Introduction
Diet-induced diabetes is one of the leading causes
of mortality worldwide. The global prevalence of diabetes is
estimated to affect 439 million adults (aged 20–79 years) by 2030
(1). Between 2010 and 2030, it has
been predicted that there will be a 69% increase in the number of
patients with diabetes in developing countries (2). Notably, it has been proposed that 40%
of patients with type 2 diabetes mellitus may develop renal
fibrosis, which is a major cause of end-stage renal disease.
However, current treatment strategies for renal fibrosis are the
same in diabetic patients as in non-diabetic patients, and they do
not address the underlying causes of renal dysfunction.
Mitochondria are the energy centers of the cell and
have been reported to serve a critical role in regulating kidney
function (3). Increasing evidence
suggests that kidney complications associated with diabetes
converge on mitochondria as an epicenter for diabetes-induced renal
fibrosis (4). The hallmarks of
renal fibrosis include glomerular cell apoptosis due to high
glucose-induced stress, and renal interstitial fibrosis caused by
accumulation of the extracellular matrix, which contributes to the
irreversible decline in renal function. Notably, mitochondrial
injury is observed under all of these situations. Mitochondria are
particularly susceptible to diabetic insults and, at the molecular
level, damaged mitochondria produce excessive reactive oxygen
species (ROS), release pro-apoptotic factors into the cytoplasm,
impair cellular energy metabolism and activate caspase-9-dependent
apoptotic signaling (5–8), thus augmenting renal injury and
promoting kidney fibrosis. Therefore, protecting mitochondrial
function and preventing mitochondria-initiated glomerular apoptosis
may delay the development of diabetes-associated renal
fibrosis.
Recently, several studies have documented the
involvement of melatonin in kidney protection (9). Melatonin presumably enters
mitochondria through melatonin receptor-dependent and -independent
manners (10,11). Measurement of the sub-cellular
distribution of melatonin revealed that the concentration of
melatonin in mitochondria greatly exceeds that in blood (12), thus suggesting that melatonin may
specifically target mitochondria. In addition, there is
accumulating evidence demonstrating the protective role of
melatonin in diabetes-associated renal fibrosis and glomerular
apoptosis (13,14). Melatonin can inhibit
nicotinamide-adenine dinucleotide oxidase activity (15), reduce Raf-1 proto-oncogene,
serine/threonine kinase/extracellular signal-regulated kinases
signaling (16), activate the
cyclic guanosine 3′,5′-monophosphate-protein kinase G axis
(17) and suppress the nuclear
receptor subfamily 4 group A member 1/DNA-dependent protein kinase,
catalytic subunit/tumor protein p53 (p53) cascade (18), thus favoring the survival of
glomeruli and inhibiting the progression of diabetes-induced renal
fibrosis. Melatonin has also been reported to protect mitochondrial
function and structure in the context of high glucose stimulus
(19,20). However, it remains unclear as to
whether melatonin has the ability to preserve mitochondrial
homeostasis during the development of diabetic renal fibrosis and,
if so, what molecular mechanisms link melatonin to mitochondrial
protection under high glucose attack.
It has previously been suggested that 5′adenosine
monophosphate-activated protein kinase (AMPK) signaling is closely
associated with diabetic renal dysfunction (20). AMPK, a sensor of cellular energy
production, is capable of regulating mitochondrial dynamics and
controlling mitophagy (21–24).
Furthermore, several studies have identified melatonin as the
upstream trigger of AMPK activation (19,25).
However, whether AMPK is required for the protective role of
melatonin in mitochondrial damage upon hyperglycemia exposure
remains unclear. The aim of the present study was to investigate
the effects of melatonin on mitochondrial homeostasis and kidney
fibrosis under diabetic stress, as well as to understand the
underlying mechanisms.
Materials and methods
Animal and cellular experiments
Male wild-type (WT) mice (n=60, weight, 250±10 g)
and AMPK knockout (AMPK−/−) mice (n=60, weight, 250±10
g) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). All
mice were housed under standard laboratory conditions (27°C, 40–60%
humidity, 12-h light/dark cycle) with free access to water and on a
standard laboratory diet. Eight-week-old WT and AMPK−/−
mice were injected with streptozotocin (STZ; 50 mg/kg/day) for 5
days (20). Subsequently,
melatonin (20 mg/kg/day; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was administered intraperitoneally into WT and
AMPK−/− mice over the course of 12 weeks. The animal
experiments were performed for a 12-week period (n=6/group). WT
mice injected with PBS were termed the control group; WT mice
injected with STZ were termed the STZ+WT group; WT mice treated
with STZ and melatonin were termed the Mel+STZ+WT group; and,
AMPK−/− mice treated with STZ and melatonin were termed
the Mel+STZ+AMPK−/− group. At the end of the experiment,
blood pressure was measured in conscious, acclimated mice using the
tail-cuff method. Kidney tissues were harvested following the
sacrifice of the mice. A part of the kidney was snap-frozen in
liquid nitrogen and the other part was fixed with 10% PBS-buffered
formalin, processed and embedded in paraffin. All experimental
protocols were approved by the Ethics Committee of Chinese PLA
General Hospital (Beijing, China).
The rat glomerular mesangial cell line, HBZY-1, was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured according to the supplier's
protocols in RPMI medium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in 5%
CO2. To mimic high glucose damage, cells were cultured
in high glucose medium (25 mmol/l), as opposed to normal glucose
medium (5.5 mmol/l), for ~12 h according to a previous study
(26). The cells were incubated
with 5 µM melatonin for ~12 h before high-glucose stress (18).
Sample preparation and histological
analysis
The kidneys were harvested, fixed for 1 h in 4%
(w/v) paraformaldehyde at room temperature, and rapidly frozen in
Optimal Cutting Temperature Compound (Agar Scientific, Ltd.,
Stansted, UK) for the preparation of cryosections (size, 4 µm)
(27). Hematoxylin and eosin
(H&E), Masson's trichrome (cat. no. KGMST-8004; Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) and periodic acid
Schiff-methenamine (PASM; cat. no. 395B; Sigma-Aldrich; Merck KGaA)
staining were performed at room temperature, following which,
samples were observed under an inverted microscope (magnification,
×40; BX51; Olympus Corporation, Tokyo, Japan).
ELISA
Blood samples from tail vein were collected before
the mice were sacrificed. Then, laminin (Mouse Laminin ELISA kit;
cat. no. ab119572; Abcam, Cambridge, UK) and pre-collagen III
(Mouse Collagen Type III ELISA kit; cat. no. MBS727234;
MyBiosource, San Diego, CA, USA) in blood samples were measured via
ELISA analysis, according to a previous study (28). Blood urea nitrogen (BUN; Mouse
Blood Urea Nitrogen ELISA kit; cat. no. MBS751125; MyBiosource) and
serum creatinine levels (Creatinine Assay kit; cat. no. ab65340;
Abcam) were determined via ELISA based on the manufacturers'
protocols.
Measurement of glucose levels and
urinary albumin
After 12 h of fasting, the glucose levels in venous
blood samples drawn from the tail vein were measured using a
glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Urine
samples were collected prior to the sacrifice of the mice (29). To collect morning spot urine
samples, animals were placed in metabolic cages at the beginning of
the light cycle and were kept for 2 h with access to water, but not
food. To obtain 24-h urine samples, animals were placed in
metabolic cages at the beginning of the light cycle and were kept
for 24 h with free access to water and on a standard laboratory
diet (30). Urinary albumin
content (Mouse Albumin ELISA kit; cat. no. ab108792; Abcam,) was
determined according to the manufacturer's protocols.
Immunofluorescence staining
The cells were first washed in cold PBS,
permeabilized with 0.1% Triton-X-100 for 10 min at 4°C and blocked
with 10% goat serum albumin (Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Subsequently, samples were
incubated with cytochrome c(cyt-c) antibody (1:1,000; cat.
no. ab133504; Abcam) and cleaved caspase-3 antibody (1:1,000; cat.
no. 9664; Cell Signaling Technology, Inc. Danvers, MA, USA),
overnight at 4°C (31). Following
three washes in PBS, Alexa-Fluor 116 488 donkey anti-rabbit
secondary antibody (1:1,000; cat. no. A-21206; Invitrogen; Thermo
Fisher Scientific, Inc.) was added to the samples for 1 h at room
temperature (32). Images were
observed with an inverted fluorescence microscope (magnification,
×40; BX51; Olympus Corporation).
Western blotting
The proteins isolated from kidney tissues with the
help of RIPA buffer (Thermo Fisher Scientific, Inc.) were from
three mice in each group. Proteins were also isolated from cells
using RIPA buffer (Thermo Fisher Scientific, Inc.). The protein
concentration was analyzed using the bicinchoninic acid protein
assay (Thermo Fisher Scientific, Inc.). In the present study, a
total of 40 µg cell proteins and 60 µg tissue proteins were
separated by 12–15% SDS-PAGE. Following electrophoresis, the
proteins were transferred onto a polyvinylidene fluoride membrane
(Roche Applied Science, Penzberg, Germany). The membranes were
subsequently blocked with 5% non-fat milk for 1 h at room
temperature prior to incubation with the primary antibodies. The
primary antibodies used were: Pro-caspase-3 (1:1,000; cat. no.
9662; Cell Signaling Technology, Inc.), cleaved caspase-3 (1:1,000;
cat. no. 9664; Cell Signaling Technology, Inc.), cellular inhibitor
of apoptosis protein 1 (c-IAP; 1:1,000; cat. no. 4952; Cell
Signaling Technology, Inc.), B-cell lymphoma 2 (Bcl2; 1:1,000, cat.
no. 3498; Cell Signaling Technology, Inc.), caspase-9 (1:1,000;
cat. no. ab32539; Abcam), transforming growth factor (TGF)β
(1:1,000; cat. no. 3711; Cell Signaling Technology, Inc.), matrix
metalloproteinase 9 (MMP9; 1:1,000; cat. no. 13667; Cell Signaling
Technology, Inc.), cyclophilin D (1:1,000; cat. no. ab181983;
Abcam), p-cyclophilin D antibody (1:1,000, bioss, cat. no.
bs-9878R), collagen I (1:1,000; cat. no. ab34710; Abcam), collagen
III (1:1,000; cat. no. ab7778; Abcam), Bcl-2-associated X protein
(Bax; 1:1,000; cat. no. ab32503; Abcam), AMPK (1:1,000; cat. no.
ab131512; Abcam), phosphorylated (p)-AMPK (1:1,000, cat. no.
ab23875; Abcam) and peroxisome proliferator-activated receptor γ
coactivator 1-α (PGC1α; 1:1,000; cat. no. 2178; Cell Signaling
Technology, Inc.) The secondary antibodies used in the present
study were: Horseradish peroxidase (HRP)-coupled secondary
antibodies (1:2,000; cat. nos. 7074 and 7076; Cell Signaling
Technology, Inc.). Band intensities were normalized to the
respective β-actin (1:2,000; cat. no. ab8224; Abcam) and/or GAPDH
(1:1,000, cat. no. 5174; Cell Signaling Technology, Inc.) internal
control signal intensity with the help of Quantity One Software
(version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
(29). Bands were detected using
an enhanced chemiluminescence substrate (Applygen Technologies,
Inc., Beijing, China). The experiment was repeated three times
(33).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate total RNA from tissues
(34). Subsequently, the Reverse
Transcription kit (Kaneka Eurogentec S.A., Seraing, Belgium) was
applied to transcribe RNA (1 µg/group) into cDNA according to the
manufacturer's protocol. RT-qPCR was performed with primers and
matched probes from the Universal Fluorescence-labeled Probe
Library (Roche Diagnostics, Basel, Switzerland) using SYBR™ Green
PCR Master Mix (Thermo Fisher Scientific, Inc.) (35). The primers used in the present
study were: Bax forward, 5′-GTCCTATTCTGATGGATCCC-3′ and reverse,
5′-GCTACGGTACCATGGCCTACG-3′; Bad, 5′-CCTAGATTCAGTGACTGAG-3′ and
reverse, 5′-ACCTAACCGATGGCGGTCGAGTGC-3′; Survivin,
5′-ATCCGTTGTCCAGTCTTAGTCTA-3′ and reverse,
5′-GGTCGGTAGATTCATTAATGAT-3′ and GAPDH forward,
5′-GCGGATGAACGTGGACGTGAC−3′ and reverse,
5′-AACGTGGTCCAGACCAATGCG-3′). The cycling conditions were: 95°C for
8 min, followed by 35 cycles of 95°C for 10 sec and 72°C for 12
sec, for telomere PCR. The mRNA ratio of the target genes to GAPDH
was calculated using the 2−ΔΔCq method (36).
Electron transport chain complex (ETC)
activity detection
Electron transport chain complex I activity
(Electron transport chain Complex I Assay kit; cat.no. MBS2540528;
MyBiosource), Electron transport chain complex II activity (Complex
II Enzyme Activity Microplate Assay kit; cat. no. ab109908; Abcam,)
and Electron transport chain complex V activities (MitoTox™ Complex
V OXPHOS Activity Assay kit; cat. no. ab109907; Abcam) were
determined according to the manufacturers' protocols.
Cellular ROS detection
To observe cellular ROS levels, the dihydroethidium
ROS probe (Molecular Probes; Thermo Fisher Scientific, Inc.) was
incubated with the cells (1×105) for ~30 min at 37°C in
the dark. Subsequently, the cells were washed with PBS to remove
the ROS probe. The cells were immediately analyzed under a
fluorescence microscope (37).
Image-Pro Plus version 6.0 (Media Cybernetics, Rockville, MD, USA)
was used to obtain the fluorescence densities, which were
normalized to that of the control group.
Mitochondrial permeability transition
pore (mPTP) opening assay, JC-1 staining and ATP production
mPTP opening is an early event in mitochondrial
apoptosis. In the present study, mPTP opening was measured via
tetramethylrhodamine ethyl ester (TMRE) fluorescence. Samples were
washed three times with PBS and were then stained with TMRE dye
(38). The baseline fluorescence
of TMRE was recorded and, after 30 min, TMRE fluorescence was
recorded again. Image-Pro Plus version 6.0 (Media Cybernetics) was
used to obtain the fluorescence densities of TRME fluorescence.
According to a previous study (39), the mPTP opening rate was determined
as the time for the fluorescence intensity to decrease by half of
the baseline fluorescence intensity.
Mitochondrial potential was assessed using a JC-1
probe, a sensitive fluorescent dye used to detect alterations in
mitochondrial potential (34).
Following treatment, cells (1×105) were incubated with
10 mg/ml JC-1 for 10 min at 37°C in the dark and monitored with a
fluorescence microscope (magnification, ×100; BX51; Olympus
Corporation). Red fluorescence was attributable to
potential-dependent dye aggregation in the mitochondria. Green
fluorescence, reflecting the monomeric form of JC-1, appeared in
the cytosol following mitochondrial membrane depolarization.
ATP production was detected to monitor mitochondrial
function. Firstly, the samples were washed three times with cold
PBS. The samples were then lysed using the RIPA Lysis Buffer
(Beyotime, China, cat. No:P0013E) and the Luciferase-based ATP
Assay kit (Beyotime Institute of Biotechnology, Haimen, China) was
used according to a previous study (25). ATP production was measured using a
microplate reader (40).
Lactate dehydrogenase (LDH) assay and
caspase-3/9 activity detection
LDH is released into the cell culture supernatant
when cell membranes rupture (36,41).
To evaluate LDH levels (cells density 1×105), an LDH
Release Detection kit (Beyotime Institute of Biotechnology) was
used according to the manufacturer's protocol.
To analyze changes in caspase-3/9, caspase-3/9
activity kits (Beyotime Institute of Biotechnology) were used,
according to the manufacturer's protocols (42). To analyze caspase-3 activity, 5 µl
4 mM DEVD-pNA substrate (final concentration, 200 µM) was added to
the cells (1×105) for 2 h at 37°C. To measure caspase-9
activity, 5 µl 4 mM LEHD-p-NA substrate (final concentration, 200
µM) was added to the samples for 1 h at 37°C. Subsequently, caspase
activity was quantified by spectrophotometric detection at 400 nm
using a microplate reader (43).
MTT and terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assays
MTT experiments were performed in 96-well plates to
analyze cellular viability. Samples (1×105) were washed
three times with PBS and 50 µl MTT reagent was added to each well.
The samples were incubated for 4 h at 37°C in a humidified
atmosphere containing 5% CO2. Subsequently, the MTT
solution was removed, 200 µl dimethyl sulfoxide was added and the
samples were incubated for 10 min at room temperature. Following
the addition of Sorensen's buffer, the absorbance at 570 nm was
measured (44).
To detect cell death, a TUNEL assay was performed
using an In Situ Cell Death Detection kit (Roche Diagnostics
GmbH), according to the manufacturer's protocol. DAPI was used to
label the nuclei at room temperature for ~30 min and the cells were
observed under an inverted fluorescence microscope (magnification,
×40; BX51; Olympus Corporation) (45).
RNA silencing assay
To inhibit the expression of AMPK and PGC1α, small
interfering (si)RNAs against AMPK and PGC1α were used to knockdown
their expression. The siRNA against AMPK and PGC1α as well as the
negative control siRNA were purchased from Yangzhou Ruibo Biotech
Co., Ltd (Yangzhou, China) (46).
The siRNA sequences were as follows: AMPK siRNA sense strand,
5′-GCTTACUGACTGACGT-3′ and antisense strand, 3′-AUGUUACCGUATTC-5′;
PGC1α siRNA sense strand, 5′-GUAGGUACTACCTA-3′ and antisense
strand, 3′-TUAUUTAGTTAACTGAT-5′; negative control siRNA sense
strand, 5′-UUACCUTUCCATGATGCT′ and antisense strand,
3′-AUGTUGAAGUTCCGT-5′. To transfect siRNA into HBZY-1 cells,
Opti-Minimal Essential Medium (Invitrogen; Thermo Fisher
Scientific, Inc.) without serum or antibiotics was used to incubate
cells for 24 h. Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was added into the
medium according to the manufacturer's protocol (47). Subsequently, siRNAs (70 nM/well of
siRNA) were added into serum-free medium and incubated with cells
for 72 h. Cells were collected and proteins were extracted
(48).
Statistical analysis
All data are expressed as the means ± standard
deviation and experiments were repeated ≥3 times in the present
study. Statistical analyses were performed with SPSS software
version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical analysis
was performed using one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Melatonin reduces diabetic renal
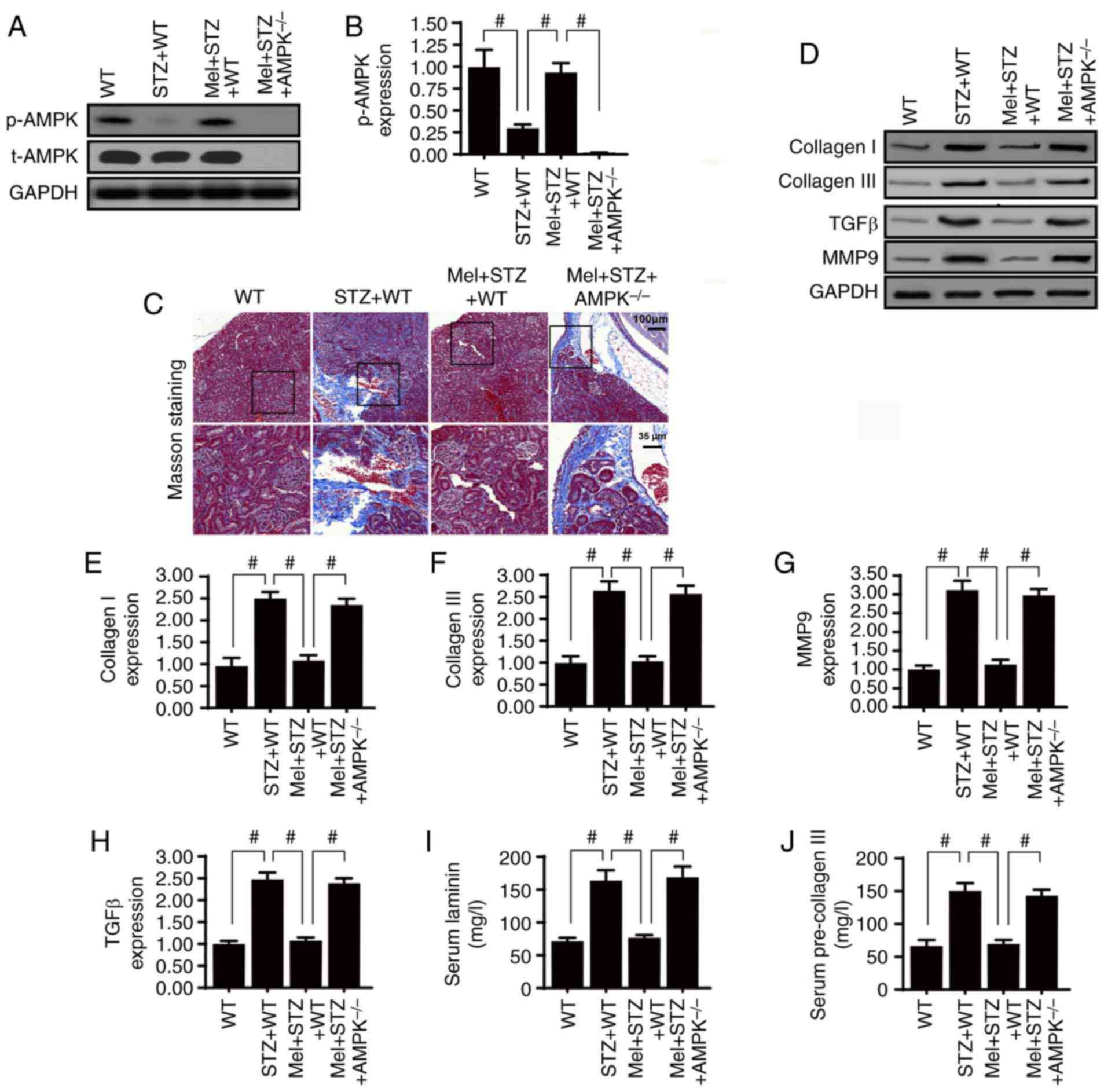
fibrosis by activating AMPK
To verify the role of AMPK in diabetic renal
fibrosis, western blotting was used to analyze AMPK expression.
Compared with the control WT mice, STZ treatment significantly
impaired AMPK activation; however, this effect was reversed by
melatonin treatment (Fig. 1A and
B). Furthermore, to confirm whether AMPK is necessary for
melatonin-induced kidney protection under diabetic stress,
AMPK−/− mice were used. As shown in Fig. 1C, renal fibrosis was observed using
Masson's trichrome staining. Compared with the control WT mice,
STZ-treated mice presented with severe kidney fibrosis and this
change was partially reversed by melatonin treatment. However, AMPK
deletion inhibited the protective effects of melatonin on renal
fibrosis. In addition, the expression levels of collagen I and
collagen III were assessed in kidney samples. Chronic hyperglycemia
significantly enhanced collagen I and collagen III expression
(Fig. 1D-F). Notably, this effect
was inhibited by melatonin, whereas absence of AMPK completely
abrogated the inhibitory effects of melatonin on collagen
accumulation.
Furthermore, signaling factors associated with
fibrosis, including TGFβ and MMP9, were more abundant in the kidney
tissues of STZ-treated mice compared with the control WT mice
(Fig. 1D, G and H). Treatment with
melatonin significantly reduced TGFβ and MMP9 expression levels;
however, the effects of melatonin were abolished by AMPK deletion.
Furthermore, the serum concentrations of laminin and pre-collagen
III were notably higher in STZ-treated mice compared with in
control WT mice (Fig 1I and J). As
expected, the high serum concentrations of laminin and pre-collagen
III were significantly reduced by melatonin supplementation,
whereas the effects of melatonin were reversed by AMPK deletion.
Taken together, these results indicated that melatonin regulated
diabetic renal interstitial fibrosis by activating AMPK.
Melatonin promotes glomerular survival
in an AMPK-dependent manner
Glomerular apoptosis is the primary factor
associated with the development of diabetic renal fibrosis;
therefore, the role of melatonin and AMPK in glomerular apoptosis
was assessed. Firstly, caspase-3 staining demonstrated that the
renal tissue in STZ-treated mice exhibited increased cleaved
caspase-3 expression compared with WT mice (Fig. 2A and B). However, melatonin
administration reduced caspase-3 expression, whereas the effects of
melatonin were suppressed in response to AMPK deficiency. Similar
results were obtained for the caspase-3 activity assay (Fig. 2C). Additionally, to investigate the
protective role of melatonin and AMPK in glomerular apoptosis,
western blotting was performed. Melatonin reduced the expression
levels of pro-apoptotic proteins (caspase-3, −9 and Bax), but
increased the expression of an anti-apoptotic factor (c-IAP), in an
AMPK-dependent manner (Fig. 2D-H).
Alterations in the expression levels of apoptosis-associated genes
were also measured (Fig. 2I-L).
The results demonstrated that the mRNA expression levels of Bcl2
and survivin were significantly reduced in the kidney tissues of
STZ-treated mice compared with in the control WT mice. Conversely,
Bax and Bcl2-associated agonist of cell death (Bad) mRNA expression
levels were increased in STZ-treated mice compared with in control
WT mice. Treatment with melatonin significantly reduced Bad and p53
mRNA expression, and increased Bcl2 and survivin mRNA expression in
STZ-treated mice in an AMPK-dependent manner.
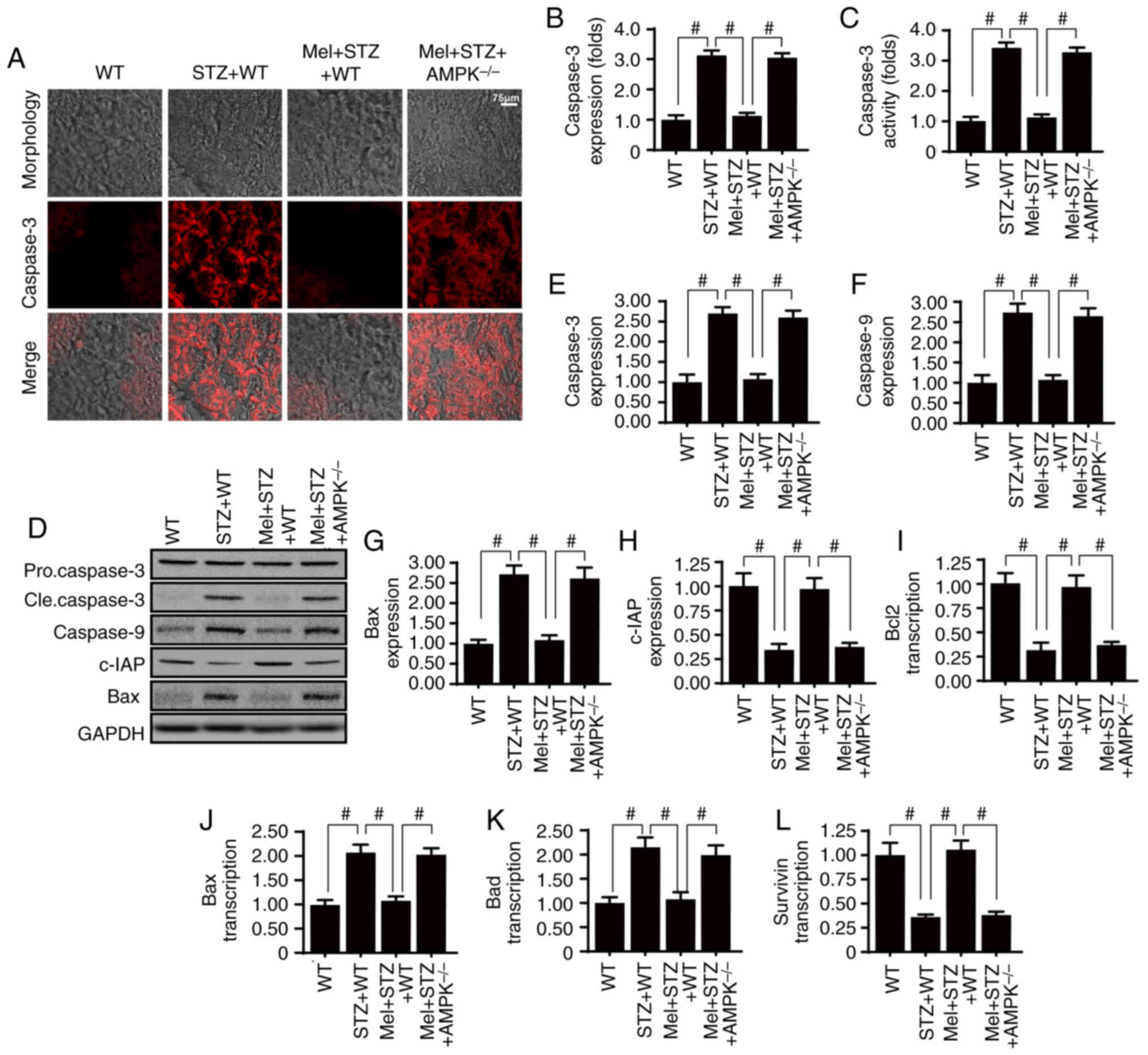 | Figure 2.Melatonin activates AMPK and
alleviates high glucose-induced glomerular apoptosis. (A and B)
Caspase-3 expression in the kidney tissues of STZ-treated mice, in
the presence or absence of melatonin. (C) Measurement of caspase-3
activity. (D-H) Western blot analysis of apoptosis-associated
proteins. (I-L) Reverse transcription-quantitative polymerase chain
reaction analysis of apoptosis-associated genes.
#P<0.05. AMPK, 5′adenosine monophosphate-activated
protein kinase; Bad, Bcl2-associated agonist of cell death; Bax,
Bcl-2-associated X protein; Bcl2, B-cell lymphoma 2; c-IAP,
cellular inhibitor of apoptosis protein 1; cle, cleaved; Mel,
melatonin; STZ, streptozotocin; WT, wild-type. |
Melatonin ameliorates structural and
functional renal damage in diabetes by upregulating AMPK
Diabetic kidney fibrosis is associated with renal
hypertrophy. To evaluate renal hypertrophy, the mice were
sacrificed and kidneys were weighed. Compared with the control WT
mice, the kidneys of STZ-treated mice weighed more; however, this
alteration was reversed by melatonin treatment (Fig. 3A). Loss of AMPK abolished the
protective role of melatonin in kidney hypertrophy. These findings
were further supported by the histopathological examination of
H&E- and PASM-stained kidney tissues. The results revealed that
STZ-treated mice exhibited moderate glomeruli atrophy and
fragmentation, epithelial desquamation, renal tubule degeneration
and kidney glomerular basement membrane thickening (Fig. 3B-F). However, these renal
histopathological alterations were diminished by melatonin
treatment in an AMPK-dependent manner.
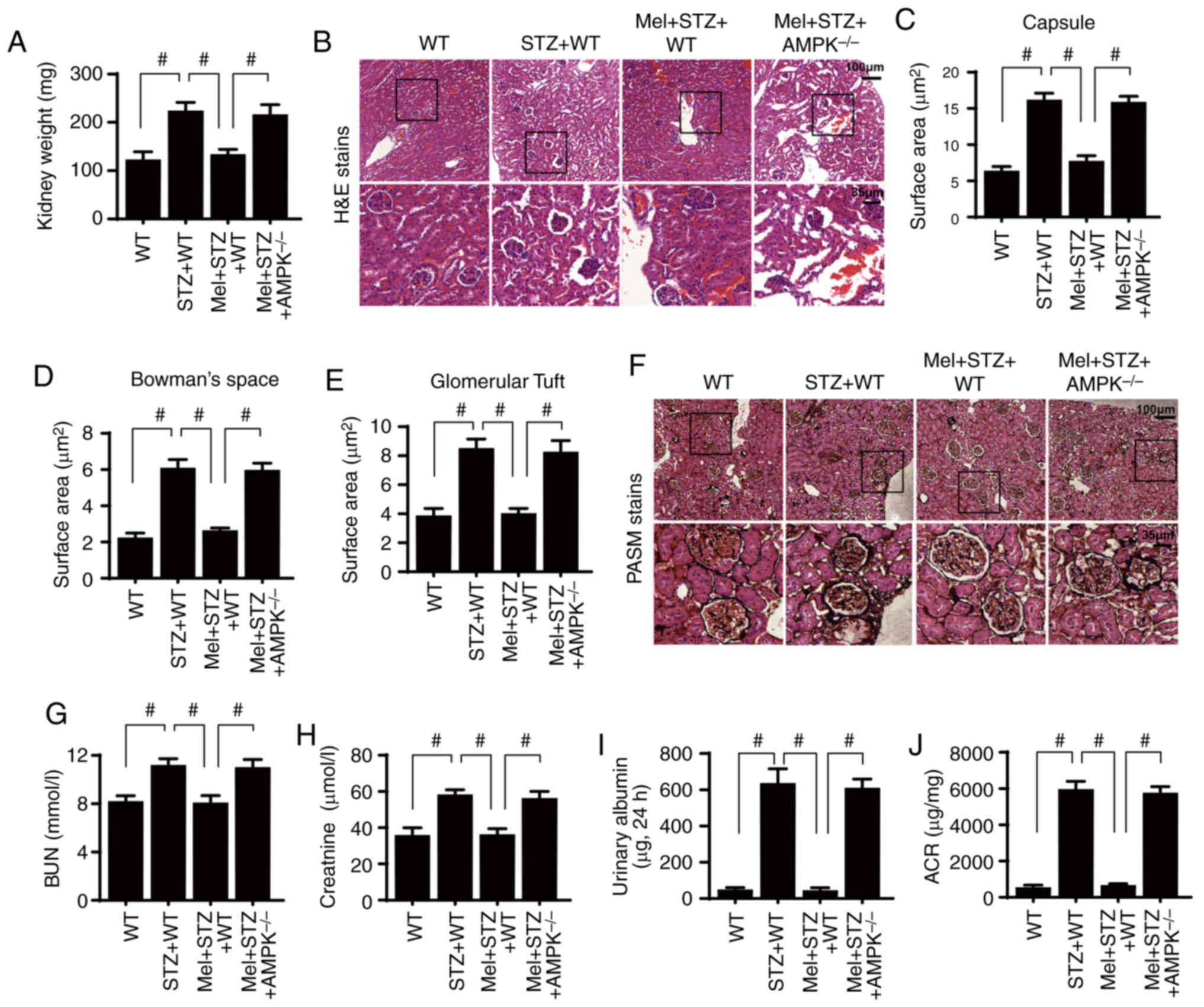 | Figure 3.Melatonin attenuates diabetic renal
injury in an AMPK-dependent manner. (A) Kidney weights of
STZ-treated mice in the presence or absence of melatonin. (B)
H&E staining of kidney sections. Surface area of the (C)
Bowman's capsule, (D) Bowman's space and (E) glomerular tuft. (F)
PASM staining of kidney tissues. (G) BUN, (H) serum creatinine, (I)
urinary albumin and (J) ACR levels were measured.
#P<0.05. ACR, albumin-to-creatinine ratio; AMPK,
5′adenosine monophosphate-activated protein kinase; BUN, blood urea
nitrogen; H&E, hematoxylin and eosin; Mel, melatonin; PASM,
periodic acid Schiff-methenamine; STZ, streptozotocin; WT,
wild-type. |
Notably, the preservation of renal structural
integrity was closely associated with an improvement in kidney
function. Renal function was assessed by determining blood urea
nitrogen (BUN) and serum creatinine levels at the end of the
experiment. Compared with the control WT mice, STZ increased the
levels of BUN (Fig. 3G) and serum
creatinine (Fig. 3H). However,
melatonin application reduced the BUN and serum creatinine levels,
potentially by restoring AMPK activity. Additionally, urinary
albumin content and the albumin-to-creatinine ratio (ACR) were
measured to determine the severity of diabetic nephropathy. As
shown in Fig. 3I-J, compared with
the control WT mice, STZ-treated mice exhibited significantly
increased urinary albumin content level and ACR. However, melatonin
administration significantly reduced the urinary albumin and ACR in
the STZ-treated mice, whereas the protective effect of melatonin
was inhibited by AMPK deletion.
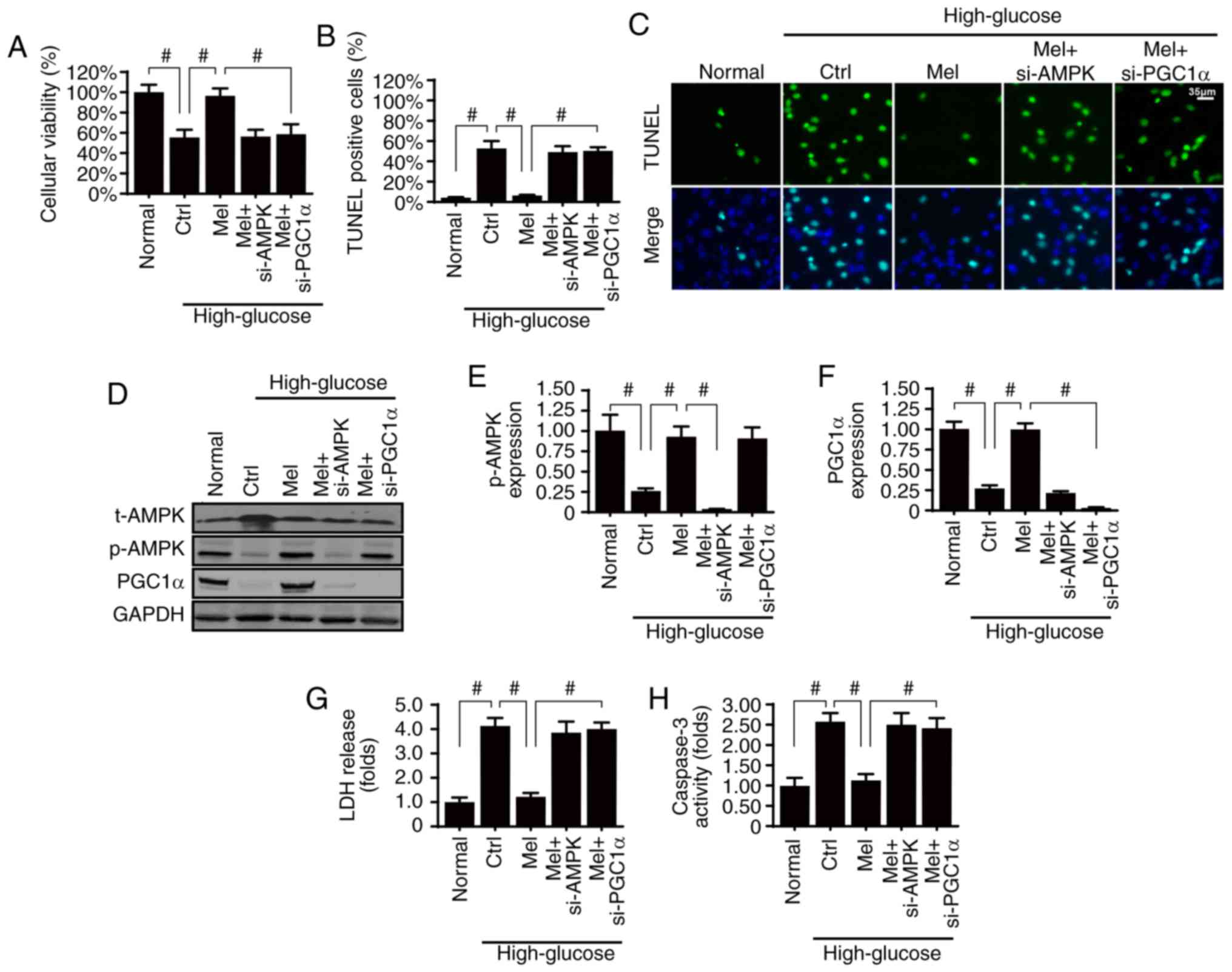
Melatonin sustains glomerular survival
by activating the AMPK/PGCα1 pathway in vitro
Diabetic renal fibrosis is involved in glomerular
apoptosis; therefore, it was of interest to investigate whether
melatonin may suppress hyperglycemia-induced glomerular damage via
AMPK. Firstly, cellular viability and apoptosis were analyzed by
MTT assay and TUNEL staining, respectively. Compared with the
normal group, high glucose treatment significantly reduced cellular
viability (Fig. 4A) and
significantly increased apoptosis of HBZY-1 cells (Fig. 4B and C). However, melatonin
treatment sustained cellular viability and suppressed
hyperglycemia-induced apoptosis, whereas silencing AMPK resulted in
a loss of these effects.
It has previously been suggested that PGCα1 is the
downstream mediator of AMPK, and activated PGCα1 favors cellular
survival via inhibition of the mitochondrial apoptosis pathway
(49). Therefore, PGCα1 activation
may be required for the kidney protection exerted by melatonin/AMPK
pathways. The present results demonstrated that PGCα1 expression
was reduced by high glucose-induced stress and was reversed back to
normal levels with melatonin treatment (Fig. 4D-F). However, silencing AMPK
abolished PGCα1 expression, thus suggesting that PGCα1 was
activated by melatonin/AMPK. To understand the role of PGCα1 in
glomerular survival, PGCα1 expression was knocked down in
melatonin-treated HBZY-1 cells (Fig.
4D-F). Following inhibition of PGCα1 expression, the protective
role of melatonin on cellular apoptosis was inhibited, as indicated
by the LDH release assay (Fig.
4G), which was similar to the results obtained in AMPK-silenced
cells. In addition, these findings were further supported by the
caspase-3 activity assay (Fig.
4H). Therefore, these data indicated that the AMPK/PGCα1
pathway was necessary for melatonin-induced glomerular survival
under high glucose treatment.
Melatonin suppresses mitochondrial
apoptosis via the AMPK/PGCα1 pathway in vitro
Considering the well-established role of
mitochondrial damage in glomerular apoptosis and diabetic renal
fibrosis, the role of the melatonin/AMPK/PGCα1 pathway in
mitochondrial homeostasis was investigated. Mitochondrial apoptosis
is activated by ROS overproduction, membrane potential collapse,
pro-apoptotic factor release and caspase family activation
(4,37). Compared with the normal group, high
glucose evoked excessive ROS production (Fig. 5A and B), which was accompanied by a
decline in mitochondrial potential (Fig. 5C and D). In addition, the
mitochondrial pro-apoptotic protein cyt-c was rapidly released into
the nucleus (Fig. 5E and F), in
turn leading to an upregulation of other pro-apoptotic proteins,
including caspase-3, caspase-9 and Bax (Fig. 5G-J). Conversely, the mitochondrial
anti-apoptotic factor Bcl2 was downregulated under high
glucose-induced stress (Fig. 5G and
K). These results indicated that mitochondrial apoptosis was
activated by hyperglycemia. Conversely, melatonin treatment
neutralized ROS generation (Fig. 5A
and B), maintained mitochondrial potential (Fig. 5C and D), suppressed cyt-c release
(Fig. 5E and F), and corrected the
imbalance between mitochondrial anti- and pro-apoptotic proteins
(Fig. 5G and K). Notably,
silencing of AMPK or PGCα1 attenuated the beneficial effects of
melatonin on mitochondrial damage. Therefore, these data indicated
that melatonin sustained mitochondrial homeostasis by activating
the AMPK/PGCα1 pathway, thereby protecting the glomerulus against
glucotoxicity.
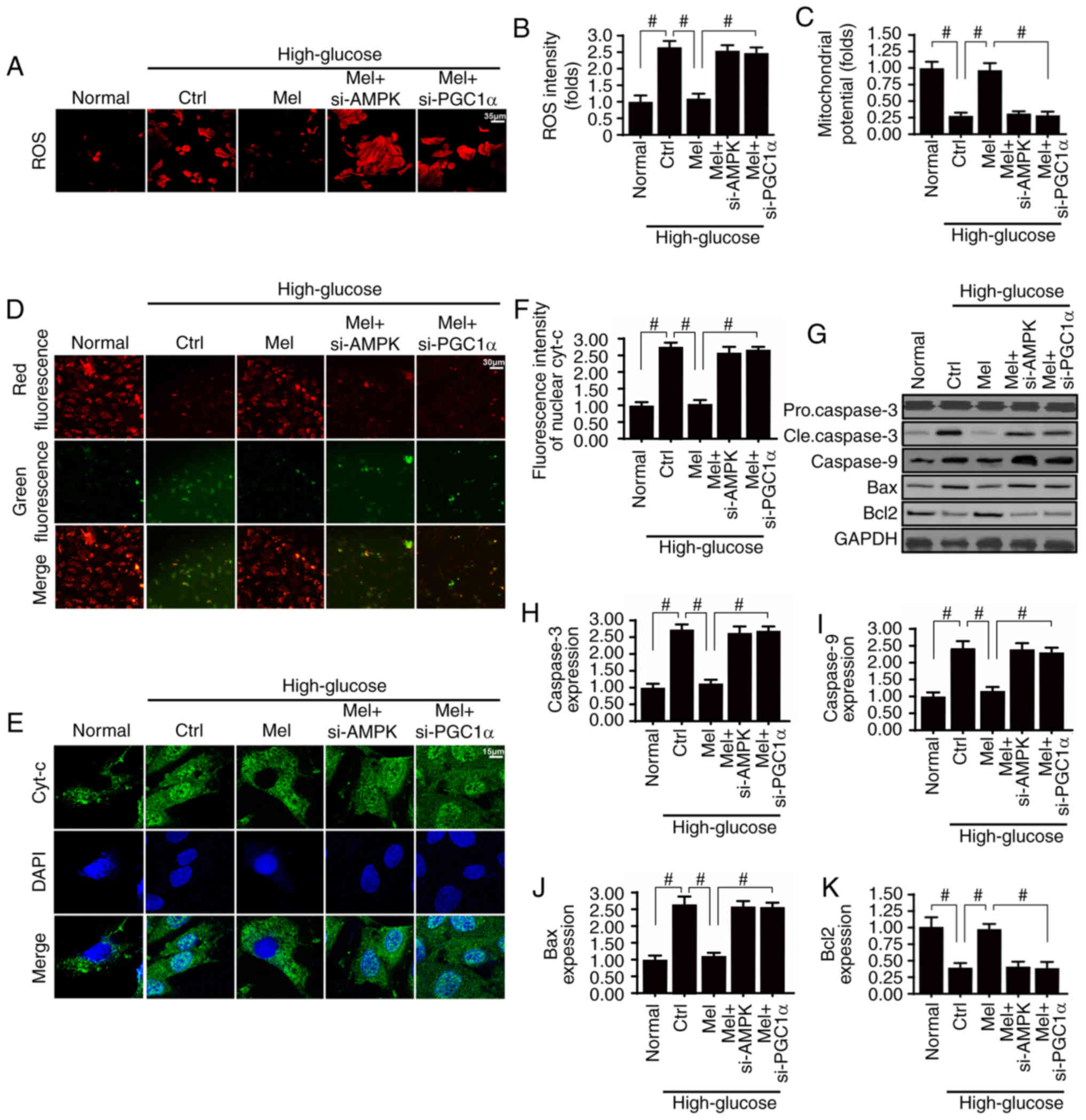 | Figure 5.Melatonin suppresses high
glucose-induced mitochondrial apoptosis by upregulating the
AMPK/PGC1α signaling pathway. (A and B) Dihydroethidium staining of
cellular ROS. (C and D) JC-1 staining was used to detect changes in
mitochondrial membrane potential. Green fluorescence indicates low
mitochondrial membrane potential and red fluorescence represents
high mitochondrial membrane potential. (E and F) Immunofluorescence
staining of cyt-c and analysis of nuclear translocation. (G-K)
Western blot analysis of apoptosis-associated proteins in HBZY-1
cells. #P<0.05. AMPK, 5′adenosine
monophosphate-activated protein kinase; Bax, Bcl-2-associated X
protein; Bcl2, B-cell lymphoma 2; cle, cleaved; Ctrl, control;
Cyt-c, cytochrome c; Mel, melatonin; PGC1α, peroxisome
proliferator-activated receptor γ coactivator 1-α; ROS, reactive
oxygen species; si, small interfering. |
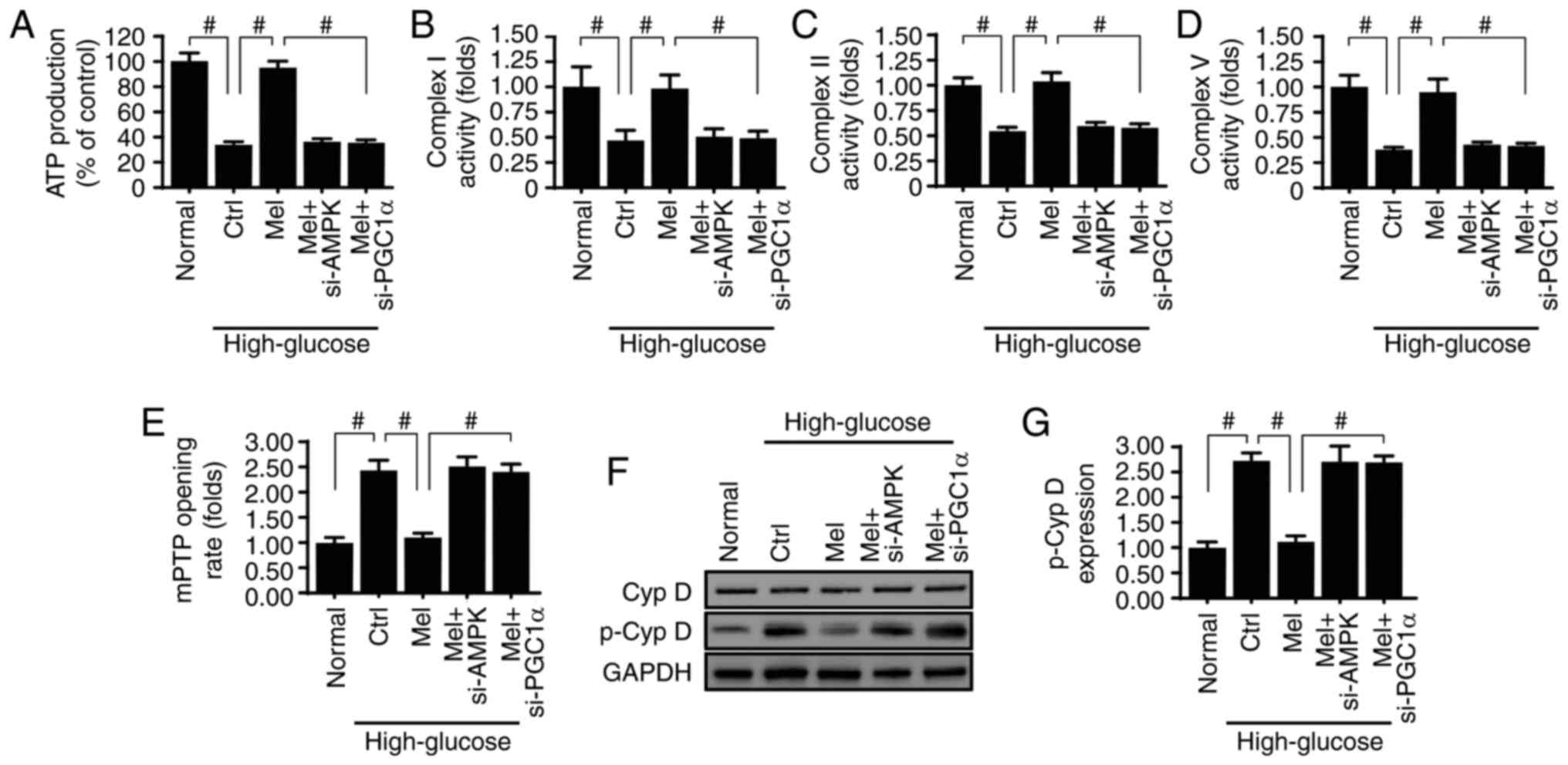
Melatonin activates the AMPK/PGCα1
pathway to preserve mitochondrial function and structure in
vitro
Besides mitochondrial apoptosis, mitochondrial
function and structure are essential for cellular viability
(10,50). The primary function of mitochondria
is to supply sufficient energy for cellular metabolism. The ATP
content was reduced by high glucose treatment (Fig. 6A) and was reversed to normal levels
in response to melatonin administration via activation of the
AMPK/PGCα1 pathway. Mechanistically, mitochondria produce ATP via
the respiratory electron-transport chain (ETC) (38,51).
The activity of ETC was decreased by high glucose treatment
(Fig. 6B-D) and was reversed to
normal levels with melatonin application. However, inhibition of
the AMPK/PGCα1 pathway abolished the protective effects of
melatonin on ETC activity.
Renal functional impairment is closely associated
with structural damage. Compared with the normal group, high
glucose medium promoted the opening of mPTP, an early indicator of
mitochondrial damage. Notably, melatonin was able to activate the
AMPK/PGCα1 pathway and thereby modify the mPTP opening rate
(Fig. 6E). At the molecular level,
mPTP opening is regulated by cyclophilin D, whereby p-cyclophilin D
enhances mPTP opening (52,53).
Based on this, the present study investigated whether melatonin and
the AMPK/PGCα1 pathway controls mPTP opening via cyclophilin D. As
shown in Fig. 6F and G,
cyclophilin D phosphorylation was increased in response to high
glucose treatment and was decreased with melatonin supplementation.
Knockdown of AMPK and PGCα1 upregulated p-cyclophilin D expression,
confirming that melatonin may maintain mitochondrial structure and
function via the AMPK/PGCα1 pathway.
Discussion
The prevalence of diabetes continues to rise and is
a global health burden. Diabetic renal fibrosis affects a
significant percentage of patients with diabetes and contributes
greatly to mortality. Previous studies have demonstrated that
mitochondrial injury serves a primary role in the development of
diabetic renal fibrosis (54,55),
which is line with the findings of the present study. In addition,
the present study further explored the mechanism by which
hyperglycemia evoked mitochondrial damage. Based on the findings,
it may be hypothesized that AMPK downregulation and PGCα1
inactivation are responsible for the hyperglycemia-induced
mitochondrial dysfunction and cellular apoptosis. Mechanistically,
suppression of the AMPK/PGCα1 pathway promoted mitochondrial
oxidative stress, triggered mitochondrial potential collapse,
enhanced pro-apoptotic cyt-c leakage into the nucleus and finally
initiated caspase-9-induced mitochondrial apoptosis in the
glomerulus. In addition, the inactive AMPK/PGCα1 pathway was
instrumental to renal dysfunction and kidney fibrosis. Therefore,
these findings comprehensively describe the causal relationship
between the AMPK/PGCα1 pathway and diabetic renal fibrosis, which
may provide a potential target to retard and/or reverse the
progression of renal fibrosis induced by glucotoxicity.
Based on the present findings, mitochondrial damage
was the initial upstream signal for glomerular injury and
subsequent renal fibrosis. This conclusion was in agreement with
previous studies (56,57). Hyperglycemia has been reported to
induce mitochondrial damage via numerous mechanisms. Hyperglycemia
causes mitochondrial fragmentation and reduces mitochondrial ATP
production. Additionally, high glucose stimulation evokes an
excessive NLR family pyrin domain containing 3-associated
inflammatory response, leading to mitochondrial dysfunction and
oxidative stress (58). Pierelli
et al (59) demonstrated
that hyperglycemia-induced downregulation of uncoupling protein 2
expression impairs mitochondrial membrane potential and induces
energy metabolism disorder. Besides, suppression of the
mitochondrial tricarboxylic acid cycle due to chronic high glucose
injury causes energy undersupply (60) and results in abnormal lipid
metabolism. Similar to these reports, the present study
demonstrated that high glucose induced excessive ROS production and
impaired the glomerular anti-oxidative system, resulting in opening
of the mPTP. Subsequently, hydrogen can be liberated, which
accounts for the reduction in mitochondrial potential and
disruption of ATP production (61,62).
The mPTP opening can also facilitate leakage of the pro-apoptotic
factor cyt-c into the cytoplasm or nucleus, where it activates the
caspase-9-associated mitochondrial apoptotic pathway (63,64).
Based on these data, the potential processes underlying
mitochondrial injury induced by glucotoxicity were determined and
these findings may further extend our understanding of
hyperglycemia-mediated mitochondrial damage.
Additionally, melatonin was demonstrated to be an
effective approach to protect mitochondria against glucotoxicity.
Notably, melatonin may directly reduce diabetic renal damage by
sustaining mitochondrial homeostasis. This notion has been
supported by ample evidence, which has documented the role of
melatonin in mitochondrial protection (27,64,65).
Melatonin suppresses excessive mitochondria fission (19), reverses protective mitophagy
(24), scavenges mitochondrial ROS
(66), alleviates mitochondrial
calcium overload (18,67), sustains mitochondrial energy
metabolism (68), modifies
mitochondrial sirtuin protein (69) and blocks the caspase-9 apoptosis
pathway (61,70). These findings are in line with the
results in the present study. However, the key finding in the
present study is that the AMPK/PGC1α pathway was required for
melatonin-mediated mitochondrial protection, glomerular survival
and renal fibrosis inhibition. Similarly, recent studies have
reported that melatonin can activate AMPK and protect cardiac
endothelial cells against reperfusion injury (25,26).
In addition, it has been demonstrated that genetic ablation of AMPK
negated the protective role of AMPK on endothelial survival and
mitochondrial protection. Other studies have also revealed that
AMPK activation by drugs, including liraglutide and empagliflozin,
inhibits the progression of diabetes (6,71).
Therefore, these findings suggested that influencing the stability
of AMPK may be a potential therapeutic tool to limit diabetes
development and progression.
In conclusion, through loss-of-function assays in
vivo and in vitro, the present study confirmed that
diabetic renal fibrosis was associated with mitochondrial damage
via a decline in AMPK/PGC1α activity. In addition, melatonin
administration was capable of rescuing the AMPK/PGC1α pathway,
preserving mitochondrial homeostasis and reducing diabetic renal
fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL conceived the project. STY, XYM, ZYG and YHL
performed the experiments. YHS and NL collected and analyzed the
data. All authors participated in discussing and revising the
manuscript.
Ethics approval and consent to
participate
The animal study was performed in accordance with
The Declaration of Helsinki. All experimental protocols were
approved by the Ethics Committee of Chinese PLA General Hospital
(Beijing, China; ethics reference no. 2017013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hung YC, Lin YC, Hsieh HM, Huang CJ and
Chiu HC: Impact of non-apnea sleep disorders on diabetic control
and metabolic outcome-A population-based cohort study. Gen Hosp
Psychiatry. 52:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaman SB, Karim MA, Hossain N, Al Kibria
GM and Islam SMS: Plasma triglycerides as a risk factor for chronic
kidney disease in type 2 diabetes mellitus: Evidence from the
northeastern of Thailand. Diabetes Res Clin Pract. 138:238–245.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu S, Gao Y, Zhou H, Kong F, Xiao F, Zhou
P and Chen Y: New insight into mitochondrial changes in vascular
endothelial cells irradiated by gamma ray. Int J Radiat Biol.
93:470–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li R, Xin T, Li D, Wang C, Zhu H and Zhou
H: Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic
fatty liver disease: The role of the ERK-CREB pathway and
Bnip3-mediated mitophagy. Redox Biol. 18:229–243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou H, Wang J, Zhu P, Hu S and Ren J:
Ripk3 regulates cardiac microvascular reperfusion injury: The role
of IP3R-dependent calcium overload, XO-mediated oxidative stress
and F-action/filopodia-based cellular migration. Cell Signal.
45:12–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou H, Yang J, Xin T, Li D, Guo J, Hu S,
Zhou S, Zhang T, Zhang Y, Han T and Chen Y: Exendin-4 protects
adipose-derived mesenchymal stem cells from apoptosis induced by
hydrogen peroxide through the PI3K/Akt-Sfrp2 pathways. Free Radic
Biol Med. 77:363–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T,
Ma Q, Han T, Zhang Y, Tian F and Chen Y: Liraglutide protects
cardiac microvascular endothelial cells against
hypoxia/reoxygenation injury through the suppression of the
SR-Ca(2+)-XO-ROS axis via activation of the
GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 95:278–292.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou H, Li D, Shi C, Xin T, Yang J, Zhou
Y, Hu S, Tian F, Wang J and Chen Y: Effects of Exendin-4 on bone
marrow mesenchymal stem cell proliferation, migration and apoptosis
in vitro. Sci Rep. 5:128982015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu
S, Ren J and Chen Y: NR4A1 aggravates the cardiac microvascular
ischemia reperfusion injury through suppressing FUNDC1-mediated
mitophagy and promoting Mff-required mitochondrial fission by CK2α.
Basic Res Cardiol. 113:232018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Randriamboavonjy V, Kyselova A, Elgheznawy
A, Zukunft S, Wittig I and Fleming I: Calpain 1 cleaves and
inactivates prostacyclin synthase in mesenteric arteries from
diabetic mice. Basic Res Cardiol. 112:102017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ronchi C, Torre E, Rizzetto R, Bernardi J,
Rocchetti M and Zaza A: Late sodium current and intracellular ionic
homeostasis in acute ischemia. Basic Res Cardiol. 112:122017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ and
Chen Y: Protective role of melatonin in cardiac
ischemia-reperfusion injury: From pathogenesis to targeted therapy.
J Pineal Res. 64:2018.doi: 10.1111/jpi.12471. View Article : Google Scholar
|
|
13
|
Motawi TK, Ahmed SA, A Hamed M, El-Maraghy
SA and M Aziz W: Melatonin and/or rowatinex attenuate
streptozotocin-induced diabetic renal injury in rats. J Biomed Res.
Nov 1–2017.(Epub ahead of print). PubMed/NCBI
|
|
14
|
Hrenak J, Paulis L, Repova K, Aziriova S,
Nagtegaal EJ, Reiter RJ and Simko F: Melatonin and renal
protection: Novel perspectives from animal experiments and human
studies (review). Curr Pharm Des. 21:936–949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garcia-Nino WR, Correa F,
Rodriguez-Barrena JI, León-Contreras JC, Buelna-Chontal M,
Soria-Castro E, Hernández-Pando R, Pedraza-Chaverri J and Zazueta
C: Cardioprotective kinase signaling to subsarcolemmal and
interfibrillar mitochondria is mediated by caveolar structures.
Basic Res Cardiol. 112:152017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Wu H, Liu N, Cao X, Yang Z, Lu B, Hu
R, Wang X and Wen J: Melatonin exerts an inhibitory effect on
insulin gene transcription via MTNR1B and the downstream Raf1/ERK
signaling pathway. Int J Mol Med. 41:955–961. 2018.PubMed/NCBI
|
|
17
|
Yu LM, Di WC, Dong X, Li Z, Zhang Y, Xue
XD, Xu YL, Zhang J, Xiao X, Han JS, et al: Melatonin protects
diabetic heart against ischemia-reperfusion injury, role of
membrane receptor-dependent cGMP-PKG activation. Biochim Biophys
Acta. 1864:563–578. 2018. View Article : Google Scholar
|
|
18
|
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S,
Wang W and Ren J: Effects of melatonin on fatty liver disease: The
role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and
mitophagy. J Pineal Res. 64:2018.doi: 10.1111/jpi.12450. View Article : Google Scholar
|
|
19
|
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D,
Zhou H and Chen Y: Melatonin protected cardiac microvascular
endothelial cells against oxidative stress injury via suppression
of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis by
activation of MAPK/ERK signaling pathway. Cell Stress Chaperones.
23:101–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou H, Wang S, Zhu P, Hu S, Chen Y and
Ren J: Empagliflozin rescues diabetic myocardial microvascular
injury via AMPK-mediated inhibition of mitochondrial fission. Redox
Biol. 15:335–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torres-Quesada O, Mayrhofer JE and Stefan
E: The many faces of compartmentalized PKA signalosomes. Cell
Signal. 37:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jovancevic N, Dendorfer A, Matzkies M,
Kovarova M, Heckmann JC, Osterloh M, Boehm M, Weber L, Nguemo F,
Semmler J, et al: Medium-chain fatty acids modulate myocardial
function via a cardiac odorant receptor. Basic Res Cardiol.
112:132017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alghanem AF, Wilkinson EL, Emmett MS,
Aljasir MA, Holmes K, Rothermel BA, Simms VA, Heath VL and Cross
MJ: RCAN1.4 regulates VEGFR-2 internalisation, cell polarity and
migration in human microvascular endothelial cells. Angiogenesis.
20:341–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou H, Zhu P, Wang J, Zhu H, Ren J and
Chen Y: Pathogenesis of cardiac ischemia reperfusion injury is
associated with CK2α-disturbed mitochondrial homeostasis via
suppression of FUNDC1-related mitophagy. Cell Death Differ.
25:1080–1093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q,
Jin Q, Cao F, Tian F and Chen Y: Melatonin protects cardiac
microvasculature against ischemia/reperfusion injury via
suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis.
J Pineal Res. 63:2017.doi: 10.1111/jpi.12413. View Article : Google Scholar :
|
|
26
|
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S,
Zhang Y, Han T, Ren J, Cao F and Chen Y: Melatonin suppresses
platelet activation and function against cardiac
ischemia/reperfusion injury via PPARγ/FUNDC1/mitophagy pathways. J
Pineal Res. 63:2017.doi: 10.1111/jpi.12438. View Article : Google Scholar :
|
|
27
|
Oanh NTK, Park YY and Cho H: Mitochondria
elongation is mediated through SIRT1-mediated MFN1 stabilization.
Cell Signal. 38:67–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fuhrmann DC and Brüne B: Mitochondrial
composition and function under the control of hypoxia. Redox Biol.
12:208–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai M, Li B, Duan W, Jing L, Zhang B,
Zhang M, Yu L, Liu Z, Yu B, Ren K, et al: Melatonin ameliorates
myocardial ischemia reperfusion injury through SIRT3-dependent
regulation of oxidative stress and apoptosis. J Pineal Res.
63:2017.doi: 10.1111/jpi.12419. View Article : Google Scholar
|
|
30
|
Zhang R and Sun Y, Liu Z, Jin W and Sun Y:
Effects of melatonin on seedling growth, mineral nutrition, and
nitrogen metabolism in cucumber under nitrate stress. J Pineal Res.
62:2017.doi: 10.1111/jpi.12403. View Article : Google Scholar
|
|
31
|
Couto JA, Ayturk UM, Konczyk DJ, Goss JA,
Huang AY, Hann S, Reeve JL, Liang MG, Bischoff J, Warman ML and
Greene AK: A somatic GNA11 mutation is associated with extremity
capillary malformation and overgrowth. Angiogenesis. 20:303–306.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Griffiths HR, Gao D and Pararasa C: Redox
regulation in metabolic programming and inflammation. Redox Biol.
12:50–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tallman KA, Kim HH, Korade Z,
Genaro-Mattos TC, Wages PA, Liu W and Porter NA: Probes for protein
adduction in cholesterol biosynthesis disorders: Alkynyl lanosterol
as a viable sterol precursor. Redox Biol. 12:182–190. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu
S, Zhu P, Wang W and Zhou H: Yap promotes hepatocellular carcinoma
metastasis and mobilization via governing
cofilin/F-actin/lamellipodium axis by regulation of
JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 14:59–71. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou H, Yang J, Xin T, Zhang T, Hu S, Zhou
S, Chen G and Chen Y: Exendin-4 enhances the migration of
adipose-derived stem cells to neonatal rat ventricular
cardiomyocyte-derived conditioned medium via the phosphoinositide
3-kinase/Akt-stromal cell-derived factor-1α/CXC chemokine receptor
4 pathway. Mol Med Rep. 11:4063–4072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salminen A, Kaarniranta K and Kauppinen A:
Integrated stress response stimulates FGF21 expression: Systemic
enhancer of longevity. Cell Signal. 40:10–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sigala F, Efentakis P, Karageorgiadi D,
Filis K, Zampas P, Iliodromitis EK, Zografos G, Papapetropoulos A
and Andreadou I: Reciprocal regulation of eNOS, H2S and
CO-synthesizing enzymes in human atheroma: Correlation with plaque
stability and effects of simvastatin. Redox Biol. 12:70–81. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma
S, Zhu H, Ren J and Zhou H: DUSP1 alleviates cardiac
ischemia/reperfusion injury by suppressing the Mff-required
mitochondrial fission and Bnip3-related mitophagy via the JNK
pathways. Redox Biol. 14:576–587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D,
Hu S, Ren J, Cao F and Chen Y: Ripk3 induces mitochondrial
apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury.
Redox Biol. 13:498–507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu S, Wang X, Geng P, Tang X, Xiang L, Lu
X, Li J, Ruan Z, Chen J, Xie G, et al: Melatonin regulates PARP1 to
control the senescence-associated secretory phenotype (SASP) in
human fetal lung fibroblast cells. J Pineal Res. 63:2017.doi:
10.1111/jpi.12405. View Article : Google Scholar
|
|
42
|
Lee K and Back K: Overexpression of rice
serotonin N-acetyltransferase 1 in transgenic rice plants confers
resistance to cadmium and senescence and increases grain yield. J
Pineal Res. 62:2017.doi: 10.1111/jpi.12392. View Article : Google Scholar
|
|
43
|
Fukumoto M, Kondo K, Uni K, Ishiguro T,
Hayashi M, Ueda S, Mori I, Niimi K, Tashiro F, Miyazaki S, et al:
Tip-cell behavior is regulated by transcription factor FoxO1 under
hypoxic conditions in developing mouse retinas. Angiogenesis.
21:203–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee HJ, Jung YH, Choi GE, Ko SH, Lee SJ,
Lee SH and Han HJ: BNIP3 induction by hypoxia stimulates
FASN-dependent free fatty acid production enhancing therapeutic
potential of umbilical cord blood-derived human mesenchymal stem
cells. Redox Biol. 13:426–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Z, Gan L, Luo D and Sun C: Melatonin
promotes circadian rhythm-induced proliferation through
Clock/histone deacetylase 3/c-Myc interaction in mouse adipose
tissue. J Pineal Res. 62:2017.doi: 10.1111/jpi.12383. View Article : Google Scholar
|
|
46
|
Le Cras TD, Mobberley-Schuman PS, Broering
M, Fei L, Trenor CC III and Adams DM: Angiopoietins as serum
biomarkers for lymphatic anomalies. Angiogenesis. 20:163–173. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vargas LA, Velasquez FC and Alvarez BV:
Compensatory role of the NBCn1 sodium/bicarbonate cotransporter on
Ca2+-induced mitochondrial swelling in hypertrophic
hearts. Basic Res Cardiol. 112:142017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pickard JM, Burke N, Davidson SM and
Yellon DM: Intrinsic cardiac ganglia and acetylcholine are
important in the mechanism of ischaemic preconditioning. Basic Res
Cardiol. 112:112017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brasacchio D, Alsop AE, Noori T, Lufti M,
Iyer S, Simpson KJ, Bird PI, Kluck RM, Johnstone RW and Trapani JA:
Epigenetic control of mitochondrial cell death through
PACS1-mediated regulation of BAX/BAK oligomerization. Cell Death
Differ. 24:961–970. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim EJ, Kim SH, Jin X, Jin X and Kim H:
KCTD2, an adaptor of Cullin3 E3 ubiquitin ligase, suppresses
gliomagenesis by destabilizing c-Myc. Cell Death Differ.
24:649–659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu
P, Ma Q, Tian F and Chen Y: Mff-dependent mitochondrial fission
contributes to the pathogenesis of cardiac microvasculature
Ischemia/reperfusion injury via induction of mROS-mediated
cardiolipin oxidation and HK2/VDAC1 Disassociation-involved mPTP
opening. J Am Heart Assoc. 6(pii): e0053282017.PubMed/NCBI
|
|
52
|
Solomon H, Brauning B, Fainer I,
Ben-Nissan G, Rabani S, Goldfinger N, Moscovitz O, Shakked Z,
Rotter V and Sharon M: Post-translational regulation of p53
function through 20S proteasome-mediated cleavage. Cell Death
Differ. 24:2187–2198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Morozzi G, Beccafico S, Bianchi R, Riuzzi
F, Bellezza I, Giambanco I, Arcuri C, Minelli A and Donato R:
Oxidative stress-induced S100B accumulation converts myoblasts into
brown adipocytes via an NF-κB/YY1/miR-133 axis and NF-κB/YY1/BMP-7
axis. Cell Death Differ. 24:2077–2088. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J,
Hu S, Chen Y and Zhang Y: Inhibitory effect of melatonin on
necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway
attenuates cardiac microvascular ischemia-reperfusion injury. J
Pineal Res. May 16;e125032018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou H, Wang S, Hu S, Chen Y and Ren J:
ER-mitochondria microdomains in cardiac Ischemia-reperfusion
injury: A fresh perspective. Front Physiol. 9:7552018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang W, Tao A, Lan T, Cepinskas G, Kao R,
Martin CM and Rui T: Carbon monoxide releasing molecule-3 improves
myocardial function in mice with sepsis by inhibiting NLRP3
inflammasome activation in cardiac fibroblasts. Basic Res Cardiol.
112:162017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Banerjee K, Keasey MP, Razskazovskiy V,
Visavadiya NP, Jia C and Hagg T: Reduced FAK-STAT3 signaling
contributes to ER stress-induced mitochondrial dysfunction and
death in endothelial cells. Cell Signal. 36:154–162. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rovira-Llopis S, Apostolova N, Bañuls C,
Muntané J, Rocha M and Victor VM: Mitochondria, the NLRP3
inflammasome, and sirtuins in type 2 diabetes: New therapeutic
targets. Antioxid Redox Signal. 29:749–791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pierelli G, Stanzione R, Forte M,
Migliarino S, Perelli M, Volpe M and Rubattu S: Uncoupling protein
2: A key player and a potential therapeutic target in vascular
diseases. Oxid Med Cell Longev. 2017:73483722017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rauckhorst AJ, Gray LR, Sheldon RD, Fu X,
Pewa AD, Feddersen CR, Dupuy AJ, Gibson-Corley KN, Cox JE, Burgess
SC and Taylor EB: The mitochondrial pyruvate carrier mediates high
fat diet-induced increases in hepatic TCA cycle capacity. Mol
Metab. 6:1468–1479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dufour F, Rattier T, Shirley S, Picarda G,
Constantinescu AA, Morlé A, Zakaria AB, Marcion G, Causse S,
Szegezdi E, et al: N-glycosylation of mouse TRAIL-R and human
TRAIL-R1 enhances TRAIL-induced death. Cell Death Differ.
24:500–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang
Z, Li Z, Feng X, Hao J, Zhang K, et al: Melatonin synergizes the
chemotherapeutic effect of 5-fluorouracil in colon cancer by
suppressing PI3K/AKT and NF-kB/iNOS signaling pathways. J Pineal
Res. 62:2017.doi: 10.1111/jpi.12380. View Article : Google Scholar
|
|
63
|
Daiber A, Oelze M, Steven S, Kroller-Schon
S and Münzel T: Taking up the cudgels for the traditional reactive
oxygen and nitrogen species detection assays and their use in the
cardiovascular system. Redox Biol. 12:35–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou H, Shi C, Hu S, Zhu H, Ren J and Chen
Y: BI1 is associated with microvascular protection in cardiac
ischemia reperfusion injury via repressing
Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis.
21:599–615. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou H, Yue Y, Wang J, Ma Q and Chen Y:
Melatonin therapy for diabetic cardiomyopathy: A mechanism
involving Syk-mitochondrial complex I-SERCA pathway. Cell Signal.
47:88–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mayo JC, Sainz RM, Gonzalez Menendez P,
Cepas V, Tan DX and Reiter RJ: Melatonin and sirtuins: A ‘not-so
unexpected’ relationship. J Pineal Res. 62:2017.doi:
10.1111/jpi.12391. View Article : Google Scholar
|
|
67
|
Lee JH, Han YS and Lee SH: Potentiation of
biological effects of mesenchymal stem cells in ischemic conditions
by melatonin via upregulation of cellular prion protein expression.
J Pineal Res. 62:2017.doi: 10.1111/jpi.12385. View Article : Google Scholar
|
|
68
|
Lee HY and Back K: Melatonin is required
for H2 O2- and NO-mediated defense signaling
through MAPKKK3 and OXI1 in Arabidopsis thaliana. J Pineal Res.
62:2017.doi: 10.1111/jpi.12379. View Article : Google Scholar
|
|
69
|
Lee MS, Yin TC, Sung PH, Chiang JY, Sun CK
and Yip HK: Melatonin enhances survival and preserves functional
integrity of stem cells: A review. J Pineal Res. 62:2017.doi:
10.1111/jpi.12372. View Article : Google Scholar
|
|
70
|
Tamura H, Kawamoto M, Sato S, Tamura I,
Maekawa R, Taketani T, Aasada H, Takaki E, Nakai A, Reiter RJ and
Sugino N: Long-term melatonin treatment delays ovarian aging. J
Pineal Res. 62:2017.doi: 10.1111/jpi.12381. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ligeza J, Marona P, Gach N, Lipert B,
Miekus K, Wilk W, Jaszczynski J, Stelmach A, Loboda A, Dulak J, et
al: MCPIP1 contributes to clear cell renal cell carcinomas
development. Angiogenesis. 20:325–340. 2017. View Article : Google Scholar : PubMed/NCBI
|