Introduction
Colorectal cancer is the third most common cancer
worldwide (1); the lifetime risk
of developing colorectal cancer is 4.7% for men and 4.4% for women.
Although the mortality rate from colorectal cancer has been
declining for several decades owing to the early diagnosis and
improved treatment, >1 million novel cases are diagnosed each
year. Therefore, it is crucial to identify novel biomarkers and
therapeutic targets for colorectal cancer to improve the prognosis
of the disease.
Sumoylation is a transient post-translational
modification process that is highly regulated by the balance
between enzyme-mediated conjugating and deconjugating activities.
Small ubiquitin-like modifier (SUMO) conjugation involves an
associated enzymatic cascade that includes an E1
ubiquitin-activating enzyme, an E2 ubiquitin-conjugating enzyme and
an E3 ubiquitin ligase (2). It has
been demonstrated that sumoylation is associated with a range of
cellular processes, including cell cycle progression, apoptosis
regulation, maintenance of genome integrity, DNA repair, cell
survival, modulation of subcellular transport and transcription
(3,4). A previous study reported that
sumoylation regulates the cell cycle in glioblastoma by stabilizing
cyclin-dependent kinase (CDK) 6 (5). Other studies have demonstrated the
interaction between CDKs and sumoylation during cell cycle
regulation (6,7). The imbalance between sumoylation and
desumoylation is associated with a variety of diseases, such as
cancer (8–10). For example, sumo-conjugating enzyme
UBC9 is upregulated in a number of types of cancer, including
prostate cancer, breast cancer, advanced melanomas and colon cancer
(11,12). UBA2/SAE2 was reported to promote
the growth of colon tumors in mice (13), and tumor aggressiveness was
associated with increased MDM2 expression induced by the
upregulation of SUMO1 in patients with oral squamous cell carcinoma
(14).
Ubiquitin-like modifier-activating enzyme 2 (UBA2),
also known as sumo-activating enzyme subunit 2 (SAE2), is a
subcomponent of the sumoylated E1 enzyme. The human UBA2 gene is
located on chromosome19q12, which is one of the most important
enzymes that directly affect the levels of sumoylation in the body
(3,15,16).
A number of previous studies have implicated UBA2 in clinical
diseases; for example, haploinsuffiency of the UBA2 gene is
associated with cutis aplasia (17,18).
In addition, UBA2 was reported to form a fusion protein with the
DNA dC>dU-editing enzyme APOBEC3G to prevent the degradation of
the APOBEC3G protein by human immunodeficiency virus (HIV)-viral
infectivity factor (19).
Therefore, UBA2 may serve an inhibitory role in HIV infectivity,
which suggested that UBA2 may exert its biological function by
stabilizing certain proteins. Recent studies have demonstrated that
UBA2 was highly expressed in certain malignant diseases, including
liver cancer (20), small cell
lung cancer (21) and gastric
cancer (22). Another study
reported that the expression levels of UBA2 were different in
metastatic colorectal cancer compared with the primary tumor
(23). However, the function and
clinical significance of UBA2 in colorectal cancer are not
clear.
In the present study, the expression level of UBA2
was examined in patients with colorectal cancer, and UBA2
expression was associated cancer stage. Furthermore, the function
of UBA2 on cell proliferation in vitro and in vivo
was examined. The results of the present study indicated that UBA2
may serve important roles in tumorigenesis and may act as a
potential therapeutic target in colorectal cancer.
Materials and methods
Patients
Colorectal cancer tissues were collected from 237
patients with colorectal cancer in Bethune First Hospital of Jilin
University (Changchun, China) between January 2009 and December
2015; patient clinicopathological characteristics are presented in
Table I. Patients who received
preoperative radiotherapy or chemotherapy and patients with
multiple tumors were excluded from the present study. The
pathological stage of cancer was determined according to the
American Joint Committee on Cancer stage. All tumors were confirmed
by a pathologist and have been collected intraoperatively. The
fresh specimens for molecular analysis were immediately frozen in
liquid nitrogen and stored in −80°C. Specimens for
immunohistochemistry (IHC) were fixed in formalin for 24 h at room
temperature and embedded in paraffin for further experiments. The
present study was approved by the Ethics Review Committee at Jilin
University. All patients were informed their participation rights
and signed the written informed consent.
 | Table I.Clinicopathological characteristics
of patients with colorectal cancer. |
Table I.
Clinicopathological characteristics
of patients with colorectal cancer.
|
| UBA2
expression |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Negative | Positive | Total | P-value |
|---|
| Number of
patients | 35 | 202 | 237 |
|
| Age (years) |
|
|
| 0.110 |
|
<61 | 20 | 86 | 106 |
|
|
≥61 | 15 | 116 | 131 |
|
| Sex |
|
|
| 0.058 |
|
Male | 24 | 98 | 122 |
|
|
Female | 11 | 104 | 115 |
|
| Tumor location |
|
|
| <0.001 |
|
Ascending colon | 6 | 87 | 93 |
|
|
Descending colon | 25 | 112 | 137 |
|
|
Transverse colon | 4 | 3 | 7 |
|
| Histological
grade |
|
|
| <0.001 |
| Well
and moderate | 14 | 177 | 191 |
|
| Poor
and other | 21 | 25 | 46 |
|
| Depth of
invasion |
|
|
| 0.546 |
|
T1-T2 | 2 | 13 | 15 |
|
| T3 | 23 | 148 | 171 |
|
| T4 | 10 | 41 | 51 |
|
| Lymph node
metastasis |
|
|
| 0.056 |
|
Absent | 14 | 116 | 130 |
|
|
Present | 21 | 86 | 107 |
|
| TNM stage |
|
|
| 0.041 |
|
I–II | 23 | 95 | 118 |
|
|
III–IV | 12 | 107 | 119 |
|
Bioinformatic analysis
Analysis of UBA2 mRNA expression levels was
performed using datasets containing 334 colorectal cancer specimens
and 28 normal colorectal specimens were obtained from the Cancer
Genome Atlas via the starBase online database (http://starbase.sysu.edu.cn) (24,25).
Total RNA extraction
Total RNA from ~100 mg tissue and 5×106
cells of 7 colorectal cancer cell lines was extracted using
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according the manufacturer's protocol.
Briefly, the frozen specimens were placed in a microcentrifuge tube
with 1 ml TRIzol and homogenized with a disposable plastic pestle.
The specimens were incubated at room temperature for 5 min, and
subsequently centrifuged at 12,000 × g for 10 min at 4°C. The
supernatants were transferred to a fresh microcentrifuge tube and
200 µl chloroform was added for phase separation. RNA was
precipitated by adding pre-chilled isopropanol. The specimens were
centrifuged at 12,000 × g for 10 min at 4°C and the pellets were
washed with 75% ethanol. The concentrations and purity of the
extracted RNA were measured on a spectrophotometer at 260 and 280
nm wavelengths.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA from frozen colorectal cancer tissues or
cell cultures was reverse transcribed into cDNA using a Reverse
Transcriptase kit (Qiagen, Inc., Valencia, CA, USA) according to
the manufacturer's protocol. UBA2 mRNA expression levels were
measured by qPCR using 12.5 µl SYBR-Green (cat. no. DRR041B; Takara
Biotechnology Co., Ltd., Dalian, China) 1 µl each primer, 2 µl cDNA
and 8.5 µl diethyl pyrocarbonate-treated water. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 15
sec, followed by 40 cycles of 95°C (5 sec) and 60°C (30 sec). The
primers used were as follows: GAPDH, forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′; UBA2, forward, 5′-CACAGGTTGCCAAGGAA-3′
and reverse, 5′-GACACTCATAACACTCGGTCA-3′; GAPDH was used as an
internal control. UBA2 mRNA expression levels were quantified using
the 2−ΔΔCq method and normalized to the expression
levels of GAPDH (26). All
experiments were performed three times in triplicate.
IHC analysis
IHC was performed to examine the protein expression
of UBA2 in colorectal cancer specimens. The specimen sections (5
µm) were deparaffinized using xylene for 5 min at room temperature
three times, which was followed by rehydration using 100% ethanol
for 10 min, 95% ethanol for 10 min and water for 10 min. Antigen
retrieval via steam treatment in citrate buffer was then performed
at 120°C for 10 min. Endogenous peroxidase activity was blocked by
3% hydrogen peroxide at room temperature for 10 min. Non-specific
protein binding was blocked by 10% normal sheep serum for 30 min at
room temperature (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China).
Sections were incubated with anti-UBA2 primary antibody (1:100;
Abcam, Cambridge, MA, USA; cat. no. ab185955) at 4°C overnight.
Subsequently, the sections were incubated with a secondary antibody
conjugated with HRP [cat. no. KIT-9706; UltraSensitive™
SP (Rabbit) IHC kit; Fuzhou Maixin Biotech Co., Ltd.] for 40 min at
room temperature. The high-sensitivity 3,3-diaminobenzidine
chromogenic substrate system was used for colorimetric
visualization. In addition, specimen sections were also stained
with 0.2% hematoxylin at room temperature for 5 min, 1% acid
alcohol at room temperature for 30 sec and 0.5% eosin at room
temperature for 1 min. The slides were examined using a light
microscope (BX53F; Olympus Corporation, Tokyo, Japan). The
paracancerous normal tissues were used as negative controls. The
magnification was ×100 and 25 visual fields were analyzed.
Cell culture
The human colorectal cancer cell lines RKO, HCT116,
HT-29, DLD-1, SW480, SW620 and LoVo were purchased from Shanghai
GeneChem Co., Ltd., (Shanghai, China). The cells were routinely
grown in RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal calf serum and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified incubator with 5% CO2.
Cell proliferation by MTT assay
RKO and HCT116 colorectal cancer cells at 70–80%
confluency were transfected with UBA2-targeting small interfering
(si)RNA (Invitrogen; Thermo Fisher Scientific, Inc.). The
UBA2-siRNA sequence was 5′-GCCCGAAACCATGTTAATAGA-3′, and a
scrambled siRNA (5′-GCCTAACTGTGTCAGAAGGAA-3′) was used as the
negative control (NC-siRNA). siRNAs and Lipofectamine®
2000 (Gibco; Thermo Fisher Scientific, Inc.) were diluted in
OPTI-MEM (Gibco; Thermo Fisher Scientific, Inc.) separately prior
to being mixed. The siRNA-Lipofectamine 2000 mixture at a ratio of
1:0.1 (pmol:µl) was incubated for 30 min at room temperature, added
to the colorectal cancer cell cultures for 6–8 h in a 37°C, 5%
CO2 incubator and replaced with fresh culture medium. A
total of 72 h post-transfection, proliferation was measured by MTT
assay. Briefly, the transfected cancer cells were seeded in 96-well
plates at a density of 1×104 cells/well. Following
overnight incubation, MTT (5 mg/ml) was added to each well without
replacing the medium. Following 4 h incubation, the medium was
aspirated followed by the addition of 100 µl dimethyl sulfoxide
into each well to dissolve the purple formazan crystals; the plates
were agitated for 5–10 min. The optical density values were
measured at 490 nm using a microplate reader. All experiments were
performed three times in triplicate.
Determination of cell colony
formation
To examine the effects of UBA2 on colony formation,
800 RKO and HCT116 colorectal cancer cells transfected with
UBA2-siRNA or NC-siRNA were suspended in culture medium containing
10% FBS and seeded in 6-well plates; cells were cultured at 37°C in
a humidified incubator with 5% CO2. Fresh complete
culture medium was changed every 3 days, and the cells were grown
for 14 days. Subsequently, the cells were washed with PBS and fixed
with 4% paraformaldehyde for 30 min at room temperature and stained
with 500 µl clean, particle-free GIEMSA staining solution for 20
min at room temperature. Colonies were washed with PBS and
air-dried. Images were captured with a digital camera. All
experiments were performed three times in triplicate.
Cell cycle analysis by
fluorescence-activated cell sorting
To determine the effects of UBA2 on the cell cycle,
RKO and HCT116 colorectal cancer cells (1×106)
transfected with UBA2-siRNA or NC-siRNA were harvested and washed
in PBS (pH=7.2–7.4) followed by fixation in pre-chilled 70% ethanol
for 1 h at −20°C. The cells were treated with RNase (2 U/ml) and
stained with propidium iodide (50 µg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in the dark for 30 min at room temperature. The
cell cycle was determined by flow cytometry analysis (NovoCyte
Benchtop flow cytometer; ACEA Biosciences, San Diego, CA, USA) and
NovoExpress software (version 1.2.1; ACEA Biosciences, San Diego,
CA, USA). All experiments were performed three times in
triplicate.
Apoptosis analysis by flow
cytometry
To determine the effects of UBA2 on apoptosis,
5×105 RKO and HCT116 colorectal cancer cells transfected
with UBA2-siRNA and NC-siRNA were harvested and washed with PBS.
The cells were washed with binding buffer at room temperature and
then incubated with Annexin V-APC (cat. no. 88-8007; eBioscience;
Thermo Fisher Scientific, Inc.) in the dark at room temperature for
15 min prior to flow cytometric analysis. All experiments were
performed three times in triplicate.
Western blotting
To detect the effects of UBA2 on target protein
expression, western blotting was performed using the primary
antibodies listed in Table II, as
described previously (2). Cells
(1×106) transfected with UBA2-siRNA and NC-siRNA were
harvested and lysed with lysis buffer (cat. no. 9803; Cell
Signaling Technology, Inc., Danvers, MA, USA). Protein
concentration was analyzed using a protein assay kit (5000007) with
bovine serum albumin standards according to the manufacturer's
instructions (5000002) (both from Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Proteins (30 µg per lane) were separated by 10
or 15% SDS-PAGE gel and transferred to a polyvinylidene difluoride
membrane. GAPDH was used as loading control. The densitometry of
protein band was analyzed with ImageJ software (version 1.48v;
National Institutes of Health, Bethesda, MD, USA).
 | Table II.Antibodies used in western
blotting. |
Table II.
Antibodies used in western
blotting.
| Protein | Vendor | Cat. no. | Dilution |
|---|
| UBA2 | Abcam (Cambridge,
MA, USA) | ab185955 | 1:500 |
| p21 | Abcam | ab227443 | 1:300 |
| p27 | Abcam | ab137736 | 1:300 |
| Cyclin B1 | Cell Signaling
Technology, Inc. (Danvers, MA, USA) | 4138 | 1:500 |
| Bcl-2 | Abcam | ab194583 | 1:300 |
| MDM2 | Abcam | ab226939 | 1:200 |
| p-AKT | Abcam | ab38449 | 1:300 |
| GAPDH | Cell Signaling
Technology, Inc. | 2118 | 1:2,000 |
In vivo xenograft
All animal experiments were performed according to
the approved protocols of the Animal Care and Use Committee at
Jilin University. Female BALB/c nude mice (6 weeks; weight, 20–22
g; n=12) were purchased from Jilin University Animal Center and
were housed under specific pathogen free conditions at 20–26°C,
40–70% humidity and a 12/12 h light/dark cycle. The animals were
acclimated for 1 week prior to start of the experiment, could move
freely and had free access to food and water. A total of 6 mice
were subcutaneously injected with 2.0×106 RKO tumor
cells transfected with UBA2-siRNA or NC-siRNA mixed with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA) into left flank of the
mice respectively to form a single tumor. Tumor growth and body
weight were monitored every 2 days for 2 weeks, and tumor volume
was measured in three dimensions using calipers on alternate days.
The tumor volume (V) was calculated using the formula: V=π/6 × L ×
W × H; where W is width, L is length and H is height.
Statistical analysis
All data were analyzed by SPSS 21.0 (IBM Corp.,
Armonk, NY, USA). Clinicopathological features were evaluated using
Pearson's χ2 test. The differences between groups were
examined by the Student's t-test or the analysis of variance
followed by a Tukey post-hoc test. For the IHC study, the Fisher's
exact test was performed for statistical analysis. Overall survival
rate was determined by the Kaplan-Meier method; log-rank test was
used for statistical analysis of survival curves. P<0.05 was
considered to indicate a statistically significant difference. All
the data are presented as the mean ± standard deviation.
Results
UBA2 expression is increased and
associated with prognosis in colorectal cancer
Colorectal cancer tissues and normal tissues were
collected from patients with colorectal cancer, and RT-qPCR was
performed to examine the UBA2 mRNA expression levels. UBA2 mRNA
expression levels were significantly higher in cancer tissues
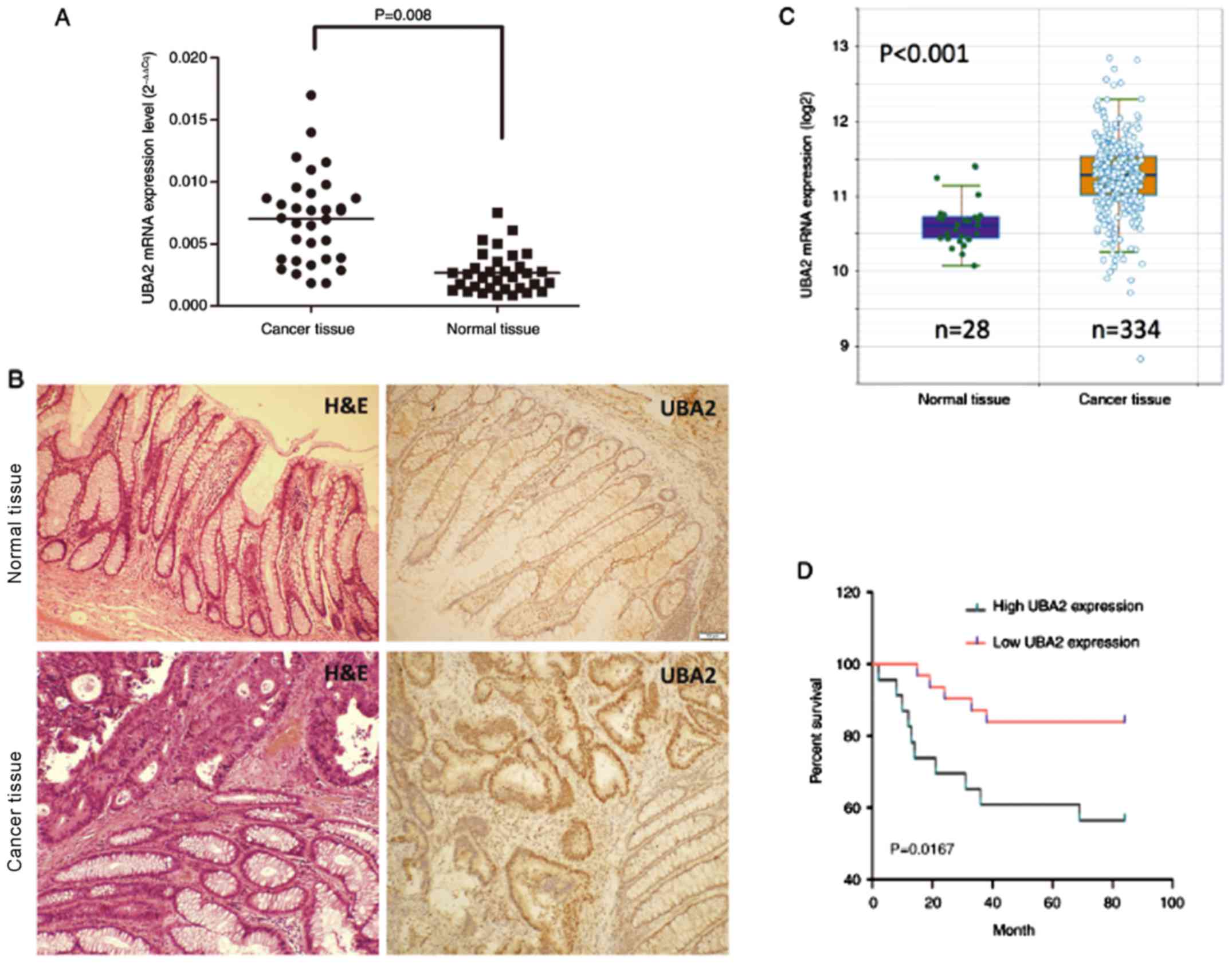
compared with the normal tissues (P=0.008; Fig. 1A). UBA2 protein expression was also
examined in the cancer tissues by IHC, which demonstrated that UBA2
expression was notably increased compared with the paracancerous
tissues (Fig. 1B). UBA2 mRNA
expression levels in 334 colorectal cancer specimens and 28 normal
colorectal specimens from the Cancer Genome Atlas obtained through
the starBase online database (http://starbase.sysu.edu.cn; Fig. 1C) were further analyzed; and the
results suggested that UBA2 mRNA expression levels were
significantly increased in cancerous tissues compared with normal
tissues (P<0.001).
To determine the effects of UBA2 expression on the
survival rate of patients with colorectal cancer, 237 colorectal
cancer patients were followed up for 84 months. Patients with
higher UBA2 protein expression levels exhibited a significantly
decreased survival rate compared with those patients expressing
lower UBA2 levels (P<0.05; Fig.
1D). In addition, UBA2 protein expression was revealed to be
associated with the location of tumor, histological grade and tumor
node and metastasis stage (Table
I). In addition, UBA2 protein expression is enhanced in the
ascending and descending colon compared with the transverse colon,
and also enhanced in tumors without lymph node metastasis compared
with tumors with lymph node metastasis (Table I). No significant associations were
made between UBA2 expression and age, sex, lymph node and depth of
invasion (Table I).
Downregulation of UBA2 inhibits
colorectal cancer cell proliferation and colony formation
To investigate the effects of UBA2 on colorectal
cancer cell proliferation, MTT and colony formation assays were
performed using colorectal cancer cells transfected with UBA2-siRNA
and NC-siRNA. First, the mRNA expression levels of UBA2 in
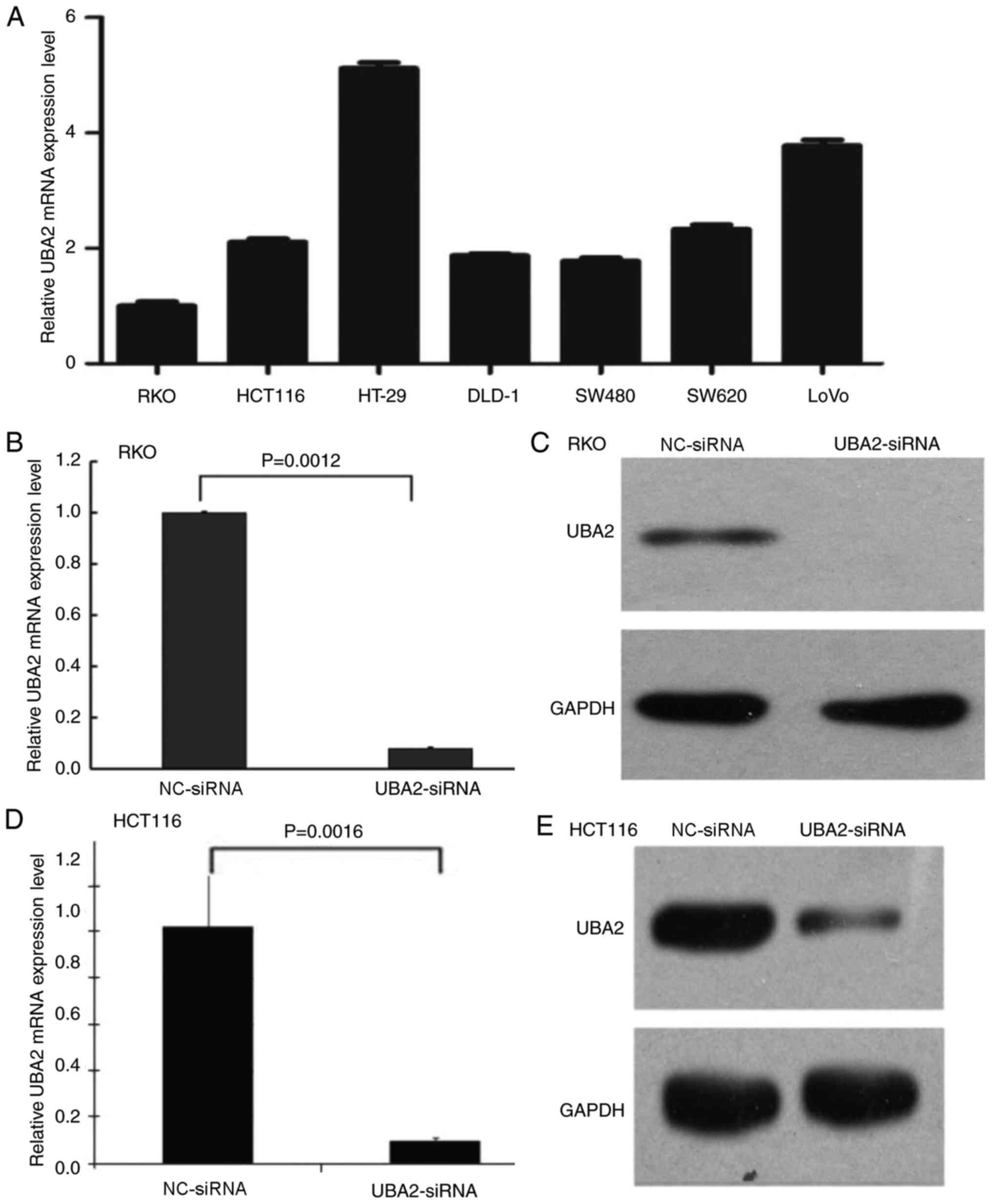
different colorectal cancer cell lines were examined (Fig. 2A); UBA2 was expressed in all seven
selected cell lines, including RKO, HCT116, HT-29, DLD-1, SW480,
SW620 and LoVo cells. In order to compare the data generated in the
present study with previous studies (27,28),
RKO and HCT116 cells were selected to determine the role of UBA2 on
cell proliferation. UBA2-siRNA transfection notably reduced the
mRNA and protein expression levels of UBA2 in RKO cells and HCT116
cells (Fig. 2B-E).
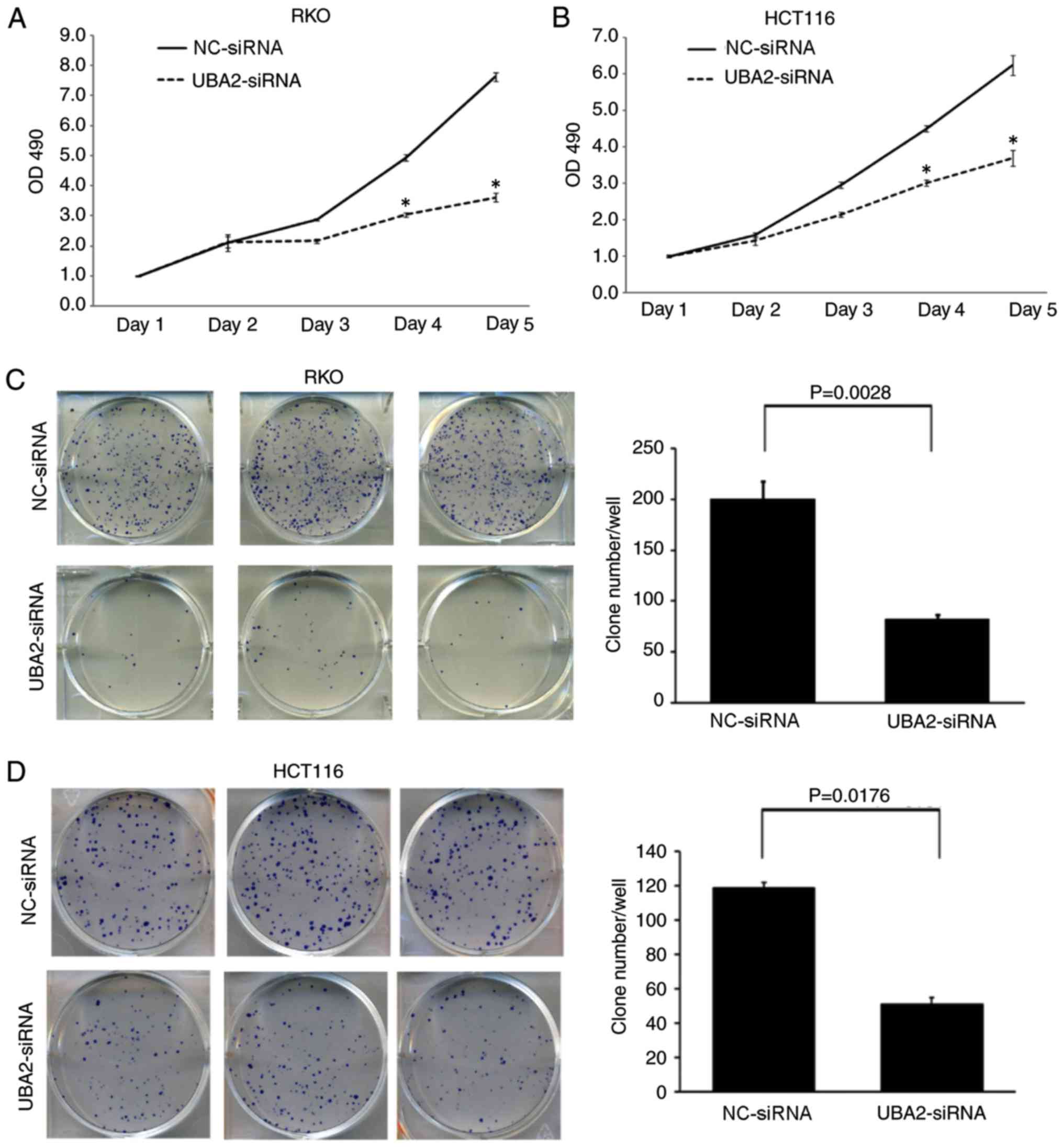
Downregulation of UBA2 significantly inhibited the
proliferation of RKO and HCT116 cells compared with
NC-siRNA-transfected cells at day 4 and day 5 time points
(P<0.05; Fig. 3A and B).
Furthermore, downregulation of UBA2 in RKO and HCT116 cells
significantly decreased the colony formation capacity compared with
the control cells (P<0.05; Fig. 3C
and D).
Downregulation of UBA2 induces cell
cycle arrest and apoptosis in colorectal cancer cells
The effects of UBA2 on the cell cycle and apoptosis
in colorectal cancer cells were examined by flow cytometry.
Compared with NC-siRNA-treated cells, RKO and HCT116 cells
transfected with UBA2-siRNA exhibited significant reductions in the
number of cells in S phase, whereas the number of G2 phase cells
was significantly increased (P<0.05; Fig. 4A-F). These results indicated that
UBA2 induced G2/M arrest in these colorectal cancer cell lines.
Downregulation of UBA2 significantly increased the apoptotic rates
in RKO cells and HCT116 cells compared with NC-siRNA-treated cells
(P<0.05; Fig. 4G-L). These
results suggested that UBA2 might inhibit apoptosis in colorectal
cells.
UBA2 promotes tumor growth in
vivo
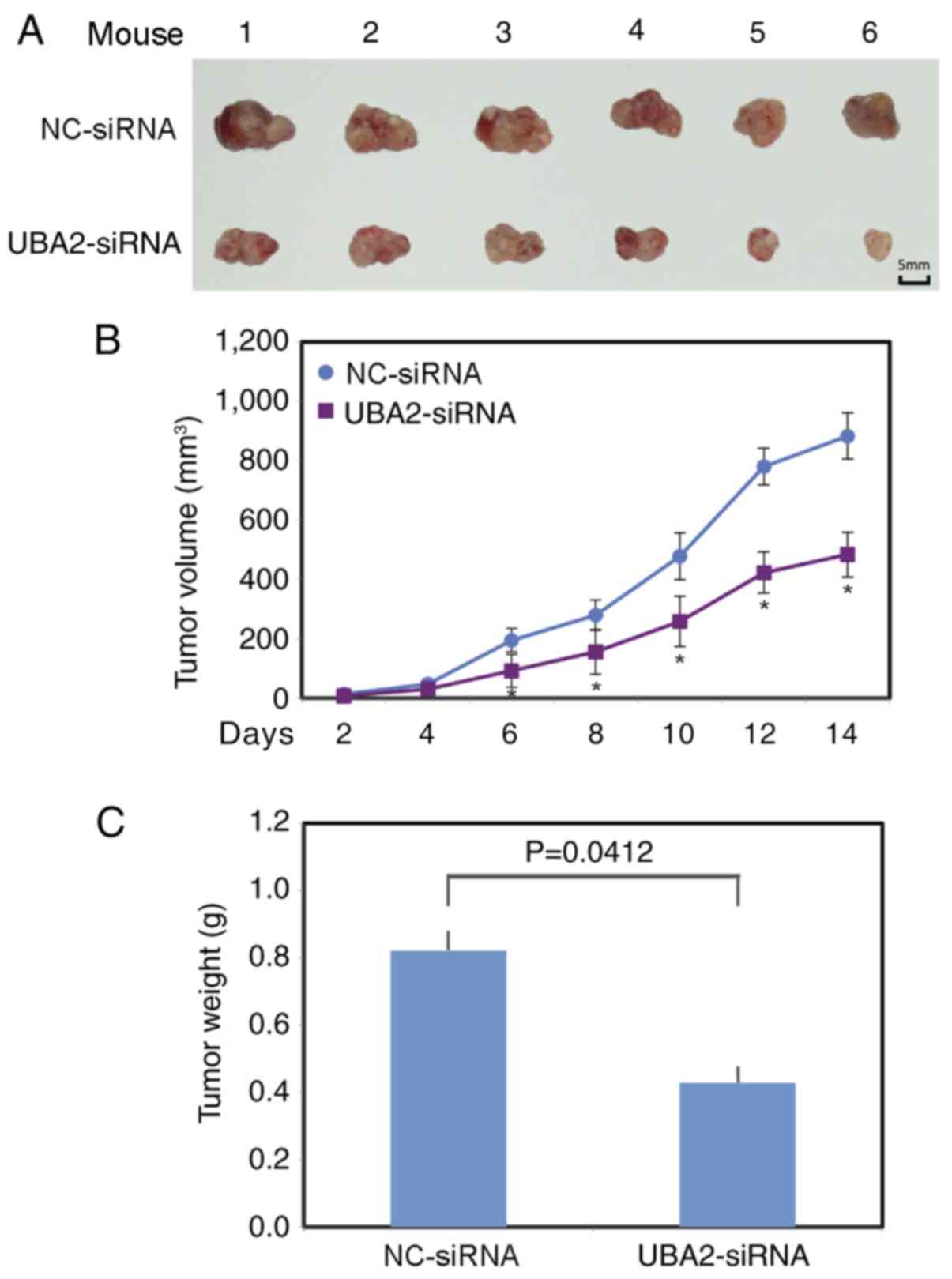
To further examine the function of UBA2 on
colorectal cancer growth, UBA2-siRNA or NC-siRNA transfected RKO
cells were injected subcutaneously into the left flank of the
athymic nude mice. Tumor volume was measured every other day. The
largest tumor diameter was ~14.9 mm in the control group and ~13 mm
in the UBA2 downregulation group. A total of 2 weeks following
xenotransplantation, the mice (weight, 25–28 g) were sacrificed and
the tumors from each animal were collected (Fig. 5A). The tumors grew significantly
more slowly in the UBA2-siRNA RKO cell group, compared with the
NC-siRNA RKO cell group (P<0.05; Fig. 5B). The average tumor weight was
also significantly lower in the UBA2-siRNA RKO cell group compared
with the NC-siRNA RKO cell group (P<0.05; Fig. 5C).
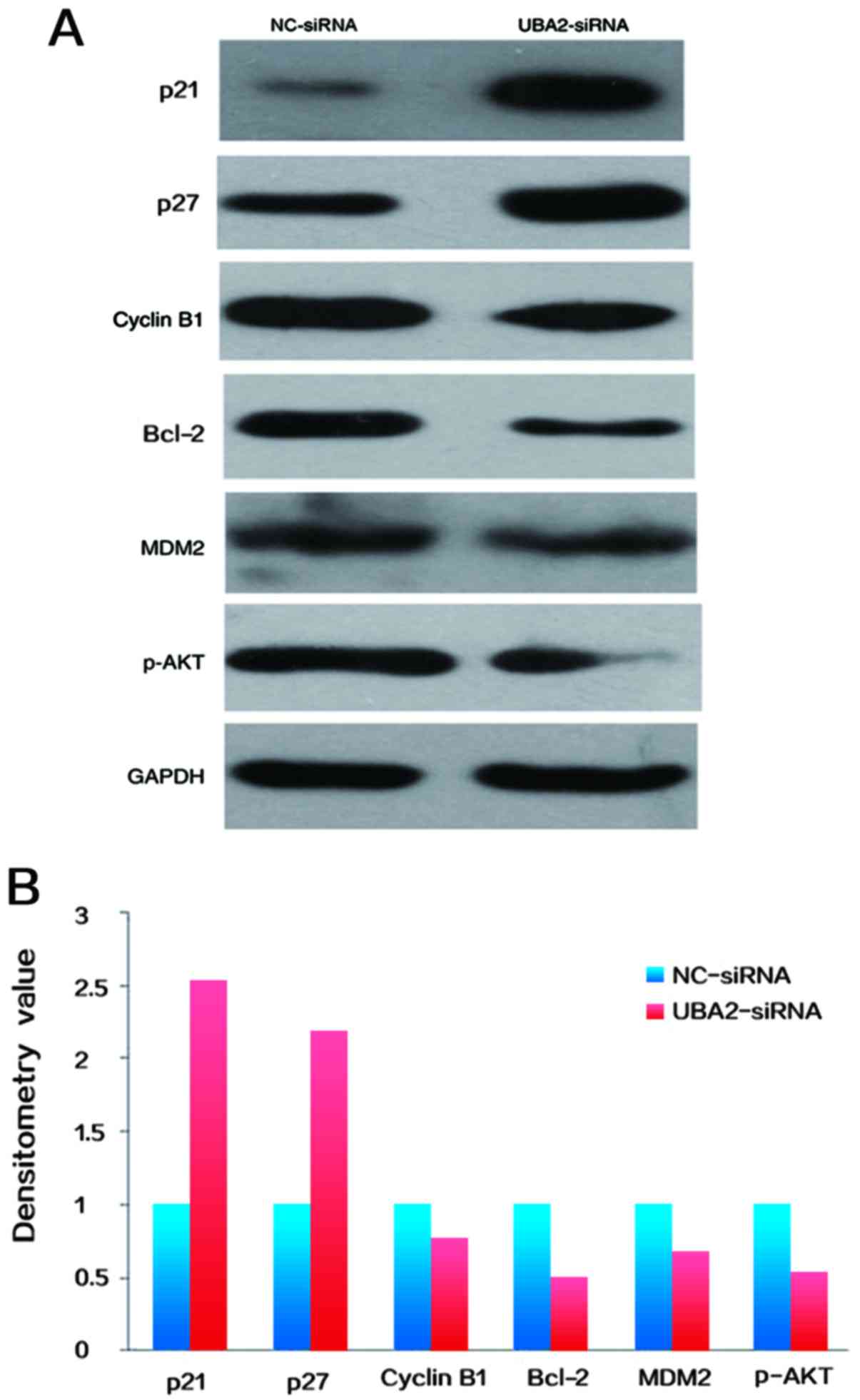
Target protein expression
To determine the potential mechanisms underlying
UBA2-mediated cell proliferation and apoptosis, the expression
levels of cell cycle associated proteins in RKO colorectal cancer
cells were examined by western blotting. As demonstrated in
Fig. 6, the expression of cyclin
B1, Bcl-2, phosphorylated protein kinase B (p-AKT) and E3
ubiquitin-protein ligase MDM2 were decreased, whereas the
expression levels of p21 and p27 were increased in colorectal
cancer cells following downregulation of UBA2, compared with
NC-siRNA transfected cells (Fig. 6A
and B).
Discussion
UBA2 forms a heterodimer with SAE1, which acts as an
E1-activating enzyme for the sumoylation of proteins (16). Adenosine triphosphate-dependent
activation is mediated by the heterodimer through a thioester bond
between sumo and a conserved active site cysteine residue on
UBA2/SAE2 (29). Previous studies
indicated that UBA2 may be a metastasis suppressor and prognostic
marker in colorectal cancer. UBA2 expression levels are decreased
in metastatic colorectal cancer cells (21–23).
Another study demonstrated that the ubiquitin-associated protein
2-like (UBAP2L) gene, located on human chromosome 1q21.3, is
associated with tumorigenesis of several types of human cancer,
including multiple myeloma (30),
liver cancer (31) and ovarian
cancer (32). Chai et al
demonstrated that UBAP2L serves important roles in colorectal
cancer cell growth and survival (33), and that knockdown of UBAP2L in
colorectal cancer cells led to suppression of proliferation, cell
cycle arrest and apoptosis through the inhibition of p38
phosphorylation, and activation of proline-rich AKT1 substrate 1,
Bcl-2-associated agonist of cell death, Bax, cleavage of
poly[ADP-ribose] polymerase-4 and caspase-3.
Results from the present study demonstrated that the
expression levels of UBA2 were increased in colorectal cancer
tissues, and UBA2 expression was associated with higher stage in
colorectal cancer and poor prognosis, which are consistent with a
previous study (23). The results
also demonstrated that UBA2 affected colorectal cancer cell
proliferation in vitro and in vivo. In addition,
downregulation of UBA2 expression induced apoptosis in colorectal
cancer cells. These results indicated that UBA2 might serve
important roles in colorectal cancer. However, normal colon cell
lines were not included as a control in the present study, which
limited our understanding of the expression level of UBA2 in
non-cancerous colon cell lines.
Recent studies have demonstrated that knockdown
sumo-conjugating enzyme UBC9 expression suppressed proliferation of
RKO and HCT116 colorectal cancer cell lines (27,28).
Similarly, knockdown UBA2 expression also suppressed proliferation
of RKO and HCT116 cells, indicating SUMO pathway serves important
roles in carcinogenesis of colorectal cancer. Knockdown of UBA2
decreased expression of cyclin B1, Bcl-2, MDM2 and p-AKT. Since
sumoylation promotes cell cycle progress in glioblastoma by
stabilizing CDK6 (5), the data
from the present study indicated that expression reduction of
cyclin B1, Bcl-2, MDM2 and p-AKT may be due to desumoylation by
UBA2 inhibition, which is required to be studied in future. To
further investigate the molecular mechanisms underlying
UBA2-mediated cell proliferation and apoptosis in colorectal
cancer, p53/MDM2/p21 signaling pathway was examined in the present
study Knockdown of UBA2 by siRNA decreased MDM2, but increased p21
and p27 protein expression in colorectal cancer cells. Cyclin B1
and cell division control 2 form a complex that promotes the
transition of cells from G2 to M phase (34). p21 and p27, downstream target
proteins of p53, act as inhibitors of cell cycle progression at the
G1, and S phase, and increased expression of such factors induces
cell cycle arrest (35,36). Consistent with the alterations in
cyclin B1, p21 and p27 expression levels, cells transfected with
UBA2-siRNA were observed to be present in the G2/M cell cycle
stage. Bcl-2 is an important member of the Bcl-2 family of
regulator proteins that regulate apoptosis, and Bcl-2
downregulation leads to the induction of apoptosis (37). The results of the present study
revealed that UBA2 silencing suppressed Bcl-2 expression and
apoptosis levels, which indicated that downregulation of Bcl-2 is
associated with UBA2-siRNA-induced apoptosis. The PTEN/PI3K/AKT
signaling pathway is an essential pathway in the regulation of
multiple biological processes, including apoptosis, cell
proliferation and metabolism (38), in which both PTEN and AKT are part
of the sumoylation pathway. The amino (N)-terminal domain of MDM2
interacts directly with the N-terminal transactivation domain of
p53 and negatively regulates p53 transcriptional activation
(39). The results suggested that
UBA2 promoted cell proliferation and inhibited apoptosis by
increasing cyclin B1, Bcl-2, p-AKT, MDM2, but decreasing p21 and
p27 expression and involving PTEN/PI3K/AKT and p53/MDM2/p21
signaling pathways in colorectal cancer cells. These data suggest
that elevated expression of UBA2 is an important contributor to the
development of colorectal cancer.
In conclusion, results from the present study
indicated that UBA2 expression may enhance colorectal cancer cell
proliferation and inhibit apoptosis. Furthermore, the expression of
UBA2 may be associated with p53/MDM2/p21 and PI3K/AKT signaling
pathways. Although UBA2 is an ubiquitin-like modifier-activating
enzyme, the present study did not establish a mechanistic link
between UBA2 and SUMO modification of proteins. Further studies are
required to determine how UBA2 silencing downregulates cyclin B1,
Bcl-2 and p-AKT levels associated with SUMO modification. UBA2 may
serve an essential role in proliferation of colorectal cancer and
may be used as a potential biomarker to predict prognosis and a
therapeutic target in colorectal cancer.
Acknowledgements
Not applicable.
Funding
The project is supported by the Young Scientists
Award in Jilin Province (grant no. 20150204006YY).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
PH, YZ, WL and YH conducted genetic analyses and
cell proliferation experiments; PH and XM designed the study and
drafted the manuscript; XS, QW, CZ and HC performed the western
blotting experiments and analyzed the results. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Review
Committee at Jilin University. All patients were informed their
participation rights and provided written informed consent (Jilin,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pichler A, Fatouros C, Lee H and
Eisenhardt N: SUMO conjugation-a mechanistic view. Biomol Concepts.
8:13–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hay RT: SUMO: A history of modification.
Mol Cell. 18:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JS, Choi HJ and Baek SH: Sumoylation
and Its contribution to cancer. Adv Exp Med Biol. 963:283–298.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellail AC, Olson JJ and Hao C: SUMO1
modification stabilizes CDK6 protein and drives the cell cycle and
glioblastoma progression. Nat Commun. 5:42342014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonne-Andrea C, Kahli M, Mechali F,
Lemaitre JM, Bossis G and Coux O: SUMO2/3 modification of cyclin E
contributes to the control of replication origin firing. Nat
Commun. 4:18502013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hendriks IA, D'Souza RC, Yang B,
Verlaan-de Vries M, Mann M and Vertegaal AC: Uncovering global
SUMOylation signaling networks in a site-specific manner. Nat
Struct Mol Biol. 21:927–936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eifler K and Vertegaal ACO:
SUMOylation-mediated regulation of cell cycle progression and
cancer. Trends Biochem Sci. 40:779–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KI and Baek SH: SUMOylation code in
cancer development and metastasis. Mol Cells. 22:247–253.
2006.PubMed/NCBI
|
|
10
|
Zhou Z, Wang M, Li J, Xiao M, Chin YE,
Cheng J, Yeh ET, Yang J and Yi J: SUMOylation and SENP3 regulate
STAT3 activation in head and neck cancer. Oncogene. 35:5826–5838.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moschos SJ, Jukic DM, Athanassiou C,
Bhargava R, Dacic S, Wang X, Kuan SF, Fayewicz SL, Galambos C,
Acquafondata M, et al: Expression analysis of Ubc9, the single
small ubiquitin-like modifier (SUMO) E2 conjugating enzyme, in
normal and malignant tissues. Hum Pathol. 41:1286–1298. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu S, Sachdeva M, Wu F, Lu Z and Mo YY:
Ubc9 promotes breast cell invasion and metastasis in a
sumoylation-independent manner. Oncogene. 29:1763–1772. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He X, Riceberg J, Pulukuri SM, Grossman S,
Shinde V, Shah P, Brownell JE, Dick L, Newcomb J and Bence N:
Characterization of the loss of SUMO pathway function on cancer
cells and tumor proliferation. PLoS One. 10:e01238822015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katayama A, Ogino T, Bandoh N, Takahara M,
Kishibe K, Nonaka S and Harabuchi Y: Overexpression of small
ubiquitin-related modifier-1 and sumoylated Mdm2 in oral squamous
cell carcinoma: Possible involvement in tumor proliferation and
prognosis. Int J Oncol. 31:517–524. 2007.PubMed/NCBI
|
|
15
|
Zhang D, Raasi S and Fushman D: Affinity
makes the difference: Nonselective interaction of the UBA domain of
Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains. J
Mol Biol. 377:162–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Truong K, Lee TD, Li B and Chen Y:
Sumoylation of SAE2 C terminus regulates SAE nuclear localization.
J Biol Chem. 287:42611–42619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Melo JB, Estevinho A, Saraiva J, Ramos L
and Carreira IM: Cutis Aplasia as a clinical hallmark for the
syndrome associated with 19q13.11 deletion: The possible role for
UBA2 gene. Mol Cytogenet. 8:212015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venegas-Vega C, Nieto-Martinez K,
Martinez-Herrera A, Gómez-Laguna L, Berumen J, Cervantes A, Kofman
S and Fernández-Ramírez F: 19q13.11 microdeletion concomitant with
ins(2;19) (p25.3;q13.1q13.4)dn in a boy: Potential role of UBA2 in
the associated phenotype. Mol Cytogenet. 7:612014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Liang D, Li JY and Zhao RY:
APOBEC3G-UBA2 fusion as a potential strategy for stable expression
of APOBEC3G and inhibition of HIV-1 replication. Retrovirology.
5:722008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu J, Chen Y, Cai L, Xu C, Zhang Y, Chen
Y, Zhang C, Zhao J, Cheng J, Xie H, et al: Functional proteomics
study reveals SUMOylation of TFII-I is involved in liver cancer
cell proliferation. J Proteome Res. 14:2385–2397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Xu Y, Pang Z, Guo F, Qin Q, Yin T,
Sang Y, Feng C, Li X, Jiang L, et al: Knockdown of SUMO-activating
enzyme subunit 2 (SAE2) suppresses cancer malignancy and enhances
chemotherapy sensitivity in small cell lung cancer. J Hematol
Oncol. 8:672015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao DF, Wang XH, Li ZY, Xing XF, Cheng
XJ, Guo T, Du H, Hu Y, Dong B, Ding N, et al: High-level SAE2
promotes malignant phenotype and predicts outcome in gastric
cancer. Am J Cancer Res. 5:140–154. 2014.PubMed/NCBI
|
|
23
|
Torres S, Garcia-Palmero I, Bartolomé RA,
Fernandez-Aceñero MJ, Molina E, Calviño E, Segura MF and Casal JI:
Combined miRNA profiling and proteomics demonstrates that different
miRNAs target a common set of proteins to promote colorectal cancer
metastasis. J Pathol. 242:39–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang JH, Li JH, Shao P, Zhou H, Chen YQ
and Qu LH: starBase: A database for exploring microRNA-mRNA
interaction maps from Argonaute CLIP-Seq and Degradome-Seq data.
Nucleic Acids Res. 39:D202–D209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bogachek MV, Park JM, De Andrade JP,
Lorenzen AW, Kulak MV, White JR, Gu VW, Wu VT and Weigel RJ:
Inhibiting the SUMO pathway represses the cancer stem cell
population in breast and colorectal carcinomas. Stem Cell Reports.
7:1140–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomasi ML, Ryoo M, Ramani K, Tomasi I,
Giordano P, Mato JM and Lu SC: Methionine adenosyltransferase α2
sumoylation positively regulate Bcl-2 expression in human colon and
liver cancer cells. Oncotarget. 6:37706–37723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okuma T, Honda R, Ichikawa G, Tsumagari N
and Yasuda H: In vitro SUMO-1 modification requires two enzymatic
steps, E1 and E2. Biochem Biophys Res Commun. 254:693–698. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawyer JR: The prognostic significance of
cytogenetics and molecular profiling in multiple myeloma. Cancer
Genet. 204:3–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu ZZ, Wang D, Cong WM, Jiang H, Yu Y,
Wen BJ, Dong H, Zhang X, Liu SF, Wang AZ, et al: Sex-related
differences in DNA copy number alterations in hepatitis B
virus-associated hepatocellular carcinoma. Asian Pac J Cancer Prev.
13:225–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naz RK and Dhandapani L: Identification of
human sperm proteins that interact with human zona pellucida3 (ZP3)
using yeast two-hybrid system. J Reprod Immunol. 84:24–31. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chai R, Yu X, Tu S and Zheng B: Depletion
of UBA protein 2-like protein inhibits growth and induces apoptosis
of human colorectal carcinoma cells. Tumour Biol. 37:13225–3522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Atherton-Fessler S, Liu F, Gabrielli B,
Lee MS, Peng CY and Piwnica-Worms H: Cell cycle regulation of the
p34cdc2 inhibitory kinases. Mol Biol Cell. 5:989–1001. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pestell RG, Albanese C, Reutens AT, Segall
JE, Lee RJ and Arnold A: The cyclins and cyclin-dependent kinase
inhibitors in hormonal regulation of proliferation and
differentiation. Endocr Rev. 20:501–534. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lloyd RV, Erickson LA, Jin L, Kulig E,
Qian X, Cheville JC and Scheithauer BW: p27kip1: A multifunctional
cyclin-dependent kinase inhibitor with prognostic significance in
human cancers. Am J Pathol. 154:313–323. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kontos CK, Christodoulou MI and Scorilas
A: Apoptosis-related BCL2-family members: Key players in
chemotherapy. Anticancer Agents Med Chem. 14:353–374. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pant V, Xiong S, Iwakuma T,
Quintás-Cardama A and Lozano G: Heterodimerization of Mdm2 and Mdm4
is critical for regulating p53 activity during embryogenesis but
dispensable for p53 and Mdm2 stability. Proc Natl Acad Sci USA.
108:11995–12000. 2011. View Article : Google Scholar : PubMed/NCBI
|