Introduction
Liver cancer is one of the most common types of
human cancer and the third most frequent cause of cancer-associated
mortality worldwide (1).
Currently, the primary method for treating liver cancer is surgical
resection. Although important progress has been made in liver
cancer treatment via surgery, chemotherapy and biological agents, a
cure is not achieved in the majority of patients, particularly for
those with metastasis (2). Liver
cancer is associated with a poor prognosis due to the high
frequency of intrahepatic and extrahepatic metastasis (3). Hepatocarcinogenesis is a multi-step
process beginning with specific genetic alterations, which
ultimately cause the malignant transformation of hepatocytes
(4). However, the mechanisms
underlying hepatocarcinogenesis remain to be fully elucidated.
Addressing the pathological mechanisms underlying the development
and progression of liver cancer is of critical importance for
developing effective therapeutic agents to treat this disease.
MicroRNAs (miRNAs) are defined as a class of small
(~20 nucleotides in length), endogenous, non-coding RNAs. They have
been investigated substantially in the last two decades and are
known to exert crucial regulatory roles by targeting mRNAs for
cleavage or translational repression (5). Emerging evidence has revealed that
miRNAs are involved in almost all cellular processes and human
diseases, particularly in cancer (6–11).
miRNAs can be characterized as either oncogenes or tumor
suppressors, and the same miRNA can have opposing functions in
different types of cancer (12–14).
Among these, miRNA-363-3p (miR-363-3p) functions as a tumor
suppressor in various types of cancer, including liver cancer
(15–17). For example, miR-363-3p inhibits
tumor growth, epithelial-to-mesenchymal transition and metastasis
in lung and colorectal cancer (18,19).
One study demonstrated that miR-363-3p was downregulated in liver
cancer, and suppressed cell proliferation, migration and invasion
by targeting specificity protein 1 (SP1) (17). However, bioinformatics analyses
suggest that one miRNA can simultaneously target several
protein-coding mRNAs (5).
Identifying more targets for miR-363-3p increases the understanding
of the role of miR-363-3p in liver cancer.
The present study focused on the functions of
miR-363-3p in liver cancer. The expression, functions and
mechanisms of miR-363-3p in liver cancer were characterized. The
results revealed that miR-363-3p can reduce the proliferation,
survival and migration of liver cancer cells by regulating the
expression of high mobility group AT-hook 2 (HMGA2). Therefore,
miR-363-3p acts as a tumor suppressor in liver cancer by targeting
HMGA2. The findings provide valuable insight into the molecular
mechanisms of hepatocarcinogenesis, and highlight the potential of
targeting the miR-363-3p/HMGA2 axis in liver cancer therapy in the
future.
Materials and methods
Tissues and patients
Tissue samples were obtained between January 2016
and December 2017 at Tianjin Medical University General Hospital
Airport Site (Tianjin, China). A summary of patients and the
relevant clinical characteristic is in Table I. Informed consent
was obtained from all 50 patients in advance. The tissues samples
were obtained during surgery and immediately frozen in liquid
nitrogen and stored at −80°C prior to use.
Cell culture
Human liver cancer cell lines (HepG2 and Huh-7) were
provided by the Cell Bank of Chinese Academy of Sciences (Shanghai,
China). All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco) with 10% fetal bovine serum (FBS; both Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
atmosphere with 5% CO2.
miRNA mimics, small interfering RNAs
(siRNAs) and transfection
The miR-363-3p mimics and control mimics were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China), and
transfected into Huh-7 or HepG2 cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The HMGA2 siRNAs and
negative control siRNA were also designed and purchased from
Shanghai GenePharma Co., Ltd. The sequences were: Negative control
siRNA: 5′-UUCUCCGAACGUGUCACGUTT-3′; HMGA2 siRNA 1#:
5′-GGCAUUCAUAUAGGAAGAACGTT-3′; HMGA2 siRNA 2#:
5′-GCUGCUAUACACAAGCAAUGCTT-3′. These were used to transfect Huh-7
or HepG2 cells using Lipofectamine® 2000 according to
the manufacturer's protocol.
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the liver cancer tissues, and cells
with or without the indicated treatments was extracted using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RT-qPCR using the SYBR-Green Realtime PCR
Master mix (Toyobo Life Science, Osaka, Japan) was performed to
quantify the levels of miR-363-3p in the liver cancer tissues and
cells transfected with miRNA mimics, under the following
conditions: 95°C for 30 sec, followed by 40 cycles at 95°C for 15
sec, and 60°C for 30 sec, and 72°C for 30 sec. For qPCR system, 2
µl diluted RT products were combined with 10 µl of 2X mix, 1 µl
forward and reverse primers (10 µM) and 6 µl ddH2O in a
final volume of 20 µl following the manufacturer's protocols. U6
was used as the internal control. RT-qPCR analysis was also
performed to quantify the levels of HMGA2 in the liver cancer
tissues, cells transfected with miRNA mimics or HMGA2 siRNAs alone,
and cells co-transfected with miRNA mimics plus HMGA2 plasmid.
β-actin was used as the internal control. The fold changes of miRNA
and mRNA levels were calculated using the 2−ΔΔCq method
(20). The primers for qPCR were
as follows: miR-363-3p forward (F), 5′-CGGCGAATTGCACGGTATCCA-3′; U6
forward (F), 5′-CGCAAGGATGACACGCAAATTCG-3′; miR-363-3p/U6 reverse
(R), 5′-CAGTGCAGGGTCCGAGGT-3′; HMGA2 F,
5′-AAAGCAGAAGCCACTGGAGAAA-3′; HMGA2 R, 5′-TTCCTCCTGAGCAGGCTTCTT-3′;
β-actin F, 5′-GATCATTGCTCCTCCTGAGC-3′; β-actin R,
5′-ACTCCTGCTTGCTGATCCAC-3′.
Colony formation assay
The Huh-7 or HepG2 cells transfected with miRNA
mimics were resuspended in DMEM with 10% FBS, seeded in 6-well
plates (5×103 cells per well) and incubated for 7 days.
The colonies were fixed with 4% formaldehyde solution and stained
with 0.25% crystal violet in PBS for 30 min. Images of the colonies
were captured using a digital camera (Canon PowerShot A2000 IS,
Canon, Inc., Tokyo, Japan). The experiments were repeated in
triplicate.
MTT assay
The Huh-7 or HepG2 cells transfected with miRNA
mimics or siRNAs alone, or co-transfected with miRNA mimic and
HMGA2 plasmid were seeded into 96-well plates at a density of
2.5×103 cells per well (six replicates). Cell viability
was assessed using an MTT assay (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Following incubation for 1, 2, 3 and 4 days,
10 µl MTT (0.5 mg/ml) was added into each well for an additional 3
h. Subsequently, 250 µl dimethyl sulfoxide was added into each well
and the absorbance at 490 nm was measured using a microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Wound-healing assay
For the wound-healing experiments, the Huh-7 or
HepG2 cells transfected with miRNA mimics or siRNAs alone, or
co-transfected with miRNA mimic plus HMGA2 plasmid were seeded into
6-well plates and cultured to confluency. Following transfection,
the cells were serum-starved and scraped using a P200 tip (0 h).
Images were captured using an inverted optical microscope 48 h
later.
Dual luciferase reporter assay
The Huh-7 or HepG2 cells were seeded in 24-well
plates and co-transfected with luciferase reporters plus miRNA
mimics. The transfection efficiency was normalized using a
Renilla luciferase reporter (pRL-CMV; Promega Corporation,
Madison, WI, USA). The reporter activity was measured using a
Luciferase Assay Detection kit (Promega Corporation) according to
the manufacturer's protocol.
Western blot analysis
Protein samples from the Huh-7 or HepG2 cells
transfected with miRNA mimics were extracted using
radioimmunoprecipitation assay reagent supplemented with protease
inhibitors (both Beyotime Institute of Biotechnology, Haimen,
China). The protein was quantified by using BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Denatured protein (40 µg)
was separated on 8% SDS-PAGE gels and transferred onto
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with a buffer containing 5% skimmed milk in
PBS with 0.05% Tween-20 for 1 h at room temperature. The
nitrocellulose membranes were incubated with primary antibody
(anti-HMGA2, 1:1,000, 7777; and anti-β-actin, 1:6,000, 4970; both
Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at
4°C. Then the membranes were incubated with peroxidase-conjugated
secondary antibodies (1:3,000; A0208; Beyotime Institute of
Biotechnology). Subsequently, the membranes were incubated with an
enhanced chemiluminescence detection system (EMD Millipore).
Target Prediction
Targets of miR-363-3p were searched on TargetScan
Release 3.1 (http://www.targetscan.org/mamm_31/) and the results
suggested that HMGA2 was a potential target of miR-363-3p. To
further confirm that HMGA2 is directly targeted by miR-363-3p, more
information about the 3′UTR of HMGA2 mRNAs was obtained on
TargetScan.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism v6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Student's
two-tailed t-test was used to evaluate the significance of the
differences between two groups. The correlation between the
expression of miR-363-3p and HMGA2 in tissues was calculated by
Spearman's rank correlation analysis. Data are presented as the
mean ± standard deviation from three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-363-3p is downregulated in liver
cancer and is associated with tumor grade
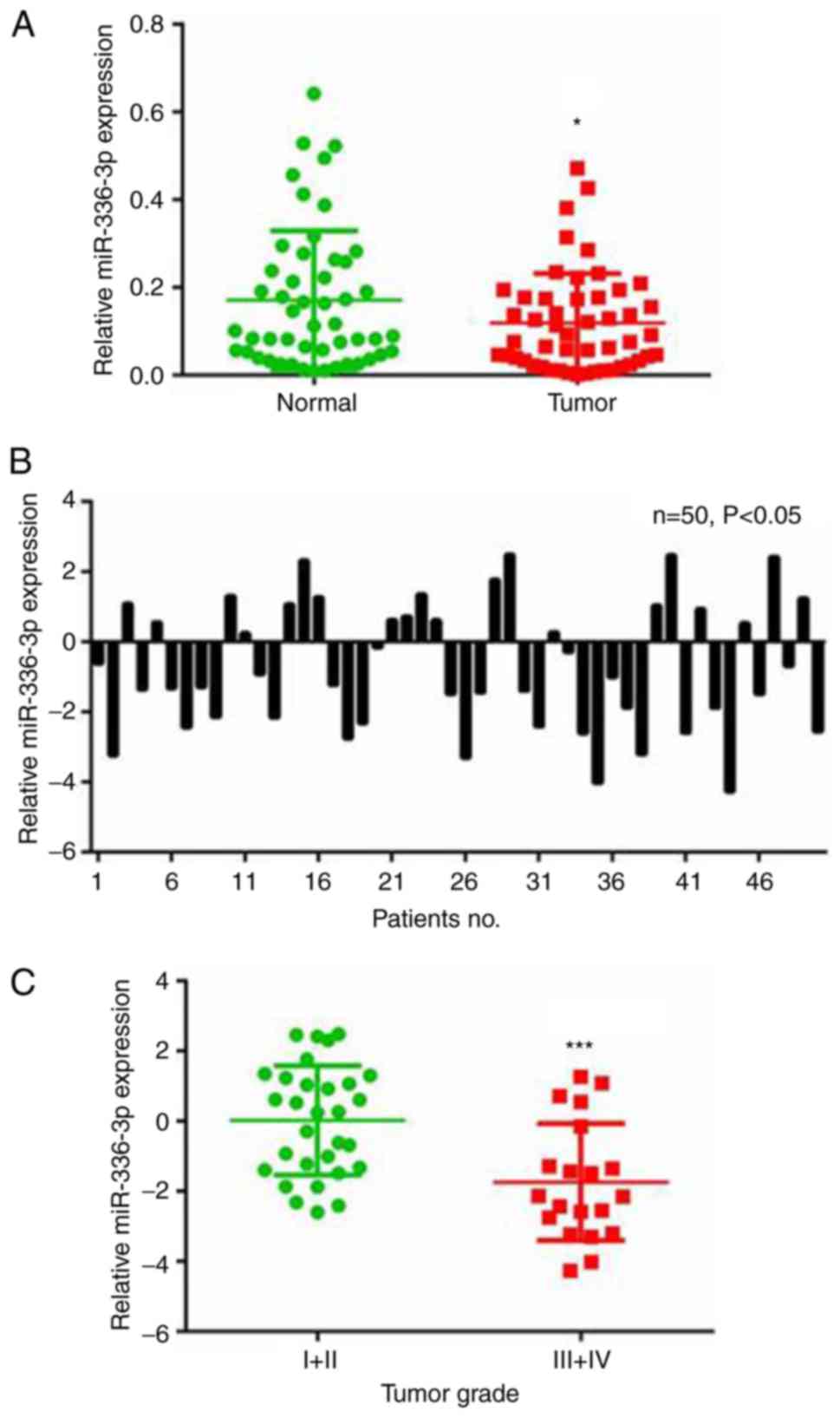
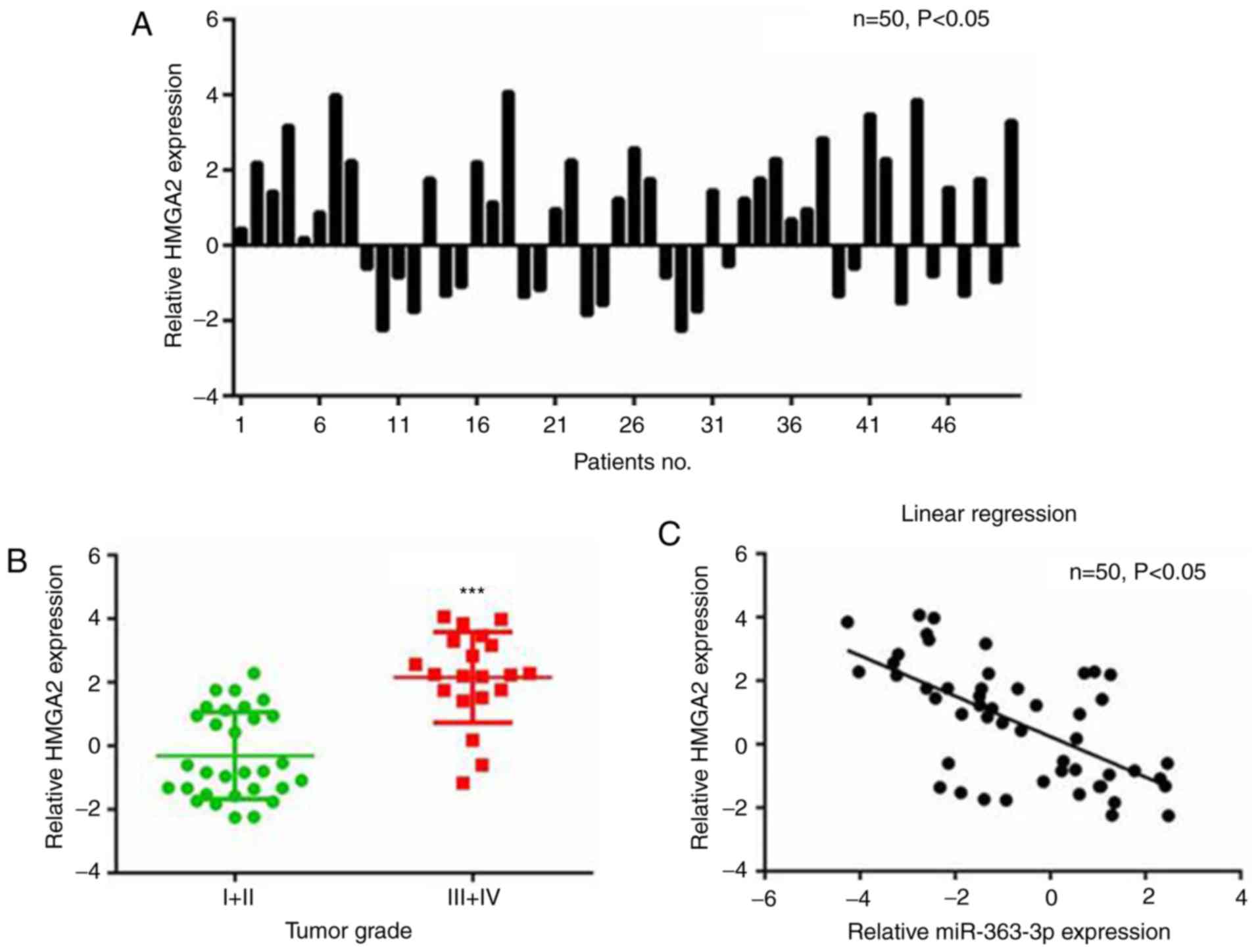
The RT-qPCR analysis demonstrated that miR-363-3p
was downregulated in the 50 liver cancer samples compared with the
paired adjacent non-cancerous liver tissue samples (Fig. 1A). Specifically, downregulation of
miR-363-3p (≤2-fold change) was observed in 50% (25/50) of all the
examined liver cancer samples (Fig.
1B). The expression of miR-363-3p in liver cancer tissues was
also analyzed with samples grouped by different tumor grades. Lower
levels of miR-363-3p were expressed in tumors with higher grades
(Fig. 1C), indicating the
potential use of miR-363-3p in liver cancer diagnosis. These
results suggest a vital tumor-suppressing role for miR-363-3p in
liver cancer.
miR-363-3p inhibits
hepatocarcinogenesis in Huh-7 and HepG2 cells
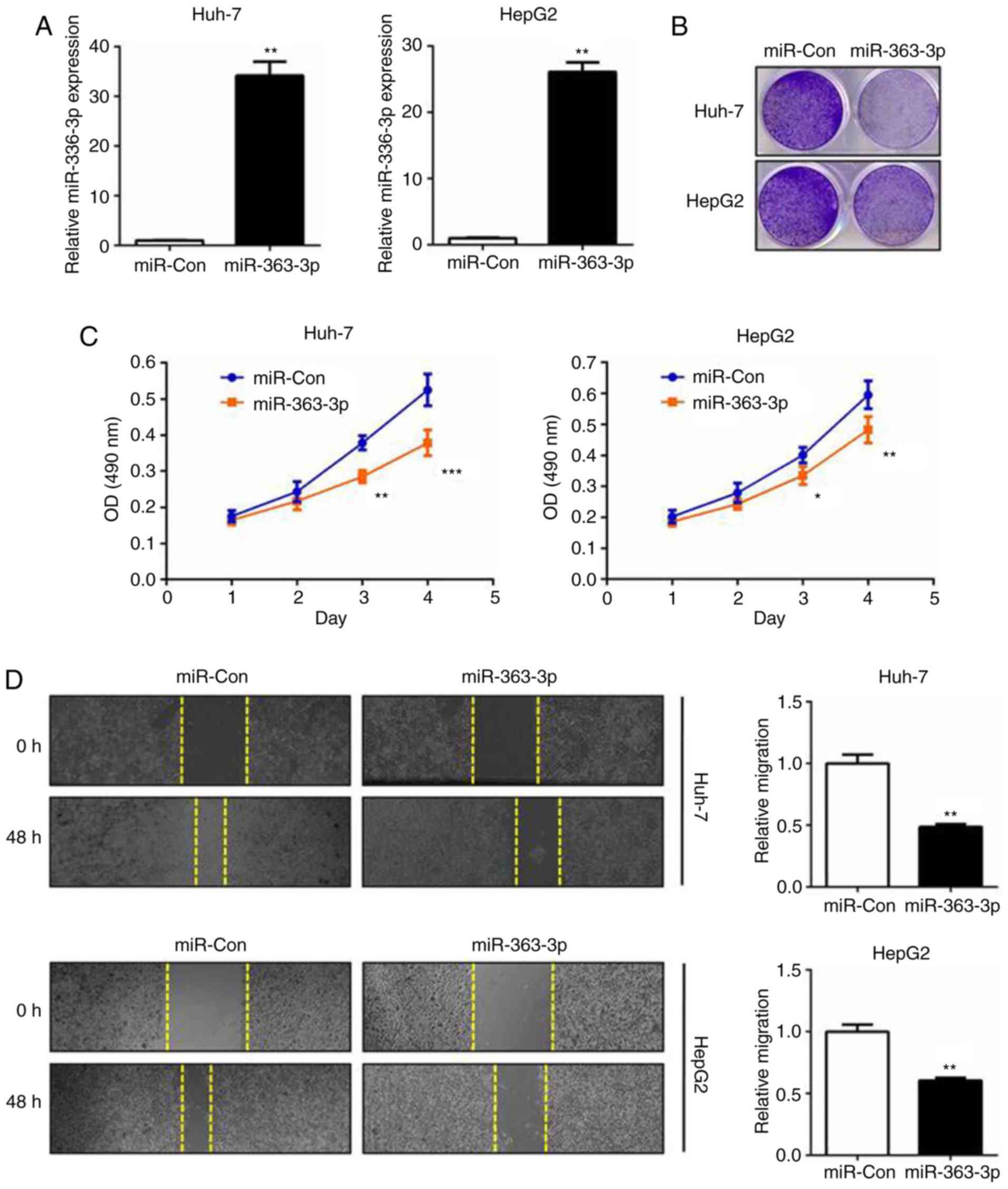
Prompted by the expression results, the role of
miR-363-3p in the cell proliferation and survival of liver cancer
was subsequently investigated. miR-363-3p mimics were transfected
into cells and RT-qPCR analysis was performed (Fig. 2A). The MTT assay demonstrated that
miR-363-3p effectively inhibited cell proliferation, and the colony
formation assay validated that miR-363-3p significantly impaired
clonogenic survival in the two liver cancer cell lines (Fig. 2B and C). In the wound-healing
experiments, miR-363-3p exerted potent anti-migration effects on
the liver cancer cells (Fig. 2D).
Collectively, these results validated that the tumor-suppressing
effect of miR-363-3p in liver cancer was mediated by inhibition of
the proliferation, survival and migration of liver cancer cells
in vitro.
HMGA2 is a direct target of miR-363-3p
in liver cancer
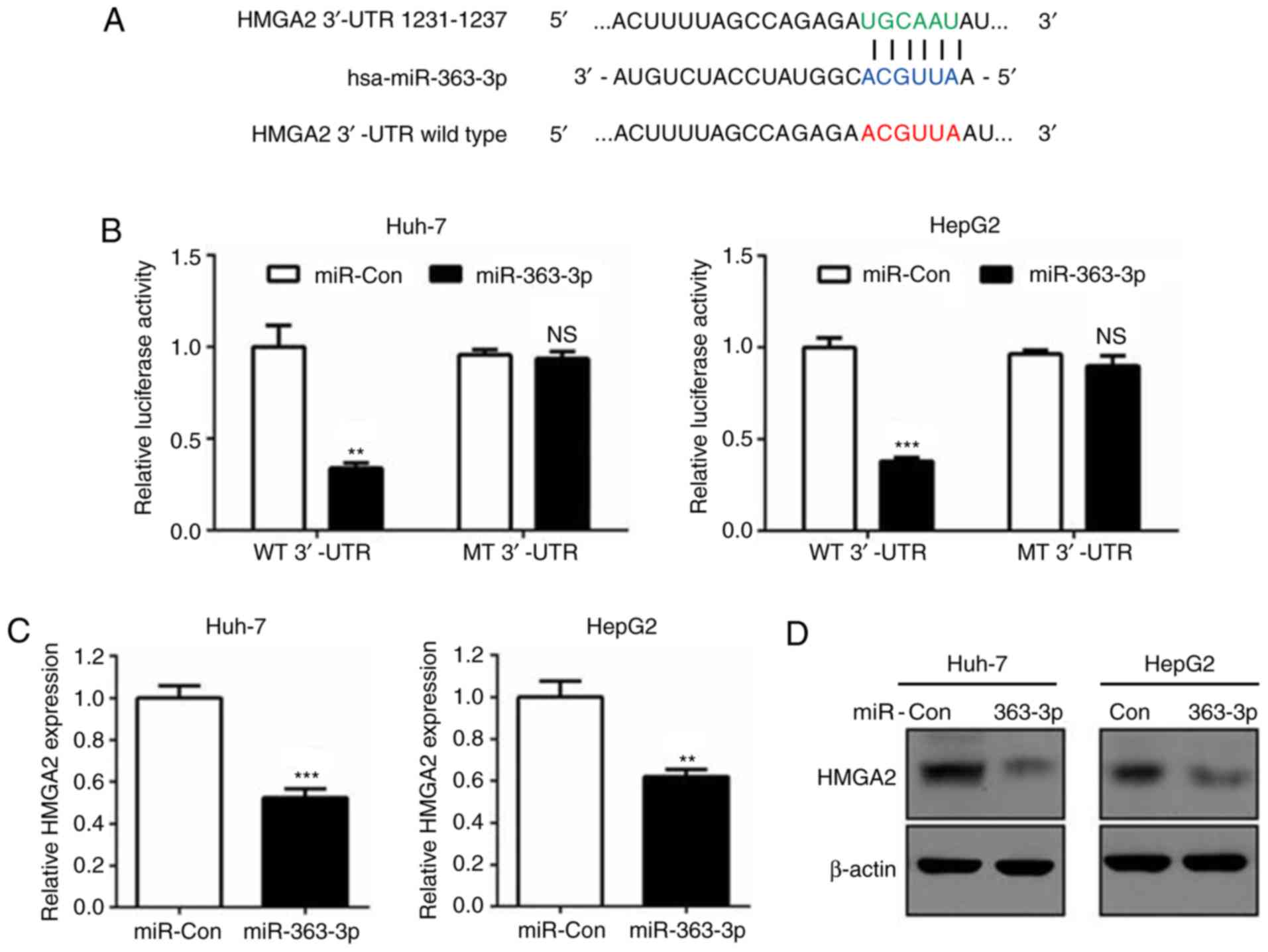
Bioinformatics prediction revealed that the
3′untranslated region (3′UTR) of the HMGA2 gene contained a
putative miR-363-3p target sequence (Fig. 3A). Dual luciferase reporter assays
further confirmed this prediction and demonstrated that HMGA2 was a
direct target of miR-363-3p, as reporters with deletions in the
miR-363-3p target bases of the 3′-UTR eliminated the silencing of
HMGA2 mRNA transcripts (Fig. 3A and
B). Additionally, RT-qPCR and western blot analysis verified
that miR-363-3p effectively reduced the mRNA and protein levels of
HMGA2 compared with those in the control group (Fig. 3C and D). Therefore, the findings
demonstrated that miR-363-3p directly targeted the HMGA2 3′-UTR and
induced its mRNA degradation in liver cancer cells.
miR-363-3p inhibits cell proliferation
and migration by targeting HMGA2
HMGA2 is known to promote proliferation and
metastasis in liver cancer and other types of cancer (21,22).
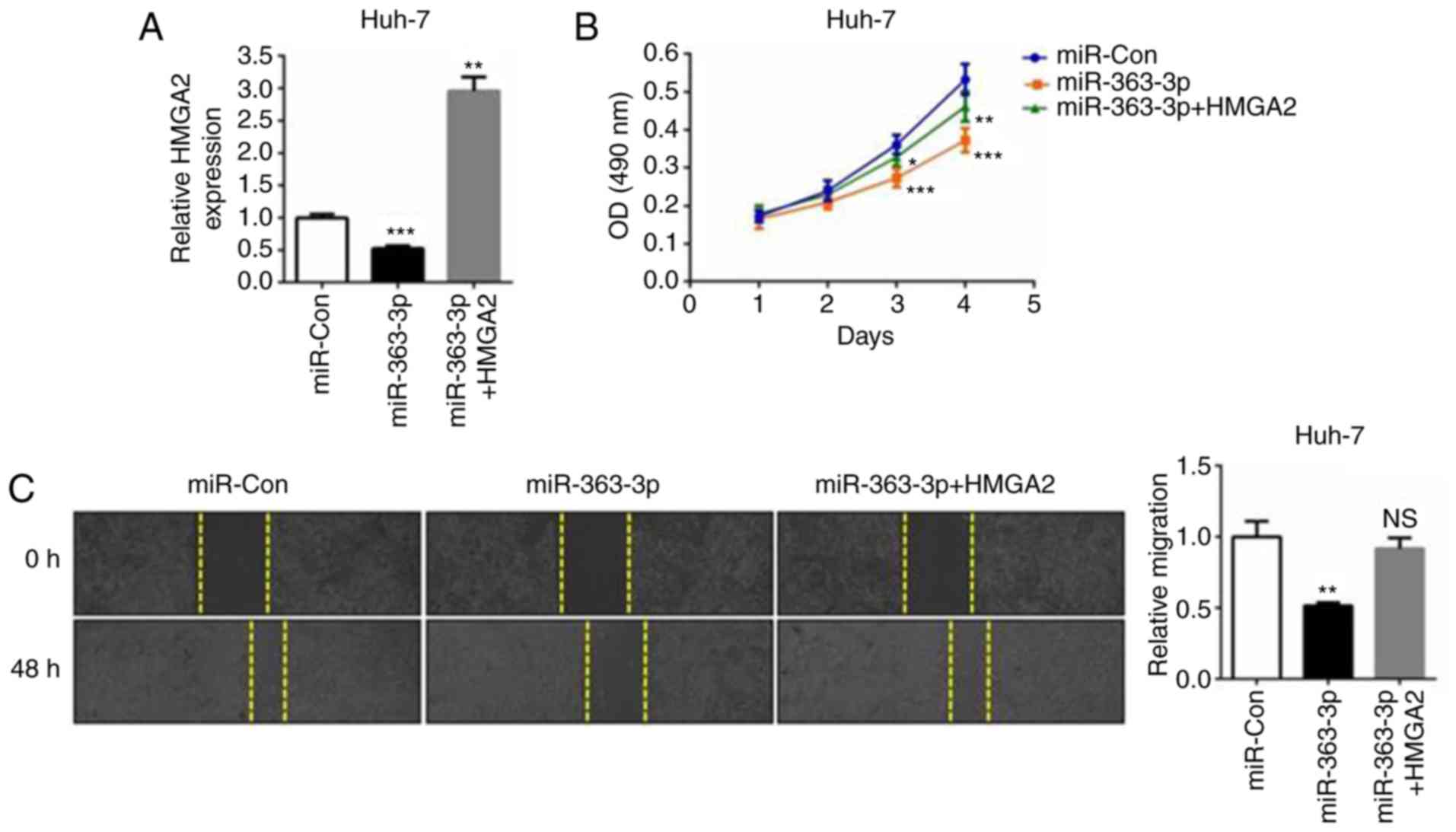
To better understand the association between HMGA2 and the
tumor-suppressing role of miR-363-3p in liver cancer cells,
miR-363-3p mimics and the HMGA2 plasmid were co-transfected into
Huh-7 cells (Fig. 4A). The MTT
assay revealed that liver cancer cell proliferation was suppressed
in the miR-363-3p-overexpressing cells, whereas the ectopic
expression of HMGA2 abrogated this inhibitory effect (Fig. 4B). The same effect was observed in
the cell migration assay; that is, the overexpression of HMGA2
reversed the anti-migration effects caused by miR-363-3p mimics
(Fig. 4C). Additionally, the
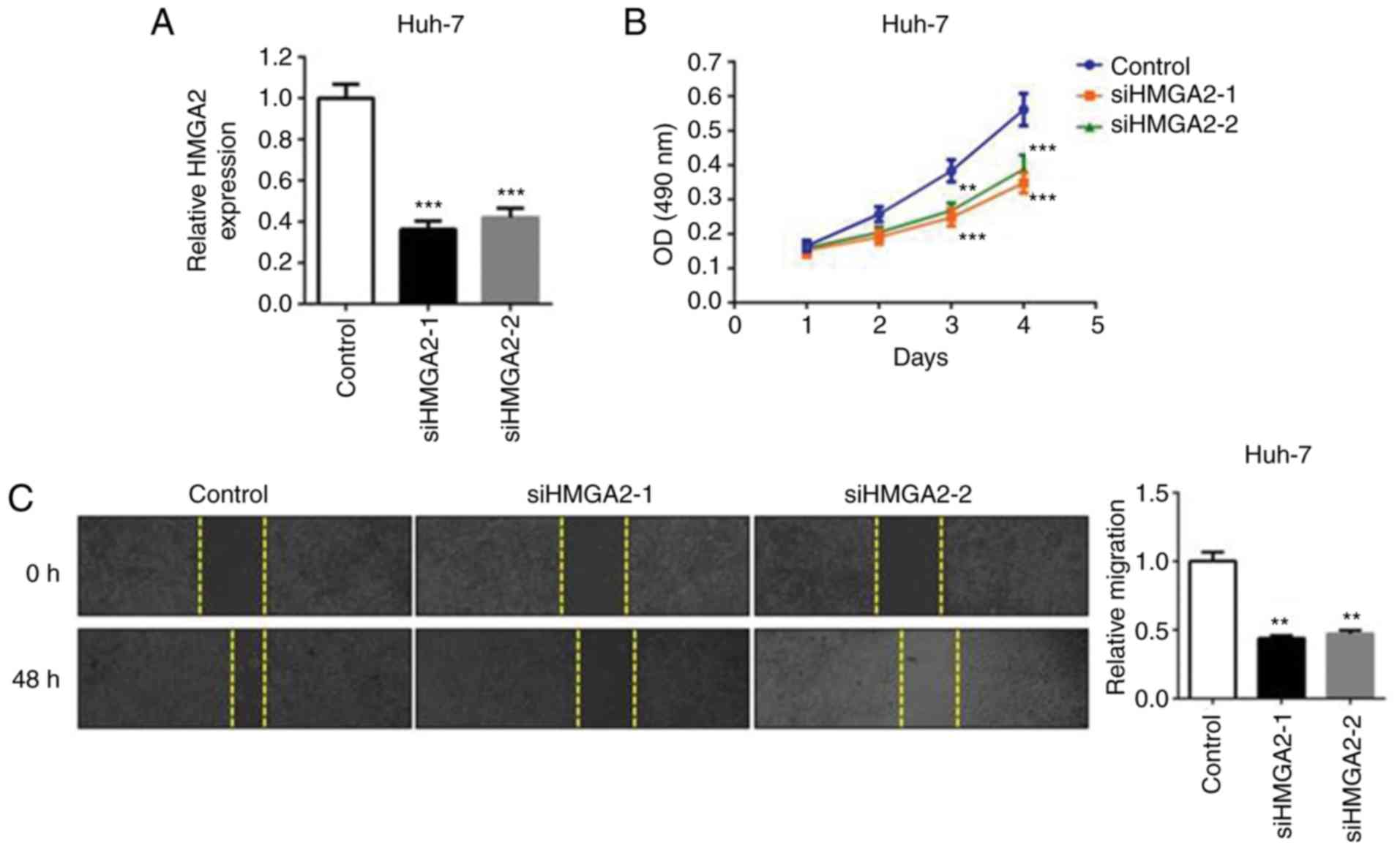
transient knockdown of endogenous HMGA2 (si-HMGA2-1 or si-HMGA2-2;
Fig. 5A) simulated the suppressive
effect of miR-363-3p mimics on the cell proliferation and migration
of liver cancer cells (Fig. 5B and
C). Therefore, the findings demonstrated that HMGA2 is a
functional target of miR-363-3p in liver cancer cells, and the
overexpression of HMGA effectively enhances cell proliferation and
migration.
Upregulated HMGA2 is correlated with
downregulated miR-363-3p in patients with liver cancer
The correlation between expression levels of HMGA2
and miR-363-3p in liver cancer tissues was analyzed. The RT-qPCR
results revealed that the mRNA levels of HMGA2 were higher in liver
cancer than in paired non-cancerous tissues (Fig. 6A), and continued to rise with
increasing tumor grade (Fig. 6B).
Furthermore, the expression of HMGA2 in liver cancer tissues was
negatively correlated with the expression of miR-363-3p (Fig. 6C). Therefore, there was a
significant negative correlation between the expression of HMGA2
and miR-363-3p in liver cancer tissues.
Discussion
In the present study, miR-363-3p was revealed to be
downregulated in liver cancer tissues. As miR-363-3p is decreased
in other types of cancer, including lung and colorectal cancer
(18,19), this finding of its downregulation
in liver cancer reinforces the evidence for its important potential
tumor suppressor role. It has been previously reported that
miR-363-3p was decreased in liver cancer and suppressed cell
proliferation, migration and invasion by targeting SP1 (17). However, the identification of HMGA2
as another target for miR-363-3p in the present study increases
current understanding of the role of miR-363-3p in liver cancer.
miR-363-3p was demonstrated to have tumor suppressor activity via
the direct targeting of HMGA2, resulting in inhibition of cell
proliferation, survival and migration in two liver cancer cell
lines, which suggests a novel mechanism of
hepatocarcinogenesis.
Regarding the transcriptional control of miR-363-3p
in liver cancer, Han et al (21) reported that c-Myc represses the
transcription of miR-363-3p by binding to the conserved region of
the miR-363-3p promoter. As c-Myc is pathologically activated in
liver cancer, the downregulation of miR-363-3p may be largely due
to the transcriptional repression caused by c-Myc.
Bioinformatics prediction and in vitro
experiments demonstrated that HMGA2 is a direct target of
miR-363-3p. HMGA2 is known to promote cancer cell proliferation and
metastasis in liver cancer and other cancer types (22,23).
HMGA proteins function as architectural factors required for
chromosome structure and are essential components of the
enhanceosome. The literature indicates that the overexpression of
HMGA in tumors is associated with a highly malignant phenotype,
resulting in poor patient prognosis (24). The findings of the present study
confirmed the overexpression of HMGA2 in liver cancer, which was
increased in parallel with the increasing tumor grade. A
significant negative correlation between the expression levels of
HMGA2 and miR-363-3p in liver cancer tissues was also established,
which provided important insight into the association between HMGA2
and miR-363-3p.
Functionally, restoring HMGA2 in
miR-363-3p-overexpressing cells significantly reversed the
tumor-suppressing effects caused by miR-363-3p mimics. Notably,
although the overexpression of HMGA2 was marked in the
co-transfection group (miR-363-3p + HMGA2), growth and migration
were not elevated in this group compared with the control group.
This may be explained by the functions of other miR-363-3p target
genes; miR-363-3p also targets SP1 in liver cancer cells (17). However, the results of the present
study adequately demonstrated that HMGA2 is an important target of
miR-363-3p in liver cancer cells. Additionally, the knockdown of
endogenous HMGA2 simulated the suppressive roles of miR-363-3p on
the proliferation and migration of liver cancer cells. These
findings were in accordance with the conclusion from a previous
study that HMGA2 is a potential oncogene and tumor biomarker in
human cancer (24); however, the
detailed mechanisms remain to be fully elucidated. Structurally,
HMGA2 protein contains DNA-binding domains and may act as a
transcriptional regulatory factor. High-throughput sequencing
methods, including RNA-Seq and chromatin immunoprecipitation-Seq,
may provide more information on the downstream gene regulation
networks that HMGA2 is involved in.
In conclusion, the findings of the present study
revealed that miR-363-3p inhibited liver cancer cell proliferation,
survival and migration by directly targeting HMGA2. Abnormal
decreases in miR-363-3p and increases of its target, HMGA2, are
important factors that may be involved in hepatocarcinogenesis and
liver cancer progression. This novel miR-363-3p/HMGA2 axis provides
insight into the mechanisms of liver cancer onset and may
facilitate drug development against liver cancer. Additionally,
miR-363-3p and HMGA2 may be effective biomarkers used for liver
cancer grading and prognosis prediction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and HL designed the study; HG and XG performed
data collection; DG performed the data analysis and YY collected
the clinical data, prepared the manuscript and approved the
publication of the final version.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of Tianjin Medical University General Hospital Airport
Site.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blum HE: Hepatocellular carcinoma: Therapy
and prevention. World J Gastroenterol. 11:7391–7400.
2005.PubMed/NCBI
|
|
3
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rapado-González Ó, Majem B, Muinelo-Romay
L, Álvarez-Castro A, Santamaría A, Gil-Moreno A, López-López R and
Suárez-Cunqueiro MM: Human salivary microRNAs in Cancer. J Cancer.
9:638–649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hosseinahli N, Aghapour M, Duijf PHG and
Baradaran B: Treating cancer with microRNA replacement therapy: A
literature review. J Cell Physiol. 233:5574–5588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma J and Li X: MicroRNAs are involved in
the toxicity of microcystins. Toxin Reviews. 36:165–175. 2017.
View Article : Google Scholar
|
|
11
|
Ma J, Li Y, Yao L and Li X: Analysis of
microRNA expression profiling involved in MC-LR-induced
cytotoxicity by high-throughput sequencing. Toxins (Basel). 9(pii):
E232017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chakraborty C, Sharma AR, Sharma G, Sarkar
BK and Lee SS: The novel strategies for next-generation cancer
treatment: miRNA combined with chemotherapeutic agents for the
treatment of cancer. Oncotarget. 9:10164–10174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vannini I, Fanini F and Fabbri M: Emerging
roles of microRNAs in cancer. Curr Opin Genet Dev. 48:128–133.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lou W, Liu J, Gao Y, Zhong G, Chen D, Shen
J, Bao C, Xu L, Pan J, Cheng J, et al: MicroRNAs in cancer
metastasis and angiogenesis. Oncotarget. 8:115787–115802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Y, Xu T, Zhou S and Cui M:
MicroRNA-363 inhibits ovarian cancer progression by inhibiting
NOB1. Oncotarget. 8:101649–101658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song B, Yan J, Liu C, Zhou H and Zheng Y:
Tumor Suppressor Role of miR-363-3p in Gastric Cancer. Med Sci
Monit. 21:4074–4080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ying J, Yu X, Ma C, Zhang Y and Dong J:
MicroRNA-363-3p is downregulated in hepatocellular carcinoma and
inhibits tumorigenesis by directly targeting specificity protein 1.
Mol Med Rep. 16:1603–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Chen T, Huang H, Jiang Y, Yang L,
Lin Z, He H, Liu T, Wu B, Chen J, et al: miR-363-3p inhibits tumor
growth by targeting PCNA in lung adenocarcinoma. Oncotarget.
8:20133–20144. 2017.PubMed/NCBI
|
|
19
|
Hu F, Min J, Cao X, Liu L, Ge Z, Hu J and
Li X: MiR-363-3p inhibits the epithelial-to-mesenchymal transition
and suppresses metastasis in colorectal cancer by targeting Sox4.
Biochem Biophys Res Commun. 474:35–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han H, Sun D, Li W, Shen H, Zhu Y, Li C,
Chen Y, Lu L, Li W, Zhang J, et al: A c-Myc-MicroRNA functional
feedback loop affects hepatocarcinogenesis. Hepatology.
57:2378–2389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao X, Dai M, Li Q, Wang Z, Lu Y and Song
Z: HMGA2 regulates lung cancer proliferation and metastasis. Thorac
Cancer. 8:501–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang W, Li J, Guo X, Zhao Y and Yuan X:
miR-663a inhibits hepatocellular carcinoma cell proliferation and
invasion by targeting HMGA2. Biomed Pharmacother. 81:431–438. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pallante P, Sepe R, Puca F and Fusco A:
High mobility group a proteins as tumor markers. Front Med
(Lausanne). 2:152015.PubMed/NCBI
|