Introduction
Osteoarthritis (OA) is a common chronic degenerative
joint disease, clinically manifesting with pain, swelling and
stiffness, and progressive OA may lead to permanent disability
(1). The clinical manifestations
of OA seriously affect the work and everyday life of patients, and
may pose a major socioeconomic burden (2,3).
With the gradual increase in the size of the elderly population,
the incidence of OA is also expected to increase (4). In order to alleviate the personal and
social burden, it is particularly important to investigate safe and
effective preventive and curative measures.
There is currently no particularly effective
treatment for OA. Common treatment methods for the relief of
symptoms, including non-steroidal anti-inflammatory drugs,
hyaluronic acid injections and arthroplasty, among others, are
associated with certain limitations (5–7). In
China, in addition to the aforementioned methods, the treatment of
OA also includes alternative methods, including Chinese herbal
medicine, acupuncture and massage therapy, among which,
electroacupuncture (EA) is often used in the management of OA.
Certain clinical and evidence-based studies have suggested that EA
can effectively relieve pain and improve joint function (8–10).
It was previously demonstrated that EA promotes the differentiation
of bone marrow-derived mesenchymal stem cells into chondrocytes
(11), promotes the proliferation
of chondrocytes (12), and reduces
the inflammatory response of chondrocytes induced by tumor necrosis
factor α (13). However, the
precise mechanism of action of EA in the treatment of OA remains to
be fully elucidated.
The principal pathological manifestations of OA are
cartilage degeneration, synovitis and osteophyte hyperplasia, among
which cartilage degeneration is the main characteristic (14). Therefore, the alleviation of
cartilage degeneration may be key to the treatment of OA. Normal
articular cartilage comprises chondrocytes and extracellular
matrix, which are in a state of dynamic equilibrium. Alterations in
chondrocyte proliferation, apoptosis and other physiological
functions may cause dynamic balance disorders, which affect
cartilage functional status (15).
Therefore, chondrocytes may represent a valuable focus of
investigation in OA research. It has been reported that levels of
nitrite, a stable end product of nitric oxide (NO) metabolism, are
elevated in serum and synovial fluid samples of OA (16). In addition, synovial cells and
cartilage cells in OA produce large amounts of NO (17). The negative effects of NO include
enhancement of matrix metalloproteinase activity, a reduction in
interleukin-1 receptor antagonist synthesis and the promotion of
apoptosis, which are closely associated with the occurrence and
development of OA (18–20). Sodium nitroprusside (SNP), which is
a widely used NO donor, is extremely unstable and produces NO when
added to chondrocyte culture fluids (21,22).
To the best of our knowledge, no previous studies have used SNP to
generate animal models of OA; however, SNP-induced chondrocytes are
common in vitro models of OA (23–25).
The present study aimed to determine whether EA may serve a
therapeutic role in OA by inhibiting SNP-induced chondrocyte
apoptosis.
Materials and methods
Animals
Chondrocytes were obtained from 4-week-old male
Sprague Dawley rats (n=30; weight, 70±10 g) purchased from Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China; permit no. SCXK
2012–0002). The rats were raised in the Animal Experimental Center
of Fujian University of Traditional Chinese Medicine (permit no.
SYXK 2014–0005; Fujian, China) at a room temperature of 24±2°C, a
relative humidity of 55±5%, a 12/12 h light/dark cycle and free
access to food and water. The present study was approved by the
Animal Care and Use Committee of Fujian University of Traditional
Chinese Medicine.
Chondrocyte acquisition and
culture
Articular cartilage cells were isolated and cultured
as previously described (26).
After the rats were euthanized, their knee joints were removed and
transferred to a clean bench for further processing. Cartilage
tissue from the rat knees was removed and rinsed three times with
PBS (HyClone; GE Healthcare Life Sciences, Logan, UT, USA). The
cartilage tissue was digested with type II collagenase
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in an incubator
(Heraeus Holding GmbH, Hanau, Germany) at 37°C and 5%
CO2 after mincing. After 90 min, the supernatant was
collected and centrifuged at 503.1 × g for 3 min to achieve cell
precipitation. The cell pellet was suspended in 4 ml Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum
(both from HyClone; GE Healthcare Life Sciences). The cell
suspension was transferred to a 25-mm2 flask, and was
then cultured in the incubator at 37°C and 5% CO2. The
cartilage tissues were digested four times repeatedly. The culture
medium was replenished every 2 days. Passages were performed when
chondrocytes had grown to 90% confluence. Second-generation
chondrocytes were employed for subsequent experiments.
Chondrocyte observation and
identification
Chondrocyte morphology on different culture days and
of different generations was observed under an inverted
phase-contrast microscope (Leica Microsystems, Inc., Wetzlar,
Germany) and images were captured. Second-generation chondrocytes
are often selected for experimentation (27); therefore, type II collagen
immunohistochemistry was applied to identify passage 2
chondrocytes. A total of 5×104 second-generation
chondrocytes per well were implanted onto a sterile round
coverglass in a 6-well plate. Chondrocytes in the 6-well plate (2
ml medium/well) were incubated for 48 h and were then randomly
divided into two groups. The positive group was treated with 100 µl
rabbit polyclonal antibody against collagen II (dilution 1:200;
cat. no. ab34712; Abcam, Cambridge, UK), whereas the negative group
was treated with 100 µl PBS. Both groups were incubated overnight
at 4°C. After incubation, the two groups of chondrocytes were
treated with a secondary antibody (cat. no. KIT-9707; MXB
Biotechnologies, Inc., Fujian, China) at 37°C for 1 h, in
accordance with the manufacturer's instructions; color was
developed using a DAB kit (cat. no. DAB-0031; MXB Biotechnologies,
Inc.); and the cells were stained with hematoxylin (Sigma-Aldrich;
Merck KGaA) for 1 min. The staining of the two groups of cells was
observed and compared under a phase-contrast microscope (Leica
Microsystems, Inc.).
Experimental grouping
A cell suspension (1×105/ml) was seeded
in 6-well plates (2 ml/well). After 72 h, the cells were randomly
divided into the following groups: i) The control group without
treatment, ii) the 1 mM SNP-treated group, iii) the group treated
with 1 mM SNP and EA for 30 min every 8 h (electrical stimulator
was obtained from Suzhou Medical Appliance Factory, Suzhou, China),
and iv) the group treated with 1 mM SNP and EA for 60 min every 8
h. The intervention time for all groups was 24 h. EA intervention
on chondrocytes was conducted as described previously (11).
DAPI staining
After treatment, the chondrocyte morphology in each
group was observed under a microscope, and the nuclear alterations
in each group were observed using DAPI staining. Initially,
chondrocytes were fixed in 1 ml 4% neutral paraformaldehyde
(HyClone; GE Healthcare Life Sciences) at 4°C for 30 min. After
washing with PBS three times, the chondrocytes were stained with
500 µl DAPI (5 µg/ml; cat. no. D1306; Thermo Fisher Scientific,
Inc.) for 5 min in the dark. After staining, the nuclear
alterations were observed and images were captured under a
fluorescence microscope (Leica Microsystems, Inc.).
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining and flow cytometry
After treatment, chondrocytes from each group were
digested with EDTA-free trypsin (Gibco; Thermo Fisher Scientific,
Inc.) and collected in separate 15-ml centrifuge tubes, in
accordance with the instructions of the apoptosis detection kit
(cat. no. KGA108; Nanjing KeyGen Biotech Co. Ltd. Nanjing, China).
A total of 5×105 chondrocytes were collected in flow
tubes and suspended in 500 µl binding buffer. Subsequently, 5 µl
Annexin V-FITC and 5 µl PI were added to the chondrocyte suspension
and the cells were incubated in the dark for 15 min at room
temperature. The chondrocyte apoptotic rate was measured using a
fluorescence-activated cell sorting (FACS) machine (BD
FACSCalibur™; BD Biosciences, San Jose, CA, USA).
JC-1 staining and flow cytometry
After treatment, chondrocytes were digested with
EDTA-free trypsin and were collected in separate 15-ml centrifuge
tubes. A total of 5×105 chondrocytes in flow tubes were
then suspended in 500 µl PBS containing JC-1 (cat. no. 420200;
Calbiochem; Merck KGaA) at 10 µg/ml. The mixture was placed in a
cell incubator at 37°C and 5% CO2 for 15 min.
Alterations in mitochondrial membrane potential were then detected
using the BD FACSCalibur™ (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After treatment, TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was applied to extract
total RNA. The PrimeScript™ RT reagent kit (cat. no. RR0047A;
Takara Bio, Inc., Otsu, Japan) was used to reverse transcribe RNA
(1 µg) into cDNA in the PCR amplification instrument according to
the manufacturer's protocol. The primer sequences were as follows:
B-cell lymphoma 2 (Bcl-2), forward 5′-TCCAGGCATCAGGTTAGTC-3′,
reverse 5′-GGTCAGTGTCCAGGTAGG-3′; Bcl-2-associated X protein (Bax),
forward 5′-TGTCAGTCCTGGCAGTCAAC-3′, reverse
5′-GGCTCAGTAGTAGGCGATGG-3′; caspase-9, forward
5′-AATGGATGTGGTGCTGTC-3′, reverse 5′-AACTGTATAGGAAGGCTGAG-3′;
caspase-3, forward 5′-TACAGGAACAGACCATAATACC-3′, reverse
5′-AGACCAGTGCTCACAAGG-3′; and β-actin, forward
5′-ACCACTGGCATTGTGATGGA-3′ and reverse 5′-CGCTCGGTCAGGATCTTCT-3′
(Sangon Biotech Co., Ltd., Shanghai, China). The qPCR SYBR Green
Master Mix (cat. no. Q111-02; Vazyme Biotech Co., Ltd., Nanjing,
China) was used to detect the respective mRNA expression levels.
The total reaction volume was 20 µl, including 10 µl qPCR SYBR
Green Master Mix, 8 µl sterilized distilled water, 1 µl cDNA, 0.5
µl forward primer and 0.5 µl reverse primer. RT-qPCR was conducted
using the 7500 Fast Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with the following reaction
conditions: Stage 1, pre-denaturation at 95°C for 3 min; stage 2,
40 cycles at 95°C for 10 sec and 60°C for 30 sec; stage 3,
dissolution curve at 95°C for 15 sec, 60°C for 60 sec and 95°C for
15 sec. The 2−ΔΔCq method (28) was applied for data analysis.
Western blot analysis
Chondrocytes from the four groups were lysed on ice
for 30 min with radioimmunoprecipitation assay lysis buffer and
phenylmethanesulfonyl fluoride (both from Beyotime Institute of
Biotechnology, Shanghai, China), in order to extract proteins. A
bicinchoninic acid kit (cat. no. P0010; Beyotime Institute of
Biotechnology) was applied to determine protein concentrations in
the different groups. Protein samples (20 µg) were separated by 12%
SDS-PAGE and were transferred to polyvinylidene fluoride membranes.
Blocking buffer (Beyotime Institute of Biotechnology) was used to
block the membranes at room temperature for 1 h. After 1 h, the
membranes were incubated with antibodies against Bax (1:5,000
dilution; cat. no. ab32503; Abcam), Bcl-2 (1:1,000 dilution; cat.
no. 2870; Cell Signaling Technology, Inc., Danvers, MA, USA) and
β-actin (1:1,000 dilution; cat. no. 8457; Cell Signaling
Technology, Inc.) at 4°C overnight, followed by incubation with
secondary antibodies (1:20,000 dilution; cat. no. AP132P; Merck
KGaA) at room temperature for 1 h. Finally, protein expression
semi-quantified using the ChemiDoc™ XRS+ system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with BeyoECL Plus (Beyotime
Institute of Biotechnology).
Colorimetric assays
Two colorimetric kits (cat. nos. ab65608 and
ab39401; Abcam) were applied to detect caspase-9 and caspase-3
activity. The experimental procedure was conducted according to the
manufacturer's protocol. After the chondrocytes were completely
lysed, the mixture was centrifuged at 10,000 × g for 1 min at 4°C
to obtain total cellular protein. The total reaction volume for
this experiment included 100 µg protein dissolved in 50 µl cell
lysis buffer, substrate, 50 µl reaction buffer and either 5 µl
LEHD-p-NA (for caspase-9 detection) or DEVD-p-NA (for caspase-3
detection; all reagents contained within relevant kits); this
reaction mixture was mixed thoroughly in 96-well plates and then
incubated in the dark at 37°C for 2 h. The optical density of the
samples was measured at 405 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Immunofluorescence staining
A total of 1×105/ml chondrocytes were
seeded in a laser confocal dish. After 72 h, the cells were treated
with either SNP or EA. Following this, chondrocytes were fixed with
500 µl formaldehyde at 4°C for 30 min and then incubated with 500
µl 0.5% Triton at room temperature for 10 min in order to increase
cell membrane permeability. After blocking with PBS containing 10%
goat serum (cat. no. SL038; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and 0.5% bovine serum albumin
(cat. no. A8010; Beijing Solarbio Science & Technology Co.,
Ltd) at room temperature for 1 h, the chondrocytes were incubated
with anti-cytochrome c (Cyt-C; 1:500 dilution; cat. no.
ab90529; Abcam) at 4°C overnight. Following this, chondrocytes were
incubated with a fluorescent secondary antibody (1:300 dilution;
cat. no. A-11008; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature and with DAPI (5 µg/ml) for 5 min at room temperature
in the dark. After three washes with PBS, the chondrocytes were
observed and images were captured under a laser scanning confocal
microscope (Olympus Corporation, Tokyo, Japan). ImageJ 1.8.0
(https://imagej.nih.gov/ij/download.html) was used to
measure the fluorescence intensity of Cyt-C in different images,
and the level of intensity per area was used to indicate the
relative expression of Cyt-C. Three visual fields from each laser
confocal dish were randomly selected for data analysis.
Statistical analysis
Each experiment was independently repeated at least
three times. Experimental data were processed and analyzed using
SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The Shapiro-Wilk
test was used to determine the normality of all groups of data. If
the data exhibited a normal distribution, they were analyzed with
one-way analysis of variance followed by least significant
difference or Games Howell post hoc tests; if not, the
Kruskal-Wallis test was used and the Mann-Whitney U with
Bonferroni's correction was applied as a post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Morphological observation of
chondrocytes isolated from the articular cartilage of rats
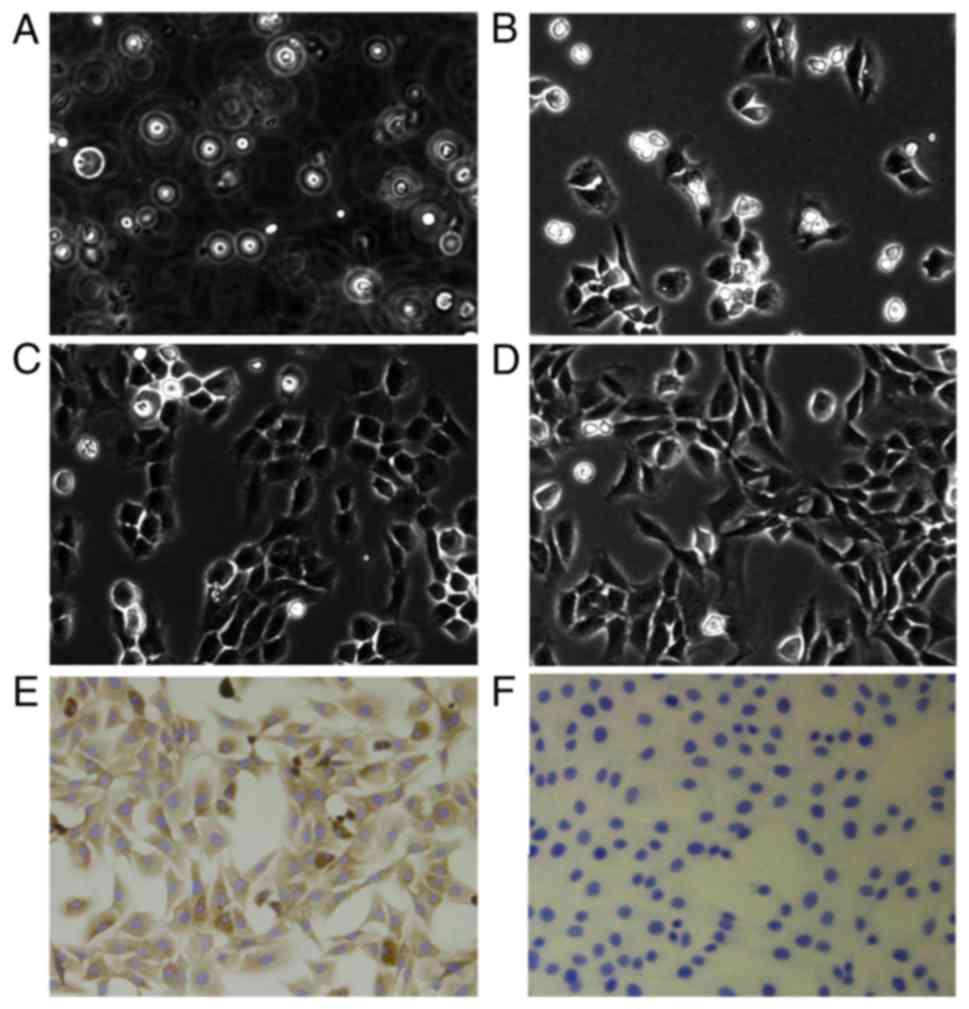
Cells that had just been extracted from rat
articular cartilage were suspended in culture medium (Fig. 1A). After 2 days, the majority of
the cells were adherent to the culture bottle and had an irregular
shape (Fig. 1B). First- and
second-generation chondrocytes after 2 days of culture assumed a
round or oval shape (Fig. 1C and
D). Briefly, the rat joint cartilage cell morphology resembled
that reported in previous studies (26,27).
Detection of type II collagen in
second-generation chondrocytes
Since type II collagen is mainly produced by
chondrocytes, type II collagen immunohistochemistry was used to
identify second-generation chondrocytes. Under an inverted phase
contrast microscope, the cytoplasm of chondrocytes in the positive
group was stained brown (Fig. 1E),
whereas the cytoplasm of the chondrocytes in the negative group was
not stained (Fig. 1F). Since
second-generation articular cartilage cells exhibited the typical
morphology of chondrocytes and abundant type II collagen, they were
employed in subsequent experiments.
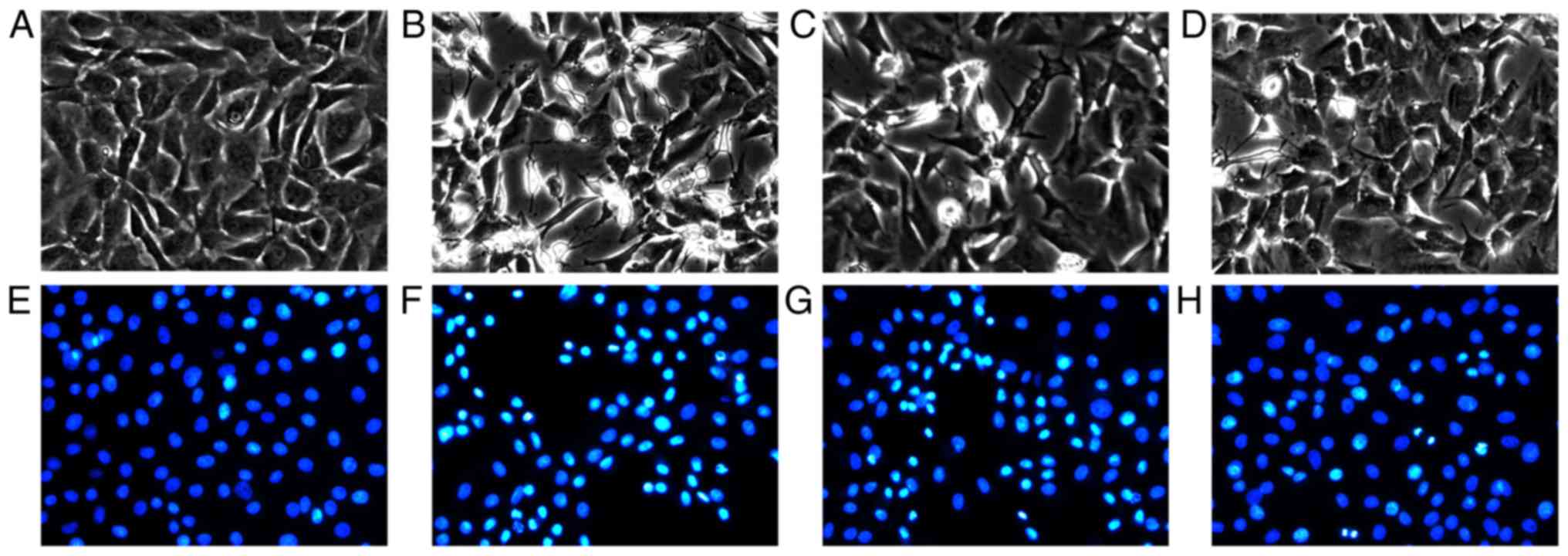
EA inhibits SNP-induced chondrocyte
apoptosis, as determined by morphological observation and DAPI
staining
Microscopic observation and DAPI staining were
performed to investigate the effects of EA on SNP-induced apoptotic
cells (Fig. 2). Chondrocyte
morphology in the normal group exhibited no obvious alterations
(Fig. 2A), and the nuclei were
stained blue, and were round- or oval-shaped (Fig. 2E). However, in the SNP-induced
group, numerous chondrocytes were observed floating in the culture
medium, and adherent cells had an irregular morphology (Fig. 2B). Compared with the normal group,
the nuclei of the SNP-treated group were significantly reduced in
size and were bright blue (Fig.
2F). Following EA treatment, there were fewer chondrocytes
floating in the culture medium (Fig.
2C and D), and the shrinking and brightness of the nuclei were
less prominent compared with in the SNP-treated group (Fig. 2G and H).
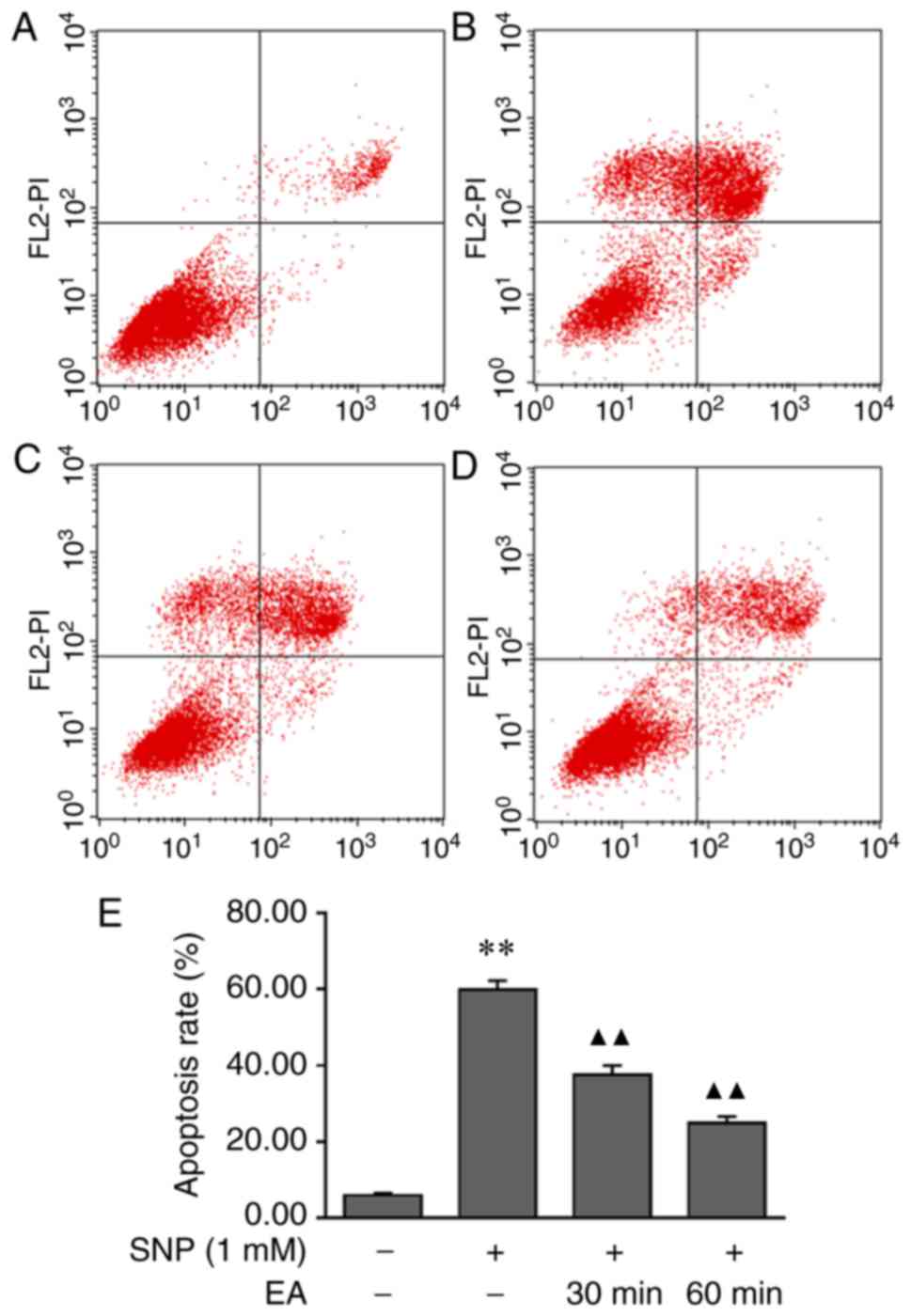
EA modulates the apoptotic rate of
SNP-induced chondrocytes
The effects of EA on chondrocyte apoptosis were
assessed by Annexin V-FITC/PI staining and flow cytometry. The
results demonstrated that the apoptotic rate of SNP-induced
chondrocytes was significantly higher compared with that of normal
chondrocytes (P<0.001). Conversely, the apoptotic rate of
chondrocytes following EA treatment was lower compared with that of
SNP-induced chondrocytes (P<0.001). These findings indicated
that EA reduced the apoptotic rate of chondrocytes (Fig. 3).
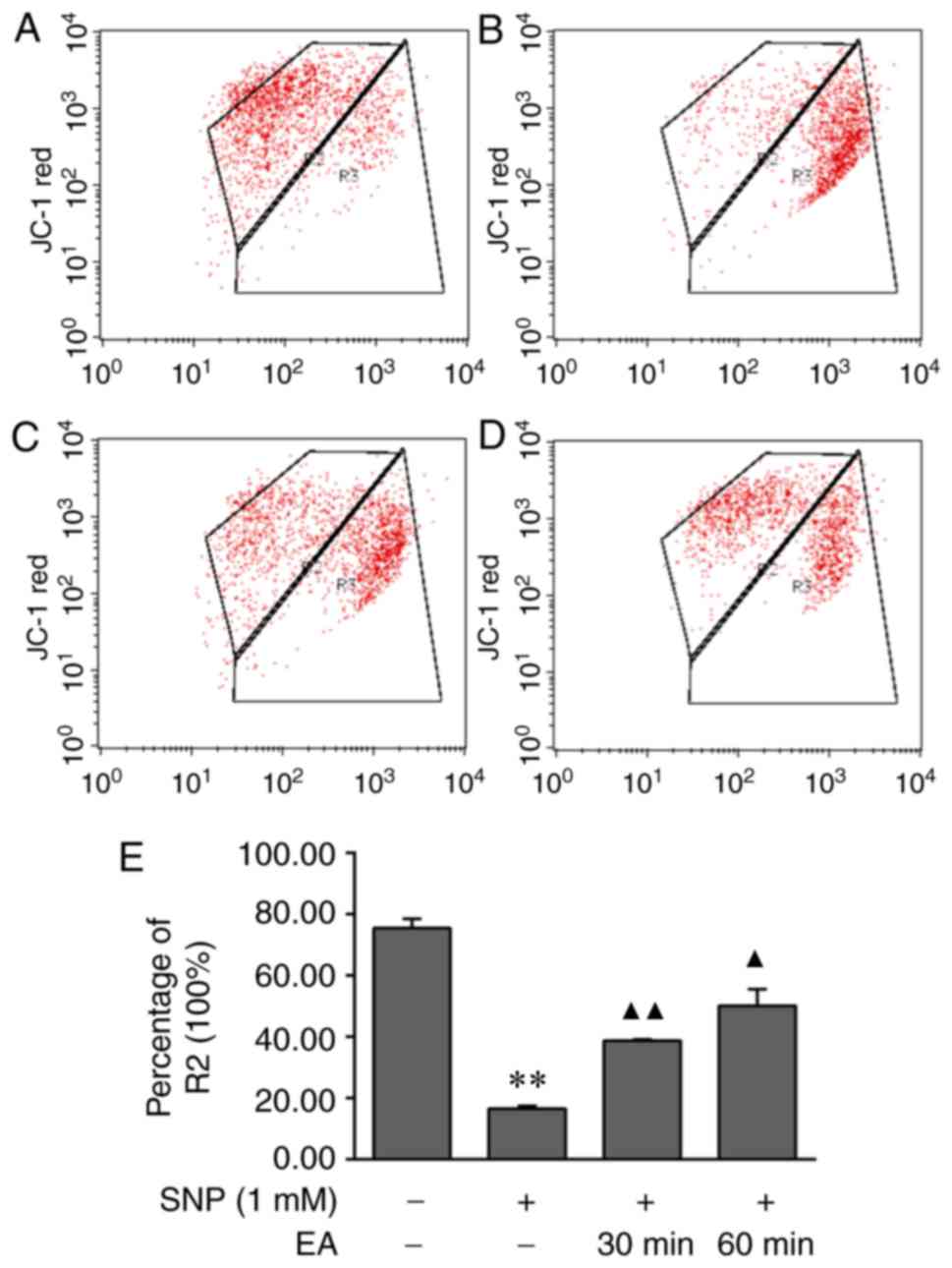
EA decelerates the reduction in
mitochondrial membrane potential in SNP-stimulated apoptotic
chondrocytes
JC-1 single-staining flow cytometry was performed to
determine the effects of EA on the mitochondrial membrane potential
of apoptotic chondrocytes. The mitochondrial membrane potential was
significantly decreased following treatment of chondrocytes with 1
mM SNP for 24 h (P=0.001). Compared with in the SNP-induced group,
the decrease in mitochondrial membrane potential was significantly
slower in the EA treatment groups (P<0.001, P=0.018). The
results demonstrated that EA increased the mitochondrial membrane
potential of apoptotic chondrocytes (Fig. 4).
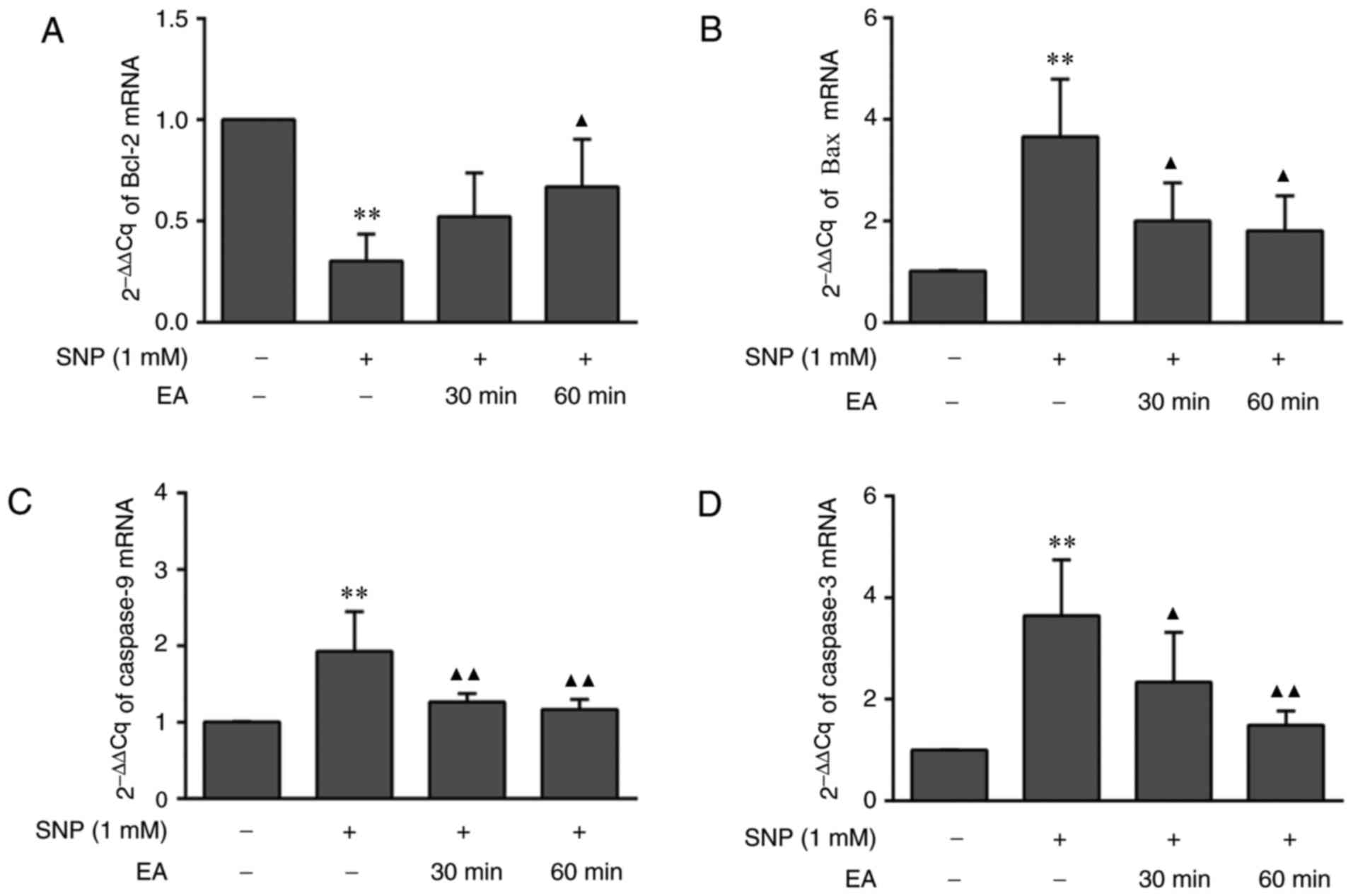
EA regulates gene and protein
expression in chondrocytes
The aforementioned results indicated that EA may
inhibit 1 mM SNP-induced chondrocyte apoptosis. To further
elucidate the mechanism of action of EA at the molecular level, the
expression levels of Bcl-2, Bax, Cyt-C, caspase-9 and caspase-3
were assessed. RT-qPCR analysis (Fig.
5) indicated that the mRNA expression levels of Bcl-2 in
SNP-induced chondrocytes were reduced compared with in normal
chondrocytes (P=0.001). Furthermore, the mRNA expression levels of
Bax (P=0.003), caspase-9 (P<0.001) and caspase-3 (P<0.001)
were significantly increased in SNP-induced chondrocytes compared
with in normal chondrocytes. Conversely, compared with in
SNP-induced chondrocytes, EA treatment for 30 and 60 min promoted
Bcl-2 mRNA expression (P=0.159, P=0.032), and reduced Bax (P=0.028,
P=0.018), caspase-9 (P=0.005, P=0.002) and caspase-3 (P=0.029,
P=0.002) mRNA expression.
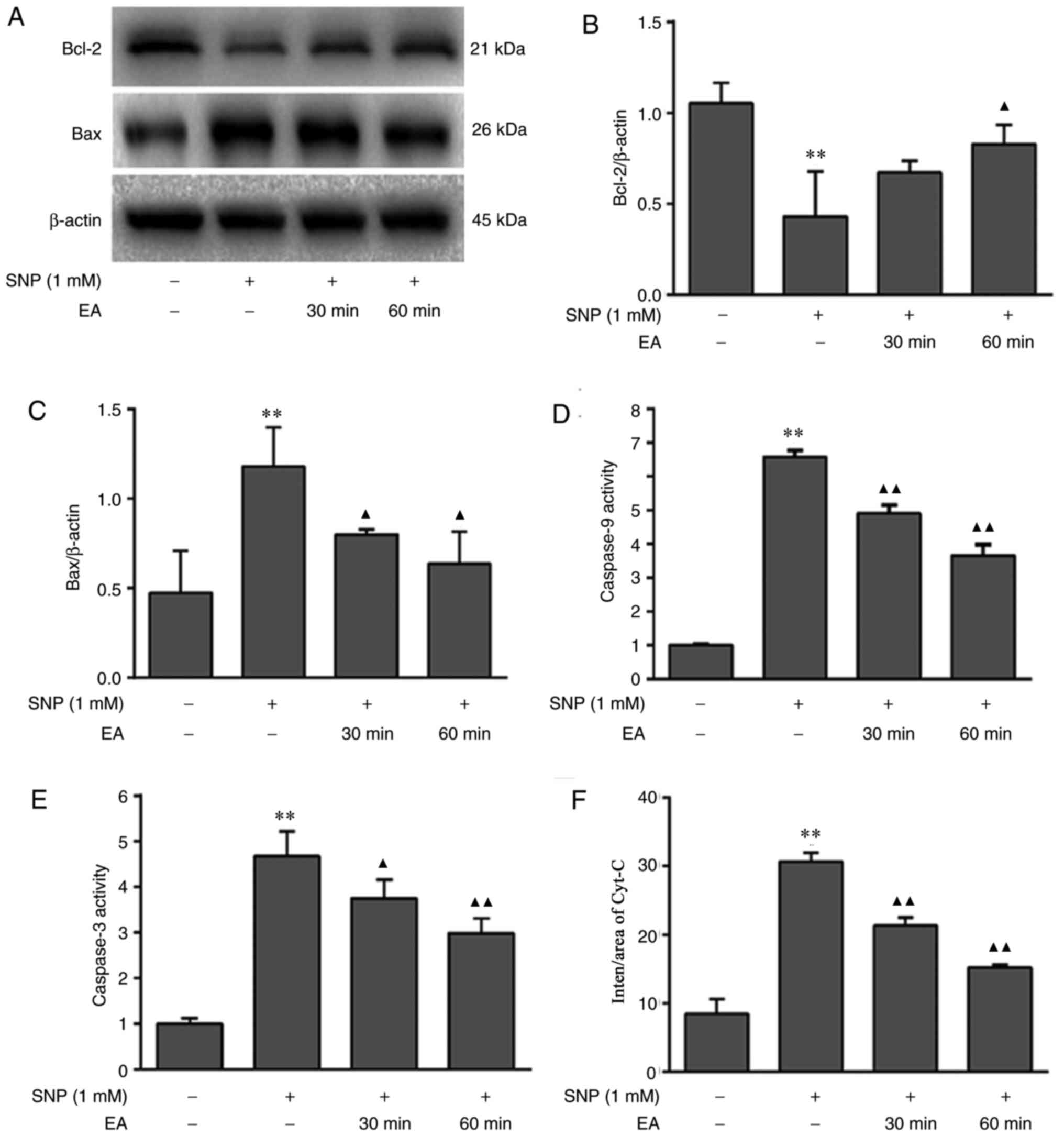
Western blotting was conducted to detect the protein
expression levels of Bcl-2 and Bax (Fig. 6A-C). In addition, caspase-9 and
caspase-3 activities were measured using colorimetric assays
(Fig. 6D and E), and
immunofluorescence staining was used to detect the expression of
Cyt-C (Fig. 6F and G). SNP
inhibited Bcl-2 protein expression (P=0.001), promoted Bax
(P=0.007) and Cyt-C (P<0.001) protein expression, and enhanced
caspase-3 (P=0.005) and caspase-9 (P=0.001) activities. Conversely,
EA treatment for 30 and 60 min increased Bcl-2 protein expression
(P=0.083, P=0.012), downregulated Bax (P=0.047, P=0.021) and Cyt-C
(both P<0.001) protein expression, and reduced caspase-9 (both
P=0.001) and caspase-3 (P=0.049, P=0.009) activities.
Discussion
The present study demonstrated that EA suppressed
SNP-mediated chondrocyte apoptosis, and exerted an inhibitory
effect on apoptosis through modulation of the mitochondrial
pathway.
Chondrocytes, which are the only type of cell
present in mature cartilage, synthesize and secrete matrix
components and fibers, thus maintaining cartilage tissue structure
and function (29). Cartilage
degeneration is the most important pathological manifestation of
OA, and chondrocyte apoptosis is closely associated with cartilage
degeneration (30). Therefore, it
has been hypothesized that effective inhibition of chondrocyte
apoptosis is key to the treatment of OA (31,32).
In previous studies, cartilage histomorphological examination
revealed that EA treatment significantly inhibits cartilage
degeneration in an ovariectomized rabbit OA model and an anterior
cruciate ligament transection rabbit OA model (33,34).
In addition, a previous TUNEL assay demonstrated that EA reduces
the rate of chondrocyte apoptosis in cartilage tissue (35), which was similar to the present
findings. However, to the best of our knowledge, the mechanism
through which EA inhibits the apoptosis of chondrocytes has not
been extensively investigated to date.
SNP is a nitrosylated sodium ferricyanide dihydrate,
which readily releases NO, as it contains an extremely unstable
nitroso group. NO, which is an important mitochondrial
apoptosis-inducing factor, may cause chondrocyte apoptosis
(21). Based on previous
experiments, 1 mM SNP was used for 24 h to induce chondrocyte
apoptosis. After treatment, it was revealed that EA improved
morphological alterations in apoptotic chondrocytes. DAPI, a
fluorescent DNA-binding dye (36),
is widely used to evaluate apoptosis. DAPI staining revealed that
the nuclei of chondrocytes treated with SNP and EA exhibited less
shrinkage, reduced brightness and higher nuclear density compared
with SNP-treated chondrocytes. Furthermore, Annexin V-FITC/PI
staining was used to assess overall chondrocyte apoptosis. When
cells undergo apoptosis, phosphatidylserine (PS), originally
located inside the lipid bilayer, is relocated to the outer surface
of the bilayer (37). Annexin-V,
which binds strongly to PS, may be used to label early apoptotic
cells (38). PI is a nucleic acid
dye that does not penetrate the intact cell membrane, but permeates
the cell membrane of late apoptotic and dead cells (39). Annexin V-FITC/PI staining is a
classic experimental method for the detection of apoptosis. In this
study, EA reduced the rate of chondrocyte apoptosis, as
demonstrated by the results of Annexin V-FITC/PI staining.
Having confirmed that EA inhibited SNP-induced
chondrocyte apoptosis, further analysis was conducted. Since SNP
has been reported to induce mitochondrial apoptosis (40), alterations in mitochondrial
membrane potential, and associated gene and protein expression
levels, were investigated. Mitochondrial membrane potential is
often detected using JC-1 (41).
In the JC-1 assay, mitochondrial membrane potential decline, which
reflects the onset of apoptosis, is indicated by the change in
fluorescence from red to green. A decrease in mitochondrial
membrane potential leads to increased membrane permeability, and
mitochondrial membrane permeability may be regulated by the Bcl-2
family (42). The Bcl-2 family
promotes and inhibits cell apoptosis through modulating
mitochondrial outer membrane permeabilization (43). Bcl-2 can suppress apoptosis by
inhibiting the increase in mitochondrial permeability (44). Conversely, Bax, another Bcl-2
family member, accelerates apoptosis. The Bax protein increases the
permeability of the mitochondrial membrane by forming activated
oligomers, promoting Cyt-C release and ultimately inducing
apoptosis (45). When cells are
stimulated by NO, mitochondrial membrane permeability increases,
which leads to Cyt-C release into the cytoplasm, triggering the
caspase cascade, namely sequential activation of caspase-9 and
caspase-3, leading to cell apoptosis (46). In the present study, EA reduced the
extent of the mitochondrial membrane potential decline, and
regulated the expression levels of Bcl-2, Bax, Cyt-C, caspase-9 and
caspase-3.
In conclusion, EA inhibited SNP-induced chondrocyte
apoptosis through regulating the mitochondrial pathway, which is
likely the mechanism of action of EA in OA treatment. However,
there were certain limitations to the present study. The
experiments were only conducted in vitro, and the results
have yet to be further validated in vivo. In addition, the
experiments regarding the inhibitory effects of EA on apoptosis
focused only on the mitochondrial pathway; therefore, more in-depth
research is required. Further in vitro and in vivo
experiments must be conducted to fully elucidate the mechanism
underlying the action of EA in the treatment of OA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81373719) and the Fujian
Provincial Development and Reform Commission (grant no.
2014-514).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
GW, XL and MW conceived and designed the study. JL,
JC, CF and XH performed the experiments. LL performed the data
analysis. JL and LL wrote the manuscript. XL and GW reviewed and
edited the manuscript. All authors approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Fujian University of Traditional Chinese
Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chu CR, Millis MB and Olson SA:
Osteoarthritis: From palliation to prevention: AOA critical issues.
J Bone Joint Surg Am. 96:e1302014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridqett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prieto-Alhambra D, Judqe A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu D, Jordan KP, Bedson J, Enqlund M,
Blyth F, Turkiewicz A, Prieto-Alhambra D and Peat G: Population
trends in the incidence and initial management of osteoarthritis:
Age-period-cohort analysis of the clinical practice research
datalink, 1992–1992. Rheumatology (Oxford). 56:1902–1917. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lanas A, Tornero J and Zamarano JL:
Assessment of gastrointestinal and cardiovascular risk in patients
with osteoarthritis who require NSAIDs: The LOGICA study. Ann Rheum
Dis. 69:1453–1458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arrich J, Piribauer F, Mad P, Schmid D,
Klaushofer K and Mullner M: Intra-articular hyaluronic acid for the
treatment of osteoarthritis of the knee: Systematic review and
meta-analysis. CMAJ. 172:1039–1043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gunaratne R, Pratt DN, Banda J, Fick DP,
Khan RJK and Robertson BW: Patient dissatisfaction following total
knee arthroplasty: A systematic review of the literature. J
Arthroplasty. 32:3854–3860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi L, Tanq Y, You Y, Qin F, Zhai L, Peng H
and Nie R: Comparing the effectiveness of electroacupuncture with
different grades of knee osteoarthritis: A prospective study. Cell
Physiol Biochem. 39:2331–2340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shim JW, Junq JY and Kim SS: Effects of
electroacupuncture for knee osteoarthritis: A systematic review and
meta-analysis. Evid Based Complement Alternat Med.
2016:34858752016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen N, Wanq J, Mucelli A, Zhang X and
Wanq C: Electro-acupuncture is benedicial for knee osteoarthritis:
The evidence from meta-analysis of randomized controlled trials. Am
J Chin Med. 45:965–985. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu G, Peng J, Wu M, Li Y, Huang Y, Lin R,
Cai Q and Liu X: Experimental study of low-frequency
electroacupunctureinduced differentiation of bone marrow
mesenchymal stem cells into chondrocytes. Int J Mol Med. 27:79–86.
2011.PubMed/NCBI
|
|
12
|
Huang Y, Wu G, Fan H, Ye J and Liu X:
Electroacupuncture promotes chondrocyte proliferation via
accelerated G1/S transition in the cell cycle. Int J Mol Med.
31:1443–1448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Shao X, Li L, Zheng C, Xu X, Hong
X, Li X and Wu M: Electroacupuncture serum inhibits TNF-α-mediated
chondrocytes inflammation via the Ras-Raf-MEK1/2-ERK1/2 signaling
pathway. Mol Med Rep. 16:5807–5814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sulzbacher L: Osteoarthritis: Histology
and pathogenesis. Wien Med Wochenschr. 163:212–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hemanth A and Anja N: Role of chondrocytes
in cartilage formation, progression of osteoarthritis and cartilage
regeneration. J Dev Biol. 3:177–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jang D and Murrell GA: Nitric oxide in
arthritis. Free Radic Biol Med. 24:1511–1519. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayashi T, Abe E, Yamate T and Jasin HE:
Nitric oxide production by superficial and deep articular
chondrocytes. Arthritis Rheum. 40:261–269. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hashimoto S, Takahashi K, Amiel D, Coutts
RD and Lotz M: Chondrocyte apoptosis and nitric oxide production
during experimentally induced osteoarthritis. Arthritis Rheum.
41:1266–1274. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abramson SB: Nitric oxide in inflammation
and pain associated with osteoarthritis. Arthritis Res Acta. 10
Suppl 2:S22008. View
Article : Google Scholar
|
|
20
|
Abramson SB: Osteoarthritis and nitric
oxide. Osteoarthritis Cartilage. 16 Suppl 2:S15–S20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blanco FJ, Ochs RL, Schwarz H and Lotz M:
Chondrocyte apoptosis induced by notric oxide. Am J Pathol.
146:75–85. 1995.PubMed/NCBI
|
|
22
|
Kim HA, Lee KB and Bae S: The mechanism of
low-concentration sodium nitroprusside-mediated protection of
chondrocytes death. Arthritis Res Ther. 7:R526–R535. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Notoya K, Jovanovic DV, Reboul P,
Martel-Pelletier J, Mineau F and Pelletier JP: The induction of
cell death in human osteoarthritis chondrocytes by nitric oxide is
related to the production of prostaglandin E2 via the induction of
cyclooxygenase-2. J Immunol. 165:3402–3410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kühn K and Lotz M: Mechanisms of sodium
nitroprusside-induced death in human chondrocytes. Rheumatol Int.
23:241–247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Todd Allen R, Robertson CM, Harwood FL,
Sasho T, Williams SK, Pomerleau AC and Amiel D: Characterization of
mature vs aged rabbit articular cartilage: Analysis of cell
density, apoptosis-related gene expression and mechanisms
controlling chondrocyte apoptosis. Osteoarthritis Cartilage.
12:917–923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Du M, Liu X, Chen W, Wu M, lin J and
Wu G: Millimeter wave treatment promotes chondrocyte proliferation
by upregulating the expression of cyclin-dependent kinase 2 and
cyclin A. Int J Mol Med. 26:77–84. 2010.PubMed/NCBI
|
|
27
|
Lin P, Weng X, Liu F, Ma Y, Chen H, Shao
X, Zheng W, Liu X, Ye H and Li X: Bushen Zhuangjin decoction
inhibits TM-induced chondrocytes apoptosis mediated by endoplasmic
reticulum stress. Int J Mol Med. 36:1519–1528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buckwalter JA and Mankin HJ: Articular
cartilage: Tissue design and chondrocyte-matrix interactons. Instr
Course Lect. 47:477–486. 1998.PubMed/NCBI
|
|
30
|
Tew SR, Kwan AP, Hann A, Thomson BM and
Archer CW: The reactions of articular cartilage to experimental
wounding: Role of apoptosis. Arthritis Rheum. 43:215–225. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hwang HS and Kim HA: Chondrocyte apoptosis
in the pathogenesis of osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gu YT, Chen J, Meng ZL, Ge WY, Bian YY,
Cheng SW, Xing CK, Yao JL, Fu J and Peng L: Research progress on
osteoarthritis treatment mechanisms. Biomed Pharmacother.
93:1246–1252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao Y, Li X, Li N and Zhou J:
Electroacupuncture protects against articular cartilage erosion by
inhibiting mitogen-activated protein kinases in a rat model of
osteoarthritis. Acupunct Med. 34:290–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qin Y, He J, Xia L, Guo H and He C:
Effects of electro-acupuncture on oestrogen levels, body weight,
articular cartilage histology and MMP-13 expression in
ovariectomised rabbits. Acupunct Med. 31:214–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang JB, Sheng XP and Fan TY: Study on the
effect of electroacupunture on knee joint chondrocyte apoptosis in
rabits with knee osteoarthritis. J Tradit Chin Orthop Traumat.
24:12–15. 2012.(In Chinese).
|
|
36
|
Kapuscinski J: DAPI: A DNA-specific
fluorescent probe. Biotech Histochem. 70:220–233. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verhoven B, Schleqel RA and Williamson P:
Mechanisms of phosphatidylserine exposure, a phagocyte recognition
signal, on apoptotic T lymphocytes. J Exp Med. 182:1597–1601. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koopman G, Reutelinqsperger CP, Kuijten
GA, Keehnen RM, Pals ST and van Oers MH: Annexin V for flow
cytometric detection of phosphatidylserine expression on B cells
undergoing apoptosis. Blood. 84:1415–1420. 1994.PubMed/NCBI
|
|
39
|
Lecoeur H: Nuclear apoptosis detection by
flow cytometry: Influence of endogenous endonucleases. Exp Cell
Res. 277:1–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brüne B: Nitric oxide: NO apoptosis or
turning it ON? Cell Death Differ. 10:864–869. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reers M, Smiley ST, Mottola-Hartshorn C,
Chen A, Lin M and Chen LB: Mitochondrial membrane potential
monitored by JC-1 dye. Methods Enzymol. 260:406–417. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Robertson JD, Zhivotovsky B, Goqvadze V
and Orrenius S: Outer mitochondrial membrane permeabilization: An
open-and-shut case? Cell Death Differ. 10:485–487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Hard Perspect Biol.
5:a0087222013.
|
|
45
|
Westphal D, Kluck RM and Dewson G:
Building blocks of the apoptotic pore: How Bax and Bak are
activated and oligomerize during apoptosis. Cell Death Differ.
21:196–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vakifahmetoqlu-Norberq H, Ouchida AT and
Norberq E: The role of mitochondria in metabolism and cell death.
Biochem Biophys Res Commun. 482:426–431. 2017. View Article : Google Scholar : PubMed/NCBI
|