Introduction
Hematological malignancies, represented by leukemia
and lymphoma, are life-threating diseases associated with a poor
prognosis. Although treatment outcome of the patients has improved
tremendously in the era of combination chemotherapy, autologous or
allogeneic hematopoietic stem cell transplantation, a significant
proportion of patients remain refractory to or relapse following
treatment and ultimately succumb to the disease. Therefore,
numerous strategies have been developed to treat such diseases
(1–3). In the past three decades,
target-based therapies have established milestones in the treatment
of leukemia. Two typical successful examples are the applications
of all-trans retinoic acid in acute promyelocytic leukemia (APL)
and tyrosine kinase inhibitor in chronic myeloid leukemia (CML)
(4,5). These impressive achievements are
inspired by the understanding of the precise mechanisms of these
two types of leukemia. There is no doubt that further research into
the pathogenesis of leukemia leading to novel therapeutic targets
will benefit patients enormously (6).
The Hippo signaling pathway is an important
modulator involved in the regulatory process of cell proliferation,
differentiation and death. It was first identified in the course of
identifying tumor suppressor genes in Drosophila, which serves a
vital role in controlling organ growth or regeneration (7,8). The
Hippo signaling pathway consists of three interactive elements: The
upstream sensor molecules, downstream transcriptional components
and core kinase parts. The core components of Hippo pathway in
mammals include mammalian STE20-Like1 (MST1)/(MST2), protein
salvador homolog 1, large tumor suppressor (LATS1/LATS2),
MOB1/MOB2, yes-associated protein (YAP) and Tafazzin (TAZ)
(9). Classical cascade signaling
activation of the Hippo pathway begins from the phosphorylation and
activation of MST1/MST2 by upstream molecules. Then, the
phosphorylated MST1/2 further activate LATS1 and LATS2 under the
help of MOB kinase activator (MOBKL)1, MOBKL2, which also
experiences activation by MST1/2 (10). Finally, the activated LATS1/2
phosphorylates the transcriptional co-activator YAP and TAZ,
binding to 14.3.3 proteins in the cytoplasm, which accelerates the
sequestration and degradation of YAP/TAZ (11,12).
Abnormal expression of YAP has been identified in
various human tumors, including renal cell carcinoma, pancreatic
cancer, breast cancer, cholangiocarcinoma and medulloblastoma
(13–17). Several groups have documented the
aberrant expression or genetic abnormalities of the Hippo pathway
in hematological malignancies, especially in acute leukemia and
lymphoproliferative neoplasms (18–22).
In the present study, YAP expression was screened in several
leukemia and lymphoma cell lines, and demonstrated high YAP
expression in Jurkat cells. To elucidate its effect on cell biology
of Jurkat cells, lentivirus mediated short hairpin RNA (shRNA)
technique that targets the silencing of YAP was performed. As
anticipated, YAP expression was significantly suppressed at the
mRNA and protein levels by the YAP-specific shRNA. Notably,
decreased leukemia cell proliferation and enhanced cell apoptosis
were demonstrated in YAP knockdown Jurkat cell line. Taken
together, these results indicate that YAP may be a potential novel
therapeutic target for certain types of leukemia.
Materials and methods
Materials
MTT was purchased from Beyotime Institute of
Biotechnology (Shanghai, China); Dulbecco's modified Eagle's medium
(DMEM) was obtained from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); Fetal bovine serum (FBS) was purchased from
Hyclone (GE Healthcare Life Sciences, Logan, UT, USA); interleukin
(IL)-3, IL-6 and stem cell factor (SCF) were purchased from R&D
Systems, Inc., (Minneapolis, MN, USA); TRIzol reagent and Lipofect
2000 were purchased from Invitrogen (Thermo Fisher Scientific,
Inc.); SYBR Green PCR Master Mix was obtained from Takara Bio,
Inc., (Otsu, Japan); pLenti6.3/V5-DEST lentivirus vector and
Packaging Mix were obtained from Invitrogen (Thermo Fisher
Scientific, Inc.); RIPA protein lysis buffer was obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany); Antibodies for
western blotting including anti-YAP, protein kinase B (AKT1),
B-cell lymphoma 2 (BCL2) and BCL2 like protein 1 were bought from
Santa Cruz Biotechnology, Inc., (Dallas, TX, USA); apoptosis
analysis kits (7-AAD, Annexin V-FITC) were obtained from Nanjing
KeyGEN Biotech Co., Ltd., (Nanjing, China) and the ECL-PLUS/kit was
obtained from GE Healthcare (Chicago, IL, USA).
Cell lines and culture
Jurkat, Daudi, SuDHL-4, NB4, HL60 and 293 cells were
obtained from Shanghai Institute of Hematology (Shanghai, China);
Cultured in DMEM supplemented with 10% heat-inactivated FBS, 100
U/ml of penicillin and 100 µg/ml of streptomycin. Hematopoietic
stem cells and progenitor cells (HSC/HPC) cells were collected from
normal murine bone marrow mononuclear cells (MNC). Briefly, mice
were sacrificed and the bone marrow was extracted under sterile
conditions from femurs and tibias. The bone marrow suspension was
filtered through a 40-µm-cell strainer and centrifuged for 5 min at
500 × g. Bone marrow mononuclear cells were washed in DMEM
supplemented with 10% FBS after lysis of red blood cells,
centrifuged, resuspended and cultured in the above medium
supplemented with IL-3 (10 ng/ml), IL-6 (10 ng/ml) and SCF (50
ng/ml) cytokine cocktail. Ethical approval was given for the
present study by ethics committee of Xinhua Hospital (Shanghai,
China). All cells were incubated in a humidified atmosphere
containing 5% CO2 at 37°C.
Cell transduction
shRNA loaded by lentivirus was used in the present
study. YAP targeting shRNA was synthesized and purchased from
NOVOBIO (Shanghai Novobio, Co., Ltd., Shanghai, China; http://www.novobiosci.com). shRNA specific sequences
for YAP located number 1,522 nt of YAP mRNA (PL/shRNA-YAP-1522):
5′-CACCGACCAATAGCTCAGATCCTTTCGAAAAAGGATCTGAGCTATTGGTC-3′; negative
control sequences (NC): 5′-TTCTCCGAACGTGTCACGT-3′. Jurkat cells
were cultured in 24-well plates. Cells were transduced by the
lentiviral supernatant harboring the NC or shRNA-YAP packaged in
293 cells, with 1 µg/ml polybrene (Shanghai GenePharma Co., Ltd.,
Shanghai, China) added to enhance transduction efficacy. Following
co-culture for 24 h, the medium was replenished. Fluorescence
density was observed under a fluorescence microscope.
Cell proliferation test
Cell proliferation was measured by MTT assay. Cells
were seeded in 96-well-plates at a density of 1×104
cells/well in 100 µl DMEM medium, cultured in a CO2
incubator for 24–72 h, washed and replenished with 100 µl fresh
medium. A total of 10 µl MTT stock solution was added to each well
and further incubated at 37°C for 4 h in a CO2
incubator. This was followed by adding 100 µl the SDS-HCl solution
to each well and further incubation at 37°C for 4 h in a
CO2 incubator. Each sample was mixed by pipetting up and
down. Absorbance was read at 570 nm. Cell proliferation rate at 24,
48 and 72 h were calculated.
RNA isolation and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
In order to quantitatively analyze mRNA expression
profile, RT-qPCR was performed. Total cell RNA was extracted with
TRIzol reagent according to manufacturer's protocol. Reverse
transcription was done with M-MLV at 25°C for 5 min, 42°C for 60
min and 72°C for 5 min. cDNA amplification was managed by
SYBR-Green PCR Master Mix kit according to the manufacturer's
protocol. Merlin, protein salvador homolog 1 (Sav1), MST1/2,
LATS1/2 and YAP genes of normal murine HSC/HPC were amplified by
different primers. Human YAP, AKT, BCL2, TP53, BCL2L1, tumor
necrosis factor receptor superfamily member 6 (FAS), caspase 8
(CASP8), FAS associated death domain (FADD), CASP3, BAX, tumor
necrosis factor receptor type 1-associated DEATH domain (TRADD)
protein genes were separately amplified using specific artificially
synthesized primers, with the homo-actin house-keeping gene serving
as internal control. Sequences of the primers used in the study are
presented in Table I. Expression
values were analyzed using the comparative Cq (2−ΔΔCq)
(23).
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| A, Mouse |
|---|
|
|---|
| Number | Gene | Primer | Primer sequence
(5′-3′) |
|---|
| 1 | Merlin | IF |
CGGACACTGGGGCTTCGGGAAACCT |
|
|
| IR |
TCCAAAATCTGCTTCTTCACCTGTA |
| 2 | SAV1 | IF |
GACTGGACAATGAGAGGGAGAAAAT |
|
|
| IR |
TGATGACTCTACTCGTTCCCAGCCA |
| 3 | MST1 | IF |
CAGAGTCAGGGCGGGAGTGTCAACG |
|
|
| IR |
AATAGTTGTCTTTCAGATCTTTGTC |
| 4 | MST2 | IF |
TAACAGATACAATGGCAAAACGCAA |
|
|
| IR |
AATGTTGGTGGTGGGTTTGTAGGGA |
| 5 | LATS1 | IF |
ATGTGGTTTATCGTTCTGAAAGCCC |
|
|
| IR |
ATGAGGGGGGAGGGGGAGTGGTGCC |
| 6 | LATS2 | IF |
CTGCCACAACTTACTCTGGAAATAG |
|
|
| IR |
TTGATAAGGTCCAAACTTCGGGGTG |
| 7 | YAP1 | IF |
CCTTCTTCAAGCCGCCTGAGCCCAA |
|
|
| IR |
AGGGATCTCAAAGGAGGACTGCCGG |
|
| B,
Human |
|
| Number | Gene | Primer | Primer sequence
(5′-3′) |
|
| 1 | YAP | IF |
TAGCCCTGCGTAGCCAGTTA |
|
|
| IR |
TCATGCTTAGTCCACTGTCTGT |
| 2 | AKT1 | IF |
GTCATCGAACGCACCTTCCAT |
|
|
| IR |
AGCTTCAGGTACTCAAACTCGT |
| 3 | BCL2 | IF |
GGTGGGGTCATGTGTGTGG |
|
|
| IR |
CGGTTCAGGTACTCAGTCATCC |
| 4 | TP53 | IF |
CAGCACATGACGGAGGTTGT |
|
|
| IR |
TCATCCAAATACTCCACACGC |
| 5 | BAX | IF |
CCCGAGAGGTCTTTTTCCGAG |
|
|
| IR |
CCAGCCCATGATGGTTCTGAT |
| 6 | BCL2L1 | IF |
GAGCTGGTGGTTGACTTTCTC |
|
|
| IR |
TCCATCTCCGATTCAGTCCCT |
| 7 | TRADD | IF |
GCTGTTTGAGTTGCATCCTAGC |
|
|
| IR |
CCGCACTTCAGATTTCGCA |
| 8 | FAS | IF |
TCTGGTTCTTACGTCTGTTGC |
|
|
| IR |
CTGTGCAGTCCCTAGCTTTCC |
| 9 | CASP8 | IF |
GTGGGGTAATGACAATCTCGG |
|
|
| IR |
TCAAAGGTCGTGGTCAAAGC |
| 10 | FADD | IF |
GCTGGCTCGTCAGCTCAAA |
|
|
| IR |
ACTGTTGCGTTCTCCTTCTCT |
| 11 | CASP3 | IF |
GAAATTGTGGAATTGATGCGTGA |
|
|
| IR |
CTACAACGATCCCCTCTGAAAAA |
| 12 | Homo-actin | IF |
AGCGAGCATCCCCCAAAGTT |
|
|
| IR |
GGGCACGAAGGCTCATCATT |
Western blot analysis
Protein was extracted using RIPA lysis buffer
according to protocol and protein concentration was quantified by
bicinchoninic acid protein assay kit. A total of 50 µg of protein
was loaded in 10% SDS-PAGE and electrophoresed, then transferred to
polyvinylidene difluoride membrane, which was further blocked and
incubated with 5% defatted milk powder for 2 h at room temperature.
This was then incubated with a specific primary antibody (YAP,
SC376830; BCL2, SC7382 and BCL2L1, SC56021 all 1:100 dilution;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) in TBS with 0.05%
Tween 20 overnight at 4°C or room temperature for 4 h, washed to
remove primary antibody and further incubated with horseradish
peroxidase (HRP)-conjugated secondary antibody (goat anti-mouse
IgG-HRP; SC2005; 1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. ECL Chemiluminescence Detection kit and X-ray
film were used for HRP-conjugated secondary antibody. The Bio-Rad
Gel Imaging System was used to analyze signals. Densitometry was
performed using ImageJ version 1.8.0 software (National Institutes
of Health, Bethesda, MD, USA).
Cell cycle analysis
For experiments on cell cycle analysis, cells were
harvested and resuspended in fresh DMEM medium, centrifuged (250 ×
g for 5 min at 4°C) and washed with PBS and fixed with cold 70%
ethanol added drop wise to the pellet while vortexing. Following
fixing for 30 min at 4°C and washing with PBS, the fixed cells were
stained with 7-AAD (DNA dye) in the presence of RNase for 30 min at
room temperature in the dark room. The samples were then analyzed
on a FACsort flow cytometer using CellQuest 1.0 software (BD
Biosciences, Franklin Lakes, NJ, USA).
Cell apoptosis analysis
In cell apoptosis experiment, cells were harvested
and suspended in fresh DMEM medium, then resuspended in
Annexin-binding buffer following centrifugation (300 × g, 5 min,
4°C) and washed according to the corresponding protocols. Annexin
V-FITC and 7-AAD working solution (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) were added to the cells and incubated in the
dark at room temperature for 15 min. Following incubation,
Annexin-binding buffer was added and the samples were kept on ice
to be analyzed as soon as possible by flow cytometry. Cell
apoptosis was measured and analyzed by CellQuest 1.0 software (BD
Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Values were expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS software
version 13.0 (SPSS, Inc., Chicago, IL, USA). Quantitative data was
analyzed by one-way analysis of variance, multiple comparisons
between different groups was performed using S-N-K method.
P<0.05 was considered to indicate a statistically significance
difference. Each experiment was repeated at least three times.
Results
YAP is highly expressed in Jurkat
cells
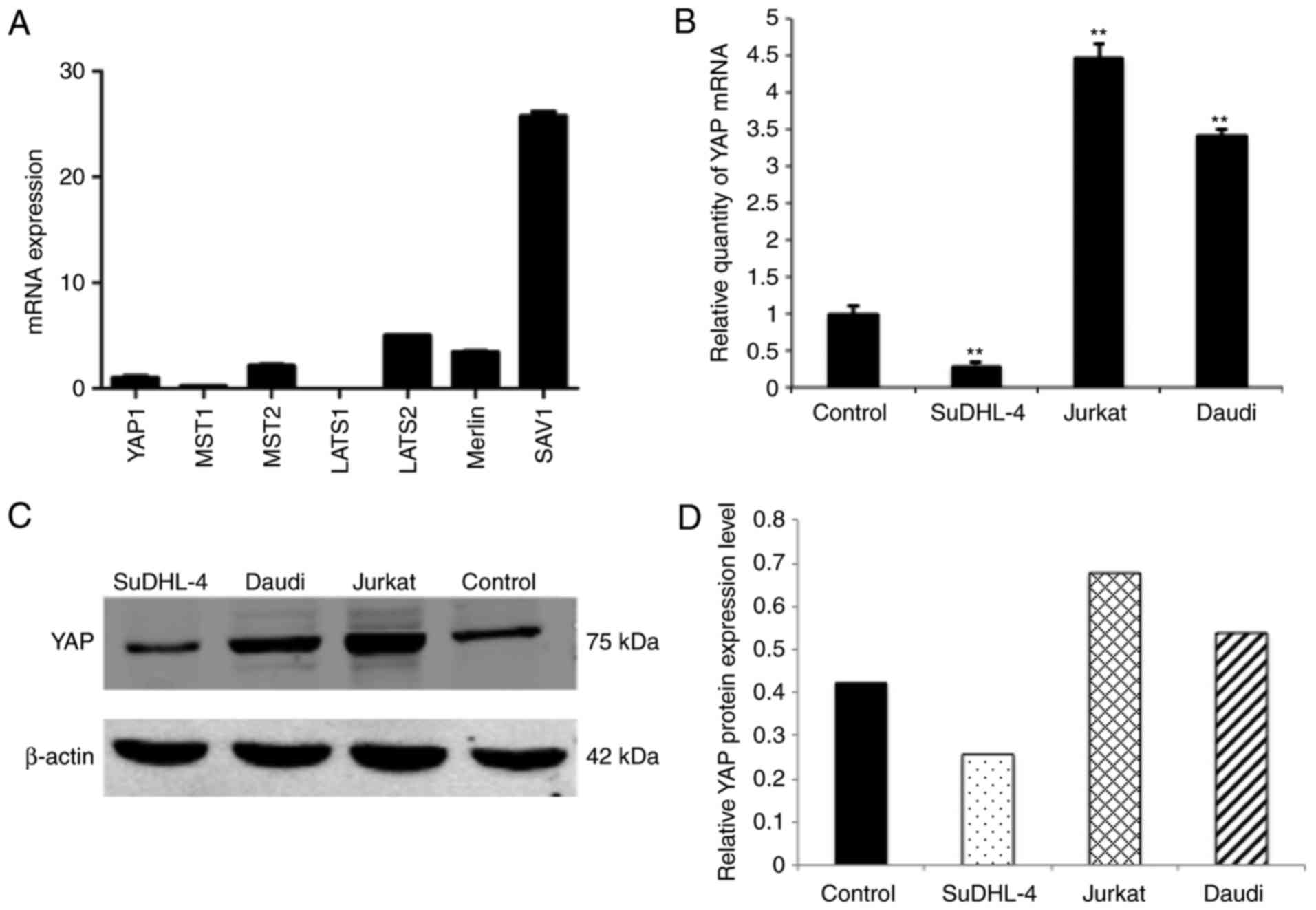
Previously the expression level of several vital
components of the Hippo signal transduction pathway has been
studied and it was demonstrated that Merlin, Sav1, MST1/2, LATS1/2
and YAP were all expressed in normal murine HSC/HPC (Fig. 1A). This provided the basis for the
hypothesis that the Hippo signaling pathway may serve certain
important roles in murine HSC/HPC function. Notably, the Hippo
signaling pathway did not influence the proliferation and colony
formation abilities when YAP was ectopically overexpressed in
HSC/HPC cells (data not shown). Therefore hematological malignant
cells were focused on in the present study. YAP expression levels
were screened in several leukemia and lymphoma cell lines including
Jurkat, Daudi, SuDHL-4, NB4 and HL60. Considering that components
of the Hippo pathway were expressed in normal human HSC/HPC, normal
human HSC/HPCs were used as a control. Compared with the control,
it was demonstrated that YAP is highly expressed in Jurkat cells at
the mRNA and protein levels (Fig.
1B-D), Daudi cells also had a relatively high expression level
of YAP while SuDHL-4 had lower expression level (Fig. 1B-D). NB4 and HL-60 had expression
level comparable to the control (data not shown).
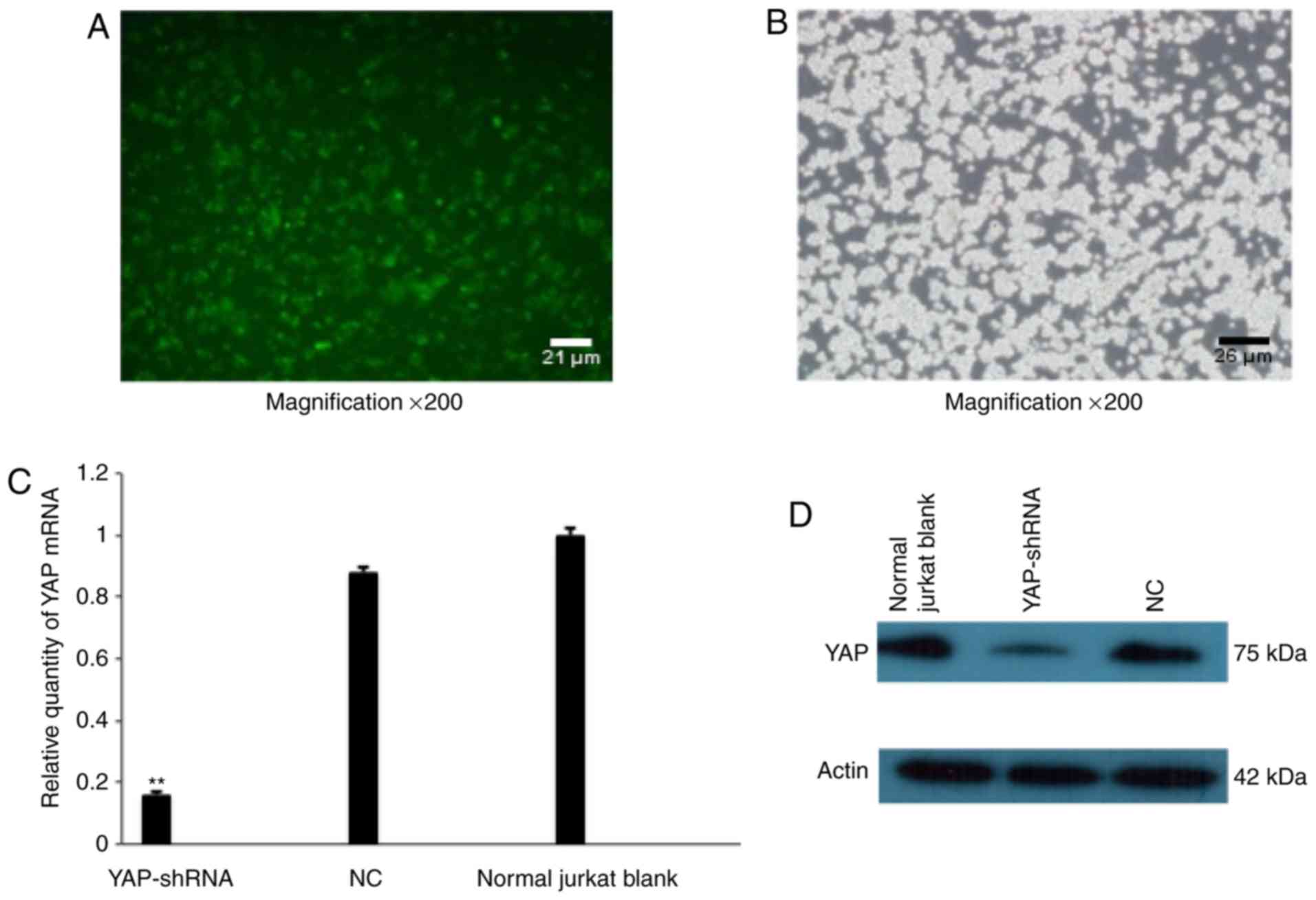
YAP knockdown in Jurkat cells
As YAP was highly expressed in Jurkat cells, the
function of YAP in Jurkat cells was further investigated, utilizing
YAP-shRNA to knockdown YAP expression in Jurkat cells. Lentivirus
harboring YAP-shRNA exhibited a high titer (5.2×108
TU/ml) following successful packaging. The lentivirus titration was
measured by calculating multiplicity of infection through
sequential dilution (data not shown). Jurkat cells were
successfully transduced by lentivirus and Jurkat cells were
observed using a fluorescence microscope system or light microscope
following transduction (Fig. 2A and
B). RT-qPCR results demonstrated that mRNA expression of YAP
was dramatically decreased in the YAP knockdown (YAP-shRNA) group
compared with the NC group, demonstrating 75% interfering efficacy
(P<0.01, Fig. 2C).
Correspondingly, the western blotting experiment further
demonstrated that YAP protein expression was markedly reduced in
these cells (Fig. 2D). All these
data demonstrated that YAP was successfully silenced by lentivirus
mediated shRNA interference.
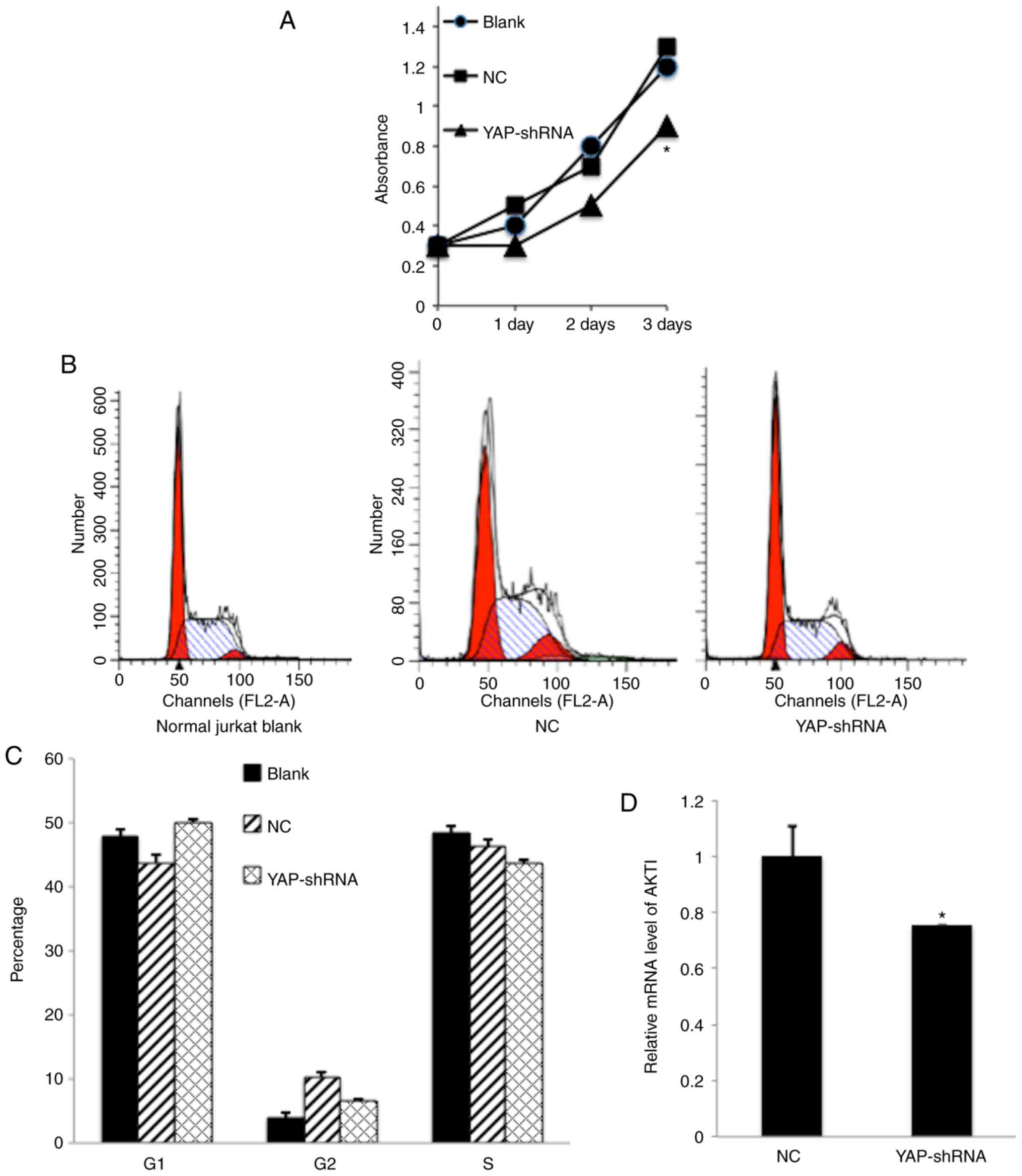
Influence of YAP silencing on
proliferation of Jurkat cells
Uncontrolled cellular proliferation is the hallmark
of leukemia cells. To check whether knockdown of YAP by shRNA
elicited a suppressive effect on Jurkat cell proliferation, an MTT
assay was utilized to investigate the effect of YAP silencing.
Compared with the NC counterpart, the proliferation rate of
YAP-shRNA Jurkat cells was affected in a time-dependent manner and
this was significant following 3 days (P<0.05; Fig. 3A). Furthermore, the cell cycle
pattern was analyzed by flow cytometry (FCM). FCM demonstrated that
knockdown of YAP in Jurkat cells boosted the percentage of
G0/G1 phase cells from 43.6–50.8% (Fig. 3B-C). Finally, the downstream genes
associated with cell proliferation or cell cycle regulation
including AKT1 and P53 were investigated. It was demonstrated that
AKT1 gene expression, which is crucial to the PI3K-AKT
proliferation pathway, was decreased in YAP-shRNA transduced Jurkat
cells compared with the NC group (Fig.
3D). These data provided strong evidence that YAP silencing
could inhibit Jurkat cell proliferation through
G0/G1 arrest.
Effect of YAP knockdown on apoptosis
of Jurkat cells
Deregulation of apoptosis is another hallmark of
leukemia cells. To investigate the effect of YAP knockdown on
Jurkat cell apoptosis. FCM analysis was performed on YAP knockdown
Jurkat cells following 7-AAD and Annexin V-FITC double staining.
Compared with the NC group, YAP knockdown significantly promoted
early apoptosis (8.05% vs. 1.93%) and late apoptosis rate (27.8%
vs. 9.9%) in Jurkat cells (P<0.01; Fig. 4A-C). Furthermore, typical
morphological features of apoptosis were also discerned in YAP
knockdown Jurkat cells (data not shown). The expression levels of
several downstream apoptosis or anti-apoptosis associated genes
including BCL2, FAS, CASP8, FADD, CASP3, BCL2L1, BAX and TRADD were
further verified. It was demonstrated that expression levels of
BCL-2 and BCL2L1 genes associated with anti-apoptosis were
significantly reduced (P<0.05; Fig.
4D and F), While no significant difference was observed in the
mRNA expression level of FAS, CASP8, FADD, CASP3, BAX and TRADD
between YAP knockdown Jurkat cells and the NC group (Fig. 4E). All these results imply that YAP
silencing induced apoptosis in Jurkat cells through deregulating
the expression of anti-apoptosis associated proteins.
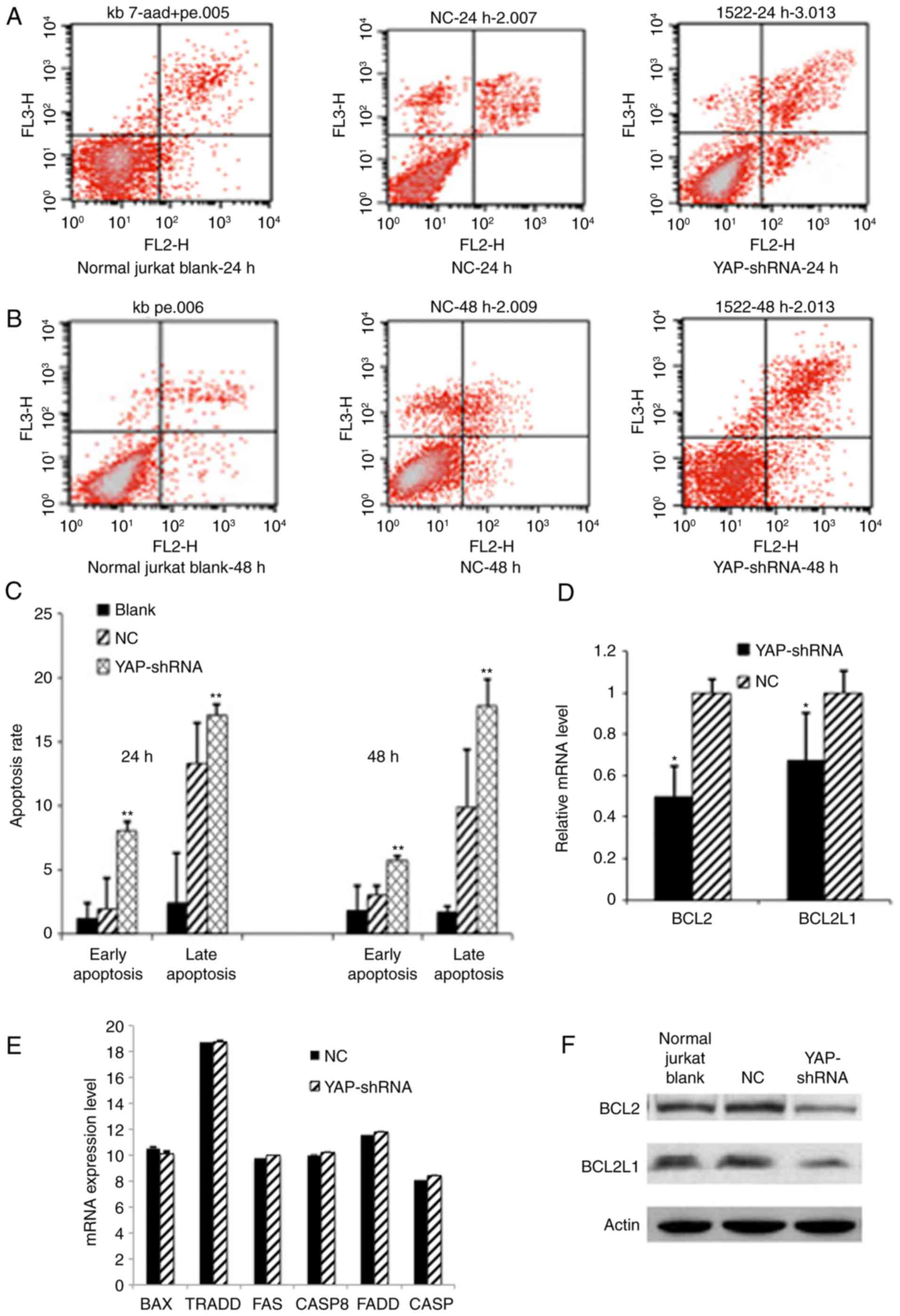 | Figure 4.YAP knockdown induced apoptosis of
Jurkat cells. (A) Apoptosis was analyzed by FCM using double
staining with FITC-labeled Annexin V and 7-AAD at 24 h. Cells
undergoing early apoptosis are Annexin V-FITC+/7-AAD-(lower right
quadrant), whereas cells undergoing late apoptosis are Annexin
V-FITC+/7-AAD+ (upper right quadrant). (B) Apoptosis analyzed by
FCM at 48 h. (C) Apoptosis as represented by a bar chart.
Quantitative data was analyzed by one-way analysis of variance and
multiple comparisons between different groups were performed using
the Student-Newman-Keuls method (**P<0.01 YAP-shRNA vs. NC
group). (D) Quantitative analysis by measuring the relative mRNA
expression levels of BCL2 or BCL2L1 to β-actin. Data are expressed
as the mean ± standard deviation. *P<0.05 vs. the NC group. (E)
Other pro-apoptotic or anti-apoptotic associated genes expression
profile. (F) Western blotting analysis of BCL2 or BCL2L1 protein
expression. FITC, fluorescein isothiocyanate; YAP, yes associated
protein; FCM, flow cytometry; BCL2, B cell lymphoma 2; BCL2L1, BCL2
lie protein 1; CASP, caspase; TRADD, tumor necrosis factor receptor
type 1-associated DEATH domain; FAS, tumor necrosis factor receptor
superfamily member 6; FADD, FAS associated death domain; NC,
negative control; sh, short hairpin. |
Discussion
Despite rapid development in drugs including
targeted therapies, hematological malignancies are still
challenging diseases to treat due to high incidence of
chemo-refractoriness and relapse. There is an urgent need to
investigate novel pathogenesis mechanisms and develop more novel
therapies. The Hippo signaling pathway contains a large group of
interactive proteins, which participate in regulating cell
proliferation, differentiation and apoptosis. Importantly,
deregulation of Hippo pathway correlates with pro-oncogenic or
anti-oncogenic processes. Function of Hippo pathway has been
clarified in a number of organs or tissues, but its function in the
hematopoietic system is still unknown. It is therefore interesting
to investigate Hippo pathway in normal hematopoiesis and
hematological malignancies.
It is controversial whether the Hippo pathway is
involved in the hematological system. Several study groups have
documented the deregulated expression profile or epigenetic
alterations of Hippo pathway in hematological malignancies,
especially in acute leukemia or lymphoproliferative neoplasms
(24,25). In acute myeloid leukemia (AML),
overexpression of the LATS2 gene was detected in 32 de novo
AML patients, suggesting that LATS2 may be associated with
leukemogenesis (18). Another
group reported that AML patients with t (8;12) translocation
generates the MST2-ETV6 fusion gene, which is a potential oncogene
(19). In acute lymphoblastic
leukemia (ALL) patients harboring t (12;21) chromosome
translocation, a vital upstream component in the Hippo pathway
named KIBBRA is demonstrated to be highly methylated and is the
main underlying leukemogenesis event in this specific subtype of
leukemia (20). The LATS2 gene is
also a methylation modification target hotspot in ALL patients; its
lower expression due to methylation predicts patients' poor
prognosis (21). In addition, in
adult T cell or natural killer leukemia/lymphoma, decreased
expression of LATS2 gives rise to lower expression of proapoptotic
genes or causes chemotherapy resistance (26). The Hippo pathway abnormality is
also seen in APL pathogenesis, because YAP is required for APL
transcriptional activation (27,28).
In mantle cell lymphoma, decreased expression of MOBKL2 and LATS2,
important component proteins of the Hippo pathway, has been
detected in certain patients and indicates poor outcome (22). MST1 deficiency is highly
predisposed to develop T-cell ALL under mutagenic stimulation.
Furthermore, MST1(−/−) mice display rapid formation of
lymphoma in a p53 knockout model and obvious chromosomal
instability has been demonstrated in MST1(−/−)
lymphocytes (29). YAP is
overexpressed in CML cells. Knockdown of YAP by siRNA or inhibiting
the function of YAP using verteporfin (VP) not only inhibits the
proliferation, induces the apoptosis of CML cells but also reduces
the expression of YAP target genes c-myc and survivin (30).
On the contrary, the ectopic overexpression of YAP
in the hematopoietic system, reported by another research group,
does not influence HSC function neither during steady state nor in
situations of hematopoietic stress (31). The author speculates that the
discrepant nature of the different tissues may explain the
mechanism of cell over-proliferation or excessive growth in solid
tissues, while not in the hematopoietic system. Machado-Neto et
al (32) demonstrated that YAP
may not correlate with hematological malignancies including AML,
ALL or myelodysplastic syndromes.
In the authors' previous study, in order to
investigate whether Hippo pathway is associated with
haematopoiesis, the expression level of several vital components of
the Hippo signal transduction pathways in normal mice was checked,
and demonstrated that Merlin, Sav1, MST1/2, LATS1/2 and YAP were
all expressed in normal murine HSC/HPC. It was therefore
hypothesized that Hippo signal pathway may serve certain important
roles in murine HSC/HPC function. Notably, it did not influence
proliferation and colony formation abilities when ectopic YAP was
overexpressed in HSC/HPC cells, which is consistent with what has
been reported by other groups (31). In the present study, hematological
malignancies were the main focus. YAP expression levels were
screened in several leukemia and lymphoma cell lines and it was
demonstrated that YAP was highly expressed in Jurkat cells at the
mRNA and protein levels. Considering that YAP has oncogeneic
features, YAP was silenced by shRNA in Jurkat cells and the
consequences were investigated. Growth arrest, inhibition of cell
proliferation, apoptosis, and differentiation are the most
well-characterized effects of tumor suppressor response, therefore
we tested for these aspects of Jurkat cells following silencing
YAP.
An MTT assay was conducted to assess cell metabolic
activity associated with cell proliferation. It was demonstrated
that Jurkat cell proliferation was weakened in YAP knockdown group,
compared with the control group. Furthermore,
G0/G1 phase cells were increased in YAP
knockdown group compared with the control group. Finally, several
downstream genes associated with cell proliferation or cell cycle
regulation were checked. It is well known that inhibition of the
PI3K-AKT pathway could inhibit proliferation of tumor cells. In the
present study the expression levels of AKT1 were demonstrated to be
decreased in the YAP knockdown group, compared with the control
group. Therefore, the results indicated that cell proliferation was
inhibited in Jurkat cells following silencing YAP by interfering
with the PI3K-AKT pathway.
FCM was also used to analyze apoptosis in the
YAP-knockdown Jurkat cells. It was demonstrated that YAP knockdown
remarkably increased early and late apoptosis in Jurkat cells. In
addition, morphological alterations of apoptotic Jurkat cells
including nuclear fragmentation or condensation could be easily
seen in the YAP silenced group, but not in the NC or normal
untreated group. Finally, the expression level of several
downstream genes associated with cell apoptosis was measured, and
decreased levels of Bcl-2 and BCL2L1 genes were observed, the two
of have anti-apoptotic properties. These results suggested that
knockdown of YAP by shRNA enhanced apoptosis through deregulating
the expression levels of pro-apoptotic or anti-apoptotic associated
genes.
Cottini et al (33) demonstrated a tumor suppressor
function for YAP in multiple myeloma, lymphoma and leukemia cell
lines. In an experimental model, damaged DNA activates apoptosis by
a p53-independent pathway in a process guided by nuclear
re-localization of the ABL1 kinase and its interaction with YAP,
demonstrating that low YAP1 levels prevent nuclear ABL1-induced
apoptosis in these hematological malignancies, supporting the
hypothesis that YAP1 is involved in the tumor suppressor process.
In contrast, the present study demonstrated that Jurkat cells
express a high level of YAP1. Furthermore, knockdown of the YAP1 in
the Jurkat cell caused growth arrest and apoptosis, indicating that
YAP1 promotes oncogenesis process. Possible explanations for the
discordance between these two different studies were analyzed: i)
The work of Cottini et al (33) has demonstrated that Jurkat cells
express a low level of YAP protein, while the cells of the present
study expressed a relatively high level. This dissimilarity may
originate from different cellular metabolic conditions, excessive
passage, mutation or other clonal evolution. ii) Indeed, the YAP
protein possesses dual functions, like a double-edged sword, as a
pro-oncogenic or anti-oncogenic molecule. Its double role seems to
be dependent on its phosphorylation pattern: Phosphorylation by
LATS1/2 at Ser127 promotes YAP sequestration in the cytoplasm and
prevents its interaction with TEAD transcription factors, while
phosphorylation by c-Abelson murine leukemia viral oncogene (ABL)
at Tyr357 upon DNA damage confers a tumor suppressive function to
YAP (25,33). Furthermore, It was also
demonstrated that YAP expression was high in Daudi cells and for
the next study YAP will be knocked down in Daudi cells to
investigate what kind of role YAP serves in these cells.
In conclusion, the current study initially revealed
a high expression level of YAP in Jurkat cells. Lentivirus mediated
shRNA decreased YAP expression in Jurkat cells. Silencing of YAP
expression by shRNA suppressed the growth and promoted apoptosis of
Jurkat cells. These results demonstrated that YAP serves an
important role in leukemogenesis, which may be a potential target
for the treatment of leukemia in the future.
Acknowledgements
The authors would like to thank all members of the
Department of Hematology, Xinhua Hospital, Affiliated to Shanghai
Jiao Tong University (SJTU) School of Medicine, for their support
and to thank Dr Yeh-ching Linn from Singapore General Hospital for
English language editing.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM made substantial contributions to the design of
the present study. RW and HY performed the experiments, data
collection and data analysis. RW and LM wrote the manuscript. JW,
XD, LC provided help in conceiving and designing the study. SH made
substantial contributions in data analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was given for the present study by
ethics committee of Xinhua hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terwilliger T and Abdul-Hay M: Acute
lymphoblastic leukemia: A comprehensive review and 2017 update.
Blood Cancer J. 7:e5772017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi Y: Current status and progress of
lymphoma management in China. Int J Hematol. 107:405–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Thé H, Pandolfi PP and Chen Z: Acute
promyelocytic leukemia: A paradigm for oncoprotein-targeted cure.
Cancer Cell. 32:552–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saglio G, Cilloni D, Rancati F and Boano
L: Glivec and CML: A lucky date. J Biol Regul Homeost Agents.
18:246–251. 2004.PubMed/NCBI
|
|
6
|
Rashidi A, Weisdorf DJ and Bejanyan N:
Treatment of relapsed/refractory acute myeloid leukaemia in adults.
Br J Haematol. 181:27–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu S, Huang J, Dong J and Pan D: Hippo
encodes a Ste-20 family protein kinase that restricts cell
proliferation and promotes apoptosis in conjunction with salvador
and warts. Cell. 114:445–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harvey K and Tapon N: The
Salvador-Warts-Hippo pathway-an emerging tumour-suppressor network.
Nat Rev Cancer. 7:182–191. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong W and Guan KL: The YAP and TAZ
transcription co-activators: Key downstream effectors of the
mammalian Hippo pathway. Semin Cell Dev Biol. 23:785–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sansores-Garcia L, Atkins M, Moya IM,
Shahmoradgoli M, Tao C, Mills GB and Halder G: Mask is required for
the activity of the Hippo pathway effector Yki/YAP. Curr Biol.
23:229–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Couzens AL, Knight JD, Kean MJ, Teo G,
Weiss A, Dunham WH, Lin ZY, Bagshaw RD, Sicheri F, Pawson T, et al:
Protein interaction network of the mammalian Hippo pathway reveals
mechanisms of kinase-phosphatase interactions. Sci Signal.
6:rs152013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandez-L A, Squatrito M, Northcott P,
Awan A, Holland EC, Taylor MD, Nahlé Z and Kenney AM: Oncogenic YAP
promotes radio resistance and genomic instability in
medulloblastoma through IGF2-mediated Akt activation. Oncogene.
31:1923–1937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao JJ, Zhao XM, Wang DL, Chen KH, Sheng
X, Li WB, Li MC, Liu WJ and He J: YAP is overexpressed in clear
cell renal cell carcinoma and its knockdown reduces cell
proliferation and induces cell cycle arrest and apoptosis. Oncol
Rep. 32:1594–1600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Liu Y, Zhang C, Niu Q, Wang H, Che
C, Xie M, Zhou B, Xu Y, Zhang Q, et al: Stiehopusjaponieus acidic
mucopolysaccharide inhibits the proliferation of pancreatic cancer
SW1990 cells through Hippo-YAP pathway. Oncotarget. 8:16356–16366.
2017.PubMed/NCBI
|
|
16
|
Kim HM, Jung WH and Koo JS: Expression of
Yes-associated protein (YAP) in metastatic breast cancer. Int J
Clin Exp Pathol. 8:11248–11257. 2015.PubMed/NCBI
|
|
17
|
Pei T, Li Y, Wang J, Wang H, Liang Y, Shi
H, Sun B, Yin D, Sun J, Song R, et al: YAP is a critical oncogene
in human cholangiocarcinoma. Oncotarget. 6:17206–17220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gholami M, Mirfakhraie R, Movafagh A,
Jalaeekhoo H, Kalahroodi R, Zare-Abdollahi D and Zare-Karizi S: The
expression analysis of LATS2 gene in de novo AML patients. Med
Oncol. 31:9612014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ogawa S, Yokoyama Y, Suzukawa K, Nanmoku
T, Kurita N, Seki M, Maie K, Suyama T, Takaiwa N, Sakata-Yanagimoto
M, et al: Identification of a fusion gene composed of a Hippo
pathway gene MST2 and a common translocation partner ETV6 in a
recurrent translocation t(8;12)(q22;p13) in acute myeloid leukemia.
Ann Hematol. 94:1431–1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hill VK, Dunwell TL, Catchpoole D, Krex D,
Brini AT, Griffiths M, Craddock C, Maher ER and Latif F: Frequent
epigenetic inactivation of KIBRA, an upstream member of the
Salvador/Warts/Hippo (SWH) tumor suppressor network, is associated
with specific genetic event in B-cell acute lymphocytic leukemia.
Epigenetics. 6:326–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiménez-Velasco A, Román-Gómez J, Agirre
X, Barrios M, Navarro G, Vázquez I, Prósper F, Torres A and
Heiniger A: Down regulation of the large tumor suppressor
2(LATS2/KPM) gene is associated with poor prognosis in acute
lymphoblastic leukemia. Leukemia. 19:2347–2350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hartmann EM, Campo E, Wright G, Lenz G,
Salaverria I, Jares P, Xiao W, Braziel RM, Rimsza LM, Chan WC, et
al: Pathway discovery in mantle cell lymphoma by integrated
analysis of high-resolution gene expression and copy number
profiling. Blood. 116:953–961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garcia-Souza LF and Oliveira MF:
Mitochondria: Biological roles in platelet physiology and
pathology. Int J Biochem Cell Biol. 50:156–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aqeilan RI: Hippo signaling: To die or not
to die. Cell Death Differ. 20:1287–1288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawahara M, Hori T, Chonabayashi K, Oka T,
Sudol M and Uchiyama T: Kpm/Lats2 is linked to chemosensitivity of
leukemic cells through the stabilization of p73. Blood.
112:3856–3866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lapi E, Di Agostino S, Donzelli S, Gal H,
Domany E, Rechavi G, Pandolfi PP, Givol D, Strano S, Lu X and
Blandino G: PML, YAP, and p73 are components of aproapoptotic
autoregulatory feedback loop. Mol Cell. 32:803–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Strano S, Fausti F, Di Agostino S, Sudol M
and Blandino G: PML Surfs into HIPPO tumor suppressor pathway.
Front Oncol. 3:362013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim TS, Lee DH, Kim SK, Shin SY, Seo EJ
and Lim DS: Mammalian sterile 20-likekinase 1 suppresses lymphoma
development by promoting faithful chromosome segregation. Cancer
Res. 72:5386–5395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Huang Z, Gao M, Huang N, Luo Z, Shen
H, Wang X, Wang T, Hu J and Feng W: Inhibition of YAP suppresses
CML cell proliferation and enhances efficacy of imatinib in vitro
and in vivo. J Exp Clin Cancer Res. 35:1342016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jansson L and Larsson J: Normal
hematopoietic stem cell function in mice with enforced expression
of the Hippo signaling effector YAP1. PLoS One. 7:e320132012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Machado-Neto JA, de Melo Campos P, Saad
Olalla ST and Traina F: YAP1 expression in myelodysplastic
syndromes and acute leukemias. Leuk Lymphoma. 55:2413–2415. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cottini F, Hideshima T, Xu C, Sattler M,
Dori M, Agnelli L, Ten Hacken E, Bertilaccio MT, Antonini E, Neri
A, et al: Rescue of Hippo coactivator YAP1 triggers DNA
damage-induced apoptosis in hematological cancers. Nat Med.
20:599–606. 2014. View
Article : Google Scholar : PubMed/NCBI
|