Introduction
Obesity is a global health problem associated with
various metabolic disorders, including diabetes, hypertension,
cardiovascular disease, and depression (1). There are two major types of
medication used to treat obese patients (2). Some anti-obesity drugs, such as
appetite suppressants, reduce food intake by regulating the
function of the central nervous system, whereas other drugs block
absorption of lipids from food in the intestine. In addition, some
candidate anti-obesity drugs directly modulate energy metabolism
without affecting the central nervous system, such as peroxisome
proliferator-activated receptor (PPAR) agonists. However, the
undesirable side effects of currently available agonists
significantly limit their use.
Natural products have been used for millennia to
treat diseases and mitigate the adverse effects of toxic substances
(3). Recently, berberine and
curcumin, which have antioxidant and anti-inflammatory properties,
have been reported to have anti-obesity effects (4–6).
Berberine and curcumin ameliorate obesity by increasing energy
expenditure. Berberine activates AMP-activated protein kinase
(AMPK) (4), a key energy sensor
that leads to reduced energy storage and increased energy
production. In addition, berberine regulates expression of
uncoupling protein 1 (UCP1), which is found in the mitochondria and
generates heat in brown adipose tissue (BAT) and white adipose
tissue (WAT) in an AMPK- and PPAR gamma coactivator-1 alpha
(PGC-1α)-dependent manner (6).
These studies suggest that therapeutic chemicals are involved in
natural products with antioxidant and anti-inflammatory
properties.
Synoviolin (Syvn1), a mammalian homolog of
Hrd1p/Der3p, is involved in the development of obesity, rheumatoid
arthritis, fibrosis, limb girdle muscular dystrophy, and liver
cirrhosis (7–11). Syvn1 was identified in rheumatoid
synovial cells (RSCs) as an endoplasmic reticulum (ER)-resident E3
ubiquitin ligase (7) that plays an
important role in RSC proliferation (12). LS-102, a Syvn1 inhibitor, repressed
proliferation of RSCs in a Syvn1-dependent manner (13). We recently demonstrated that global
elimination of Syvn1 in post-neonatal mice was associated
with weight loss and reduced white adipose tissue (14). Adipose tissue from Syvn1
knockout mice showed significant up-regulation of PGC-1β-target
genes, as well as a significant increase in the number of
mitochondria, mitochondrial respiration, and basal energy
expenditure. Syvn1 interacts with PGC-1β and negatively regulates
its function. Therefore, we propose that knockout or inhibition of
Syvn1 leads to stabilization of PGC-1β, enhancing energy
expenditure. These results suggest that Syvn1 is a therapeutic
target for anti-obesity drugs. However, natural products that
inhibit Syvn1 activity have not been found.
To identify Syvn1 inhibitors with antioxidant and
anti-inflammatory properties in this study, we performed a
screening of natural products based on their inhibitory effects on
RSC proliferation. We found that walnut extract inhibited Syvn1
activity, indicating that walnut extract could be used to treat
patients with obesity.
Materials and methods
Ethical considerations
All human experimental protocols in the present
study (no. 2728, 2729, 3758, 3759) were approved by the Ethics
Review Committee of Tokyo Medical University (Tokyo, Japan). RA
patients received stable doses of methotrexate (6–10 mg/week)
before joint replacement surgery. Written informed consent was
obtained from all patients prior to the collection of joint tissue
samples. All procedures involving animals were performed in
accordance with institutional and national guidelines for animal
experimentation, and were approved by the Institutional Animal Care
and Use Committee of Tokyo Medical University (no. S-28038,
S-28040).
Mice
Mice were kept in SPF under conditions (20–26°C
temperature; 40–65% humidity) on a 12 h light/12 h dark cycle. F-1
Foods (5.1% fat, 21.3% protein) were purchased from Funabashi farm
(Chiba, Japan). Mice had free access to water bottles. Tamoxifen
(Tam)-inducible Syvn1 knockout mice (CAG-Cre-ER;
Syvn1flox/flox) was generated previously
(14). To isolate MEFs, embryos
were isolated at E13.5, and the head and internal (including
reproductive) organs were removed. The remaining tissue was
physically dissociated and incubated in trypsin at 37°C for 15 min.
Cells were resuspended in DMEM and plate the cells in 10 cm tissue
culture dishes. On the next day, medium was changed and cells were
expanded for two passages before freezing.
Plasmids, antibodies, and walnut
extract
The Syvn1 (NM_032431) and PGC-1β (NM_133249)
plasmids are described in the literature (7,14,15).
The following antibodies were used: Anti-HA (3F10) (Roche Molecular
Biochemicals, Indianapolis, IN, USA). Polyclonal antiserum against
GST was generated by immunizing rats with purified GST. Anti-PGC-1β
antibody has been described previously (14). Walnut extracts were prepared from
the branches of walnut trees by the standard ethanol extraction
method (16). Briefly, the
air-dried walnut branches were milled into fine powder in the
blender and the fibrous powder of walnut branches was extracted
twice, on each occasion with 60% ethyl alcohol at room temperature
for 24 h. The combined ethanol extract was filtered, and the
filtrate was concentrated to dryness under reduced pressure in a
rotary evaporator. The ethanol extract was freeze-dried. Without
any further purification, the plant crude ethanol extract was used
in our study. Aliquot portions of the ethanol extract was dissolved
in DMSO for use of our experiments.
Cell culture and assessment of cell
proliferation
Rheumatoid synovial cells were obtained by standard
methods (17). Briefly, the tissue
was minced into small pieces and digested with collagenase
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The single-cell
suspension was incubated overnight, and then floating cells were
removed, and adherent cells were cultured in dishes. RSCs and MEFs,
which were derived from Tam-inducible Syvn1 knockout mice
(CAG-Cre-ER; Syvn1flox/flox), were
cultured in Dulbecco's modified Eagle's medium (DMEM) as previously
described (14). RSC proliferation
was measured with DMSO or walnut extract treatment (1, 3.3, 10,
33.3 µg/ml) for 3 days using the Cell Counting Kit-8 (Dojindo,
Tokyo, Japan). MEFs were treated with DMSO or Tamoxifen (2.5 µM)
for 2 days, and were then treated with DMSO or walnut extract (50
µg/ml) for 3 days. Electron microscopic analysis was then
performed.
GST pull-down assay
The GST pull-down assay was performed as previously
described (14,18). Briefly, GST-Syvn1ΔTM and MBP-PGC-1β
(1–367) were expressed and purified using glutathione sepharose
beads and amylose beads, respectively (GE Healthcare Life Sciences,
Little Chalfont, UK). GST-Syvn1ΔTM was incubated with MBP-PGC-1β
bound to resin in 1 ml buffer A (20 mM Tris-HCl, pH 8.0; 100 mM
NaCl; 1 mM ethylenediaminetetraacetic acid (EDTA); 1 mM
dithiothreitol (DTT); 0.1% Nonidet P-40 (NP-40); 5% glycerol; 1 mM
Na3VO4; 5 mM NaF; 1 µg/ml aprotinin; and 1
µg/ml leupeptin) for 4 h at 4°C. After washing the beads with
buffer A, bound proteins were fractionated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by
western blotting.
In vitro ubiquitination assay
In vitro ubiquitination assays were performed
as previously described (14).
Briefly, GST-PGC-1β (1–367) was incubated with 0.75 µg HA-Ub, 125
ng E1 (Biomol International, Plymouth Meeting, PA, USA), 150 ng
UbcH5c, and 150 ng MBP-Syvn1ΔTM in reaction buffer (50 mM Tris-HCl,
pH 7.5; 5 mM MgCl2; 0.6 mM DTT; and 2 mM ATP) at 37°C
for 2 h. Glutathione sepharose was added to the solution, after
which the mixture was washed with GST wash buffer (50 mM Tris-HCl,
pH 7.5; 0.5 M NaCl; 1% Triton X-100; 1 mM EDTA; 1 mM DTT; and
protease inhibitors). Ubiquitinated PGC-1β was analyzed by western
blotting using anti-PGC-1β antibodies.
Ubiquitination assay
In vivo ubiquitination assays were performed
as previously described (14).
Briefly, 293T cells were transfected with HA-PGC-1β, FLAG Ub, or
Syvn1 expression plasmids. Cells were treated with DMSO or walnut
extract (50 µg/ml) for 3 days. Cells were lysed in lysis buffer (50
mM HEPES, pH 7.9; 150 mM KCl; 1 mM phenylmethanesulfonyl fluoride,
1% Triton X-100; 10% glycerol; and protease inhibitors). Lysates
were mixed with 1 µg anti-HA antibody conjugated to protein
G-sepharose beads. After a 4-h incubation at 4°C, beads were washed
three times with lysis buffer. Bound proteins were fractionated by
SDS-PAGE and analyzed by immunoblotting.
MitoTracker staining
For analysis of mitochondria using MitoTracker Red
(Molecular Probes, Eugene, OR, USA), MEFs were treated with DMSO or
walnut extract (50 µg/ml) for 3 days. Mitochondria were stained
with the MitoTracker Red probe for 30 min at 37°C according to the
manufacturer's protocol. Nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI). The intensity of staining by
mitotracker was measured (n=8).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Tam-inducible Syvn1 knockout MEFs were treated with
DMSO (WT MEFs) or Tam (Syvn1 knockout MEFs) for 2 days, and then WT
MEFs and Syvn1 knockout MEFs were treated with DMSO or walnut
extract (50 µg/ml) for 3 days. Total RNA from MEFs treated with
DMSO or walnut extract was purified by using ISOGEN (Nippon Gene,
Tokyo, Japan) according to the manufacturer's instructions and
reverse transcribed by using ReverTra Ace with random primers
(Toyobo, Osaka, Japan). RT-qPCR was performed by using LightCycler
480 Probes Master (Roche Diagnostics, Mannheim, Germany) and the
Step One Plus Detection System (Applied Biosystems; Life
Technologies Japan, Tokyo, Japan). The thermocycling conditions
were as follows: Initial denaturation at 95°C for 10 min, followed
by 45 cycles of denaturation at 95°C for 10 sec, annealing at 60°C
for 20 sec and extension at 72°C for 1 sec. Expression levels were
determined relative to that of 18sRNA (RSCs) or ACTB
(MEFs). Primers and probes used in the present study are shown in
Table I. Relative expression was
determined using the 2−∆∆Cq method (19).
 | Table I.Primers and probes for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers and probes for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Type | Primer (5′-3′) | Probe no. |
|---|
| SYVN1 | Forward |
ccagtacctcaccgtgctg | 16 |
|
| Reverse |
tctgagctagggatgctggt |
|
| 18sRNA | Forward |
gcaattattccccatgaacg | 48 |
|
| Reverse |
gggacttaatcaacgcaagc |
|
| MCAD | Forward |
tcttgctggaaatgatcaaca | 88 |
|
| Reverse |
gggctctgtcacacagtaagc |
|
| Atp5b | Forward |
tgagagaggtcctatcaaaacca | 15 |
|
| Reverse |
cctttatcccagtcaccagaa |
|
| ACTB | Forward |
ctaaggccaaccgtgaaaag | 64 |
|
| Reverse |
accagaggcatacagggaca |
|
RNA interference assay
siRNAs for Syvn1 were previously described (14). Transfection with siRNAs (20 µM) was
performed by using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Total RNA from RSCs was purified 2 days after
transfection using ISOGEN (Nippon Gene, Tokyo, Japan) according to
the manufacturer's instructions, and reverse transcribed using
ReverTra Ace with random primers (Toyobo).
Statistical analysis
All data are expressed as the mean ± standard
deviation and were analyzed using Excel Statistics 2012 version
1.00 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Differences between two groups were examined by Student's t-test.
One-way analysis of variance with Tukey-Kramer post hoc analysis
was used to determine correlations in datasets containing multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Screening of natural products for
Syvn1 inhibitors
Syvn1 is a crucial factor involved in RSC
proliferation (7,13,20).
To identify Syvn1 inhibitors in natural products, we tested the
effects of natural products on RSC proliferation with or without
Syvn1. At first, we performed knockdown experiments with control
siRNA (siControl) or siRNA for Syvn1 (siSyvn1). RT-qPCR showed that
siSyvn1 induced 60% repression of Syvn1 expression (Fig. 1A). Walnut extract inhibited
proliferation in a concentration-dependent manner in two RSC lines
treated with siControl (Fig. 1B).
Whereas, the inhibitory effect was attenuated in siSyvn1-treated
cells (Fig. 1B). In the case of
patient 1, walnut extract did not significantly have any effect
(n.s.). In the case of patient 2, walnut extract had still
inhibited cell growth, however, the strength of the effect was
reduced as compared to control siRNA-treated cells.
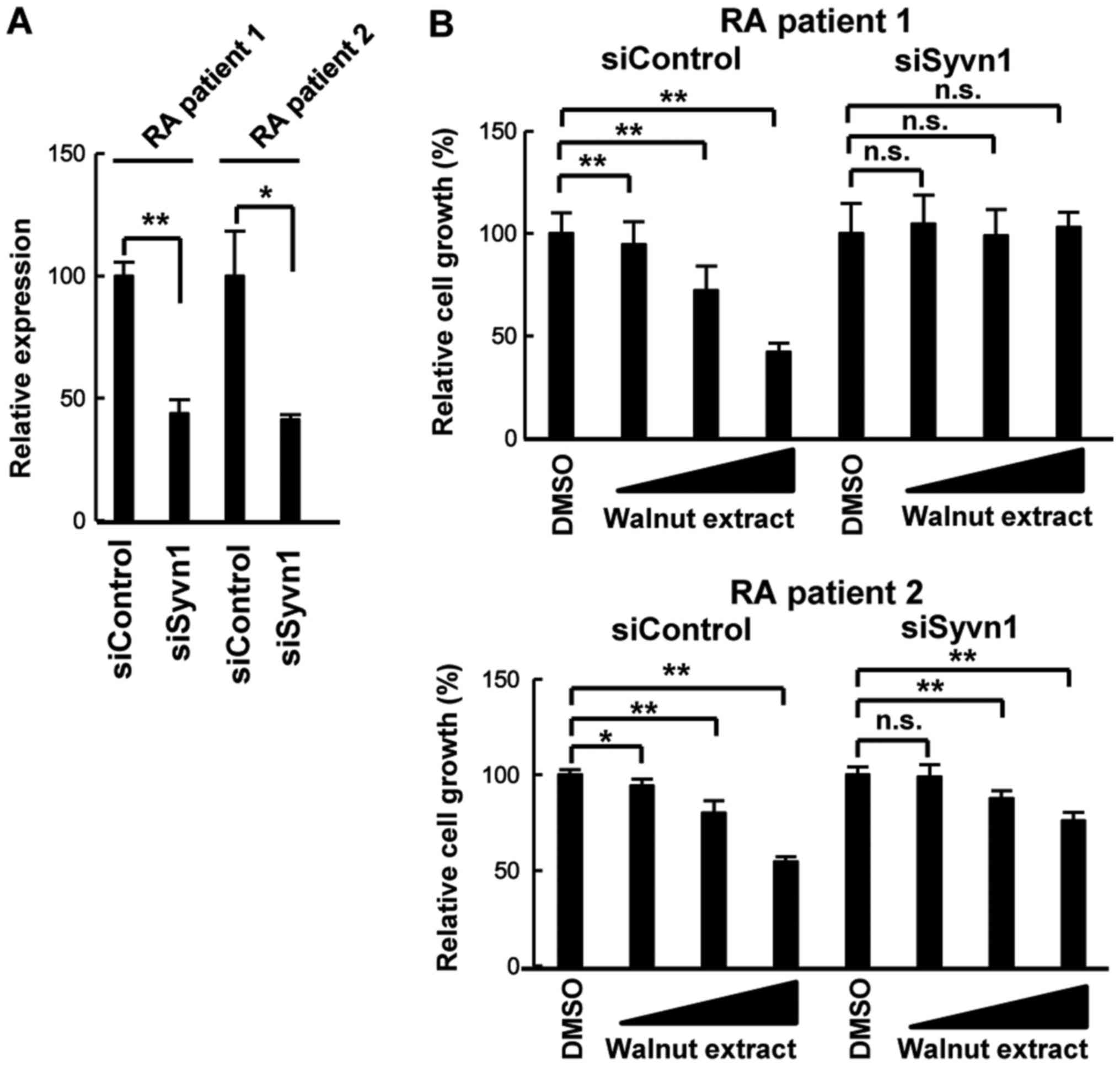 | Figure 1.Effect of walnut extract on RSC
growth. (A) Effect of Syvn1 knockdown by siRNA. RSCs derived from
two patients with RA were transiently transfected with control
siRNA (siControl) or siRNA for Syvn1 (siSyvn1). Following 2 days,
total RNA were purified and reverse transcription-quantitative
polymerase chain reaction was performed. Individual measurements
were standardized using 18S RNA, and the average for siControl was
set to 100. (B) RSCs were transiently transfected with siControl or
siSyvn1. Following 2 days, RSCs were treated with walnut extract
(1, 3.3, and 10 µg/ml) for 3 days. Data are presented as the mean ±
standard deviation (n=3). *P<0.05 and **P<0.01, as indicated.
n.s., not significant; DMSO, dimethyl sulfoxide; RSC, rheumatoid
synovial cell; RA, rheumatoid arthritis; si-/siRNA, small
interfering RNA; Syvn1, Synoviolin. |
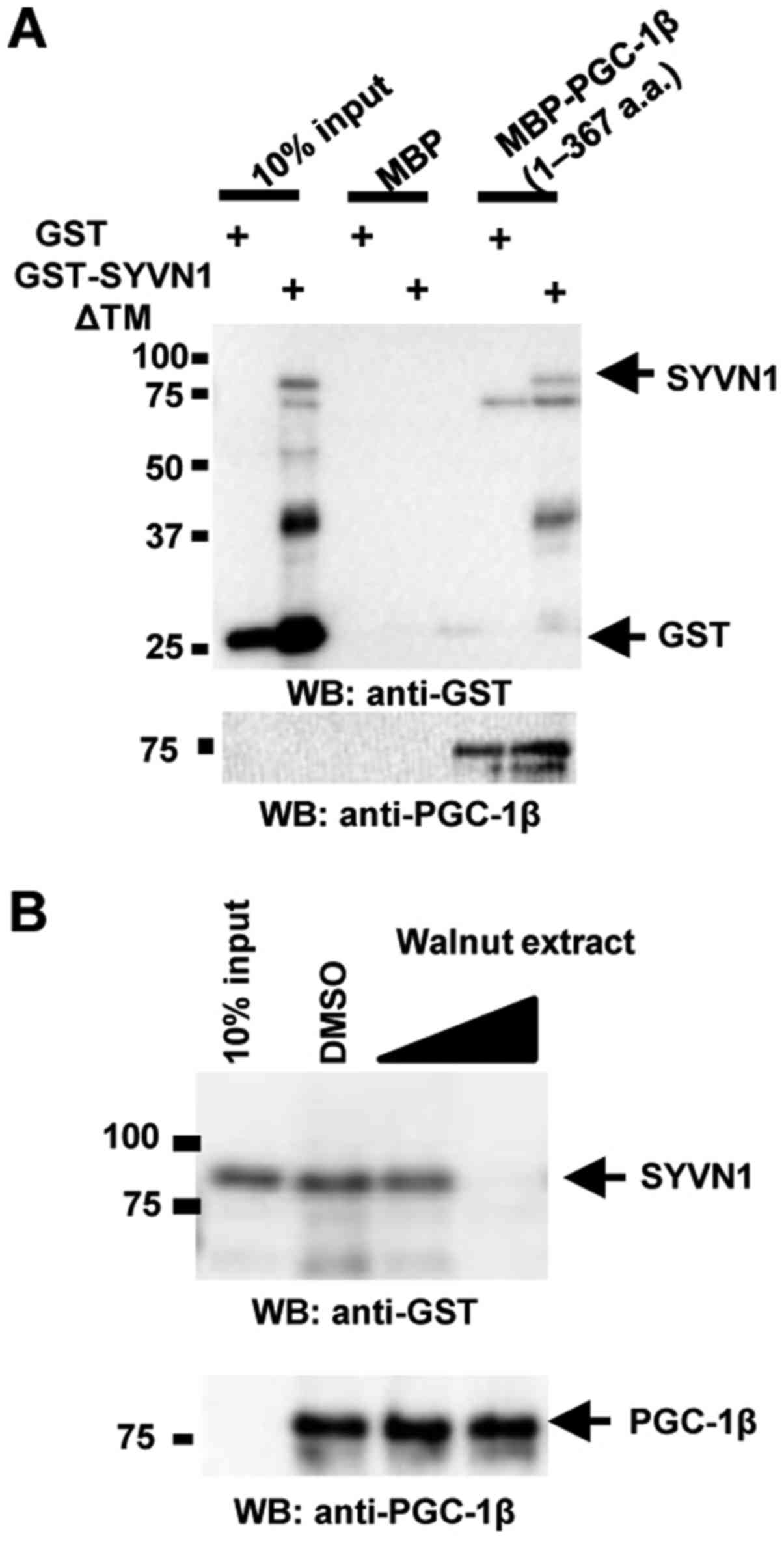
Regulation of Syvn1-PGC-1β interaction
by walnut extract
Syvn1 negatively regulates PGC-1β activity via
direct interaction with PGC-1β in vitro and in vivo
(14). To determine whether walnut
extract inhibits the interaction of Syvn1 with PGC-1β, we performed
in vitro binding assays using glutathione
S-transferase-tagged Syvn1 lacking the transmembrane domain
(GST-Syvn1ΔTM) and maltose binding protein-tagged PGC-1β (amino
acids 1–367) (MBP-PGC-1β (1–367)). As previously reported (14), MBP-PGC-1β (1–367) directly bound to
GST-Syvn1ΔTM (Fig. 2A). MBP-PGC-1β
(1–367) did not bind to GST, and GST-Syvn1ΔTM did not bind to MBP
(Fig. 2A). Walnut extract
inhibited the interaction of Syvn1 and PGC-1β in a
concentration-dependent manner (Fig.
2B).
Inhibition of PGC-1β ubiquitination by
walnut extract
Syvn1 ubiquitinates PGC-1β in vitro and in
vivo, negatively regulating PGC-1β abundance (14). To investigate ubiquitination of
PGC-1β by Syvn1 in the presence of walnut extract, we performed an
in vitro assay of ubiquitination with MBP-Syvn1ΔTM and
GST-PGC-1β (1–367) in the presence of ATP, hemagglutinin-tagged
ubiquitin (HA-Ub), E1, and E2 (UbcH5c) (14). As previously reported (14), Syvn1 induced polyubiquitination of
PGC-1β in vitro, and polyubiquitination of PGC-1β was not
observed in the absence of ATP or Syvn1. Walnut extract inhibited
PGC-1β polyubiquitination (Fig.
3A). To examine the effect of walnut extract in vivo, we
performed in vivo ubiquitination assay. FLAG-tagged Ub and
HA-PGC-1β were coexpressed with Syvn1 in HEK 293T cells and cells
were treated with DMSO or walnut extract (50 µg/ml) for 3 days. The
ubiquitination of PGC-1β was observed in Syvn1-expressing cells
(DMSO-treated cells). The treatment with walnut extract decreased
the ubiquitination of PGC-1β in Syvn1-expressing cells (Fig. 3B). Walnut extract did not inhibit
autoubiquitination of Syvn1 (Fig.
3C). The effect of walnut extract was also examined in
vivo. FLAG-tagged Ub (FLAG Ub) and Syvn1/HA were coexpressed in
HEK 293T cells and cells were treated with DMSO or walnut extract
(50 µg/ml) for 3 days. Autoubiquitination of Syvn1 was not
inhibited in walnut extract-treated cells (Fig. 3D).
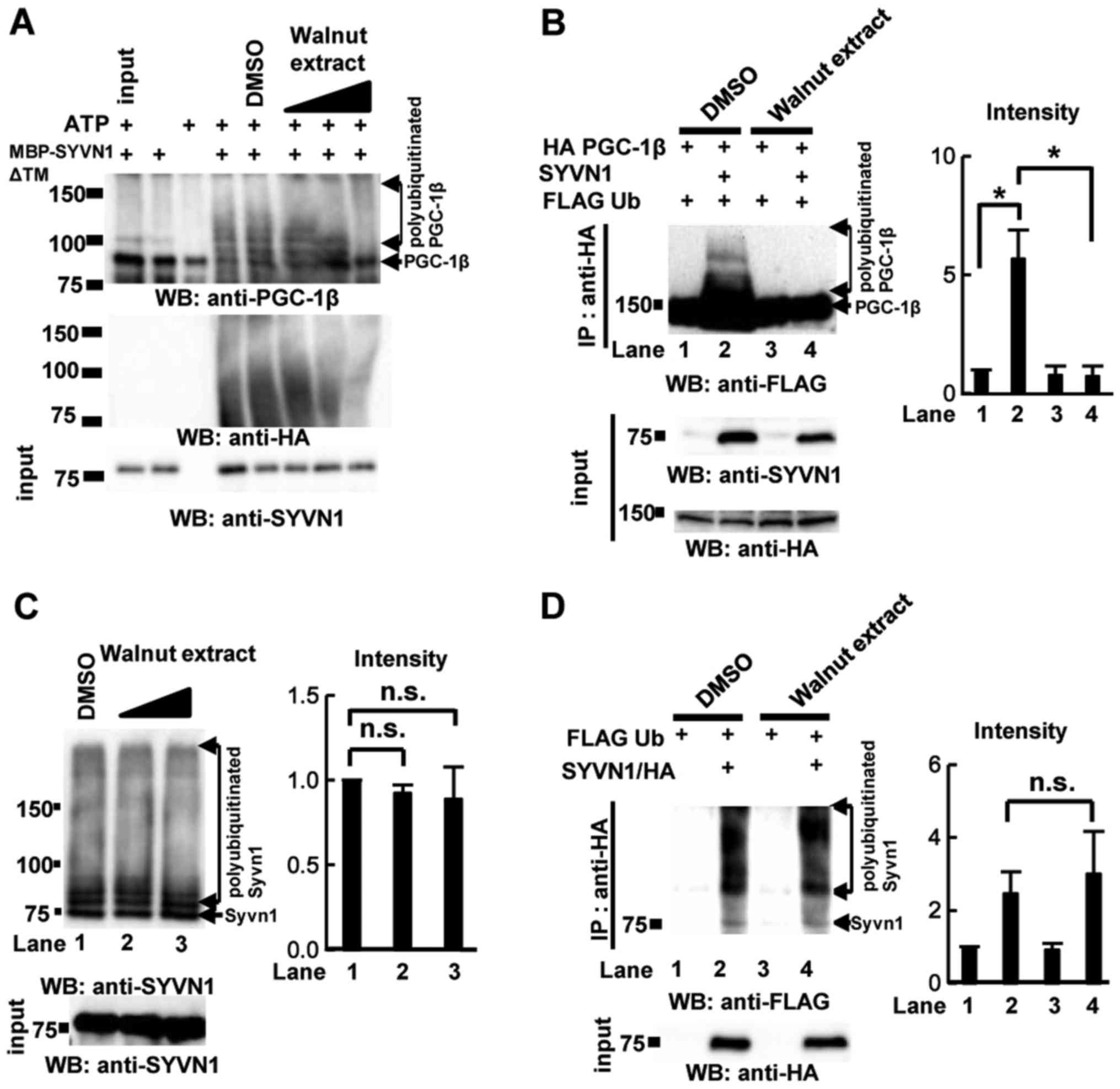 | Figure 3.Effect of walnut extract on the
polyubiquitination of PGC-1β. (A) In vitro ubiquitination
assays were performed with MBP-SYVN1ΔTM, GST-PGC-1β (1–367), E1 and
E2 enzymes, and HA-Ub in the presence of DMSO or walnut extract
(0.5, 5 or 50 µg/ml). WB was performed using anti-PGC-1β antibodies
and anti-SYVN1 antibodies. (B) In vivo ubiquitination assays
were performed. 293T cells were transfected with HA PGC-1β, FLAG Ub
and/or SYVN1 expression plasmids and cells were treated with DMSO
and walnut extract (50 µg/ml) for 3 days. Whole cell extracts were
immunoprecipitated with anti-HA antibody. WB was performed using
anti-FLAG and anti-HA antibodies. Quantification of the data is
presented. *P<0.05, as indicated. (C) Effect of walnut extract
on SYVN1 autoubiquitination. In vitro ubiquitination assays
were performed with GST-SYVN1ΔTM, E1 and E2 enzymes, and HA-Ub in
the presence of DMSO or walnut extract (0.5, 5 or 50 µg/ml). WB was
performed using anti-SYVN1 antibodies. Quantification of the data
is presented. (D) In vivo ubiquitination assays were
performed. 293T cells were transfected with FLAG Ub, and SYVN1/HA
expression plasmids and cells were treated with DMSO and walnut
extract (50 µg/ml) for 3 days. Whole cell extracts were
immunoprecipitated with anti-HA antibody. WB was performed using
anti-FLAG and anti-HA antibodies. Quantification of the data is
presented. The positions of molecular weight standards (in kDa) are
indicated to the left of each image. Data are expressed as the mean
± standard deviation (n=3). HA, hemagglutinin; Ub, ubiquitin; DMSO,
dimethyl sulfoxide; WB, western blotting; Syvn1, Synoviolin;
PGC-1β, PGC-1β, peroxisome proliferator-activated receptor
coactivator 1β; ATP, mitochondrial adenosine triphosphate synthase;
GST, glutathione S-transferase; TM, transmembrane domain; MBP,
maltose binding protein; n.s., not significant. |
Effects of walnut extract on PGC-1β
function
PGC-1β is a coactivator of several transcription
factors, including PPARα, and is implicated in various biological
processes, including mitochondrial biogenesis (21,22).
LS-102 exposure increases the number of mitochondria in cultured
cells (14). To investigate the
effect of walnut extract on regulation of mitochondria by PGC-1β,
we performed mitochondrial staining using MitoTracker with mouse
embryonic fibroblasts (MEFs) (14). MitoTracker staining showed
increased mitochondria in MEFs treated with walnut extract compared
with MEFs treated with DMSO (Fig.
4A). We used electron microscopy and counted mitochondria. The
cells treated with walnut extract had significantly more
mitochondria than the cells treated with dimethyl sulfoxide (DMSO)
did (Fig. 4B and C). In addition,
the number of mitochondria in Syvn1 knockout MEFs treated with DMSO
(KO+DMSO) increased compared to that in wildtype MEFs treated with
DMSO. However, walnut extract produced no additional effect on the
number of mitochondria in the Syvn1 KO MEFs (KO+Walnut extract).
Furthermore, the expression of PGC-1β target genes, medium chain
acyl-coenzyme A dehydrogenase (MCAD) and mitochondrial ATP synthase
β subunit (ATP5b), was also induced in MEFs treated with walnut
extract and in Syvn1 KO MEFs treated with DMSO (Fig. 4D). However, walnut extract produced
no additional effect on the induction of MCAD and ATP5b in the
Syvn1 KO MEFs (Fig. 4D).
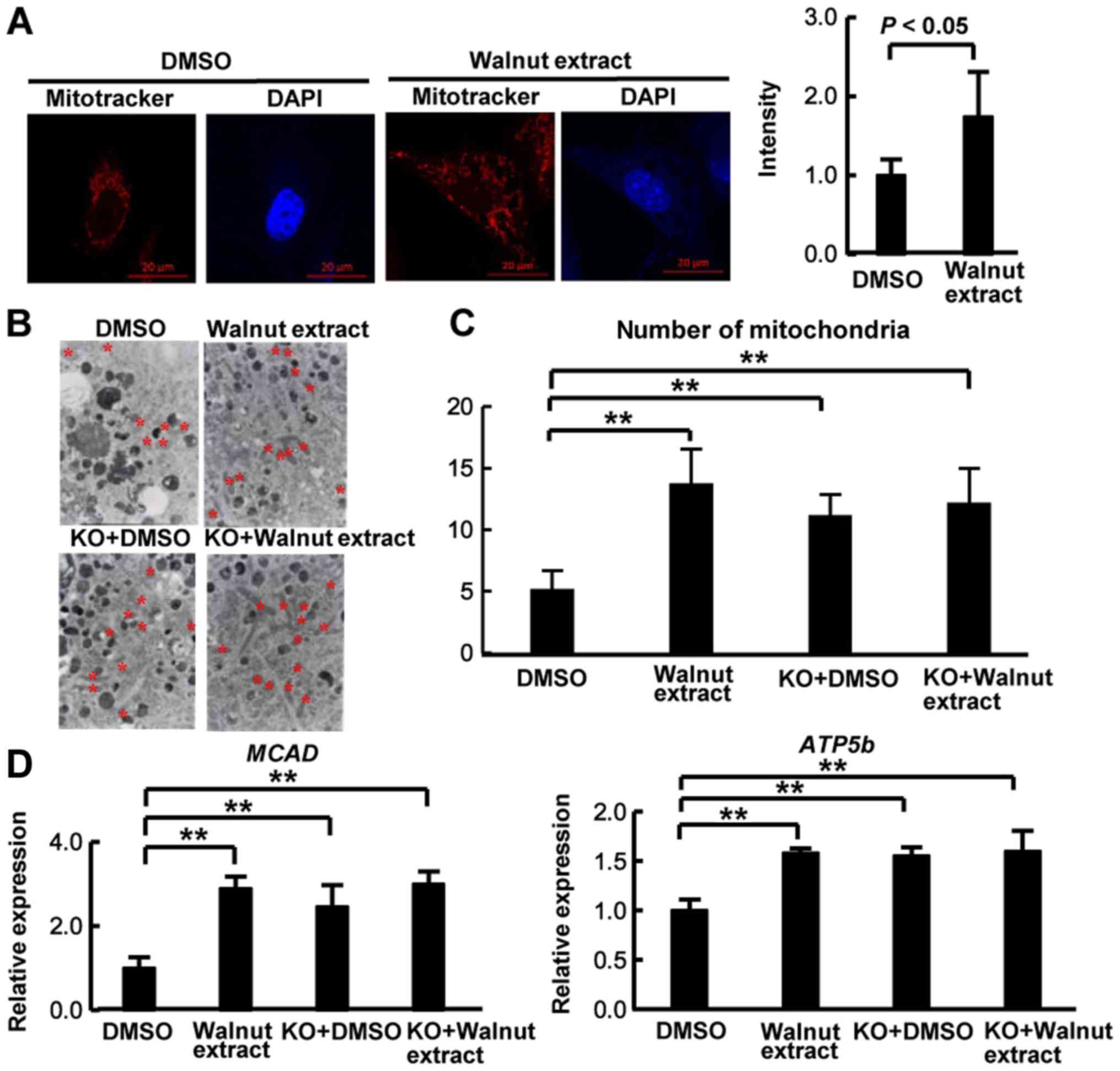 | Figure 4.Effect of walnut extract on the
number of mitochondria. (A) MEFs were treated with DMSO or walnut
extract (50 µg/ml) for 3 days. Cells were immunostained with
MitoTracker Red (red), and DAPI (nuclei, blue); scale bars, 20 µm.
The intensity of staining was measured by MitoTracker. Data were
expressed as the mean ± standard deviation (n=8). (B)
Representative electron micrographs of walnut extract-treated cells
(50 µg/m) are presented. Increased mitochondrial volume can be
observed in the large cytoplasmic areas in MEFs (mitochondria are
indicated by red asterisks). Magnification, ×10,000. (C) The number
of mitochondria in the area (1,000×1,000 pixels) was measured. Data
were expressed as the mean ± standard deviation (n=13). (D) Total
RNA was isolated from wild-type MEFs and Syvn1 knockout MEFs
treated with DMSO or walnut extract, and reverse
transcription-quantitative polymerase chain reaction was performed.
Individual measurements were standardized using β-actin, and then
the average DMSO value was set to 1. Data were expressed as the
mean ± standard deviation (n=6). **P<0.01, as indicated. MEFs,
mouse embryonic fibroblasts; DMSO, dimethyl sulfoxide; Syvn1,
Synoviolin; KO, Syvn1 knockout mice; MCAD, medium chain
acyl-coenzyme A dehydrogenase; ATP5b, mitochondrial adenosine
triphosphate synthase β subunit. |
Discussion
The development of Syvn1 inhibitors is an active
field of study because they have the potential to treat patients
with several diseases, including rheumatoid arthritis, fibrosis,
liver cirrhosis, and obesity (7–10,13).
In a previous study, we demonstrated that the Syvn1 inhibitor,
LS102, suppressed weight gain in a mouse model of obesity via
inhibition of PGC-1β polyubiquitination by Syvn1 (14). In this study, we showed that walnut
extract, a natural product, inhibits Syvn1 activity. Walnut extract
inhibited the interaction between Syvn1 and PGC-1β and repressed
polyubiquitination of PGC-1β by Syvn1. Taken together, these
results suggest that walnut extract has anti-obesity activity.
Selectivity and specificity are important
characteristics for targeted drugs. We identified LS-102 as an
inhibitor of autoubiquitination of Syvn1 via a high-throughput
screening (13) and demonstrated
its inhibitory effect on the E3 ligase activity of Syvn1. LS-102
suppressed polyubiquitination of target proteins of Syvn1,
including nuclear factor erythroid 2-related factor 2 (NRF2), V247M
α-sarcoglycan mutant, and PGC-1β (10,11,13).
Interestingly, Syvn1 interacts with NRF2 and V247M α-sarcoglycan
mutant through proline-rich domains at the C-terminus, whereas
Syvn1 binds to PGC-1β via the Syvn1 unique (SyU) domain (10,11,13,14).
Walnut extract did not have an inhibitory effect on
autoubiquitination of Syvn1. However, walnut extract decreased
polyubiquitination of PGC-1β by inhibiting the interaction of Syvn1
and PGC-1β. Therefore, walnut extract may specifically target the
SyU domain of Syvn1. These results indicate that walnut extract
might improve obesity by selectively inhibiting the interaction of
Syvn1 and PGC-1β.
The mitochondrion is an important organelle involved
in cellular energy control that has been reported to be involved in
the process of obesity and chronic inflammation (23,24).
PGC-1β plays an important role in mitochondrial biogenesis and
energy metabolism, including β-oxidation of fatty acids (25). Overexpression of PGC-1β results in
increased numbers of mitochondria and increased mitochondrial
respiratory function (26). PGC-1β
transgenic mice show high energy expenditure and resistance to
obesity (27). In addition, PGC-1β
attenuated inflammation. PGC-1β diminishes the increase in
proinflammatory mediators, such as interleukin-6 (IL-6) and
macrophage inflammatory protein 1-alpha (MIP1α), by repressing the
activity of nuclear factor-κB (NF-κB) (28). These studies indicate that PGC-1β
has anti-obesity and anti-inflammatory properties. In this study,
we found that walnut extract inhibited the negative regulation of
PGC-1β activity by Syvn1, suggesting that walnut extract activates
PGC-1β. Our results suggest that walnut extract may attenuate not
only obesity, but also diseases involving chronic inflammation.
Further analysis with disease state model will be need to determine
whether walnut extract will be helpful in several disease with
chronic inflammation. Future studies will be aimed at identifying
the bioactive constituents in walnut extract that are responsible
for its inhibitory effect on Syvn1.
Acknowledgements
The authors thank Mr. S. Shibata (Tokyo Medical
University, Tokyo, Japan) for their technical assistance. The
authors would also like to thank all of the members of Dr.
Nakajima's laboratory and Dr. Khin Thuzar Wynn (Yangon Speciality
Hospital, Yangon, Myanmar).
Funding
The present study was funded in part by grants from
the Naito Foundation, Natural Science Scholarship Daiichi-Sankyo
Foundation of Life Science, Mitsubishi, Tanabe Pharma Corporation,
Bureau of Social Welfare and Public Health, Academic Contribution
of Pfizer, Eisai, Santen Pharmaceutical, Abbvie, Takeda Science
Foundation, AstraZeneca (R&D Grant 2013) and ONO Medical
Research Foundation. The present study was also supported partly by
funds provided through a MEXT-Supported Program of the Strategic
Research Foundation at Private Universities (grant no. S1411011;
2014–2018) from the Ministry of Education, Culture, Sports, Science
and Technology of Japan, as well as the Japan Society for the
Promotion of Science KAKENHI (grant nos. 23659176, 26670479,
26461478 and 16H05157) and Industry-University Cooperation
(BioMimetics Sympathies Inc.).
Availability of data and materials
All data analyzed in this study are included in this
article.
Authors' contributions
HF, SA, KN, NY and TN conceived the project and
designed the experiments. HF, SA and TN performed the experiments
and analyzed the data. HF and TN wrote the manuscript. All authors
discussed the results and commented on the manuscript.
Ethics approval and consent to
participate
All human experimental protocols in the present
study (nos. 2728 and 2729, 3758, 3759) were approved by the Ethics
Review Committee of Tokyo Medical University (Tokyo, Japan).
Written informed consent was obtained from all of the patients
prior to the collection of joint tissue samples.
Patient consent for publication
Consent for publication was obtained from all of the
patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Syvn1
|
synoviolin
|
|
PGC-1β
|
peroxisome proliferator-activated
receptor coactivator 1β
|
|
RSCs
|
rheumatoid synovial cells
|
|
SPF
|
specific pathogen-free
|
References
|
1
|
Tsai AG, Williamson DF and Glick HA:
Direct medical cost of overweight and obesity in the USA: A
quantitative systematic review. Obes Rev. 12:50–61. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gautron L, Elmquist JK and Williams KW:
Neural control of energy balance: Translating circuits to
therapies. Cell. 161:133–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Y, Xun K, Wang Y and Chen X: A
systematic review of the anticancer properties of berberine, a
natural product from Chinese herbs. Anticancer Drugs. 20:757–769.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ,
Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al: Berberine, a natural
plant product, activates AMP-activated protein kinase with
beneficial metabolic effects in diabetic and insulin-resistant
states. Diabetes. 55:2256–2264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ejaz A, Wu D, Kwan P and Meydani M:
Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and
angiogenesis and obesity in C57/BL mice. J Nutr. 139:919–925. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Zhang H, Li B, Meng X, Wang J,
Zhang Y, Yao S, Ma Q, Jin L, Yang J, et al: Berberine activates
thermogenesis in white and brown adipose tissue. Nat Commun.
5:54932014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amano T, Yamasaki S, Yagishita N,
Tsuchimochi K, Shin H, Kawahara K, Aratani S, Fujita H, Zhang L,
Ikeda R, et al: Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel
pathogenic factor for arthropathy. Genes Dev. 17:2436–2449. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasegawa D, Fujii R, Yagishita N,
Matsumoto N, Aratani S, Izumi T, Azakami K, Nakazawa M, Fujita H,
Sato T, et al: E3 ubiquitin ligase synoviolin is involved in liver
fibrogenesis. PLoS One. 5:e135902010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Shen Y, Ding Y, Liu Y, Su D and
Liang X: Hrd1 participates in the regulation of collagen I
synthesis in renal fibrosis. Mol Cell Biochem. 386:35–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu T, Zhao F, Gao B, Tan C, Yagishita N,
Nakajima T, Wong PK, Chapman E, Fang D and Zhang DD: Hrd1
suppresses Nrf2-mediated cellular protection during liver
cirrhosis. Genes Dev. 28:708–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bianchini E, Fanin M, Mamchaoui K, Betto R
and Sandona D: Unveiling the degradative route of the V247M
α-sarcoglycan mutant responsible for LGMD-2D. Hum Mol Genet.
23:3746–3758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki S, Yagishita N, Tsuchimochi K,
Nishioka K and Nakajima T: Rheumatoid arthritis as a
hyper-endoplasmic-reticulum-associated degradation disease.
Arthritis Res Ther. 7:181–186. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yagishita N, Aratani S, Leach C, Amano T,
Yamano Y, Nakatani K, Nishioka K and Nakajima T: RING-finger type
E3 ubiquitin ligase inhibitors as novel candidates for the
treatment of rheumatoid arthritis. Int J Mol Med. 30:1281–1286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujita H, Yagishita N, Aratani S,
Saito-Fujita T, Morota S, Yamano Y, Hansson MJ, Inazu M, Kokuba H,
Sudo K, et al: The E3 ligase synoviolin controls body weight and
mitochondrial biogenesis through negative regulation of PGC-1β.
EMBO J. 34:1042–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamasaki S, Yagishita N, Sasaki T,
Nakazawa M, Kato Y, Yamadera T, Bae E, Toriyama S, Ikeda R, Zhang
L, et al: Cytoplasmic destruction of p53 by the endoplasmic
reticulum-resident ubiquitin ligase ‘Synoviolin’. EMBO J.
26:113–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ojewole JA: Analgesic, antiinflammatory
and hypoglycaemic effects of ethanol extract of Zingiber officinale
(Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res.
20:764–772. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakajima T, Aono H, Hasunuma T, Yamamoto
K, Shirai T, Hirohata K and Nishioka K: Apoptosis and functional
Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum.
38:485–491. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita H, Fujii R, Aratani S, Amano T,
Fukamizu A and Nakajima T: Antithetic effects of MBD2a on gene
regulation. Mol Cell Biol. 23:2645–2657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakajima T, Aono H, Hasunuma T, Yamamoto
K, Maruyama I, Nosaka T, Hatanaka M and Nishioka K: Overgrowth of
human synovial cells driven by the human T cell leukemia virus type
I tax gene. J Clin Invest. 92:186–193. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scarpulla RC: Transcriptional paradigms in
mammalian mitochondrial biogenesis and function. Physiol Rev.
88:611–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C and Lin JD: PGC-1 coactivators in
the control of energy metabolism. Acta Biochim Biophys Sin
(Shanghai). 43:248–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bournat JC and Brown CW: Mitochondrial
dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes.
17:446–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Felice FG and Ferreira ST:
Inflammation, defective insulin signaling, and mitochondrial
dysfunction as common molecular denominators connecting type 2
diabetes to Alzheimer disease. Diabetes. 63:2262–2272. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Puigserver P, Wu Z, Park CW, Graves R,
Wright M and Spiegelman BM: A cold-inducible coactivator of nuclear
receptors linked to adaptive thermogenesis. Cell. 92:829–839. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
St-Pierre J, Lin J, Krauss S, Tarr PT,
Yang R, Newgard CB and Spiegelman BM: Bioenergetic analysis of
peroxisome proliferator-activated receptor gamma coactivators
1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol
Chem. 278:26597–26603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamei Y, Ohizumi H, Fujitani Y, Nemoto T,
Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O and Kakizuka A:
PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand,
whose expression induces a high-energy expenditure and antagonizes
obesity. Proc Natl Acad Sci USA. 100:12378–12383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eisele PS, Salatino S, Sobek J, Hottiger
MO and Handschin C: The peroxisome proliferator-activated receptor
γ coactivator 1α/β (PGC-1) coactivators repress the transcriptional
activity of NF-κB in skeletal muscle cells. J Biol Chem.
288:2246–2260. 2013. View Article : Google Scholar : PubMed/NCBI
|