Introduction
Proliferation of mesangial cells (MCs) and
accumulation of extracellular matrix (ECM) are critical to
glomerulosclerosis (GS), which serves as an end-stage event in
chronic renal failure (1–3). Sclerotic glomeruli are characterized
by loss of functional cells and the overexpression of
non-functional matrix, commonly composed of type I collagen (COL-1)
and fibronectin (FN) (4–6). These factors initially form in the
mesangium, and the response may be enhanced by intraglomerular
hypertension (7–9) or certain chemical cytokines (10–13).
Intraglomerular hypertension, which is attributed to amplified
cyclic stretch, causes mechanical damage to MCs (7–9).
Certain chemical cytokines, including transforming growth factor
(TGF)-β1, cause MCs to undergo a phenotype transformation to
resemble fibroblasts, accompanied by the infiltration of
inflammatory cells, causing glomerular scarring. This type of
scarring eventually results in glomerular sclerosis (14). In patients with glomerulotubular
imbalance, when glomerular filtration rate (GFR) declines to 10% of
normal values, renal replacement therapy is required, as currently
there is no specific treatment to reverse the progression.
Prostaglandin E2 (PGE2) has multiple biological
effects in organ fibrosis. In the kidney, through its four
receptors (EP1, EP2, EP3 and EP4), there is also a dual regulatory
role of PGE2 (15–19). The present study focused on EP4,
the role of which still remains controversial, particularly in the
regulation of renal diseases, depending on the cell type and
context. Our previous study identified that EP4 plays a maladaptive
role in MC injury associated with chronic kidney disease (20–22).
Consistent with these findings, inflammation and albuminuria was
minimized through cyclooxygenase-2 (COX-2)/EP4 suppression in
streptozotocin-induced diabetic mice (23,24).
In addition, diabetes-induced COL-1 and connective tissue growth
factor (CTGF) expression was markedly increased in EP4
agonist-treated mice (24,25). However, Vukicevic et al
(25), argued that both EP2 and
EP4 receptor agonists prevent the progression of chronic renal
failure in unilateral ureteral obstruction models. EP2 and EP4,
which both bind to G stimulatory (Gs) protein to increase cyclic
adenosine 5′-monophosphate (cAMP) levels, are present in the
medulla and glomerulus, respectively (26,27).
It is reported that EP2 primarily increases the level of cAMP and
delivers a protective effect in the regulation of tissue fibrosis,
but EP4 is not thought to have the same effect (28–30).
Therefore, it was hypothesized that EP4 may be involved in a
different signaling pathway.
TGF-β1, a fibrotic stimulus, is able to activate
Smad proteins (Smad2 or Smad3) directly to cause renal fibrosis
(31–34). Mitogen-activated protein kinase
(MAPK) pathways, which comprise extracellular signal-regulated
kinase (ERK), c-JUN N-terminal kinase (JNK) and p38 signaling, have
been reported to play a role in inflammatory regulation (35,36),
and also contribute to the amplification of transmitted signals to
promote cellular processes, including proliferation and
differentiation, either in the cytoplasm or nucleus (37,38).
Thus, the current study aimed to investigate two areas: Firstly,
whether there is a cross-talk effect between Smad3 and MAPKs in
glomerulosclerosis; and secondly, whether the phosphorylation of
MAPKs may be mediated by EP4 receptors.
In the present study, 5/6 nephrectomy was performed
to examine the effect of EP4 on glomerulosclerosis. Primary MCs of
different genotypes were also cultured to detect possible signaling
pathways. Briefly, the aim of the current study was to investigate
whether EP4 is required for mesangium fibrosis.
Materials and methods
Experimental animals
The experimental animals were provided by the Animal
Experimentation Committee of Beijing University Health Science
Center (Beijing, China). The mice were C57/BL6 type and bred in a
specific-pathogen-free environment. The animal protocol in this
study was approved by the Beijing University Animal Care and Use
Committee. Wild-type (WT), EP4 heterozygotes (EP4+/−)
and EP4flox/flox (with a conditional knock-out EP4 gene
sequence between LoxP sites flanking exon 2 of the EP4 gene) male
mice aged 8–12 weeks were used for experiments.
EP4flox/flox mice were transfected with Cre adenovirus
to knock out EP4 receptors (23).
All mice were kept in an air-conditioned environment (20°C, 50%
humidity) with a 12-h light/dark cycle and had free access to food
and water prior to and following surgery. The mice were euthanized
with 150 mg/kg pentobarbital by intraperitoneal injection.
5/6 nephrectomy
A total of 40 C57/BL6 mice weighing 25–35 g were
included in the experiment, and were not limited in eating and
drinking the night prior to surgery. The 5/6 nephrectomy was
performed according to our previous study (39). The mice were divided into four
groups (n=10 per group): 1, WT Control (CON) group; 2, WT 5/6
Nephrectomy (Nx) group; 3, EP4+/− CON group; 4,
EP4+/− 5/6 Nx group.
Sample collection
Prior to sacrifice, WT CON, WT 5/6 Nx,
EP4+/− CON and EP4+/− 5/6 Nx mice (n=10 in
each group) were placed individually in metabolic cages for 24-h
urine collection. Urine samples were centrifuged at 10,000 × g for
10 min at room temperature and the supernatants were stored at
−20°C until analysis. Prior to sacrifice, the mice were
anaesthetized with 1% pentobarbital (40 mg/kg) via intraperitoneal
injection for serum and kidney collection. Blood samples (~0.8 ml)
were collected from the postcava in heparinized tubes and
centrifuged at 5,000 × g for 15 min to obtain serum for the
measurement of serum creatinine, urea nitrogen and urine protein.
The kidneys were quickly removed and either frozen immediately in
liquid nitrogen or fixed with 4% buffered formalin. Residual
tissues and serum were preserved at −80°C. Finally, animals were
sacrificed as aforementioned.
Albumin and creatinine concentrations were
determined using Exocell assays, according to the manufacturer's
instructions (Exocell, Inc., Philadelphia, PA, USA).
Histology and
immunohistochemistry
Kidneys were fixed with 10% neutral buffered
formalin and processed for histology or immunostaining using
standard techniques. Histological sections (5-µm thick) were
prepared and stained with anti-FN (Abcam, Cambridge, MA, USA),
anti-COI-I, anti-COX-2, anti-EP4, anti-CTGF (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), anti-phosphorylated (p)-Smad3, anti-p-p38
and anti-ERK (Cayman Chemical Company, Ann Arbor, MI, USA)
overnight at 4°C, and incubated at 37°C for 1 h. Then Secondary
antibodies were added. For staining quantification (FN, COI-I,
COX-2, and CTGF), Six golmeruli per mouse from each group and 3
mice per group were randomly and blindly selected and analyzed. The
percentage of the positively stained area was measured by using
ImageJ sofware. The degree of fibrosis was quantified in trichrome
sections by assessing the surface area of the cortical area
(avoiding great vessels and glomeruli) as a ratio of total surface
area at ×400 magnification. All measurements and quantification
were performed in a random blinded manner using an Olympus BX50
microscope (Olympus Corporation, Tokyo, Japan), a Micropublisher
3.3 RTV camera (Q-Imaging, Surrey, BC, Canada), and NIS Elements
Imaging software (Nikon Instruments, Inc., Melville, NY, USA).
Culture of primary MCs
MC culture was performed according to the protocol
in our previous study (21). The
primary mice MCs at passages 8 to 10 were used, and were cultured
at 37°C in a humidified incubator containing 5% CO2,
with the addition of 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Then, WT cells were divided
into the following groups: 1, WT + AD-GFP group; 2) WT + AD-GFP+
TGF-β1 group; 3, WT + AD-EP4 group; 4, WT + AD-EP4 + TGF-β1 group.
EP4flox/flox cells were also divided into four groups:
1, EP4flox/flox + AD-GFP group; 2)
EP4flox/flox + AD-GFP + TGF-β1 group; 3,
EP4flox/flox + AD-CRE group; 4, EP4flox/flox
+ AD-CRE + TGF-β1 group. Prior to the experiments, the cells were
incubated in serum-free medium for 24 h. The optimum dose and
reaction time of TGF-β1 (10 ng/ml, 24 h) was based on a previous
study (14). Each individual assay
was repeated at least three times with different cell
preparations.
Adenoviral constructs and infection of
cultured mouse MCs
AD-CRE and AD-EP4 were generated by the Shanghai
GenePharma Co., Ltd., (Shanghai, China). Linearized recombinant
adenoviral plasmid was transfected into AD-293 cells to obtain a
primary viral stock, which was amplified and purified. For
optimization of infection conditions, differentiated mouse MCs were
infected with AD-CRE at a multiplicity of infection (MOI) of 5 or
AD-EP4 at an MOI of 10 for 72 h.
Western blot analysis
Cell lysis buffer was added to the wells and the
plate was placed on ice for 30 min. Then, cells treated as
described above were scraped, and cell lysate was removed to 1.5 ml
EP tubes and centrifuged for 15 min (4°C, 12,000 rpm). Protein
concentration in the supernatant was determined by BCA assay
(Pierce; Thermo Fisher Scientific, Inc.). Samples were diluted in
the loading buffer and boiled for 5 min. Then, 130 mg of each
sample was separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to a PVDF membrane
for 2 h. The membrane was blocked at room temperature for 1 h in 5%
(w/v) non-fat dry milk. The PVDF membrane was then incubated with
primary antibodies (rabbit anti-p-Smad3, rabbit anti-Smad3, rabbit
anti-p-p38, rabbit anti-p38, rabbit anti-p-ERK, rabbit anti-ERK,
mouse anti-p-JNK and mouse anti-JNK; 1:1,000; Cayman Chemical
Company) at 4°C overnight.
Subsequent to stimulation by TGF-β1 (10 ng/ml) for
24 h, the medium of some dishes was removed and 1 mM PD98059, 10 mM
P38ML3404 or 2 mM SP600125 was added for 30 min to block ERK, P38
or JNK signaling, respectively. The control cells were treated with
vehicle (DMSO), then the PVDF membrane was incubated with primary
antibodies (mouse anti-FN, mouse anti-COI-I, rabbit anti-COX2,
mouse anti-CTGF 1:1,000; Rockland Immunochemicals, Inc., Pottstown,
PA, USA) at 4°C overnight. The membrane was washed with
Tris-buffered saline with Tween, incubated with DyLight 800-labeled
antibody to mouse IgG (1:5,000) or rabbit IgG for 2 h, and the
membrane was scanned using the Bio-Rad Imaging System (Bio-Rad
Laboratories, Inc.) for semi-quantitative analysis. EIF5 was used
as a loading control.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Data were analyzed by Student's t-test (paired groups)
or one-way analysis of variance, followed by Bonferroni's post-hoc
test for multiple comparisons. Statistics were performed using
GraphPad Pro (GraphPad, San Diego, CA, USA) P<0.05 was
considered to indicate a statistically significant difference.
Results
EP4 knockout confers protection in
response to 5/6 nephrectomy
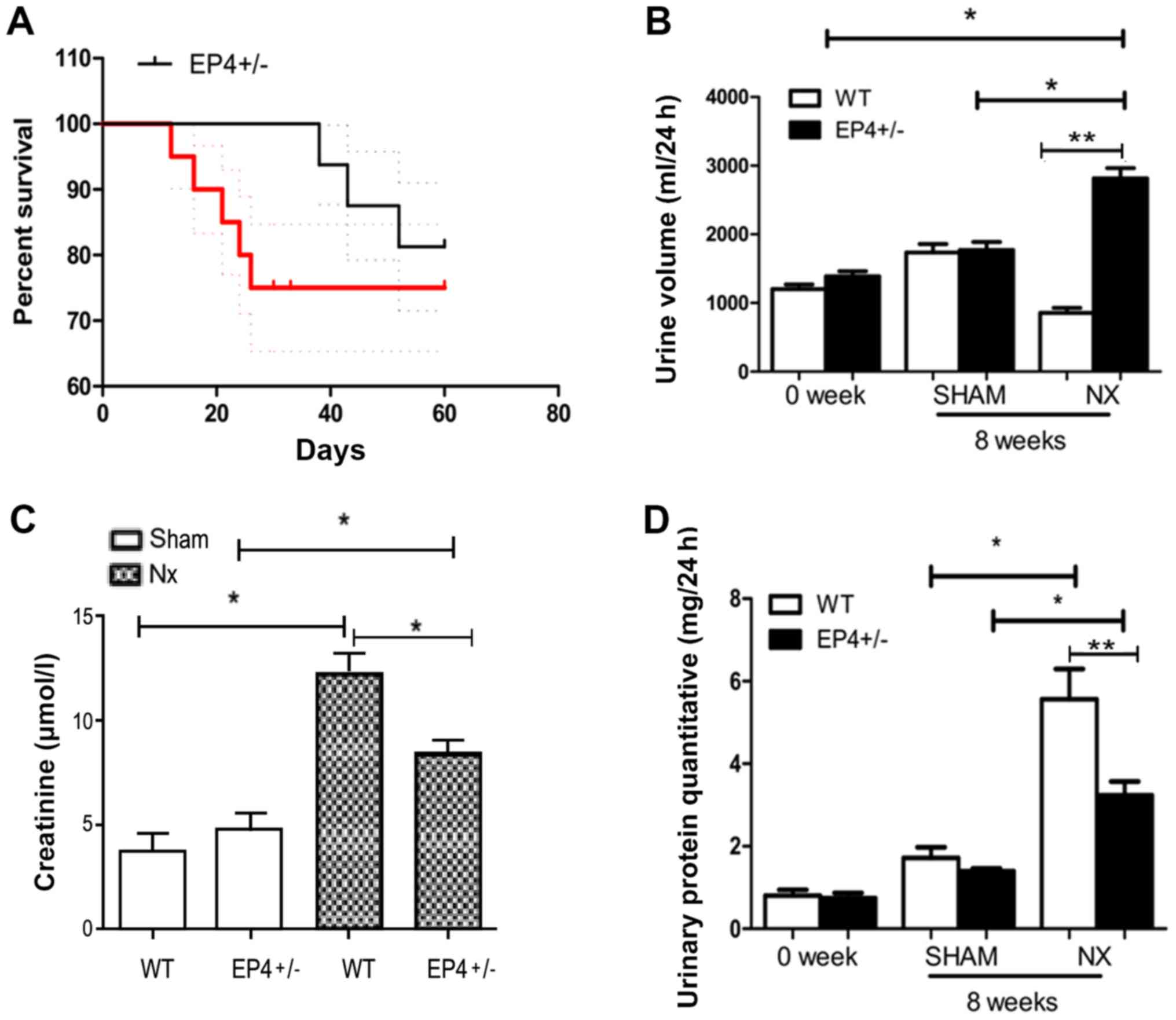
To assess the protective effect of EP4 knockout in
response to 5/6 nephrectomy, the survival rate of EP4+/−
and WT mice was compared (Fig. 1).
There was a significant difference in the survival rate between
EP4+/− and WT mice (Fig.
1A). The WT group was severely polyuric, excreting almost
three-fold the volume excreted by the litter-matched
EP4+/− mice at 8 weeks post-surgery (Fig. 1B). Serum creatinine levels were
lower in EP4+/− mice compared with WT mice at 8 weeks
post-surgery (Fig. 1C).
Furthermore, mice in both groups exhibited massive proteinuria at 8
weeks post-surgery. Compared with EP4+/− mice, WT mice
presented two-fold proteinuria (Fig.
1D).
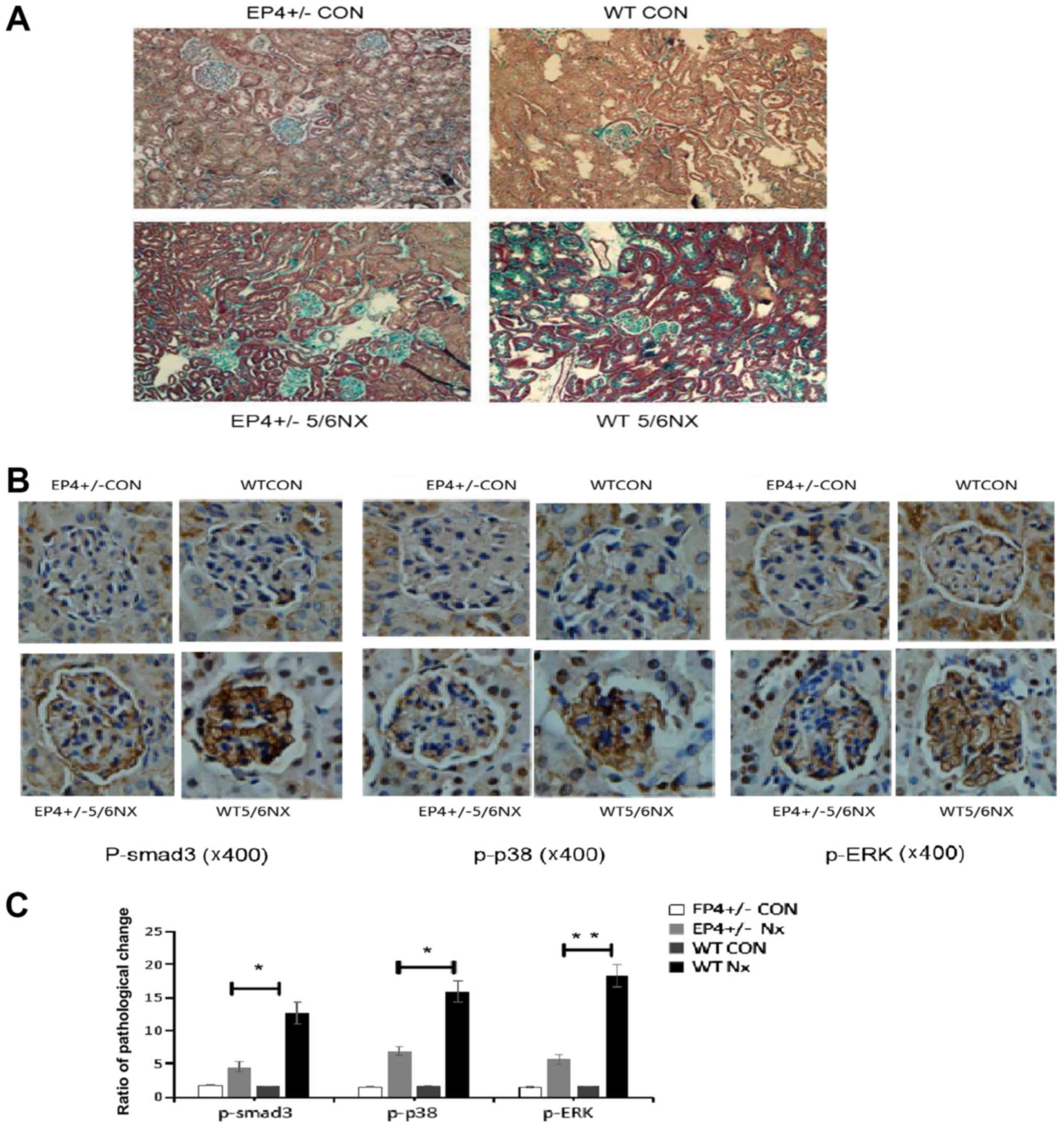
EP4 knockout reduces GS following 5/6
nephrectomy
To determine whether EP4 knockout reduces GS induced
by 5/6 nephrectomy, Masson trichrome staining was used to detect
the level of GS. GS following nephrectomy was obviously evident in
the WT group at 8 weeks post-surgery, compared with a less obvious
change in the EP4+/− group (Fig. 2A). The expression of p-Smad3, p-ERK
and p-p38 was markedly increased in the 5/6 nephrectomy group
compared with the EP4+/− group (Fig.
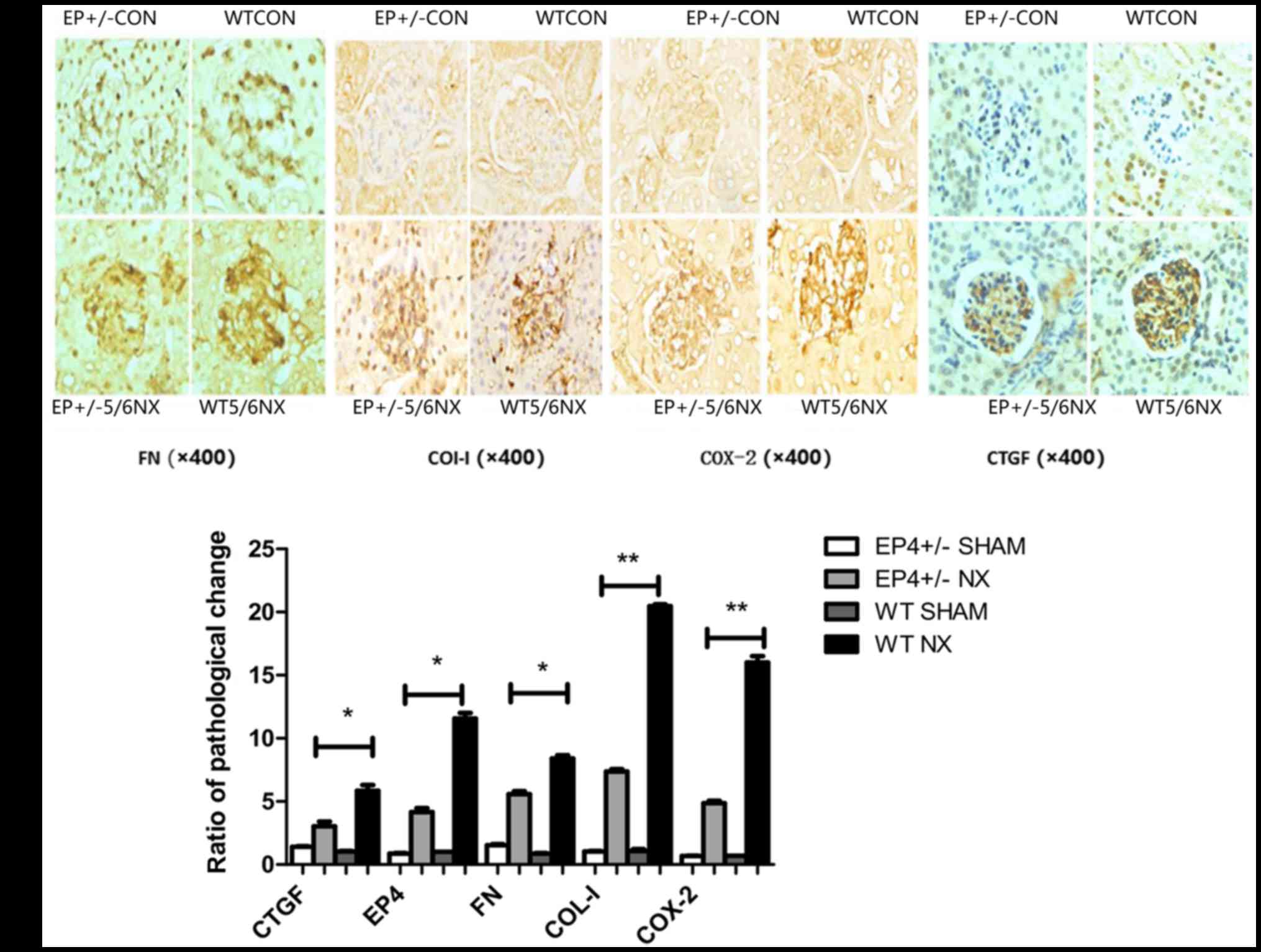
2B and C). A decrease in COX-2, FN, COI-I and CTGF was detected
in EP4+/− mice compared with WT mice. These indicators were
markedly increased at 8 weeks subsequent to 5/6 nephrectomy,
particularly in the WT group (Fig. 3A
and B).
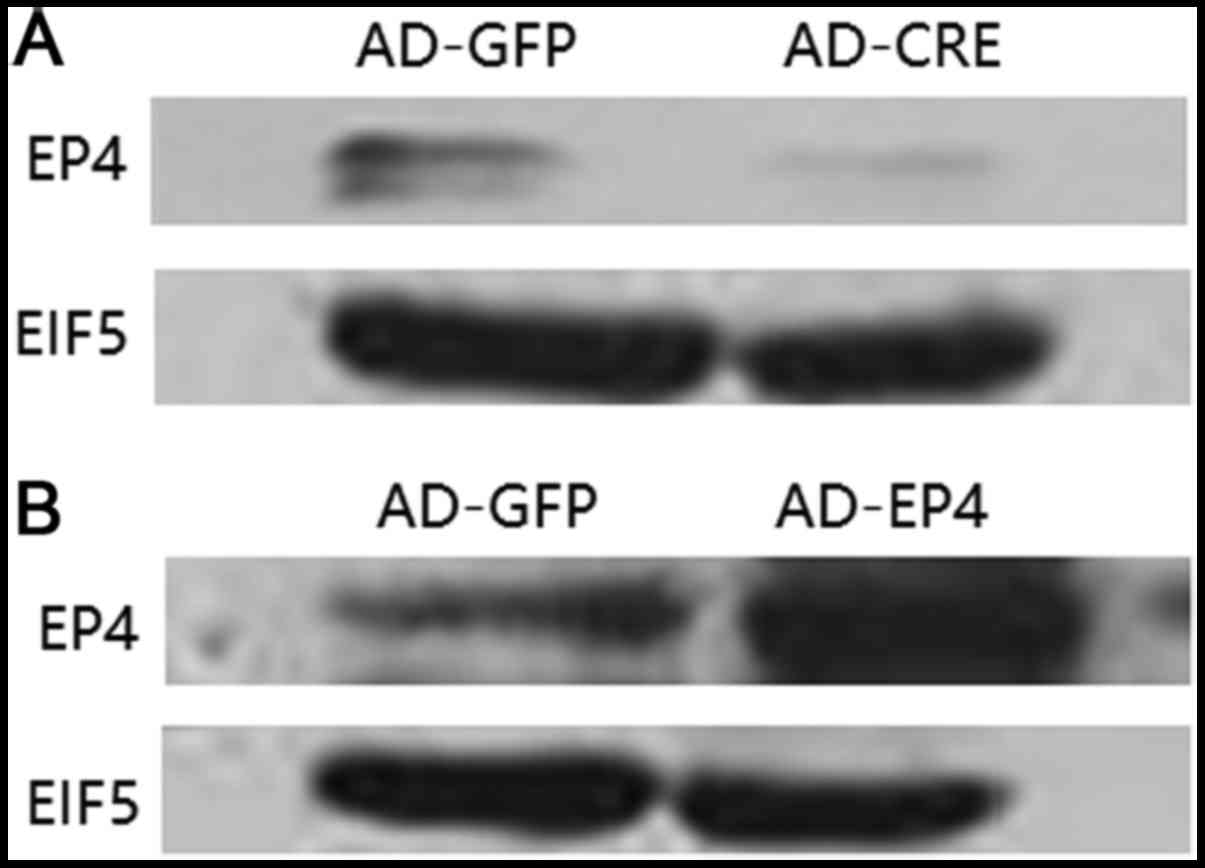
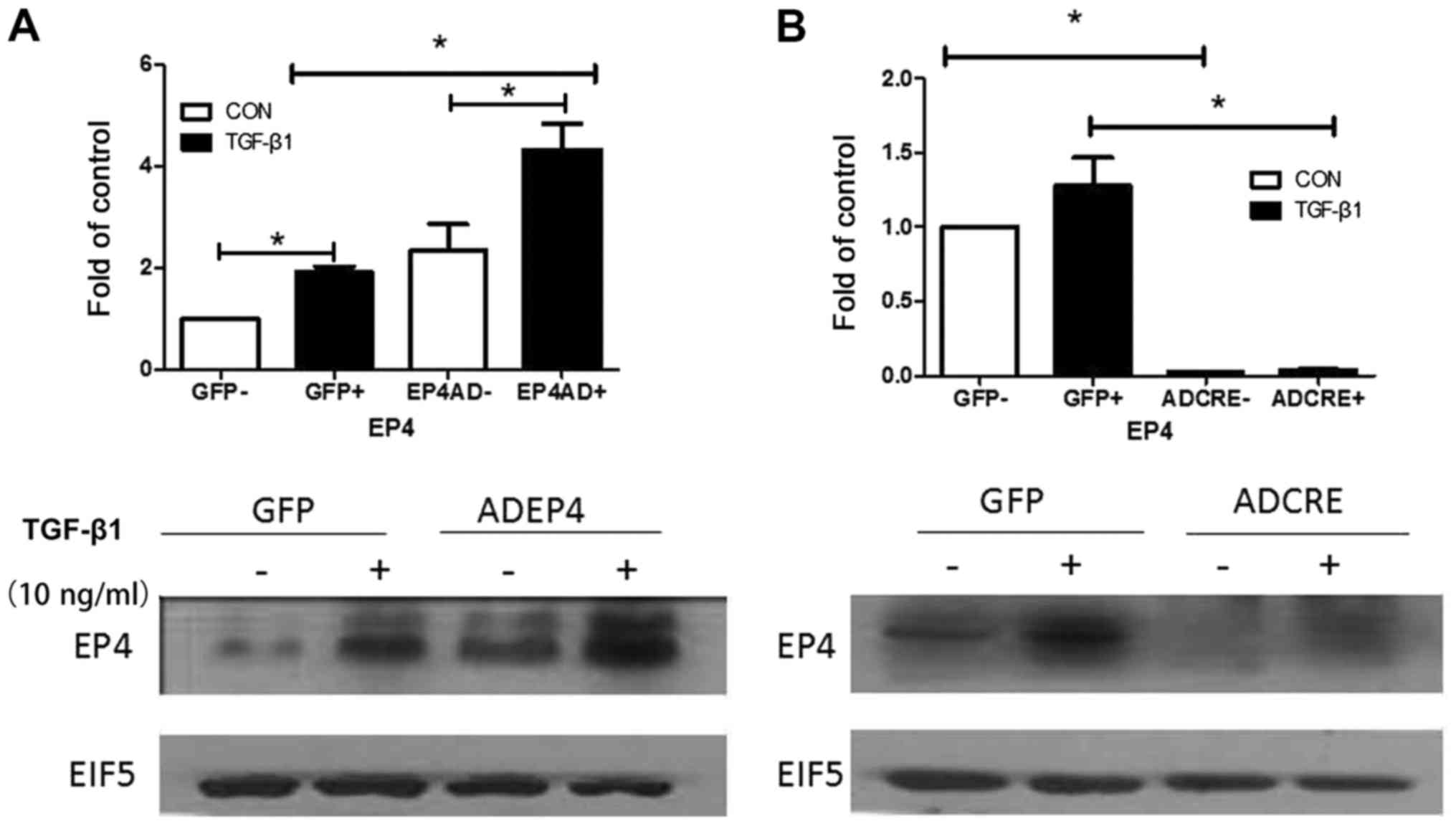
Expression of EP4 protein infected
with AD-EP4 in WT mouse MCs and with AD-CRE in
EP4Flox/Flox mouse MCs
To confirm that AD-EP4 had been successfully
transfected in WT mouse MCs, and AD-CRE had been successfully
transfected in EP4Flox/Flox mouse MCs (LoxP sequences
were introduced at both ends of the EP4 gene, allowing CRE
recombinant enzyme to remove the EP4 gene), western blotting was
performed to evaluate EP4 protein expression. Expression of EP4
protein is increased following infection with AD-EP4 in WT mouse
MCs, compared with the AD-GFP group. It is opposite following
infection with AD-CRE in EP4Flox/Flox mouse MCs (Fig. 4A and B).
Expression of EP4 protein is increased
following infection with AD-EP4 in WT mouse MCs induced by
TGF-β1
Following infection with AD-EP4 (MOI=10) in WT mice
MCs treated with 10 ng/ml TGF-β1 for 12 h, the expression of EP4
protein markedly increased compared with the AD-GFP group (control
group, gene recombinant adenovirus with green fluorescent protein;
Fig. 5A and B).
Expression of EP4 protein is decreased
following infection with AD-CRE in EP4Flox/Flox mouse
MCs induced by TGF-β1
AD-CRE had been transfected in
EP4Flox/Flox mouse MCs treated with 10 ng/ml TGF-β1 for
12 h western blotting was performed to evaluate EP4 protein
expression. Following infection with AD-CRE (MOI=10), the
expression of EP4 protein decreased markedly compared with the
AD-GFP groups (control group, gene recombinant adenovirus with
green fluorescent protein; Fig. 5C and
D).
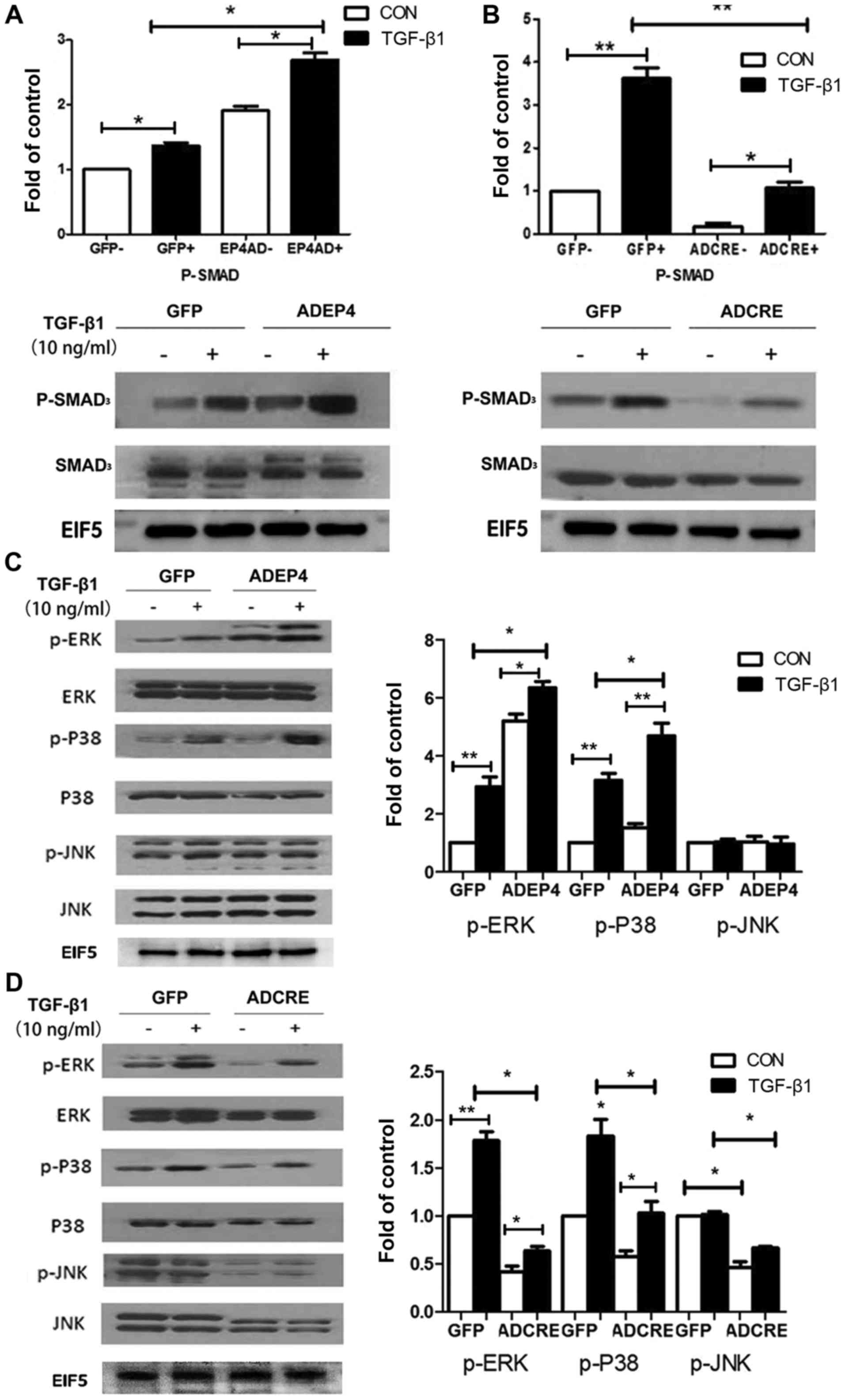
EP4 knockout reduces Smad3, ERK and
P38 phosphorylation in MCs induced by TGF-β1
Smad signaling is generally considered to be the
classical mechanism for fibrosis in physiological change induced by
TGF-β1 (32). To confirm the
fibrosis effect in MCs induced by TGF-β1, the level of Smad3 was
detected and it was identified that TGF-β1 effectively enhanced the
phosphorylation level of Smad3 in both MC genotypes. Compared with
GFP-treated WT cells, AD-EF4 transfection increased the level of
phosphorylation, while AD-CRE administered in
EP4flox/flox MCs downregulated the level of p-Smad3
(Fig. 6A and B).
Furthermore, previous research has indicated that
there is cross-talk between Smad and MAPK signaling (32). In addition, although both EP2 and
EP4 couple with Gs protein to upregulate the level of cAMP, it is
EP2 that primarily increases cAMP level (25). Therefore, the current study aimed
to reveal the possible mechanism of the maladaptive role of EP4 in
MC injury. It was identified that there was a marked increase in
phosphorylation of ERK and P38 when MCs were stimulated by TGF-β1,
while JNK signaling did not exhibit phosphorylation. The
phosphorylation of ERK and P38 was markedly enhanced with the
introduction of AD-EP4, and markedly reduced with the introduction
of AD-CRE, compared with the respective GFP-treated cells. However,
there was no significant change in the level of p-JNK (Fig. 6C and D).
To confirm the critical role of MAPK signaling in
the regulation of MC injury, three corresponding signaling
inhibitors against ERK (PD98059), P38 (ML3404) and JNK (SP600125)
were used to block their effects. The expression of COI-I and COX-2
was inhibited ~2-fold by PD98059 in comparison with the control
group. The level of CTGF was primarily reduced by ML3404. FN
expression was reduced upon administration of PD98059 and
ML3404.SP600125 did not exhibit any effects (Fig. 7).
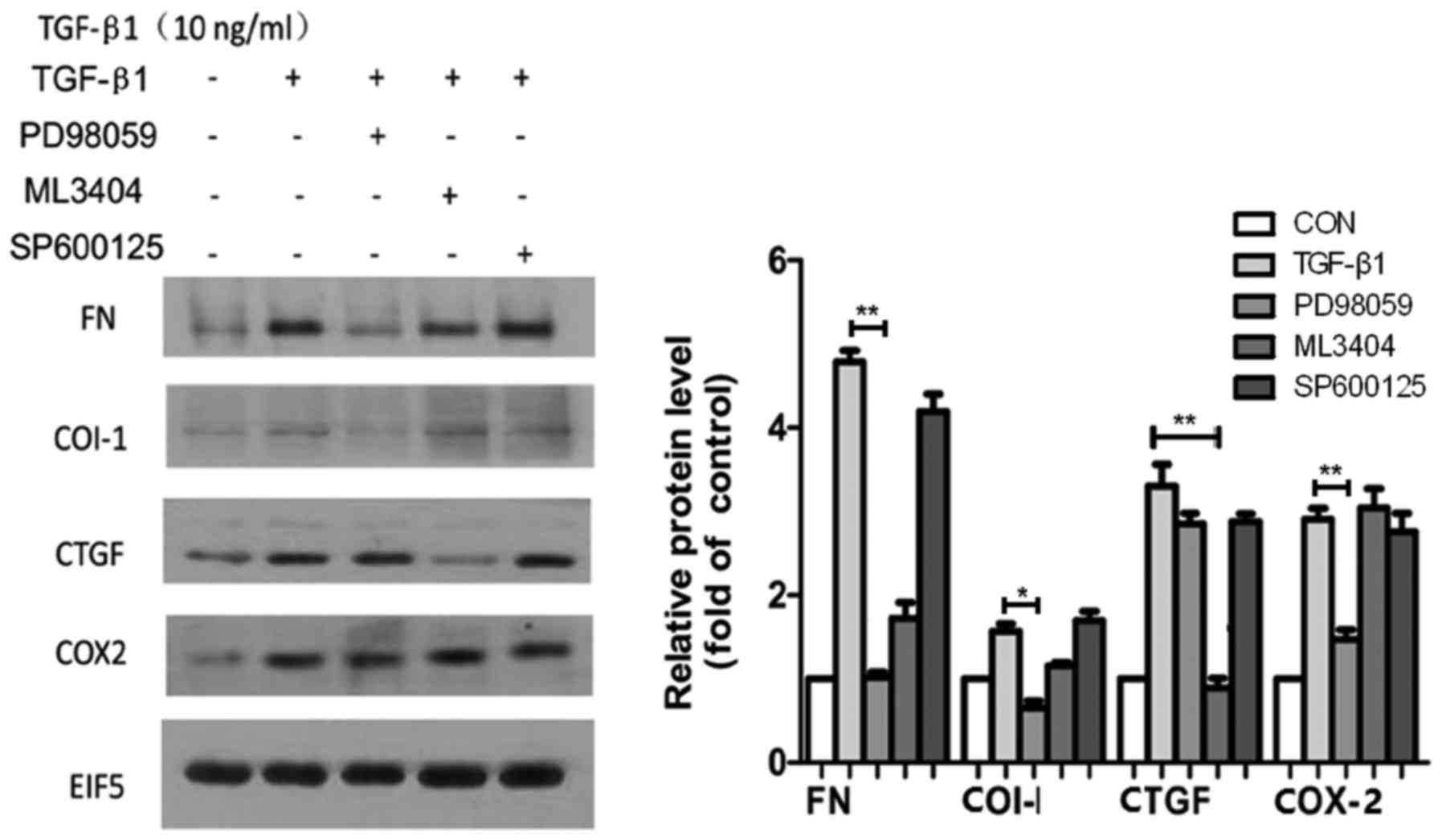 | Figure 7.Following treatment with 10 ng/ml
TGF-β1 for 24 h, the expression of FN, COI-I, COX-2 and CTGF
protein increased in MCs of WT mice, compared with the control
group. Inhibitors of ERK, p38 MAPK and JNK (PD98059, ML3404 and
SP600125, respectively) were added to MCs for 30 min prior to
treatment with 10 ng/ml TGF-β1 for 24 h. The expression of FN and
COI-I decreased following treatment with PD98059, and the
expression of CTGF decreased following treatment with ML3404.
However, no obvious differences were observed following treatment
with SP600125. *P<0.05, **P<0.01. MCs, mesangial cells; WT,
wild type. |
Discussion
PGE2 is widely secreted and plays a significant role
in pathological processes. Our previous research indicated that
conditional deletion of EP4 from MCs conferred partial protection
from glomerular damage, and such protection against mesangial ECM
accumulation primarily involves the COX2-PGE2-EP4 system (22). However, these findings contrast
with those of Vukicevic et al (25). The current findings strongly
indicate that EP4 plays a role in the injury of MCs and development
of glomerular sclerosis. Therefore, the EP4 receptor may exert
pleiotropic effects on kidney injury, depending on the specific
tissue or cell type. In the present study, subtotal-nephrectomy
(5/6) was also performed to further confirm the effect of EP4. This
model showed that mesangial cell hyperplasia appeared from the
second week, and this becomed more serious after 8 week. We
analyzed the potential signaling pathway of such damage.
As EP4−/− mice inevitably suffer from
neonatal patent ductus arteriosus (19), heterozygotic mice were used to
establish the model in the current study. Partial EP4 silencing was
efficient in the current research, as the level of cAMP decreased
significantly compared with WT mice (27). The knockdown of EP4 resulted in
higher survival rate and suitable regulation of urine osmotic
pressure, suggesting the receptor's possible maladaptive effect in
the balance between glomerular infiltration and tubular
re-absorption. This imbalance also led to secondary hypertension,
as indicated by renal artery stenosis (RAS), and it interacted with
mesangium expansion in the progression of glomerular sclerosis. It
has been reported that the stimulation of EP4 receptors could
exacerbate glomeruli sclerosis associated with enhanced glomerular
capillary pressure (40). Hartono
demonstrated that db/db mice with RAS develop diffuse mesangial
sclerosis (41). These reports are
consistent with the current findings.
Recent studies have reported that Gq-dependent
signaling induces COX-2 expression in podocytes, and its
upregulation is associated with PGE2 synthesis (42). Our previous study confirmed the
synergistic effect of COX-2 in MCs injury induced by TGF-β1
(20).
In the present study, the level of COX-2 in
intravital glomeruli was measured, and pathological changes were
evaluated to indicate the maladaptive role of COX-2 in 5/6
nephrectomy. The results indicated that glomerular COX-2 induction
coupled with enhanced PGE2 synthesis required the activation of
EP4. Although it has been indicated that the administration of EP4
agonist intravenously may assist in preserving renal blood flow
(18), to the best of our
knowledge, the expression of EP4 in resident glomeruli has not been
studied in detail previously. In the current study, EP4 level was
highest in WT 5/6 nephrectomy mice, which indicated a specific role
of EP4 in PGE2/COX-2-associated injury.
Smads mediate the signal transduction from TGF-β1.
Smad3 is well known as a key regulator of fibrosis in many organ
systems, and this is supported by the findings that mice lacking
Smad3 are protected against fibrosis in multiple disease models
(32–34). MAPK signaling is able to regulate
fibrosis either independently or through cross-talk with Smad
pathways (36–38). It has been reported that both
TGF-β1/Smad and ERK/MAPK signaling act as pro-fibrotic pathways
accelerated by blood glucose fluctuation (43–45).
In the present study, the regulatory effect of MAPKs in MC injury
induced by TGF-β1 was evaluated. The effect was also validated via
the introduction of MAPK pathway inhibitors. The results indicated
that ECM accumulation was primarily mediated by Smad3, ERK/MAPK and
P38 signaling. Subsequently, to confirm the role of EP4 in MC
damage, AD-CRE and AD-EP4 were introduced. These two compounds have
been well documented in our previous research (20–22).
The level of phosphorylated Smad3, ERK and P38, but not JNK, were
obviously enhanced and reduced in MCs transfected with AD-EP4 and
AD-CRE, respectively.
In conclusion, EP4 enhancement could accelerate MC
damage induced by TGF-β1 with increasing ECM protein synthesis.
ERK, P38 and TGF-β1/Smad3 signaling appear to play a key role in
the injury. If the two pathways interact with each other, it is our
next work. The maladaptive effect of EP4 is also manifested in
vivo, with glomerulo-tubular imbalance, higher
reno-hypertension and glomerular sclerosis. The current findings on
EP4 identify a novel potential target for treatment in order to
delay the progression of glomerulopathy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81170656), Key
Projects of Science and Technology Development Funds of Nantong
(grant no. MS32015018) and the Fifth ‘226 Project’ Research
Projects of Nantong (grant no. 2016-1-23).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJC and TYP performed the experiments, participated
in collecting data and drafted the manuscript. NFG and BCW
performed statistical analysis and participated in study design.
XLC designed the experiments. JHW performed the animal experiments.
XLC and JHW helped to draft the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal protocol in this study was approved by
the Beijing University Animal Care and Use Committee (Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu C, Sun L, Xiao L, Han Y, Fu X, Xiong X,
Xu X, Liu Y, Yang S, Liu F and Kanwar YS: Insights into the
mechanisms involved in the expression and regulation of
extracellular matrix proteins in diabetic nephropathy. Curr Med
Chem. 22:2858–2870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo F, Wang Q, Zhou Y, Wu L, Ma X, Liu F,
Huang F and Qin G: Lentiviral vector-mediated FoxO1 overexpression
inhibits extracellular matrix protein secretion under high glucose
conditions in mesangial cells. J Cell Biochem. 117:74–83. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng FF, Xiao ZL, Chen HM, Chen Y, Zhou J,
Yu H and Zhang BF: Parathyroid hormone inhibits TGF-β/Smad
signaling and extracellular matrix proteins upregulation in rat
mesangial cells. Biochem Biophys Res Commun. 478:1093–1098. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen FQ, Wang QY, Wei GZ, Ma XY, Ma DW,
Deng WW and Sun WB: Effects of mycophenolate mofetil on the
expression of monocyte chemoattractant protein-1 and fibronectin in
high glucose cultured human mesangial cells. Genet Mol Res.
13:3154–3161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang P, Zhang Y, Jiang T and Zhang N:
Effects of p38 MAPK signaling pathway and aldose reductase on
transforming growth factor-β1 induced expression of fibronectin in
cultured human mesangial cells. Zhonghua Bing Li Xue Za Zhi.
44:778–782. 2015.(Iin Chinese). PubMed/NCBI
|
|
6
|
Zhu L, Qi XY, Aoudjit L, Mouawad F,
Baldwin C, Nattel S and Takano T: Nuclear factor of activated T
cells mediates RhoA-induced fibronectin upregulation in glomerular
podocytes. Am J Physiol Renal Physiol. 304:F849–F862. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohara N, Hanyu O, Hirayama S, Nakagawa O,
Aizawa Y, Ito S and Sone H: Hypertension increases urinary
excretion of immunoglobulin G, ceruloplasmin and transferrin in
normoalbuminuric patients with type 2 diabetes mellitus. J
Hypertens. 32:432–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Thai K, Kepecs DM and Gilbert RE:
Sodium-Glucose linked cotransporter-2 inhibition does not attenuate
disease progression in the rat remnant kidney model of chronic
kidney disease. PLoS One. 11:e01446402016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan T, Gao B, Chen G, Chen X, Janssen M,
Uttarwar L, Ingram AJ and Krepinsky JC: Colchicine attenuates renal
injury in a model of hypertensive chronic kidney disease. Am J
Physiol Renal Physiol. 305:F1466–F1476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Har R, Scholey JW, Daneman D, Mahmud FH,
Dekker R, Lai V, Elia Y, Fritzler ML, Sochett EB, Reich HN and
Cherney DZ: The effect of renal hyperfiltration on urinary
inflammatory cytokines/chemokines in patients with uncomplicated
type 1 diabetes mellitus. Diabetologia. 56:1166–1173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Izzedine H, Escudier B, Lhomme C, Pautier
P, Rouvier P, Gueutin V, Baumelou A, Derosa L, Bahleda R,
Hollebecque A, et al: Kidney diseases associated with anti-vascular
endothelial growth factor (VEGF): An 8-year observational study at
a single center. Medicine (Baltimore). 93:333–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Batal I, De Serres SA, Mfarrej BG, Grafals
M, Pinkus GS, Kalra A, Weins A, Bijol V, Rennke HG, Guleria I, et
al: Glomerular inflammation correlates with endothelial injury and
with IL-6 and IL-1β secretion in the peripheral blood.
Transplantation. 97:1034–1042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zelveian PA and Dgerian LG: The main
pathophysiological mechanisms of kidney injury in obstructive sleep
apnea syndrome. Ter Arkh. 86:100–105. 2014.(In Russian). PubMed/NCBI
|
|
14
|
Zhang S, Zhang M, Huang H, Zhou S, Du Y,
Yi X and Luo J: High glucose-induced Matrilin-2 expression in mouse
mesangial cells was mediated by transforming growth factor beta 1
(TGF-β1). Biochem Biophys Res Commun. 474:303–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akaba T, Komiya K, Suzaki I, Kozaki Y,
Tamaoki J and Rubin BK: Activating prostaglandin E2 receptor
subtype EP4 increases secreted mucin from airway goblet cells. Pulm
Pharmacol Ther. 48:117–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin Y, Smith C, Hu L, Coutant DE,
Whitehurst K, Phipps K, McNearney TA, Yang X, Ackermann B, Pottanat
T and Landschulz W: LY3127760, a selective prostaglandin E4 (EP4)
receptor antagonist and celecoxib: A comparison of pharmacological
profiles. Clin Transl Sci. 11:46–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujioka H, Funabashi T and Akema T:
Prostaglandin E2 modulates presynaptic regulation of GnRH neurons
via EP4 receptors in accordance with estrogen milieu. Neuroscience.
360:139–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thieme K, Majumder S, Brijmohan AS, Batchu
SN, Bowskill BB, Alghamdi TA, Advani SL, Kabir MG, Liu Y and Advani
A: EP4 inhibition attenuates the development of diabetic and
non-diabetic experimental kidney disease. Sci Rep. 7:34422017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong YA, Yang KJ, Jung SY, Park KC, Choi
H, Oh JM, Lee SJ, Chang YK, Park CW, Yang CW, et al: Paricalcitol
pretreatment attenuates renal ischemia-reperfusion injury via
prostaglandin E2 receptor EP4 pathway. Oxid Med Cell Longev.
2017:50319262017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xi PP, Xu YY, Chen XL, Fan YP and Wu JH:
Role of the prostaglandin E2 receptor agonists in TGF-β1-induced
mesangial cell damage. Biosci Rep. 36:pii: e00383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Jiang D, Wang J, Chen X, Xu X, Xi
P, Fan Y, Zhang X and Guan Y: Prostaglandin E2 EP1 receptor
enhances TGF-β1-induced mesangial cell injury. Int J Mol Med.
35:285–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang GX, Xu YY, Fan YP, Wang J, Chen XL,
Zhang YD and Wu JH: A maladaptive role for EP4 receptors in mouse
mesangial cells. PLoS One. 9:e1040912014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mohamed R, Jayakumar C, Ranganathan PV,
Ganapathy V and Ramesh G: Kidney proximal tubular
epithelial-specific overexpression of netrin-1 suppresses
inflammation and albuminuria through suppression of COX-2-mediated
PGE2 production in streptozotocin-induced diabetic mice. Am J
Pathol. 181:1991–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohamed R, Jayakumar C and Ramesh G:
Chronic administration of EP4-selective agonist exacerbates
albuminuria and fibrosis of the kidney in streptozotocin-induced
diabetic mice through IL-6. Lab Invest. 93:933–945. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vukicevic S, Simic P, Borovecki F,
Grgurevic L, Rogic D, Orlic I, Grasser WA, Thompson DD and Paralkar
VM: Role of EP2 and EP4 receptor-selective agonists of
prostaglandin E(2) in acute and chronic kidney failure. Kidney Int.
70:1099–1106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olesen ET, Moeller HB, Assentoft M,
MacAulay N and Fenton RA: The vasopressin type 2 receptor and
prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma
membrane targeting through a cAMP-independent pathway. Am J Physiol
Renal Physiol. 311:F935–F944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang HH, Young SH, Sinnett-Smith J, Chou
CE, Moro A, Hertzer KM, Hines OJ, Rozengurt E and Eibl G:
Prostaglandin E2 activates the mTORC1 pathway through an
EP4/cAMP/PKA- and EP1/Ca2+-mediated mechanism in the human
pancreatic carcinoma cell line PANC-1. Am J Physiol Cell Physiol.
309:C639–C649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rumzhum NN and Ammit AJ: Prostaglandin E2
induces expression of MAPK phosphatase 1 (MKP-1) in airway smooth
muscle cells. Eur J Pharmacol. 782:1–5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu C, Zhu P, Wang W, Li W, Shu Q, Chen
ZJ, Myatt L and Sun K: Inhibition of lysyl oxidase by prostaglandin
E2 via EP2/EP4 receptors in human amnion fibroblasts: Implications
for parturition. Mol Cell Endocrinol. 424:118–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shishikura K, Horiuchi T, Sakata N, Trinh
DA, Shirakawa R, Kimura T, Asada Y and Horiuchi H: Prostaglandin E2
inhibits neutrophil extracellular trap formation through production
of cyclic AMP. Br J Pharmacol. 173:319–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang HW, Shi L, Xu YP, Qin XY and Wang QZ:
Oxymatrine inhibits renal fibrosis of obstructive nephropathy by
downregulating the TGF-β1-Smad3 pathway. Ren Fail. 38:945–951.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hong F, Wu N, Ge Y, Zhou Y, Shen T, Qiang
Q, Zhang Q, Chen M, Wang Y, Wang L and Hong J: Nanosized titanium
dioxide resulted in the activation of TGF-β/Smads/p38MAPK pathway
in renal inflammation and fibration of mice. J Biomed Mater Res A.
104:1452–1461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu W, Huang YR, Wan YG, Yang HM, Mao ZM,
Yang JJ, Shi G and Sun W: Effects and mechanisms of UCG
ameliorating renal interstitial fibrosis by regulating
TGF-β1/SnoN/Smads signaling pathway in renal failure rats. Zhongguo
Zhong Yao Za Zhi. 41:2291–2297. 2016.(In Chinese). PubMed/NCBI
|
|
34
|
Loboda A, Sobczak M, Jozkowicz A and Dulak
J: TGF-β1/Smads and miR-21 in renal fibrosis and inflammation.
Mediators Inflamm. 2016:83192832016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Wang B, Huang P, Wang H, Xu K, Wang
X, Xu L and Guo Z: Microcystin-LR promotes cell proliferation in
the mice liver by activating Akt and p38/ERK/JNK cascades.
Chemosphere. 163:14–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Zhang L, Zhang M, Li R, Li Y, Hu X,
Wang S and Bao Z: Characterization of three mitogen-activated
protein kinases (MAPK) genes reveals involvement of ERK and JNK,
not p38 in defense against bacterial infection in Yesso scallop
Patinopecten yessoensis. Fish Shellfish Immunol. 54:507–515. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Costa AP, Lopes MW, Rieger DK, Barbosa SG,
Goncalves FM, Xikota JC, Walz R and Leal RB: Differential
activation of mitogen-activated protein kinases, ERK 1/2, p38(MAPK)
and JNK p54/p46 during postnatal development of rat hippocampus.
Neurochem Res. 41:1160–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Munoz L, Yeung YT and Grewal T: Oncogenic
Ras modulates p38 MAPK-mediated inflammatory cytokine production in
glioblastoma cells. Cancer Biol Ther. 17:355–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li X, Xue C, Wang L, Tang D, Huang J, Zhao
Y, Chen Y, Zhao D, Shi Q, Wang Y and Shu B: Osteoprotective effects
of osthole in a mouse model of 5/6 nephrectomy through inhibiting
osteoclast formation. Mol Med Rep. 14:3769–3776. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stitt-Cavanagh EM, Faour WH, Takami K,
Carter A, Vanderhyden B, Guan Y, Schneider A, Breyer MD and Kennedy
CR: A maladaptive role for EP4 receptors in podocytes. J Am Soc
Nephrol. 21:1678–1690. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hartono SP, Knudsen BE, Lerman LO, Textor
SC and Grande JP: Combined effect of hyperfiltration and renin
angiotensin system activation on development of chronic kidney
disease in diabetic db/db mice. BMC Nephrol. 15:582014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Fields TA, Pazmino K, Dai Q,
Burchette JL, Howell DN, Coffman TM and Spurney RF: Activation of
Galpha q-coupled signaling pathways in glomerular podocytes
promotes renal injury. J Am Soc Nephrol. 16:3611–3622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiao K, Cao S, Jiao L, Song Z, Lu J and Hu
C: TGF-β1 protects intestinal integrity and influences Smads and
MAPK signal pathways in IPEC-J2 after TNF-α challenge. Innate
Immun. 23:276–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Zhao Z, Liu J, Huang N, Long D, Wang
J, Li X and Liu Y: MEK/ERK and p38 MAPK regulate chondrogenesis of
rat bone marrow mesenchymal stem cells through delicate interaction
with TGF-beta1/Smads pathway. Cell Prolif. 43:333–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo B, Inoki K, Isono M, Mori H, Kanasaki
K, Sugimoto T, Akiba S, Sato T, Yang B, Kikkawa R, et al:
MAPK/AP-1-dependent regulation of PAI-1 gene expression by TGF-beta
in rat mesangial cells. Kidney Int. 68:972–984. 2005. View Article : Google Scholar : PubMed/NCBI
|