Introduction
Osteoarthritis (OA) is reported to afflict nearly
250 million people in the whole world and is the most common
arthritis (1,2). Still, OA remains a critical challenge
socially and medically due to the lack of optimal therapeutic
methods (3). Till now, important
advances have been made in the prevention and treatment of OA
(4,5). The specific etiology of OA is
complicated and unclear where multiple intertwining factors may
take part in, for example, aging, trauma, obesity and heredity
predisposition (6,7). The basic features of OA includes
degradation of cartilage and synovitis which lead to pain,
stiffness and abnormal joint structure (8). It remains undefined currently
although the pathogenesis of OA has been paid much attention to.
However, increasing evidence has identified the critical role of
chondrocyte in OA initiation and progression (9). It has been reported that
chondroapoptosis and chondrosenescence could be the two specific
hallmarks of OA (10,11). A growing body of evidence reveals
that chondrocyte apoptosis plays a key role in the destruction of
cartilage (11). Therefore,
identification of the underlying mechanisms of chondroapoptosis may
provide a promising therapy for the management of OA.
Long noncoding RNAs (lncRNAs) refer to a kind of
non-protein coding, longer than 200 nucleotides transcripts
(12). In recent years, lncRNAs
have been reported to modulate various biological and pathological
processes, such as cell cycle, cell apoptosis, epigenetics and
multiple cancers (13–15). Interestingly, lncRNAs also
participate in the progression of OA (14). For example, the overexpression of
lncRNA-CIR increases the expression of matrix-degrading enzymes and
contributes to extracellular matrix degradation in OA (16). LncRNA plasmacytoma variant
translocation 1 (PVT1) promotes the apoptosis of chondrocytes in OA
by acting as a sponge for miR-488-3p (17). LncRNA-p21 has been reported to play
roles in malignant and benign diseases. It is indicated that
lncRNA-p21 could affect the growth of breast cancer cells by
regulating the G1/S checkpoint and modulating energy metabolism of
neoplasm cells (18,19). In osteosarcoma, lncRNA-p21 inhibits
the proliferation of osteosarcoma cells by regulating
miR-130b/PTEN/AKT signaling pathway (20).
Chondrocytes, the only kind of cell in articular
cartilage, are important for the maintenance of the cartilage
homeostasis. Apoptosis is a physiological process, and the purpose
is to remove harmful, damaged or unwanted cells (21). Recent report has demonstrated that
the apoptosis of chondrocytes is related to the development and
progression of OA (22). Several
molecules considered to be markers of chondrocyte apoptosis, such
as bcl-2, bax, and so on (23). In
addition, inflammation process of chondrocytes also affects cell
survival and take parts in maintaining cell homeostasis (24).
Till now, the role of lncRNA-p21 in OA remains
unclear. According to reported papers, we assume it play an
important role in OA. Therefore, the current study is designed to
investigate the role of lncRNA-p21 in OA and the underlying
mechanism. The aim of the current study is to investigate the role
of lncRNA-p21 in OA and its underling mechanism which helps to
better understand the development of OA and its treatment.
Materials and methods
Chondrocyte culture
A total of 20 OA articular cartilages were collected
from patients during the surgery of total knee arthroplasty and 20
normal articular cartilages were obtained from non-OA patients with
femoral neck fracture from Second Affiliated Hospital, Zhejiang
University School of Medicine. The diagnosis of OA followed
radiographic images and the American College of Rheumatology
criteria. All experiments were completely approved by the Ethics
Committee of Second Affiliated Hospital (Zhejiang University School
of Medicine, Hangzhou, China). All patients signed informed consent
forms. The cartilage samples were cut into small pieces and
predigested in trypsin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 10 min and the digested for 16 h with type II
collagenase (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in DMEM
with 10% FBS. Then the suspension was strained with nylon cell
strainer (100 µm) and washed with Dulbecco's modified Eagle's
medium (DMEM) (Invitrogen; Thermo Fisher Scientific, Inc.). The
isolated chondrocytes were cultured in DMEM supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2 as reported (25).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cartilages (directly
from cartilage samples) and chondrocytes (isolated chondrocytes)
with TRIzol regent (Invitrogen; Thermo Fisher Scientific, Inc.).
Quantitation of RNAs was performed on Prism 7500 real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using
SYBR Premix Ex Taq (Takara Bio, Inc., Shiga, Japan). The results
were analyzed using the 2−ΔΔCq method as reported
(26). The primes used are listed
below: lncRNA-p21: Forward, 5′-TGTTGCATTGTTGCATCATC-3′; reverse,
5′-TTTCTTCCAGTGGTGAGTGG-3′. miR-451: forward,
5′-TAGCAAGAGAACCATTACCA-3′; reverse, 5′-GAACATGTCTGCGTATCTC-3′.
Transfection
Chondrocytes were transfected with miR-451 mimics,
miR-451 inhibitor, pcDNA3.1(+)-p21 or si-p21 (GenePharma, Shanghai,
China) with Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) when chondrocytes reached 70% confluence. Cells
were harvested 48 h after transfection.
Cell viability assay
CCK-8 assay was performed to detect cell viability
according to the manufacturer's protocol (27). Chondrocytes were seeded in 96-well
plates after transfection. DMEM (100 µl) and CCK-8 regent (10 µl,
Dojindo, Kumamoto, Japan) were added to 96-well plates. After
incubation at 37°C for one hour, the absorbance was detected with
spectrophotometer at 450 nm and cell viability was calculated. The
experiment was conducted in triplicate.
Flow cytometry analysis
The apoptosis rate of cells was measured by Annexin
V-FITC Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ,
USA) as reported (28). Briefly
speaking, cells were harvested 48 after transfection and
resuspended in 1X binding buffer after washed with cold PBS for
twice. Then 5 µl PI and 5 µl FITC were added and incubated for 15
min at room temperature in the dark. The apoptotic cells were
detected by flow cytometry. The FlowJo 7.6 software was used to
analyze flow cytometry data. Each experiment was conducted in
triplicate.
Western blot analysis
Western blot analysis was done as reported (27). Chondrocytes were harvested after
transfection for 48 h and lysed with RIPA (Beyotime, Shanghai,
China) supplemented. Protein concentration was quantified with the
BCA kit (Beyotime) and 30 µg of protein was loaded to SDS-PAGE gels
for electrophoresis and transferred to PVDF membranes. The
membranes were blocked in 5% fat-free milk for one hour at room
temperature and incubated in primary antibodies at 4°C overnight.
Then membranes were incubated with horse radish peroxidases
(HRP)-conjugated secondary antibodies (1:4,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) for one hour at room
temperature and detected with an ECL detection system (Thermo
Fisher Scientific, Inc.). Anti-Bcl-2 antibody, anti-Bax antibody
and anti-GAPDH antibody were purchased from Cell Signaling
Technology, Inc. All primary antibodies were monoclonal and at a
dilution of 1:1,000. The relative expression levels of proteins
were normalized with GAPDH. All experiments were repeated for three
times.
Statistical analysis
Data were expressed as mean ± standard deviation.
Statistical analysis was performed using GraphPad Prism 6.0
(GraphPad Software Inc., La Jolla, CA, USA). Student's t-test or
analysis of variance (ANOVA) was used to conduct statistical
comparisons after performing tests to verify the normal
distribution of the data and confirming that the variances were
homogeneous. Student's t-test was used to determine the significant
differences between two groups. ANOVA followed by Bonferroni post
hoc test were used to determine the significant differences among
multiple groups. P<0.05 was considered to indicate a as
statistically significant difference.
Results
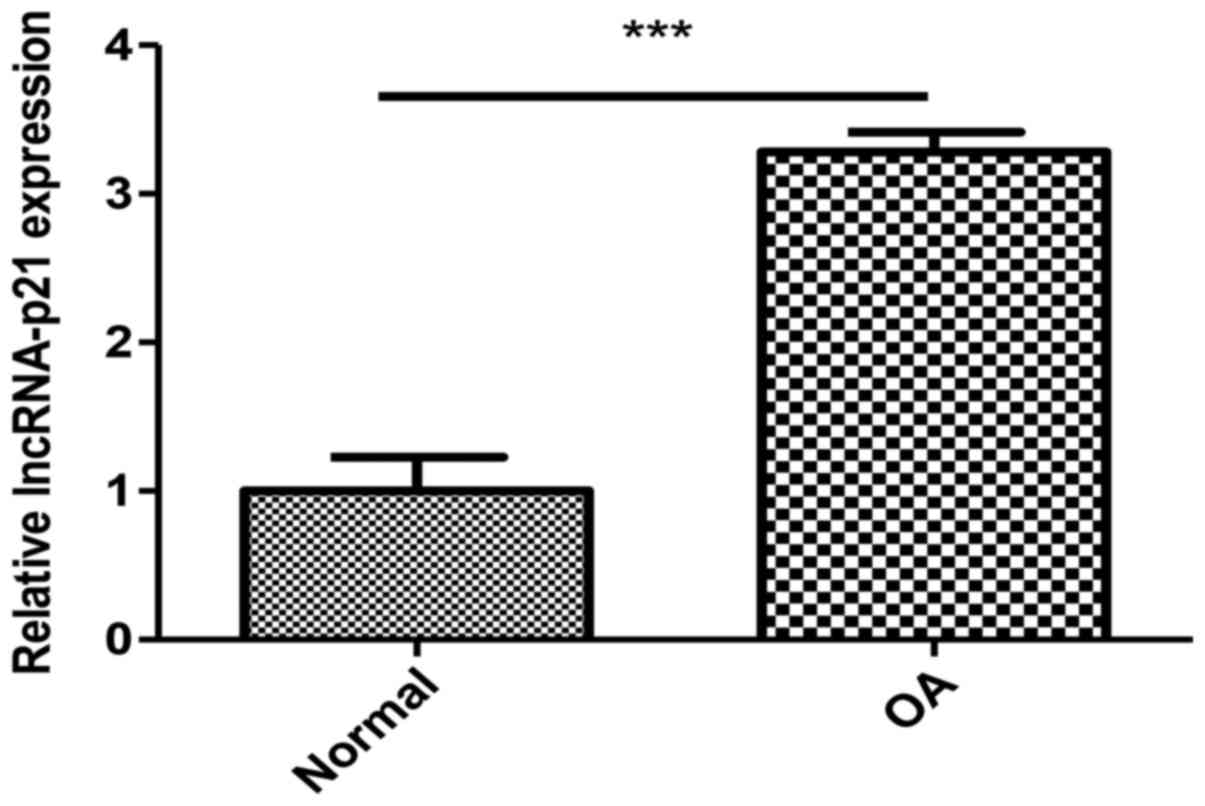
The expression of lncRNA-p21 is
upregulated in OA chondrocytes
To determine the potential role of lncRNA-p21 in OA,
we compared the expression level of lncRNA-p21 in cartilage samples
of OA patients and normal people. Results showed that the
expression level of lncRNA-p21 were significantly upregulated in OA
compared with the normal (Fig.
1).
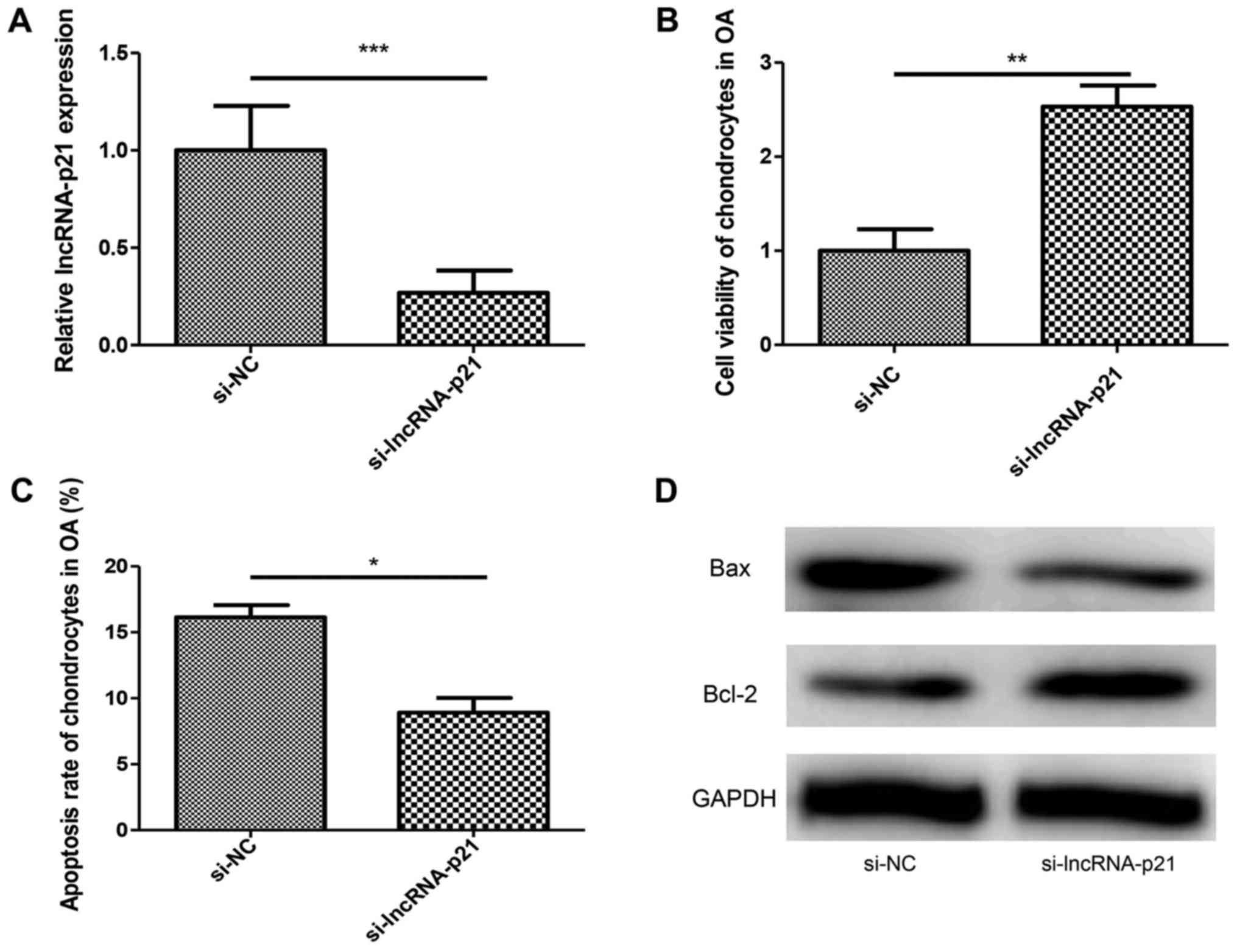
Downregulation of lncRNA-p21 inhibits
apoptosis of chondrocytes in OA
Chondrocytes apoptosis plays a critical role in
cartilage degradation in OA. In order to investigate the function
of lncRNA-p21 in chondrocytes apoptosis, si-lncRNA-p21 was
transfected into chondrocytes of OA. RT-qPCR was performed to
confirm the silencing effect (Fig.
2A). Results showed silencing of lncRNA-p21 increased cell
viability and inhibited the apoptosis rate of chondrocytes in OA
(Fig. 2B and C) compared with the
control group. At the same time, the downregulation of Bax and
upregulation of Bcl-2 induced by the silencing of lncRNA-p21 was
identical to the flow cytometry analysis (Fig. 2D). These results showed that
lncRNA-p21 promoted apoptosis of chondrocytes in OA.
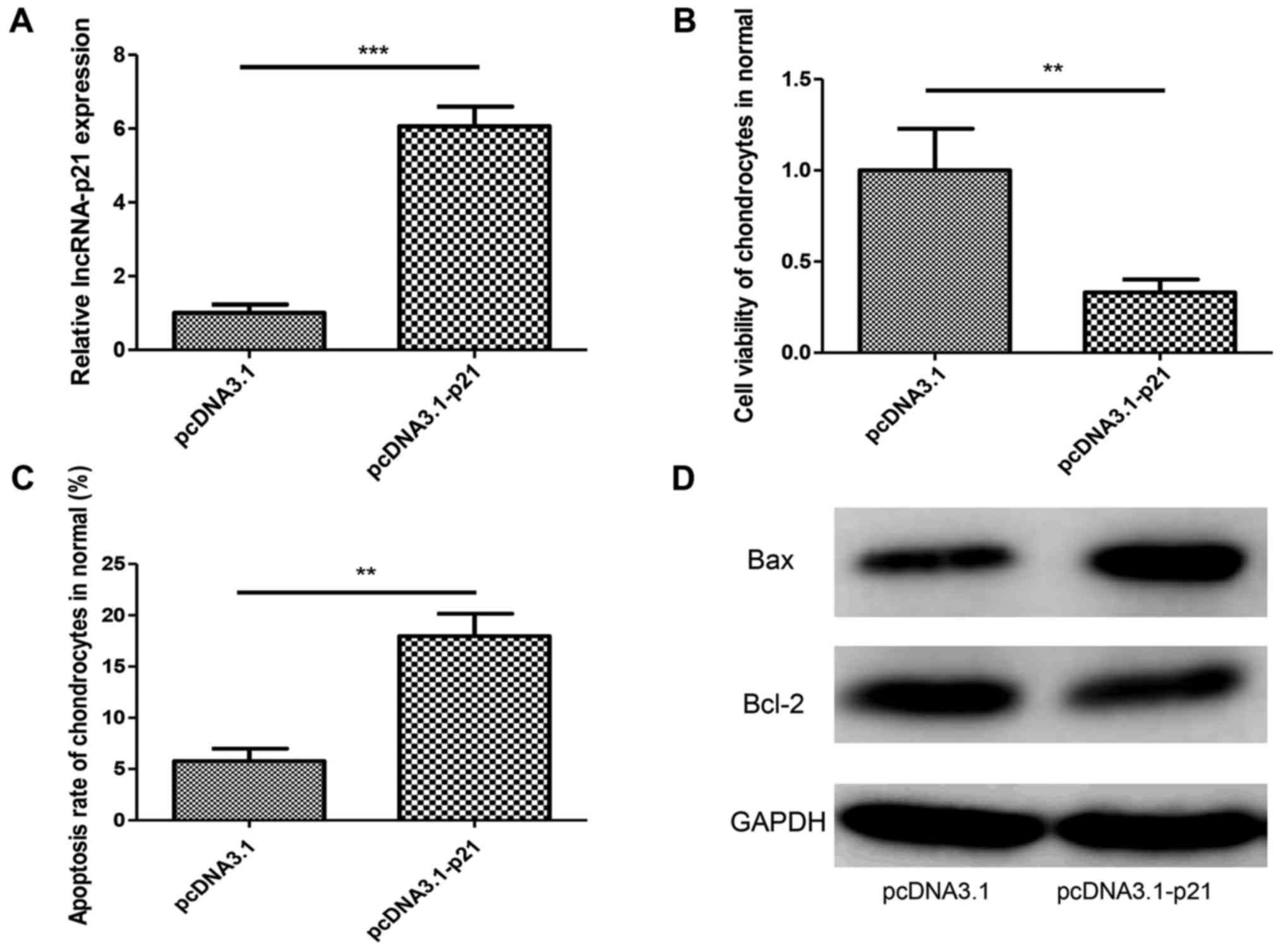
Overexpression of lncRNA-p21 promotes
the apoptosis of chondrocytes in normal cartilage
To confirm the role of lncRNA-p21 in chondrocyte
apoptosis further, pcDNA3.1 (+)-p21 or negative control plasmid was
transfected into normal chondrocytes isolated from normal
cartilage. RT-qPCR was performed to confirm the overexpression
effect (Fig. 3A). We found that
lncRNA-p21 overexpression decreased cell viability and increased
the apoptosis rate of chondrocytes in OA (Fig. 3B and C). Identically, lncRNA-p21
overexpression upregulated Bax and downregulated Bcl-2 (Fig. 3D). These results further confirmed
the pro-apoptotic effect of lncRNA-p21 in chondrocytes.
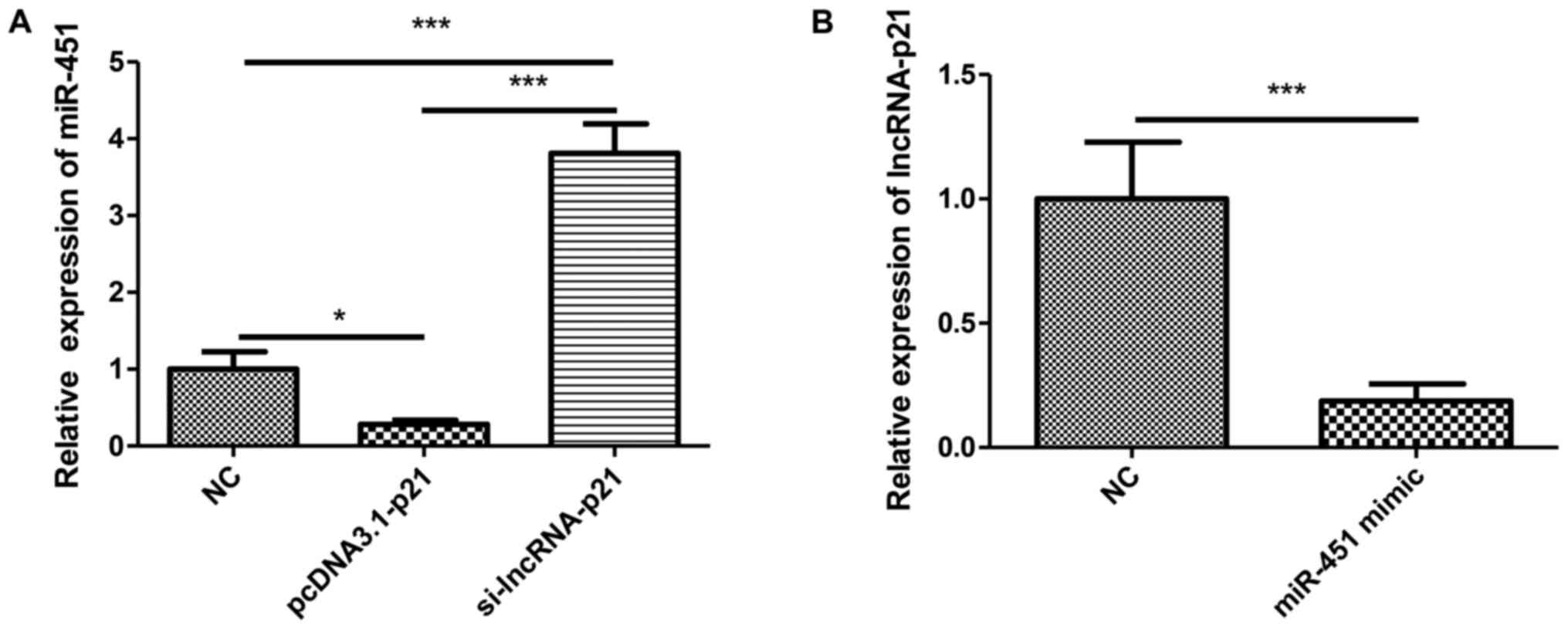
LncRNA-p21 interacts with miR-451 in
OA
Although it was indicated above that lncRNA-p21
regulate the chondrocyte apoptosis in OA, the underlying mechanism
remains unclear. A growing studies indicated that lncRNAs exert
their biological or pathological functions by interacting with
miRNAs. We reasoned that lncRNA-p21 regulates chondrocyte apoptosis
by interacting with miRNA. MiR-451 was reported to inhibit the
proliferation and promote apoptosis of osteosarcoma. Whether
miR-451 plays a role in OA is unknown. To investigate whether
lncRNA-p21 could exert its function by acting as a sponge for
miR-451, we transfected chondrocytes of OA with pcDNA3.1(+)-p21 or
si-lncRNA-p21 and detected the expression of miR-451. Results
showed that the overexpression of lncRNA-p21 suppressed the
expression of miR-451 while the silencing of lncRNA-p21 reversed
this effect (Fig. 4A). Then
miR-451 mimic or negative control was transfected into chondrocytes
of OA, the expression of lncRNA-p21 was suppressed by miR-451
overexpression (Fig. 4B). These
results indicated that lncRNA-p21 functioned by interacting with
miR-451.
LncRNA-p21 promotes chondrocytes
apoptosis through miR-451 in OA and normal
Given that lncRNA-p21 regulates the chondrocytes
apoptosis in OA and interacts with miR-451, we reasoned that
miR-451 might be involved in the regulation of chondrocytes
apoptosis in OA. To investigate this speculation, miR-451 inhibitor
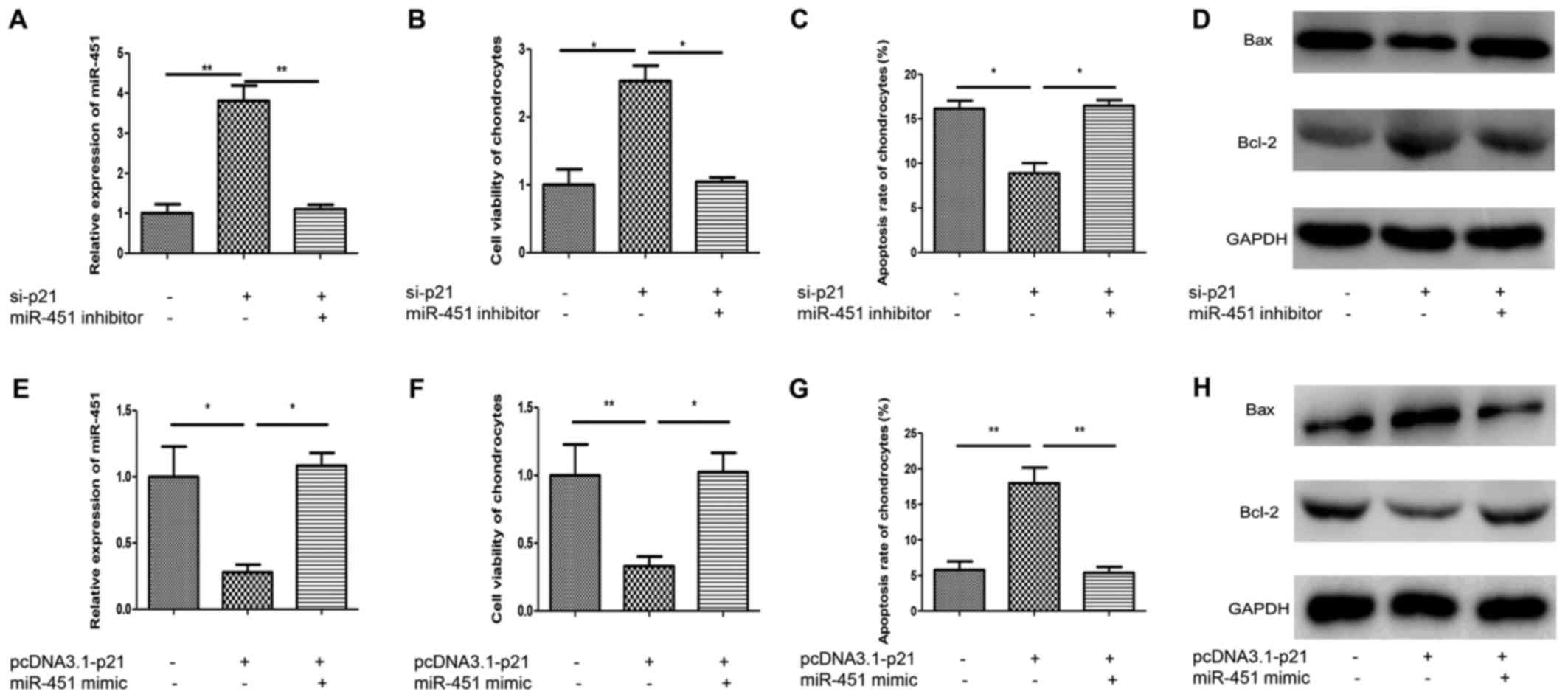
was transfected into lncRNA-p21-silencing chondrocytes of OA.
MiR-451 inhibitor effectively inhibited the upregulation effect of
si-p21 on miR-451 (Fig. 5A).
Interestingly, the increased cell viability and decreased apoptosis
rate induced by lnvRNA-p21 silence was abolished by the miR-451
inhibitor (Fig. 5B and C). Also,
miR-451 inhibitor reversed the Bcl-2 upregulation and Bax
downregulation effect exerted by lncRNA-p21 silence (Fig. 5D). To further investigate the role
of miR-451 in normal chondrocytes, miR-451 mimic was transfected
into lncRNA-p21 overexpression chondrocytes isolated from normal
cartilage. MiR-451 mimic effectively increased the downregulation
effect of pcDNA3.1-lncRNA-p21 on miR-451 (Fig. 5E). The decreased cell viability and
increased apoptosis rate induced by overexpression of lncRNA-p21
was abolished by the miR-451 mimic (Fig. 5F and G). Meanwhile, miR-451 mimic
reversed the Bcl-2 downregulation and Bax upregulation effect
exerted by lncRNA-p21 overexpression (Fig. 5H). These results indicated that
miR-451 was involved in lncRNA-p21-regulated chondrocytes
apoptosis.
Discussion
OA is characterized by degradation of articular
cartilage and often afflict the facets of various joints, such as
knee, hip and elbow. With the progression of chronic inflammation,
the degradation of articular and degeneration and deformation of
joints occur. The chronic pain and regression of joints
significantly reduce the quality of life of patients. In recent
years, the biological roles including in regulating OA of miRNAs
have been investigated extensively. However, lncRNAs, as novel and
potentially valuable non-coding RNAs, are yet to be investigated
(29). An increasing evidence
supports that lncRNAs may regulate OA pathogenesis via competing
endogenous RNA to act as sponges for miRNAs (30). Kang et al (31) reported that PCGM1, acting as a
sponge for miR-770, promoted the proliferation of synoviocytes in
OA. For another example, UFC1 stimulated chondrocytes proliferation
in OA by interacting with miR-34a (32). In the current study, by loss of
function and gain of function experiment, we found that the
overexpression of lncRNA-p21 suppressed the expression of miR-451
while the silence of lncRNA-p21 upregulated the expression of
miR-451. Further study indicated that miR-451 regulated the
apoptosis of chondrocytes in OA and normal. Our results were in
accordance with the previous published studies that lncRNAs possess
the endogenous RNAs competing activities.
LncRNA-p21 have been reported to possess various
biological functions in malignant tumors and non-tumor disorders.
In various tumors, lncRNA-p21 exhibits anti-cancer abilities and
inhibits tumor cell viability. For example, lncRNA-p21 suppressed
the proliferation of hepatocellular carcinoma cells by activating
the endoplasmic reticulum stress, thereby alleviate sorafenib
resistance (33). In prostate
cancer, the overexpression of lncRNA-p21 promoted the apoptosis of
cancer cells mediated by p53 (34). What's more, lncRNA-p21
downregulated the expression of β-catenin in colon cancer cells and
glioma cells by which inhibiting the cell viability and enhancing
radiotherapy effects (35,36). Han and Liu (20) reported that the overexpression of
lncRNA-p21 effectively controlled proliferation of osteosarcoma
cells by the activation of PTEN/pAKT signaling pathway. However,
the role of lncRNA-p21 in OA remains unclear. Our study revealed
that lncRNA-p21 promoted the apoptosis of chondrocytes in OA via
acting as a sponge for miR-451.
In conclusion, results from the study indicated that
lncRNA-p21 promoted the apoptosis of chondrocytes in OA via acting
as a sponge for miR-451. It opens up new horizons for the
development and progression of OA. Meanwhile, it adds new
interpretation of biological functions of lncRNAs, especially
lncRNA-p21. We propose that lncRNA-p21 may be a promising molecular
target for the therapy of OA.
Acknowledgements
The authors would like to acknowledge GenePharma
Co., Ltd. (Shanghai, China) for assisting with the design and
synthesis of the small interfering RNA.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LT and ZL were responsible for the conception,
design and acquisition of data. JD and GZ were conducted the
analysis and interpretation of data. LT was involved in drafting
the manuscript and critically revising it for important
intellectual content. ZL gave final approval of the version to be
published.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of The Second Affiliated Hospital, Zhejiang University
School of Medicine (Zhejiang, China). All patients signed informed
consent forms.
Patient consent for publication
All patients signed informed consent forms for the
publication of any associated images/data in the manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Corciulo C, Lendhey M, Wilder T, Schoen H,
Cornelissen AS, Chang G, Kennedy OD and Cronstein BN: Endogenous
adenosine maintains cartilage homeostasis and exogenous adenosine
inhibits osteoarthritis progression. Nat Commun. 8:150192017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iezaki T, Ozaki K, Fukasawa K, Inoue M,
Kitajima S, Muneta T, Takeda S, Fujita H, Onishi Y, Horie T, et al:
ATF3 deficiency in chondrocytes alleviates osteoarthritis
development. J Pathol. 239:426–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rufino AT, Rosa SC, Judas F, Mobasheri A,
Lopes MC and Mendes AF: Expression and function of K(ATP) channels
in normal and osteoarthritic human chondrocytes: Possible role in
glucose sensing. J Cell Biochem. 114:1879–1889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delgado-Enciso I, Paz-Garcia J,
Rodriguez-Hernandez A, Madrigal-Perez VM, Cabrera-Licona A,
Garcia-Rivera A, Soriano-Hernandez AD, Cortes-Bazan JL,
Galvan-Salazar HR, Valtierra-Alvarez J, et al: A promising novel
formulation for articular cartilage regeneration: Preclinical
evaluation of a treatment that produces SOX9 overexpression in
human synovial fluid cells. Mol Med Rep. 17:3503–3510.
2018.PubMed/NCBI
|
|
5
|
Cui GH, Wang YY, Li CJ, Shi CH and Wang
WS: Efficacy of mesenchymal stem cells in treating patients with
osteoarthritis of the knee: A meta-analysis. Exp Ther Med.
12:3390–3400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldring MB: Update on the biology of the
chondrocyte and new approaches to treating cartilage diseases. Best
Pract Res Clin Rheumatol. 20:1003–1025. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and Duong LT: Characterization of articular cartilage
and subchondral bone changes in the rat anterior cruciate ligament
transection and meniscectomized models of osteoarthritis. Bone.
38:234–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allen KD, Choong PF, Davis AM, Dowsey MM,
Dziedzic KS, Emery C, Hunter DJ, Losina E, Page AE, Roos EM, et al:
Osteoarthritis: Models for appropriate care across the disease
continuum. Best Pract Res Clin Rheumatol. 30:503–535. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldring MB: The role of the chondrocyte
in osteoarthritis. Arthritis Rheum. 43:1916–1926. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang HS and Kim HA: Chondrocyte apoptosis
in the pathogenesis of osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frank S, Aguirre A, Hescheler J and Kurian
L: A lncRNA perspective into (Re)building the heart. Front Cell Dev
Biol. 4:1282016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su Y, Wu H, Pavlosky A, Zou LL, Deng X,
Zhang ZX and Jevnikar AM: Regulatory non-coding RNA: new
instruments in the orchestration of cell death. Cell Death Dis.
7:e23332016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J, Ahn C, Chun CH and Jin EJ: A long
non-coding RNA, GAS5, plays a critical role in the regulation of
miR-21 during osteoarthritis. J Orthop Res. 32:1628–1635. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colombo T, Farina L, Macino G and Paci P:
PVT1: A rising star among oncogenic long noncoding RNAs. Biomed Res
Int. 2015:3042082015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Q, Zhang X, Dai L, Hu X, Zhu J, Li L,
Zhou C and Ao Y: Long noncoding RNA related to cartilage injury
promotes chondrocyte extracellular matrix degradation in
osteoarthritis. Arthritis Rheumatol. 66:969–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Li S, Luo Y, Liu Y and Yu N: LncRNA
PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as
a sponge for miR-488-3p. DNA Cell Biol. 36:571–580. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dimitrova N, Zamudio JR, Jong RM, Soukup
D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA and Jacks
T: LincRNA-p21 activates p21 in cis to promote Polycomb target gene
expression and to enforce the G1/S checkpoint. Mol Cell.
54:777–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang F, Zhang H, Mei Y and Wu M:
Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the
Warburg effect. Mol Cell. 53:88–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han W and Liu J: LncRNA-p21 inhibited the
proliferation of osteosarcoma cells via the miR-130b/PTEN/AKT
signaling pathway. Biomed Pharmacother. 97:911–918. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Musumeci G, Loreto C, Carnazza ML and
Martinez G: Characterization of apoptosis in articular cartilage
derived from the knee joints of patients with osteoarthritis. Knee
Surg Sports Traumatol Arthrosc. 19:307–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Musumeci G, Loreto C, Leonardi R,
Castorina S, Giunta S, Carnazza ML, Trovato FM, Pichler K and
Weinberg AM: The effects of physical activity on apoptosis and
lubricin expression in articular cartilage in rats with
glucocorticoid-induced osteoporosis. J Bone Miner Metab.
31:274–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Musumeci G, Castrogiovanni P, Trovato FM,
Weinberg AM, Al-Wasiyah MK, Alqahtani MH and Mobasheri A:
Biomarkers of chondrocyte apoptosis and autophagy in
osteoarthritis. Int J Mol Sci. 16:20560–20575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Musumeci G, Castrogiovanni P, Loreto C,
Castorina S, Pichler K and Weinberg AM: Post-traumatic caspase-3
expression in the adjacent areas of growth plate injury site: A
morphological study. Int J Mol Sci. 14:15767–15784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Zhu H, Yan X, Gu H, Gu Z and Liu F:
Endoplasmic reticulum stress participates in the progress of
senescence and apoptosis of osteoarthritis chondrocytes. Biochem
Biophys Res Commun. 491:368–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu J, Zheng Y, Zhang H and Sun H:
Targeting cancer cell metabolism: The combination of metformin and
2-Deoxyglucose regulates apoptosis in ovarian cancer cells via p38
MAPK/JNK signaling pathway. Am J Transl Res. 8:4812–4821.
2016.PubMed/NCBI
|
|
28
|
Zhu J, Zheng Y, Zhang H, Zhu J and Sun H:
Low concentration of chloroquine enhanced efficacy of cisplatin in
the treatment of human ovarian cancer dependent on autophagy. Am J
Transl Res. 9:4046–4058. 2017.PubMed/NCBI
|
|
29
|
Perry RB and Ulitsky I: The functions of
long noncoding RNAs in development and stem cells. Development.
143:3882–3894. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tay Y, Karreth FA and Pandolfi PP:
Aberrant ceRNA activity drives lung cancer. Cell Res. 24:259–260.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang Y, Song J, Kim D, Ahn C, Park S, Chun
CH and Jin EJ: PCGEM1 stimulates proliferation of osteoarthritic
synoviocytes by acting as a sponge for miR-770. J Orthop Res.
34:412–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang G, Wu Y, Xu D and Yan X: Long
noncoding RNA UFC1 promotes proliferation of chondrocyte in
osteoarthritis by acting as a sponge for miR-34a. DNA Cell Biol.
35:691–695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang N, Fu Y, Zhang H, Sima H, Zhu N and
Yang G: LincRNA-p21 activates endoplasmic reticulum stress and
inhibits hepatocellular carcinoma. Oncotarget. 6:28151–28163.
2015.PubMed/NCBI
|
|
34
|
Wang X, Ruan Y, Wang X, Zhao W, Jiang Q,
Jiang C, Zhao Y, Xu Y, Sun F, Zhu Y, et al: Long intragenic
non-coding RNA lincRNA-p21 suppresses development of human prostate
cancer. Cell Prolif. 50:e123182017. View Article : Google Scholar
|
|
35
|
Wang G, Li Z, Zhao Q, Zhu Y, Zhao C, Li X,
Ma Z, Li X and Zhang Y: LincRNA-p21 enhances the sensitivity of
radiotherapy for human colorectal cancer by targeting the
Wnt/β-catenin signaling pathway. Oncol Rep. 31:1839–1845. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang W, Yu H, Shen Y, Liu Y, Yang Z and
Sun T: MiR-146b-5p overexpression attenuates stemness and
radioresistance of glioma stem cells by targeting
HuR/lincRNA-p21/β-catenin pathway. Oncotarget. 7:41505–41526.
2016.PubMed/NCBI
|