Introduction
Peripheral arterial disease (PAD) is caused by
atherosclerotic occlusion of the arteries to the lower extremities.
PAD affects >200 million people worldwide and puts them at risk
of lower extremity amputation and mortality (1–3). As
total occlusions along the path of the major inflow arteries to the
lower extremities are common in patients with PAD, the blood flow
that is able to be delivered to the distal tissue becomes dependent
on the extent of neovascularization in the ischemic leg (4–6). In
the ischemic muscle, reactive oxygen species (ROS) impair
ischemia-stimulated angiogenesis and perfusion recovery (7–10).
Gene delivery of ROS scavengers was demonstrated to improve
perfusion recovery and reduce tissue loss in an experimental PAD
model (11,12).
Molecular hydrogen (H2), a physiological
regulatory gas molecule, is able to neutralize numerous types of
cytotoxic ROS and therefore acts as an antioxidant within the body
(13). Clinical trials have
demonstrated that H2 therapy improves the outcome of a
variety of diseases, including cerebral ischemia (14,15)
and diabetes (16). However, to
the best of our knowledge, the effects of H2 on PAD have
not been studied. The present study investigated the hypothesis
that H2 therapy may improve angiogenesis and perfusion
recovery by neutralizing ROS in experimental PAD.
Materials and methods
Hindlimb ischemia (HLI) model and
H2 treatment
A total of 36 male Balb/c mice (25–30 g) were used
in the present study; the mice were housed in a specific
pathogen-free laboratory environment with free access to food and
water under a 12-h light/dark cycle, an ambient temperature of
21±2°C and a constant humility of 50±10%. Following induction of
anesthesia (30–40 mg/kg pentobarbital via an intraperitoneal
injection), unilateral femoral artery ligation and excision were
performed on the left side of the mice, as described previously
(17,18). Immediately following surgery,
hydrogen-saturated water (1.6 ppm or 0.8 mM) or dehydrogenized
water was supplied to the mice (n=18 per group), and the water was
changed daily to ensure adequate H2 levels in the
drinking water. Hydrogen-saturated water was prepared daily using
an AquelaBlue electrolysis instrument (MiZ Co., Ltd, Kanagawa,
Japan), as described previously (19,20).
All procedures involving animal use conformed with the Guide for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health (Bethesda, MD, USA), and the protocol was
approved by the Institutional Animal Care Committee of Wuhan
University (Wuhan, China).
Perfusion recovery
Perfusion flow in the ischemic and contralateral
non-ischemic limbs was measured as described previously, with the
use of a laser Doppler perfusion imaging system (Perimed AB,
Järfälla, Sweden) (17,18). Perfusion was expressed as the ratio
of the left (ischemic) to the right (non-ischemic) hind limb and
was performed on days 0, 7, 14 and 21 following surgery. In mice
that developed autoamputation (2 in the hydrogen-saturated water
group and 6 in the control group), the perfusion ratio obtained
from the limb prior to autoamputation was used.
Immunofluorescence
For the assessment of capillary density, ischemic
gastrocnemius muscle sections from mice treated with
hydrogen-saturated water or dehydrogenized water at 21 days
post-HLI were used for immunofluorescent staining, as described
previously (17,21). Anti-platelet endothelial cell
adhesion molecule (CD31) antibody (rat anti-mouse CD31; 550274;
1:100; BD Pharmingen; BD Biosciences, San Jose, CA, USA) was
applied to acetone-fixed (−20°C for 30 min) cryosections (7 µm) of
ischemic gastrocnemius muscle specimens at 4°C overnight in
blocking solution (1% goat serum in saline solution; Wuhan Boster
Biological Technology Co., Ltd., Wuhan, China). Following rinsing
with PBS, Alexa Fluor 488 phalloidin (for muscle fiber staining;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the secondary
reagent goat anti-rat Alexa Fluor 555 (1:100; A-21434; Thermo
Fisher Scientific, Inc.) were applied for 1 h at room temperature.
Secondary antibody only, without primary antibody, was used as a
negative control to assess non-specific binding. Stained sections
were examined at ×200 magnification using an Olympus IX71
high-magnification microscope (Olympus Corporation, Tokyo, Japan).
Capillary densities were analyzed by counting five random
high-power fields (magnification, ×200), and were expressed as the
number of CD31+ cells per muscle fiber area, as
described previously (17,22).
For the assessment of arteries, α-smooth muscle
actin (1:50; Wuhan Boster Biological Technology Co., Ltd.) was
applied to the abductor muscle in the ischemic side 21 days after
HLI. A total of five microscopic fields were randomly selected and
counted in three tissue sections from each animal. Artery density
was expressed as the number of arteries counted in ×200 high-power
magnification fields.
Biochemical assay
Malondialdehyde (MDA) in the mouse ischemic muscle
homogenates was measured using thiobarbituric acid, as previously
described (23). The absolute
amount of MDA was read from a standard curve prepared from serial
dilutions of the primary standard. Activation of nitric oxide
(NO)-sensitive guanylyl cyclase (GC) leads to enhanced production
of the intracellular messenger cyclic guanosine monophosphate
cyclic (cGMP) (24). Therefore,
tissue cGMP levels in the ischemic muscle were measured in order to
assess tissue NO bioactivity using a cGMP Parameter Assay Kit
(KGE3; R&D Systems, Minneapolis, MN).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated and used for qPCR, as
previously described (21,25). qPCR was performed using
primers/probes for arginase-1 (arg1), tumor necrosis factor
(tnf) and 18S rRNA from Applied Biosystems, Thermo Fisher
Scientific, Inc. (Hs00163660_m1, Hs00174128_m1 and #Hs03003631_g1,
respectively). Quantitative normalization of cDNA in each sample
was performed using the expression of 18S rRNA as an internal
control. The generated Cq value of each gene was normalized to the
respective Cq value of 18S rRNA (ΔCq). Each gene was further
normalized to the average ΔCq value of its control group (ΔΔCq).
The final fold expression changes were calculated using the
equation 2−ΔΔCq (26).
Cell culture and in-vitro angiogenesis
assay
Human umbilical vein endothelial cells (HUVECs) were
purchased from Cyagen Biosciences (Santa Clara, CA, USA), and grown
in standard endothelial cell growth medium (Cell Applications,
Inc., San Diego, CA, USA) with 10% fetal bovine serum (FBS; Wuhan
Boster Biological Technology Co., Ltd.). To generate bone
marrow-derived macrophages (BMDMs), femurs from four 2-month-old
male Balb/c mice (18–22 g, purchased from the animal center of
Wuhan University, China), which were housed in the same conditions
as those mentioned above, were flushed with sterile Dulbecco's
modified Eagle's medium (DMEM; Wuhan Boster Biological Technology
Co., Ltd.). Following lysis of the red blood cells, total bone
marrow cells were plated in a Petri dish in DMEM medium with 10%
FBS and 100 ng/ml macrophage colony-stimulating factor (R&D
systems, Inc., Minneapolis, MN, USA) and were allowed to
differentiate for 7 days.
The in-vitro angiogenesis assay was performed
as previously described (27).
Briefly, HUVECs were plated at a density of 1×104
cells/well in 96-well dishes that were coated with growth
factor-reduced Matrigel (BD Biosciences). The cells were exposed to
hypoxia serum starvation conditions (HSS; mimic in vivo
ischemia) for 12 h with H2-saturated endothelial
starvation medium or dehydrogenized endothelial starvation medium
to assess tube formation. Hydrogen-saturated medium was prepared
daily using an AquelaBlue electrolysis instrument (MiZ Co., Ltd.,
Kanagawa, Japan). Each condition was applied to eight wells. The
degree of tube formation was determined by measuring the length of
the tubes and the number of loops in each well under ×40
magnification using Image J version 6.0 (National Institutes of
Health).
For the assessment of macrophage polarization, bone
marrow-derived macrophages (BMDMs) were treated with
H2-saturated or dehydrogenized medium under HSS for 24
h. Arg1 and tnf mRNA expression levels were measured
as the markers for M2/M1 polarization by qPCR, respectively.
Intracellular ROS assay
Intracellular ROS production was detected using the
nonfluorescent cell permeating compound,
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), as previously
described (28). In the presence
of ROS, DCFH-DA is hydrolyzed to the fluorescent product DCF.
HUVECs were incubated with 10 µM DCFH-DA for 45 min at 37°C, and
the fluorescence intensity of DCFH was read at 525 nm emission when
excited at 488 nm in a 96-well plate reader. Results are expressed
as a percentage of the control (cells cultured under normoxic
conditions) fluorescence intensity.
Statistical analysis
All results are presented as the mean ± standard
error and statistical analysis was performed using SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA). All the in vitro
studies were repeated 3 times. An unpaired t-test was used for
comparison between two groups, and comparisons in experiments with
≥3 groups were performed using one-way analysis of variance and the
Tukey post-hoc test. Differences in necrosis rate were analyzed by
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hydrogen-saturated water improves
perfusion recovery, angiogenesis and arteriogenesis in experimental
PAD
Balb/c mice receiving hydrogen-saturated water
exhibited improved perfusion recovery at 14 (49.2±2.5% vs.
38.7±2.6%; P<0.01) and 21 (67.9±3.8% vs. 50.5±4.3%; P=0.012)
days post HLI (Fig. 1A), and less
tissue necrosis (4 out of 18 vs. 10 out of 18, P=0.02) compared
with those receiving dehydrogenized water, 2 mice in
hydrogen-saturated water group and 6 in control group developed
auto-amputation in the first 7 days after HLI. The capillary
density was determined in the ischemic gastrocnemius muscle, and
mice receiving hydrogen-saturated water had a higher capillary
density compared with those receiving dehydrogenized water (2.2±0.2
vs. 0.96±0.1 capillaries/fiber; n=10 per group; P<0.01) 21 days
post-HLI (Fig. 1B). As Fig. 1C demonstrates, 21 days post-HLI,
mice received hydrogen-saturated water had a higher artery density
(2.4±0.3 vs. 1.0±0.06 capillaries/fiber; n=10 per group; P=0.01) in
the abductor muscle from the ischemic limb.
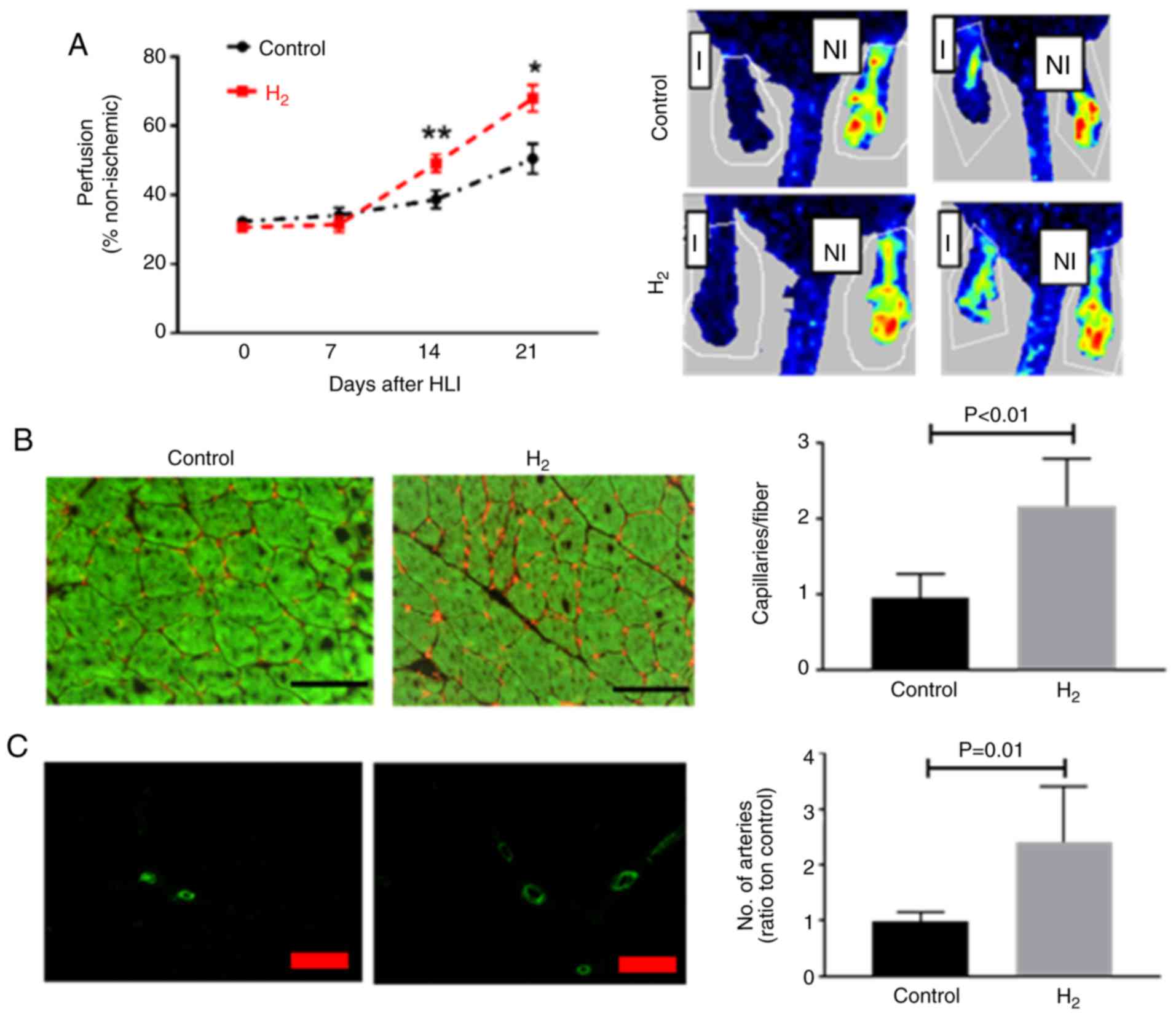 | Figure 1.H2-saturated water
improves perfusion recovery following HLI. (A) Laser Doppler
imaging indicated a significant increase in perfusion recovery in
Balb/c mice treated with H2-saturated water on days 14
and 21 following HLI (n=10 per group). (B) At day 21 following HLI,
the ischemic gastrocnemius muscle from mice treated with
H2-saturated water had a significantly higher capillary
density (CD31, red) when compared with those treated with
dehydrogenized water (n=8 mice/group). Scale bar, 60 µM. (C) Artery
density (α-smooth muscle actin, green) in the abductor muscle from
mice treated with H2-saturated water was higher compared
with the control mice (n=8 per group). Scale bar, 100 µM. Data are
presented as the mean ± standard error of the mean. *P<0.05,
**P<0.01 vs. respective control. H2, hydrogen
molecule-saturated water; control, mice with dehydrogenized water.
HLI, hindlimb ischemia; I, ischemic; NI, non-ischemic. |
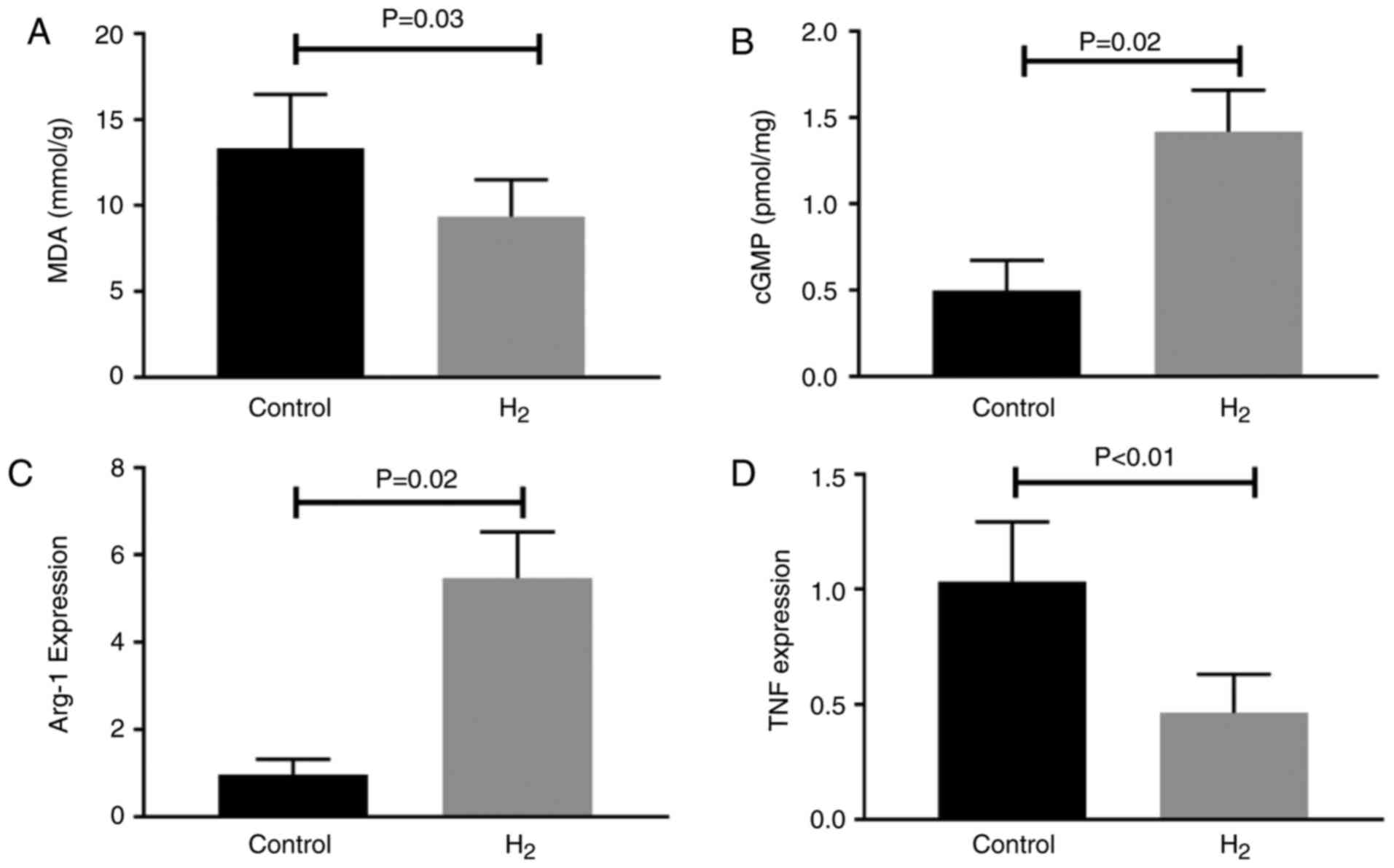
Hydrogen-saturated water decreases MDA
levels, increases cGMP levels and promotes M2-like macrophage
polarization in ischemic muscle
Day 7 following HLI was selected to study the
molecular alterations since, although hydrogen-saturated water
improved the long-term (14 and 21 days post-HLI) outcome in
experimental PAD, it did not alter perfusion recovery at this time
point. Lipid peroxidation, established by measuring MDA, is widely
used to assess ROS bioactivity in tissues (29). In the present study,
hydrogen-saturated water significantly decreased MDA levels in the
ischemic muscle 7 days subsequent to HLI (Fig. 2A).
NO exerts its effects via stimulation of
NO-sensitive GC in blood vessels, which leads to enhanced
production of the intracellular messenger cGMP (30). Levels of cGMP, the product of
NO-activated GC, were assessed in the present study. cGMP levels
were 2.8-fold higher (P<0.01) in ischemic hind-limbs from the
hydrogen-saturated water group compared with the control group,
thus providing evidence that H2 increases NO bioactivity
in ischemic muscles (Fig. 2B).
Macrophages have been reported to modulate
arteriogenesis and angiogenesis, which are important vascular
remodeling processes following PAD. In the present study,
arg1 and tnf, as the respective markers for M2-like
and M1-like macrophages, were measured. In the ischemic muscle from
experimental PAD, H2-saturated water significantly
increased the mRNA expression levels of arg-1 (Fig. 2C) and decreased the mRNA expression
levels of tnf (Fig.
2D).
Hydrogen-saturated water decreases ROS
levels and increases the levels of cGMP and angiogenesis in
endothelial cells and promotes M2-like-macrophage polarization in
vitro
In the cultured endothelial cells under HSS
conditions, hydrogen-saturated medium resulted in a significant
increase in tube formation as indicated by increased tube length
and number of loops (Fig. 3A).
Compared with normoxic conditions, ROS levels were increased, as
indicated by DCFH-DA assay, under HSS; however, hydrogen-saturated
medium significantly decreased ROS levels in HUVECs (Fig. 3B). cGMP levels were detected in the
cultured endothelial cell lysates, and hydrogen-saturated medium
was found to increase cGMP levels (Fig. 3C).
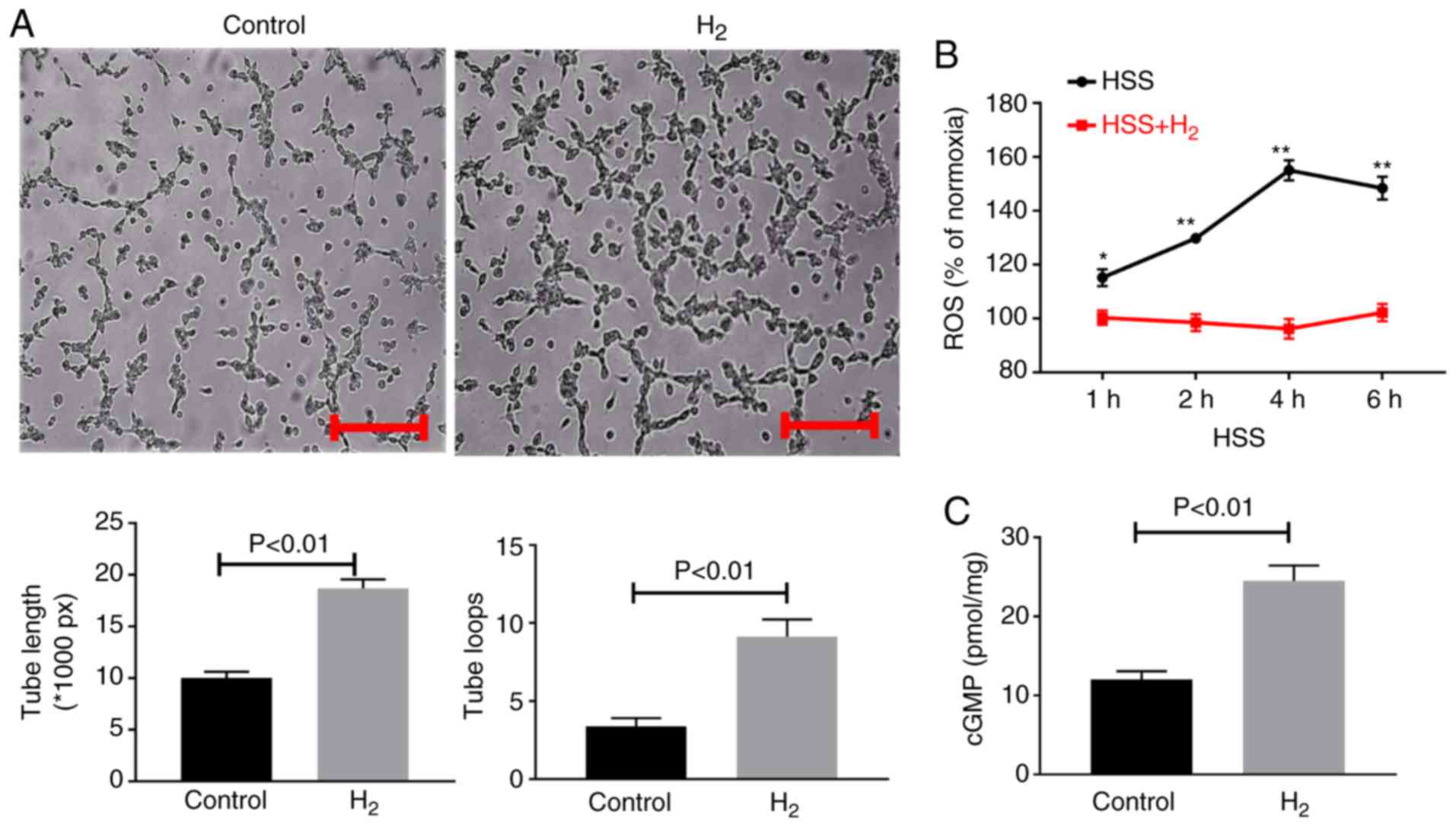 | Figure 3.H2-saturated medium
increases tube formation and cellular cGMP levels and decreases
cellular ROS levels in cultured HUVECs. (A) HUVECs were plated on
Matrigel with reduced growth factors and incubated for 12 h in HSS
conditions with H2-saturated medium or medium without
H2. H2 treatment resulted in enhanced tube
formation, which was quantified as total length of the cords per
visual field, and total loops per visual field as represented by
the bar graph. Scale bar, 100 µm. (B) H2-saturated
medium resulted in reduced ROS levels as indicated by
2′,7′-dichlorodihydrofluorescein diacetate staining in cultured
HUVECs 1–6 h under HSS. *P<0.05, **P<0.01 vs. respective
control. (C) H2-saturated medium resulted in increased
cellular cGMP levels 24 h under HSS. Data are representative of 2–3
separate batches of HUVECs (n=8–12 per group). Data are presented
as the mean ± standard error of the mean. H2, hydrogen
molecule-saturated medium; control, medium without H2.
ROS, reactive oxygen species; cGMP, cyclic guanine monophosphate;
HUVEC, human umbilical vein endothelial cell; HSS, hypoxia serum
starvation. |
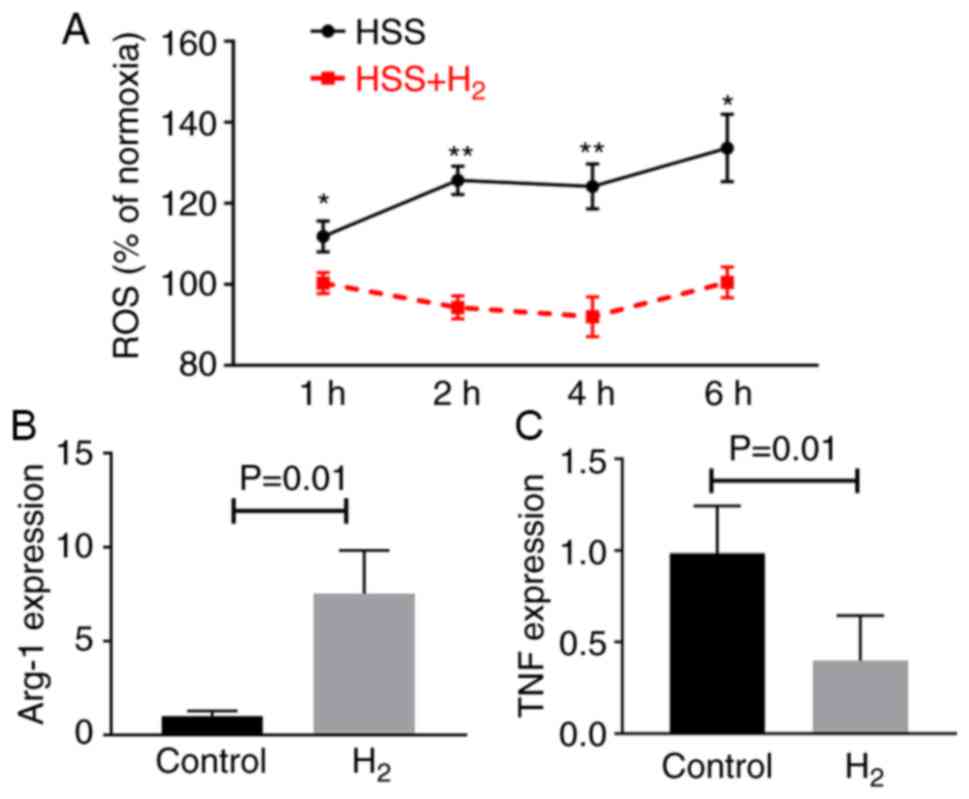
Consistent with the findings in the endothelial
cells, HSS increased ROS levels in the cultured BMDMs.
H2-saturated water significantly reduced ROS levels in
the BMDMs 1–6 h after exposure to HSS (Fig. 4A), which led to increased
expression levels of the M2-like macrophage marker (arg1)
and decreased expression levels of the M1-like macrophage marker
(tnf) (Fig. 4B and C).
These findings are consistent with our in vivo findings in
the mouse ischemic muscle.
Discussion
To best of our knowledge, this is the first study to
demonstrate that H2 improves perfusion recovery
angiogenesis and arteriogenesis in experimental PAD. Furthermore,
it was identified that H2-saturated water is sufficient
to neutralize ROS in ischemic muscle tissue, cultured endothelial
cells and macrophages under simulated ischemia, and subsequently
increases NO bioactivity and promotes M2-like macrophage
polarization.
The most important finding of the present study is
that molecular hydrogen improves perfusion recovery, angiogenesis
and arteriogenesis following PAD. Currently, there is no known
pharmaceutical therapy that is able to improve blood perfusion in
the ischemic limbs of patients with PAD (2,6).
Hydrogen gas inhalation or hydrogen-saturated water has been widely
used in a variety of clinical conditions, including strokes, and
its efficacy and safety in patients has been demonstrated in
previous studies (31,32). In line with these studies, the
present study demonstrated that oral administration of
H2 was effective for the treatment of PAD, which is a
convenient method for clinical use. Thus, the findings of the
present study may initiate the clinical study of H2 in
patients with PAD.
Another important finding of the present study is
that H2-saturated water increased NO bioavailability in
ischemic muscle tissue, as indicated by higher cGMP levels in
ischemic muscles that received treatment with molecular hydrogen.
Neovascularization is a physiological repair process that primarily
depends on NO, a bioactive gas that induces multiple pathways in
order to promote angiogenesis and tissue repair (30,33).
However, NO is not stable and may be converted to peroxynitrite in
the presence of ROS (34). Unlike
NO, peroxynitrite does not promote angiogenesis. H2 is
known to neutralize the most cytotoxic ROS, including ·OH and ONOO-
(13,35). In the present study, H2
decreased MDA levels in the ischemic muscle and ROS levels in
endothelial cells and macrophages. These results indicated that
H2 decreases the levels of ROS in experimental PAD, and
subsequently leads to increased bioavailability of NO.
In experimental PAD, monocyte/macrophage recruitment
into tissue supplied by the occluded vessel may induce potent
effects on neovascularization and tissue repair. The ability of
macrophages to modulate these processes is dependent on their
polarization state. M2-like macrophages serve critical roles in
inflammation resolution by secreting growth factors that induce
arteriogenesis and angiogenesis (36). In the present study, H2
increased the levels of M2-like macrophages, which may be explained
by their effects on ROS neutralization as ROS elevation has been
reported to induce macrophage M1 polarization through activation of
hypoxia-inducible factor 1 (37).
In conclusion, hydrogen-saturated water improves
perfusion recovery and increases angiogenesis and arteriogenesis by
neutralizing ROS, at least partially, in endothelial cells and
macrophages under ischemia. The present study may initiate further
experimental and clinical studies that examine pharmaceutical
approaches towards the treatment of PAD.
Acknowledgements
The authors would like to thank Dr Mingjie Yuan
(Department of Cardiology, Renmin Hospital of Wuhan University,
Hubei, China) for his comments on the writing of this
manuscript.
Funding
The present study was supported by a Grant from the
Planned Science and Technology Project of Hubei Province, China
(grant no. 2006A301A04).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF conceived the project and designed experiments.
JF, JZ, CC, HL, LW and YZ performed the experiments. JZ, CC and JF
wrote and edited the manuscript and all authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures involving animal use conformed with
the Guide for the Care and Use of Laboratory Animals published by
the US National Institutes of Health (Bethesda, MD, USA), and the
protocol was approved by the Institutional Animal Care Committee of
Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guerchet M, Aboyans V, Mbelesso P, Mouanga
AM, Salazar J, Bandzouzi B, Tabo A, Clément JP, Preux PM and
Lacroix P: Epidemiology of peripheral artery disease in elder
general population of two cities of Central Africa: Bangui and
Brazzaville. Eur J Vasc Endovasc Surg. 44:164–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Criqui MH and Aboyans V: Epidemiology of
peripheral artery disease. Circ Res. 116:1509–1526. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fowkes FG, Aboyans V, Fowkes FJ, McDermott
MM, Sampson UK and Criqui MH: Peripheral artery disease:
Epidemiology and global perspectives. Nat Rev Cardiol. 14:156–170.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Annex BH and Beller GA: Towards the
development of novel therapeutics for peripheral artery disease.
Trans Am Clin Climatol Assoc. 127:224–234. 2016.PubMed/NCBI
|
|
5
|
Ko SH and Bandyk DF: Therapeutic
angiogenesis for critical limb ischemia. Semin Vasc Surg. 27:23–31.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Annex BH: Therapeutic angiogenesis for
critical limb ischaemia. Nat Rev Cardiol. 10:387–396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gardner AW, Montgomery PS, Zhao YD,
Silva-Palacios F, Ungvari Z, Csiszar A and Sonntag WE: Association
between daily walking and antioxidant capacity in patients with
symptomatic peripheral artery disease. J Vasc Surg. 65:1762–1768.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loffredo L, Marcoccia A, Pignatelli P,
Andreozzi P, Borgia MC, Cangemi R, Chiarotti F and Violi F:
Oxidative-stress-mediated arterial dysfunction in patients with
peripheral arterial disease. Eur Heart J. 28:608–612. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loffredo L, Pignatelli P, Cangemi R,
Andreozzi P, Panico MA, Meloni V and Violi F: Imbalance between
nitric oxide generation and oxidative stress in patients with
peripheral arterial disease: Effect of an antioxidant treatment. J
Vasc Surg. 44:525–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muller MD, Drew RC, Blaha CA, Mast JL, Cui
J, Reed AB and Sinoway LI: Oxidative stress contributes to the
augmented exercise pressor reflex in peripheral arterial disease
patients. J Physiol. 590:6237–6246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HW, Lin A, Guldberg RE, Ushio-Fukai M
and Fukai T: Essential role of extracellular SOD in reparative
neovascularization induced by hindlimb ischemia. Circ Res.
101:409–419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saqib A, Prasad KM, Katwal AB, Sanders JM,
Lye RJ, French BA and Annex BH: Adeno-associated virus serotype
9-mediated overexpression of extracellular superoxide dismutase
improves recovery from surgical hind-limb ischemia in BALB/c mice.
J Vasc Surg. 54:810–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shui M, Liu X, Zhu Y and Wang Y: Exogenous
hydrogen sulfide attenuates cerebral ischemia-reperfusion injury by
inhibiting autophagy in mice. Can J Physiol Pharmacol.
94:1187–1192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Zhang L, Zhao W and Liu T: The
protective effects of hydrogen on HO-1 expression in the brainafter
focal cerebral ischemia reperfusion in rats. Turk J Med Sci.
46:1534–1539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Liu J, Jin K, Xu H, Wang C, Zhang
Z, Kong M, Zhang Z, Wang Q and Wang F: Subcutaneous injection of
hydrogen gas is a novel effective treatment for type 2 diabetes. J
Diabetes Investig. 9:83–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hazarika S, Farber CR, Dokun AO,
Pitsillides AN, Wang T, Lye RJ and Annex BH: MicroRNA-93 controls
perfusion recovery after hindlimb ischemia by modulating expression
of multiple genes in the cell cycle pathway. Circulation.
127:1818–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dokun AO, Keum S, Hazarika S, Li Y,
Lamonte GM, Wheeler F, Marchuk DA and Annex BH: A quantitative
trait locus (LSq-1) on mouse chromosome 7 is linked to the absence
of tissue loss after surgical Hindlimb ischemia. Circulation.
117:1207–1215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kishimoto Y, Kato T, Ito M, Azuma Y,
Fukasawa Y, Ohno K and Kojima S: Hydrogen ameliorates pulmonary
hypertension in rats by anti-inflammatory and antioxidant effects.
J Thorac Cardiovasc Surg. 150:645–654, e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakai Y, Sato B, Ushiama S, Okada S, Abe K
and Arai S: Hepatic oxidoreduction-related genes are upregulated by
administration of hydrogen-saturated drinking water. Biosci
Biotechnol Biochem. 75:774–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hazarika S, Dokun AO, Li Y, Popel AS,
Kontos CD and Annex BH: Impaired angiogenesis after Hindlimb
ischemia in type 2 diabetes Mellitus: Differential regulation of
vascular endothelial growth factor receptor 1 and soluble vascular
endothelial growth factor receptor 1. Circ Res. 101:948–956. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meisner JK, Song J, Annex BH and Price RJ:
Myoglobin overexpression inhibits reperfusion in the ischemic mouse
hindlimb through impaired angiogenesis but not arteriogenesis. Am J
Pathol. 183:1710–1718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papastergiadis A, Mubiru E, Van Langenhove
H and De Meulenaer B: Malondialdehyde measurement in oxidized
foods: Evaluation of the spectrophotometric thiobarbituric acid
reactive substances (TBARS) test in various foods. J Agric Food
Chem. 60:9589–9594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denninger JW and Marletta MA: Guanylate
cyclase and the. NO/cGMP signaling pathway. Biochim Biophys Acta.
1411:334–350. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hazarika S, Angelo M, Li Y, Aldrich AJ,
Odronic SI, Yan Z, Stamler JS and Annex BH: Myocyte specific
overexpression of myoglobin impairs angiogenesis after hind-limb
ischemia. Arterioscl Throm Vas Biol. 28:2144–2150. 2008. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang T, Cunningham A, Dokun AO, Hazarika
S, Houston K, Chen L, Lye RJ, Spolski R, Leonard WJ and Annex BH:
Loss of interleukin-21 receptor activation in hypoxic endothelial
cells impairs perfusion recovery after hindlimb ischemia.
Arterioscl Throm Vas Biol. 35:1218–1225. 2015. View Article : Google Scholar
|
|
28
|
Wu D and Yotnda P: Production and
detection of reactive oxygen species (ROS) in cancers. J Vis Exp.
pii: 3357. 2011.doi: 10.3791/3357. View
Article : Google Scholar
|
|
29
|
Pirinccioglu AG, Gokalp D, Pirinccioglu M,
Kizil G and Kizil M: Malondialdehyde (MDA) and protein carbonyl
(PCO) levels as biomarkers of oxidative stress in subjects with
familial hypercholesterolemia. Clin Biochem. 43:1220–1224. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murohara T and Asahara T: Nitric oxide and
angiogenesis in cardiovascular disease. Antioxid Redox Signal.
4:825–831. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakayama M, Itami N, Suzuki H, Hamada H,
Osaka N, Yamamoto R, Tsunoda K, Nakano H, Watanabe K, Zhu WJ, et
al: Possible clinical effects of molecular hydrogen (H2) delivery
during hemodialysis in chronic dialysis patients: Interim analysis
in a 12 month observation. PLoS One. 12:e01845352017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishimaki K, Asada T, Ohsawa I, Nakajima
E, Ikejima C, Yokota T, Kamimura N and Ohta S: Effects of molecular
hydrogen assessed by an animal model and a randomized clinical
study on mild cognitive impairment. Curr Alzheimer Res. 15:482–492.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schleicher M, Yu J, Murata T, Derakhshan
B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang
PL and Sessa WC: The Akt1-eNOS axis illustrates the specificity of
kinase-substrate relationships in vivo. Sci Signal. 2:ra412009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohta S: Hydrogen gas and hydrogen water
act as a therapeutic and preventive antioxidant with a novel
concept. Nihon Ronen Igakkai Zasshi. 45:355–362. 2008.(In
Japanese). PubMed/NCBI
|
|
36
|
Takeda Y, Costa S, Delamarre E, Roncal C,
de Oliveira Leite R, Squadrito ML, Finisguerra V, Deschoemaeker S,
Bruyère F, Wenes M, et al: Macrophage skewing by Phd2
haplodeficiency prevents ischaemia by inducing arteriogenesis.
Nature. 479:122–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Covarrubias A, Byles V and Horng T: ROS
sets the stage for macrophage differentiation. Cell Res.
23:984–985. 2013. View Article : Google Scholar : PubMed/NCBI
|