Introduction
Due to lifestyle changes, the prevalence of type 2
diabetes mellitus (T2DM) in China is increasing; the incidence rate
of T2DM is 12% (1,2) and complications associated with T2DM,
such as diabetes-associated cognitive decline (DACD), are
subsequently becoming increasingly prominent. DACD has received
increased attention due to its severe effects on health and the
quality of life of patients, including memory deficits and
neurasthenia (2).
Nuclear factor-κB (NF-κB) combines with NF-κB
inhibitor α (IκBa) in the cytoplasm in its inactive state during
T2DM/DADC. When activated, NF-κB dissociates from IκBa, which
exposes the localization sequence of NF-κB. Subsequently, NF-κB
translocates to the nucleus to regulate transcription (3,4). It
induces the expression of proinflammatory factors, including
interleukin (IL)-1, intercellular adhesion molecule 1 and tumor
necrosis factor α (3,4).
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a key anti-oxidative transcription factor in T2DM/DADC (5). Early prevention and treatment of
diabetic cognitive dysfunction may delay and reduce the incidence
of dementia, thus improving the quality of life of patients with
diabetes (6).
Sirtuin 1 (SIRT1) is an NAD+-dependent
protein deacetylase that is involved in cell differentiation,
aging, apoptosis, physiological rhythms, metabolic regulation,
oxidative stress and numerous other important biological processes.
Furthermore, it has an important biological role in transcriptional
regulation (7). Studies have
demonstrated that SIRT1 is required for the maintenance of normal
cognitive function and synaptic plasticity regulation. It is also
reported to exhibit neuroprotective effects against Alzheimer's
disease and other degenerative disease, through thermoregulation,
reduction of Aβ protein deposition and antioxidative,
anti-inflammatory and antiapoptotic mechanisms (8). The present study investigated the
effect and underlying mechanisms of the SIRT1 agonist, SRT1720, on
cognitive decline in a rat model of T2DM.
Materials and methods
Experimental animals
Male Sprague-Dawley rats (n=40; weight, 210±20 g; 8
weeks old) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd (Beijing, China) and housed at 23±2°C
with 50–60% humidity on a 12 h light/dark cycle, free access to
food and water access, with n=3 rats/cage. The present study was
approved by the Ethics Committee of the Chinese PLA General
Hospital (Beijing, China). Rats of the control group were subjected
to a single intraperitoneal injection of normal saline. Rats of
T2DM model were subjected to a single intraperitoneal injection of
streptozotocin saline (STZ; 65 mg/kg, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) dissolved in 100 mM sodium citrate buffer (pH
4.5) to induce T2DM. The body weight of the T2DM rats was
monitored. The fasting blood glucose (FBG) of the T2DM rats was
confirmed with an Optium Xceed FBG meter (Abbott Pharmaceutical
Co., Ltd., Lake Bluff, IL, USA). Rats with blood glucose levels
>300 mg/dl were considered diabetic and used in subsequent
experiments. The dose of SRT1720 was selected based on a recent
study (9). At 8 weeks after the
development of diabetes, rats were divided into four groups: Normal
control rats (n=6), T2DM rats (n=8), T2DM rats treated with 25
mg/kg SRT1720 (MedChemExpress, Monmouth Junction, NJ, USA) (n=8)
and T2DM rats treated with 50 mg/kg SRT1720 (n=8). Treatment group
rats were administered SRT1720 via gavage for 4 weeks. Rats of the
normal control group were treated with saline. Rats were
anesthetized with an intraperitoneal injection of pentobarbital (35
mg/kg) and sacrificed by decapitation. Hippocampal samples were
collected and saved at −80°C.
Morris water maze test
After the 4 week SRT17200 treatment period, the
effect of SRT1720 on cognitive function was evaluated with the
Morris water maze test. Rats were trained twice a day every day for
5 days and the test was performed in a blind fashion. Swimming was
video tracked and latency, path length, swim speed and the
cumulative distance from the platform were recorded. Mean swim
latency was evaluated on each day. Following a probe trial, the
mean time spent in the correct quadrant containing the platform and
the mean number of times that mice crossed the former platform
position during 60 sec was determined for day 5.
PC12 diabetic cell model
PC12 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
antibiotics at 37°C in 5% CO2. PC12 cells were treated
with 25 mg/ml glucose for 24 h at 37°C. Cells were divided into
four groups: Control group (25 mg/ml glucose only), SRT1720 (10 µM)
group, SRT1720 (10 µM) + Nrf2 agonist (curcumin, 25 µM,
MedChemExpress) group and SRT1720 (10 µM) + NF-κB inhibitor
(JSH-23, 2 µM, MedChemExpress) group. Groups were treated for 24 h
at 37°C.
Measurement of oxidative stress and
inflammation
Hippocampal samples or PC12 cells were homogenized
and protein was extracted with radioimmunoprecipitation assay
(RIPA) lysis buffer (BestBio, Shanghai, China). Levels of
glutathione (GSH, S0053), GSH peroxidase (GSH-PX, S0056),
superoxide dismutase (SOD, S0109), malondialdehyde (MDA, S0131),
IL-1β (PI303) and IL-6 (PI328) were detected by their respective
enzyme-linked immunosorbent assay (ELISA) kits (Beyotime Institute
of Biotechnology, Nanjing, China), according to the manufacturer's
protocols. Absorbency was measured at 450 nm.
Western blot analysis
Hippocampus Samples or PC12 cell were homogenized
and dissociated in RIPA lysis buffer (Beyotime Institute of
Biotechnology) and protein concentration was quantified via a
Bicinchoninic Acid assay (Beyotime Institute of Biotechnology).
Equal amounts of protein (50 µg) were separated by 12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. Subsequently,
membranes were blocked in Tris buffered saline with 0.1% Tween-20
(TBST) containing 5% milk prior for 1 h at 37°C to incubation with
the following primary antibodies overnight at 4°C: Anti-NF-κB p65
(1:1,000; sc-71677), anti-endothelial nitric oxide synthase (eNOS,
1:1,000; sc-136977), anti-peroxisome proliferator-activated
receptor γ (PPARγ, 1:1,000; sc-1981), anti-AMP-activated protein
kinase (AMPK, 1:1,000; cat. no. sc-74461), anti-heat shock 70 kDa
protein (HSP70, 1:1,000; sc-6242), anti-SIRT1 (1:1,000; sc-135791),
anti-Nrf2 (1:1,000; sc-722), anti-heme oxygenase 1 (HO-1, 1:1,000;
sc-136960) and anti-β-actin (1:5,000; sc-1615; all Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following three washes with
TBST, the membrane was incubated with goat anti-rabbit
IgG-horseradish peroxidase (1:5,000; sc-2004; Santa Cruz
Biotechnology, Inc.) at room temperature for 2 h with shaking
according to the manufacturer's protocols. Bands were visualized
using BeyoECL Plus (Beyotime Institute of Biotechnology) and
densitometry analysis was performed with Image Lab 3.0 (Bio-Rad
Laboratories, Inc.).
Caspase-3 activity
Hippocampal samples or PC12 cell were homogenized
and dissociated in RIPA lysis buffer. Activity was determined with
a Caspase-3 Activity kit (cat. no. C1115; Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. The
sample mixture was incubated at 37°C for 120 min and absorbance
values were measured at 405 nm.
Statistical analysis
All data are presented as the mean ± standard
deviation (n=3). All statistical tests were conducted using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Statistical significance was
analyzed using one-way analysis of variance followed by Dunnett's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
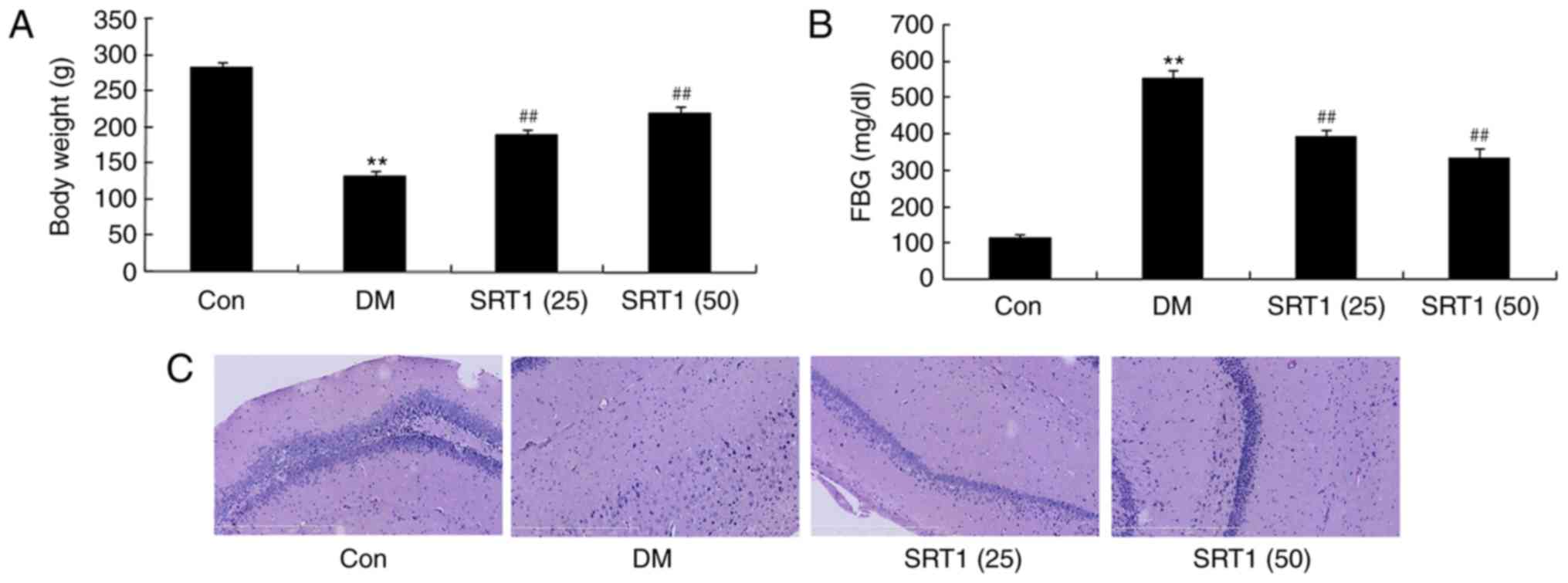
SRT1720 increases body weight and
reduces FBG
The body weight of the DM group was significantly
decreased compared with the control group. Notably, at the end of
SRT1720 treatment, the body weight of both SRT1720-treated groups
was significantly increased compared with the DM group (Fig. 1A). The FBG of DM rats was
significantly higher compared normal control rats. SRT1720
treatment at both concentrations significantly decreased FBG
compared with the DM group (Fig.
1B). In the DM group, the number of neurocyte was reduced,
compared with in the control group; treatment with SRT1720 appeared
to have increased the number of neurocyte compared with the DM
group (Fig. 1C). These results
indicated that SRT1720 treatment may increase body weight and
reduce FBG in T2DM rats.
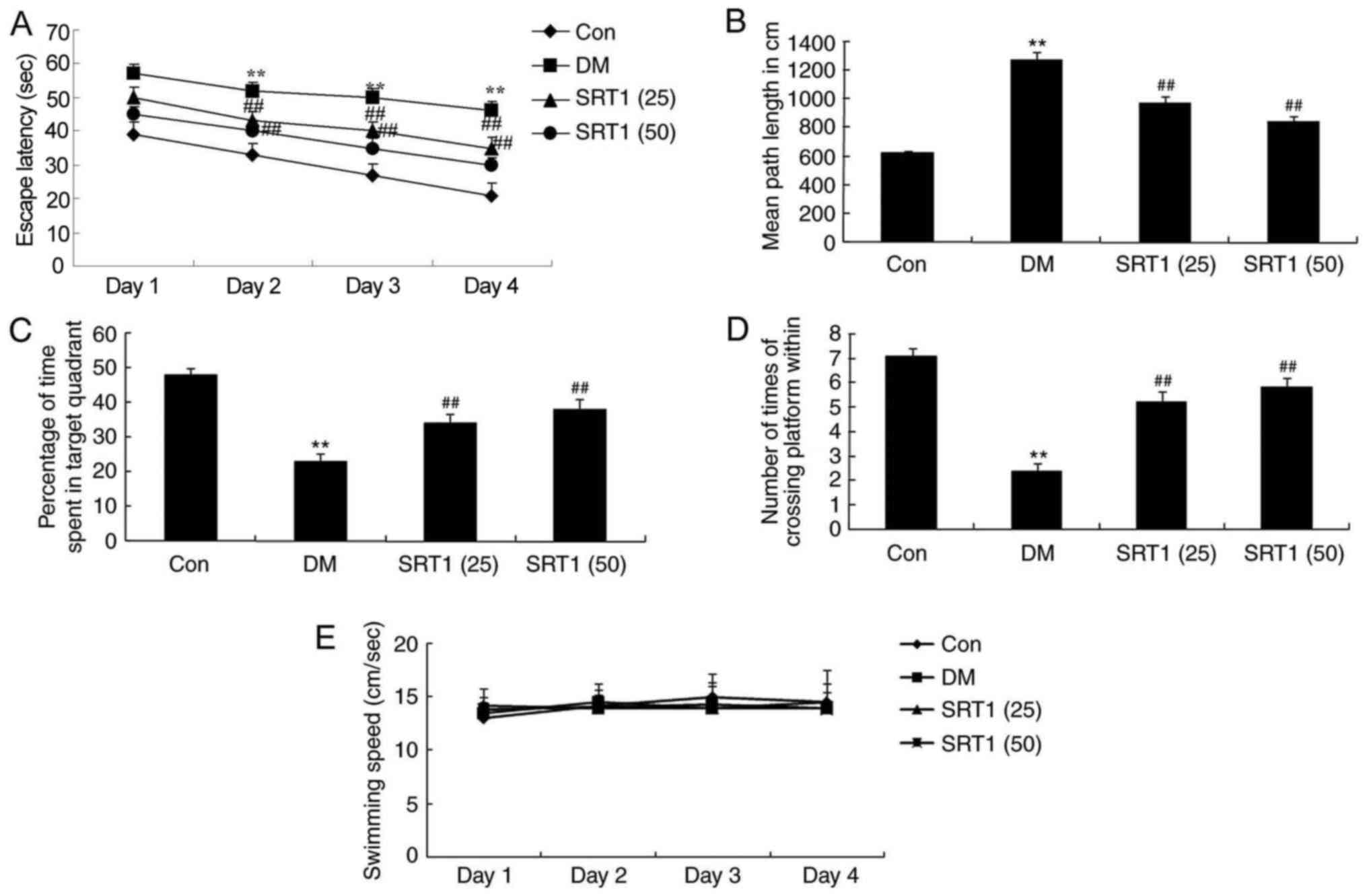
SRT1720 improves cognitive
function
In the Morris water maze testing, a significantly
increased escape latency time was recorded in DM rats after 2–4
days of training, compared with control rats (Fig. 2A). Compared with the DM group,
SRT1720 treatment significantly reduced the escape latency time
(Fig. 2A). Additionally, the mean
path length was notably increased in the DM group compared with the
control group rats after 5 days of training; SRT1720 treatment
reversed this effect and the mean path length was significantly
reduced compared with the DM rats (Fig. 2B). Furthermore, on day 5, DM rats
spent significantly less time in the target quadrant compared with
the control group rats (Fig. 2C).
The frequency that the animals crossed the former platform location
was also markedly reduced in DM rats compared with control rats
(Fig. 2D). SRT1720 treatment in DM
rats significantly reversed these alterations to results that were
similar to those of the control group rats (Fig. 2C and D). No significant difference
in swimming speed was determined among experimental groups
(Fig. 2E). Taken together, these
results demonstrated that SRT1720 may reduce cognitive impairment
in rats with T2DM.
SRT1720 reverses DM-induced
alterations in GSH-PX, GSH, SOD and MDA levels
Compared with the control group, the results of
ELISA demonstrated that the levels of GSH-PX (Fig. 3A), GSH (Fig. 3B) and SOD (Fig. 3C) were notably reduced in DM rats.
However, SRT1720 treatment significantly increased these levels
compared with the DM group (Fig.
3A-C). By contrast, MDA levels were significantly increased in
DM rats compared with the control group, and SRT1720 treatment
markedly reduced this MDA content in DM rats (Fig. 3D). These results indicate that
SRT1720 may reduce the level of oxidative stress in rats with
T2DM.
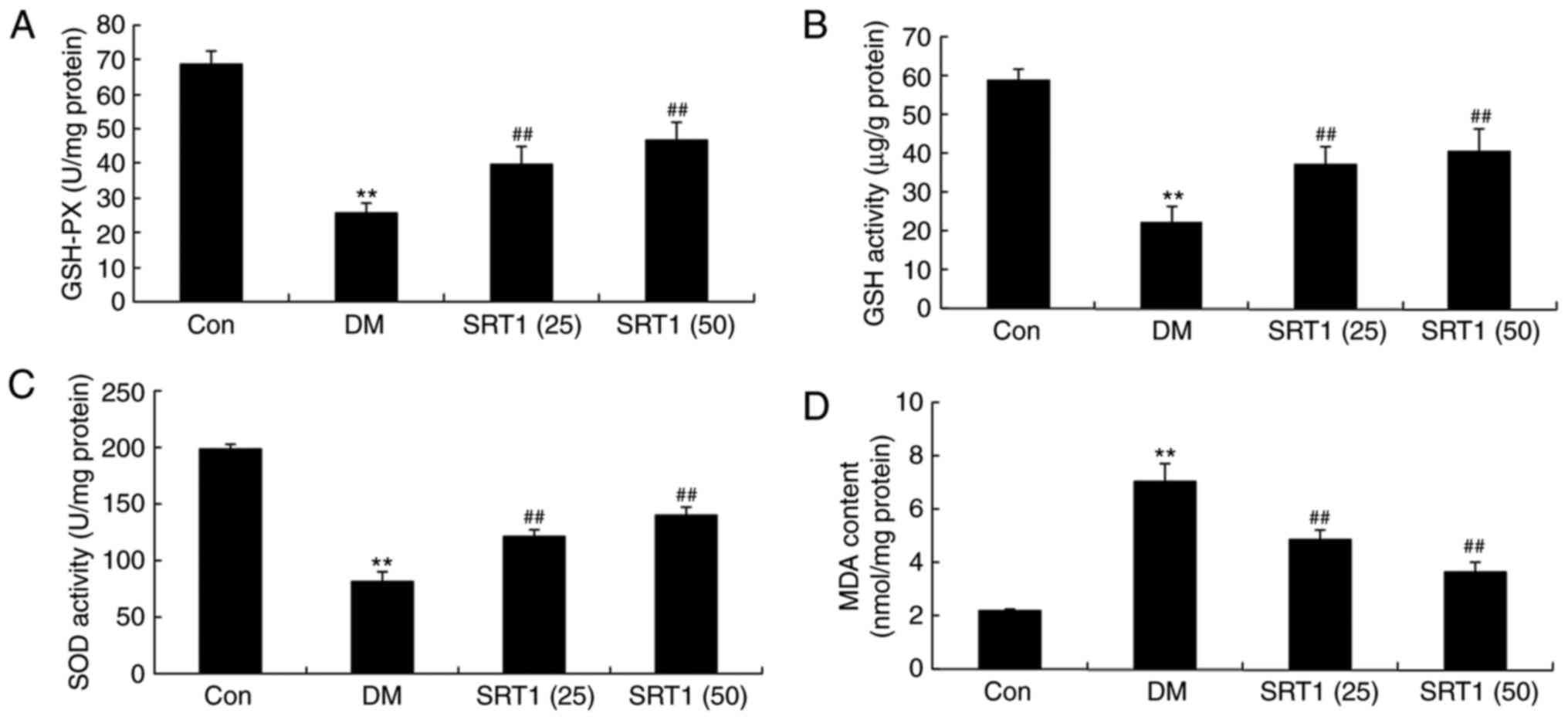 | Figure 3.Effect of SRT1720 on DM-induced
alterations in GSH-PX, GSH, SOD and MDA levels in hippocampal
tissue of a rat model of type 2 DM. ELISA was performed to
determine the levels of (A) GSH-PX, (B) GSH, (C) SOD and (D) MDA in
control, DM and SRT1720-treated DM rats. **P<0.01 vs. control
group; ##P<0.01 vs. DM group. DM, diabetes mellitus;
GSH, glutathione; GSH-PX, glutathione peroxidase; SOD, superoxide
dismutase; MDA, malondialdehyde; Con, control; SRT1 (25), 25 mg/kg SRT1720; SRT1 (50), 50
mg/kg SRT1720. |
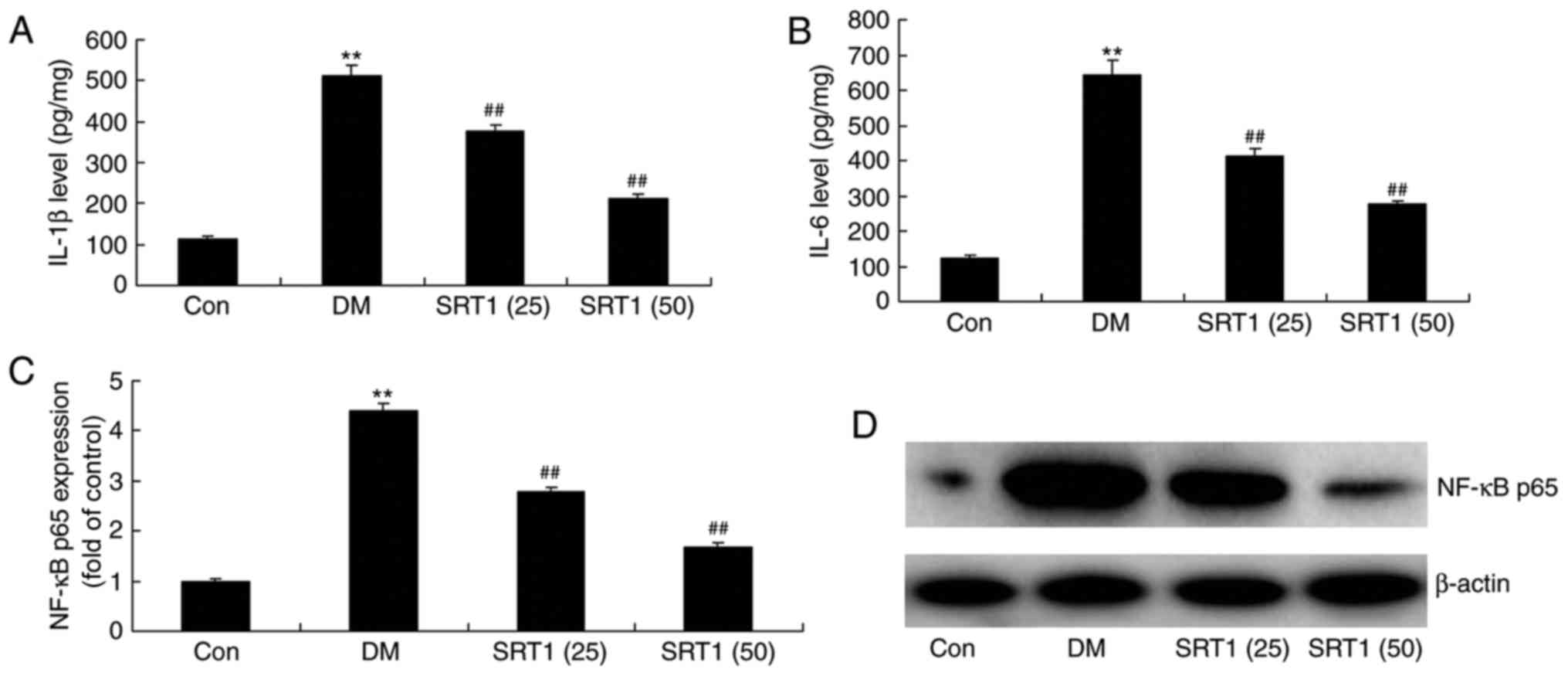
SRT1720 reduces NF-κB p65, IL-1β and
IL-6 expression in DM rats
Compared with the control group, ELISA results
demonstrated that IL-1β (Fig. 4A)
and IL-6 (Fig. 4B) expression was
significantly increased in DM rats. In addition, western blot
analysis demonstrated that the protein expression of NF-κB p65 in
DM rats was also significantly increased compared with the control
group (Fig. 4C and D). SRT1720
treatment significantly reversed these effects and the expression
of these proteins was significantly reduced compared with DM rats.
These results indicate that SRT1720 may reduce inflammation in T2DM
rats.
SRT1720 increases eNOS, PPARγ and AMPK
expression
The protein expression of eNOS, PPARγ and AMPK was
detected by western blot analysis (Fig. 5A). Compared with the control group,
eNOS (Fig. 5A and B), PPARγ
(Fig. 5A and C) and AMPK (Fig. 5A and D) protein expression was
significantly reduced in the DM model rats. However, SRT1720
treatment significantly increased eNOS, PPARγ and AMPK protein
expression in DM rats.
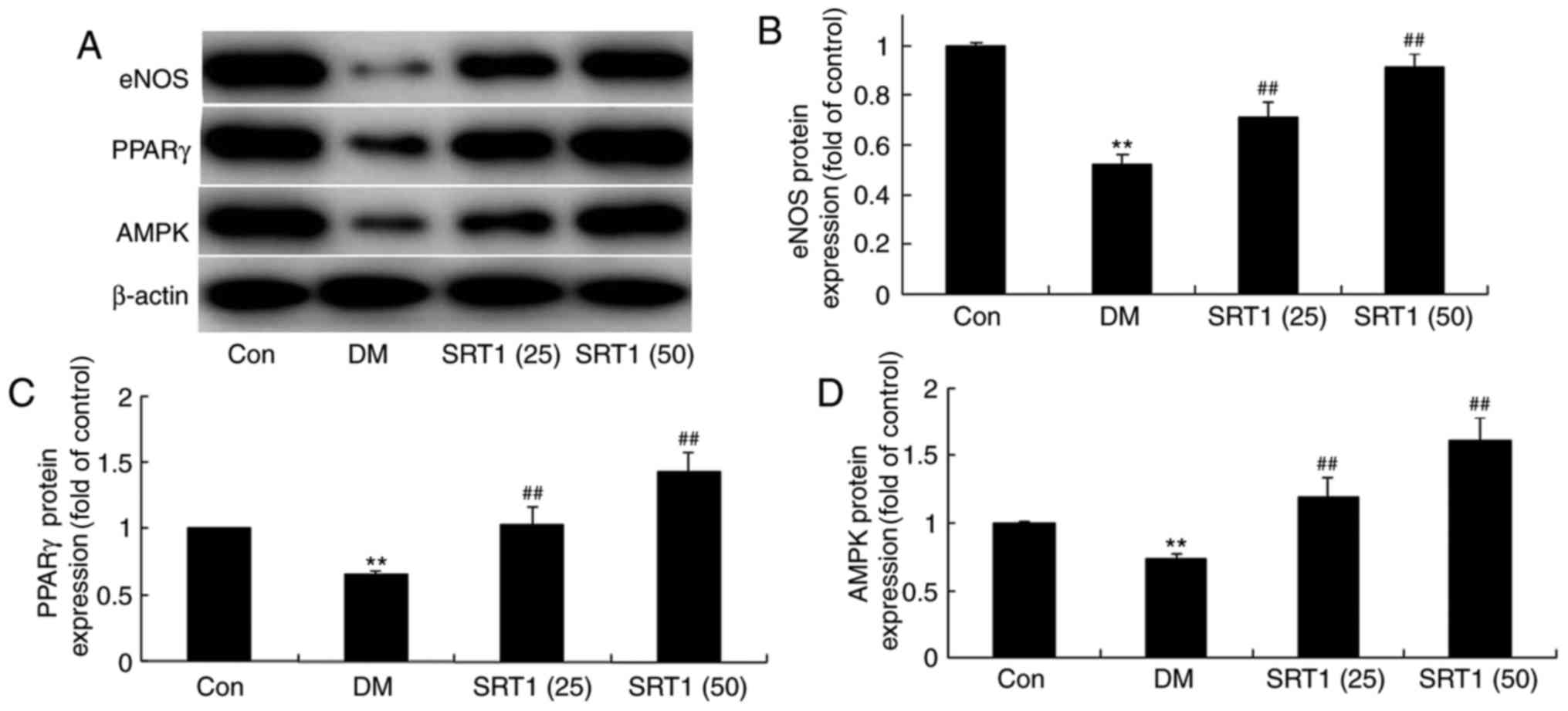 | Figure 5.Effect of SRT1720 on DM-induced
alterations in hippocampal tissue of DM rats. (A) Protein
expression levels of eNOS, PPARγ and AMPK were detected by western
blot analysis. Densitometric analysis of western blotting results
was performed to quantify the protein levels of (B) eNOS, (C) PPARγ
and (D) AMPK in control, DM and SRT1720-treated DM rats. The
results demonstrated that the expression of eNOS, PPARγ and AMPK
was increased in the SRT1720-treated groups compared with the DM
group. **P<0.01 vs. control group; ##P<0.01 vs. DM
group. DM, diabetes mellitus; eNOS, endothelial nitric oxide
synthase; PPARγ, peroxisome proliferator-activated receptor γ;
AMPK, AMP-activated protein kinase; Con, control; SRT1 (25), 25 mg/kg SRT1720; SRT1 (50), 50
mg/kg SRT1720. |
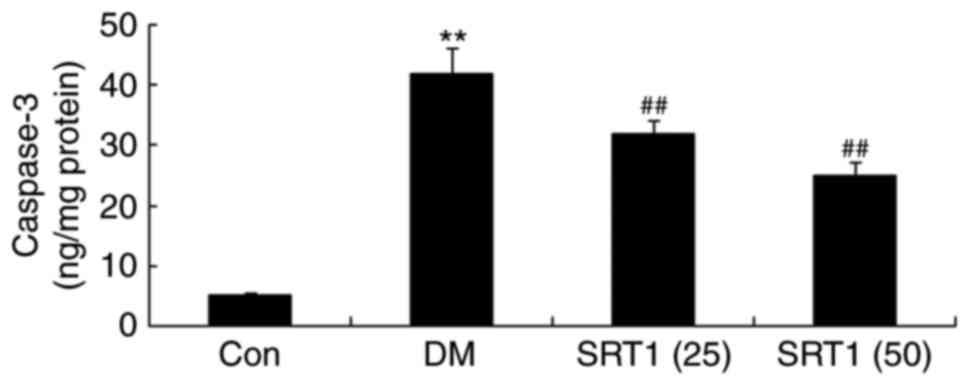
SRT1720 decreases the activity of
caspase-3
Caspase-3 activity was significantly upregulated in
DM rats compared with the control group. Additionally, compared
with the DM group, caspase-3 activity was markedly downregulated in
the SRT1720 treatment groups (Fig.
6).
Effect of SRT1720 on the protein
expression of HSP70, SIRT1, Nrf2 and HO-1
The protein expression of HSP70, SIRT1, Nrf2 and
HO-1 was determined by western blot analysis (Fig. 7A). It was revealed that HSP70
expression was significantly increased (Fig. 7B), while SIRT1 (Fig. 7C), Nrf2 (Fig. 7D) and HO-1 (Fig. 7E) expression was markedly
suppressed, in the DM group compared with the control group.
Furthermore, compared with the DM group, HSP70 expression was
significantly reduced (Fig. 7B),
and SIRT1, Nrf2 and HO-1 expression was markedly increased, in the
SRT1720 treatment groups. These results indicate that SRT1720 may
reduce cognitive decline in diabetic rats through antioxidative and
anti-inflammatory mechanisms, potentially via a SIRT1/Nrf2-NF-κB
signaling pathway.
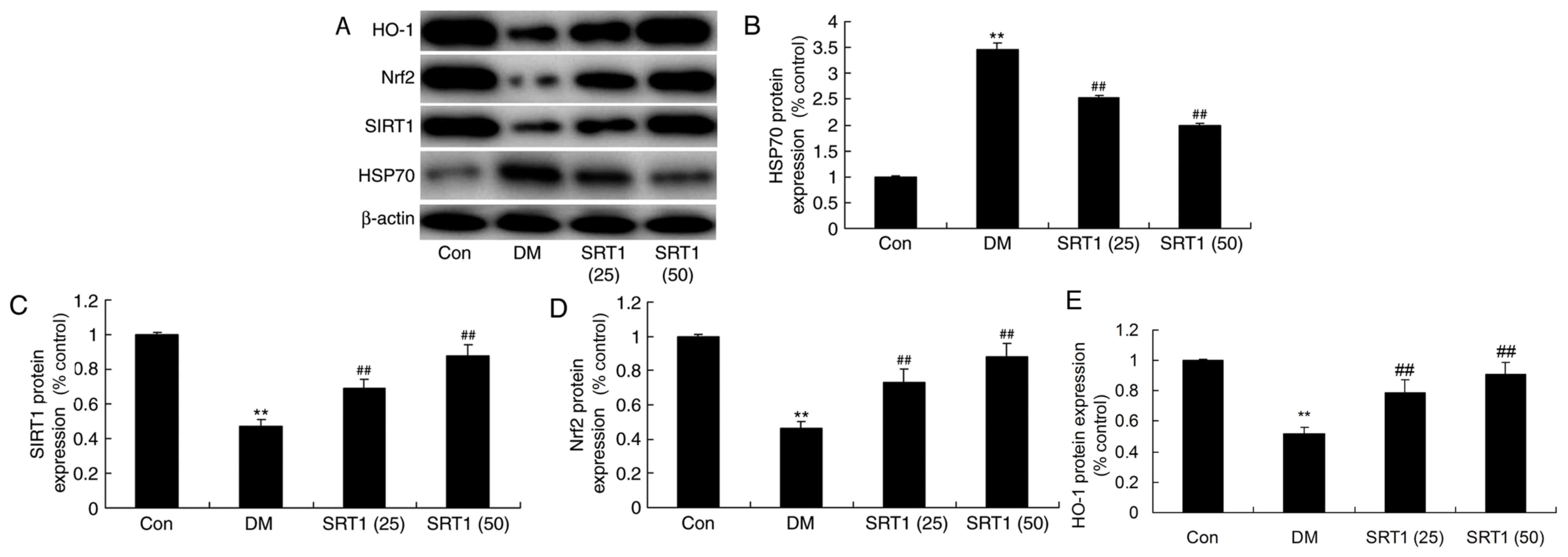 | Figure 7.Effect of SRT1720 on DM-induced
alterations in HSP70, SIRT1, Nrf2 and HO-1 protein expression in
hippocampal tissue. (A) Protein expression levels of HSP70, SIRT1,
Nrf2 and HO-1 were detected by western blot analysis. Densitometric
analysis of western blotting results was performed to quantify the
protein levels of (B) HSP70, (C) SIRT1, (D) Nrf2 and (E) HO-1 in
control, DM and SRT1720-treated DM rats. The results demonstrated
that alterations observed in DM rats compared with control rats
were reversed by SRT1720 treatment. **P<0.01 vs. control group;
##P<0.01 vs. DM group. DM, diabetes mellitus; HSP70,
heat shock 70 kDa protein; SIRT1, sirtuin 1; Nrf2, nuclear factor
erythroid 2-related factor 2; HO-1, heme oxygenase 1; Con, control;
SRT1 (25), 25 mg/kg SRT1720; SRT1
(50), 50 mg/kg SRT1720. |
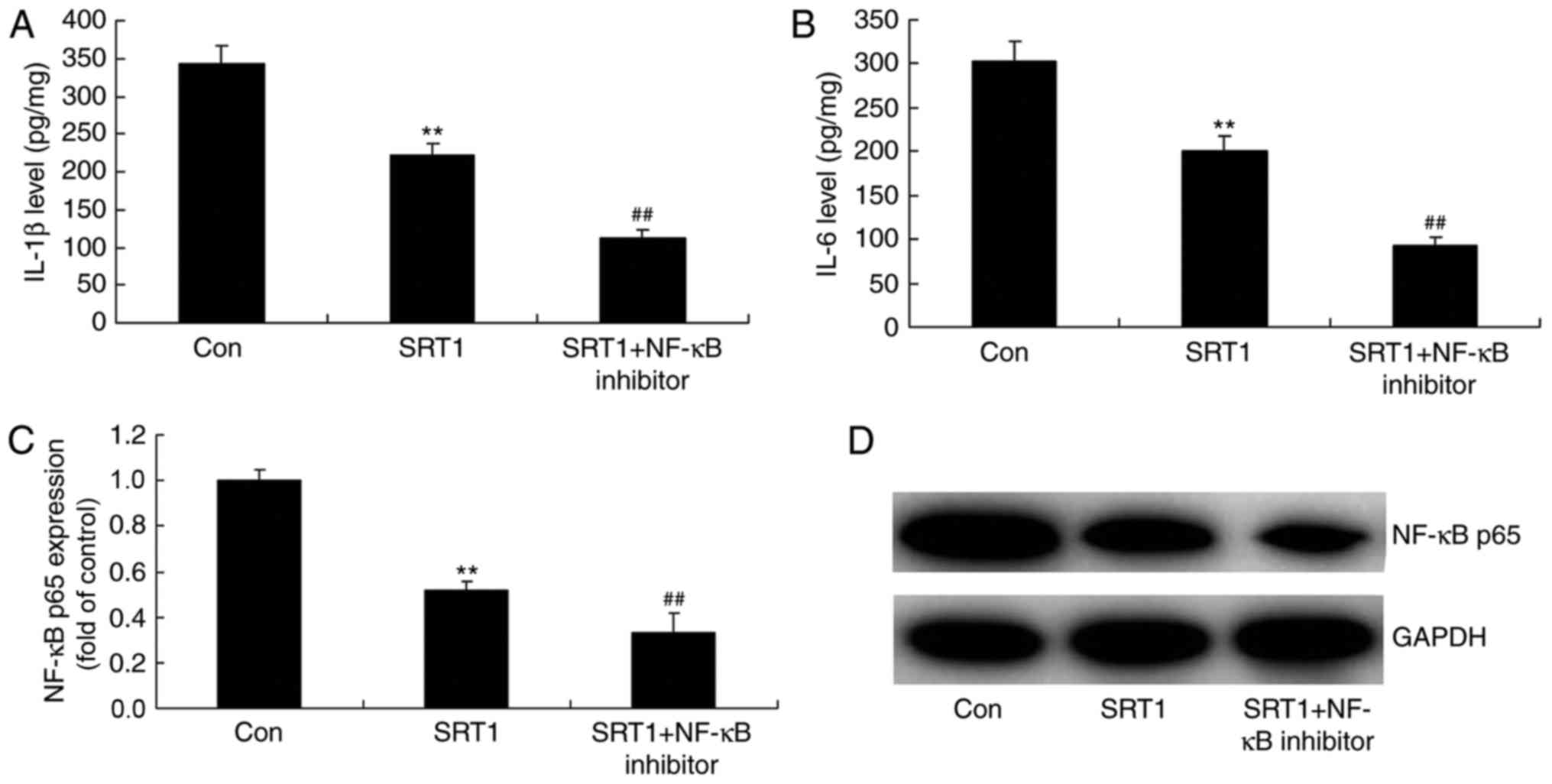
NF-κB inhibitor enhances the
anti-inflammatory effects of SRT1720
To identify the role of NF-κB in the
anti-inflammatory effects of SRT1720, the protein expression of
NF-κB, IL-1β and IL-6 was analyzed in a PC12 diabetic cell model
treated with an NF-κB inhibitor (Fig.
8). ELISA was performed to measure IL-1β (Fig. 8A) and IL-6 (Fig. 8B) levels, and western blotting was
performed to measure NF-κB p65 levels (Fig. 8C and D). The results demonstrated
that SRT1720 treatment significantly reduced NF-κB p65, IL-1β and
IL-6 expression compared with control diabetic cells. Furthermore,
compared with SRT1720 treatment alone, combination treatment with
an NF-κB inhibitor further decreased the expression of these
proteins in PC12 diabetic model cells.
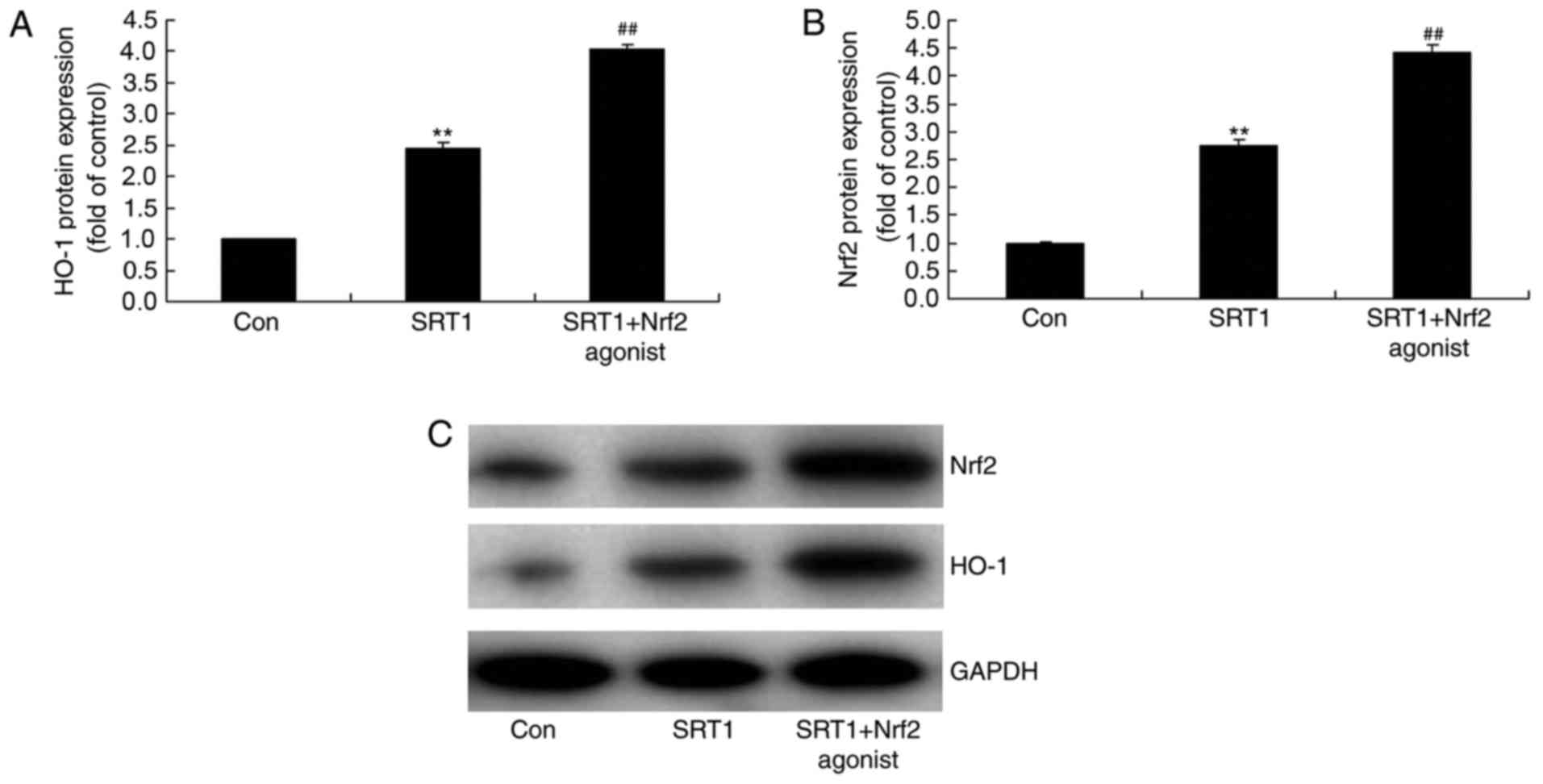
Nrf2 enhances the antioxidative
effects of SRT1720
To identify the role of Nrf2 in the antioxidative
effects of SRT1720, the protein expression of HO-1 and Nrf2 was
detected in a PC12 diabetic cell model treated with Nrf2 agonist
using western blot analysis (Fig.
9). Compared with the control diabetic cells, SRT1720 treatment
significantly increased the protein expression of HO-1 and Nrf2,
while combined treatment with SRT1720 and Nrf2 agonist further
increased the expression of Nrf2 and HO-1 (Fig. 9). Additionally, the levels of
GSH-PX (Fig. 10A), GSH (Fig. 10B), SOD (Fig. 10C) and MDA (Fig. 10D) were detected by ELISA in a
PC12 diabetic cell model treated with Nrf2 agonist. Compared with
the control group, GSH-PX, GSH and SOD levels were significantly
increased in the SRT1720 treatment group (Fig. 10A-C), an effect that was enhanced
in the combination treatment group. By contrast, MDA levels were
significantly decreased in PC12 cells treated with SRT1720,
compared with control group, and combination treatment with Nrf2
agonist further decreased MDA levels (Fig. 10D).
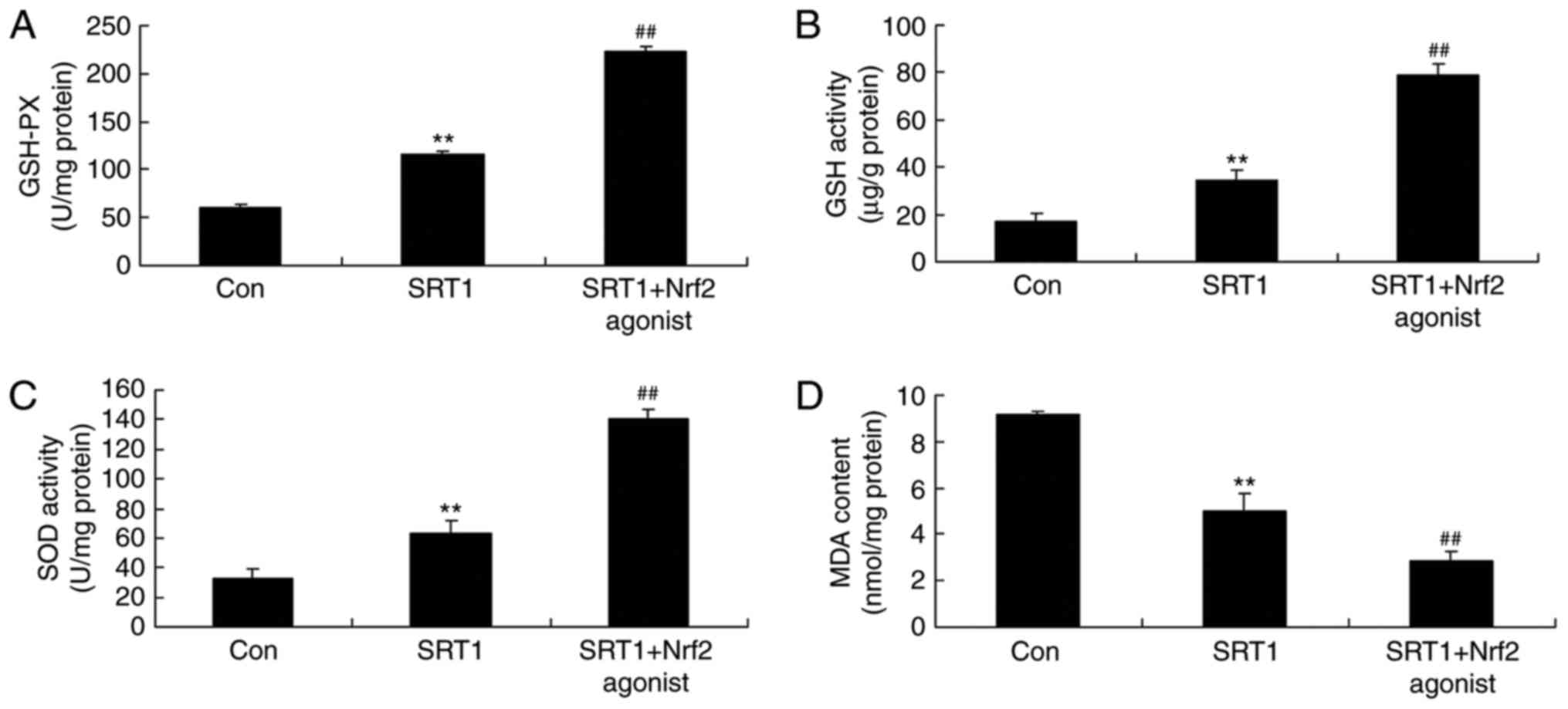 | Figure 10.Effect of a Nrf2 agonist on GSH-PX,
GSH, SOD and MDA levels in PC12 diabetic model cells treated with
SRT1720. ELISA was performed to determine the levels of (A) GSH-PX,
(B) GSH, (C) SOD and (D) MDA in control, SRT1720-treated and
SRT1720 + Nrf2 agonist-treated PC12 diabetic model cells. Curcumin
was employed as the Nrf2 agonist. **P<0.01 vs. control group;
##P<0.01 vs. SRT1 group. Nrf2, nuclear factor
erythroid 2-related factor 2; GSH, glutathione; GSH-PX, glutathione
peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde; Con,
control; SRT1, 50 mg/kg SRT1720; SRT1 + Nrf2 agonist, 50 mg/kg
SRT1720 + 25 µM curcumin. |
Discussion
Population ageing and geriatric disease have become
important social and medical problems as social economy and medical
science have advanced (10). Among
the various types of geriatric disease, the incidence of cognitive
impairment has distinctly increased, leading to severe impairments
in the quality of life of patients and heavy burdens to the
families of patients and society (11). The present study demonstrated that
SRT1720 reversed reductions in body weight, reduced FBG and
improved cognitive function in a rat model of T2DM. Furthermore,
SRT1720 upregulated GSH-PX, GSH and SOD levels, and downregulated
levels of MDA, in DM rats. Consistent with the findings of the
present study, Ding et al (12) reported that improvements observed
in rat cognitive deficits following hyperbaric oxygen
preconditioning was mediated by SIRT1. These results demonstrate
that SRT1720 may have potential as a novel drug for cognitive
impairment in diabetes.
NF-κB is a major immunomodulatory factor that has
important roles in cells and peripheral body fluids, and is among
the strongest immunomodulatory factors within the body (13). T2DM pathogenesis typically involves
b cell injury in the islet of Langerhans, which is caused by the
chronic activation of nonspecific immunity by increased blood
glucose, saturated fatty acid and adipose tissue levels (14). In the present study, the rat
tissues were analyzed by western blotting. The results of the
present study revealed that SRT1720 significantly downregulated
NF-κB and upregulated eNOS expression in DM rats.
AMPK is involved in the regulation of
glycometabolism and fat metabolism, and leads to effects on various
functions, including energy metabolism and signal transduction
(15). AMPK activity is regulated
and controlled by the AMP/ATP ratio (16). The present study revealed that
SRT1720 markedly increased PPARγ and AMPK protein expression, and
reduced caspase-3 activity, in DM rats. Yang et al (15) demonstrated that the upregulation of
SIRT1-AMPK ameliorated liver injury in hepatic stellate cells
through PPARγ expression. AMPK activity is a major regulator of
metabolic homeostasis, which is regulated by reactive oxygen
species (17). The present study
revealed that SRT1720 increased eNOS and AMPK expression in DM
rats. Similar results were reported by Liu et al (18), who demonstrated that HSP70
protected mice against lung ischemia/reperfusion injury through the
SIRT1/AMPK/eNOS signaling pathway.
The antioxidative and anti-neurotoxic effects of
Nrf2 have been widely recognized (5,19).
Additionally, therapy targeting the kelch-like ECH-associated
protein 1 (Keap1)-Nrf2-antioxidant response element (ARE) signaling
pathway has become the focus of research at present (19). It has been suggested that
inhibiting the Keap1-Nrf2-ARE pathway may result in endothelial
dysfunction, vascular endothelial dysfunction and insulin
resistance (19). Therefore,
regulation of Nrf2 expression is expected to be a potential means
for the prevention and treatment of diabetes and its complications.
The present study demonstrated that SRT1720 treatment significantly
reversed the inhibition of Nrf2 and HO-1 expression observed in DM
rats. Furthermore, when SRT1720 was combined with a Nrf2 agonist,
curcumin, Nrf2 and HO-1 expression was further induced in PC12
cells treated with SRT1720. Additionally, the Nrf2 agonist
increased the levels of GSH-PX, GSH and SOD, and inhibited MDA
levels, in PC12 cells treated with SRT1720. Xue et al
(20) reported that SIRT1 may be
involved in a Nrf2/antioxidant defense pathway against transient
focal cerebral ischemia. SRT1720 may also regulate the
Nrf2/HO-1/antioxidant pathway in diabetic cognitive impairment. Liu
et al (21) demonstrated
that licochalcone A reduced oxygen-glucose deprivation/reperfusion
damage by attenuating oxidative stress injury and the inflammatory
response via SIRT1/Nrf2 signaling in rat primary cortical neurons.
The results of the present study revealed that SRT1720 may regulate
the SIRT1/Nrf2 pathway to inhibit oxidative stress and cognitive
dysfunction in diabetes.
SIRT1 is essential for normal cognitive function and
synaptic plasticity (22). It has
been demonstrated that SIRT1-knockout mice exhibit immediate memory
defects, short-term and long-term associative memory impairment,
dendritic tree branching of hippocampal neuron and reductions in
neurite length and complexity. This suggests that SIRT1 may be
essential in normal spatial learning and the regulation of synaptic
plasticity (23). Long-term
calorie restriction has been reported to inhibit NF-κB, thus
reducing its proinflammatory effect (24). The present study revealed that an
NF-κB inhibitor, JSH-23, further suppressed NF-κB expression, as
well as IL-1β and IL-6 levels, in PC12 cells treated with SRT1720.
Liu et al (25) reported
that SIRT1 may mediate NF-κB in human alveolar epithelial cells.
Taken together, these results demonstrate that SRT1720 may regulate
the inflammatory defense system to suppress NF-κB and subsequently
reduce cognitive impairment in diabetes.
In conclusion, the results of the present study
indicate that SRT1720 may possess antioxidant and anti-inflammatory
properties and may reduce cognitive decline through a
Nrf2-NF-κB-dependent mechanism in T2DM rats. These findings
indicate that SRT1720 may have potential as a drug for the
treatment of cognitive decline in diabetes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QZ made substantial contributions to the design of
the study; FW, YS, RZ and XG performed the experiments; QZ and FW
analyzed the data; QZ wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chinese PLA General Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shikora S, Toouli J, Herrera MF, Kulseng
B, Zulewski H, Brancatisano R, Kow L, Pantoja JP, Johnsen G,
Brancatisano A, et al: Vagal blocking improves glycemic control and
elevated blood pressure in obese subjects with type 2 diabetes
mellitus. J Obes. 2013:2456832013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fonseca VA, Ferrannini E, Wilding JP,
Wilpshaar W, Dhanjal P, Ball G and Klasen S: Active- and
placebo-controlled dose-finding study to assess the efficacy,
safety and tolerability of multiple doses of ipragliflozin in
patients with type 2 diabetes mellitus. J Diabetes Complications.
27:268–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang L, Stone RC, Stojadinovic O, Ramirez
H, Pastar I, Maione AG, Smith A, Yanez V, Veves A, Kirsner RS, et
al: Integrative analysis of miRNA and mRNA paired expression
profiling of primary fibroblast derived from diabetic foot ulcers
reveals multiple impaired cellular functions. Wound Repair Regen.
24:943–953. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Sun XJ, Chen J, Hu ZW, Wang L, Gu
DM and Wang AP: Increasing the miR-126 expression in the peripheral
blood of patients with diabetic foot ulcers treated with maggot
debridement therapy. J Diabetes Complications. 31:241–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madhyastha R, Madhyastha H, Pengjam Y,
Nakajima Y, Omura S and Maruyama M: NFkappaB activation is
essential for miR-21 induction by TGFβ1 in high glucose conditions.
Biochem Biophys Res Commun. 451:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang T, He R, Zhao J, Mei JC, Shao MZ, Pan
Y, Zhang J, Wu HS, Yu M, Yan WC, et al: Negative pressure wound
therapy inhibits inflammation and upregulates activating
transcription factor-3 and downregulates nuclear factor-kB in
diabetic patients with foot ulcerations. Diabetes Metab Res Rev.
33:2017.doi: 10.1002/dmrr.2871. View Article : Google Scholar
|
|
7
|
Bhat MA and Gandhi G: Elevated oxidative
DNA damage in patients with coronary artery disease and its
association with oxidative stress biomarkers. Acta Cardiol. 1–8.
2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang B, Xu B, Zhao H, Wang YB, Zhang J, Li
CW, Wu Q, Cao YK, Li Y and Cao F: Dioscin protects against coronary
heart disease by reducing oxidative stress and inflammation via
Sirt1/Nrf2 and p38 MAPK pathways. Mol Med Rep. 18:973–980.
2018.PubMed/NCBI
|
|
9
|
Milne JC, Lambert PD, Schenk S, Carney DP,
Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al: Small
molecule activators of SIRT1 as therapeutics for the treatment of
type 2 diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilmot EG, Davies MJ, Edwardson CL, Gorely
T, Khunti K, Nimmo M, Yates T and Biddle SJ: Rationale and study
design for a randomised controlled trial to reduce sedentary time
in adults at risk of type 2 diabetes mellitus: Project stand
(Sedentary Time ANd diabetes). BMC Public Health. 11:9082011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah PS, Todkar JS and Shah SS:
Effectiveness of laparoscopic sleeve gastrectomy on glycemic
control in obese Indians with type 2 diabetes mellitus. Surg Obes
Relat Dis. 6:138–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding P, Ren D, He S, He M, Zhang G, Chen
Y, Sang H, Peng Z and Yan W: Sirt1 mediates improvement in
cognitive defects induced by focal cerebral ischemia following
hyperbaric oxygen preconditioning in rats. Physiol Res.
66:1029–1039. 2017.PubMed/NCBI
|
|
13
|
Palmer R, Nyman E, Penney M, Marley A,
Cedersund G and Agoram B: Effects of IL-1β-blocking therapies in
type 2 diabetes mellitus: A quantitative systems pharmacology
modeling approach to explore underlying mechanisms. CPT
Pharmacometrics Syst Pharmacol. 3:e1182014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raja L, Palanivelu S and Panchanatham S:
Anti-inflammatory property of Kalpaamruthaa on myocardium in type 2
diabetes mellitus induced cardiovascular complication.
Immunopharmacol Immunotoxicol. 35:119–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Zhao P, Wan D, Zhou Q, Wang C, Shu
G, Mei Z and Yang X: Antidiabetic effect of methanolic extract from
berberis julianae Schneid. via activation of AMP-activated protein
kinase in type 2 diabetic mice. Evid Based Complement Alternat Med.
2014:1062062014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russo GL, Russo M and Ungaro P:
AMP-activated protein kinase: A target for old drugs against
diabetes and cancer. Biochem Pharmacol. 86:339–350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang RH, Lin J, Hou XH, Cao R, Yu F, Liu
HQ, Ji AL, Xu XN, Zhang L and Wang F: Effect of docosahexaenoic
acid on hippocampal neurons in high-glucose condition: Involvement
of PI3K/AKT/nuclear factor-kB-mediated inflammatory pathways.
Neuroscience. 274:218–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Xu J, Fang C, Shi C, Zhang X, Yu B
and Yin Y: Over-expression of heat shock protein 70 protects mice
against lung ischemia/reperfusion injury through SIRT1/AMPK/eNOS
pathway. Am J Transl Res. 8:4394–4404. 2016.PubMed/NCBI
|
|
19
|
Kuo YR, Chien CM, Kuo MJ, Wang FS, Huang
EY and Wang CJ: Endothelin-1 expression associated with lipid
peroxidation and nuclear factor-kB activation in type 2 diabetes
mellitus patients with angiopathy and limb amputation. Plast
Reconstr Surg. 137:187e–195e. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ,
Li T, Fan J, Peng ZW and Yan WJ: Nrf2/antioxidant defense pathway
is involved in the neuroprotective effects of Sirt1 against focal
cerebral ischemia in rats after hyperbaric oxygen preconditioning.
Behav Brain Res. 309:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Ma Y, Wei X and Fan T:
Neuroprotective effect of licochalcone A against oxygen-glucose
deprivation/reperfusion in rat primary cortical neurons by
attenuating oxidative stress injury and inflammatory response via
the SIRT1/Nrf2 pathway. J Cell Biochem. 119:3210–3219. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsarouhas K, Tsitsimpikou C, Papantoni X,
Lazaridou D, Koutouzis M, Mazzaris S, Rezaee R, Mamoulakis C,
Georgoulias P, Nepka C, et al: Oxidative stress and kidney injury
in trans-radial catheterization. Biomed Rep. 8:417–425.
2018.PubMed/NCBI
|
|
23
|
Prasad K and Dhar I: Oxidative stress as a
mechanism of added sugar-induced cardiovascular disease. Int J
Angiol. 23:217–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bicer M, Senturk T, Yanar M, Tutuncu A,
Oral AY, Ulukaya E, Serdar Z and Signak IS: Effects of off-pump
versus on-pump coronary artery bypass grafting: Apoptosis,
inflammation and oxidative stress. Heart Surg Forum. 17:E271–E276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Yang T, Sun T and Shao K:
SIRT1-mediated regulation of oxidative stress induced by
Pseudomonas aeruginosa lipopolysaccharides in human alveolar
epithelial cells. Mol Med Rep. 15:813–818. 2017. View Article : Google Scholar : PubMed/NCBI
|