women with osteoporosis:
Randomized, double-blind, placebo-controlled trial with open-label
extension. Yonsei Med J. 57:905–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tüzün Ş, Akyüz G, Eskiyurt N, Memiş A,
Kuran B, İçağasıoğlu A, Sarpel T, Özdemir F, Özgirgin N, Günaydın
R, et al: Impact of the training on the compliance and persistence
of weekly bisphosphonate treatment in postmenopausal osteoporosis:
A randomized controlled study. Int J Med Sci. 10:1880–1887. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You L, Gu W, Chen L, Pan L, Chen J and
Peng Y: MiR-378 overexpression attenuates high glucose-suppressed
osteogenic differentiation through targeting CASP3 and activating
PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 7:7249–7261.
2014.PubMed/NCBI
|
|
17
|
Shrivastava S, Jeengar MK, Reddy VS, Reddy
GB and Naidu VG: Anticancer effect of celastrol on human triple
negative breast cancer: Possible involvement of oxidative stress,
mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp Mol
Pathol. 98:313–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adami G, Orsolini G, Adami S, Viapiana O,
Idolazzi L, Gatti D and Rossini M: Effects of TNF inhibitors on
parathyroid hormone and Wnt signaling antagonists in rheumatoid
arthritis. Calcif Tissue Int. 99:360–364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liou SF, Hsu JH, Chu HC, Lin HH, Chen IJ
and Yeh JL: KMUP-1 Promotes osteoblast differentiation through cAMP
and cGMP pathways and signaling of BMP-2/Smad1/5/8 and
Wnt/β-catenin. J Cell Physiol. 230:2038–2048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen J, James AW, Zhang X, Pang S, Zara
JN, Asatrian G, Chiang M, Lee M, Khadarian K, Nguyen A, et al:
Novel Wnt regulator NEL-like molecule-1 antagonizes adipogenesis
and augments osteogenesis induced by bone morphogenetic protein 2.
Am J Pathol. 186:419–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rawadi G, Vayssiere B, Dunn F, Baron R and
Roman-Roman S: BMP-2 controls alkaline phosphatase expression and
osteoblast mineralization by a Wnt autocrine loop. J Bone Miner
Res. 18:1842–1853. 2003. View Article : Google Scholar : PubMed/NCBI
|