Introduction
Neuropathic pain (NP) is a complex, chronic pain
state that is characterized by hyperalgesia, allodynia and
spontaneous pain; it occurs as a consequence of mechanical nerve
injury that can occur during the progression of cancer, multiple
sclerosis and stroke (1). It is
currently an important clinical problem that lacks an effective
treatment. At present, the commonly used analgesics, especially
opioid drugs, do not completely reduce symptoms of chronic pain and
have various side effects, including respiratory depression, the
development of drug tolerance and addiction (2). However, the broad range of receptors
and signal transduction pathways that may be involved in this
process provide a wealth of research opportunities. The current
evidence has revealed that spinal microglia are critically involved
in the development and maintenance of NP, with two members of the
Toll-like receptor (TLR) family serving a pivotal role. For
neuropathy, the most relevant region of TLR expression is on
microglia (3). Therefore, a number
of studies are investigating more effective and sustained
treatments targeting the TLR family.
TLR4, a membrane-spanning receptor protein, is
closely associated with chronic nociceptive responses in the
central nervous system, as determined previously in animal models
of NP (4,5). In pain-associated neuropathy mouse
models, thermal hyperalgesia and mechanical allodynia were reduced
by the administration of FP-1, a potent TLR4 antagonist (6). Furthermore, induced hypersensitivity
has been reported to be decreased in TLR4 deficient mice (6). Therefore, blocking the TLR4 signaling
pathway represents a potentially effective method for curing
NP.
Based on previous studies, the availability of TLR4
receptor antagonists is non-specific. Furthermore, the increasing
body of information on the RNA interference (RNAi) technique means
it is now possible to precisely knockdown relevant genes (7,8).
Therefore, it seems plausible to investigate a novel method for the
treatment of NP by targeting the TLR4 receptor. It has been
reported that downregulation of the GluN2B receptor by intrathecal
injection of small interfering (si)RNA reduced formalin-induced
nociception in rats, which supported the notion of treating NP
using the RNAi technique (8).
However, siRNA alone is unstable due to its tendency to degrade
(9), therefore, a vector is
required to express the siRNA. In the author's previous study, a
lentivirus system was introduced as a tool for the expression of
siRNA. The results revealed that intrathecal injection of the
lentivirus-mediated siRNA against GluN2B reduced the nociception of
NP rats for 5 weeks (10), which
provided a vehicle for expressing siRNA in treating NP.
In the present study, a lentivirus system was
introduced in order to express TLR4 siRNA. To control the timing
and levels of target gene expression, a tetracycline inducible
system was applied to regulate TLR4 expression. The
anti-nociceptive effect of TLR4 siRNA under the regulation of
doxycycline (Dox) was observed in a rat model of chronic
constriction injury (CCI).
Materials and methods
Production of the LvOn-siTLR4
lentivirus
LvOn-siTLR4, a tetracycline inducible lentivirus
expressing siRNA, was produced using 293T cells (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Red fluorescence protein
(RFP), a reporter protein, was introduced into the lentiviral
system in order to detect the number of lentiviruses and to trace
the location of the virus. The siRNA (5′-GUCUCAGAUAUCUAGAUCU-3′)
targeting the TLR4 receptor gene (GenBank accession NM_019178) was
screened and tested as described in the author's previous study
(9). Based on the sequences of the
lentivirus and the principle of ‘Tuschl’, the inducible lentivirus
LvOn-siTLR4 was produced as described previously (11) and was confirmed by an
immunofluorescence assay for RFP under an Olympus fluorescence
microscope (Olympus Corporation, Tokyo, Japan) (9). Briefly, target sequences were
chemically synthesized and were then cloned into plasmid pLenR-TRIP
(Genomeditech, Shanghai, China) and named pLenR-TRIP-TLR4. To
produce recombinant lentivirus LvOnsiTLR4 (lentivirus expressing
the TLR4 siRNA), pRsv-REV (4,174 bp; 5.04 µg), pMDlg-pRRE (8,895
bp; 7.57 µg) and pMD2G (5,824 bp; 3.79 µg) were co-transfected into
293T cells (2–2.5×106) with Lipofectamine
2000™ (Invitrogen; Thermo Fisher Scientific, Inc.). A
total of ~48 h following transfection, the lentivirus was
harvested, and activity measurement was performed following a
further 24 h. The final titer of the virus was adjusted to
1×109 TU/ml.
Animals and CCI surgery
Male Sprague-Dawley (SD) rats weighing 200–250 g
(n=180; aged 6–7 weeks old) were obtained from the Shanghai
Experimental Animal Center, The Chinese Academy of Sciences
(Shanghai, China). Animals were provided food and water ad
libitum and housed at a temperature of 23–25°C) and 45–55%
humidity, which was maintained on a 12/12 h light/dark cycle. All
animal experiments were approved by the Administrative Committee of
Experimental Animal Care and Use of the Second Military Medical
University (Shanghai, China), and conformed to the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (NIH Publications No. 8023, revised 1978). The CCI
procedure was performed as described previously (12). The right sciatic nerve was exposed
at the mid-thigh level following the rats were anesthetized with 40
mg/kg of sodium pentobarbital (intraperitoneal injection). The
sciatic nerve was loosely ligated with 4-0 chromic gut threads at 4
sites, 1 mm apart, allowing the nerve diameter to reduce slightly.
The sciatic nerve was exposed but not ligated in the sham group.
The rats were then individually housed following recovery from
anesthesia and monitored three times a day.
Lumbar subarachnoid
catheterization
Chronic indwelling catheters were implanted in the
lumbar subarachnoid space on the same day as the CCI surgery. A
PE-10 catheter (BD Biosciences, Franklin Lakes, NJ, USA) was
carefully inserted into the subarachnoid space between lumbar
vertebrae 5 (L5) and L6 (13).
Successful implantation was confirmed by observing the tail-flick
reflex and cerebrospinal fluid flow from the tip of the catheter.
The external part of the catheter was protected according to the
Milligan's method (14). A
lidocaine test was performed to determine the position and
functionality of the catheter in the subarachnoid space.
Intrathecal delivery of
lentivirus
Rats were randomly divided into 6 groups (n=30 per
group): A sham group [Sham surgery + normal saline (NS)], a CCI
group (CCI surgery), an Lv-mismatch group (CCI + Lv-mismatch), an
LvOn-siTLR4 group (CCI + LvOn-siTLR4 + NS), a Dox group (CCI + Dox)
and an LvOn-siTLR4 with Dox group (CCI + LvOn-siTLR4 + Dox). The
lentivirus Lv-mismatch expressing scrambled siRNA
(TTCTCCGAACGTGTCACGT) was used as a control. Following CCI, rats in
the LvOn-siTLR4 and LvOn-siTLR4 with Dox groups were administered
the LvOn-siTLR4 virus (1×107 TU/10 µl) intrathecally.
The same titer of Lv-mismatch was applied intrathecally to the
Lv-mismatch group. NS of equal volume was administered
intrathecally to the rats of the NS groups. In the Dox and
LvOn-siTLR4 with Dox groups, Dox was given orally in water (200
ng/ml).
Evaluation of thermal
hyperalgesia
Thermal hyperalgesia was evaluated by paw withdrawal
latency (PWL) to radiant heat as previously described (15,16).
PWL was measured one day prior to and 1, 3, 5 and 7 days following
intrathecal administration of the lentivirus. Rats were placed in
an inverted clear plexiglass cage (23×18×13 cm) on a 3-mm-thick
glass plate for 30 min to acclimate to the surroundings. A radiant
heat source consisting of a high-intensity projection lamp bulb was
positioned under the glass floor beneath the right hind paw. The
radiant heat source was placed 40 mm below the floor and projected
through a 5×10 mm aperture at the top of a movable case. A digital
timer automatically detected the time from stimuli to PWL.
Detection was carried out twice on each rat with a 5-min interval.
A cut-off time of 20 sec was set to avoid damage to the hind
paw.
Evaluation of mechanical
allodynia
Mechanical allodynia was evaluated by the paw
withdrawal threshold (PWT) as described previously (17). The PWT was assessed using an
electronic von Frey filament (Von Frey anesthesiometer; IITC Life
Science, Woodland Hills, CA, USA) following PWL detection, on the
same day. Rats were placed on a wire mesh platform covered with a
transparent plastic dome for 30 min to acclimate to the
environment. A single rigid filament (nociceptive stimulus)
connected to a transducer was applied perpendicularly to the medial
surface of the hind paw with increasing force. The endpoint was
confirmed as paw withdrawal accompanied by head turning, biting
and/or licking. The required pressure was indicated in grams and
was considered to be the value of PWT. Each rat was tested three
times and the averages were considered to be the final results.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the L4-5 segments of the spinal cord
was extracted on the 3rd day following intrathecal injection of the
lentivirus. The RNA was treated with DNase I for 30 min at 37°C
prior to RT-qPCR. RT-qPCR was performed using PrimeScript™ RT
reagent Kit (cat. no. RR037A, Takara Bio, Inc., Otsu, Japan) and
SYBR® Premix Ex Taq™ II (cat. no. RR820L; Takara Bio,
Inc.). PCR was performed using the following thermocycling
conditions: initial 30 sec denaturation at 95°C for 30 sec;
followed by 40 cycles at 95°C for 5 sec and 60°C for 20 sec. The
RT-qPCR primers of TLR4 were as follows: 5′-TCTCCAGGAAGGCTTCCAC-3′
(forward) and 5′-GGCGATACAATTCGACCTGC-3′ (reverse). The RT-qPCR
primers of GAPDH were as follows: 5′-GCAAGTTCAACGGCACAG-3′
(forward) and 5′-GCCAGTAGACTCCACGACAT-3′ (reverse). The Real-time
PCR Detection System (Roche Diagnostics, Basel, Switzerland)
continually monitored the increase in fluorescence, which was
directly proportional to the PCR product. The relative expression
level of TLR4 was normalized to GAPDH. The data were analyzed with
the 2−∆∆Cq formula (18).
Western blot assay
The proteins from the L4-5 segments of the spinal
cord were prepared on the 3rd day following injection as
previously described (19,20). Protein concentrations were
determined using a Bicinchoninic Acid Assay Protein Assay kit
(Thermo Fisher Scientific, Inc.). Proteins (30 µg) were separated
by 8% SDS-PAGE gel and transferred onto a nitrocellulose membrane.
The nitrocellulose membrane was blocked with 5% non-fat dry milk
for 2 h at room temperature followed with a primary antibody
against TLR4 (1:500; cat. no. ab13556; Abcam, Cambridge, MA, USA)
at 4°C overnight, and then with secondary antibody (goat anti-mouse
IgG; 1:1,000; cat. no. ab6789; Abcam) conjugated with horseradish
peroxidase for 2 h at room temperature. The proteins were detected
using Pierce ECL Plus Western Blotting Substrate (cat. no. 32134;
Thermo Fisher Scientific, Inc.). β-actin (Sigma-Aldrich-Merck KGaA,
Darmstadt, Germany; 1:500) was used as a loading control.
Densitometry analysis was performed using ImageJ software (version
1.8.0; National Institutes of Health, Bethesda, MD, USA).
ELISA
The samples from the spinal tissue (L4-5) were
prepared on the day following evaluating mechanical allodynia.
Total protein concentrations were determined by Bradford assay and
the results were then adjusted for sample size. ELISAs for tumor
necrosis factor (TNF)-α (cat. no. 900-K73) and interleukin (IL)-1β
(cat. no. 900-K91) were carried out following the manufacturer's
protocol (Peprotech EC Ltd., London, UK) (11). The results were obtained by
analyzing the standard curves.
Statistical analysis
The behavioral and ELISA data are presented as the
mean ± standard error of the mean (SEM) of 6 rats per group. Tests
were performed on six groups (sham, CCI, CCI + Lv-mismatch, CCI +
LvOn-siTLR4 + NS, CCI + Dox and CCI + LvOn-siTLR4 + Dox) one day
prior to and 1, 3, 5 and 7 days following intrathecal
administration of the lentivirus. All assays were performed in
triplicate. Intergroup differences were statistically analyzed by
two-way analysis of variance (ANOVA) followed by Tukey's post hoc
test for multiple comparisons. The RT-qPCR data are presented as
the mean ± SEM and represent the normalized averages that were
derived from six samples for each group. The protein results are
presented as fold-changes compared with the sham group. The data
are presented as the mean ± SEM and represent the normalized
averages that were derived from six samples for each group.
Intergroup differences, in the RT-qPCR and protein expression data,
were analyzed by one-way ANOVA followed by Tukey post hoc analysis.
All data were analyzed with GraphPad Prism software (version 5;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction of lentivirus
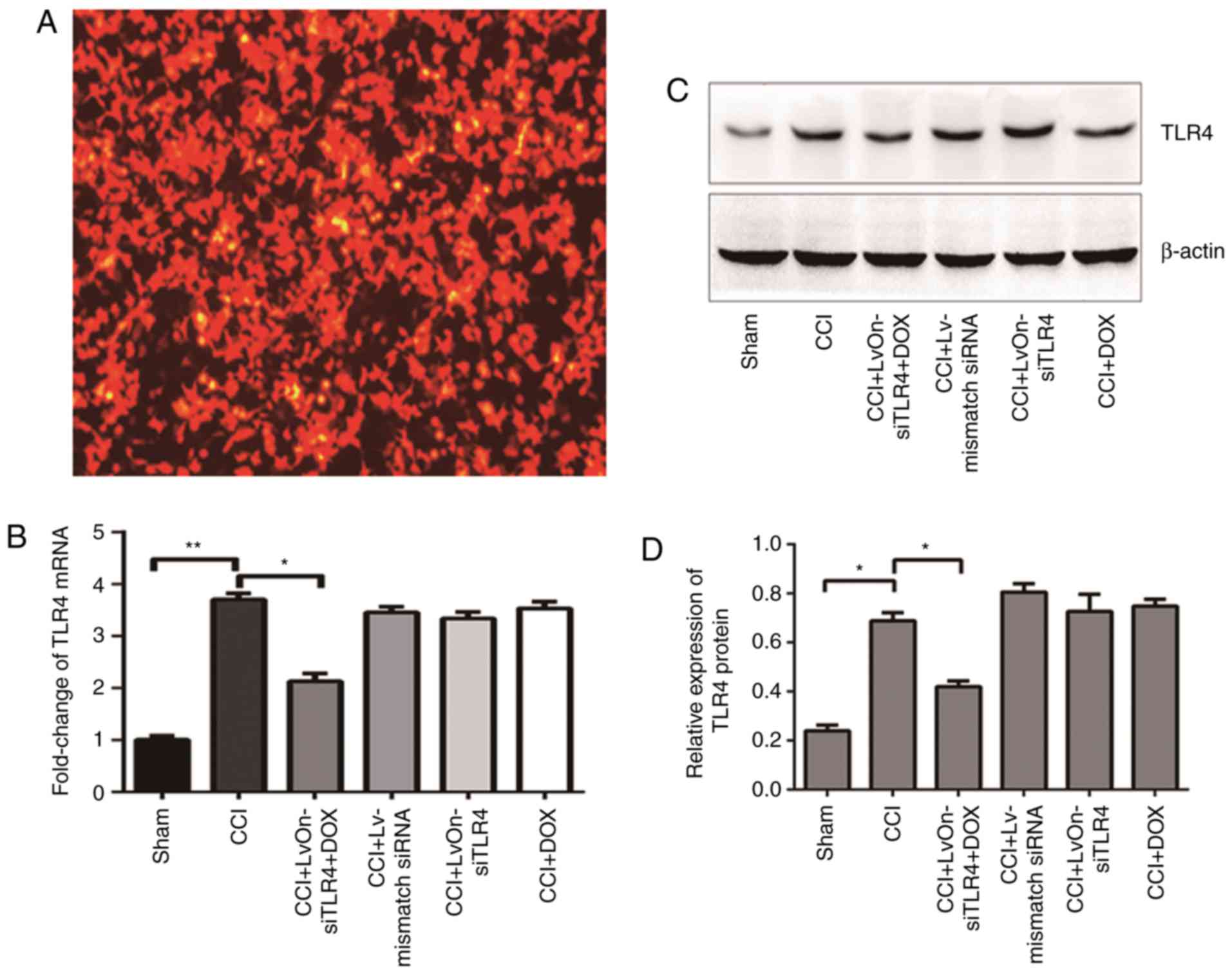
The location of the lentivirus could be tracked by
RFP expression due to the lentiviral vector system. As presented in
Fig. 1A, red fluorescence was
observed in the 293T cells, which suggested that the constructed
recombinant lentivirus LvOn-siTLR4 was successfully constructed and
transfected into 293T cells; and red fluorescence was also used as
a tracer for the subsequent experiments.
LvOn-siTLR4 with Dox decreases TLR4
expression in CCI rats
The mRNA and protein expression of TLR4 were
detected on the 3rd day following injection. As presented in
Fig. 1B, the expression of TLR4
mRNA increased significantly in the rats that received the CCI
procedure when compared with the sham group (P<0.01, n=6).
Compared with the Lv-mismatch siRNA group, TLR4 mRNA expression
decreased in the LvOn-siTLR4 with Dox group (P<0.05, n=6),
suggesting that the siRNA used in the present study was effective.
In contrast to the LvOn-siTLR4 and Dox groups, the TLR4 mRNA
expression decreased in the LvOn-siTLR4 with Dox group (P<0.05,
n=6), which indicated that the downregulation was Dox-induced, but
Dox alone was not effective. The western blot assay (Fig. 1C) demonstrated similar results as
the protein expression of TLR4 increased in the rats that underwent
the CCI procedure compared with the rats of the sham group. The
protein expression of TLR4 was downregulated in the LvOn-siTLR4
with Dox group when compared with the other four groups that
received CCI surgery (P<0.05, n=6), which suggested that the
siRNA expressed by the lentivirus interfered with the expression of
TLR4 and was induced by oral Dox administration (Fig. 1D).
TNF-a and IL-1β expression
TNF-α and IL-1β expression increased in the dorsal
spinal cord of CCI rats as presented in Fig. 2. No significant differences in
TNF-α and IL-1β expression were detected in the CCI, Dox,
LvOn-siTLR4 and Lv-mismatch siRNA groups (P>0.05). When compared
with these four groups, TNF-α and IL-1β were significantly lower in
the LvOn-siTLR4 with Dox group (P<0.05, n=6), indicating that
TNF-α and IL-1β were downregulated as TLR4 was decreased by siRNA
application.
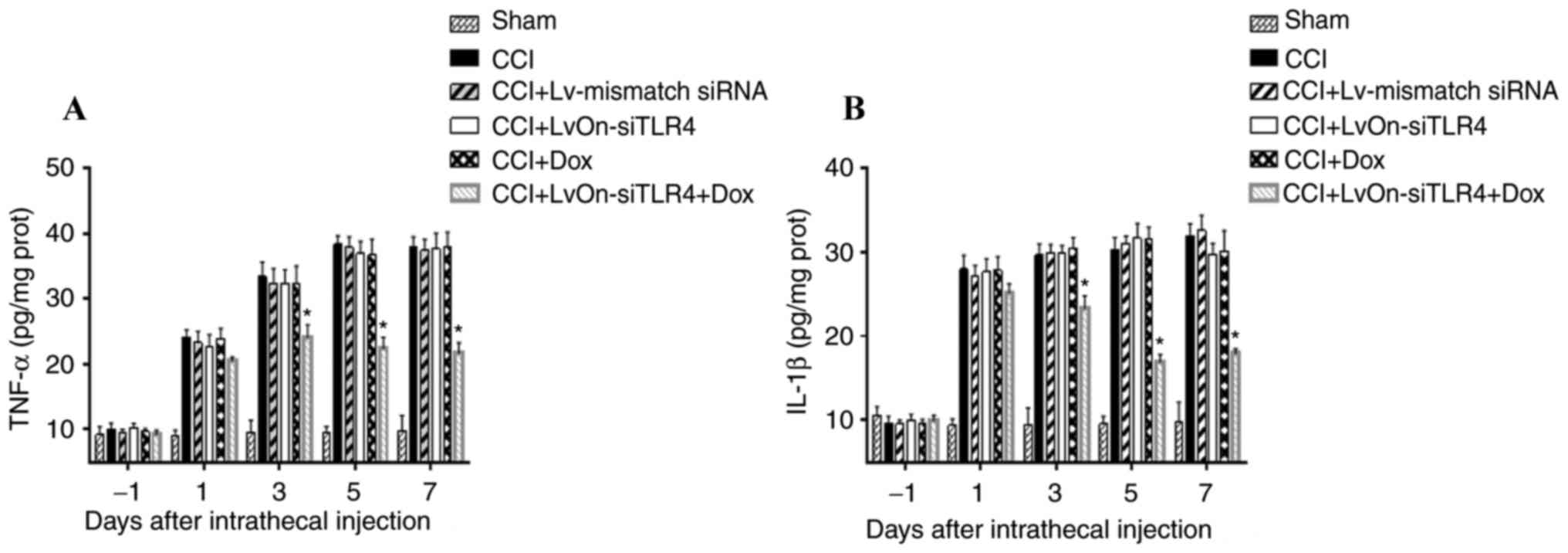 | Figure 2.Expression of TNF-α and IL-1β. (A)
Expression of TNF-α. TNF-α increased in the spinal cord of the CCI
group. No significant difference in TNF-α expression was detected
in the CCI, Dox, LvOn-siTLR4 and Lv-mismatch siRNA groups
(P>0.05). TNF-α levels were significantly decreased on the 3rd,
5th and 7th day in the LvOn-siTLR4 with Dox group (n=6). (B)
Expression of IL-1β. CCI-induced IL-1β expression in spinal cord
tissues was increased. IL-1β production in the LvOn-siTLR4 with Dox
group was significantly decreased on the 3rd, 5th and 7th day
(n=6). *P<0.05 vs. CCI group, Dox group, LvOn-siTLR4 group and
Lv-mismatch siRNA group. TLR4, Toll-like receptor 4; si, small
interfering RNA; TNF-α, tumor necrosis factor-α; IL-1β,
interleukin-1β; CCI, chronic constriction injury; ANOVA, analysis
of variance; Dox, doxycycline; Lv, lentivirus. |
LvOn-siTLR4 with Dox attenuates NP in
CCI rats
To examine the impact of LvOn-siTLR4 on the
nociception of NP rats, PWT and PWL were used to measure mechanical
allodynia and thermal hyperalgesia, respectively. Compared with the
sham group, rats in the CCI, LvOn-siTLR4 and Lv-mismatch siRNA
groups presented with a reduction in mechanical allodynia and
thermal hyperalgesia (Fig. 3;
P<0.05, n=6). In addition, when compared with these three groups
PWT (Fig. 3A) and PWL (Fig. 3B) in the LvOn-siTLR4 with Dox group
decreased on 1, 3, 5 and 7 days post-injection (P<0.05, n=6).
Furthermore, there were no significant differences in the PWT and
PWL between the Dox and CCI groups (P>0.05), indicating that Dox
did not contribute to the alterations in pain threshold.
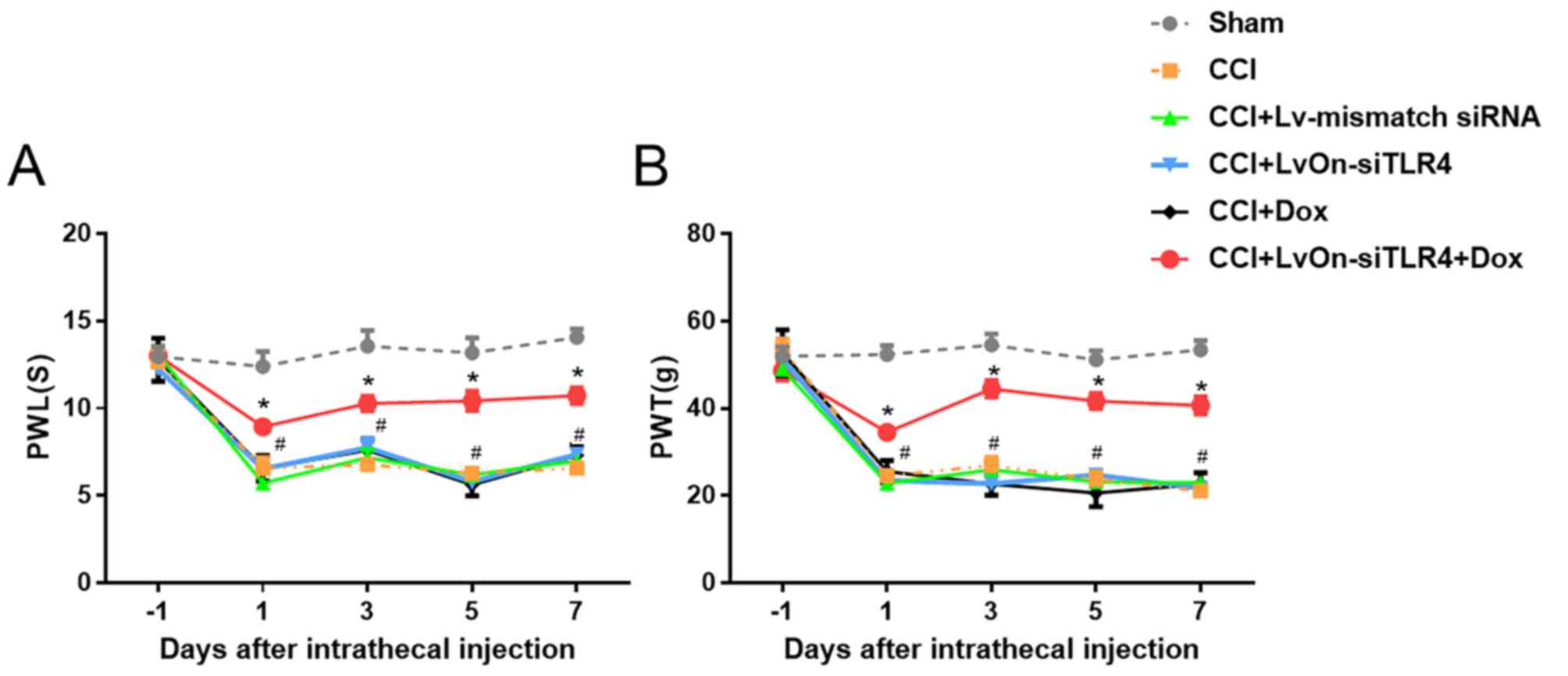 | Figure 3.Impact of LvOn-siTLR4 on PWL and PWT
in CCI rats. On the 1st, 3rd, 5th and 7th day following intrathecal
injection, CCI rats that received intrathecal LvOn-siTLR4 with oral
Dox, exhibited significantly attenuated (A) thermal hyperalgesia
and (B) mechanical allodynia. *P<0.05 vs. CCI group, Dox group,
LvOn-siTLR4 group and Lv-mismatch siRNA group. TLR4, Toll-like
receptor 4; si-, small interfering RNA; PWL, paw withdrawal
latency; PWT, paw withdrawal threshold; CCI, chronic constriction
injury; ANOVA, analysis of variance; Dox, Doxycycline; Lv,
lentivirus. |
Discussion
In the present study, a tetracycline inducible
lentivirus-expressing siRNA against TLR4 in NP was investigated in
CCI rats. The results revealed that intrathecal injection of the
lentivirus LvOn-siTLR4 with oral administration of Dox markedly
decreased the expression of TLR4 and downregulated TNF-α and IL-1β
in the spinal cord. Furthermore, the thermal and mechanical pain
hypersensitivity induced by CCI was effectively alleviated by
LvOn-siTLR4 (with oral administration of Dox). In addition, siRNA
expression was controlled by oral administration of Dox. These
results suggested that the inducible lentivirus-mediated siRNA
targeting TLR4 may be applied for NP in an experimental setting.
With more comprehensive experiments, a novel method to treat NP
using LvOn-siTLR4 may be developed.
In recent years, TLR4 has been considered to serve
an increasing number of important roles in chronic pain and
pruritus (21). Activated TLR4
interacts with myeloid differentiation primary-response protein 88
(22,23) and leads to the translocation of
nuclear factor (NF)-κB to the nucleus, which results in the release
of inflammatory factors including TNF-α and IL-1β (24,25).
A number of factors in the TLR4 signaling pathway, including NF-κB
and TNF-α, are involved in the development of central pain
sensitization. Downregulation of TLR4 by an antagonist or siRNA,
leading to a decrease in the expression of these pain-associated
factors, has been reported to relieve pain hypersensitivity in
different chronic pain models (11,17).
The NP rat model of CCI used in the present study demonstrated
similar results as decreasing the expression of TLR4 attenuated
hyperalgesia and allodynia in the rats. Therefore, TLR4 presents a
potential target for NP treatment.
The RNAi technique is a useful tool in gene therapy
as it precisely targets therapeutics for any specific subtype
(26). It utilizes double-stranded
RNAs to form RNA duplexes of specific structure and length, which
degrade homologous sequences of mRNA to siRNA and induce
sequence-specific gene silencing (27,28).
In the application of this technique, the efficiency, specificity
and stability of siRNA in target cells should be taken into
consideration (28,29). The TLR4 siRNA used in the present
study has been observed to have specificity and efficiency in
downregulating TLR4 and alleviating NP in CCI rats (9). The lentivirus LvOn-siTLR4 employed in
the present study was also revealed to have the ability to express
stable siRNA in rats. It was successfully transfected into the
dorsal horn and persisted for 5 weeks following the intrathecal
injection into rats in a bone cancer pain model in the authors'
previous study (11). In this
case, the lentivirus demonstrated potential in treating NP in an
efficient, specific and stable manner. In the NP model of CCI rats,
hyperalgesia and allodynia were reduced in the present study
following injection with the virus, which suggested that it
exhibited a certain validity in applying the virus LvOn-siTLR4 for
NP treatment.
In the present study, a tetracycline-regulated
system (Tet-on) was introduced into the lentivirus to control the
level of TLR4 expression. In this typical Tet-on system, a
Tet-regulated transactivator (tTA) was addressed by inserting a
tetracycline repressor into a herpes simplex virus VP 16
transactivation domain (29). With
the presence of Tet or Dox, the tTA would not bind to the operator
sequences and therefore, led to the activation of transcription
(30,31). In contrast, the tTA would bind to
the operator sequences and inhibit transcriptional activation
without Dox. The level of TLR4 expression was not affected. Through
this system, downregulation of TLR4 is controlled by Tet or Dox. In
the present study, the decrease in TLR4 was only observed in the
LvOn-siTLR4 with Dox group, while rats in the LvOn-siTLR4 group
demonstrated no regulatory effect on TLR4, which indicated that the
expression of TLR4 siRNA was controlled by Dox. Therefore, this
provides a method of controlling the timing and levels of TLR4
through oral Dox application in order to maintain the protein
concentrations within a therapeutic window for clinical usage
(11).
In conclusion, the TLR4 siRNA expressed by the
lentivirus effectively and stably reduced TLR4 expression in the
spinal cord of CCI rats. The Tet-on system was utilized to induce
the expression TLR4 in the present study. The inducible lentivirus
LvOn-siTLR4 reduced the thermal hyperalgesia and mechanical
allodynia of CCI rats by inhibiting TLR4 in the spinal cord, which
may present a novel strategy for NP treatment in the future.
However, the dose-dependent manner of Dox in regulating TLR4 was
not investigated in the present study and therefore, further
studies are required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81671082) to
Feixiang Wu and the Xinchen Foster Fund for Anesthesiologists in
Shanghai to Feixiang Wu.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YS, FW, YL, WY and YZ conceived and designed the
study. YL, YZ, RP, MC, XW and EK performed the experiments and
analyzed the data. YL and YZ wrote the manuscript. WY, YS and FW
reviewed and edited the manuscript. All authors read and approved
the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Administrative Committee of Experimental Animal Care and Use of
Second Military Medical University (Shanghai, China), and conformed
to the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publications No. 8023, revised 1978).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dworkin RH, Turk DC, Katz NP, Rowbotham
MC, Peirce-Sandner S, Cerny I, Clingman CS, Eloff BC, Farrar JT,
Kamp C, et al: Evidence-based clinical trial design for chronic
pain pharmacotherapy: A blueprint for ACTION. Pain. 152 3
Suppl:S107–S115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ivanova JI, Birnbaum HG, Yushkina Y, Sorg
RA, Reed J and Merchant S: The prevalence and economic impact of
prescription opioid-related side effects among patients with
chronic noncancer pain. J Opioid Manag. 9:239–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jurga AM, Rojewska E, Piotrowska A, Makuch
W, Pilat D, Przewlocka B and Mika J: Blockade of Toll-like
receptors (TLR2, TLR4) attenuates pain and potentiates
buprenorphine analgesia in a rat neuropathic pain model. Neural
Plast. 2016:52387302016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma YQ, Chen YR, Leng YF and Wu ZW:
Tanshinone IIA downregulates HMGB1 and TLR4 expression in a spinal
nerve ligation model of neuropathic pain. Evid Based Complement
Alternat Med. 2014:6395632014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hutchinson MR, Zhang Y, Brown K, Coats BD,
Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier
SF, et al: Non-stereoselective reversal of neuropathic pain by
naloxone and naltrexone: Involvement of Toll-like receptor 4
(TLR4). Eur J Neurosci. 28:20–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bettoni I, Comelli F, Rossini C, Granucci
F, Giagnoni G, Peri F and Costa B: Glial TLR4 receptor as new
target to treat neuropathic pain: Efficacy of a new receptor
antagonist in a model of peripheral nerve injury in mice. Glia.
56:1312–1319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaur IP and Sharma G: siRNA: A new
approach to target neuropathic pain. BioDrugs. 26:401–412. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu F, Liu Y, Lv X, Miao X, Sun Y and Yu W:
Small interference RNA targeting TLR4 gene effectively attenuates
pulmonary inflammation in a rat model. BioMed Res Int.
2012:4064352012.
|
|
9
|
Wu FX, Bian JJ, Miao XR, Huang SD, Xu XW,
Gong DJ, Sun YM, Lu ZJ and Yu WF: Intrathecal siRNA against
Toll-like receptor 4 reduces nociception in a rat model of
neuropathic pain. Int J Med Sci. 7:251–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu F, Pan R, Chen J, Sugita M, Chen C, Tao
Y, Yu W and Sun Y: Lentivirus mediated siRNA against GluN2B Subunit
of NMDA receptor reduces nociception in a rat model of neuropathic
pain. Biomed Res Int. 2014:8716372014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan R, Di H, Zhang J, Huang Z, Sun Y, Yu W
and Wu F: Inducible lentivirus-mediated siRNA against TLR4 reduces
nociception in a rat model of bone cancer pain. Mediators Inflamm.
2015:5238962015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge Y, Wu F, Sun X, Xiang Z, Yang L, Huang
S, Lu Z, Sun Y and Yu WF: Intrathecal infusion of hydrogen-rich
normal saline attenuates neuropathic pain via inhibition of
activation of spinal astrocytes and microglia in rats. PLoS One.
9:e974362014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milligan ED, Hinde JL, Mehmert KK, Maier
SF and Watkins LR: A method for increasing the viability of the
external portion of lumbar catheters placed in the spinal
subarachnoid space of rats. J Neurosci Methods. 90:81–86. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu F, Miao X, Chen J, Sun Y, Liu Z, Tao Y
and Yu W: Down-regulation of GAP-43 by inhibition of caspases-3 in
a rat model of neuropathic pain. Int J Clin Exp Pathol. 5:948–955.
2012.PubMed/NCBI
|
|
17
|
Lan LS, Ping YJ, Na WL, Miao J, Cheng QQ,
Ni MZ, Lei L, Fang LC, Guang RC, Jin Z and Wei L: Down-regulation
of Toll-like receptor 4 gene expression by short interfering RNA
attenuates bone cancer pain in a rat model. Mol Pain. 6:22010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
An G, Lin TN, Liu JS, Xue JJ, He YY and
Hsu CY: Expression of c-fos and c-jun family genes after focal
cerebral ischemia. Ann Neurol. 33:457–464. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu F, Miao X, Chen J, Liu Z, Tao Y, Yu W
and Sun Y: Inhibition of GAP-43 by propentofylline in a rat model
of neuropathic pain. Int J Clin Exp Pathol. 6:1516–1522.
2013.PubMed/NCBI
|
|
21
|
Liu T, Han Q, Chen G, Huang Y, Zhao LX,
Berta T, Gao YJ and bJi RR: Toll-like receptor 4 contributes to
chronic itch, alloknesis, and spinal astrocyte activation in male
mice. Pain. 157:806–817. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vijayan V, Khandelwal M, Manglani K, Gupta
S and Surolia A: Methionine down-regulates TLR4/MyD88/NF-κB
signalling in osteoclast precursors to reduce bone loss during
osteoporosis. Br J Pharmacol. 171:107–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wieland CW, Florquin S, Maris NA, Hoebe K,
Beutler B, Takeda K, Akira S and van der Poll T: The
MyD88-dependent, but not the MyD88-independent, pathway of TLR4
signaling is important in clearing nontypeable haemophilus
influenzae from the mouse lung. J Immunol. 175:6042–6049. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin J, Wang H, Li J, Wang Q, Zhang S, Feng
N, Fan R and Pei J: κ-Opioid receptor stimulation modulates
TLR4/NF-κB signaling in the rat heart subjected to
ischemia-reperfusion. Cytokine. 61:842–848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song Y, Shi Y, Ao LH, Harken AH and Meng
XZ: TLR4 mediates LPS-induced HO-1 expression in mouse liver: Role
of TNF-α and IL-1β. World J Gastroenterol. 9:1799–1803. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ichim TE, Li M, Qian H, Popov IA, Rycerz
K, Zheng X, White D, Zhong R and Min WP: RNA Interference: A potent
tool for gene-specific therapeutics. Am J Transplant. 4:1227–1236.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elbashir SM, Lendeckel W and Tuschl T: RNA
interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev.
15:188–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lambeth LS and Smith CA: Short hairpin
RNA-mediated gene silencing. Methods Mol Biol. 942:205–232. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Welman A, Barraclough J and Dive C:
Tetracycline regulated systems in functional oncogenomics. Transl
Oncogenomics. 2:17–33. 2007.PubMed/NCBI
|
|
30
|
Pluta K, Luce MJ, Bao L, Agha-Mohammadi S
and Reiser J: Tight control of transgene expression by lentivirus
vectors containing second-generation tetracycline-responsive
promoters. J Gene Med. 7:803–817. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moriyama H, Moriyama M, Sawaragi K, Okura
H, Ichinose A, Matsuyama A and Hayakawa T: Tightly regulated and
homogeneous transgene expression in human adipose-derived
mesenchymal stem cells by lentivirus with tet-off system. PLoS One.
8:e662742013. View Article : Google Scholar : PubMed/NCBI
|