Introduction
Wound healing is a dynamic and coordinated process
consisting of a sequence of cell and tissue repair that is promoted
by multiple growth factors, including vascular endothelial growth
factor (VEGF), endothelial growth factor (EGF), fibroblast growth
factor and platelet-derived growth factor (PDGF) (1,2). The
human wound healing process may be divided into three or four
distinct phases in a coordinated series of events that includes
inflammation, proliferation and extracellular matrix (ECM)
remodeling (3). In addition,
neoangiogenesis, re-epithelization and the production of novel ECM
are vital to the wound healing process (4).
Polydeoxyribonucleotide (PDRN) is an Oncorhynchus
mykiss (salmon trout) or Oncorhynchus keta (chum salmon)
sperm DNA polymer with a specific molecular weight between 50 and
1,500 kDa with biological therapeutic activity (5). This molecular weight range of PDRN
[classic or conventional PDRN] has been applied to promote tissue
regeneration in various pathological conditions (6–8). The
final PDRN products are produced by controlled processes that may
contain sources of purine and pyrimidine
deoxynucleosides/deoxynucleotides. The classic PDRN molecular
weight of ~132 kDa is formed from purification and sterilization
processes (5,9).
Classic PDRN is an A2A receptor agonist
(10,11). Multiple in vitro and in
vivo studies demonstrated that PDRN serves as an
anti-inflammatory and tissue-regenerating agent. Treatment with
classic PDRN significantly improved clinical symptoms by perturbing
cytokine signaling in a collagen-induced osteoarthritis model.
Classic PDRN upregulates VEGF, collagen and cellular tumor antigen
p53; it functions as a negative regulator for tumor necrosis
factor-α, high mobility group protein B1 and metalloproteinase in
tissue regeneration (7,9). Notably, previous clinical studies
have demonstrated that PDRN promotes rapid autologous skin graft
healing and corneal epithelium regeneration following
photorefractive keratectomy, and alleviates pain and disability in
patients with rotator cuff disease (12–14).
Furthermore, classic PDRN improves wound closure and promotes
re-epithelialization in patients with refractory diabetic foot
ulcers (15).
Accumulating evidence suggests that PDRN is a safe
and immune response-free substance in multiple clinical settings
(15,16). A recent study reported that O.
keta- and O. mykiss-derived PDRN injections had the same
wound healing effects in full-thickness animal models (17). However, the optimal molecular
weight of PDRNs for improving the quality and efficacy of wound
regeneration has not been determined. In the present study, it was
examined whether PDRNs across a range of sizes promoted healing
following experimental injury, and whether this effect results from
migration processes.
Materials and methods
Animal model
The experimental protocol used in the present study,
including the use of animals, was approved by the Institutional
Animal Care and Use Committee, Yonsei University Wonju College of
Medicine (Wonju, Korea; approval no. YWC-160504-1). Male hairless
mice (SHK1; n=32) aged 8 weeks and weighing 25–30 g were obtained
from Orient Bio, Inc. (Seongnam, Korea). Mice were randomly divided
into four groups: Control (n=8); low (low-PDRN; <50 kDa; n=8);
middle (classic PDRN; 50–1,500 kDa; n=8); and high (high-PDRN;
>1,500 kDa; n=8) molecular weight PDRNs. All PDRN products were
obtained from Pharma Research Products Co., Ltd. (Pangyo, Korea).
All animals were provided with standard laboratory food and water
ad libitum. The mice were raised at a constant temperature
(22±3°C) and relative humidity (50±10%) in a 12 h light/dark cycle.
Wound production was performed under general anesthetic isoflurane
inhalation. Under aseptic conditions, a 4 mm biopsy punch (Kai
Europe GmbH, Solingen, Germany) was used to produce wounds in the
skin of the mice. Following the procedure, mice were treated with a
daily intraperitoneal (i.p.) administration of PDRNs at a dose of 8
mg/kg (day 0 to 6). The control group received i.p. injection of
vehicle (0.9% NaCl) at the same volume and time as the treatment
group.
Cell culture
A stable human fibroblast cell line (CCD-986SK;
Korean Cell Line Bank, Seoul, Korea) was cultured in 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin and streptomycin in high glucose Dulbecco's modified
Eagle's medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) in a 37°C humidified atmosphere of 5% CO2.
In vitro scratch assay
The human fibroblast cells were seeded at
1×106 cells/well in a 6-well plate and grown to full
confluence. Cells were scratched with a 200 µl pipette tip across
the center of the wells. In order to distinguish cell migration
from proliferation, all wound-healing assays were performed with or
without anti-tumor drug mitomycin C (cat. no. M4287; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) treatment, at a final concentration
of 5 µg/ml. Images were captured using a light microscope (×100),
24 h after treatment at 37°C with the drug (time 0 was the initial
time point). The healing area (%) was measured using Image J
software version 1.8 (National Institutes of Health, Bethesda, MD,
USA).
Western blotting
Cells were incubated in 1% FBS and 1% antibiotics in
high glucose DMEM for 48 h, treated at 37°C with PDRNs (50 µg/ml)
or EGF (30 ng/ml) for 24 h, and subsequently washed with PBS and
lysed with radioimmunoprecipitation assay buffer (Intron
Biotechnology, Inc., Seongnam, Korea) containing protease and
phosphatase inhibitors (Roche Diagnostics, Basel, Switzerland).
Extracts were isolated by centrifugation at 4°C and 12,000 × g for
15 min. Protein concentration was measured using the Bio-Rad
Protein Assay reagent (Bio-Rad Laboratories, Hercules, CA, USA).
Protein (10 µg/lane) were loaded and electrophoresis was conducted
on 10% SDS-PAGE, and transferred to polyvinylidene difluoride
membranes. The membranes were blocked in 5% bovine serum albumin
(Bioshop Canada, Inc., Burlington, ON, Canada) in Tris-buffered
saline containing 0.1% Tween-20 for 1 h at room temperature. The
blocked membranes were incubated with primary antibodies overnight
at 4°C with agitation, followed by incubation with a secondary
antibody of horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (1:3,000; cat. no. sc-2357; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
The blots were visualized using the Chemiluminescence Western Blot
Detection System (BioSpectrum® 600 Imaging System; UVP,
LLC, Upland, CA, USA). Primary antibodies against phosphorylated
(p)-c-Jun N-terminal kinase (JNK; 1:1,000, cat. no. 9251S), total
(t)-JNK (1:1,000; cat. no. 9252S), p-focal adhesion kinase (FAK;
1:1,000; cat. no. 8556S), t-FAK (1:1,000; cat. no. 13009S),
p-extracellular signal-regulated kinase (ERK)1/2 (1:3,000; cat. no.
9101S) and t-ERK1/2 (1:3,000; cat. no. 9102S) were acquired from
Cell Signaling Technology, Inc. (Danvers, MA, USA), and an antibody
against α-tubulin (1:1,000; cat. no. sc-8035) was obtained from
Santa Cruz Biotechnology, Inc.
Immunohistochemistry
For immunohistochemistry, mice were sacrificed at
days 3 and 7 and tissues were harvested from normal and wounded
areas. All tissues were fixed in 4% paraformaldehyde for at least
48 h at room temperature. Following fixation, tissues were
dehydrated in graded ethanol from 70, 80, 90, 95 to 100%, cleared
in xylene and embedded in paraffin. Sections (4 µm) were mounted on
glass slides at 45°C, dewaxed using xylene, rehydrated with graded
ethanol from 100, 95, 90, 80 and 70% to distilled water and stained
at room temperature with hematoxylin and eosin (H&E) or
Masson's Trichrome. As part of the histological evaluation, all
slides were examined by a pathologist without knowledge of
treatments under a light microscope with magnification, ×20-100.
The area of collagen portion (%) was measured using Image J
software.
Statistical analysis
All experiments were repeated at least three times.
Data analysis was performed with the Prism software (version 5;
GraphPad Software, Inc., La Jolla, CA, USA). All data are presented
as the mean ± standard error of the mean. Statistical comparisons
between two groups were determined using a two-tailed unpaired
Student's t-test. Multiple comparisons were determined using
one-way analysis of variance followed by Tukey's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of PDRN size on wound closure
in mice skin
Multiple previous studies have demonstrated that
PDRNs promote wound healing in various animal models (6,10,18–20).
To evaluate the effect of PDRNs on wound healing, 8-week old
hairless (SKH1) mice were given different sizes of PDRNs daily for
a week. Based on previous results of in vivo pharmacological
tests (21), 8 mg/kg doses of
PDRNs were administered (6,19).
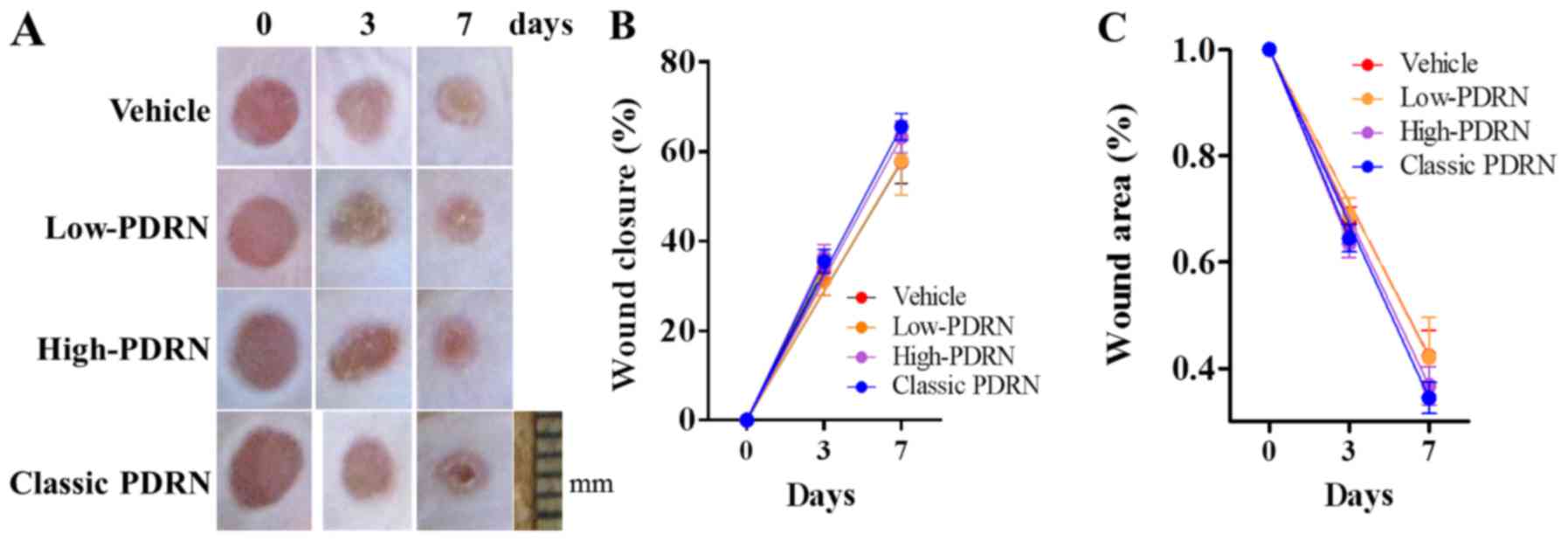
To evaluate wound closure, images were taken of PDRN-treated mouse
skin at different healing stages (days 3 and 7). Administration of
classic PDRN demonstrated no significant differences in wound
closure or wound area compared with low- and high-PDRNs (Fig. 1).
Optimal PDRN improves the quality of
wound closure in mice
Although differences in PDRN molecular weight did
not affect the apparent surface wound closure, it did affect the
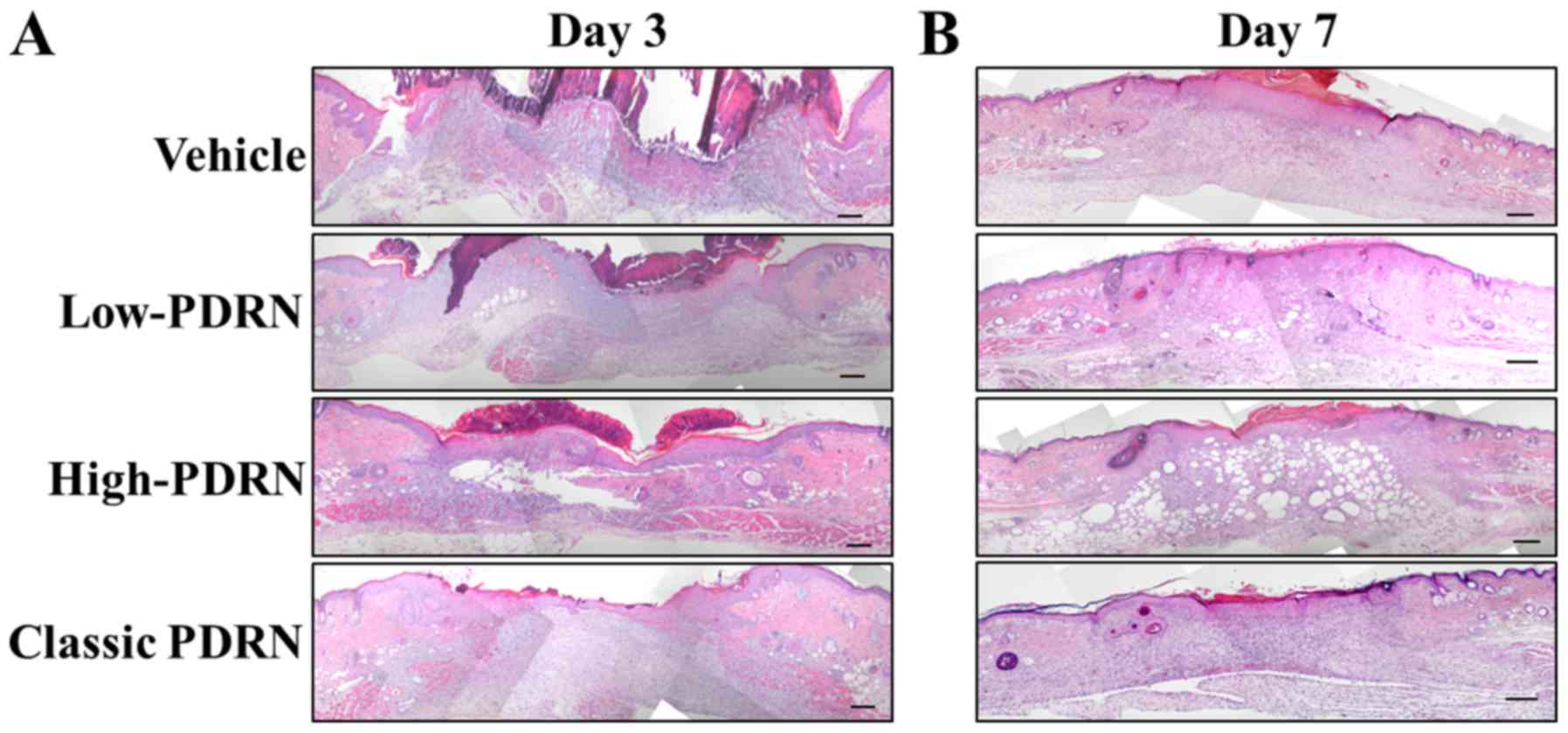
quality of wound repairing. To analyze wound healing quality
following treatment with PDRNs, the tissue repair process was
monitored by H&E staining. Among all the treatments, classic
PDRN-treated mice demonstrated the best wound closure quality
(Fig. 2). Although the wound
closure was similar among the different groups, treatment with
classic PDRN resulted in less lipid accumulation and a normal wound
healing process. This result suggests that among the DNA mixtures,
classic PDRN improved the quality of wound regeneration in mice the
most.
Treatment with PDRN increases the
collagen portion in wounded skin
The normal wound healing process primarily
progresses based on the balance between collagen synthesis and
collagen catabolism by matrix metalloproteinases (MMPs), and the
adult wound bed predominantly contains type I collagen deposition
during ECM remodeling (22). To
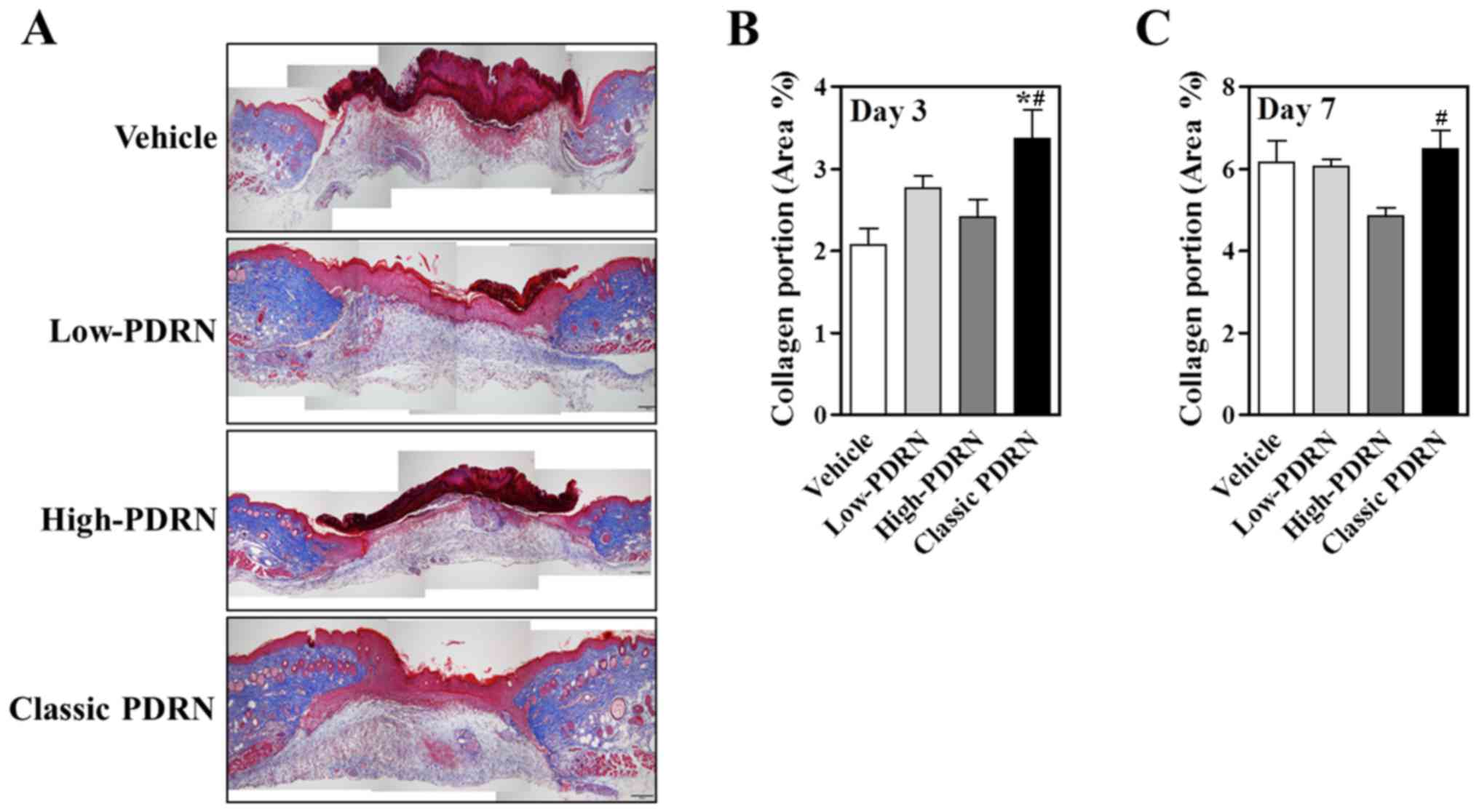
determine whether classic PDRN affected collagen fiber
accumulation, Masson's Trichrome staining was examined to analyze
collagen composition. On day 3, the collagen portion was
significantly increased in classic PDRN-treated mouse skin compared
with skin treated with other sizes of PDRN (Fig. 3A and B). At day 7, the majority of
the PDRN-treated groups demonstrated similar collagen composition
(Fig. 3C). However, compared with
the other groups, the classic PDRN-treated group demonstrated
increased collagen production and a decreased wound area; whereas,
the other groups exhibited blood clots and were still in the early
stages of wound healing. This data strongly supports the hypothesis
that classic PDRN facilitates accumulation of collagen in earlier
wound healing stages compared with other DNA mixtures.
Effect of PDRNs on migration in human
fibroblasts
Although PDRN has been implicated in the process of
repair and regeneration, the underlying mechanisms remain largely
elusive. Wound healing involves cell migration and proliferation
during wound closure. The mitogen-activated protein kinase (MAPK)
pathway serves an important role in the regulation of cell
migration and wound healing (23).
Signaling by JNK is required for wounded epithelial cells to close
the wound (24). Furthermore,
extracellular signals stimulate cellular processes, including
migration and proliferation, which are stimulated by FAK (25). To determine the mechanism
underlying PDRN-mediated wound healing and determine whether it is
mediated through the activation of FAK and JNK, FAK and JNK
activation with vehicle and the different PDRN treatments (50
µg/ml) were examined (Fig. 4A).
Classic PDRN induced the phosphorylation of JNK 24 h after
treatment. FAK and ERK phosphorylation increased in the high and
classic PDRN treatments compared with the vehicle treatment.
However, the total forms additionally increased; FAK and ERK were
not significantly activated by high and classic PDRN (Fig. 4B). These results suggest that
classic PDRN activates JNK, which may be important for the classic
PDRN-mediated wound healing process. To determine whether classic
PDRN-induced wound closure was mediated by proliferation or
migration, an in vitro scratch assay was conducted and
subsequently incubated with EGF (a known promoter of wound
healing), PDRNs or vehicle with mitomycin C, a known inhibitor of
proliferation, for 24 h. Images were captured from the initial
point of cell migration at 0 h and at a scratch after 18 h
(Fig. 4C). In the presence of
vehicle, the wound area gradually closed and the rate of wound
closure was significantly increased with EGF or classic PDRN
treatment, suggesting that classic PDRN accelerated wound closure
in human fibroblasts (Fig. 4D).
EGF increased cell proliferation significantly compared with
vehicle without mitomycin C; whereas, treatment with PDRNs did not
affect proliferation (data not shown). This result suggests that
classic PDRN may promote wound healing by increasing cell migration
to attempt closure of the wound bed in human fibroblasts.
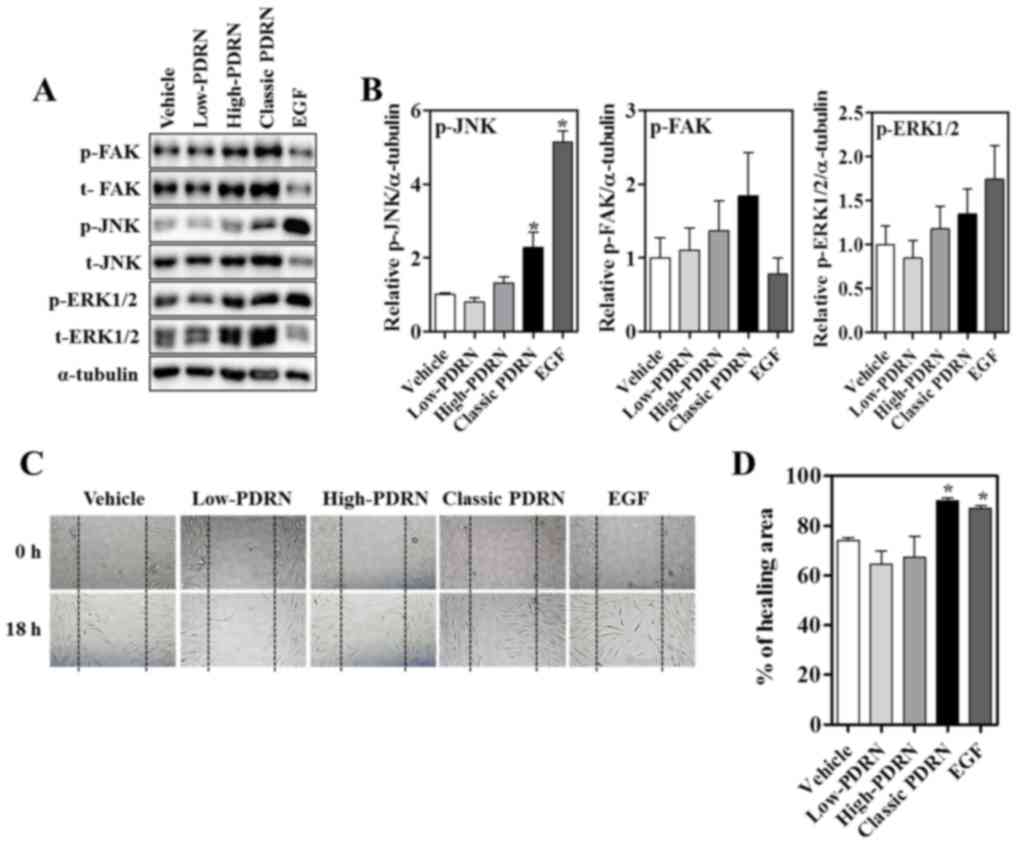 | Figure 4.Effect of PDRNs on migration in human
fibroblasts. (A) Human fibroblasts were treated with EGF and PDRNs
for 24 h and subjected to western blotting with p-FAK, p-ERK,
p-JNK, total form of FAK, ERK, JNK and α-tubulin antibodies. (B)
Quantitative densitometry analysis for p-JNK, p-FAK and p-ERK1/2
expression. Data are presented as the mean ± standard error of the
mean. *P<0.05 vs. vehicle. (C) Human fibroblasts were treated
with vehicle, PDRNs (50 µg/ml) or EGF (30 ng/ml) following initial
scratching to induce a wound. Representative images of wounds in
human fibroblasts were taken at baseline (0 h) and 18 h later. (D)
Quantified percentage of the wound healing area. Data are presented
as the mean ± standard error of the mean. *P<0.05 vs. vehicle.
PDRNs, polydeoxyribonucleotides; classic PDRN, classic
polydeoxyribonucleotide; EGF, epidermal growth factor; p,
phosphorylated; t, total; FAK, focal adhesion kinase; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase. |
Discussion
Polydeoxyribonucleotide is a DNA mixture with
molecular weights ranging between 50 and 1,500 kDa, produced by
regulated purification and sterilization processes from
Oncorhynchus mykiss (salmon trout) or Oncorhynchus
keta (chum salmon) sperm DNA (9). Multiple previous studies have
demonstrated that classic PDRN is associated with tissue repair, in
addition to anti-ischemia and anti-inflammation responses (26–28).
In the present study, it was investigated whether a diverse range
of DNAs may exert similar effects under pathophysiological
conditions. The effect of diversely sized DNAs (<50 kDa;
50–1,500 kDa; >1,500 kDa), including classic PDRN, has not been
studied. The wound healing effect of differently sized PDRNs was
examined and it was identified that classic PDRN stimulated
positive factors associated with migration in the wound bed and a
well ordered wound healing process.
A number of previous studies have demonstrated that
classic PDRN functions as a growth promoter in the regeneration of
various tissues, including wounded skin, and in osteoarthritis and
ischemic-reperfusion injuries (10,29,30).
Based on the beneficial effects of PDRN, the effects of different
molecular size DNA mixtures on wound healing were investigated.
Epithelial cells begin to proliferate at the wound margin following
injury, and epithelial migration may be subsequently triggered by
growth factors and chemokines, including VEGF, PDGF and
transforming growth factor-β (31). In the wound-healing phase,
granulation tissue begins the remodeling process via collagen,
fibronectin and hyaluronic acid (31). It was identified that among the
sizes examined, classic PDRN not only improved wound closure
quality; however, additionally promoted the accumulation of
collagen. The wound bed exhibited increased re-epithelialization
subsequent to wounding, following treatment with classic PDRN
compared with the other groups.
Furthermore, proliferation and migration promote the
wound healing process. JNK, FAK and/or MAPK signaling promote cell
proliferation and migration (23–25,32).
Promotion of cell motility is a well-known function of FAK.
Treatment with PDRN induced an increase in the expression of the
phosphorylated and total forms of FAK and ERK. Although there were
no differences in the expression levels of activated FAK and ERK,
PDRN may still regulate FAK and ERK expression. Treatment with EGF,
a promoter of proliferation and migration, demonstrated
phosphorylation of JNK and ERK. According to the present results,
EGF stimulates JNK and ERK signaling to promote wound-healing
processes in human fibroblasts. Notably, the observed increased JNK
activity and wound closure effect with mitomycin C supports the
hypothesis that the beneficial effects of classic PDRN on wound
healing may be mediated by accelerating cell migration in human
fibroblasts. Compared with low or high molecular weight PDRNs,
middle range classic PDRN demonstrated better efficacy in wound
healing processes. Numerous previous studies have reported that
classic PDRN regulates cell proliferation, in addition to growth
factor and pro-inflammatory signaling (33–35).
Therefore, the wound healing ability of classic PDRN is not due to
environmental improvement; however, rather more likely to be a
result of numerous factors, including growth factors and chemokines
during the wound healing process. JNK phosphorylation leads to
transcription factors and target gene activation, including MMPs,
and thereby promote wound healing (32). Although the present study
demonstrated classic PDRN-mediated regulation of multiple signaling
cascades, including total proteins of FAK and ERK, its downstream
effectors and specific pathways linking to wound healing remain to
be determined. In addition, how classic PDRN exerted pleotropic
effects on the wound healing process requires further study.
Therefore, future studies are required to examine the mechanism by
which classic PDRN promotes wound healing via regulating multiple
downstream effectors, including MMPs.
In conclusion, all PDRNs are derived from the same
DNA and may share the same biological effects on cell migration and
proliferation. In the present study, it was demonstrated that a
broad middle molecular size of PDRN ranging between 50–1,500 kDa
improved the quality of wound regeneration by promoting migration
in a mouse skin wound model. Notably, classic PDRN-stimulating JNK
signaling is critical for cell migration to improve the healing
process. Collectively, the present results provide a novel
perspective on the most effective molecular size of PDRN and the
signaling for tissue repair, and suggest a novel potential drug to
aid in the wound healing process.
Acknowledgements
All polydeoxyribonucleotide products were provided
by Pharma Research Products Co., Ltd. (Pangyo, Korea).
Funding
The present study was supported by the Medical
Research Center Program (grant no. 2017R1A5A2015369) and the Basic
Science Research Program (grant nos. 2015R1D1A1A01060454 and
2017R1D1A3B03031760) through the National Research Foundation of
Korea.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KHH, JHK and EYP designed the study, conducted the
experiments, analyzed the data and participated in writing the
paper; KHH and EYP conducted and analyzed the in vivo wound
healing experiment; JHK conducted and analyzed the in vitro
experiments; SKC designed and supervised the entire project and
wrote the final manuscript. All authors read, commented and
approved the paper.
Ethics approval and consent to
participate
The experimental protocol used in the present study,
including the use of animals, was approved by the Institutional
Animal Care and Use Committee, Yonsei University Wonju College of
Medicine (Wonju, Korea; approval no. YWC-160504-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wahl SM, Wong H and McCartney-Francis N:
Role of growth factors in inflammation and repair. J Cell Biochem.
40:193–199. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greenhalgh DG, Sprugel KH, Murray MJ and
Ross R: PDGF and FGF stimulate wound healing in the genetically
diabetic mouse. Am J Pathol. 136:1235–1246. 1990.PubMed/NCBI
|
|
3
|
Gilmore MA: Phases of wound healing.
Dimens Oncol Nurs. 5:32–34. 1991.PubMed/NCBI
|
|
4
|
Yu A, Niiyama H, Kondo S, Yamamoto A,
Suzuki R and Kuroyanagi Y: Wound dressing composed of hyaluronic
acid and collagen containing EGF or bFGF: Comparative culture
study. J Biomater Sci Polym Ed. 24:1015–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tonello G, Daglio M, Zaccarelli N,
Sottofattori E, Mazzei M and Balbi A: Characterization and
quantitation of the active polynucleotide fraction (PDRN) from
human placenta, a tissue repair stimulating agent. J Pharm Biomed
Anal. 14:1555–1560. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galeano M, Bitto A, Altavilla D, Minutoli
L, Polito F, Calò M, Lo Cascio P, Stagno d'Alcontres F and
Squadrito F: Polydeoxyribonucleotide stimulates angiogenesis and
wound healing in the genetically diabetic mouse. Wound Repair
Regen. 16:208–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Altavilla D, Squadrito F, Polito F, Irrera
N, Calò M, Lo Cascio P, Galeano M, La Cava L, Minutoli L, Marini H
and Bitto A: Activation of adenosine A2A receptors restores the
altered cell-cycle machinery during impaired wound healing in
genetically diabetic mice. Surgery. 149:253–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bitto A, Polito F, Altavilla D, Minutoli
L, Migliorato A and Squadrito F: Polydeoxyribonucleotide (PDRN)
restores blood flow in an experimental model of peripheral artery
occlusive disease. J Vasc Surg. 48:1292–1300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Squadrito F, Bitto A, Irrera N, Pizzino G,
Pallio G, Minutoli L and Altavilla D: Pharmacological activity and
clinical use of PDRN. Front Pharmacol. 8:2242017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veronesi F, Dallari D, Sabbioni G, Carubbi
C, Martini L and Fini M: Polydeoxyribonucleotides (PDRNs) from skin
to musculoskeletal tissue regeneration via adenosine A2A
receptor involvement. J Cell Physiol. 232:2299–2307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thellung S, Florio T, Maragliano A,
Cattarini G and Schettini G: Polydeoxyribonucleotides enhance the
proliferation of human skin fibroblasts: Involvement of A2
purinergic receptor subtypes. Life Sci. 64:1661–1674. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valdatta L, Thione A, Mortarino C, Buoro M
and Tuinder S: Evaluation of the efficacy of
polydeoxyribonucleotides in the healing process of autologous skin
graft donor sites: A pilot study. Curr Med Res Opin. 20:403–408.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Zheng Z, Kang JS, Kim DY, Oh SH
and Cho SB: Therapeutic efficacy of autologous platelet-rich plasma
and polydeoxyribonucleotide on female pattern hair loss. Wound
Repair Regen. 23:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon YC, Lee DH, Lee MY and Yoon SH:
Polydeoxyribonucleotide injection in the treatment of chronic
supraspinatus tendinopathy: A case-controlled, retrospective,
comparative study with 6-month follow-up. Arch Phys Med Rehabil.
98:874–880. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Squadrito F, Bitto A, Altavilla D,
Arcoraci V, De Caridi G, De Feo ME, Corrao S, Pallio G, Sterrantino
C, Minutoli L, et al: The effect of PDRN, an adenosine receptor A2A
agonist, on the healing of chronic diabetic foot ulcers: Results of
a clinical trial. J Clin Endocrinol Metab. 99:E746–E753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Caridi G, Massara M, Acri I, Zavettieri
S, Grande R, Butrico L, de Franciscis S and Serra R: Trophic
effects of polynucleotides and hyaluronic acid in the healing of
venous ulcers of the lower limbs: A clinical study. Int Wound J.
13:754–758. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim S, Kim J, Choi J, Jeong W and Kwon S:
Polydeoxyribonucleotide improves peripheral tissue oxygenation and
accelerates angiogenesis in diabetic foot ulcers. Arch Plast Surg.
44:482–489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong W, Yang CE, Roh TS, Kim JH, Lee JH
and Lee WJ: Scar prevention and enhanced wound healing induced by
polydeoxyribonucleotide in a rat incisional wound-healing model.
Int J Mol Sci. 18:pii: E1698. 2017. View Article : Google Scholar
|
|
19
|
Polito F, Bitto A, Galeano M, Irrera N,
Marini H, Calò M, Squadrito F and Altavilla D:
Polydeoxyribonucleotide restores blood flow in an experimental
model of ischemic skin flaps. J Vasc Surg. 55:479–488. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JH, Han JW, Byun JH, Lee WM, Kim MH
and Wu WH: Comparison of wound healing effects between
Oncorhynchus keta-derived polydeoxyribonucleotide (PDRN) and
Oncorhynchus mykiss-derived PDRN. Arch Craniofac Surg.
19:20–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon JW, Lee JI, Shin HP, Cha JM, Joo KR,
Kim SH, Ko IG, Jin JJ, Kim SE and Kim CJ: Adenosine
A2A-receptor agonist polydeoxyribonucleotide promotes
gastric ulcer healing in Mongolian gerbils. Anim Cells Syst.
18:399–406. 2014. View Article : Google Scholar
|
|
22
|
Bohn G, Liden B, Schultz G, Yang Q and
Gibson DJ: Ovine-based collagen matrix dressing: Next-generation
collagen dressing for wound care. Adv Wound Care (New Rochelle).
5:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kajanne R, Miettinen P, Mehlem A, Leivonen
SK, Birrer M, Foschi M, Kähäri VM and Leppä S: EGF-R regulates MMP
function in fibroblasts through MAPK and AP-1 pathways. J Cell
Physiol. 212:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gazel A, Banno T, Walsh R and Blumenberg
M: Inhibition of JNK promotes differentiation of epidermal
keratinocytes. J Biol Chem. 281:20530–20541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: in command and control of cell motility. Nat
Rev Mol cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong EK, Jang HJ, Kim SS, Lee SY, Oh MY,
Kim HJ, Eom DW, Ham JY and Han DJ: Protective effect of
polydeoxyribonucleotide against renal ischemia-reperfusion injury
in mice. Transplant Proc. 48:1251–1257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JY, Pak CS, Park JH, Jeong JH and Heo
CY: Effects of polydeoxyribonucleotide in the treatment of pressure
ulcers. J Korean Med Sci. 29 Suppl 3:S222–S227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bitto A, Polito F, Irrera N, D'Ascola A,
Avenoso A, Nastasi G, Campo GM, Micali A, Bagnato G, Minutoli L, et
al: Polydeoxyribonucleotide reduces cytokine production and the
severity of collagen-induced arthritis by stimulation of adenosine
A(2A) receptor. Arthritis Rheum. 63:3364–3371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buffoli B, Favero G, Borsani E, Boninsegna
R, Sancassani G, Labanca M, Rezzani R, Nocini PF, Albanese M and
Rodella LF: Sodium-DNA for bone tissue regeneration: An
experimental study in rat calvaria. Biomed Res Int.
2017:73209532017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noh TK, Chung BY, Kim SY, Kim SY, Lee MH,
Kim MJ, Youn CS, Lee MW and Chang SE: Novel anti-melanogenesis
properties of polydeoxyribonucleotide, a popular wound healing
booster. Int J Mol Sci. 17:14482016. View Article : Google Scholar :
|
|
31
|
Bao P, Kodra A, Tomic-Canic M, Golinko MS,
Ehrlich HP and Brem H: The role of vascular endothelial growth
factor in wound healing. J Surg Res. 153:347–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stevens LJ and Page-McCaw A: A secreted
MMP is required for reepithelialization during wound healing. Mol
Biol Cell. 23:1068–1079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bitto A, Oteri G, Pisano M, Polito F,
Irrera N, Minutoli L, Squadrito F and Altavilla D: Adenosine
receptor stimulation by polynucleotides (PDRN) reduces inflammation
in experimental periodontitis. J Clin Periodontol. 40:26–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guizzardi S, Galli C, Govoni P, Boratto R,
Cattarini G, Martini D, Belletti S and Scandroglio R:
Polydeoxyribonucleotide (PDRN) promotes human osteoblast
proliferation: A new proposal for bone tissue repair. Life Sci.
73:1973–1983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Belletti S, Uggeri J, Gatti R, Govoni P
and Guizzardi S: Polydeoxyribonucleotide promotes cyclobutane
pyrimidine dimer repair in UVB-exposed dermal fibroblasts.
Photodermatol Photoimmunol Photomed. 23:242–249. 2007. View Article : Google Scholar : PubMed/NCBI
|