Introduction
Conflicting relationship has been demonstrated
between glaucoma and diabetes mellitus (DM). For example, some
previous studies showed that DM increases a risk for development of
glaucoma (1,2). Conversely, other studies suggested
that DM prevented glaucoma occurrence (3,4).
Moreover, a recent study showed that DM is associated with
glaucoma, but this association disappeared after adjustment for
triglyceride levels (5). In the
histological level, previous studies reported that short-term
hyperglycemia (HG) preserves retinal structure in a transient high
intraocular pressure-induced ischemic rat model (6) and a common carotid artery occlusion
rat model (7). Other study
demonstrated that HG condition prevents axon loss in an ocular
hypertension rat model (8).
Furthermore, a recent study has shown that subconjunctival applied
glucose partially preserves retinal ganglion cell (RGC) somata in
the transient high intraocular pressure-induced ischemic rat model
and transiently increases contrast sensitivity in human subjects
with severe primary open-angle glaucoma (9).
Our previous study found that short-term HG
ameliorates tumor necrosis factor (TNF)-induced axon loss (10). Since a close relationship between
TNF and glaucoma has been implicated (11–16),
this TNF-mediated axon loss model may be helpful to clarify the
molecular events by which axons are degenerated in RGCs (17). In optic nerves, the short-term HG
enhances autophagy machinery (10). Autophagy plays central roles in the
pathophysiology of several human diseases (18) and its impaired condition has been
linked to neurodegenerative diseases (19–21).
Among the autophagy-related (Atg) genes, microtubule-associated
protein light chain 3 (LC3)/Atg8 is known as a maker for
autophagosomes (22).
Beclin-1/Atg6 constitutes Beclin-1 complex which is necessary for
autophagic function (23).
Up-regulation of Beclin-1 was shown in RGCs after optic nerve
transection in rats (24). In
addition, increased Beclin-1 protein levels were shown in retinal
samples in the rat hypertensive glaucoma model (25) and a monkey hypertensive glaucoma
model (26). However, its
expression and localization in optic nerve have not yet to be
demonstrated. In the present study, we tested whether Beclin-1 is
involved in the ameliorative effect of short-term HG against axon
loss caused by TNF.
Materials and methods
Animals
The present study used 8-week-old male Wistar rats
and was approved by Ethics Committee of the Institute of
Experimental Animals of St. Marianna University School of Medicine.
The rats were maintained in the controlled rooms (23±1°C; humidity
at 55±5%; light on 06:00 to 18:00).
Streptozotocin-induced hyperglycemic
(HG) rat model
Single i.p. administration of physiological saline
solution (PSS) or 60 mg/kg streptozotocin (STZ; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) was carried out for the
normoglycemic (NG) rats or the hyperglycemic (HG) rats,
respectively. The plasma glucose levels were measured using a
glucometer (Johnson & Johnson, Tokyo, Japan) 4 days after
intraperitoneal injection. We only included the individuals as the
HG group when plasma glucose exceeded 250 mg/dl. The plasma glucose
levels of HG groups were 441.5±71.3, 435.0±32.3, and 432.1±73.3
mg/dl, immunoblot analysis, immunohistochemical analysis, and
morphometric analysis studies, respectively.
Intravitreal injection
Intravitreal administration of 10 ng TNF (2 µl) was
carried out into the right eye of rats under anesthetization with a
combination of ketamine and xylazine. The left eye was received an
intravitreal administration of phosphate-buffered saline (PBS).
These intravitreal administrations were carried out 4 days
following i.p. injection of PSS or STZ. In the HG group, a
simultaneous intravitreal administration of 50 pmol Beclin-1 siRNA
(Cell Signaling Technology, Inc., Danvers, MA, USA) with TNF was
carried out into the right eyes.
Immunoblot analysis
Thirty-six rats (NG: 18 rats; HG: 18 rats) were
euthanatized 1 week after intravitreal administrations for
immunoblot analysis. Four mm optic nerves from immediately behind
the eye ball were homogenized in protein extraction buffer. Since
the optic nerve pieces were small, each sample included two optic
nerves. Equal amount of proteins (3 µg) determined by the Bradford
assay was applied and loaded. Then, samples were transferred to
PVDF membranes. After blocking, membranes were exposed with the
primary antibodies: Anti-Beclin-1 antibody for overnight (1:200;
Medical & Biological Laboratories, Co., Ltd., Nagoya, Japan) or
anti-β-actin antibody for 2 h (1:500; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). After three times washing, membranes were
reacted with the secondary antibodies: Rabbit IgG or mouse IgG.
Immunoblots were evaluated with the Amersham ECL detection system
(GE Healthcare Life Sciences, Little Chalfont, UK).
Immunohistochemical analysis
Six rats were used for immunohistochemical analysis.
One week following intravitreal administration, optic nerve samples
were immersed in 10% neutral-buffered formalin. Paraffinized cross
sections were made in 2 µm thick and incubated with 1% bovine
serum. The primary antibodies were anti-Beclin-1 antibody (1:100;
Medical & Biological Laboratories, Co., Ltd.), glial fibrillary
acidic protein (GFAP, a marker of astrocytes; 1:200; Agilent
Technologies, Inc., Santa Clara, CA, USA), and neurofilament-L (a
marker of neurons; 1:100; Agilent Technologies, Inc.). The
secondary antibodies were FITC-labeled and rhodamine-labeled IGG.
The slides were mounted in 4′,6-diamidino-2-phenylindole-including
medium (Vector Laboratories, Ltd., Peterborough, UK).
Morphometric analysis
Fourteen rats (NG: 4 rats; HG:10 rats) were
euthanatized 2 weeks after intravitreal administration for axon
morphometric analysis (10,27).
Optic nerve samples were immersed in Karnovsky's solution for
overnight. After embedded in plastic blocks, cross thin sections
were made beginning 1 mm from the eye ball. The sections were
stained with 1% paraphenylen-diamine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Five images (center and periphery in quadrant
per optic nerve) were acquired and quantified using an
image-processing software (Aphelion, ADCIS, Hérouville Saint-Clair,
France). The average of axon number in each optic nerve was
expressed as the number per mm2.
Statistical analysis
Data are presented as mean ± standard error of the
mean. Differences among groups were analyzed using one-way analysis
of variance with Dunnett's post hoc test. JMP v12.0.1 software (SAS
Institute, Inc., Cary, NC, USA) was used for statistical analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of TNF and HG on Beclin-1
levels in Optic Nerve
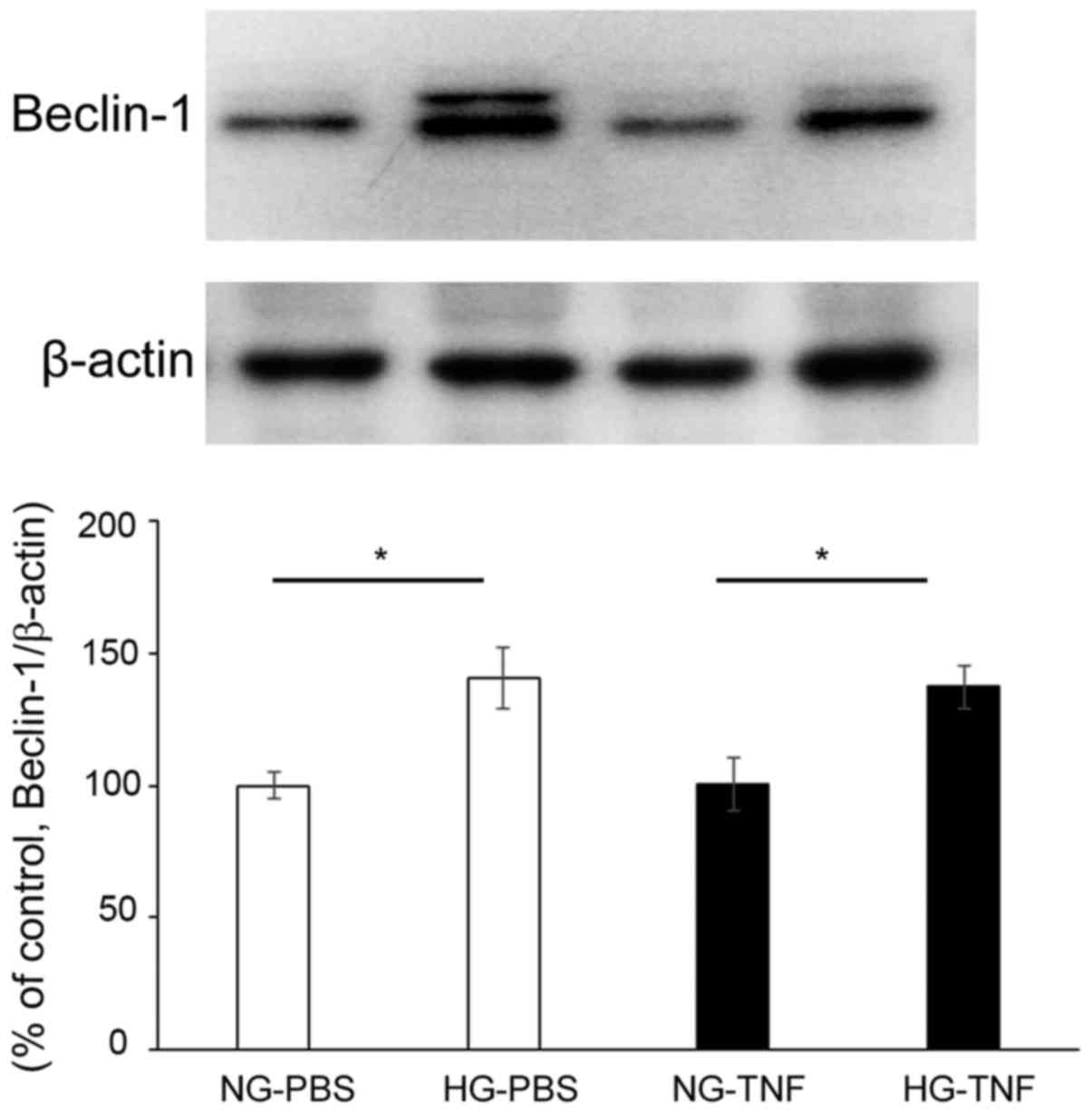
The current study found that HG condition markedly
increases Beclin-1 protein levels in optic nerves (Fig. 1). These significant increments were
seen in both PBS-treated and TNF-treated eyes (Fig. 1). However, intravitreal
administration of TNF did not alter the Beclin-1 expression in both
NG and HG conditions.
Localization of Beclin-1 in optic
nerve
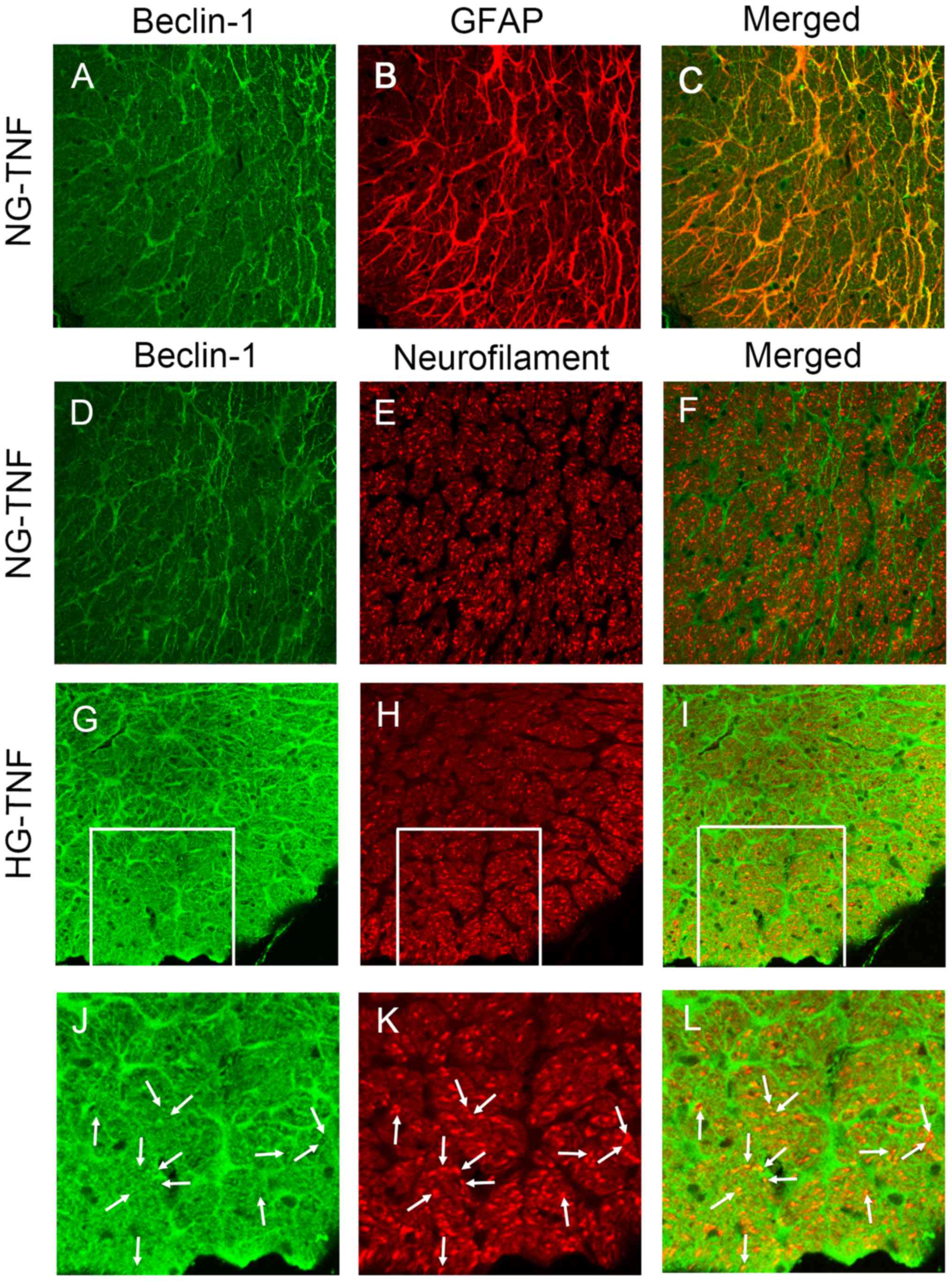
In cross sections, immunohistochemical study
revealed abundant colocalization of Beclin-1 and GFAP in the optic
nerves in NG group (Fig. 2A-C).
Although immunoreactivity pattern of Beclin-1 is different from
that of neurofilament (Fig. 2D-F),
some immunoreactivities of Beclin-1 were apparently colocalized
with those of neurofilament in the HG group (Fig. 2G-L). These findings suggest that
Beclin-1 is present mainly in glial cells, but partially in
neurofilament, and that the expression of Beclin-1 may be
upregulated by HG.
Effect of HG and Beclin-1 siRNA on
Axonal Loss induced by TNF
Consistent with our previous findings (10), the current study showed that
STZ-induced HG condition appeared a significant ameliorative effect
on axonal loss caused by TNF (Fig. 3B,
C, E). Since we found a significant upregulation of Beclin-1
protein level in the HG group, we examined whether Beclin-1 siRNA
alters this protective effect. Noticeable degenerative changes were
seen in the HG-TNF with Beclin-1 siRNA treatment group (Fig. 3D). Although no significant
difference in the axon number was seen in between the HG-TNF group
and the HG-TNF with Beclin-1 siRNA treatment group, no significant
difference was also seen in between the NG-TNF group and the HG-TNF
with Beclin-1 siRNA treatment group (Fig. 3E). These observations suggested
that the protective effect of HG was only partially suppressed by
Beclin-1 siRNA.
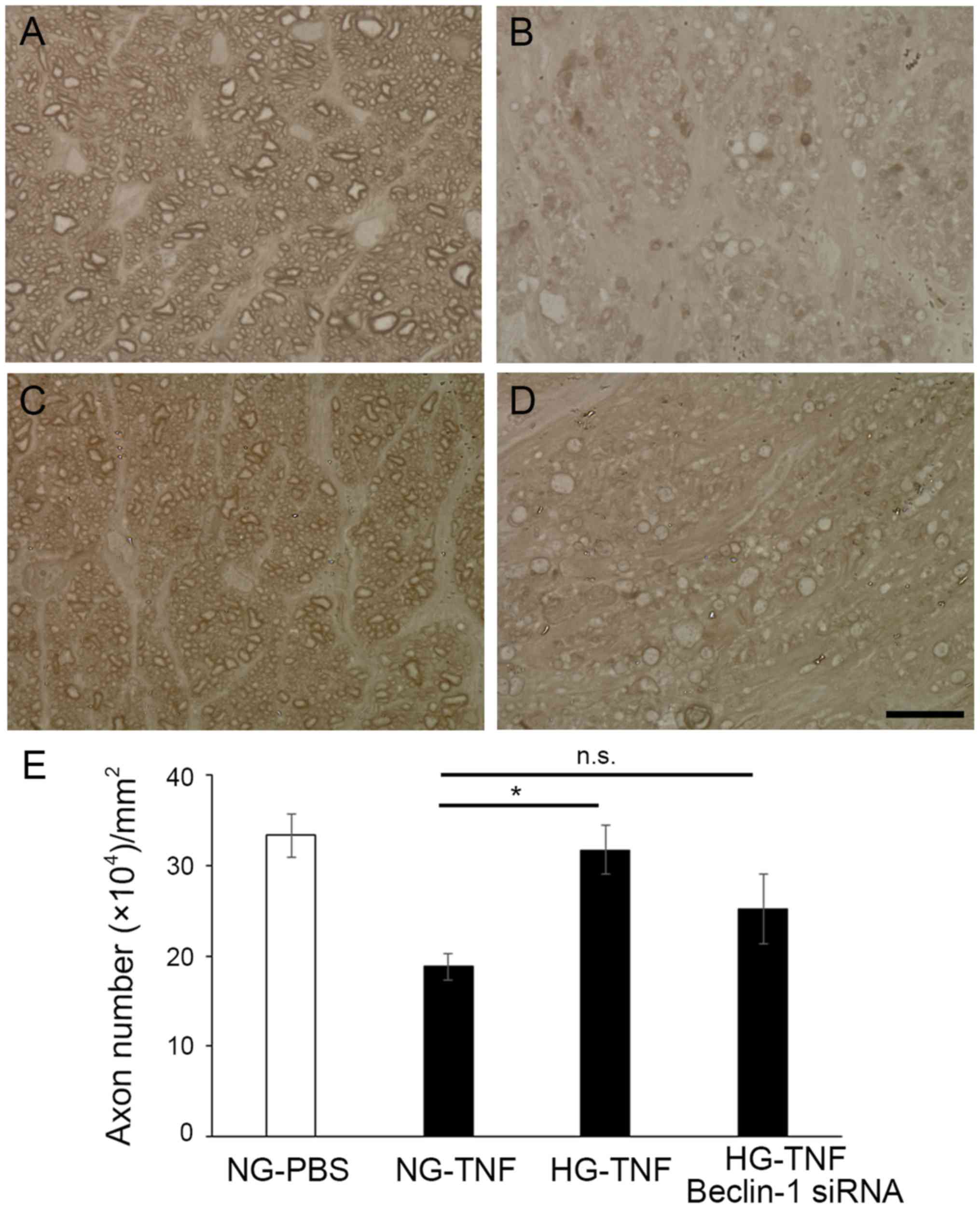 | Figure 3.Beclin-1 siRNA only partially
suppresses the axonal-protective effects of short-term HG.
Histological results of cross sectioned optic nerves in the (A)
NG-PBS, (B) NG-TNF, (C) HG-TNF, or (D) HG-TNF + 50 pmol Beclin-1
siRNA groups. Scale bar=10 µm; magnification, ×100. (E) Comparison
of axon numbers as shown by computer-assisted image analysis
(Aphelion imaging software). Five different areas of 1,446.5
µm2 each (totaling 7,232.5 µm2) from each eye
were used for analysis (n=4-5 per group). *P<0.05, as indicated.
HG, hyperglycemia; TNF, tumor necrosis factor; NG, normoglycemia;
siRNA, small interfering RNA; n.s., not significant. |
Discussion
Opposite autophagic status was demonstrated in
skeletal muscle between in the glucose-infusion HG rat and the
STZ-induced HG rat (28). In
STZ-induced HG rats, low insulin level prevented the m-TOR
signaling, thereby leading to enhancement of autophagy (28). Consistent with this finding, our
previous study found enhanced autophagy in optic nerve in the
STZ-induced HG rats (10). In
addition, a recent study demonstrated enhanced autophagy in
hippocampus with ischemia in the STZ-induced HG rats (29). In the current study, remarkable
increase in Beclin-1 expression was found in the optic nerve in the
STZ-induced HG rats. It has been shown that Beclin-1 plays an
essential role for the formation of autophagosomes in a HT22
hippocampal cells (30). That
study also demonstrated that Beclin-1 is necessary for upregulation
of LC3-II (30). Moreover, some
recent studies demonstrated increased Beclin-1 protein levels in
the hippocampus in STZ-induced HG rats (31) and in the retina in STZ-induced HG
mice (32). Therefore,
upregulation of Beclin-1 can be observed in several types of
neuronal tissue as well as optic nerve under STZ-induced HG
condition. Thus, we next examined the localization of Beclin-1 in
optic nerve.
Although LC3 immunoreactivity exists in nerve fiber
in optic nerve (10), Beclin-1
immunoreactivity is present mainly in glia in optic nerve.
Consistently, a previous study indicated that Beclin-1 expression
was found in the primary astrocytes (33). On the other hand, because it was
reported that Beclin-1 presents RGC bodies (24), and we observed partial
colocalization of Beclin-1 and neurofilaments, it is likely that
Beclin-1 also exists in neurons. In addition, it was shown that
Beclin-1 mainly expressed in neuronal cells and scarcely expressed
in GFAP-positive astrocytes in mouse cerebral cortex slices
(34). It was also shown that the
colocalization of Beclin-1 and neuronal cells was observed in rat
brain slices (35). Therefore, the
distribution of Beclin-1 may vary depending on the type of neurons,
but it may be present both in neurons and glia. It was assumed that
intravitreal administration of siRNA downregulates the protein
level of optic nerve which exists in neurofilaments (27). Our current morphometric analysis
showed that the intravitreal administration of Beclin-1 siRNA
failed to abolish the protective effect of HG. It is reasonable to
speculate that intravitreal administration of Beclin-1 siRNA may
affect the Beclin-1 expression in RGC bodies and their axons but
not glial cells in optic nerve. Because it is difficult to
distinguish the change in Beclin-1 protein level between intraaxon
and glia in optic nerve after intravitreal injection of siRNA, this
method (i.e., local knockdown) may have a limitation to address the
role of protein which exists both in axon and glia. Nonetheless,
since no significant difference in the axon number was seen in
between the NG-TNF group and the HG-TNF with Beclin-1 siRNA
treatment group, one hypothesis posits that Beclin-1 inside axons
can play some roles for protective effect of HG. A protective role
of Beclin-1 has been shown in a mouse neurodegeneration model
(36). Further studies will be
necessary to elucidate the detail role of Beclin-1 in axonal
degeneration.
In conclusion, our findings suggest that Beclin-1
exists both in neurons and glia in optic nerve and enhanced
Beclin-1 may be at least partially associated with axonal
protection by HG induction.
Acknowledgements
The authors would like to thank Ms. Yukari Hara
(Department of Ophthalmology, St. Marianna University School of
Medicine, Kanagawa, Japan) and Ms. Chizuko Sasaki (Institute for
Ultrastructural Morphology, St. Marianna University School of
Medicine, Kanagawa, Japan) for their helpful assistance.
Funding
The present study was supported by Grants-in-Aid in
Japan (grant nos. 15K10908 and 17K11469).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS, YK and CT performed experiments. KS, YK, CT and
HT conceived and designed the research, and analyzed the data. KS,
YK and HT wrote and revised the article. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Institute of Experimental Animals of St. Marianna
University Graduate School of Medicine (approval no: 1610004).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao D, Cho J, Kim MH, Friedman DS and
Guallar E: Diabetes, fasting glucose, and the risk of glaucoma: A
meta-analysis. Ophthalmology. 122:72–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou M, Wang W, Huang W and Zhang X:
Diabetes mellitus as a risk factor for open-angle glaucoma: A
systematic review and meta-analysis. PLoS One. 9:e1029722014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gordon MO, Beiser JA, Brandt JD, Heuer DK,
Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK II,
Wilson MR and Kass MA: The Ocular Hypertension Treatment Study:
Baseline factors that predict the onset of primary open-angle
glaucoma. Arch Ophthalmol. 120:714–720; discussion 829–829. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akkaya S, Can E and Öztürk F: Comparison
of optic nerve head topographic parameters in patients with primary
open-angle glaucoma with and without diabetes mellitus. J Glaucoma.
25:49–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ko F, Boland MV, Gupta P, Gadkaree SK,
Vitale S, Guallar E, Zhao D and Friedman DS: Diabetes, triglyceride
levels, and other risk factors for glaucoma in the national health
and nutrition examination survey 2005–2005. Invest Ophthalmol Vis
Sci. 57:2152–2157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Casson RJ, Chidlow G, Wood JP and Osborne
NN: The effect of hyperglycemia on experimental retinal ischemia.
Arch Ophthalmol. 122:361–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holman MC, Chidlow G, Wood JP and Casson
RJ: The effect of hyperglycemia on hypoperfusion-induced injury.
Invest Ophthalmol Vis Sci. 51:2197–2207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ebneter A, Chidlow G, Wood JP and Casson
RJ: Protection of retinal ganglion cells and the optic nerve during
short-term hyperglycemia in experimental glaucoma. Arch Ophthalmol.
129:1337–1344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibeeb O, Chidlow G, Han G, Wood JP and
Casson RJ: Effect of subconjunctival glucose on retinal ganglion
cell survival in experimental retinal ischaemia and contrast
sensitivity in human glaucoma. Clin Exp Ophthalmol. 44:24–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sase K, Kitaoka Y, Munemasa Y, Kojima K
and Takagi H: Axonal protection by short-term hyperglycemia with
involvement of autophagy in TNF-induced optic nerve degeneration.
Front Cell Neurosci. 9:4252015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan X, Tezel G, Wax MB and Edward DP:
Matrix metalloproteinases and tumor necrosis factor alpha in
glaucomatous optic nerve head. Arch Ophthalmol. 118:666–673. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan L and Neufeld AH: Tumor necrosis
factor-alpha: A potentially neurodestructive cytokine produced by
glia in the human glaucomatous optic nerve head. Glia. 32:42–50.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tezel G and Wax MB: Increased production
of tumor necrosis factor-alpha by glial cells exposed to simulated
ischemia or elevated hydrostatic pressure induced apoptosis in
cocultured retinal ganglion cells. J Neurosci. 20:8693–8700. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tezel G, Li LY, Patil RV and Wax MB:
TNF-alpha and TNF-alpha receptor-1 in the retina of normal and
glaucomatous eyes. Invest Ophthalmol Vis Sci. 42:1787–1794.
2001.PubMed/NCBI
|
|
15
|
Kang JH, Wiggs JL and Pasquale LR: A
nested case control study of plasma ICAM-1, E-selectin and TNF
receptor 2 levels, and incident primary open-angle glaucoma. Invest
Ophthalmol Vis Sci. 54:1797–1804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sawada H, Fukuchi T, Tanaka T and Abe H:
Tumor necrosis factor-alpha concentrations in the aqueous humor of
patients with glaucoma. Invest Ophthalmol Vis Sci. 51:903–906.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitaoka Y, Kitaoka Y, Kwong JM,
Ross-Cisneros FN, Wang J, Tsai RK, Sadun AA and Lam TT:
TNF-alpha-induced optic nerve degeneration and nuclear
factor-kappaB p65. Invest Ophthalmol Vis Sci. 47:1448–1457. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frake RA, Ricketts T, Menzies FM and
Rubinsztein DC: Autophagy and neurodegeneration. J Clin Invest.
125:65–74. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Menzies FM, Fleming A and Rubinsztein DC:
Compromised autophagy and neurodegenerative diseases. Nat Rev
Neurosci. 16:345–357. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Puorro G, Marsili A, Sapone F, Pane C, De
Rosa A, Peluso S, De Michele G, Filla A and Saccà F: Peripheral
markers of autophagy in polyglutamine diseases. Neurol Sci.
39:149–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wirawan E, Lippens S, Vanden Berghe T,
Romagnoli A, Fimia GM, Piacentini M and Vandenabeele P: Beclin1: A
role in membrane dynamics and beyond. Autophagy. 8:6–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH, Munemasa Y, Kwong JM, Ahn JH,
Mareninov S, Gordon LK, Caprioli J and Piri N: Activation of
autophagy in retinal ganglion cells. J Neurosci Res. 86:2943–2951.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park HY, Kim JH and Park CK: Activation of
autophagy induces retinal ganglion cell death in a chronic
hypertensive glaucoma model. Cell Death Dis. 3:e2902012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng S, Wang M, Yan Z, Tian Z, Chen H,
Yang X and Zhuo Y: Autophagy in retinal ganglion cells in a rhesus
monkey chronic hypertensive glaucoma model. PLoS One. 8:e771002013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kitaoka Y, Munemasa Y, Hayashi Y,
Kuribayashi J, Koseki N, Kojima K, Kumai T and Ueno S: Axonal
protection by 17β-estradiol through thioredoxin-1 in tumor necrosis
factor-induced optic neuropathy. Endocrinology. 152:2775–2785.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lv P, Huang J, Yang J, Deng Y, Xu J, Zhang
X, Li W, Zhang H and Yang Y: Autophagy in muscle of
glucose-infusion hyperglycemia rats and streptozotocin-induced
hyperglycemia rats via selective activation of m-TOR or FoxO3. PLoS
One. 9:e872542014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia L, Lei Z, Shi Z, Guo D, Su H, Ruan Y
and Xu ZC: Enhanced autophagy signaling in diabetic rats with
ischemia-induced seizures. Brain Res. 1643:18–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fekadu J and Rami A: Beclin-1 deficiency
alters autophagosome formation, lysosome biogenesis and enhances
neuronal vulnerability of HT22 hippocampal cells. Mol Neurobiol.
53:5500–5509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma LY, Lv YL, Huo K, Liu J, Shang SH, Fei
YL, Li YB, Zhao BY, Wei M, Deng YN and Qu QM: Autophagy-lysosome
dysfunction is involved in Aβ deposition in STZ-induced diabetic
rats. Behav Brain Res. 320:484–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Piano I, Novelli E, Della Santina L,
Strettoi E, Cervetto L and Gargini C: Involvement of autophagic
pathway in the progression of retinal degeneration in a mouse model
of diabetes. Front Cell Neurosci. 10:422016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pereira GJ, Tressoldi N, Hirata H,
Bincoletto C and Smaili SS: Autophagy as a neuroprotective
mechanism against 3-nitropropionic acid-induced murine astrocyte
cell death. Neurochem Res. 38:2418–2426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Xu XB, You WW, Lin XX, Li CT, Qian
HR, Zhang LH and Yang Y: The cytoplasmic nuclear shuttling of
Beclin 1 in neurons with Alzheimer's disease-like injury. Neurosci
Lett. 661:63–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiao L, Fu J, Xue X, Shi Y, Yao L, Huang
W, Li J, Zhang D, Liu N, Tong X, et al: Neuronalinjury and roles of
apoptosis and autophagy in a neonatal rat model of
hypoxia-ischemia-induced periventricular leukomalacia. Mol Med Rep.
17:5940–5949. 2018.PubMed/NCBI
|
|
36
|
Pickford F, Masliah E, Britschgi M, Lucin
K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E,
Levine B and Wyss-Coray T: The autophagy-related protein beclin 1
shows reduced expression in early Alzheimer disease and regulates
amyloid beta accumulation in mice. J Clin Invest. 118:2190–2199.
2008.PubMed/NCBI
|