Introduction
Lung cancer is among the most prevalent types of
cancer worldwide and represents a serious threat to human health
(1). The most common subtype of
lung cancer is non-small cell lung cancer (NSCLC), which comprises
~85% of all lung cancer cases (2).
Due to a lack of early screening and diagnosis based on specific
biological markers, and the unapparent clinical symptoms of early
lung cancer, the majority of lung cancer cases are diagnosed at a
late stage (3). The 5-year
survival rate for lung cancer is <10% (4). Therefore, it is important to identify
novel biomarkers to provide more accurate diagnosis and
individualized treatment regimens for patients with lung
cancer.
MicroRNAs (miRNA/miRs) are non-coding
single-stranded RNAs of ~19–22 nucleotides in length. Numerous
studies have demonstrated that miRNAs are involved in the processes
of tumorigenesis and tumor development, and serve similar oncogenic
roles in cell colonization, apoptosis, migration and other
biological processes (5–9). Recent studies have indicated that a
number of miRNAs, including miR-126 (10,11)
and miR-16-1 (12), are involved
in the initiation and progression of NSCLC through the regulation
of target genes. miR-939 promotes the proliferation of human
ovarian cancer cells by repressing adenomatous polyposis coli
like-2 expression (13). Decreased
expression of miR-939 contributes to the chemoresistance and
metastasis of gastric cancer via dysregulation of sodium-dependent
phosphate transport protein 2B and the RAF proto-oncogene
serine/threonine-protein kinase/dual specificity mitogen-activated
protein kinase kinase/extracellular signal-regulated kinase
pathway. mir-939 is also involved in the regulation of breast
cancer. Notably, when up taken in exosome-releasing triple negative
breast cancer cells, researchers demonstrated that inhibiting
miR-939 expression caused downregulation of vascular endothelial
cadherin expression (14). Using a
number of bioinformatics software packages and platforms, Ma et
al (15) reported that miR-939
was the most connected miRNA that regulated a large number of
genes, and was involved in lung cancer and the nervous system via
functional annotation analysis.
The aim of the present study was to observe the
function of miR-939 in NSCLC and its mechanism of action, in order
to provide data to potentially aid in the early diagnosis and
clinical treatment of NSCLC. Additionally, the present study aimed
to provide a theoretical basis for the development of novel drugs
against genes associated with NSCLC.
Materials and methods
Clinical samples
NSCLC samples and the adjacent tissues were obtained
from 60 patients (27 males/33 females; age range, 40–70 years) who
underwent surgery at the First Affiliated Hospital of Nanjing
Medical University (Nanjing, China) from March 2014 to July 2016.
Patients who had received radiotherapy and/or chemotherapy were
excluded. The specimens were immediately frozen in liquid nitrogen
and stored at −80°C until further analysis. Approval to conduct
human experiments was obtained from the Ethical Committee at the
First Affiliated Hospital of Nanjing Medical University and all
patients enrolled in the study signed consent forms. All clinical
procedures were conducted in accordance with the guidelines of the
Ethical Committee of the First Affiliated Hospital of Nanjing
Medical University.
Cell culture
H1299, SPCA1, A549, H358 and H1650 cells were
purchased from the Chinese Academy of Sciences (Shanghai, China).
The 16HBE human normal lung cell line was obtained from our
laboratory. All cell lines were maintained in Dulbecco's modified
Eagle's medium or RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2
atmosphere.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of both tissues and cell lines were
extracted from the cell lines and clinical samples with a TRIzol
kit (Thermo Fisher Scientific, Inc.), used according to the
manufacturer's protocol. RNA quality was confirmed with a NanoDrop
300 spectrophotometer (Thermo Fisher Scientific, Inc.). RT was
performed using a SuperScript II first-strand cDNA synthesis kit
(Thermo Fisher Scientific, Inc.). RT was performed at 42°C for 40
min, followed by 70°C for 15 min. The following PCR cycling
conditions were used: 95°C for 5 min; followed by 45 cycles of 95°C
for 45 sec; 60°C for 60 sec; 72°C for 45 sec. PCR for the detection
of miR-939 was performed with an ABI Prism 7900 detection system
(Thermo Fisher Scientific, Inc.) using a TaqMan MicroRNA Assay
(Thermo Fisher Scientific, Inc.). The primer sequences were as
follows: miR-939 forward, TGGGGAGCTGAGGCTCTG and reverse,
AGTGCAGGGTCCGAGGTATT; miR-939 RT Primer:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACCCC; GAPDH forward,
AACTTTGGCATTGTGGAAGG and reverse, CACATTGGGGGTAGGAACAC. miR-939
expression was normalized to the expression level of GAPDH.
Relative miR-939 expression was analyzed by the 2−ΔΔCq
method (16).
Transfection
Human miR-939 inhibitor and inhibitor negative
control (inhibitor NC) (miR-939 mimics sense,
UGGGGAGCUGAGGCUCUGGGGGUG and mimics antisense,
CCCCCAGAGCCUCAGCUCCCCAUU; mimics NC sense, UUCUCCGAACGUGUCACGUTT
and mimics NC antisense, ACGUGACACGUUCGGAGAATT; inhibitor,
CACCCCCAGAGCCUCAGCUCCCCA and inhibitor NC, CAGUACUUUUGUGUAGUACAA)
oligonucleotides were synthesized by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The NSCLC cell lines, H1299 and SPCA1, were
transfected with the miR-939 inhibitor and NC using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) at
a final concentration of 100 nM. Between 2 and 5 days following
transfection, NSCLC cells were harvested and RT-qPCR was performed
to determine transfection efficiency.
Proliferation experiment
Cell Counting Kit-8 (CCK-8) experiments were used to
detect cell proliferative ability. According to the kit
instructions, CCK-8 reagent (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to 4×103 transfected H1299 or
SPCA1 cells/well in a 96-well plate, which were incubated at 37°C
for 2 h. The optical density of the wells was evaluated at 450 nm
with a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). According to the manufacturer's protocol, a
5-ethynyl-29-deoxyuridine (EdU) assay (Guangzhou RiboBio Co, Ltd.,
Guangzhou, China) was additionally performed to detect cell
proliferation. In brief, 24 h after transfection, H1299 or SPCA1
cells were incubated with EdU (50 µM) for 2 h at 37°C. Apollo
staining (Guangzhou RiboBio Co, Ltd., Guangzhou, China; 400 µl for
30 min at room temperature) and DAPI (Guangzhou RiboBio Co, Ltd.)
staining (400 µl for 30 min at room temperature) were performed and
the EdU positive cells were evaluated with a fluorescence
microscope (×200; Nikon Corporation, Tokyo, Japan). The EdU
incorporation rate was calculated as the ratio of EdU-positive
cells, to the total number of DAPI-positive cells (blue).
Transwell assay
Cell invasion was assessed using a 24-well, 8-mm
pore size (EMD Millipore, Billerica, MA, USA) and BioCoat Matrigel
(BD Biosciences, San Jose, CA, USA). Briefly, transfected H1299 and
SPCA1 cells (1×105 cells/well) were plated in the upper
chamber in DMEM with 1% FBS. The lower chamber was filled with DMEM
with 10% FBS as a chemoattractant. At 24 h following incubation at
37°C in a 5% CO2 atmosphere, the upper chamber was
removed. Cells that had invaded into the lower chamber were fixed
with 70% ethanol at 4°C for 15 min and stained with hematoxylin at
room temperature for 15 min. In each well, cell numbers
(5×104) in five random rectangular fields were counted
under a light microscope (×200), and the average value was used to
express the invasive ability of the cells, and then normalized to
the cell numbers in control wells. Images were obtained using
NIS-Elements Viewer software through a Nikon microscope (Nikon
Corporation, Tokyo, Japan).
Western blot analysis
Total protein of transfected H1299 and SPCA1 were
extracted and quantified using a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology, Haimen, China). Cells
were lysed in ice-cold radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology) with 10 nM
phenylmethylsulfonyl fluoride for 30 min at 4°C and collected to
extract total protein. The proteins (10 µl) were separated by 10%
SDS-PAGE and transferred to polyvinylidene difluoride membranes.
The membranes were blocked with 5% fat-free milk at room
temperature for 1 h, incubated with mouse monoclonal anti-tissue
inhibitor of metalloproteinases 2 (TIMP2; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-21735) and
anti-GAPDH antibodies (1:1,000; Santa Cruz Biotechnology, Inc.;
cat. no. sc-32233). Mouse IgGk binding protein horseradish
peroxidase-conjugated secondary antibody (1:1,000; Santa Cruz
Biotechnology, Inc.; cat. no. sc-516102) was added for 2 h at room
temperature. Bands were visualized with the Pierce ECL Western
Blotting substrate (Thermo Fisher Scientific, Inc.) and exposed in
a Molecular Imager ChemiDoc XRS system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). According to the manufacturer's protocol, the
analysis was performed using Image Lab (version 5.2.1; Bio-Rad
Laboratories, Inc.) and GAPDH was used as the normalization.
Luciferase reporter assay
Using the prediction programs miRDB (mirdb.org) and TargetScan Human (www.targetscan.org), potential targets of miR-939 were
predicted. The binding sites or mutant (mut) sequence of the TIMP2
3′-untranslated region (UTR) was inserted into the KpnI and
SacI sites of the pGL3 promoter vector (GenScript,
Piscataway, NJ, USA). H1299 cells (5×105 cells/well)
were plated onto 6-well plates and were transfected with 100 ng
pGL3-TIMP2 or pGL3-TIMP2-mut and miR-939 mimics (50 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The 3′-UTR of TIMP2 was determined to contain a
binding site for miR-939. After 24 h, the luciferase activity of
the constructs was evaluated according to the ratio of firefly
fluorescence to Renilla fluorescence using a Luciferase
Reporter Assay system (Promega Corporation, Madison, WI, USA).
Statistical analysis
Statistical analysis was performed with Stata
software (version 11.0; StataCorp LP, College Station, TX, USA).
The data were presented as mean ± standard deviation of three
independent experiments. The Student's t-test, or two-way analysis
of variance followed by Fisher's Least Significant Difference test
were used to analyze statistical differences. Additionally, the
analysis of statistical differences in the clinical factors of
patients with NSCLC was conducted using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-939 is upregulated in NSCLC
tissues
To investigate the underlying function of miR-939
expression in NSCLC progression, clinical specimens were collected
from patients with NSCLC, and miR-939 expression was assessed by
comparing cancer specimens with adjacent normal lung tissues.
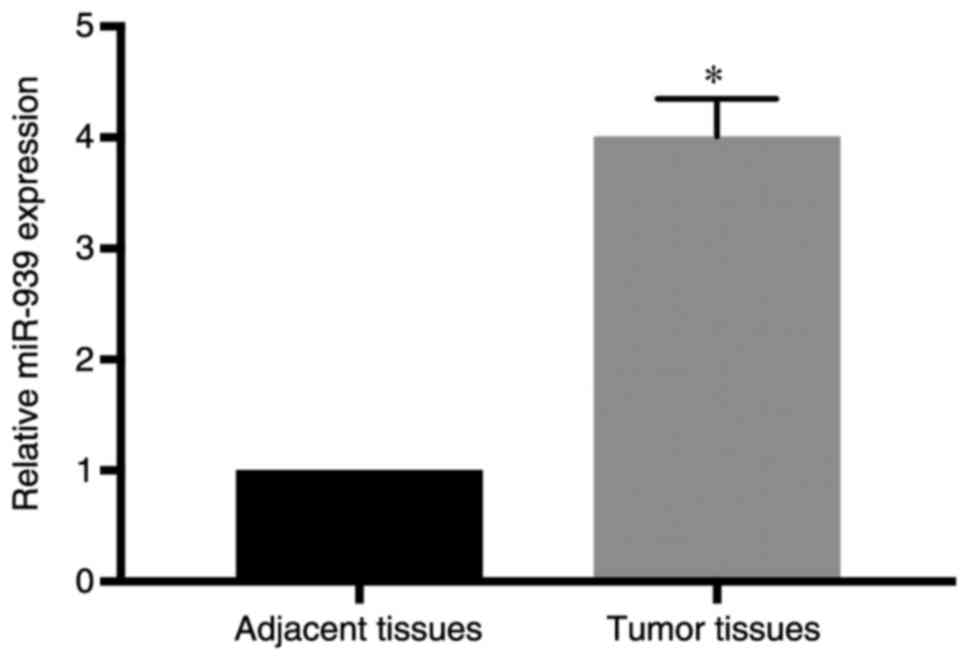
RT-qPCR analysis demonstrated that miR-939 was expressed at a
higher level in the cancer specimens compared with its expression
in the adjacent normal tissues (P<0.05; Fig. 1). In addition, in the analysis of
the clinical characteristics of the patients with NSCLC (Table I), it was observed that increased
miR-939 expression was significantly associated with higher tumor
stage (stages III–IV; P=0.008), increased tumor size (>3 cm;
P=0.015) and lymphatic metastasis (P=0.001). However, its abnormal
expression was not associated with patient age, sex or smoking
history. These findings indicated that miR-939 may be involved in
the development of NSCLC, and thus subsequent experiments aimed to
clarify its potential function.
 | Table I.Association between miR-939 expression
and the clinicopathological characteristics of patients with
non-small cell lung cancer. |
Table I.
Association between miR-939 expression
and the clinicopathological characteristics of patients with
non-small cell lung cancer.
|
|
| Expression of
miR-939, n |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Patients, n | High | Low | P-value |
|---|
| Total | 60 | 30 | 30 |
|
| Age, years |
|
|
| 0.793 |
| ≤50 | 25 | 12 | 13 |
|
|
>50 | 35 | 18 | 17 |
|
| Sex |
|
|
| 0.436 |
| Male | 27 | 12 | 15 |
|
|
Female | 33 | 18 | 15 |
|
| Smoke |
|
|
| 0.796 |
|
Yes | 31 | 15 | 16 |
|
| No | 29 | 15 | 14 |
|
| TNM stage |
|
|
| 0.008a |
|
I–II | 36 | 13 | 23 |
|
|
III–IV | 24 | 17 | 7 |
|
| Tumor size |
|
|
| 0.015a |
| <3
cm | 39 | 15 | 24 |
|
| >3
cm | 21 | 15 | 6 |
|
| Lymphatic
metastasis |
|
|
| 0.001a |
| No | 38 | 13 | 25 |
|
|
Yes | 22 | 17 | 5 |
|
miR-939 expression is increased in
NSCLC cell lines
The present study focused on investigating the
potential function of miR-939 in vitro. miR-939 expression
was detected in NSCLC cell lines (H1299, SPCA1, A549, H358 and
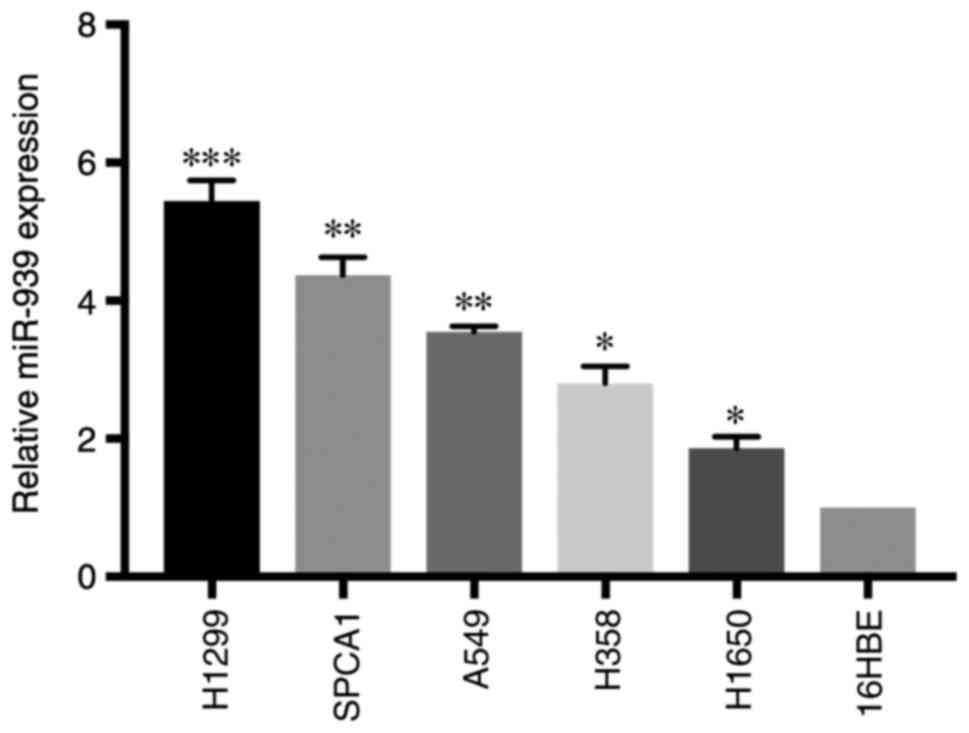
H1650) and a normal lung cell line (16HBE). RT-qPCR analysis
demonstrated that miR-939 expression was significantly increased in
all NSCLC cell lines compared with that in the 16HBE cell line
(P<0.05; Fig. 2).
miR-939 downregulation inhibits NSCLC
cell line proliferation
Subsequently, based on the abnormal expression of
miR-939 among the NSCLC cell lines, H1299 and SPCA1 cells that
demonstrated the highest miR-939 expression were transfected with
an miR-939 inhibitor to detect the regulatory mechanisms affected
by miR-939 knockdown in NSCLC development in vitro, with
inhibitor negative control (NC)-transfected cells as the control.
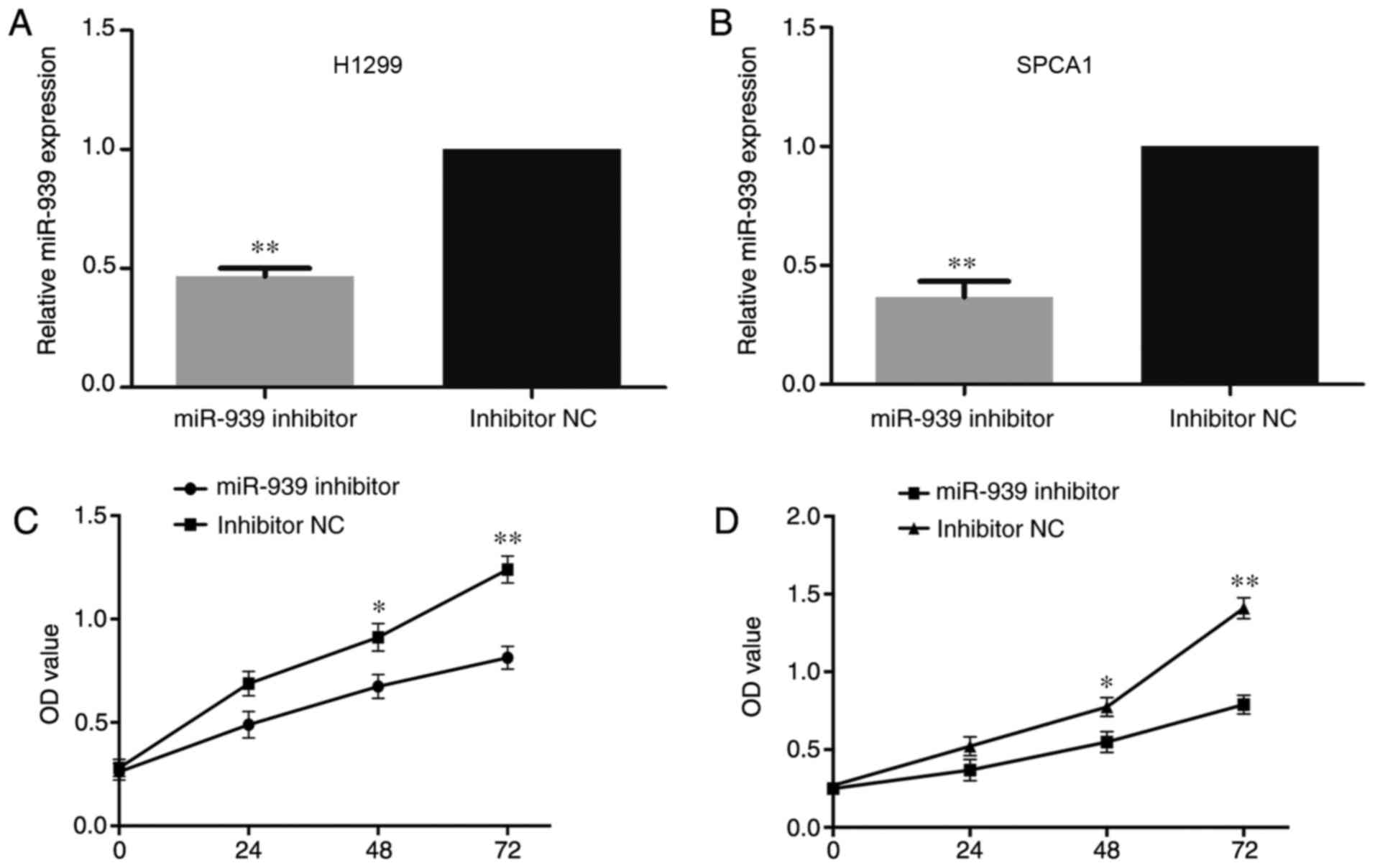
Following transfection of the cells with miR-939 inhibitor or
inhibitor NC, it was identified via RT-qPCR that miR-939 was
significantly downregulated in the miR-939-silenced cells compared
with its expression in the inhibitor NC group (P<0.01; Fig. 3A and B).
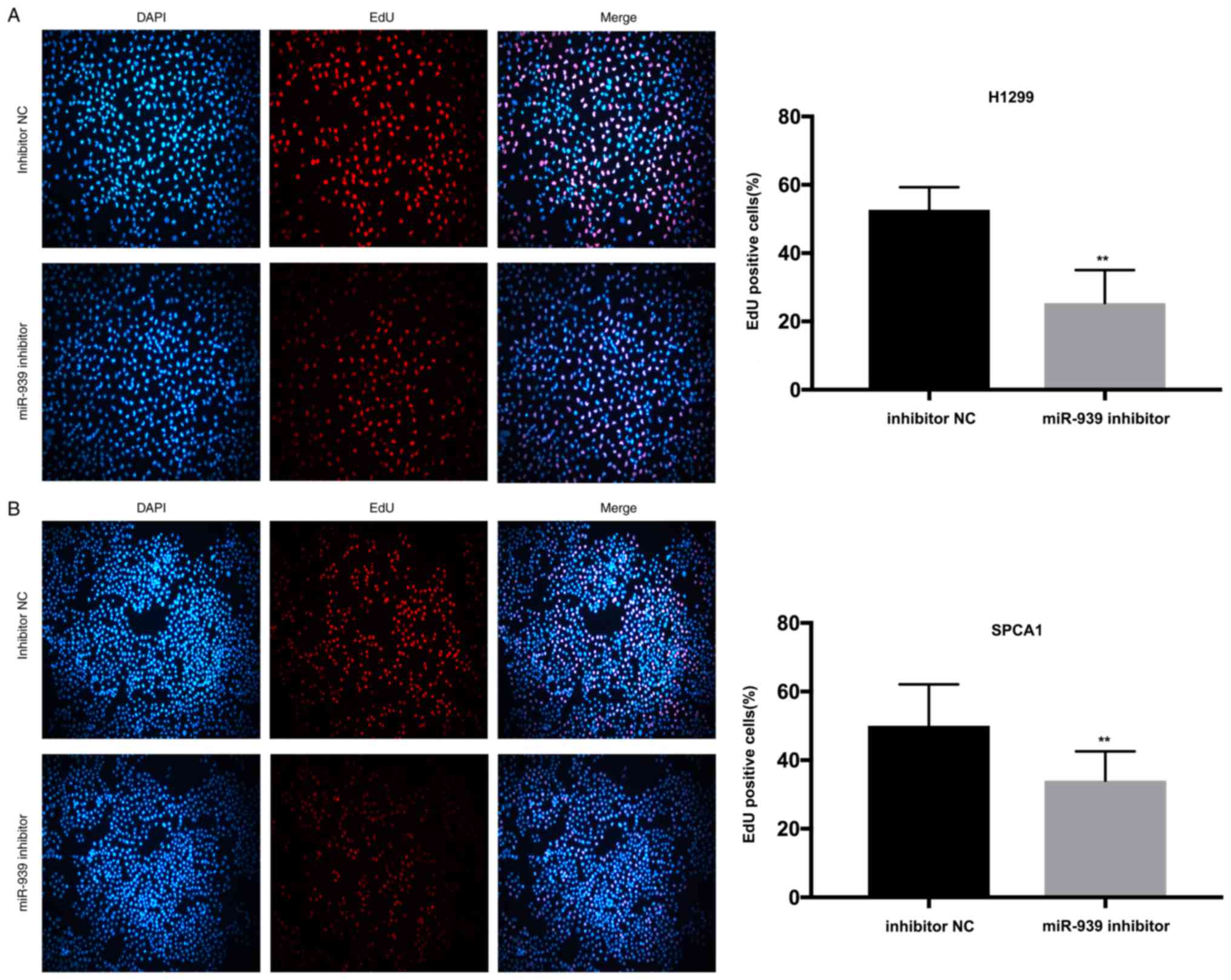
Additionally, to investigate the influence of
miR-939 on the cell proliferative ability, CCK-8 and EdU assays
were performed. In the H1299 and SPCA1 cell lines, the CCK-8
(Fig. 3C and D, respectively) and
EdU (Fig. 4A and B, respectively)
assays demonstrated that cell proliferative ability was markedly
decreased by the silencing of miR-939 expression.
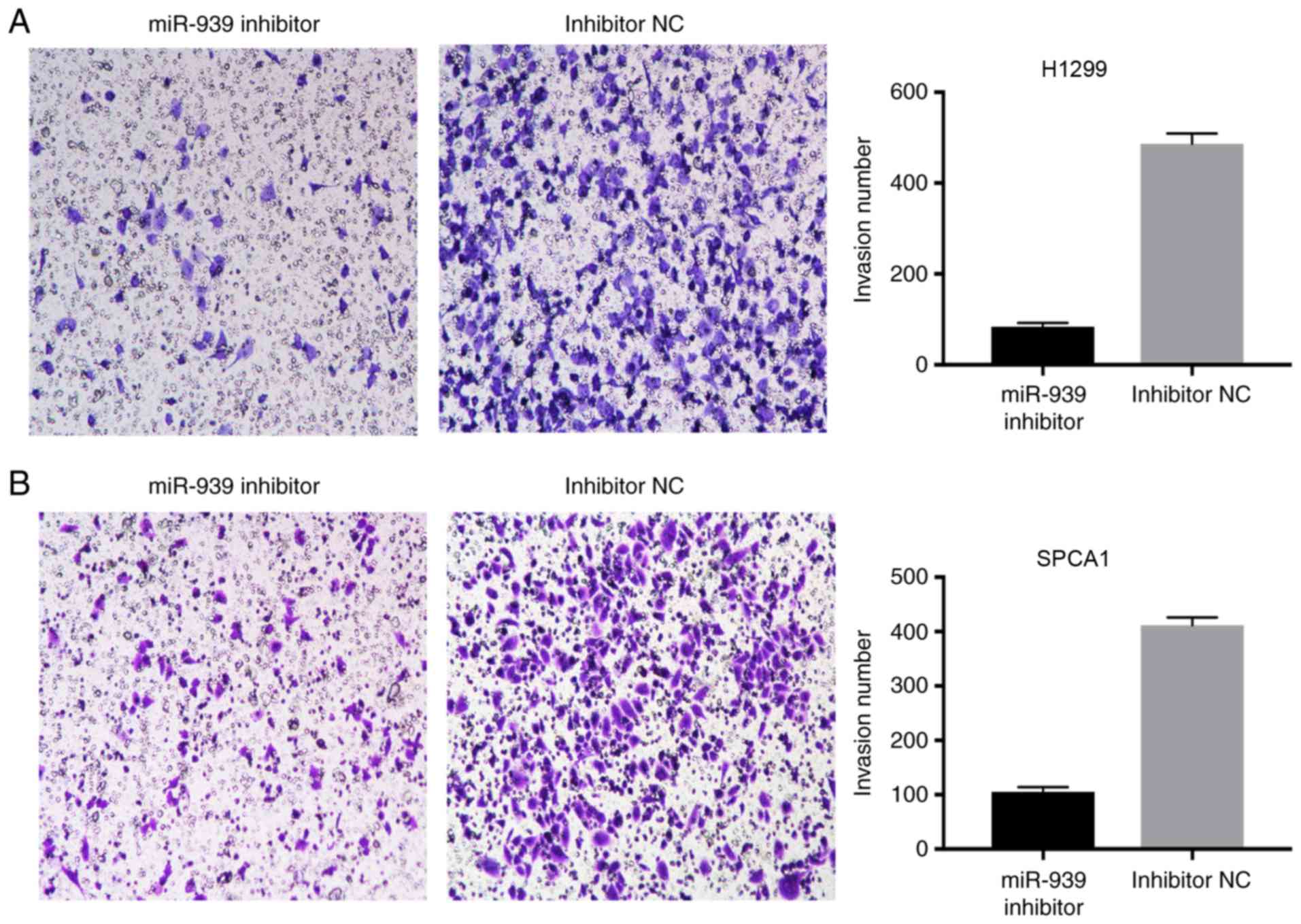
miR-939 downregulation inhibits NSCLC
invasion
The effect of miR-939 downregulation on the invasion
of H1299 and SPCA1 cells was also examined. A Transwell assay
demonstrated that compared with the cells transfected with
inhibitor NC, a reduced number of cells invaded into the lower
chambers in the miR-939 inhibitor group (Fig. 5A and B). This data suggested that
the knockdown of miR-939 markedly reduced NSCLC invasion.
miR-939 regulates TIMP2 by binding to
its 3′-UTR
In order to define whether TIMP2 had a binding site
for miR-939, cells were transfected with miR-939 mimics (Fig. 6A) and, using the prediction
programs miRDB and TargetScan Human, the underlying target genes of
miR-939 were verified. The 3′-UTR of TIMP2 was identified to have a
binding site for miR-939 (Fig.
6B). To determine whether miR-939 directly targets TIMP2 in
NSCLC cells, a luciferase reporter assay was conducted. The results
demonstrated a reduction in luciferase activity following
co-transfection with miR-939 mimics (P<0.01), whereas
co-transfection with NC had no significant effect on luciferase
activity (Fig. 6C), which
indicated that miR-939 regulated TIMP2 by binding to its
3′-UTR.
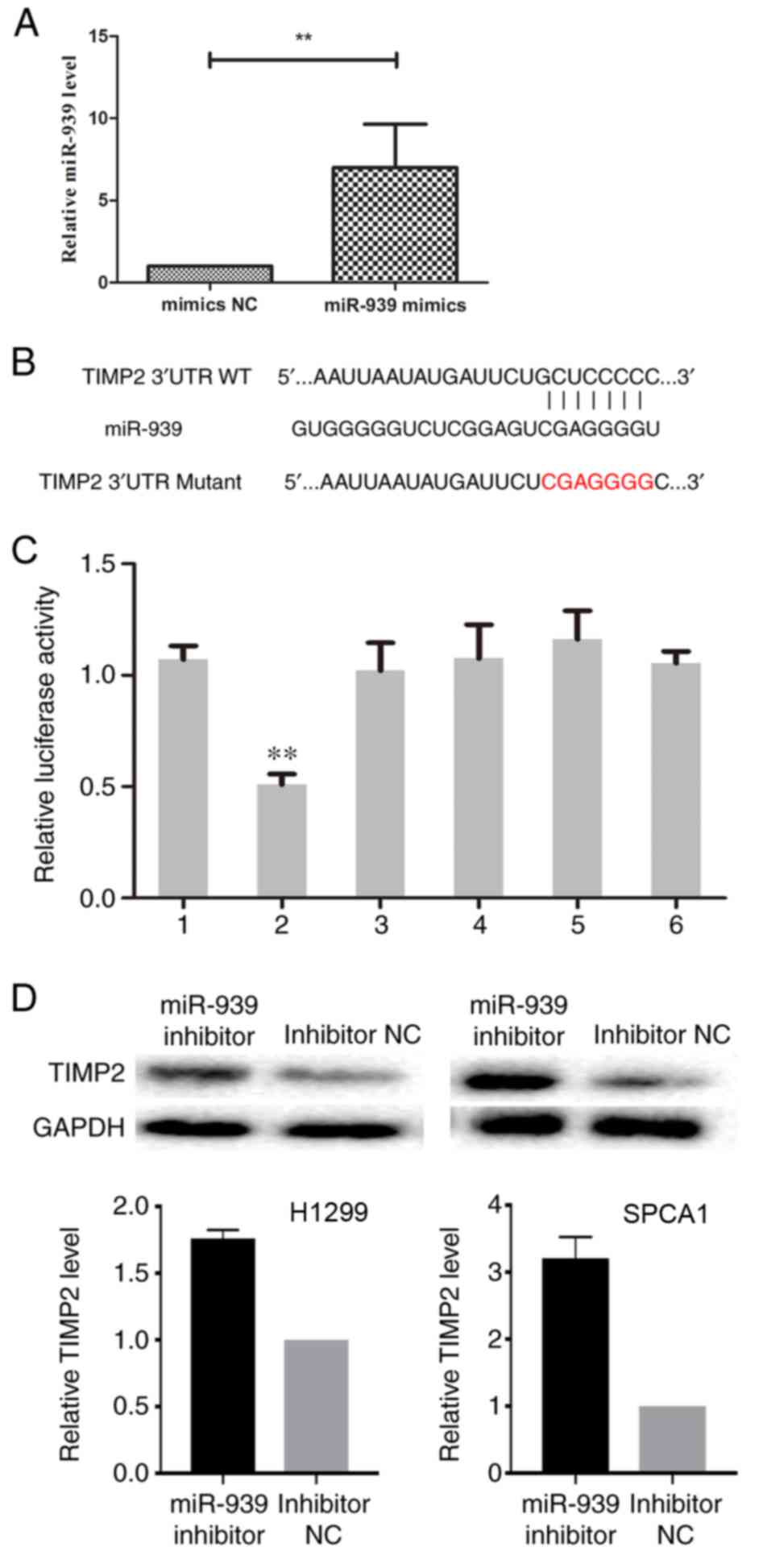 | Figure 6.TIMP2 is negatively regulated by
miR-939. (A) Successful transfection efficiency was determined by
reverse transcription-quantitative polymerase chain reaction.
**P<0.01. (B) In the prediction programs miRDB and TargetScan
Human, the 3′-UTR of TIMP2 was indicated to have a binding site for
miR-939. (C) Relative luciferase activity was evaluated. 1,
pGL3-TIMP2; 2, pGL3-TIMP2+miR-939 mimics; 3, pGL3-TIMP2+NC; 4,
pGL3-TIMP2 Mutant; 5, pGL3-TIMP2 Mutant+miR-939 mimics; 6,
pGL3-TIMP2 Mutant+NC. **P<0.01 vs. group 1. (D) The relative
protein expression of TIMP2 in H1299 (left) and SPCA1 (right) cells
was detected by western blot analysis. miR, microRNA; NC, negative
control; TIMP2, TIMP metallopeptidase inhibitor 2; UTR,
untranslated region; WT, wild-type. |
Subsequently, the effect of miR-939 downregulation
on TIMP2 expression was assessed. Western blot analysis indicated
that the silencing of miR-939 increased the protein expression
level of TIMP2 in H1299 and SPCA1 cells (Fig. 6D). Collectively, these findings
demonstrated that miR-939 was able to negatively regulate TIMP2 by
binding to its 3′-UTR, although this requires further
investigation.
Discussion
Numerous studies have reported that miRNAs function
as oncogenes or tumor suppressors in the progression of different
types of cancer. Lu et al (17) reported that miRNA-221 expression
was increased in renal cell carcinoma, and promoted cell
proliferation, migration and invasion by regulating TIMP2. In
gastric cancer, miR-29a repressed cell migration and invasion by
modulating roundabout homolog 1 (18). Low-level expression of miR-329 has
been implicated in gastric cancer invasion and growth by targeting
T cell lymphoma invasion and metastasis 1 (19). In addition, a number of key miRNAs
have been established as biomarkers for early diagnosis and
prognosis in a range of malignancy types (5). For instance, the downregulation of
miR-197 predicted a poor prognosis for patients with esophageal
cancer (20), whereas miR-126 and
miR-200c dysregulation has been implicated in the prognosis of
patients with NSCLC (21).
In the present study, RT-qPCR analysis demonstrated
that the upregulation of miR-939 occurred in NSCLC cell lines and
clinical specimens, thus indicating a potential prognostic marker
for patients with NSCLC. Furthermore, RT-qPCR analysis identified
that differential expression of miR-939 in NSCLC was significantly
associated with the patient clinical factors of tumor stage, tumor
size and lymphatic metastasis status. In addition, the functional
role of miR-939 was investigated in vitro. On silencing of
miR-939 in the NSCLC cell lines H1299 and SPCA1, inhibition of cell
proliferation and invasion was observed. Collectively, these data
demonstrated that miR-939 may be involved in the development of
NSCLC, although this requires further investigation.
It is established that miRNAs regulate certain
target genes by binding to their 3′-UTRs (22–24).
miR-124 suppressed gastric cancer development by directly targeting
enhancer of zeste homolog 2 (25);
whereas, miR-26a, serving as a tumor suppressor, repressed cell
proliferation by directly binding the high-mobility group AT-hook 2
3′-UTR in gallbladder cancer (26). Additionally, using the prediction
programs miRDB and TargetScan Human, the underlying target genes of
miR-939 were verified in the present study. A luciferase reporter
assay identified a reduction in luciferase activity following
co-transfection with miR-939 mimics, while co-transfection with the
negative control did not significantly alter the luciferase
activity, indicating that miR-939 regulated TIMP2 by binding to its
3′UTR. Subsequently, the effect of miR-939 downregulation on TIMP2
expression was examined. Western blot analysis indicated that the
silencing of miR-939 increased the protein expression level of
TIMP2.
Lang et al (27) reported miR-429 downregulates TIMP2
expression to promote the proliferation and metastasis of NSCLC
cells. Wang et al (28)
reported that microRNA-15b promotes proliferation and invasion of
NSCLC by directly targeting TIMP2. Finally, another study reported
that miRNA-221 serves an oncogenic role by directly targeting TIMP2
in NSCLC (29).
The development and progression of lung cancer is
not determined by a single or a few factors; rather, it is affected
by a number of genes and signaling pathways (30,31).
The present study was limited due to the sample size and cell line
type, and therefore a larger sample size is required. Further
experiments are required in order to examine the association
between miR-939 and invasion or migration in order to discover any
potential regulatory role of miR-939 in the development of lung
cancer.
In conclusion, the present study demonstrated that
miR-939 was able to regulate NSCLC cell proliferation and invasion.
Additionally, miR-939 negatively regulated TIMP2 by binding to its
3′UTR. Therefore, miR-939 may be a potential target in the
treatment of NSCLC, although this required further
investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author, on reasonable
request.
Authors' contributions
YC and LW designed the experiments. AC and SL
performed the experiments. XL analyzed the data. All authors have
read and approved the manuscript.
Ethics approval and consent to
participate
Approval to conduct human experiments was obtained
from the Ethical Committee at the First Affiliated Hospital of
Nanjing Medical University (Nanjing, China) and all patients
enrolled in the study signed consent forms. All clinical procedures
were conducted in accordance with the guidelines of the Ethical
Committee of the First Affiliated Hospital of Nanjing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gompelmann D, Eberhardt R and Herth FJ:
Advanced malignant lung disease: What the specialist can offer.
Respiration. 82:111–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Sui J, Shen X, Li C, Yao W, Hong
W, Peng H, Pu Y, Yin L and Liang G: Differential expression
profiles of microRNAs as potential biomarkers for the early
diagnosis of lung cancer. Oncol Rep. 37:3543–3553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Cos Sanchez J, Sojo Gonzalez MA,
Montero MV, Calvo Perez MC, Vicente MJ and Valle MH: Non-small cell
lung cancer and silent brain metastasis. Survival and prognostic
factors. Lung Cancer. 63:140–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang SM and Lee HJ: MicroRNAs in human
lung cancer. Exp Biol Med (Maywood). 239:1505–1513. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding XM: MicroRNAs: Regulators of cancer
metastasis and epithelial-mesenchymal transition (EMT). Chin J
Cancer. 33:140–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin CW, Chang YL, Chang YC, Lin JC, Chen
CC, Pan SH, Wu CT, Chen HY, Yang SC, Hong TM and Yang PC:
MicroRNA-135b promotes lung cancer metastasis by regulating
multiple targets in the Hippo pathway and LZTS1. Nat Commun.
4:18772013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Huang R, Wang L, Hao J, Zhang Q,
Ling R and Yun J: microRNA-762 promotes breast cancer cell
proliferation and invasion by targeting IRF7 expression. Cell
Prolif. 48:643–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang CZ, Yuan P and Li Y: MiR-126
regulated breast cancer cell invasion by targeting ADAM9. Int J
Clin Exp Pathol. 8:6547–6553. 2015.PubMed/NCBI
|
|
11
|
Kong R, Ma Y, Feng J, Li S, Zhang W, Jiang
J, Zhang J, Qiao Z, Yang X and Zhou B: The crucial role of miR-126
on suppressing progression of esophageal cancer by targeting
VEGF-A. Cell Mol Biol Lett. 21:32016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang W, Tong JH, Lung RW, Dong Y, Zhao J,
Liang Q, Zhang L, Pan Y, Yang W, Pang JC, et al: Targeting of YAP1
by microRNA-15a and microRNA-16-1 exerts tumor suppressor function
in gastric adenocarcinoma. Mol Cancer. 14:522015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ying X, L-Ya Q, Feng Z, Yin W and Ji-Hong
L: MiR-939 promotes the proliferation of human ovarian cancer cells
by repressing APC2 expression. Biomed Pharmacother. 71:64–69. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Modica M, Regondi V, Sandri M, Iorio
MV, Zanetti A, Tagliabue E, Casalini P and Triulzi T: Breast
cancer-secreted miR-939 downregulates VE-cadherin and destroys the
barrier function of endothelial monolayers. Cancer Lett.
384:94–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma R, Wang C, Wang J, Wang D and Xu J:
miRNA-mRNA interaction network in non-small-cell lung cancer.
Interdiscip Sci. Apr 11–2015.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu GJ, Dong YQ, Zhang QM, Di WY, Jiao LY,
Gao QZ and Zhang CG: miRNA-221 promotes proliferation, migration
and invasion by targeting TIMP2 in renal cell carcinoma. Int J Clin
Exp Pathol. 8:5224–5229. 2015.PubMed/NCBI
|
|
18
|
Liu X, Cai J, Sun Y, Gong R, Sun D, Zhong
X, Jiang S, He X, Bao E, Yang L and Li Y: MicroRNA-29a inhibits
cell migration and invasion via targeting Roundabout homolog 1 in
gastric cancer cells. Mol Med Rep. 12:3944–3950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J
and Feng F: By downregulating TIAM1 expression, microRNA-329
suppresses gastric cancer invasion and growth. Oncotarget.
6:17559–17569. 2015.PubMed/NCBI
|
|
20
|
Wang TY, Liu SG, Zhao BS, Qi B, Qin XG and
Yao WJ: Implications of microRNA-197 downregulated expression in
esophageal cancer with poor prognosis. Genet Mol Res. 13:5574–5581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MK, Jung SB, Kim JS, Roh MS, Lee JH,
Lee EH and Lee HW: Expression of microRNA miR-126 and miR-200c is
associated with prognosis in patients with non-small cell lung
cancer. Virchows Archiv. 465:463–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia Y and Gao Y: MicroRNA-181b promotes
ovarian cancer cell growth and invasion by targeting LATS2. Biochem
Biophys Res Commun. 447:446–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X,
Jin X and Tian X: MicroRNA-183 functions as the tumor suppressor
via inhibiting cellular invasion and metastasis by targeting MMP-9
in cervical cancer. Gynecol Oncol. 141:166–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang
C, Wu S, Yu D, Huang Z, Liu F, Luo Q, et al: MicroRNA-33b inhibits
breast cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci
Rep. 5:99952015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie
Y, Li S, Tang G, Tang H and He X: MicroRNA-124 inhibits
proliferation and induces apoptosis by directly repressing EZH2 in
gastric cancer. Mol Cell Biochem. 392:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou H, Guo W, Zhao Y, Wang Y, Zha R, Ding
J, Liang L, Hu J, Shen H, Chen Z, et al: MicroRNA-26a acts as a
tumor suppressor inhibiting gallbladder cancer cell proliferation
by directly targeting HMGA2. Int J Oncol. 44:2050–2058. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lang Y, Xu S, Ma J, Wu J, Jin S, Cao S and
Yu Y: MicroRNA-429 induces tumorigenesis of human non-small cell
lung cancer cells and targets multiple tumor suppressor genes.
Biochem Biophys Res Commun. 450:154–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Zhan Y, Jin J, Zhang C and Li W:
MicroRNA-15b promotes proliferation and invasion of nonsmall cell
lung carcinoma cells by directly targeting TIMP2. Oncol Rep.
37:3305–3312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin Z, Xu M and Li P: miRNA-221 acts as an
oncogenic role by directly targeting TIMP2 in non-small-cell lung
carcinoma. Gene. 620:46–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ai X, Mao F, Shen S, Shentu Y, Wang J and
Lu S: Bexarotene inhibits the viability of non-small cell lung
cancer cells via slc10a2/PPARγ/PTEN/mTOR signaling pathway. BMC
Cancer. 18:4072018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv M and Wang L: Comprehensive analysis of
genes, pathways, and TFs in nonsmoking Taiwan females with lung
cancer. Exp Lung Res. 41:74–83. 2015. View Article : Google Scholar : PubMed/NCBI
|