Introduction
Pancreatic cancer (PaC) is one of the most
aggressive and lethal types of tumor, and is the fourth leading
cause of cancer-associated mortality worldwide (1). Despite improvement in cancer
treatment, the prognosis for patients with PaC remains poor, with a
5-year survival rate of ~5% (2).
It has been predicted that, with its current rise in incidence, PaC
will become the second leading cause of cancer-associated mortality
by 2030 (3). The majority of
patients with PaC are not suitable for surgical resection due to
local advancement and metastasis, and the current chemotherapeutic
regimens for advanced PaC are limited (4). The high proclivity for partial
invasion and early progression to distant metastases leads to the
poor outcome in PaC (5), thus
resulting in the high mortality rate of this malignancy.
Furthermore, the current biomarkers poorly predict prognosis of
patients with metastatic PaC (6).
Therefore, for the clinical treatment of this disease, the
selection and identification of valid and reliable biomarkers as
prognostic indicators for patients with PaC are important.
Circulating tumor cells (CTCs) are tumor cells that
induce metastasis, which is responsible for the majority of cases
of cancer-associated mortality (7). CTCs have been widely recognized as
tumor- or metastasis-derived cells, which move into the circulatory
system from the primary tumor site during surgical resection and
lead to disease recurrence in patients with cancer (8–11).
CTCs have already been widely used as a biomarker for the
assessment of cancer prognosis and response to therapy (12). Previous studies have reported that
CTC counts have an association with the prognosis and development
of numerous metastatic diseases, including breast, colon, prostate
and lung cancers (13–17). Therefore, studying CTCs may improve
understanding of the potential mechanisms underlying tumorigenesis
and metastasis, offer promising clinical trials for patients with
metastatic cancer, and supply a target for designing effective and
individualized cancer therapies.
Receptor tyrosine kinase-like orphan receptor 1
(ROR1) is an embryonic protein, and a member of the ROR
receptor tyrosine kinase family, which serves key roles in cell
differentiation and proliferation, angiogenesis, tumor migration
and metastasis (18–23). Numerous studies have indicated that
ROR1 is expressed at high levels in various blood and solid
malignancies; however, it is lowly expressed in normal adult
tissues (24–27). In this regard, ROR1 protein may be
an ideal drug target for cancer therapy; however, the underlying
functions of ROR1 in PaC have yet to be elucidated.
On the basis of these findings, the present study
explored the association between ROR1 and PaC, and revealed
that ROR1 was increased in PaC tissues compared with in
noncancerous tissues. In addition, the mRNA expression levels of
ROR1 were increased in CTCs compared with in peripheral
blood cells from patients with PaC. Furthermore, the proliferative
and invasive capacity was increased in CTCs with high levels of
ROR1 compared with in PANC-1 and SW-1990 cells. Furthermore, the
present study revealed that downregulating ROR1 expression
suppressed the invasive ability of CTCs. In addition, the results
demonstrated that the epithelial-mesenchymal transition (EMT)
process may participate in the metastasis of CTCs from primary PaC
tissue. Taken together, these results suggested that ROR1
may be a novel genetic modifier for the progression of PaC.
Materials and methods
PaC tissues and blood samples
A total of 25 (male; mean age, 52.6; age range 35–70
years) human PaC tissue and paired adjacent noncancerous pancreatic
tissue samples were acquired after written informed consent was
obtained from the participating patients. The patients did not
receive partial or systemic treatment prior to tissue sampling, and
agreed to receive these treatments at the First Affiliated Hospital
of Soochow University (Suzhou, China) between June 2006 and January
2017. Paired adjacent noncancerous pancreatic tissues (≤5 cm from
the tumor site) were obtained from patients during surgery. All
tissue specimens were immediately snap-frozen in liquid nitrogen
following surgery. In addition, peripheral blood specimens were
obtained from patients with PaC using vacutainer tubes containing
the anticoagulant EDTA. All peripheral blood samples were stored at
4°C and were processed within 3 days. The present study was
approved by the Ethics Review Committee of the First Affiliated
Hospital of Soochow University.
PaC cell culture
Human PaC cell lines PANC-1 and SW-1990, and the
normal human pancreatic cell line HPDE6-C7 were used in the present
study from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). All cells were seeded in Dulbecco's modified
Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with penicillin (100 U/ml),
streptomycin (100 µg/ml) and 10% fetal bovine serum (FBS) (Gibco;
Thermo Fisher Scientific, Inc.), and were cultured at 37°C in a
humidified incubator containing 5% CO2.
Isolation and culture of CTCs
Peripheral blood cells (PBCs) and CTCs were isolated
and cultured with Isolation of Peripheral Blood Mononuclear Cells
kit (Qiagen, Inc., Valencia, CA, USA) and the CellSearch
Circulating Tumor Cell kit (Menarini Silicon Biosystems, Inc.,
Bologna, Italy) according to the manufacturer's protocol,
respectively. The peripheral blood samples from patients with PaC
were pooled into 10 ml vacutainer tubes containing the
anticoagulant EDTA on ice. The 2-ml peripheral blood cells were
lysed in a conical tube containing 13 ml 1X red blood cell (RBC)
lysis buffer (Beijing Leagene Biotech Co., Ltd., Beijing, China)
for 10 min at room temperature, and were then centrifuged at 800 ×
g for 8 min at room temperature. Subsequently, the cells were
washed twice by resuspending the pellets in 6 ml 1X PBS and were
centrifuged at 800 × g for 3 min at room temperature. Finally, the
remaining cells were cultured in RPMI 1640 medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% FBS
and incubated in a cell incubator containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from PaC tissues or cells
and peripheral blood cells using a HP Total RNA kit (Omega Bio-Tek,
Inc., Norcross, GA, USA) and Blood RNA kit (Omega Bio-Tek, Inc.),
respectively, according to the manufacturer's protocols. The RNA
levels were analyzed using a NanoDrop spectrophotometer (NanoDrop;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA). RNA was
reverse transcribed into cDNA with an M-MLV First Strand kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The mRNA expression
levels of ROR1 were quantified using a Platinum SYBR Green
qPCR SuperMix-UDG kit (Invitrogen; Thermo Fisher Scientific, Inc.)
on an ABI Prism 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Sequences of the primers were as follows:
ROR1 (forward), 5′-AGATCACAGCTGCCTTCACTAT-3′; ROR1
(reverse), 5′-GACATTCTCCAGGATTTCACAT-3′; β-actin (forward),
5′-GGCGGCACCACCATGTACCCT-3′; β-actin (reverse),
5′-AGGGGCCGGACTCGTCATACT-3′. The thermocycling conditions were as
follows: 95°C for 10 min, followed by 40 cycles of 50°C for 20 sec
and 60°C for 1 min. Quantification cycle (Cq) values of ROR1
mRNA were equilibrated to the internal control β-actin mRNA.
Relative expression was calculated using the ΔΔCq method (28). All experiments were performed in
triplicate.
Western blot analysis
Protein lysates from cells and tissues were obtained
using radioimmunoprecipitation lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA), which contained protease and
phosphatase inhibitors (Sangon Biotech Co., Ltd., Shanghai, China).
Protein concentrations were measured using NanoDrop technology
(NanoDrop; Thermo Fisher Scientific, Inc.). Protein products (25
µg) were separated by 10% SDS-PAGE and were electrophoretically
transferred onto a nitrocellulose membrane (EMD Millipore,
Billerica, MA, USA). The membrane was then incubated with primary
antibodies at 4°C overnight after blocking with 1.5% bovine serum
albumin (Beyotime Institute of Biotechnology, Shanghai, China),
followed by incubation with secondary antibodies at room
temperature for 2 h. An Pierce™ ECL Western Blotting substrate
(Pierce; Thermo Fisher Scientific, Inc.) was used to detect protein
bands, which were analyzed using Quantity One 4.6 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin was used to
normalize target proteins. Antibodies employed in western blotting
were: Rabbit anti-ROR1 (1:200, cat. no. ab15148; Abcam, Cambridge,
UK), rabbit anti-E-cadherin (1:800, cat. no. ab15148; Abcam),
rabbit anti-N-cadherin (1:1,000, cat. no. ab18203; Abcam), rabbit
anti-β-actin (1:2,000, cat. no. ab8227; Abcam) and mouse
anti-rabbit secondary antibodies (1:2,000, cat. no. sc-2357; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA).
RNA interference
The small interfering RNA (siRNA) sequence that
directly targets human ROR1 and the scrambled sequence used
as a corresponding negative control (NC) were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). The target siRNA
sequence for ROR1 was (5′-3′): Sense,
UGAACCAAUGAAUAACAUCdTdT and antisense, GAUGUUAUUCAUUGGUUCAdTdT. The
NC sequence was (5′-3′): Sense, UUCUCCGAACGUGUCACGUTT and
antisense, ACGUGACACGUUCGGAGAATT. CTC cells were seeded into
24-well plates at a density of 5×104 cells/well. On the
following day, CTCs were transfected with a mixture containing 100
pmol siRNA and Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol,
incubated in a humidified atmosphere containing 95% air and 5%
CO2 at 37°C. The cells were harvested after 72 h and
Transwell assays were performed.
MTT assay
The proliferation of PANC-1 and SW-1990 cells, as
well as CTCs obtained from patient blood samples, was assessed
using MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
according to the manufacturer's protocol. A total of 3,000
cells/well were seeded in a 96-well plate and were incubated for 4
days in a humidified atmosphere containing 95% air and 5%
CO2 at 37°C. Subsequently, 10 µl MTT reagent was added
to each well and cultured at 37°C for 2 h in darkness. Dimethyl
sulfoxide was added to dissolve the crystals, and the absorbance
was measured at 570 nm every 24 h. Three independent experiments
were performed.
Transwell assay
Cell invasion assays were performed using Transwell
plates (BD Biosciences, Franklin Lakes, NJ, USA) Cell invasion
assays were performed in 24-well Transwell chambers containing
polycarbonate filters with 8 mm pores coated with Matrigel.
According to the manufacturer's protocol, 5×104 cells
were suspended in DMEM containing 1% FBS and were placed into the
top chamber, which contained a Matrigel-coated filter. DMEM
supplemented with 10% FBS was added to the bottom chamber to be
used as a chemoattractant. After 48 h at 37°C, the DMEM was
discarded and cells adhering to the upper side of the membrane were
removed with a cotton swab. Cells that had invaded onto the lower
side of the membrane were stained with 1% crystal violet for 30 min
at room temperature and observed by a light microscope
(magnification, ×100; Olympus Corporation, Tokyo, Japan). Invasive
cells were stained and counted in at least three microscopic fields
(magnification, ×100). The experiments were independently repeated
three times.
Statistical analysis
Differences between two groups were analyzed using
Student's t-test (two-tailed). Statistical analyses were conducted
using GraphPad Prism 5.02 (GraphPad Software, Inc., La Jolla, CA,
USA) and SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
Representative data from three independent experiments were
presented as the means ± standard deviation or means ± standard
error. P<0.05 was considered to indicate a statistically
significant difference.
Results
ROR1 expression is upregulated in PaC
tissues and CTCs
Numerous studies have indicated that ROR1 is
increased in several types of blood and solid malignancies
(18,27). To investigate whether the
expression levels of ROR1 were elevated in PaC, ROR1
expression was detected in 25 paired PaC tissues and adjacent
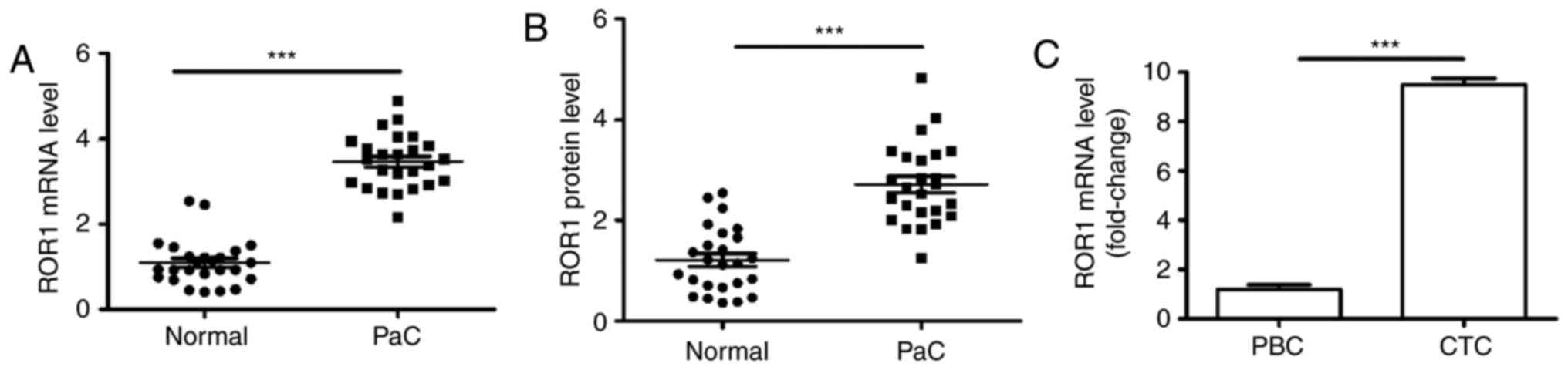
noncancerous tissues using RT-qPCR and western blotting. The mRNA
expression levels of ROR1 in PaC tissues were significantly
increased compared with in paired noncancerous pancreatic tissues
(Fig. 1A). Furthermore, the
protein expression levels of ROR1 were detected, and the results
demonstrated that PaC tissues exhibited significantly increased
ROR1 protein expression compared with in the paired noncancerous
pancreatic tissues (Fig. 1B).
CTCs, also known as metastasis-derived cells, move into the
circulatory system from the primary tumor site. Therefore, the
difference in ROR1 mRNA expression between CTCs and PaC
blood cells was investigated. The results indicated that the
abundance of ROR1 mRNA in CTCs was ~10-fold higher compared
with in peripheral blood cells obtained from patients with PaC
(Fig. 1C). These findings
indicated that ROR1 expression was increased in PaC,
particularly in CTCs.
Proliferative and invasive ability of
CTCs is stronger than PANC-1 and SW-1990 cells
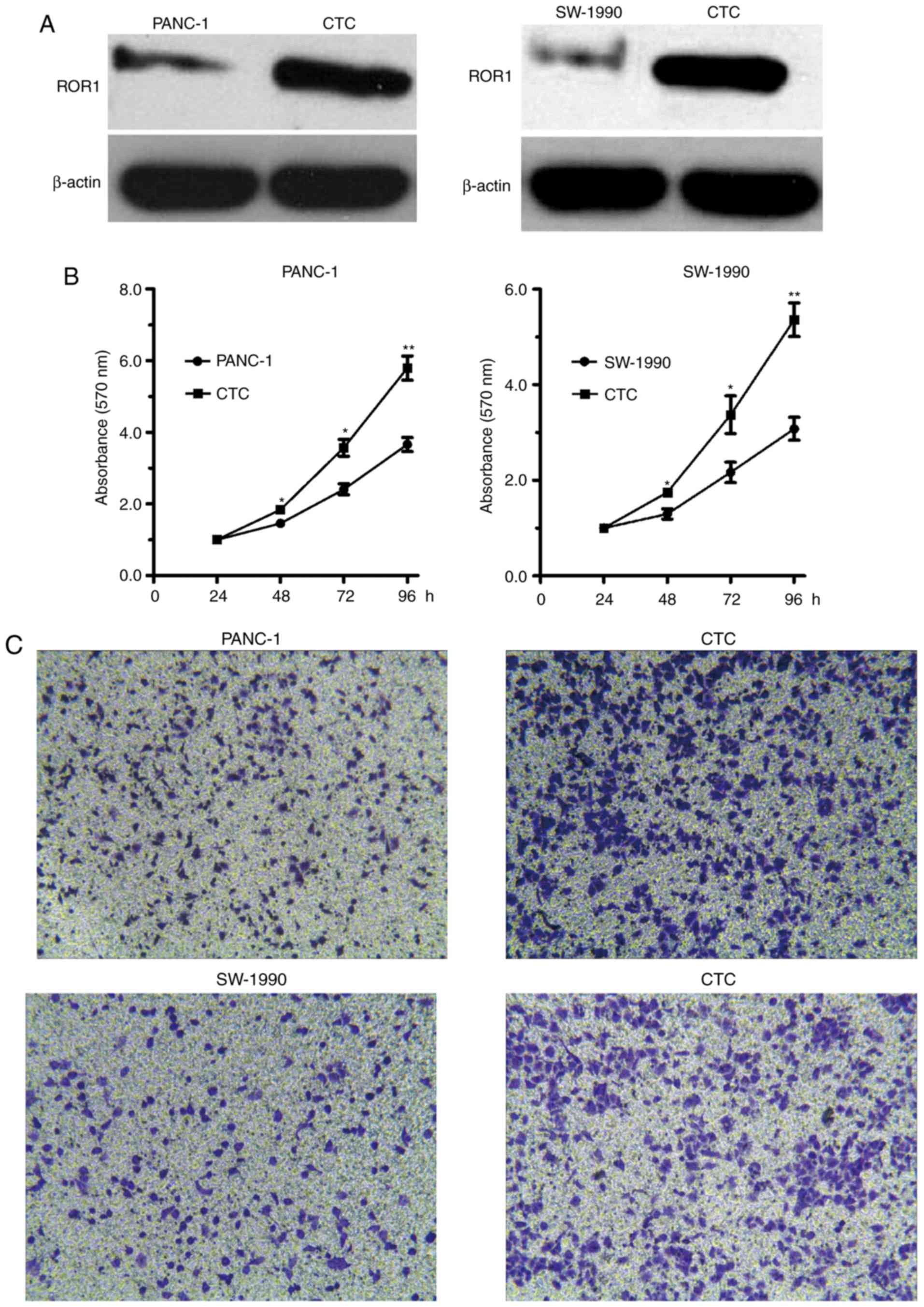
Increased ROR1 expression serves key roles in cell
proliferation and invasion (18,27).
To investigate the role of ROR1 in PaC cells, and in CTC
growth and invasion, MTT and Transwell assays were performed.
Initially, the expression levels of ROR1 were detected in CTCs and
PANC-1 and SW-1990 cells by western blotting. As illustrated in
Fig. 2A, ROR1 expression was
markedly increased in CTCs compared with in PANC-1 and SW-1990
cells. Furthermore, compared with in the PANC-1 and SW-1990 cells,
CTCs with high ROR1 expression exhibited significantly higher cell
viability (Fig. 2B). Consistent
with the MTT assay results, CTCs with high ROR1 expression
displayed enhanced cell invasive ability compared with PANC-1 and
SW-1990 cells (Fig. 2C). Taken
together, these results suggested that ROR1 may serve key
roles in the ability of CTCs to mediate PaC growth and
invasion.
Knockdown of ROR1 suppresses CTC
invasion via regulation of the EMT process
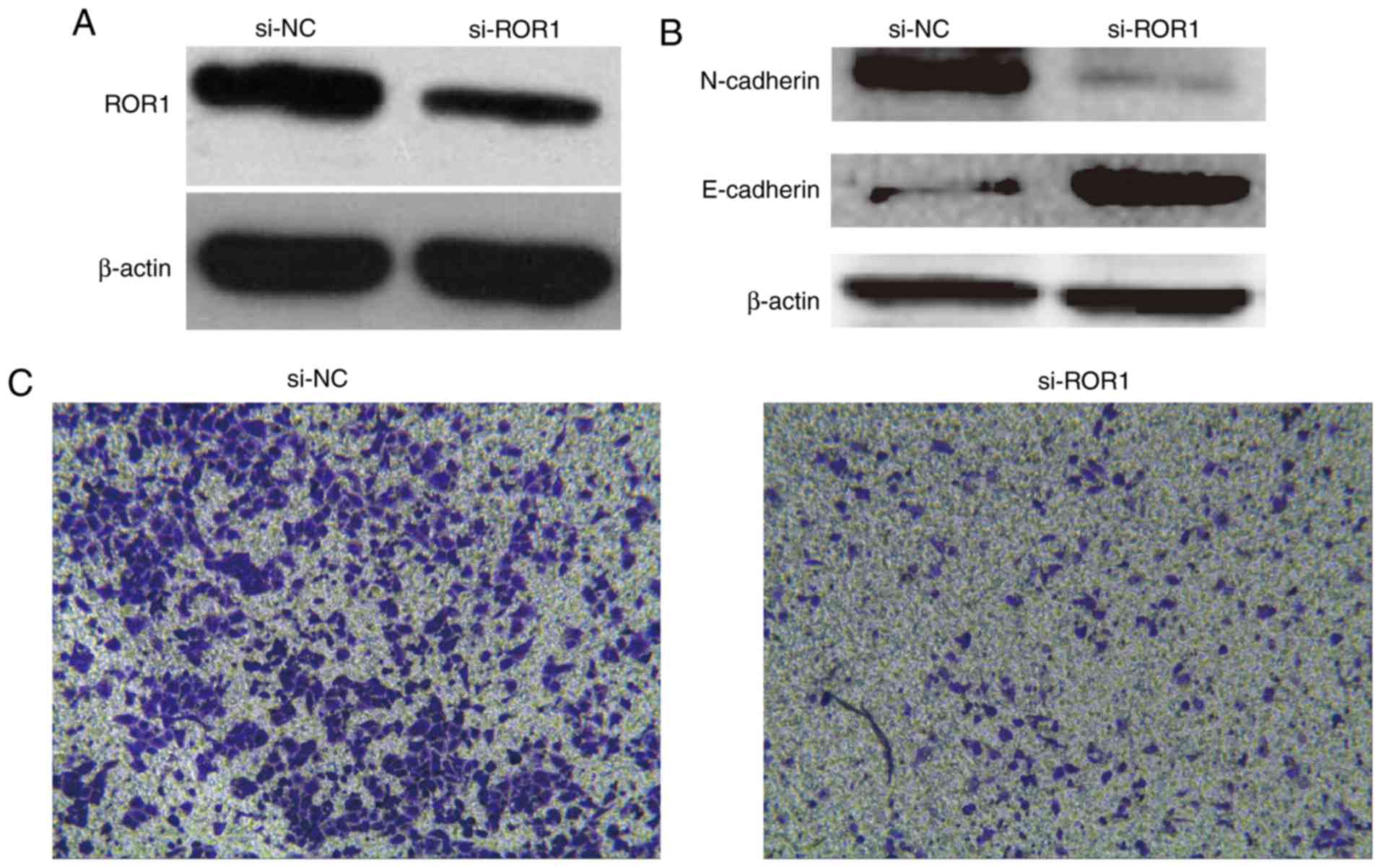
In order to further demonstrate the role of
ROR1 in PaC metastasis, ROR1 was knocked down in CTCs
by siRNA (Fig. 3). Western blot
analysis demonstrated that ROR1 expression in ROR1
siRNA-transfected CTCs was markedly reduced compared with in NC
siRNA-transfected CTCs (Fig. 3A).
Subsequently, a Transwell assay was performed to determine the
invasive ability of ROR1 siRNA-transfected CTCs and NC
siRNA-transfected CTCs cells. Knockdown of ROR1 expression
markedly prevented CTC invasion compared with in the control cells
(Fig. 3C).
The present study explored the mechanism by which
ROR1 regulates cell invasion. EMT serves a key role in tumor
cell invasion and metastasis (29). To investigate whether the typical
molecular markers of EMT were altered, the protein expression
levels of E-cadherin and N-cadherin were examined by western
blotting. ROR1 knockdown by siRNA increased E-cadherin
protein expression, but decreased N-cadherin protein expression in
CTCs (Fig. 3B). Taken together,
these results indicated that knockdown of ROR1 in CTCs
markedly decreased the invasive ability by regulating the EMT
process.
Discussion
In the present study, ROR1 expression was
examined in PaC tissues and CTCs, and the roles of ROR1 were
detected in proliferation and invasion of PaC cells and CTCs. In
PaC samples, ROR1 was upregulated compared with in the paired
noncancerous pancreatic tissues. Notably, the mRNA expression
levels of ROR1 were increased in CTCs compared with in
peripheral blood cells from patients with PaC. It was also
demonstrated that ROR1 levels were markedly increased in CTCs
compared with in PANC-1 and SW-1990 cells. The CTCs, which were
obtained from PaC blood samples and contained higher ROR1 levels,
possessed stronger proliferative and invasive capabilities compared
with the PaC cells, and knockdown of ROR1 by siRNA reduced
the invasive ability of CTCs. In addition, E-cadherin expression
was increased and N-cadherin expression was decreased when ROR1 was
knocked down in CTCs. Therefore, the EMT process may participate in
the metastasis of CTCs from primary PaC tissue. These findings,
combined with those of previous studies (24–27),
indicated that ROR1 may be a novel tool for the treatment of
patients with PaC.
CTCs serve a crucial role in cancer development and
metastasis. For example, Chang et al (30), reported that tumor marker detection
could complement CTC enumeration in predicting progression in
patients with metastatic castration-resistant prostate cancer. The
basic biological mechanisms of CTCs in facilitating cancer
development and metastasis have attracted much attention (31,32).
It is of great importance that highly specific molecular markers
for CTC-targeted cancer therapy are identified. An increasing
number of studies have reported on the expression and function of
ROR1 (24–27); however, its regulatory role in CTCs
from PaC remains largely unknown.
ROR1 is critically involved in the
development and progression of various human cancers. In addition,
ROR1 has been reported to act as a promoter of stem cell
tumorigenicity in ovarian cancer, which could be used as an
indirect antagonist when its expression is decreased (33–35).
In the present study, CTCs exhibited a higher proliferative and
invasive potential compared with PANC-1 and SW-1990 cells, thus
suggesting that ROR1 may promote the tumorigenic process in
PaC cells. In support of this, Cui et al (24), reported that ROR1 stimulates
leukemia-cell activation and enhances disease progression in
patients. In addition, Gentile et al (23), reported that ROR1 serves a
key role in the malignant phenotypes maintained by the MET
proto-oncogene, receptor tyrosine kinase.
In recent years, numerous methods have been applied
to detect and quantify CTCs. Furthermore, the number and phenotype
of CTCs, which have many similar biological characteristics, can
provide information on patient prognosis and treatment efficacy
(36–39). The present study demonstrated that
the expression of ROR1 was upregulated in PaC tissues.
Notably, the abundance of ROR1 mRNA in CTCs from patients
with PaC was ~10-fold higher compared with its abundance in
peripheral blood cells. These findings are in accordance with
previous studies, which demonstrated that ROR1 levels are
increased in PaC tissues compared with in corresponding normal
tissues (20,22–24).
However, there are some contradictory findings with regards to
ROR1 expression in other types of cancer. For example,
Balakrishnan et al (40),
reported that ROR1 is overexpressed in normal tissues
compared with cancer tissues, including parathyroid tissues,
pancreatic islets, and regions of the esophagus, stomach and
duodenum, with resultant toxicity. These results could be explained
by the finding that alterations in metabolic gene expression
induced by cancer are heterogeneous in different tumor types
(41). PaC is usually treated with
interventional therapy, chemotherapy and radiotherapy; however, the
efficacy is poor. More researchers have begun to regard molecular
targeted therapy as a study focus for the acquisition of an
in-depth understanding of the molecular mechanism underlying PaC.
The present results indicated that ROR1 could be knocked
down by siRNA, which resulted in prevention of the invasion of CTCs
in PaC. A previous study suggested that the number of CTCs in the
peripheral blood of patients with several types of metastatic
carcinoma is positively correlated with poor clinical prognosis
(42). The present results
suggested that ROR1 may exhibit potential for use as a
screening tool to optimize the outcome of patients with PaC, and to
aid the decision of what population should be screened given the
relatively low incidence, but poor survival associated with PaC.
Suppressing ROR1 may be a potential therapeutic approach in
PaC.
In recent years, EMT, which serves a key role not
only in embryonic development but also in tumor cell invasion and
metastasis, has become a focus of attention (28). In the process of EMT, cell-cell
junctions, polarity and epithelial cell markers are lost, which is
accompanied by the gain of mesenchymal markers, and a motile and
invasive phenotype, which may initiate tumor metastasis (43). Wang et al demonstrated that
microRNA (miR)-30a inhibits EMT and metastasis by targeting
ROR1, and that the downregulation of ROR1 by the
overexpression of miR-30a could elevate E-cadherin levels and
reduce N-cadherin expression in breast cancer (44). The present study revealed that when
ROR1 was lowly expressed, the mesenchymal marker N-cadherin was
also expressed at low levels, whereas the epithelial marker
E-cadherin was highly expressed. These findings are consistent with
those of previous studies and further indicated that ROR1
may facilitate PaC metastasis by modulating the EMT process.
In conclusion, the present findings demonstrated
that ROR1 may be associated with the metastasis of PaC. This
study furthers the understanding of the biological regulation of
CTCs and suggests a novel rationale and therapeutic strategy for
the diagnosis and treatment of ROR1-positive CTCs. However,
the sample size was relatively small; therefore, there is a need to
further validate these results in a larger sample size. In
addition, the molecular mechanisms underlying the effects of ROR1
on PaC metastasis requires further research.
Acknowledgements
Not applicable.
Funding
The present study was funded by Healthy Life Science
and Technology Projects in Jiangsu Province (grant no.
BL201204).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
GLX and JS wrote the main manuscript. GLX, JS, WSW
and YHX performed the experiments. GLX, WSW and CFN designed the
study. WSW and CFN analyzed the data. All authors reviewed and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of the First Affiliated Hospital of Soochow University.
Written informed consent was obtained from the participating
patients.
Patient consent for publication
Informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Puleo FR, Maréchal R, Demetter P, Bali MA,
Calomme A, Closset J, Bachet JB, Deviere J and Van Laethem JL: New
challenges in perioperative management of pancreatic cancer. World
J Gastroenterol. 21:2281–2293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ying H, Dey P, Yao W, Kimmelman AC,
Draetta GF, Maitra A and DePinho RA: Genetics and biology of
pancreatic ductal adenocarcinoma. Genes Dev. 30:355–385. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moyer MT and Gaffney RR: Pancreatic
adenocarcinoma. N Engl J Med. 371:21402014.PubMed/NCBI
|
|
5
|
Huang S, Zheng J, Huang Y, Song L, Yin Y,
Ou D, He S, Chen X and Ouyang X: Impact of S100A4 expression on
clinicopathological characteristics and prognosis in pancreatic
cancer: A Meta-analysis. Dis Markers. 2016:81373782016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ni S, Wang H, Zhu X, Wan C, Xu J, Lu C,
Xiao L, He J, Jiang C, Wang W and He Z: CBX7 suppresses cell
proliferation, migration, and invasion through the inhibition of
PTEN/Akt signaling in pancreatic cancer. Oncotarget. 8:8010–8021.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen XX and Bai F: Single-cell analyses of
circulating tumor cells. Cancer Biol Med. 12:184–192.
2015.PubMed/NCBI
|
|
8
|
Hardingham JE, Grover P, Winter M, Hewett
PJ, Price TJ and Thierry B: Detection and clinical significance of
circulating tumor cells in colorectal cancer-20 years of progress.
Mol Med. 21 Suppl 1:S25–S31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okegawa T, Itaya N, Hara H, Tambo M and
Nutahara K: Epidermal growth factor receptor status in circulating
tumor cells as a predictive biomarker of sensitivity in
castration-resistant prostate cancer patients treated with
docetaxel chemotherapy. Int J Mol Sci. 17:pii: E2008. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mostert B, Sieuwerts AM, Kraan J, Bolt-de
Vries J, van der Spoel P, van Galen A, Peeters DJ, Dirix LY,
Seynaeve CM, Jager A, et al: Gene expression profiles in
circulating tumor cells to predict prognosis in metastatic breast
cancer patients. Ann Oncol. 26:510–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mego M, Giordano A, De Giorgi U, Masuda H,
Hsu L, Giuliano M, Fouad TM, Dawood S, Ueno NT, Valero V, et al:
Circulating tumor cells in newly diagnosed inflammatory breast
cancer. Breast Cancer Res. 17:22015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mego M, Gao H, Cohen EN, Anfossi S,
Giordano A, Tin S, Fouad TM, De Giorgi U, Giuliano M, Woodward WA,
et al: Circulating tumor cells (CTCs) are associated with
abnormalities in peripheral blood dendritic cells in patients with
inflammatory breast cancer. Oncotarget. 8:35656–35668. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao W, Yuan H, Jing F, Wu S, Zhou H, Mao
H, Jin Q, Zhao J, Cong H and Jia C: Analysis of circulating tumor
cells from lung cancer patients with multiple biomarkers using
high-performance size-based microfluidic chip. Oncotarget.
8:12917–12928. 2017.PubMed/NCBI
|
|
14
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chikaishi Y, Yoneda K, Ohnaga T and Tanaka
F: EpCAM-independent capture of circulating tumor cells with a
‘universal CTC-chip’. Oncol Rep. 37:77–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joosse SA, Gorges TM and Pantel K:
Biology, detection, and clinical implications of circulating tumor
cells. EMBO Mol Med. 7:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aggarwal C, Meropol NJ, Punt CJ, Iannotti
N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell
E, et al: Relationship among circulating tumor cells, CEA and
overall survival in patients with metastatic colorectal cancer. Ann
Oncol. 24:420–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Yang H, Chen T, Luo Y, Xu Z, Li Y
and Yang J: Silencing of receptor tyrosine kinase ROR1 inhibits
tumor-cell proliferation via PI3K/AKT/mTOR signaling pathway in
lung adenocarcinoma. PLoS One. 10:e01270922015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi T, Yanagisawa K, Sugiyama R,
Hosono Y, Shimada Y, Arima C, Kato S, Tomida S, Suzuki M, Osada H
and Takahashi T: NKX2-1/TITF1/TTF-1-induced ROR1 is required to
sustain EGFR survival signaling in lung adenocarcinoma. Cancer
Cell. 21:348–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Green JL, Kuntz SG and Sternberg PW: Ror
receptor tyrosine kinases: Orphans no more. Trends Cell Biol.
18:536–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui B, Zhang S, Chen L, Yu J, Widhopf GF
II, Fecteau JF, Rassenti LZ and Kipps TJ: Targeting ROR1 inhibits
epithelial-mesenchymal transition and metastasis. Cancer Res.
73:3649–3660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukuda T, Chen L, Endo T, Tang L, Lu D,
Castro JE, Widhopf GF II, Rassenti LZ, Cantwell MJ, Prussak CE, et
al: Antisera induced by infusions of autologous Ad-CD154-leukemia B
cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a.
Proc Natl Acad Sci USA. 105:3047–3052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gentile A, Lazzari L, Benvenuti S,
Trusolino L and Comoglio PM: Ror1 is a pseudokinase that is crucial
for Met-driven tumorigenesis. Cancer Res. 71:3132–3141. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui B, Ghia EM, Chen L, Rassenti LZ,
DeBoever C, Widhopf GF II, Yu J, Neuberg DS, Wierda WG, Rai KR, et
al: High-level ROR1 associates with accelerated disease progression
in chronic lymphocytic leukemia. Blood. 128:2931–2940. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henry C, Llamosas E, Knipprath-Meszaros A,
Schoetzau A, Obermann E, Fuenfschilling M, Caduff R, Fink D, Hacker
N, Ward R, et al: Targeting the ROR1 and ROR2 receptors in
epithelial ovarian cancer inhibits cell migration and invasion.
Oncotarget. 6:40310–40326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borcherding N, Kusner D, Liu GH and Zhang
W: ROR1, an embryonic protein with an emerging role in cancer
biology. Protein Cell. 5:496–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henry CE, Llamosas E, Djordjevic A, Hacker
NF and Ford CE: Migration and invasion is inhibited by silencing
ROR1 and ROR2 in chemoresistant ovarian cancer. Oncogenesis.
5:e2262016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gal A, Sjöblom T, Fedorova L, Imreh S,
Beug H and Moustakas A: Sustained TGF beta exposure suppresses Smad
and non-Smad signalling in mammary epithelial cells, leading to EMT
and inhibition of growth arrest and apoptosis. Oncogene.
27:1218–1230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang K, Kong YY, Dai B, Ye DW, Qu YY,
Wang Y, Jia ZW and Li GX: Combination of circulating tumor cell
enumeration and tumor marker detection in predicting prognosis and
treatment effect in metastatic castration-resistant prostate
cancer. Oncotarget. 6:41825–41836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pantel K and Alix-Panabieres C: Functional
studies on viable circulating tumor cells. Clin Chem. 62:328–334.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamilton G and Rath B: Detection of
circulating tumor cells in non-small cell lung cancer. J Thorac
Dis. 8:1024–1028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Cui B, Lai H, Liu G, Ghia EM,
Widhopf GF II, Zhang Z, Wu CC, Chen L, Wu R, et al: Ovarian cancer
stem cells express ROR1, which can be targeted for
anti-cancer-stem-cell therapy. Proc Natl Acad Sci USA.
111:17266–17271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reinholz MM, Kitzmann KA, Tenner K,
Hillman D, Dueck AC, Hobday TJ, Northfelt DW, Moreno-Aspitia A, Roy
V, LaPlant B, et al: Cytokeratin-19 and mammaglobin gene expression
in circulating tumor cells from metastatic breast cancer patients
enrolled in North Central Cancer Treatment Group trials,
N0234/336/436/437. Clin Cancer Res. 17:7183–7193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hojjat-Farsangi M, Moshfegh A,
Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A and Mellstedt
H: The receptor tyrosine kinase ROR1-an oncofetal antigen for
targeted cancer therapy. Semin Cancer Biol. 29:21–31. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Farace F, Massard C, Vimond N, Drusch F,
Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L,
Planchard D, et al: A direct comparison of CellSearch and ISET for
circulating tumour-cell detection in patients with metastatic
carcinomas. Br J Cancer. 105:847–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van de Stolpe A, Pantel K, Sleijfer S,
Terstappen LW and den Toonder JM: Circulating tumor cell isolation
and diagnostics: Toward routine clinical use. Cancer Res.
71:5955–5960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Danila DC, Anand A, Sung CC, Heller G,
Leversha MA, Cao L, Lilja H, Molina A, Sawyers CL, Fleisher M and
Scher HI: TMPRSS2-ERG status in circulating tumor cells as a
predictive biomarker of sensitivity in castration-resistant
prostate cancer patients treated with abiraterone acetate. Eur
Urol. 60:897–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barriere G, Fici P, Gallerani G, Fabbri F,
Zoli W and Rigaud M: Circulating tumor cells and epithelial,
mesenchymal and stemness markers: Characterization of cell
subpopulations. Ann Transl Med. 2:1092014.PubMed/NCBI
|
|
40
|
Balakrishnan A, Goodpaster T,
Randolph-Habecker J, Hoffstrom BG, Jalikis FG, Koch LK, Berger C,
Kosasih PL, Rajan A, Sommermeyer D, et al: Analysis of ROR1 protein
expression in human cancer and normal tissues. Clin Cancer Res.
23:3061–3071. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu J, Locasale JW, Bielas JH, O'Sullivan
J, Sheahan K, Cantley LC, Vander Heiden MG and Vitkup D:
Heterogeneity of tumor-induced gene expression changes in the human
metabolic network. Nat Biotechnol. 31:522–529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Qiu H, Tang R, Song H, Pan H, Feng
Z and Chen L: miR-30a inhibits epithelialmesenchymal transition and
metastasis in triple-negative breast cancer by targeting ROR1.
Oncol Rep. 39:2635–2643. 2018.PubMed/NCBI
|