Introduction
Glioma has become the most frequent primary brain
cancer among adults worldwide and contributes to a large amount of
cancer-related deaths every year (1). Surgery combined with chemotherapy and
radiotherapy is the major approach for glioma patients. However,
resistance to chemoradiotherapy greatly attenuated the outcome. The
five-year overall survival in glioma patients was very
unsatisfactory (2). Most glioma
patients died within 2 years after diagnosed (3). Therefore, it is urgently required to
understand the molecular mechanism underlying chemoresistance of
gliomas cells.
microRNAs (miRNAs/miRs) belong to a group of small
noncoding RNAs with a length of about 18–24 nucleotides (4). Evidences show that miRNAs could
regulate gene expression via binding to target complementary site
in the 3′-UTR region of specific mRNAs (5). More and more studies indicate that
miRNAs possess extremely important functions in various biological
processes, such as proliferation, death and migration (6,7).
Differential expression of miRNAs in tumor and matched normal
tissues has been observed in many cancers, such as glioma (8). In the past years, increasing reports
show that miRNAs take part in the chemoresistance of glioma. For
instance, miR-21 contributes to the resistance of glioma cells to
camustine through inhibiting Spry2 mRNA levels (9). Both miR-324-5p and mIR-524-5p target
EZH2 to facilitate the growth and temozolomide resistance of glioma
cells (10). miR-136 was reported
to inhibit cisplatin chemosensitivity of glioma cells by
suppressing E2F1 expression (11).
Additionally, Let-7b regulates glioblastoma cell sensitivity to
chemotherapy by targeting Cyclin D1 (12). Thus, it is critical to understand
the correlation between miRNA and glioma chemoresistance.
miR-501-3p has been shown to regulate tumor
development in hepatocellular carcinoma (13) and cervical cancer (14). Howbeit the function of miR-501-3p
in glioma is elusive. In the present study, we found that
miR-501-3p expression was significantly downregulated in glioma
tissues compared to matched normal tissues. Functionally, we found
that miR-501-3p targeted MYCN, which contributes to the
cisplatin-resistance of glioma cells. Overexpression of MYCN
reversed the effects of miR-501-3p mimics on glioma cell resistance
to cisplatin. In sum, our study demonstrated that miR-501-3p might
be a potential target to solve cisplatin resistance in glioma.
Materials and methods
Patient tissues
A total of 31 pairs of glioma tissues and adjacent
normal tissues were obtained between 2014 and 2016 from The Third
People's Hospital of Linyi (Linyi, China). All samples were frozen
in liquid nitrogen at −80°C until use. This study was approved by
the ethics committee of The third People's Hospital of Linyi.
Written informed consent was obtained from each enrolled
patient.
Cell culture and transfection
The human glioma cell line U251 was purchased from
American Type Culture Collection (ATCC). The cisplatin-resistant
U251 cell line (U251/DDP) was obtained through adding increasing
concentrations (from 0.1 to 10 µg/ml) of cisplatin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) to the culture medium until the
cell proliferation ability was similar to wild-type U251 cells. All
cells were cultured in DMEM medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% FBS (GGibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml streptomycin
and 100 U/ml penicillin according to the manufacturer's instruction
and maintained at 37°C in a 5% CO2 humidified
incubator.
miR-501-3p mimics and negative controls (NC) were
purchased from GenePharma (Shanghai, China). MYCN coding sequence
was cloned into pCDNA3 vector. miR-501-3p mimics, pCDNA2-MYCN and
corresponding controls were transduced into glioma cells using
Lipofectamine 2000 Reagent (Invitrogen) according to the
manufacturer's protocol.
Cell proliferation
For cell viability analysis, 3×103 U251
or U251/DDP cells transfected with miR-501-3p or control per well
were seeded into 96-well plate and cultured for indicative times.
Then MTT solution (0.5 µg/ml) (Sigma-Aldrich; Merck KGaA) was added
for cellular viability analysis and incubated for 4 h at 37°C,
followed by addition with 150 µl DMSO (Sigma-Aldrich; Merck KGaA).
Immediately, the plate was vortexed for 30 min and absorbance at
570 nm was measured by spectrophotometer.
In vitro invasion assays
For transwell invasion assay, 1×104 U251
or U251/DDP cells in 200 µl DMEM medium were seed into the upper
Matrigel-coated chamber with 8 µm pores in diameter (Corning
Incorporated, Corning, NY, USA) of the 24-well plate. The lower
chamber contains 600 µl DMEM medium supplemented with 10% FBS.
After culture for 24 h, the cells in upper chamber were scrapped.
Then the invasive cells in the lower chamber was fixed with
methanol for 1 h and stained with 0.1% crystal violet for 15 min.
Invasive cells were photographed using a light microscope (Olympus
Corporation, Tokyo, Japan) at 100× magnifications.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues or cultured
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Then 1 µg RNA templates were added for cDNA generation using
a Reverse Transcription System kit (Takara Biotechnology Co., Ltd.,
Dalian, China). miR-501-3p and MYCN levels were analyzed ultilizing
SYBR® Premix Ex TaqTM (Takara Biotechnology Co., Ltd.)
and the TaqMan miRNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) on ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.), respectively. We used
GAPDH as a normalized control for MYCN and U6 as a control for
miR-501-3p. The results were calculated according to the
2−ΔΔCq method (15).
Caspase-3/7 activity analysis
We used the Caspase-Glo3 assay kit (Promega
Corporation, Madison, WI, USA) to analyze Caspase-3/7 activity in
U251 and U251/DDP cells according to the manufacturer's
instructions.
Luciferase reporter assay
To obtain the MYCN-wild-type (MYCN-WT) reporter, we
cloned the 3′-UTR sequence of MYCN containing the binding site for
miR-501-3p into pmiR-Reporter vector (Promega Corporation). As for
the MYCN-mutant (MYCN-Mut) reporter, the putative binding site for
miR-501-3p was mutated using Quik-Change™ Site-Directed Mutagenesis
kit (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA).
For dual luciferase reporter assay, 50 ng WT or mutant reporter
plasmid and miR-501-3p mimics or negative control were transduced
into U251/DDP cells in the 24-well plate using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instruction. 24 h later after transfection, cells
were harvested and assayed with the Dual-Luciferase Reporter Assay
kit (Promega Corporation) according to the manufacturer's
instruction.
Statistical analysis
All statistical analyses were performed ultilizing
SPSS 20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism (version
6.0; GraphPad Software, Inc., La Jolla, CA, USA). Student's t-test
and one-way ANOVA followed by Tukey's post hoc test were used to
analyze 2 or multiple groups, respectively, for statistical
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-501-3p expression patterns in
glioma
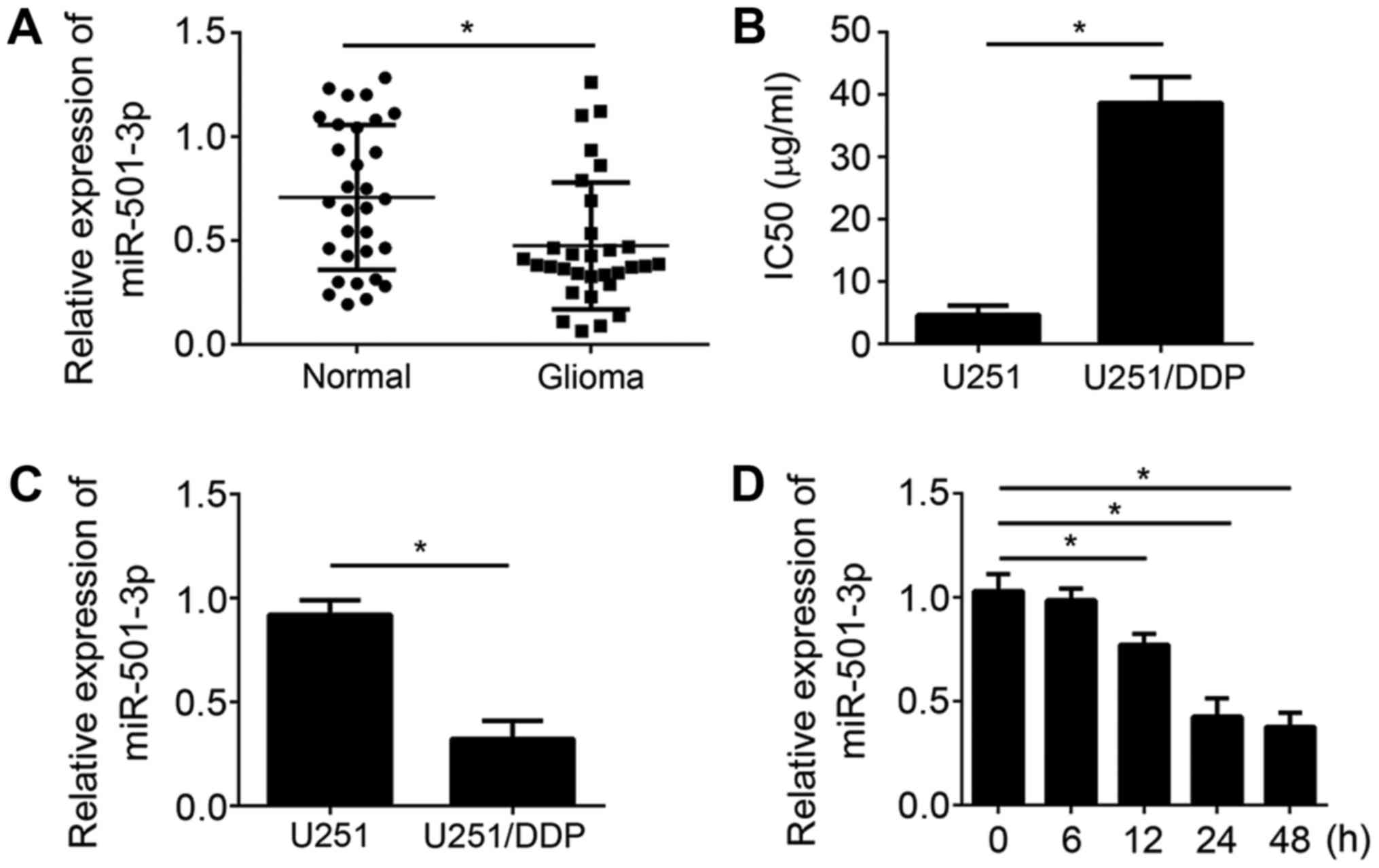
To analyze the function of miR-501-3p in glioma for
drug resistance, we first checked the levels of miR-501-3p in pairs
of glioma tissues and matched normal tissues by RT-qPCR. The
results indicated that miR-501-3p expression was significantly
downregulated in glioma tissues compared to adjacent normal tissues
(Fig. 1A). Then, we generated a
cisplatin-resistant glioma cell line named U251/DDP. We measured
the IC50 for WT U251 and U251/DDP cells. The results indicated that
the IC50 in U251/DDP cells was significantly higher than that in WT
cells (Fig. 1B). We measured the
expression of miR-501-3p in U251 and U251/DDP cells by RT-qPCR and
found that miR-501-3p expression was lower in U251/DDP cells than
that in U251 cells (Fig. 1C),
suggesting miR-501-3p might regulate the resistance to cisplatin.
Thus, we further assessed the effects of cisplatin on miR-501-3p
expression in U251 cells. miR-501-3p expression was significantly
decreased in a time-dependent manner after treatment with cisplatin
(5 µg/ml) (Fig. 1D). Above data
indicated that miR-501-3p might regulate glioma cell resistance to
cisplatin.
miR-501-3p inhibits proliferation and
invasion of U251/DDP cells while promoting apoptosis
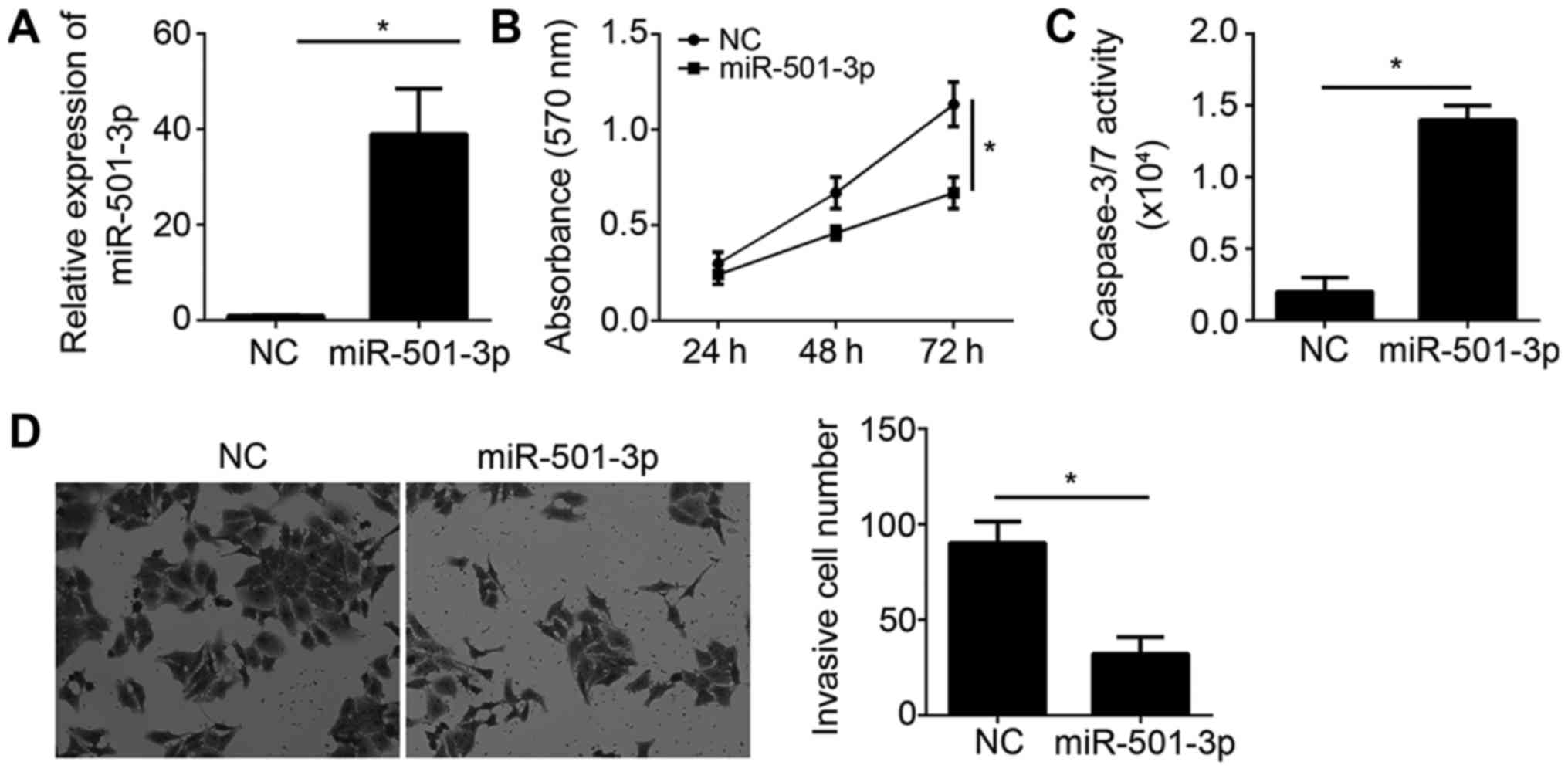
To determine the function of miR-501-3p on
cisplatin-resistant glioma cells, we transfected U251/DDP cells
with 50 nM miR-501-3p mimics or negative control (NC). 48 h later,
we measured miR-501-3p expression and found that miR-501-3p was
significantly upregulated in U251/DDP cells transfected with
miR-501-3p mimics (Fig. 2A). Then,
we examined cell proliferation by MTT assay. The results
illustrated that miR-501-3p overexpression significantly inhibited
cell proliferation (Fig. 2B).
Besides, miR-501-3p ectopic expression promoted the apoptosis of
U251/DDP cells as shown by increased caspase-3/7 activity (Fig. 2C). Moreover, we examined the effect
of miR-501-3p on cell invasion by transwell assay. The results
showed that overexpression of miR-501-3p reduced the invasive cell
number in U251/DDP cells (Fig.
2D). Collectively, our data indicated that miR-501-3p served as
a tumor suppressor and might be related with cisplatin resistance
of glioma cells.
miR-501-3p sensitizes glioma cells to
cisplatin
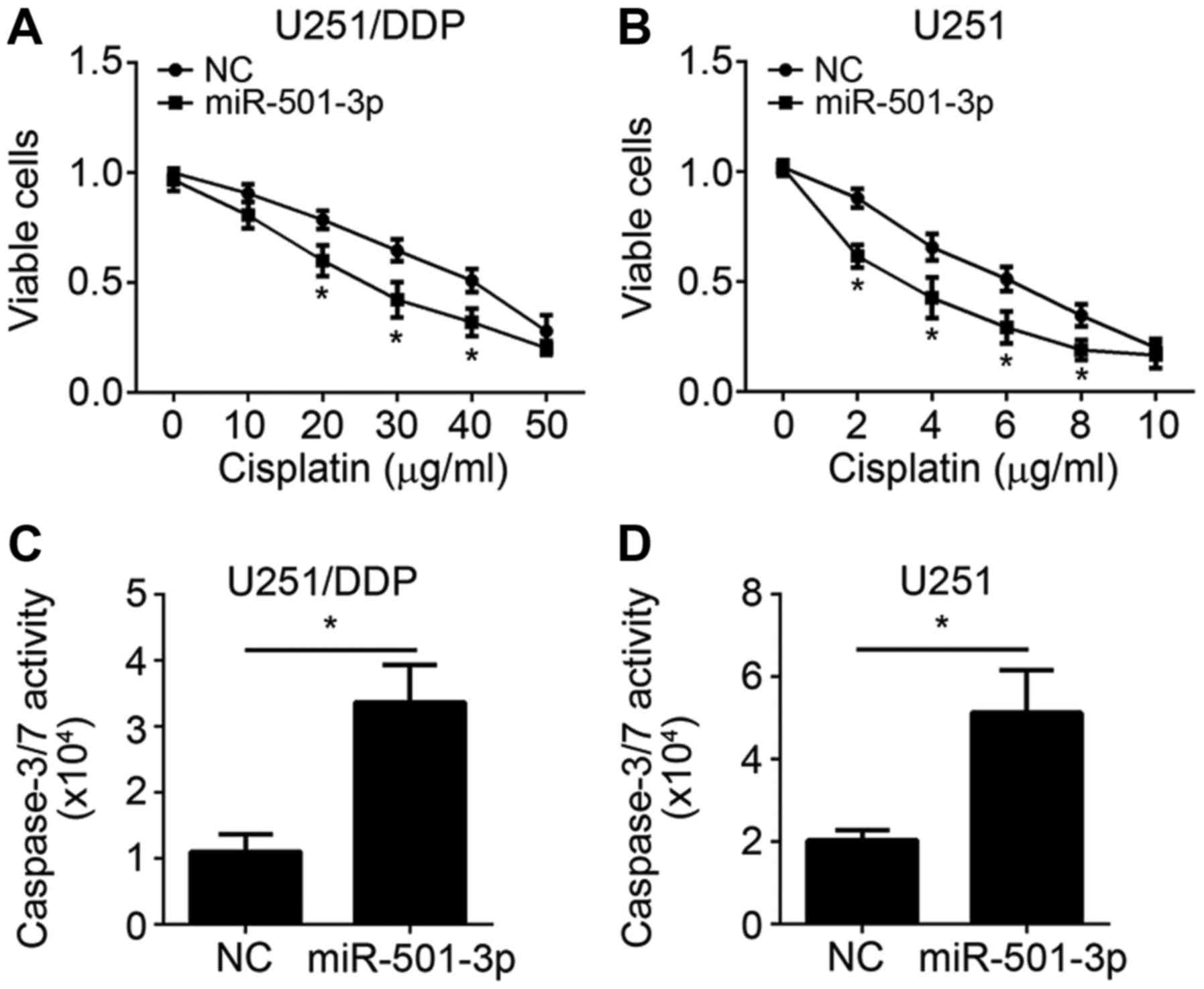
To explore the role of miR-501-3p on cisplatin
resistance, miR-501-3p mimics was transduced into U251 and U251/DDP
cells. Firstly, we conducted MTT assay to assess the effect of
miR-501-3p on glioma cell proliferation. After transfection with
miR-501-3p for 24 h, cisplatin was added and incubated for another
24–72 h. Then cell viability was measured. The results of MTT assay
revealed that miR-501-3p significantly enhanced the suppressive
effect of cisplatin on both U251 and U251/DDP cell proliferation
(Fig. 3A and B). Moreover, the
caspase-3/7 activity assay showed that an upregulated activity of
caspase-3/7 in miR-501-3p mimic-transfected U251 and U251/DDP cells
was observed when exposed to cisplatin (Fig. 3C and D). Taken together, these
results suggested that miR-501-3p promoted the sensitivity of
glioma cells to cisplatin.
MYCN is a target of miR-501-3p
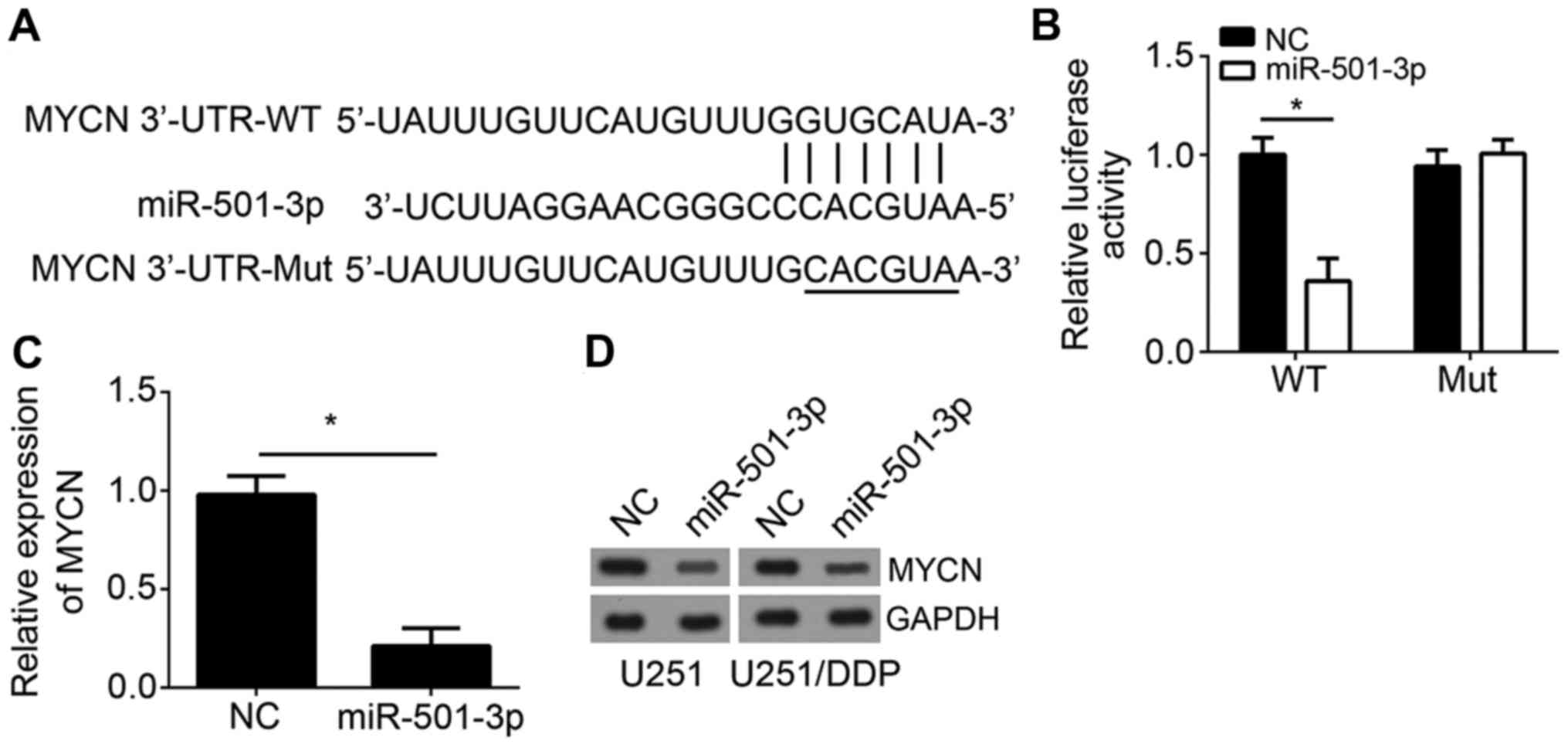
It has been acknowledged that miRNAs play roles via
regulating target gene expression (13). Hence, we predicted the targets of
miR-501-3p by TargetScan. We identified MYCN as the most potential
target of miR-501-3p. There was a potential binding site of
miR-501-3p of MYCN in the 3′-UTR of MYCN (Fig. 4A). To validate it, we performed
luciferase reporter assay. The 3′-UTR region containing the WT or
mutant (Mut) potential binding site was cloned into pmiR-Reporter
vector. The results showed that overexpression of miR-501-3p
significantly repressed the luciferase activity of MYCN-WT in
U251/DDP cells but not MYCN-Mut (Fig.
4B), suggesting that there was a direct interaction between
MYCN and miR-501-3p. Furthermore, RT-qPCR analysis showed that
overexpression of miR-501-3p inhibited the mRNA level of MYCN in
U251/DDP cells (Fig. 4C).
Consistently, western blot result indicated that ectopic expression
of miR-501-3p led to reduced protein levels of MYCN in both U251
and U251/DDP cells (Fig. 4D).
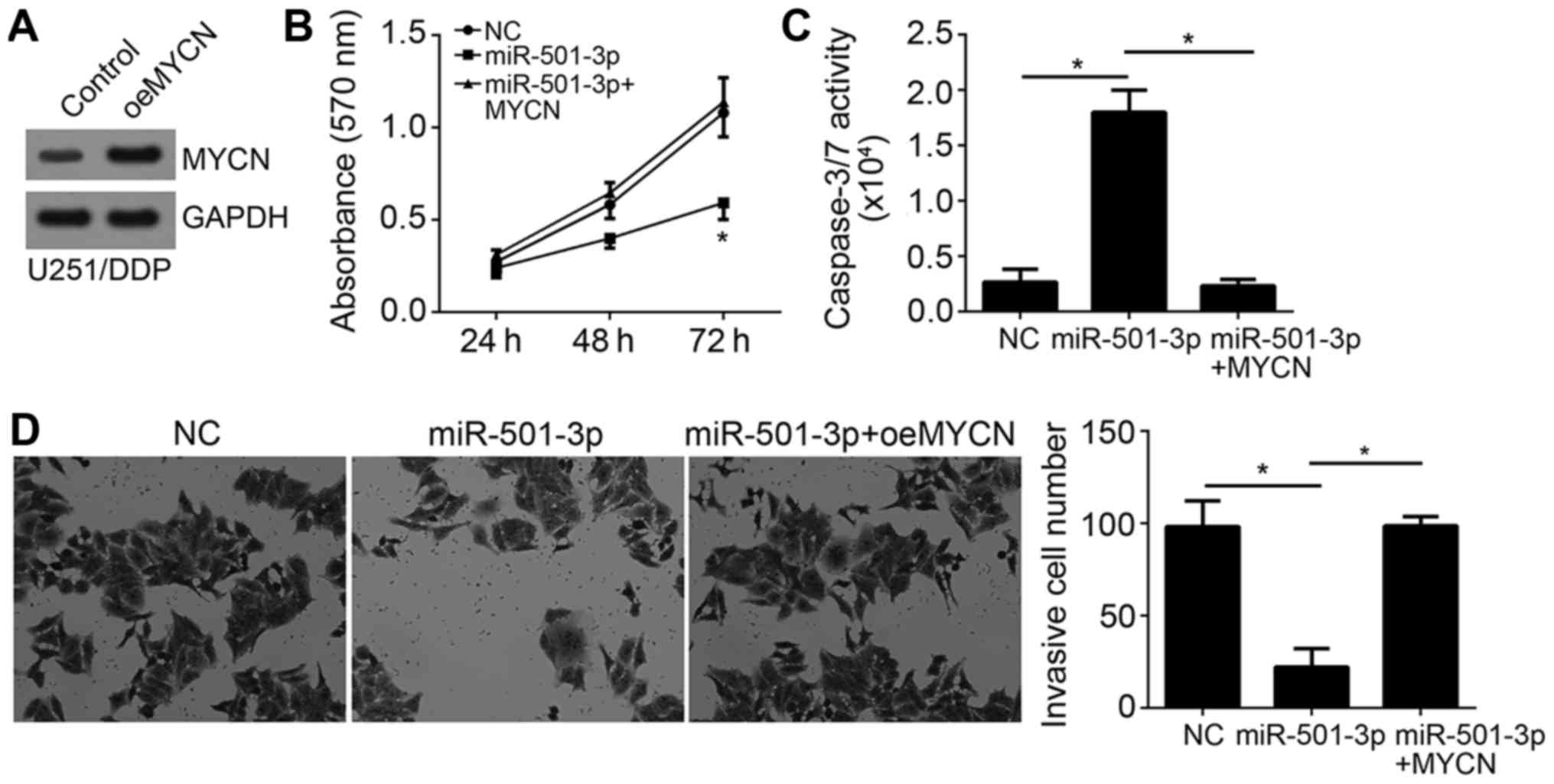
Ectopic expression of MYCN reverses
the effects of miR-501-3p
To explore whether MYCN expression is responsible
for the function of miR-501-3p in glioma cells, we overexpressed
MYCN in U251/DPP cells (Fig. 5A).
Then we performed MTT assay and found that restoration of MYCN
significantly increased cell proliferation in U251/DDP cells
transfected with miR-501-3p mimics (Fig. 5B). Besides, re-expression of MYCN
abrogated the pro-apoptotic effect of miR-501-3p on U251/DDP cells
(Fig. 5C). Furthermore, transwell
assay also showed that MYCN overexpression rescued the invasive
ability of U251/DPP cells (Fig.
5D). Taken together, these results demonstrated that MYCN was a
functional target of miR-501-3p in glioma.
Discussion
Resistance to chemotherapeutic agents remains a
major problem for the treatment of glioma patients. The molecular
mechanism underlying resistance to chemotherapeutic agents is
largely unknown. In the present study, we explored the role of
miR-501-3p on cisplatin resistance in glioma cells. Our study is
the first time to show that miR-501-3p was significantly
downregulated in glioma tissues and involved in tumor
chemoresistance. We found that miR-501-3p expression was further
decreased by cisplatin treatment. Through MTT, caspase-3/7 activity
and transwell assays, we showed that miR-501-3p overexpression
suppressed cisplatin-resistant glioma cell proliferation and
invasion while promoting apoptosis. Moreover, we for the first time
illustrated that miR-501-3p enhanced the sensitivity of glioma
cells to cisplatin by targeting the 3′-UTR region of MYCN mRNA.
Taken together, these results demonstrated that miR-501-3p might be
a promising target for overcoming the resistance of glioma to
chemotherapeutic agents.
Previous studies have demonstrated that aberrant
expression of miRNAs is implicated in the process of
chemoresistance in glioma. For instance, miR-223 influences
glioblastoma cell growth and TMZ resistance by modulating PI3 K/Akt
singaling (16). miR-101 regulates
glioblastoma cell resistance to temozolomide through inhibiting
GSK3β (17). miR-29c decreased the
resistance of glioma cells to temozolomide via inhibiting
O6-methylguanine-DNA methyltransferases (18). miR-873 sensitizes glioma cell to
cisplatin by inhibiting Bcl-2 (1).
miR-203 is involved in chemoresistance in human glioblastoma
through targeting SNAI2 (19).
Furthermore, other studies also indicated that miR-139 and miR-136
were underexpressed in glioma tissues and enhances
cisplatin-induced or temozolomide-induced apoptosis of glioma
(20). These evidences together
showed that dysregulated miRNA expression might affect the response
of glioma cells to chemotherapy. To date, the role of miR-501-3p is
poorly understood. Luo et al showed that miR-501-3p inhibits
liver ancer growth and metastasis via targeting LIN7A (13). Sanches et al indicated that
miR-501overexpression enhances proliferation and metastasis of
cervical cancer cells through inhibiting CYLD (14). However, the role of miR-501-3p in
glioma requires investigation. In our study, we showed that
miR-501-3p was downregulated in glioma tissues compared to adjacent
normal tissues and further descreased in cisplatin-resistant glioma
cell line. Our results also demonstrated that miR-501-3p regulates
the sensitivity of glioma cells to cisplatin. Nevertheless, the
mechanism regulating miR-501-3p downregulation during cisplatin
treatment in glioma remains to be determined. Moreover, to better
confirm the correlation between miR-501-3p expression and cisplatin
resistance, it is meanful to obtain more clinical data of the
glioma patients. Furthermore, whether every patient with low
miR-501-3p expression shows cisplatin resistance requires
investigation in future.
MYCN is a classical oncogene in various cancers,
such as hepatocellular carcinoma (21) and prostate cancer (22). Besides, MYCN is also reported to be
an oncogenic driver and serve as a worst prognostic biomarker in
neuroblastoma (23,24). Bjerke et al showed that
upregulation of MYCN promotes glioblastoma progression (25). In our study, we utilized luciferase
reporter assay to validate that MYCN was a direct target of
miR-501-3p. Overexpression of MYCN could significantly offset the
miR-501-3p-induced inhibition of cisplatin-resistant glioma cells
on cell proliferation and invasion. However, more genes targeted by
miR-501-3p require to be investigated further.
In conclusion, we for the first time demonstrated
that miR-501-3p was underexpressed in cisplatin-resistant glioma
cells and promotes the sensitization of cisplatin through targeting
MYCN. Our findings suggest that miR-501-3p might serve as a
promising biomarker and target for cisplatin-resistant glioma
therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CZ designed this study, performed the experiments,
and analyzed and interpreted the results. XW conceived this study
and wrote this manuscript. FY, YL, YS and XL performed certain
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for the
present study was approved by the Institutional Ethics Committee of
The third People's Hospital of Linyi and all enrolled patients
signed a written informed consent document.
Patient consent for publication
All patients within this study provide consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen X, Zhang Y, Shi Y, Lian H, Tu H, Han
S, Peng B, Liu W and He X: miR-873 acts as a novel sensitizer of
glioma cells to cisplatin by targeting Bcl-2. Int J Oncol.
47:1603–1611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han L, Liu D, Li Z, Tian N, Han Z, Wang G,
Fu Y, Guo Z, Zhu Z, Du C and Tian Y: HOXB1 is a tumor suppressor
gene regulated by miR-3175 in glioma. PLoS One. 10:e01423872015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bageritz J, Puccio L, Piro RM, Hovestadt
V, Phillips E, Pankert T, Lohr J, Herold-Mende C, Lichter P and
Goidts V: Stem cell characteristics in glioblastoma are maintained
by the ecto-nucleotidase E-NPP1. Cell Death Differ. 21:929–940.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan L, Yuan P, Yuan H, Wang Z, Run Z,
Chen G, Zhao P and Xu B: miR-542-3p inhibits colorectal cancer cell
proliferation, migration and invasion by targeting OTUB1. Am J
Cancer Res. 7:159–172. 2017.PubMed/NCBI
|
|
5
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujii T, Shimada K, Tatsumi Y, Hatakeyama
K, Obayashi C, Fujimoto K and Konishi N: microRNA-145 promotes
differentiation in human urothelial carcinoma through
down-regulation of syndecan-1. BMC Cancer. 15:8182015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hata A and Lieberman J: Dysregulation of
microRNA biogenesis and gene silencing in cancer. Sci Signal.
8:re3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai NS, Wu DG, Fang XG, Lin YC, Chen SS,
Li ZB and Xu SS: Serum microRNA-210 as a potential noninvasive
biomarker for the diagnosis and prognosis of glioma. Br J Cancer.
112:1241–1246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang GB, Liu JH, Hu J and Xue K: miR-21
enhanced glioma cells resistance to carmustine via decreasing Spry2
expression. Eur Rev Med Pharmacol Sci. 21:5065–5071.
2017.PubMed/NCBI
|
|
10
|
Zhi T, Yu T, Pan M, Nie E, Wu W, Wang X,
Liu N, You Y, Wang Y and Zhang J: EZH2 alteration driven by
microRNA-524-5p and microRNA-324-5p promotes cell proliferation and
temozolomide resistance in glioma. Oncotarget. 8:96239–96248. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Yang Y, Chen B, Lu P, Zhan L, Yu
Q, Cao K and Li Q: miR-136 targets E2F1 to reverse cisplatin
chemosensitivity in glioma cells. J Neurooncol. 120:43–53. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Y, Yan K, Fang J, Qu Q, Zhou M and
Chen F: Let-7b expression determines response to chemotherapy
through the regulation of cyclin D1 in glioblastoma. J Exp Clin
Cancer Res. 32:412013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo C, Yin D, Zhan H, Borjigin U, Li C,
Zhou Z, Hu Z, Wang P, Sun Q, Fan J, et al: microRNA-501-3p
suppresses metastasis and progression of hepatocellular carcinoma
through targeting LIN7A. Cell Death Dis. 9:5352018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanches JGP, Xu Y, Yabasin IB, Li M, Lu Y,
Xiu X, Wang L, Mao L, Shen J, Wang B, et al: miR-501 is upregulated
in cervical cancer and promotes cell proliferation, migration and
invasion by targeting CYLD. Chem Biol Interact. 285:85–95. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang BS, Luo QZ, Han Y, Huang D, Tang QP
and Wu LX: miR-223/PAX6 axis regulates glioblastoma stem cell
proliferation and the chemo resistance to TMZ via regulating
PI3K/Akt pathway. J Cell Biochem. 118:3452–3461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian T, Mingyi M, Qiu X and Qiu Y:
MicroRNA-101 reverses temozolomide resistance by inhibition of
GSK3β in glioblastoma. Oncotarget. 7:79584–79595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao S, Yang Z, Qiu X, Lv R, Liu J, Wu M,
Liao Y and Liu Q: miR-29c contribute to glioma cells temozolomide
sensitivity by targeting O6-methylguanine-DNA methyltransferases
indirectely. Oncotarget. 7:50229–50238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao H, Bai Y, Qiu S, Zheng L, Huang L,
Liu T, Wang X, Liu Y, Xu N, Yan X and Guo H: miR-203 downregulation
is responsible for chemoresistance in human glioblastoma by
promoting epithelial-mesenchymal transition via SNAI2. Oncotarget.
6:8914–8928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: miR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin XY, Suzuki H, Honda M, Okada H, Kaneko
S, Inoue I, Ebisui E, Hashimoto K, Carninci P, Kanki K, et al:
Prevention of hepatocellular carcinoma by targeting MYCN-positive
liver cancer stem cells with acyclic retinoid. Proc Natl Acad Sci
USA. 115:4969–4974. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Liu B, Wu W, Li L, Broom BM,
Basourakos SP, Korentzelos D, Luan Y, Wang J, Yang G, et al:
Targeting the MYCN-PARP-DNA damage response pathway in
neuroendocrine prostate cancer. Clin Cancer Res. 24:696–707. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang T, Liu L, Chen X, Shen Y, Lian G,
Shah N, Davidoff AM, Yang J and Wang R: MYCN drives glutaminolysis
in neuroblastoma and confers sensitivity to an ROS augmenting
agent. Cell Death Dis. 9:2202018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Estiar MA, Javan F, Zekri A, Mehrazin M
and Mehdipour P: Prognostic significance of MYCN gene amplification
and protein expression in primary brain tumors: Astrocytoma and
meningioma. Cancer Biomark. 19:341–351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bjerke L, Mackay A, Nandhabalan M, Burford
A, Jury A, Popov S, Bax DA, Carvalho D, Taylor KR, Vinci M, et al:
Histone H3.3. mutations drive pediatric glioblastoma through
upregulation of MYCN. Cancer Discov. 3:512–519. 2013. View Article : Google Scholar : PubMed/NCBI
|