Introduction
Multipotent mesenchymal stromal cells (MSCs) are
found in mammalian stromal tissue compartments (1). The umbilical cord, bone marrow and
adipose tissue are most commonly used as a source of MSCs (2). The International Society for Cellular
Therapy has proposed several criteria (3) to define MSCs based on their plastic
adherent growth, subsequent expansion and in vitro and in
vivo differentiation multipotency (osteoblasts, chondrocytes
and adipocytes). The phenotypic definition requires the expression
of cell surface markers cluster of differentiation (CD)73, CD90 and
CD105 in addition to the lack of expression of hematopoietic
lineage markers, including CD11b, CD14, CD19, CD34 and CD45, and
human leukocyte antigen (HLA)-DR.
The bladder consists of a urothelial layer, the
lamina propria, a layer of stromal cells and submucosal, smooth
muscle and serous layers (4,5).
Basal cells, which are a type of stem cells capable of renewing and
differentiating into intermediate and superficial cells, exist in
the adult urothelium. CD44 is a basal cell surface marker (6) and is also a major surface receptor of
hyaluronic acid, which is involved in various cellular functions
including cell proliferation, differentiation, migration,
presentation of cytokines and chemokines, and signaling for cell
survival (7). Studies have
demonstrated that MSCs also express CD44 (8–10).
However, MSCs have not yet been described in the normal human
bladder.
Tissue engineering offers a promising alternative
technique for urethral reconstruction. This process involves
biodegradable scaffolds that can be used to seed cells to promote
bladder reconstruction (11). The
present study provided evidence that there is a small number of
MSC-like cells in the bladder, which the present study termed
‘human bladder-derived MSC-like cells’ (hBSCs). Cell culture
experiments show that hBSCs can be cultured to a large number of
cells. These cells possess the capacity to differentiate into
osteogenic, adipogenic and chondrogenic cells. In addition, hBSCs
expressed MSC markers. Following induction with appropriate media
in vitro, hBSCs differentiated into bladder-related cell
types. These findings reinforce the understanding of MSCs and may
inspire future research on the role of hBSCs in urological tissue
engineering applications.
Materials and methods
Primary isolation of hBSCs
Human BSCs were isolated from 10 bladder samples
collected from seven individuals with urinary bladder cancer (five
men and two women; tumor stage, T2-T3; two tissue samples were
taken from three of the bladders) ranging between 38 and 65 years
of age at Fuzhou General Hospital (between February 2014 and
November 2014). These patients had undergone whole bladder removal
surgery and the tumors had not spread to adjacent tissues from the
site of origin. During bladder resection, the bladder was infused
with epirubicin. Normal bladder tissue was cut >2 cm away from
the tumor. Following organ removal, 0.5–0.8 cm3 of
normal bladder tissue was pulled from the internal face of the
bladder. The tissues were stored in antibiotics-containing
phosphate-buffered saline (PBS) for ≤2 h prior to isolation. In
order to facilitate single cell extraction and reduce epidermal
cell contamination, the middle tissue of the bladder was taken. The
inner dense mucosa tissue and the outer bladder wall were not
included. The tissues were then cut into small pieces. The trimmed
tissue was washed three times in PBS containing antibiotics (300
µg/ml penicillin and streptomycin; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) to remove red blood cells, and minced
thoroughly using curved scissors. The fragments were reincubated in
collagenase solution (1 mg/ml type IV collagen; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 1 h at 37°C. The supernatant
containing single cells was transfer to a new centrifuge tube, then
centrifuged at 300 × g for 5 min at room temperature. The pellet
was resuspended in 10 ml of growth medium for MSCs (12), containing low-glucose Dulbecco's
modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS;
HyClone; GE Healthcare Life Sciences), 1% MEM non-essential amino
acids (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
100 µg/ml penicillin/streptomycin, and plated in a
75-cm2 culture flask. After 48 h, non-adherent cells
were removed and maintained in a humidified atmosphere of 5%
CO2 at 37°C. Once the cells achieved 70–80% confluence,
they were propagated by trypsinization. All cell isolation steps
were carried out following the receipt of informed consent from the
patients. The isolation and use of human tissues were approved by
the Fuzhou General Hospital IRB (Fuzhou, China) with written
consent (no. 2014-012).
Fluorescence-activated cell sorting
(FACS)
As is standard practice at our centers (Fujian
Provincial Key Laboratory of Transplant Biology and the Organ
Transplant Institute, Fuzhou General Hospital) (13,14),
cell surface marker analysis was performed. Cultured
bladder-derived cells at passages 3 were trypsinized and stained
with specific human antibodies labeled for CD11b-phycoerythrin (PE)
(cat. no. 557321), CD13-PE (cat. no. 347837), CD14-PE (cat. no.
555398), CD29-PE (cat. no. 555443), CD31-PE (cat. no. 340297),
CD34-PE/CD45-fluorescein isothiocyanate (FITC) (cat. no. 341071),
CD73-PE (cat. no. 550257), CD90-PE (cat. no. 555596), CD105-PE
(cat. no. 560839), HLA-ABC-PE (cat. no. 555553), and HLA-DR-PE
(cat. no. 347367) (all 1:100; BD Pharmingen; BD Biosciences,
Franklin Lakes, NJ, USA); CD19-PE-cy7 (cat. no. E10328-1633),
CD44-PE (cat. no. E01238-1632) (both 1:100; eBioscience; Thermo
Fisher Scientific, Inc.); PE-(cat. no. 555749) or
PE/FITC-conjugated isotype control antibodies (cat. no. 349526)
(1:100; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) and
PE-cy7-conjugated isotype control antibodies (1:100; cat. no.
E10143-1633, eBioscience; Thermo Fisher Scientific, Inc.) were used
to determine background fluorescence. Following staining, the cells
were analyzed using a FACS analytical fluorescence-activated cell
sorter (FACSAria II; BD Biosciences).
Differentiation potential of hBSCs
Osteogenic induction
hBSCs were seeded at a density of 4,000
cells/cm2 in a 6-well culture dish in MSC medium
(low-glucose DMEM containing 10% FBS). After 24 h of culture, the
media was replaced with StemPro osteogenic supplements (Gibco;
Thermo Fisher Scientific, Inc.) for 14 additional days (15). The medium was replaced every two to
three days. Osteogenic differentiation was assessed by 0.2%
Alizarin red staining at room temperature (16). The cells were observed using an
inverted microscope (X81; Olympus Corporation, Tokyo, Japan;
magnification, ×100).
Adipogenic induction
hBSCs were plated at a density of 8,000
cells/cm2. After 24 h of culture, the medium was
replaced with StemPro adipogenic supplements (Gibco; Thermo Fisher
Scientific, Inc.) for 14 additional days (17). Cells were fixed with 4%
paraformaldehyde for 15 min at room temperature and stained by Oil
Red O (0.5% Oil red O in isopropyl alcohol) for 30 min at room
temperature (18). The cells were
observed using an inverted microscope (X81; Olympus Corporation;
magnification, ×100).
Chondrogenic induction
Chondrogenic supplements were used to generate a
cell solution of 1.6×107 viable cells/ml. To generate
micromass cultures, 5-µl droplets of cell solution were seeded in a
6-well culture plate for 2 h. Then 2 ml of StemPro chondrogenic
supplements (Gibco; Thermo Fisher Scientific, Inc.) was added
(19). After 14 days, cells were
fixed with 4% paraformaldehyde for 15 min at room temperature and
stained by 1% Alcian blue for 30 min at room temperature (20). The cells were observed using an
inverted microscope (X81; Olympus Corporation, Tokyo, Japan;
magnification, ×100).
Urothelial induction
hBSCs were seeded in a plate at 3,000
cells/cm2. After 24 h, the medium was replaced for 14
days with a mixture of media containing 49% embryo fibroblast
medium (EFM) (Lonza Group, Ltd., Basel, Switzerland) (19) and 49% keratinocyte serum-free
medium (21) (Gibco; Thermo Fisher
Scientific, Inc.) with 2% FBS and 30 ng/ml epidermal growth factor
(Gibco; Thermo Fisher Scientific, Inc.), per the protocol reported
by Bharadwaj et al (22).
Endothelial induction
hBSCs were plated at a density of 5,000
cells/cm2 and grown for 2 days. Endothelial basal medium
(Lonza Group, Ltd.) containing 50 ng/ml vascular endothelial growth
factor was used to culture hBSCs for 14 days for induction
(22).
Smooth muscle cell induction
hBSCs were seeded in a 6-well culture plate at 2,000
cells/cm2. After 24 h, the media was replaced with
smooth muscle differentiation medium containing 45% high-glucose
DMEM and 45% EFM with 10% FBS, 2.5 ng/ml transforming growth factor
β 1 and 5 ng/ml platelet-derived growth factor-BB (PeproTech, Inc.,
Rocky Hill, NJ, USA) (22). Cell
morphology was evaluated for ≤14 days.
Cells that were continuously cultured in the growth
medium were assayed together with the induced cells and used as a
negative control for each of the differentiation experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from each type of induced and non-induced
control cell was extracted using TRIzol® (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
purity and concentration were detected by spectrophotometer
(Nanodrop 2000c; Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). cDNA (3 µg) was synthesized by reverse-transcription using a
First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol (1 h at
42°C). qPCR was performed with the SYBR Green PCR Master Mix on an
ABI 7900 Real-time PCR (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and was run for 40 cycles under the following
conditions: 94°C for 15 sec, 58°C for 15 sec and 72°C for 30 sec.
Specific primer sequences for human alkaline phosphatase,
runt-related transcription factor 2 (RUNX2), peroxisome
proliferator-activated receptor (PPAR)γ, CCAAT-enhancer-binding
protein (C/EBP)α, SRY-Box (Sox)9, collagen II, uroplakin-Ia,
cytokeratin (CK)-7, von Willebrand factor (vWF), CD31, desmin,
smoothelin and actin are provided in Table I. Actin was used as an endogenous
control. Relative fold-changes in mRNA expression were calculated
using the 2−ΔΔCq formula (23). The assay was replicated six times
for each sample.
 | Table I.Details of primers used for gene
expression analysis and their expected product size. |
Table I.
Details of primers used for gene
expression analysis and their expected product size.
| Target gene | Forward primer (5′
to 3′) | Reverse primer (5′
to 3′) | Amplicon (bp) |
|---|
| hALP |
CCACGTCTTCACATTTGGTG |
AGACTGCGCCTGGTAGTTGT | 196 |
| hRunx2 |
TCTGGCCTTCCACTCTCAGT |
GACTGGCGGGGTGTAAGTAA | 161 |
| hPPARG |
GAGCCCAAGTTTGAGTTTGC |
CTGTGAGGACTCAGGGTGGT | 198 |
| hCEBPA |
TGGACAAGAACAGCAACGAG |
TTGTCACTGGTCAGCTCCAG | 130 |
| hSox9 |
AGTACCCGCACTTGCACAAC |
CGTTCTTCACCGACTTCCTC | 177 |
| hCol-2 |
TCACGTACACTGCCCTGAAG |
CTATGTCCATGGGTGCAATG | 126 |
| hUPK1A |
GATCACCAAGCAGATGCTGA |
CAGTCCATGGGACCAGATGT | 123 |
| hCK7 |
GGCTGAGATCGACAACATCA |
GCTTCACGCTCATGAGTTCC | 191 |
| hvWF |
AGTGTGCCTGCAACTGTGTC |
CCACAGGGTAGATGGTGCTT | 144 |
| hCD31 |
GGTTCTGAGGGTGAAGGTGA |
TTGCAGCACAATGTCCTCTC | 97 |
| hDesmin |
CAGTGGCTACCAGGACAACA |
CTCAGAACCCCTTTGCTCAG | 238 |
| hSMTN |
CCTGGTGCACAACTTCTTCC |
TACACGCACTTCCAGTCAGG | 174 |
| hActin |
AGCGAGCATCCCCCAAAGTT |
GGGCACGAAGGCTCATCATT | 285 |
Immunofluorescence
Differentiated cells were fixed with 4%
paraformaldehyde overnight at 4°C, washed with PBS and
permeabilized with 1% Triton X-100 in PBS. Cells were blocked with
PBS containing 5% bovine serum albumin (Sangon Biotech Co., Ltd.,
Shanghai, China) and 0.5% Triton X-100 for 30 min at 25°C. The
urothelium-specific marker uroplakin-Ia (1:300; cat. no. orb186483;
Biorbyt Ltd., Cambridge, UK) was assessed and vWF (1:500; cat. no.
ab154193; Abcam, Cambridge, UK) was assessed for endothelial
differentiation. The smooth muscle-like cell (SMC)-specific marker
desmin (1:500; cat. no. ab32362; Abcam) was used. Antibodies were
diluted in blocking buffer and applied to slides overnight at 4°C.
The sections were then incubated with Dylight 594-conjugated IgG
secondary antibodies (1:100; cat. no. 35560; Thermo Fisher
Scientific, Inc.) for 30 min at 37°C and counterstained with DAPI
(Sigma-Aldrich, Merck KGaA) for 5 min at room temperature. Slides
were analyzed using an epifluorescence microscope (X81; Olympus
Corporation; magnification, ×400). Surface marker assays were
performed ≥3 times to ensure consistent results.
Determination of telomerase
activity
Cell extracts from 1×105 hBSCs (passage
3) were assayed using a TRAPeze RT Telomerase Detection kit (Merck
KGaA), according to the manufacturer's protocols. In brief, frozen
samples were lysed on ice with the TRAPeze 1X CHAPS lysis buffer
(EMD Millipore, Billerica, MA, USA) to release cellular proteins.
Telomerase activity was measured with 1 µg of protein lysate using
the TRAPeze RT telomerase detection kit (EMD Millipore). Briefly,
PCR was performed with a volume of 12.5 µl, including 2.5 µl of 5X
TRAPeze RT reaction mixture, 8.8 µl of nuclease-free water, 0.2 µl
of Taq polymerase and 1 µl of protein sample. A series of diluted
TSR8 control templates was used as a standard curve. The ABI 7900
PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.)
was used for PCR amplification. Cycling conditions included one
cycle at 30°C for 30 min and 95°C for 2 min, followed by 45 cycles
at 94°C for 15 sec, 59°C for 1 min and 45°C for 10 sec. Analyses
were performed in triplicate. The log10 for each well of
each reaction was calculated. In Excel 2016 (MSO, 16.0.4549.1000;
Microsoft Corporation, Redmond, WA, USA), different concentrations
of TSR8 data points were fitted to a linear regression plot
according to the curve fitting option. The linear equation obtained
from the data curve fitting is used to infer the enzyme
concentration of the experimental sample. Telomerase-positive
samples were provided by the kit. CHAPS lysis buffer (EMD
Millipore) was used as the negative control. Human bone
marrow-derived MSCs (hBM-MSCs, passage 3) and umbilical cord
Wharton's jelly-derived MSCs (hUC-MSCs, passage 4) were used as
control cells for hBSCs. The growth media for hBM-MSCs and hUC-MSCs
were similar to that for hBSCs.
Statistical analysis
SPSS software version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Data were expressed as the
mean ± standard deviation (SD). Data were analyzed using one- or
two-way analysis of variance, followed by Bonferroni's multiple
comparison tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
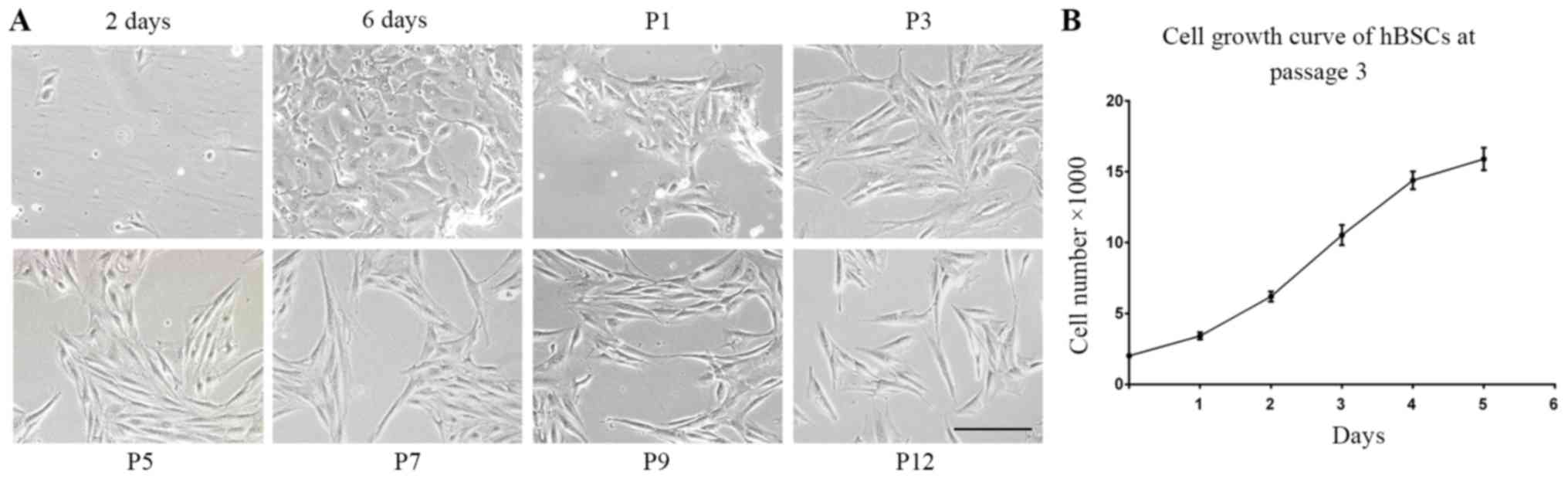
Isolation and expansion of hBSCs
A total of 10 bladder samples were collected from 7
individuals. Following 2 days of culture, 99% of the cells did not
attach to plates and non-adherent cells were removed when the
medium was replaced with fresh. After 6 days of plating, certain
single cells became a cluster of cells that appeared compact and
uniform. hBSCs did not exhibit a homogeneous appearance in primary
culture. However, these cells became symmetrical, spindle-shaped
cells (MSC-like cells) following the first propagation and no
epidermal-like cells appeared. This appearance was consistently
observed at every subsequent passage (Fig. 1A). A high yield of cells was
achieved from these cells. In ~2 weeks, the cells were expanded to
~5 million cells in 3, 75-cm2 plates at passage 1.
Average population doubling time was 28 h in growth medium. These
cells demonstrated normal exponential cell growth patterns with a
steady increase in number during a 6-day culture period (Fig. 1B).
Cell surface markers
FACS was used to assess cell surface marker
expression. All cell lines demonstrated similar results. hBSCs
consistently expressed MSC-like cell surface markers (CD13, CD29,
CD44, CD73, CD90 and CD105), but not hematopoietic markers (CD11b,
CD14, CD19, CD34 and CD45), endothelial cell markers (CD31), or
HLA-DR (Fig. 2). HLA-ABC was
expressed at a lower level. CD44 was expressed in MSCs and
considered a cell surface marker for urothelial basal cells
(6). In the hBSC populations, CD44
was expressed and its mean expression was high.
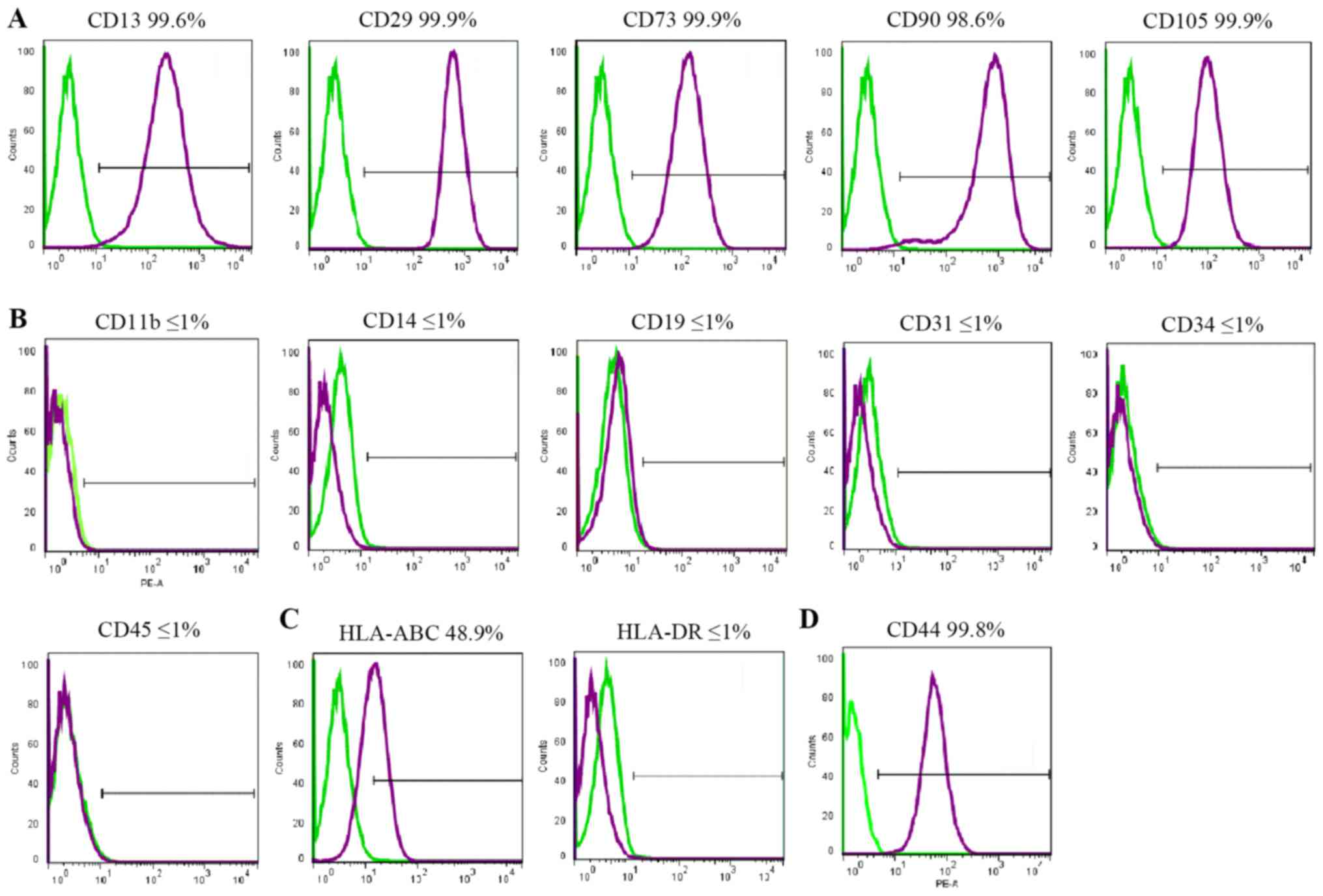 | Figure 2.Immunophenotypes of hBSCs. hBSCs at
passage 3 were labeled with antibodies against (A) mesenchymal stem
cell markers (CD13, CD29, CD73, CD90 and CD105), (B) hematopoietic
stem cell markers (CD14, CD19, CD31, CD34 and CD45), (C) HLA-ABC
and HLA-DR, and (D) CD44, and then analyzed by
fluorescence-activated cell sorting. Representative histograms are
demonstrated (purple). The respective isotype control is indicated
by a green line. hBSCs, human bladder-derived mesenchymal stromal
cells; CD, cluster of differentiation; HLA, human leukocyte
antigen. |
Osteogenic, adipogenic and
chondrogenic differentiation capacity of hBSCs
MSCs can be induced to differentiate into
osteoblasts, adipocytes and chondrocytes under specific culture
conditions (1). In the hBSC
populations of the present study, osteogenic differentiation could
be induced, as demonstrated by Alizarin red S staining for calcium
deposition. Osteogenically-induced hBSCs expressed osteoblastic
gene markers alkaline phosphatase and RUNX2 (P<0.05; Fig. 3A; 34.2±2.6-fold and 5.2±1.4-fold,
respectively). Adipogenic-differentiated hBSCs were positive for
Oil Red O staining. Induction of the adipogenic markers PPARγ and
C/EBPα increased in adipogenically-induced cells (P<0.05;
Fig. 3B; 3.8±1.1-fold and
17.6±2.3-fold, respectively). Chondrogenic-differentiated hBSCs
were examined with Alcian blue staining. Differentiated hBSCs
expressed the chondrogenic lineage markers Sox9 and collagen II
(P<0.05; Fig. 3C, 13.2±1.8-fold
and 3.4±1.6-fold, respectively). These results demonstrated that
hBSCs could differentiate into the 3 above-mentioned cell lineages.
This differentiation capacity of BSCs was similar to that of MSCs.
Therefore, this class of bladder-derived cells were designated as
MSC-like cells.
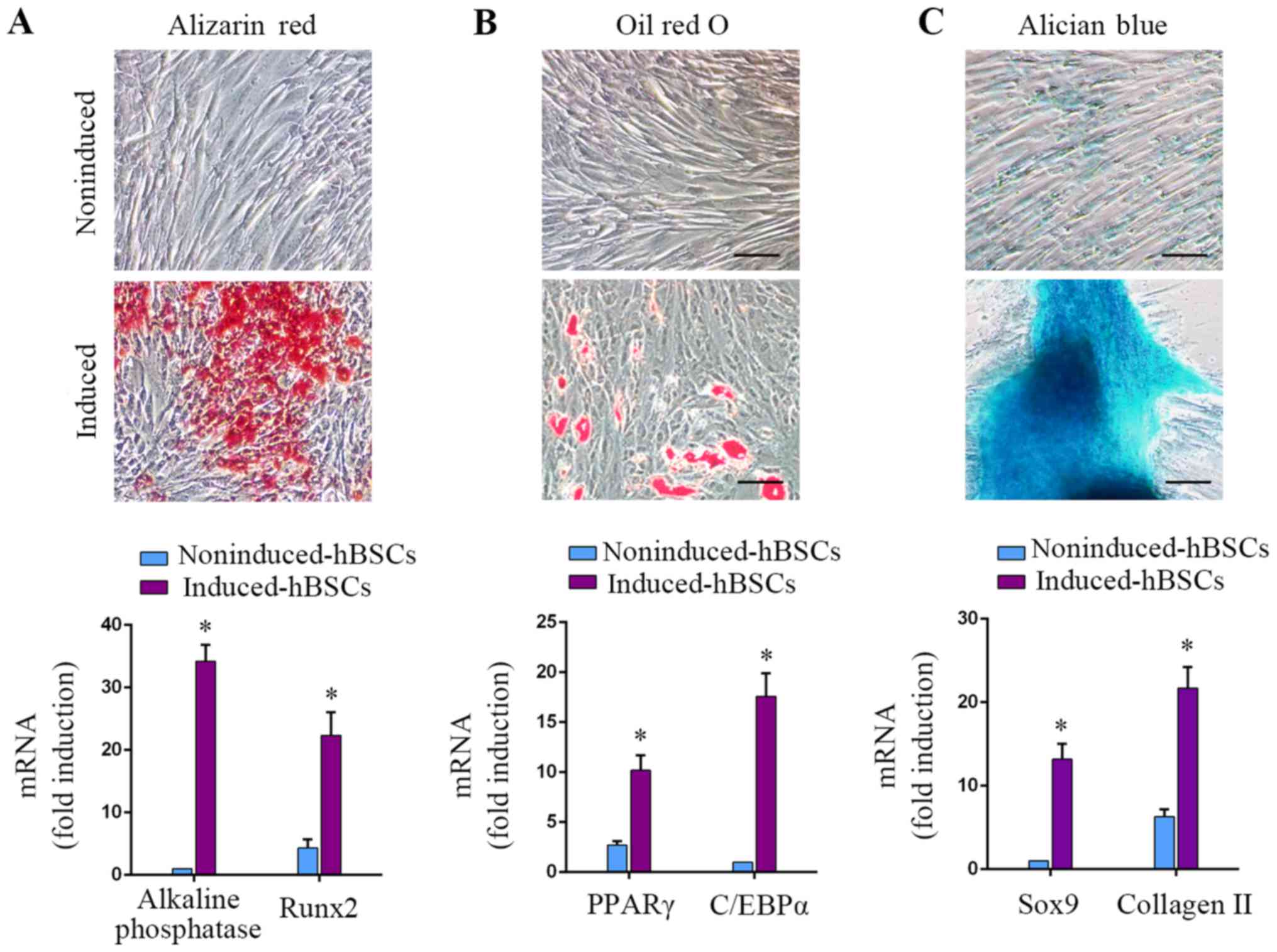 | Figure 3.hBSCs were induced to differentiate
into osteogenic, adipogenic and chondrogenic lineages using
specific media cocktails. hBSCs cultured in different induction
media were assessed for differentiation into 3 lineages. (A)
Osteogenic differentiation, day 14: Alizarin red staining for
alkaline phosphatase activity. RT-qPCR analysis for
lineage-specific transcripts alkaline phosphatase and RUNX2. Error
bars indicate the SD of 6 biological replicates. (B) Adipogenic
differentiation, day 14: Oil red O staining for lipid droplets.
RT-qPCR analysis for lineage-specific transcripts PPARγ and C/EBPα.
Error bars indicate the SD of 6 biological replicates. (C)
Chondrogenic differentiation, day 14: Alcian blue staining for
glycosaminoglycans. RT-qPCR analysis for lineage-specific
transcripts Sox9 and collagen II. Error bars indicate the SD of 6
biological replicates. *P<0.05 vs. noninduced-hBSCs. hBSCs,
human bladder-derived mesenchymal stromal cells; RUNX2,
runt-related transcription factor 2; PPAR, peroxisome
proliferator-activated receptor; C/EBP, CCAAT-enhancer-binding
protein; Sox, SRY-Box; SD, standard deviation; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
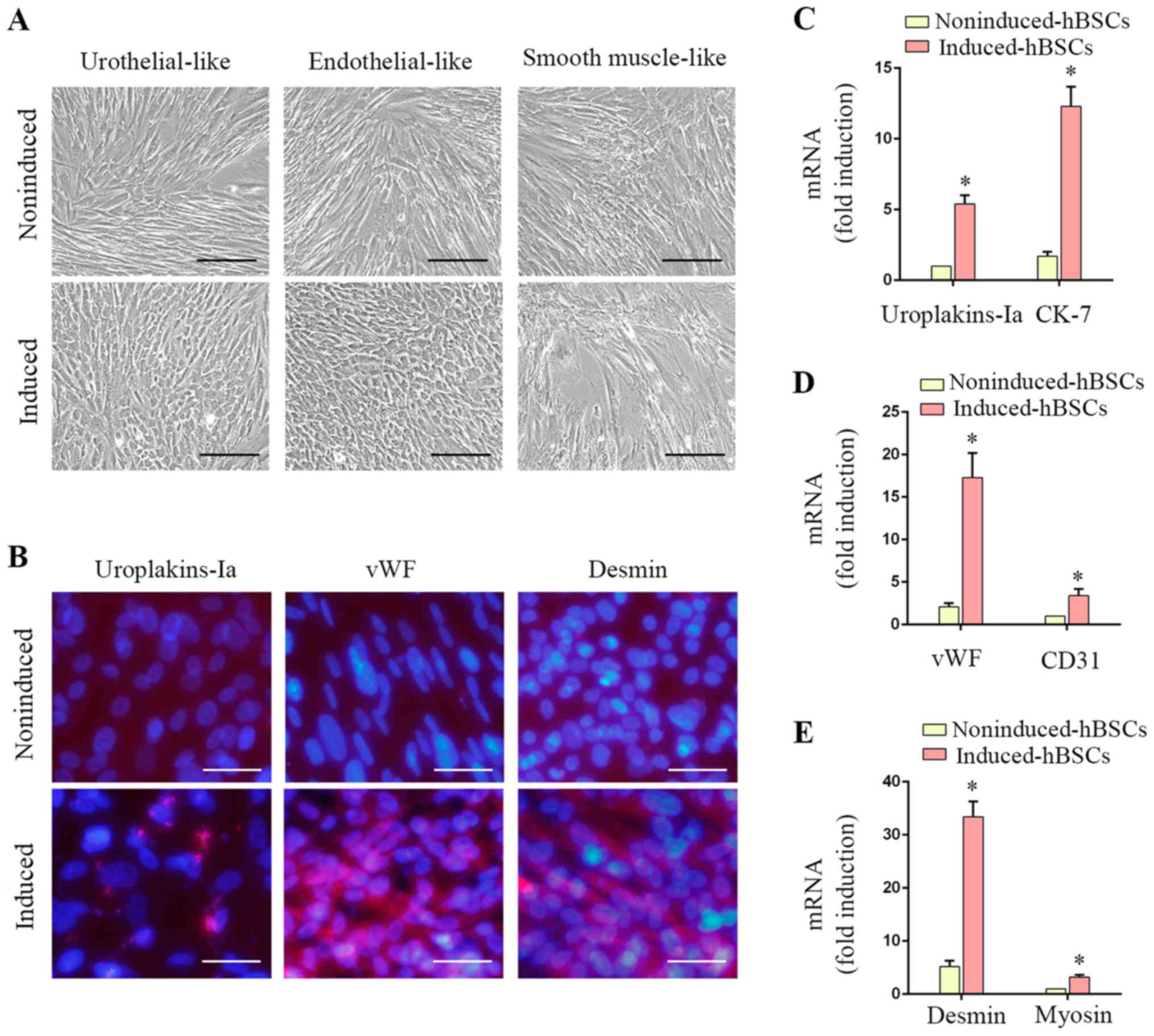
Differentiation of hBSCs into
urothelial, endothelial and smooth muscle cells in vitro
hBSCs demonstrated varying degrees of
differentiation potential as presented by positive expression of
urothelial, endothelial and SMC lineage-specific markers (Fig. 4). Urothelial-differentiated hBSCs
became ‘rice-grain’ shaped (Fig.
4A). Immunofluorescence revealed that ~37.4±2.3% of the induced
cells expressed the urothelial-specific protein uroplakin-Ia
(Fig. 4B). Transcripts of
uroplakin-Ia and CK-7 were significantly increased in induced hBSCs
(P<0.05; Fig. 4C, 5.4±0.6-fold
and 7.2±1.1-fold, respectively). Endothelial-differentiated hBSCs
became ‘cobble-stone’ shaped (Fig.
4A). Induced hBSCs expressed the endothelial cell-specific
protein marker vWF (Fig. 4B) and
gene transcripts (vWF and CD31) were significantly increased
(P<0.05; Fig. 4D; 8.2±1.5-fold
and 3.5±0.8-fold, respectively). SMC-differentiated hBSCs became
elongated (Fig. 4A) and ~84.1±5.2%
of the induced cells expressed desmin (Fig. 4B). SMC-specific gene transcripts
(desmin and myosin) were induced (P<0.05; Fig. 4E; 6.4±1.2-fold and 3.3±0.4-fold,
respectively). These data suggested that hBSCs are a potential
source for urological tissue reconstruction.
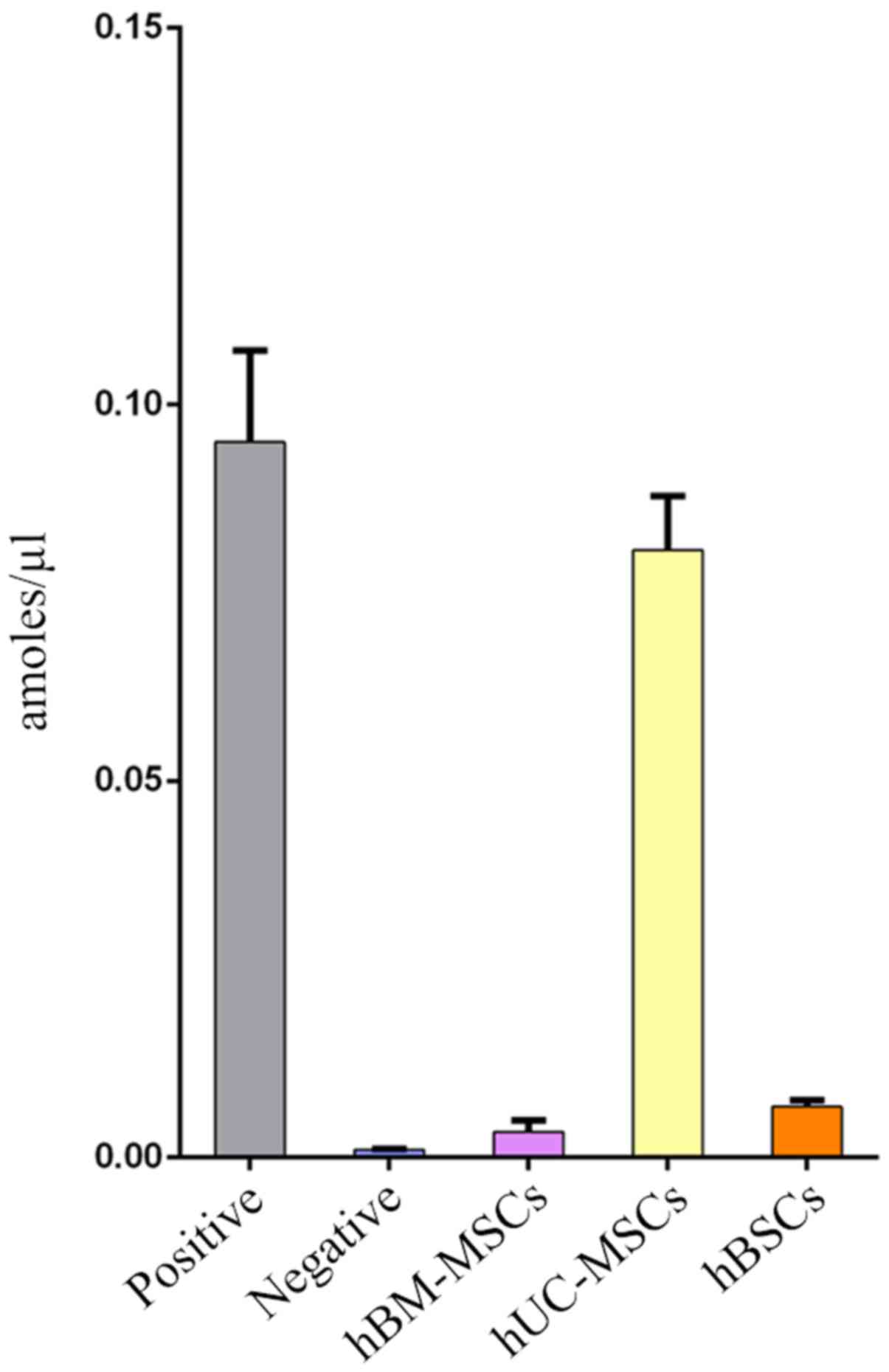
Telomerase activity of hBSCs
In the present study, telomerase was detected in 10
lines of hBSC samples at passage 4. hBM-MSCs and hUC-MSCs at the
same passage were used as controls. Fig. 5 demonstrates that the average
telomerase activity of adult hBSCs was lower compared with hUC-MSCs
but higher compared with hBM-MSCs. Telomerase activity is
correlated with lifespan and replicative capacity in cell lines
(24). In the present study, hBSCs
could proliferate for ≤20 generations (data not shown). This is far
higher than the proliferative potential of hBM-MSCs, which usually
stop growing prior to passage 10 (25). Although the cells can be passaged
for ~20 generations, as the number of passages increases, they may
still exhibit senescence and be unable to be immortalized.
Discussion
The latest discoveries in urological tissue
engineering have demonstrated that bladder regeneration can be
achieved using autologous bladder cells (11), embryonic stem cells (26) and several types of MSCs, including
those derived from bone marrow (27), skeletal muscle (28) and adipose tissue (29–31).
The present study identified that MSC-like cells exist in human
bladder tissues. They express MSC surface markers, including CD13,
CD29, CD73, CD90, CD105 and CD44, and exhibit telomerase activity.
Importantly, these culture-expanded cells possess multipotent
differentiation capacity. In addition to osteogenic, adipogenic and
chondrogenic cells, hBSCs give rise to multiple lineages that
express urothelium, endothelial and smooth muscle cell markers
in vitro, suggesting that this type of progenitor cells can
be planted in the cellular matrix to repair damaged tissues.
Identification of the sources of hBSCs may provide a better
understanding of their biological role. hBM-MSCs and urothelial
basal cells could potentially be the origin of BSCs. The present
study provided no evidence to suggest that hBSCs are actually
hBM-MSCs. The maximum number of passages for hBM-MSCs is ~10
(24) while for hBSCs, it could
reach ~20. The evidence from the current study suggested that hBSCs
may originate from urothelial basal cells, as these cells expressed
CD44. Since basal cells can self-renew, proliferate and
differentiate into intermediate and superficial cells, including
umbrella cells, basal cells are referred to as urothelial
progenitor cells or stem cells. Perivascular cells are another
possible origin of MSCs (32).
Human perivascular cells can be sorted from diverse human tissues
and long-term culture produces adherent, multilineage progenitor
cells with MSC characteristics.
The urothelial, endothelial and SMC differentiation
ability of hBSCs was demonstrated by the present study, suggesting
that hBSCs can be an alternative to the urological tissue biopsies
used in cell therapy. Notably, an advantage to using hBSCs is their
expansion property. It was demonstrated that one bladder sample
(~0.5 cm3) cultured for 4 weeks would yield a sufficient
quantity of cells at below passage 5 for differentiation and tissue
engineering. hBSC can be planted on biological materials in the
same way as other MSC cells (33)
and can be used for tissue damage repair following
differentiation.
The adult bladder is composed of the urothelium,
lamina propria, muscularis propria and perivesical soft tissues
(11). Future studies may attempt
to scrape the bladder tissues more precisely to identify the layer
that is the main source of hBSCs. In addition, the hBSCs in the
present study were obtained from non-carcinoma biopsy of urothelial
bladder cancer patients. The interaction between hBSCs and
urothelial cancer cells still requires study. The limitation of
hBSC applications is mainly in cell acquisition. Autologous tissue
cell separation is more traumatic. In view of the evidence that
hBSC and MSC are similar and that the expression of HLA class 2
antigen is extremely low, allogeneic cells can be used. However,
there is little chance of obtaining normal bladder tissue with
informed consent. Donation after Cardiac Death Organ donation may
be the way to obtain normal bladder tissue in the future.
In conclusion, the present study highlighted that
the progenitor cells in human bladder tissues are MSC-like cells,
which possess the capacity to expand and differentiate into
multiple cells under directed treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by The
National Natural Science Foundation of China (grant no.
81570748).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and JMT designed the experiments. LFZ, HYD, LZ
and YMC performed the experiments. LFZ provided the patient
samples. JL and LZ analyzed the data. JL and YMC wrote the
manuscript.
Ethics approval and consent to
participate
The isolation and use of human tissues were approved
by the Fuzhou General Hospital IRB, (Fuzhou, China), with written
consent (2014–12).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ding DC, Shyu WC and Lin SZ: Mesenchymal
stem cells. Cell Transplant. 20:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner W, Wein F, Seckinger A, Frankhauser
M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W
and Ho AD: Comparative characteristics of mesenchymal stem cells
from human bone marrow, adipose tissue, and umbilical cord blood.
Exp Hematol. 33:1402–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horwitz EM, Le Blanc K, Dominici M,
Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS and
Keating A: International Society for Cellular Therapy:
Clarification of the nomenclature for MSC: The international
society for cellular therapy position statement. Cytotherapy.
7:393–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong-You-Cheong JJ, Woodward PJ, Manning
MA and Sesterhenn IA: From the archives of the AFIP: Neoplasms of
the urinary bladder: Radiologic-pathologic correlation.
Radiographics. 26:553–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng L, Montironi R, Davidson DD and
Lopez-Beltran A: Staging and reporting of urothelial carcinoma of
the urinary bladder. Mod Pathol. 22 Suppl 2:S70–S95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Desai S, Lim SD, Jimenez RE, Chun T, Keane
TE, McKenney JK, Zavala-Pompa A, Cohen C, Young RH and Amin MB:
Relationship of cytokeratin 20 and CD44 protein expression with
WHO/ISUP grade in pTa and pT1 papillary urothelial neoplasia. Mod
Pathol. 13:1315–1323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu H, Mitsuhashi N, Klein A, Barsky LW,
Weinberg K, Barr ML, Demetriou A and Wu GD: The role of the
hyaluronan receptor CD44 in mesenchymal stem cell migration in the
extracellular matrix. Stem Cells. 24:928–935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bian XH, Zhou GY, Wang LN, Ma JF, Fan QL,
Liu N, Bai Y, Guo W, Wang YQ, Sun GP, et al: The role of
CD44-hyaluronic acid interaction in exogenous mesenchymal stem
cells homing to rat remnant kidney. Kidney Blood Press Res.
38:11–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma F, Chen D, Chen F, Chi Y and Han Z,
Feng X, Li X and Han Z: Human umbilical cord mesenchymal stem cells
promote breast cancer metastasis by interleukin-8- and
interleukin-6-dependent induction of CD44(+)/CD24(−) cells. Cell
Transplant. 24:2585–2599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Becker C and Jakse G: Stem cells for
regeneration of urological structures. Eur Urol. 51:1217–1228.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akram KM, Samad S, Spiteri MA and Forsyth
NR: Mesenchymal stem cells promote alveolar epithelial cell wound
repair in vitro through distinct migratory and paracrine
mechanisms. Respir Res. 14:92013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Wang Q, Huang L, Dong H, Lin L, Lin
N, Zheng F and Tan J: Palmitate causes endoplasmic reticulum stress
and apoptosis in human mesenchymal stem cells: Prevention by AMPK
activator. Endocrinology. 153:5275–5284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan J, Wu W, Xu X, Liao L, Zheng F,
Messinger S, Sun X, Chen J, Yang S, Cai J, et al: Induction therapy
with autologous mesenchymal stem cells in living-related kidney
transplants: A randomized controlled trial. JAMA. 307:1169–1177.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oikonomopoulos A, van Deen WK, Manansala
AR, Lacey PN, Tomakili TA, Ziman A and Hommes DW: Optimization of
human mesenchymal stem cell manufacturing: The effects of
animal/xeno-free media. Sci Rep. 5:165702015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gregory CA, Gunn WG, Peister A and Prockop
DJ: An Alizarin red-based assay of mineralization by adherent cells
in culture: Comparison with cetylpyridinium chloride extraction.
Anal Biochem. 329:77–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swioklo S, Constantinescu A and Connon CJ:
Alginate-encapsulation for the improved hypothermic preservation of
human adipose-derived stem cells. Stem Cells Transl Med. 5:339–349.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramirez-Zacarias JL, Castro-Muñozledo F
and Kuri-Harcuch W: Quantitation of adipose conversion and
triglycerides by staining intracytoplasmic lipids with oil red O.
Histochemistry. 97:493–497. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Nyman JS, Ono K, Stevenson DA,
Yang X and Elefteriou F: Mice lacking Nf1 in osteochondroprogenitor
cells display skeletal dysplasia similar to patients with
neurofibromatosis type I. Hum Mol Genet. 20:3910–3924. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogawa R, Mizuno H, Hyakusoku H, Watanabe
A, Migita M and Shimada T: Chondrogenic and osteogenic
differentiation of adipose-derived stem cells isolated from GFP
transgenic mice. J Nippon Med Sch. 71:240–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Itoh M, Kiuru M, Cairo MS and Christiano
AM: Generation of keratinocytes from normal and recessive
dystrophic epidermolysis bullosa-induced pluripotent stem cells.
Proc Natl Acad Sci USA. 108:8797–8802. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bharadwaj S, Liu G, Shi Y, Wu R, Yang B,
He T, Fan Y, Lu X, Zhou X, Liu H, et al: Multipotential
differentiation of human urine-derived stem cells: Potential for
therapeutic applications in urology. Stem Cells. 31:1840–1856.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bodnar AG, Ouellette M, Frolkis M, Holt
SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S and
Wright WE: Extension of life-span by introduction of telomerase
into normal human cells. Science. 279:349–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vidal MA, Walker NJ, Napoli E and
Borjesson DL: Evaluation of senescence in mesenchymal stem cells
isolated from equine bone marrow, adipose tissue, and umbilical
cord tissue. Stem Cells Dev. 21:273–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bajada S, Mazakova I, Richardson JB and
Ashammakhi N: Updates on stem cells and their applications in
regenerative medicine. J Tissue Eng Regen Med. 2:169–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim A, Yu HY, Heo J, Song M, Shin JH, Lim
J, Yoon SJ, Kim Y, Lee S, Kim SW, et al: Mesenchymal stem cells
protect against the tissue fibrosis of ketamine-induced cystitis in
rat bladder. Sci Rep. 6:308812016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corona BT, Ward CL, Baker HB, Walters TJ
and Christ GJ: Implantation of in vitro tissue engineered muscle
repair constructs and bladder acellular matrices partially restore
in vivo skeletal muscle function in a rat model of volumetric
muscle loss injury. Tissue Eng Part A. 20:705–715. 2014.PubMed/NCBI
|
|
29
|
Sakuma T, Matsumoto T, Kano K, Fukuda N,
Obinata D, Yamaguchi K, Yoshida T, Takahashi S and Mugishima H:
Mature, adipocyte derived, dedifferentiated fat cells can
differentiate into smooth muscle-like cells and contribute to
bladder tissue regeneration. J Urol. 182:355–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou X, Shi C, Chen W, Chen B, Jia W, Guo
Y, Ma C, Ye G, Kang J and Dai J: Transplantation of human
adipose-derived mesenchymal stem cells on a bladder acellular
matrix for bladder regeneration in a canine model. Biomed Mater.
11:0310012016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiu X, Zhang S, Zhao X, Fu K and Guo H:
The therapeutic effect of adipose-derived mesenchymal stem cells
for radiation-induced bladder injury. Stem Cells Int.
2016:36790472016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crisan M, Yap S, Casteilla L, Chen CW,
Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al: A
perivascular origin for mesenchymal stem cells in multiple human
organs. Cell Stem Cell. 3:301–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu S, Liu Y, Bharadwaj S, Atala A and
Zhang Y: Human urine-derived stem cells seeded in a modified 3D
porous small intestinal submucosa scaffold for urethral tissue
engineering. Biomaterials. 32:1317–1326. 2011. View Article : Google Scholar : PubMed/NCBI
|