Introduction
Acute myeloid leukaemia (AML) is a type of
haematological malignancy caused by the malignant transformation of
bone-marrow-derived, self-renewing stem cells or myeloid
progenitors (1). AML is
characterised by cells with unlimited proliferation ability,
impaired apoptosis and the accumulation of differentiation-arrested
myeloid progenitor cells (2,3). AML
is the most common type of leukaemia arising in infancy and
childhood, and accounts for 15–20% cases of acute leukaemia in
children (4). At present,
chemotherapy, targeted therapy and hematopoietic stem cell
transplantation serve as the primary therapeutic methods for
patients with AML (5). Notable
advancements have been achieved in the diagnosis and therapy of
AML; however, the long-term survival of patients with AML remains
unsatisfactory (6). Chromosomal
aberrations and gene mutations have been associated with the
initiation and progression of AML; however, the detailed mechanisms
underlying the pathogenesis of AML remain unclear (7). Therefore, the mechanisms underlying
AML leukemogenesis and development require further investigation to
identify potential therapeutic targets for the treatment of
patients with AML and improve the therapeutic outcomes of patients
with this particular type of malignancy.
MicroRNAs (miRNAs) are a large family of endogenous,
non-coding and short RNA molecules composed of 18–24 nucleotides
(8). miRNAs typically regulate
gene expression by preferentially interacting with the
3′-untranslated regions (3′-UTRs) of their target genes, thereby
inducing mRNA degradation or the inhibition of translation
(9). miRNAs serve a principal role
in regulating various physiological processes, including cellular
growth, differentiation, metabolism, the cell cycle and apoptosis
(10–12). Substantial evidence has
demonstrated that miRNAs are differently expressed in
aapproximately all types of human malignancies, including AML
(13–15). In AML, aberrantly expressed miRNAs
contribute to the genesis and development of AML by affecting a
variety of pathological processes, including cell proliferation,
cycle, apoptosis, chemotherapy resistance and autophagy (16–18).
Therefore, AML-associated miRNAs require investigation to develop
novel diagnostic biomarkers and effective therapeutic targets for
the treatment of patients with AML.
miR-339-5p-5p (miR-339-5p) is dysregulated in
numerous types of cancer, including non-small cell lung cancer
(19), hepatocellular carcinoma
(20) and ovarian cancer (21). This dysregulation has been
associated with tumorigenesis and tumour development (19,20,22).
In addition, miR-339-3p expression is decreased in AML (23); however, the expression, roles and
detailed mechanism of miR-339-5p in AML require further
investigation. In the present study, miR-339-5p was demonstrated to
inhibit cell proliferation, induce cell cycle arrest and promote
cell apoptosis in AML by directly targeting sex-determining region
Y-related high-mobility group box 4 (SOX4). The results of the
present study may provide insight into the therapeutic potential of
miR-339-5p as a novel effective target for the treatment of
patients with AML.
Materials and methods
Ethics approval and clinical
specimens
The present study was approved by the Ethics
Committee of the Yidu Central Hospital of Weifang (Weifang, China).
Written informed consent was obtained from all patients enrolled in
the present study. Clinical specimens were used in accordance with
the Declaration of Helsinki. Bone marrow samples were collected
from 35 patients with AML (19 males, 16 females; age range, 26–54
years) and 19 healthy controls (12 males, 7 females; age range,
23–47 years) in the Yidu Central Hospital of Weifang from February
2014 to October 2016. Healthy bone marrow tissues were collected
form healthy transplantation donors. Patients had not received
chemotherapy, targeted therapy or hematopoietic stem cell
transplantation prior to bone marrow aspiration. Patiens diagnosed
with AMLs that had not been treated with chemotherapy, targeted
therapy or hematopoietic stem cell transplantation were enrolled in
the present study.
Cell lines
Three AML cell lines (HL-60, THP-1 and Kasumi-1) and
a normal bone marrow cell line, HS-5, were obtained from the
American Type Culture Collection (Manassas, VA, USA), and were
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin
and 100 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). All cell lines were cultured at 37°C in a
humidified atmosphere with 5% CO2 and 95% air.
Cell transfection
Synthetic miR-339-5p mimics and negative control
miRNA mimics (miR-NC) were purchased from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). The miR-339-5p mimics sequence was
5′-UCCCUGUCCUCCAGGAGCUCACG-3′ and the miR-NC sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. The SOX4 overexpression plasmid
lacking 3′-UTR, pCMV-SOX4 and an empty plasmid, pCMV, were produced
by GeneCopoeia, Inc. (Rockville, MD, USA). Cells were plated into
six-well plates at a density of 6×105 cells/well one
night prior to transfection. Cells were transfected with miR-339-59
mimics (100 pmol), miR-NC (100 pmol), pCMV (4 µg) or pCMV-SOX4 (4
µg) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Co-transfection of miR-339-59 mimics (100 pmol) and
pCMV (4 µg) or pCMV-SOX4 (4 µg) was additionally conducted using
Lipofectamine® 2000, according to the manufacturer's
protocols. At 8 h post-transfection, the cell culture medium
containing Lipofectamine® 2000 was discarded, and fresh
DMEM containing 10% FBS was added into each well. Cells were grown
at 37°C in a humidified atmosphere containing 5% CO2 and
95% air. In the present study, HL-60 and THP-1 cells were selected
for functional analysis as the two cell lines exhibited relatively
lower miR-339-5p expression. Successful transfection was determined
by detecting miR-339-5p and SOX4 expression following transfection
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis, respectively. RT-qPCR and
western blot analysis were performed 48 and 72 h after
transfection, respectively. Following 24 h transfection, a Cell
Counting kit-8 (CCK-8) assay was conducted. Cell cycle and
apoptosis assays were conducted 48 h post-transfection.
RNA isolation and RT-qPCR
Ficoll-Paque Plus (GE Healthcare, Chicago, IL, USA)
was utilised to isolate mononuclear cells from the bone marrow
samples. Total RNA was extracted from the cultured cell lines and
mononuclear cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Subsequently, the quality and concentration of total RNA
was evaluated using a NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). For the
determination of miR-339-5p expression levels, first-strand
complementary DNA (cDNA) was prepared from total RNA using a TaqMan
MicroRNA RT kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols. Subsequently, 400
ng cDNA was subjected to qPCR using a TaqMan MicroRNA Assay kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). To quantify
the levels of SOX4 mRNA expression, a PrimeScript RT Reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China) was used to produce
cDNA, according to the manufacturer's protocol. Subsequently, qPCR
was conducted using a SYBR Premix Ex Taq kit (Takara Biotechnology
Co., Ltd., Dalian, China). The thermocycling conditions of qPCR
were as follows: 5 min at 95°C, followed by 40 cycles of 95°C for
30 sec and 65°C for 45 sec. The relative expression levels of
miR-339-5p and SOX4 mRNA were analysed using the 2−ΔΔCq
method (24) and normalised to U6
and GAPDH, respectively. RT-qPCR analysis was performed on an
Applied Biosystems 7500 Sequence Detection system (Thermo Fisher
Scientific, Inc.). The primers were designed as follows: miR-339-5p
forward, 5′-ACACTCCAGCTGGGTCCCTGTCCTCCAGGAG-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′
and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; SOX4 forward,
5′-CTTGACATGATTAGCTGGCATGATT-3′ and reverse,
5′-CCTGTGCAATATGCCGTGTAGA-3′; and GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. Each sample was analyzed in
triplicate.
CCK-8 assay
Cell proliferation was determined using a CCK-8
assay. Transfected cells were harvested after 24 h incubation at
37°C, and seeded into 96-well plates at a density of 3,000
cells/well. In the present study, four time points were selected:
0, 24, 48 and 72 h following incubation. At each time point, 10 µl
CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was added to each well, respectively; cells were incubated
at 37°C for another 2 h. Finally, the optical density was measured
at a wavelength of 450 nm using a Synergy™ Multi-Mode
Microplate Reader (Biotek Instruments, Winooski, VT, USA).
Cell cycle assay
Transfected cells were collected at 48 h
post-transfection, washed with cold PBS and fixed in 70% ethanol at
4°C for 1 h. Following centrifugation at 157 × g at 4°C for 5 min,
the supernatant was discarded, and the cells were washed three
times with cold PBS. Prior to detection, 50 µl RNase 1 (100 µg/ml)
was added to ensure that only the DNA was stained, and this
procedure was performed for 10 min at room temperature.
Subsequently, cells were stained at 37°C for 30 min with 25 µl
propidium iodide solution diluted in 425 µl cell staining buffer
(both from BioLegend, San Diego, CA, USA), according to the
manufacturer's protocol. The cell cycle was detected using a flow
cytometer (FACScan; BD Biosciences, Franklin Lakes, NJ, USA), and
analysed with CellQuest version 5.1 (BD Biosciences).
Cell apoptosis assay
After 48 h post-transfection, cells were collected
using EDTA-free trypsin (Gibco; Thermo Fisher Scientific, Inc.) and
washed three times with cold PBS. Subsequently, an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(BioLegend) was utilized to detect cell apoptosis, according to the
manufacturer's protocols. Transfected cells (1.5×106)
were re-suspended in 100 µl binding buffer, followed by incubation
with 5 µl Annexin V-FITC and 5 µl propidium iodide in the dark for
15 min at room temperature. Finally, cell apoptosis rate was
detected using a flow cytometer (FACScan), and analyzed with
CellQuest version 5.1 (both BD Biosciences, Franklin Lakes, NJ,
USA).
Bioinformatics analysis
TargetScan7.1 (http://www.targetscan.org/) was used to predict the
putative targets of miR-339-5p.
Dual-luciferase reporter assay
SOX4 was predicted as a potential target of
miR-339-5p using the bioinformatics tools. The fragments of the
SOX4 3′-UTR containing wild-type (Wt) or mutant (Mut) binding sites
of miR-339-5p were chemically produced by Shanghai GenePharma Co.,
Ltd., and inserted into pGL3 luciferase reporter vectors (Promega
Corporation, Madison, WI, USA) and named as pGL3-SOX4-3′-UTR Wt and
pGL3-SOX4-3′-UTR Mut, respectively. Cells were inoculated into
24-well plates with a density of 1.0×105 cells/well one
day prior to transfection. miR-339-5p mimics (50 pmol) or miR-NC
(50 pmol) were co-transfected with pGL3-SOX4-3′-UTR Wt (0.2 µg) or
pGL3-SOX4-3′-UTR Mut (0.2 µg) into cells using
Lipofectamine® 2000, according to the manufacturer's
protocol. After 48-h transfection, Renilla and firefly
luciferase activities were evaluated using a Dual-Luciferase
Reporter Assay kit (Promega Corporation). Renilla luciferase
activity was employed for normalisation of luciferase activity.
Western blot analysis
Total protein was extracted from cultured cells or
bone marrow samples using radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Shanghai, China). A
Bicinchoninic Acid assay kit (Beyotime Institute of Biotechnology)
was used to evaluate protein concentration. Equal amounts of
proteins (30 µg) were separated via 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% non-fat dry milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies overnight at 4°C and subsequently washed three times
with Tris-buffered saline containing 0.1% Tween-20 (TBST).
Subsequently, the membranes were incubated with a
horseradish-peroxidase-conjugated secondary antibody (1:5,000; cat.
no. ab6789; Abcam, Cambridge, UK) for 2 h at room temperature and
washed three times with TBST. Target protein signals were
visualised using Amersham Enhanced Chemilumiscence Western Blotting
Detection Reagent (GE Healthcare) according to the manufacturer's
protocols. The primary antibodies used in the present study
included mouse anti-human monoclonal SOX4 (1:500; cat. no. ab70598)
and mouse anti-human monoclonal GAPDH antibody (1:500; cat. no.
ab8245; both Abcam); GADPH was used as the internal reference.
Protein expression was quantified using Quantity One software
version 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
and analyzed with SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Each experiment was repeated at least three times. Differences
between groups were determined using Student's t-test or one-way
analysis of variance for multiple comparisons followed by Tukey's
post-hoc test. Spearman's correlation analysis was performed to
assess the correlation between miR-339-5p and SOX4 mRNA expression
levels in AML. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-339-5p is downregulated in
AML
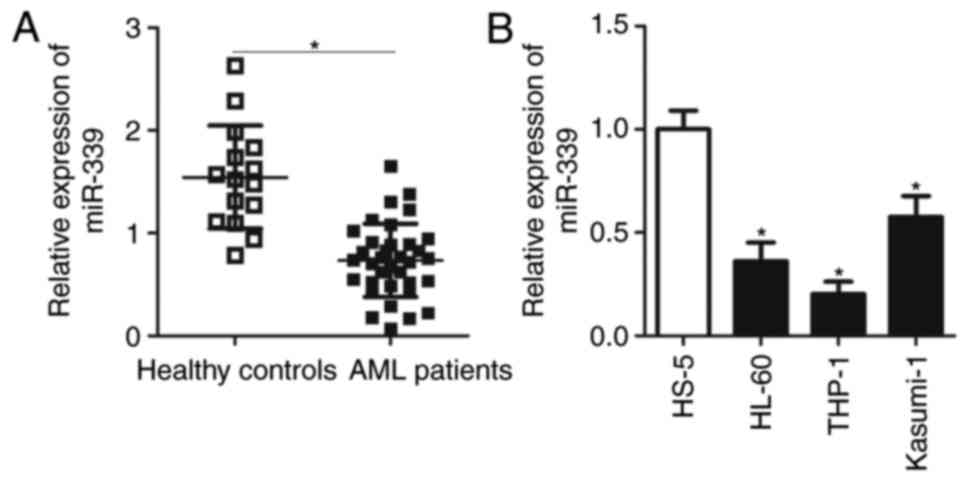
To assess the cellular functions of miR-339-5p in
AML, the expression levels of miR-339-5p in the bone marrow of 35
patients with AML and 19 healthy controls were analysed. RT-qPCR
analysis demonstrated that miR-339-5p was significantly
downregulated in patients with AML compared with in healthy
controls (Fig. 1A; P<0.05). The
expression levels of miR-339-5p were detected in three AML cell
lines (HL-60, THP-1 and Kasumi-1) and in a normal bone marrow cell
line HS-5. The expression levels of miR-339-5p in the three AML
cell lines were significantly lower compared with HS-5 cells
(Fig. 1B; P<0.05). These
results suggested that miR-339-5p may serve crucial roles in the
development of AML.
miR-339-5p overexpression inhibits the
proliferation of AML cells
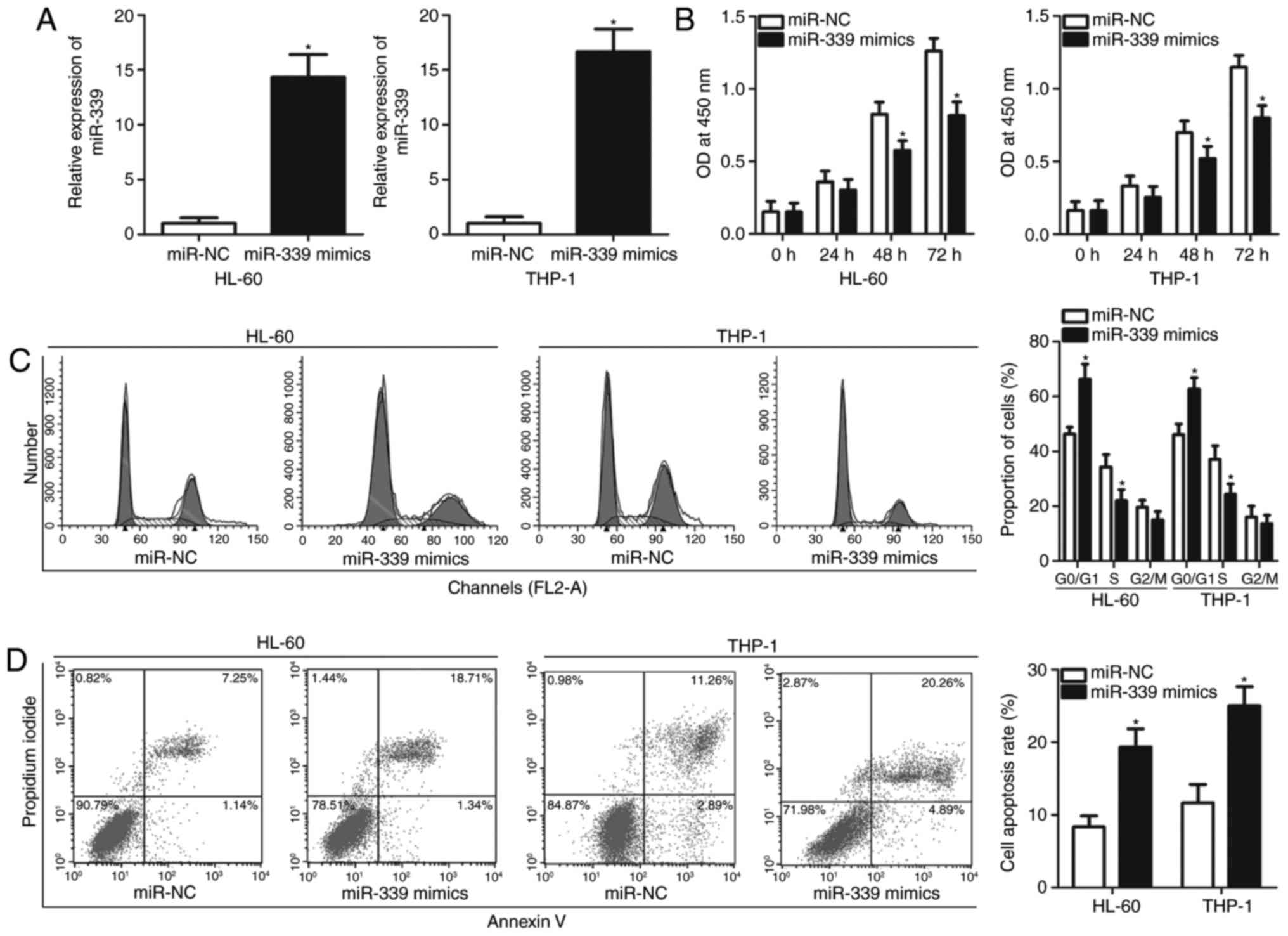
As it was determined that miR-339-5p is expressed at
low levels in AML in the present study, whether miR-339-5p serves a
tumour-suppressing role in AML progression was investigated. To
confirm this hypothesis, miR-339-5p mimics or miR-NC were
transfected into HL-60 and THP-1 cells, and the results of RT-qPCR
demonstrated that the miR-339-5p expression levels were
significantly upregulated in HL-60 and THP-1 cells transfected with
miR-339-5p mimics compared with the control (Fig. 2A; P<0.05). The effects of
miR-339-5p overexpression on AML cell proliferation was determined
via a CCK-8 assay. As presented in Fig. 2B, ectopic miR-339-5p expression
significantly inhibited the proliferation of HL-60 and THP-1 cells
relative to those of the miR-NC groups at 48 and 72 h (P<0.05).
Alterations in proliferation were primarily associated with cell
cycle progression, suggesting that cell cycle arrest may inhibit
the cell cycle. Cell cycle analysis demonstrated that HL-60 and
THP-1 cells transfected with miR-339-5p mimics exhibited a
significantly higher percentage of cells in the
G0/G1 phase and lower percentage of cells in
the S phase compared with cells transfected with miR-NC (Fig. 2C; P<0.05). A cell apoptosis
assay was conducted to examine the biological functions of
miR-339-5p in AML cell apoptosis. The results demonstrated that
restoration of miR-339-5p expression increased the percentage of
apoptotic rate in HL-60 and THP-1 cells compared with cells
transfected with miR-NC (Fig. 2D;
P<0.05). These results suggested that miR-339-5p may inhibit AML
cell proliferation by inducing cell apoptosis and cell cycle
arrest.
SOX4 serves as a direct target gene of
miR-339-5p in AML
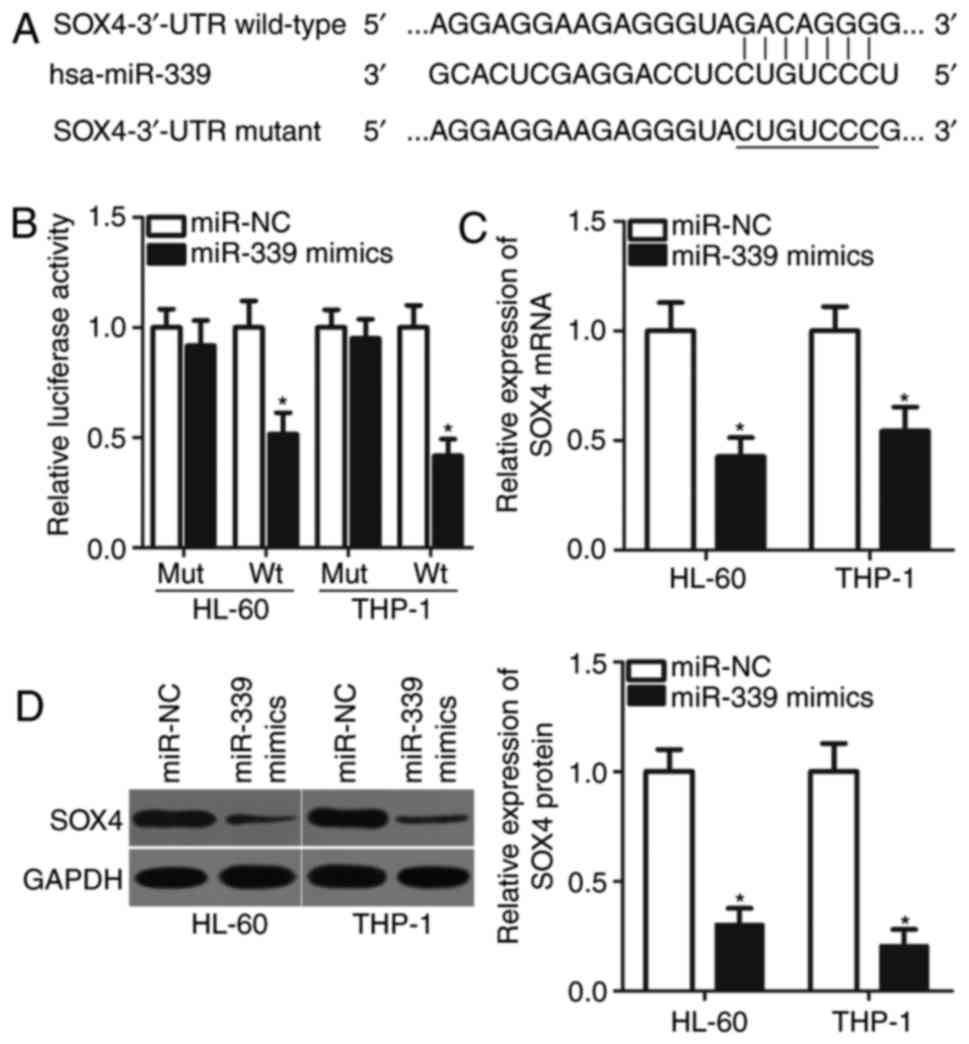
It was identified that miRNAs serve their roles by
directly interacting with the 3′-UTR of target genes and induce
mRNA degradation or the inhibition of translation (9). Therefore, bioinformatics analysis was
performed to predict the potential targets of miR-339-5p in the
present study. SOX4, a key component of AML oncogenesis and
progression (25–28), was predicted as a putative target
of miR-339-5p (Fig. 3A) and was
selected for subsequent analysis. A dual-luciferase reporter assay
was used to verify the direct interaction between miR-339-5p and
the 3′-UTR of SOX4. The data demonstrated that miR-339-5p
significantly decreased the luciferase activities of miR-399-50
mimics-transfected HL-60 and THP-1 cells containing the Wt 3′-UTR
of SOX4 compared with in the corresponding control (P<0.05),
whereas the luciferase activity of the plasmid containing the Mut
3′-UTR of SOX4 remained notably unaltered (Fig. 3B). To evaluate the regulatory
effects of miR-339-5p on the endogenous expression levels of SOX4,
RT-qPCR and western blot analysis were performed to measure SOX4
expression in HL-60 and THP-1 cells transfected with miR-339-5p
mimics or miR-NC. As presented in Fig.
3C and D, the mRNA (P<0.05) and protein (P<0.05)
expression levels of SOX4 in HL-60 and THP-1 cells transfected with
miR-339-5p mimics were significantly lower compared with in cells
transfected with miR-NC. Therefore, SOX4 may serve as a direct
target of miR-339-5p in AML.
miR-339-5p is negatively correlated
with SOX4 expression in AML
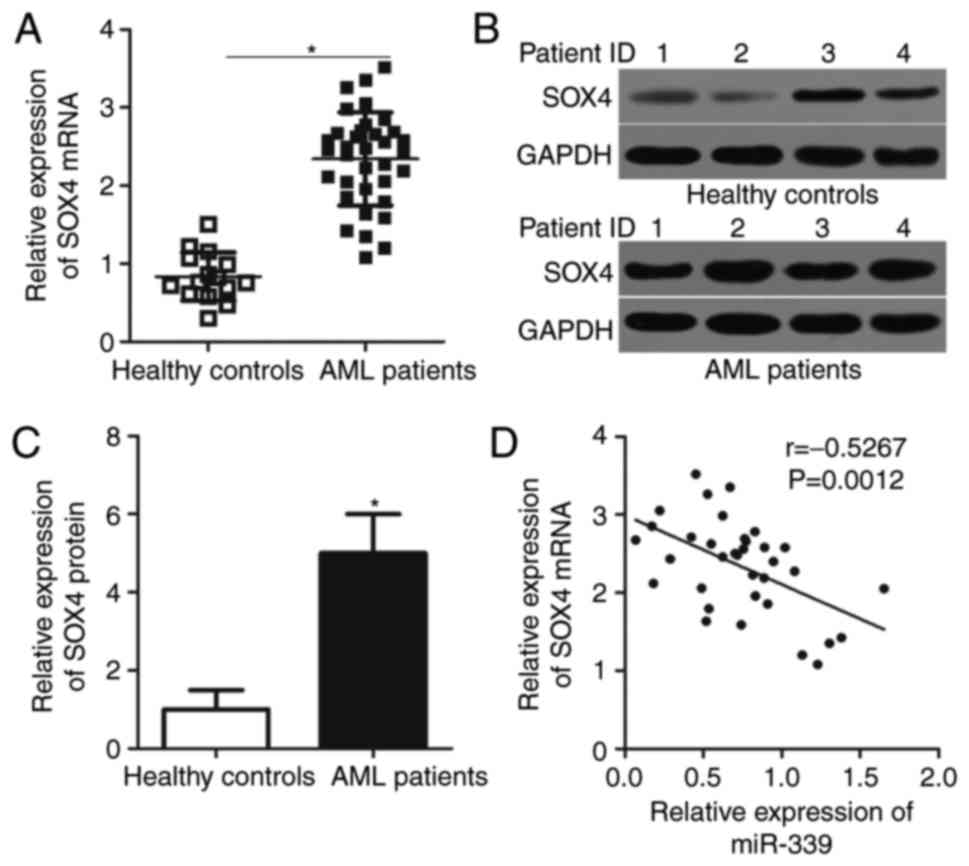
To clarify the association between miR-339-5p and
SOX4 in AML, the present study investigated SOX4 expression in bone
marrow samples of 35 patients with AML and 19 healthy controls.
RT-qPCR analysis demonstrated that SOX4 mRNA was significantly
overexpressed in patients with AML compared with in healthy
controls (Fig. 4A; P<0.05). The
protein expression levels of SOX4 in patients with AML were
significantly higher compared with the healthy controls (Fig. 4B and C; P<0.05). Spearman's
correlation analysis suggested that miR-339-5p was inversely
correlated with SOX4 mRNA expression in AML samples (Fig. 4D; r=−0.5267, P=0.0012). These
results further suggested that SOX4 is a direct target of
miR-339-5p in AML.
SOX4 overexpression partially rescues
the inhibitory effects of miR-339-5p on AML cells
Following the determination of SOX4 as a target of
miR-339-5p, the present study investigated whether SOX4 mediates
the tumour-suppressing roles of miR-339-5p in AML. HL-60 and THP-1
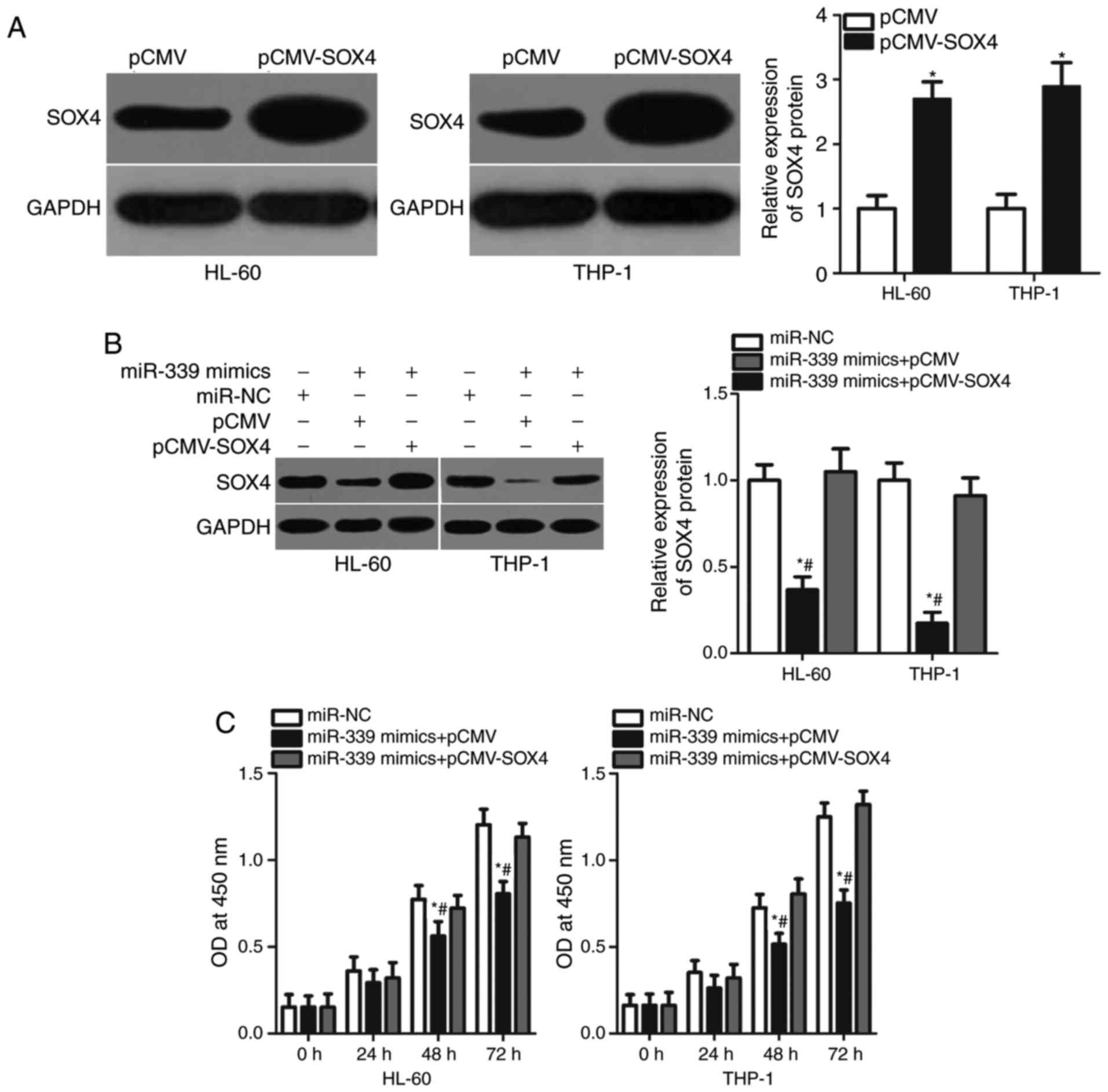
cells were transfected with pCMV or pCMV-SOX4. The results
demonstrated that SOX4 expression was significantly upregulated in
pCMV-SOX4-transfected HL-60 and THP-1 cells compared with cells
transfected with pCMV (Fig. 5A;
P<0.05). Subsequently, HL-60 and THP-1 cells were co-transfected
with miR-339-5p mimics and pCMV or pCMV-SOX4 lacking 3′-UTR. After
72-h transfection, western blot analysis suggested that the
downregulation of SOX4 expression in HL-60 and THP-1 cells induced
by miR-339-5p overexpression was recovered following
co-transfection with pCMV-SOX4 (Fig.
5B; P<0.05). In functional assays, the restoration of SOX4
expression significantly reversed the effects of exogenous
miR-339-5p on the proliferation at 48 and 72 h (Fig. 5C; P<0.05), the cell cycle
(Fig. 5D; P<0.05) and apoptosis
(Fig. 5E; P<0.05) of HL-60 and
THP-1 cells. Overall, these results suggested that the
tumour-suppressing effects of miR-339-5p on AML cells were
associated with downregulated SOX4 expression.
Discussion
In recent decades, miRNAs have been considered novel
gene regulators, whereas deregulated miRNAs serve key roles in the
initiation and progression of AML (29–31).
Therefore, the roles of differently expressed miRNAs in AML require
extensive investigation to gain insight into potential treatments
for patients with AML. miR-339-5p has been studied in acute
lymphoblastic leukaemia (32);
however, its expression profile in AML remains unknown. In the
present study, miR-339-5p expression was significantly decreased in
AML samples and cell lines. In vitro analyses demonstrated
that resumption of miR-339-5p expression reduced cell
proliferation, induced cell cycle arrest and promoted apoptosis in
AML in the present study. SOX4 was determined to be a direct target
of miR-339-5p in AML and was overexpressed in AML samples. This
overexpression was inversely correlated with miR-339-5p expression.
In addition, the present study conducted a series of rescue
experiments, which revealed that the restoration of SOX4 expression
inhibited the effects of miR-339-5p overexpression on cell
proliferation, the cell cycle and apoptosis of AML. These results
demonstrated that miR-339-5p may serve tumour-suppressing roles in
AML progression by directly targeting SOX4, suggesting that
miR-339-5p may be considered as a therapeutic target for the
treatment of patients with AML.
The dysregulation of miR-339-5p has been identified
in a variety of human cancer (19–21).
FomiR-339-5p was observed to be downregulated in non-small cell
lung cancer tissues and cell lines (19). Reduced miR-339-5p expression has
been associated with tumour-node-metastasis staging and lymph node
metastasis (19). The expression
levels of miR-339-5p in hepatocellular carcinoma were lower
compared with tumour tissues and cell lines. Compared with in
patients with hepatocellular carcinoma and high miR-339-5p
expression levels, those with lower miR-339-5p expression levels
present poorer prognosis (20).
miR-339-5p expression levels have been proposed as an independent
prognostic factor for patients with hepatocellular carcinoma
(20). Low expression levels of
miR-339-5p were also reported in ovarian (21), gastric (33) and breast cancer (34). In the present study, it was
demonstrated that miR-339-5p expression was decreased in AML
samples and cell lines. These results suggested that miR-339 is
frequently downregulated in human malignancies and may be
associated with the pathogenesis of these specific types of
tumour.
It was demonstrated that differentially expressed
miRNAs are closely correlated with the carcinogenesis and
progression of numerous human cancer types (19,20,35,36).
For instance, upregulation of miR-339-5p inhibited the cell
proliferation, migration and invasion of lung cancer (19,35).
Zhou et al (36) observed
that miR-339-5p overexpression decreased the growth, colony
formation and metastasis of colorectal cancer cell in vitro
and suppressed tumour growth in vivo. Wang et al
(20) observed that the ectopic
expression of miR-339-5p restricted the invasive ability of
hepatocellular carcinoma cells. Shan et al (21) demonstrated that the resumption of
miR-339-5p expression suppressed cell motility in ovarian cancer.
Shen et al (33) identified
that miR-339-5p restoration reduced the cell proliferation,
migration and invasion of gastric cancer in vitro and
tumorigenicity in vivo. Wu et al (34) demonstrated that miR-339-5p
overexpression reduced the cell metastatic ability of breast
cancer. In the present study, it was observed that miR-339-5p
inhibited cell proliferation, induced cell cycle arrest and
promoted apoptosis in AML. These results suggested that miR-339-5p
may be a potential therapeutic target for the treatment of patients
with these specific types of tumour.
Previous studies have validated a number of
miR-339-5p targets, including S-phase kinase-associated protein 2
in lung cancer (35), phosphatase
of regenerating liver-1 in colorectal cancer (36), nucleus accumbens associated 1
(21) and B-cell lymphoma 6 in
ovarian cancer (21) and NOVA
alternative splicing regulator 1 in gastric cancer (33). SOX4, a member of the SOX family
(37), was observed to be a direct
and functional target of miR-339-5p in AML in the present study.
Increasing evidence suggests that SOX4 is aberrantly and highly
expressed in numerous types of human cancers, including gastric
cancer (38), hepatocellular
carcinoma (39), colorectal cancer
(40), osteosarcoma (41) and cervical cancer (42). SOX4 serves oncogenic roles in the
onset and development of tumours by affecting cell proliferation,
apoptosis, the cell cycle, migration and metastasis (43–45).
SOX4 expression is additionally upregulated in the bone marrow of
patients with AML as determined in the preset study. Compared with
in patients with low levels of SOX4 expression, those with high
expression levels exhibited lower rates of remission and shorter
overall survival (25).
Functionally, SOX4 performs important roles in the formation and
progression of AML, regulating cell self-renewal, differentiation
and proliferation (26–28). In view of these important roles, in
AML, the miR-339-5p/SOX4 signalling pathway may represent a
potential therapeutic target for treating patients with AML.
To summarize, miR-339-5p was downregulated in AML
samples and cell lines. miR-339-5p overexpression inhibited cell
proliferation, induced cell cycle arrest and increased apoptosis of
AML by directly targeting SOX4. The association between miR-339-5p
and the prognosis of patients with AML was not investigated in the
present study. In addition, the association between miR-339-5p and
the clinicopathological features of patients with AML was not
examined. These limitations of the present study may be resolved in
future investigations; however, understanding the mechanism
underlying the tumour-suppressing roles of miR-339-5p in AML may
provide novel insight into the progression of AML. miR-339-5p may
be considered a potential therapeutic target for the management of
patients with AML.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LQ made substantial contributions to the design of
the present study. XS, HL and TL performed the functional
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Yidu Central Hospital of Weifang, and was
performed in accordance with the Declaration of Helsinki and the
guidelines of the Ethics Committee of Yidu Central Hospital of
Weifang. Written informed consent was obtained from all patients
for the use of their clinical tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ziai JM and Siddon AJ: Education Committee
of the Academy of Clinical Laboratory Physicians and Scientists:
Pathology consultation on gene mutations in acute myeloid leukemia.
Am J Clin Pathol. 144:539–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Estey EH: Acute myeloid leukemia: 2013
update on risk-stratification and management. Am J Hematol.
88:318–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medinger M, Lengerke C and Passweg J:
Novel prognostic and therapeutic mutations in acute myeloid
leukemia. Cancer Genomics Proteomics. 13:317–329. 2016.PubMed/NCBI
|
|
5
|
Chiu CF, Weng JR, Jadhav A, Wu CY,
Sargeant AM and Bai LY: T315 decreases acute myeloid leukemia cell
viability through a combination of apoptosis induction and
autophagic cell death. Int J Mol Sci. 17:pii: E1337. 2016.
View Article : Google Scholar
|
|
6
|
Liu X, Liao W, Peng H, Luo X, Luo Z, Jiang
H and Xu L: miR-181a promotes G1/S transition and cell
proliferation in pediatric acute myeloid leukemia by targeting ATM.
J Cancer Res Clin Oncol. 142:77–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Estey EH: Acute myeloid leukemia: 2014
update on risk-stratification and management. Am J Hematol.
89:1063–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alemdehy MF and Erkeland SJ: MicroRNAs:
Key players of normal and malignant myelopoiesis. Curr Opin
Hematol. 19:261–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagai H, Hasegawa S, Uchida F, Terabe T,
Kanno Ishibashi N, Kato K, Yamagata K, Sakai S, Kawashiri S, Sato
H, et al: MicroRNA-205-5p suppresses the invasiveness of oral
squamous cell carcinoma by inhibiting TIMP-2 expression. Int J
Oncol. 52:841–850. 2018.PubMed/NCBI
|
|
14
|
Du B, Wu D, Yang X, Wang T, Shi X, Lv Y,
Zhou Z, Liu Q and Zhang W: The expression and significance of
microRNA in different stages of colorectal cancer. Medicine
(Baltimore). 97:e96352018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Q, Luan J, Li N, Zhang Z, Zhu X, Zhao
L, Wei R, Sun L, Shi Y, Yin X, et al: MicroRNA-181 as a prognostic
biomarker for survival in acute myeloid leukemia: A meta-analysis.
Oncotarget. 8:89130–89141. 2017.PubMed/NCBI
|
|
16
|
Liu L, Ren W and Chen K: MiR-34a promotes
apoptosis and inhibits autophagy by targeting HMGB1 in acute
myeloid leukemia cells. Cell Physiol Biochem. 41:1981–1992. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao Y, Deng T, Su C and Shang Z: MicroRNA
217 inhibits cell proliferation and enhances chemosensitivity to
doxorubicin in acute myeloid leukemia by targeting KRAS. Oncol
Lett. 13:4986–4994. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wallace JA and O'Connell RM: MicroRNAs and
acute myeloid leukemia: Therapeutic implications and emerging
concepts. Blood. 130:1290–1301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Zhao W, Bao P, Li C, Ma XQ, Li Y and
Chen LA: miR-339-5p inhibits cell migration and invasion in vitro
and may be associated with the tumor-node-metastasis staging and
lymph node metastasis of non-small cell lung cancer. Oncol Lett.
8:719–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YL, Chen CM, Wang XM and Wang L:
Effects of miR-339-5p on invasion and prognosis of hepatocellular
carcinoma. Clin Res Hepatol Gastroenterol. 40:51–56. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan W, Li J, Bai Y and Lu X: miR-339-5p
inhibits migration and invasion in ovarian cancer cell lines by
targeting NACC1 and BCL6. Tumour Biol. 37:5203–5211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou C, Lu Y and Li X: miR-339-3p inhibits
proliferation and metastasis of colorectal cancer. Oncol Lett.
10:2842–2848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barrera-Ramirez J, Lavoie JR, Maganti HB,
Stanford WL, Ito C, Sabloff M, Brand M, Rosu-Myles M, Le Y and
Allan DS: Micro-RNA profiling of exosomes from marrow-derived
mesenchymal stromal cells in patients with acute myeloid leukemia:
Implications in leukemogenesis. Stem Cell Rev. 13:817–825. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu JW, Hsieh MS, Hou HA, Chen CY, Tien HF
and Lin LI: Overexpression of SOX4 correlates with poor prognosis
of acute myeloid leukemia and is leukemogenic in zebrafish. Blood
Cancer J. 7:e5932017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fung TK, Leung AY and So CW: Sox4you: A
new player in C/EBPα leukemia. Cancer Cell. 24:557–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernando TR, Contreras JR, Zampini M,
Rodriguez-Malave NI, Alberti MO, Anguiano J, Tran TM, Palanichamy
JK, Gajeton J, Ung NM, et al: The lncRNA CASC15 regulates SOX4
expression in RUNX1-rearranged acute leukemia. Mol Cancer.
16:1262017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Alberich-Jorda M, Amabile G, Yang
H, Staber PB, Di Ruscio A, Welner RS, Ebralidze A, Zhang J,
Levantini E, et al: Sox4 is a key oncogenic target in C/EBPα mutant
acute myeloid leukemia. Cancer Cell. 24:575–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gabra MM and Salmena L: microRNAs and
acute myeloid leukemia chemoresistance: A mechanistic overview.
Front Oncol. 7:2552017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Chen H, Bai J and He A: MicroRNA:
An important regulator in acute myeloid leukemia. Cell Biol Int.
41:936–945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yeh CH, Moles R and Nicot C: Clinical
significance of microRNAs in chronic and acute human leukemia. Mol
Cancer. 15:372016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mosakhani N, Missiry ME, Vakkila E,
Knuutila S and Vakkila J: Low expression of miR-18a as a
characteristic of pediatric acute lymphoblastic leukemia. J Pediatr
Hematol Oncol. 39:585–588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen B, Zhang Y, Yu S, Yuan Y, Zhong Y, Lu
J and Feng J: MicroRNA-339, an epigenetic modulating target is
involved in human gastric carcinogenesis through targeting NOVA1.
FEBS Lett. 589:3205–3211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu ZS, Wu Q, Wang CQ, Wang XN, Wang Y,
Zhao JJ, Mao SS, Zhang GH, Zhang N and Xu XC: MiR-339-5p inhibits
breast cancer cell migration and invasion in vitro and may be a
potential biomarker for breast cancer prognosis. BMC Cancer.
10:5422010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren H, Zhang Y and Zhu H: MiR-339
depresses cell proliferation via directly targeting S-phase
kinase-associated protein 2 mRNA in lung cancer. Thorac Cancer.
9:408–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou C, Liu G, Wang L, Lu Y, Yuan L, Zheng
L, Chen F, Peng F and Li X: MiR-339-5p regulates the growth, colony
formation and metastasis of colorectal cancer cells by targeting
PRL-1. PLoS One. 8:e631422013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi S, Cao X, Gu M, You B, Shan Y and You
Y: Upregulated expression of SOX4 is associated with tumor growth
and metastasis in nasopharyngeal carcinoma. Dis Markers.
2015:6581412015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang CL, Hseu YC, Lin YF, Hung ST, Tai C,
Uen YH and Lin KY: Clinical and prognostic association of
transcription factor SOX4 in gastric cancer. PLoS One.
7:e528042012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng JH, Jian ZX, Jin HS, Chen SC and
Wang GY: Expression of SOX4 gene and early recurrence of
hepatocellular carcinoma: Their relationship and the clinical
significance. Nan Fang Yi Ke Da Xue Xue Bao. 30:818–819. 2010.(In
Chinese). PubMed/NCBI
|
|
40
|
Wang B, Li Y, Tan F and Xiao Z: Increased
expression of SOX4 is associated with colorectal cancer
progression. Tumour Biol. 37:9131–9137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bao ZQ, Zhang CC, Xiao YZ, Zhou JS, Tao YS
and Chai DM: Over-expression of Sox4 and β-catenin is associated
with a less favorable prognosis of osteosarcoma. J Huazhong Univ
Sci Technolog Med Sci. 36:193–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun R, Jiang B, Qi H, Zhang X, Yang J,
Duan J, Li Y and Li G: SOX4 contributes to the progression of
cervical cancer and the resistance to the chemotherapeutic drug
through ABCG2. Cell Death Dis. 6:e19902015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aaboe M, Birkenkamp-Demtroder K, Wiuf C,
Sørensen FB, Thykjaer T, Sauter G, Jensen KM, Dyrskjøt L and
Ørntoft T: SOX4 expression in bladder carcinoma: Clinical aspects
and in vitro functional characterization. Cancer Res. 66:3434–3442.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zou J and Xu Y: MicroRNA-140 inhibits cell
proliferation in gastric cancer cell line HGC-27 by suppressing
SOX4. Med Sci Monit. 22:2243–2252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng Q, Wu J, Zhang Y, Liu X, Xu N, Zuo F
and Xu J: SOX4 promotes melanoma cell migration and invasion though
the activation of the NF-κB signaling pathway. Int J Mol Med.
40:447–453. 2017. View Article : Google Scholar : PubMed/NCBI
|