Introduction
Periodontitis and chronic apical periodontitis often
cause periodontal tissue destruction and severe alveolar bone
defects (1). In periodontally
compromised teeth, when partial or full socket wall destruction is
evident, connective tissue will grow into the extraction site and
lead to a deficient ridge (1).
Periodontitis is widely treated with instant implant technology in
clinical practice (2). However,
periodontal tissue destruction and alveolar bone defects
significantly weaken the stability and shorten the service life of
dental implants (2). To improve
the success rate of dental implants, concentrated growth factor
(CGF) can be used to promote periodontal tissue regeneration and
osteogenic differentiation (3).
CGF is a gel-like substance, obtained by centrifugation of venous
blood, which is rich in growth factors and fibrin (4–8), and
CGF combined with bone graft material promotes immediate
periodontal tissue regeneration and osteogenic differentiation
(8,9).
Previous studies have reported that platelet-rich
plasma (PRP) and platelet-rich fibrin (PRF) in the first and second
phases promote tissue regeneration, inhibit infection, regulate
inflammation and reduce postoperative reactions (9–14).
CGF is a third-phase plasma extract, consisting of multiple growth
factors (4–8). Due to the unique structure of CGF
fibrin, it can replace the diaphragm used in guiding bone
regeneration to a certain extent (8,9,15).
CGF has been widely studied in the fields of oral implantation,
extraction site preservation, jaw cyst treatment, and fracture
healing promotion (4–8,16,17).
CGF exudate (CGFe) is extracted from CGF and used in
research labs to study the effects of CGF. Previous studies have
shown that CGFe significantly shortens the time for osteogenesis in
the operational area and distinctly improves bone formation quality
(8,9). CGFe has been demonstrated to
stimulate the proliferation of human periodontal ligament
fibroblasts (PDLFs) (17).
However, these previous studies were performed without the
influence of inflammatory factors, and therefore did not
sufficiently reflect the clinical environment. Particularly, the
effect of CGFe on enhancing the proliferation of hPDLCs in the
presence of tumor necrosis factor (TNF)-α-induced inflammation has
not yet been investigated, to the best of our knowledge.
TNF-α is a pro-inflammatory cytokine that is a
critical pathological factor for the development of apical
inflammation, which significantly inhibits osteogenic
differentiation (16,18–21).
As TNF-α-induced inflammation is commonly encountered in clinical
practice (16,20), it is of great clinical interest to
evaluate the effects of CGFe in the presence of TNF-α.
In the present study, the effects of CGFe on hPDLC
proliferation and osteogenic differentiation were investigated in
an inflammatory environment, stimulated by TNF-α. Specifically, the
effects of CGFe on alkaline phosphatase (ALP) activity,
mineralization, and osteocalcin (OCN), runt-related
transcription factor 2 (RUNX2), and Osterix (OSX)
gene expression in hPDLCs under TNF-α-induced inflammatory
conditions were examined.
Materials and methods
Preparation of CGFe
The present study was approved by the Ethics
Committee of the Jilin University Health Science Center (Changchun,
China). In accordance with this committee, CGFe was obtained from
three healthy male donors who had visited the outpatient clinic at
the Health Center of Jilin University (Changchun, China) from
August 2017 to March 2018. They were non-smokers and non-drinkers
aged from 22 to 30 years old, and their informed consent was
obtained. The experiments described below were carried out under
similar conditions and procedures as the previous study (11).
Venous blood samples from the donors were used to
produce human CGF according to a previously described protocol
(22). In brief, blood samples
were centrifuged at 750 × g for 12 min at 4°C. Between the
acellular plasma and red blood cells (RBCs), a white CGF clot
formed. The CGF clot was held with sterile forceps, separated from
the RBCs using scissors, placed on the grid of an endo box and
compressed by the endo box cover. After 1 min of applied pressure,
the CGF clot was converted into CGF membrane, and the CGF exudate
(CGFe) was collected in the tray of the endo box.
The CGFe was centrifuged at 500 × g for 5 min at 4°C
to remove RBCs. Then, the CGFe was precipitated and filtered using
a 0.22 µm sterile syringe filter unit (Merck KGaA, Darmstadt,
Germany). The pooled CGFe samples were stored at −80°C prior to
use. The original concentration of CGFe was defined as 100%, and a
50% concentration of CGFe was obtained by dilution with Minimal
Essential Medium-α (α-MEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and used in subsequent experiments.
Human periodontal ligament cell
(hPDLC) culture
In total, 10 healthy and noncarious premolars from
three male and two female donors aged from 13 to 18 years old who
had received orthodontic treatment at the Oral and Maxillofacial
Surgery Department of the Stomatology School of Jilin University
(Changchun, China) were obtained with informed consent. Periodontal
ligaments were gently scraped from the middle third of the
tooth-root surface with a sharp scalpel, minced with ophthalmic
scissors and rinsed with α-MEM. These explants were cultured in
α-MEM supplemented with 10% fetal bovine serum (FBS; GE Healthcare
Life Sciences, Chicago, IL, USA), 1% streptomycin and penicillin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2. Examination by inverted light microscopy (IX73;
Olympus Corporation, Tokyo, Japan) at magnifications of ×20 or ×40
was performed daily, and the medium was replaced every 3 days. Once
cells reached 80% confluence, they were transferred to a 75
cm2 flask, and this was defined as passage one. The same
procedure was performed repeatedly to produce multiple passages.
Cells prior to passage 6 were used in the present study.
Immunocytochemistry staining
hPDLCs at passage 3 were seeded into several 24-well
plates at a density of 1×104/well and covered in advance
with circular coverslips (diameter, 14 mm) and incubated for 48 h
at 37°C. Cells were then rinsed three times with 0.01 M PBS and
fixed with 4% paraformaldehyde for 20 min at room temperature.
Following a further wash with PBS, 0.25% Triton X-100 was added
into the 24-well plates, which were incubated at 37°C for 15 min.
Endogenous peroxidase activity was eliminated by incubation with 3%
H2O2 for 10 min at room temperature. Then,
hPDLCs were incubated with 1% bovine serum albumin (Gibco; Thermo
Fisher Scientific, Inc.) and 22.52 mg/ml glycine in PBS + 0.1%
Tween-20 for 30 min at room temperature. Next, cells were incubated
with anti-vimentin (1:100; cat. no. ab24525; Abcam, Cambridge, MA,
USA) and anti-cytokeratin (1:200; cat. no. AM06387SU-N; OriGene
Technologies, Inc., Beijing, China) primary antibodies overnight at
4°C. The SP immunohistochemistry assay kit (cat. no. SP9001;
ZSGB-BIO; OriGene Technologies, Inc., Beijing, China) was used for
immunocytochemical staining according to the manufacturer's
instructions, and a 3,3′-diaminobenzidine was used to stain
positive cells. An inverted microscope (IX73; Olympus Corporation,
Tokyo, Japan) was used to view the cells at magnifications of ×20
or ×40.
Cell Counting Kit (CCK)-8 assay
The objective of this experiment was to examine the
effect of CGFe on the proliferation of hPDLCs in vitro. CGFe
was obtained according to a previous protocol (16). The CCK-8 assay (Dojindo
Laboratories, Kumamoto, Japan) was used to determine the effects of
CGFe on hPDLC proliferation.
hPDLCs (2×103/well) were seeded into a
96-well plates containing complete medium (α-MEM supplemented with
10% FBS, 1% streptomycin and penicillin) with 10% FBS and incubated
for 24 h at 37°C. Then, hPDLCs were exposed to CGFe (concentration
50%), TNF-α (10 ng/ml), or CGFe+TNF-α for 24, 48 or 72 h at 37°C,
respectively. There was one additional control group with no CGFe
or TNF-α treatment (complete medium with 10% FBS only). Cell
proliferation was determined using the CCK-8 kit at the specified
time-points. Kit reagent (10 µl) was added to the culture medium in
each well. After a 90 min incubation at 37°C, absorbance at 450 nm
was detected using an automatic microplate reader (Infinite 200
PRO; Tecan Group Ltd., Mannedorf, Switzerland). A well containing
complete medium and CCK-8 solution without seeding cells was used
as a further control. The assay was performed in duplicate, and the
experiments were repeated six times under the same conditions.
ALP activity assay
hPDLCs (500 µl; 1×104/well) were seeded
into 24-well plates and incubated for 24 h at 37°C. Next, the cells
were exposed to CGFe, TNF-α or CGFe+TNF-α for 7 or 14 days. At the
given time-points, cells were lysed with 0.1% Triton X-100, and the
lysates were centrifuged at 8000 × g for 10 min at 4°C. The
supernatant (50 µl/well) was added to 96-well plates, and ALP
activity was examined with a ALP assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's protocols. The optical density (OD) values were read
at 520 nm with an automatic microplate reader (Infinite 200 PRO;
Tecan Group, Ltd.).
Osteogenic differentiation induction
and Alizarin Red S staining
hPDLCs (500 µl; 1×104/well) were seeded
into 24-well plates in standard medium (α-MEM supplemented with 10%
FBS, 1% streptomycin and penicillin) until 60–70% confluence was
reached. The medium was then replaced with four different media: i)
Mineral induction medium (MM; α-MEM medium containing 10% FBS, 50
mg/ml ascorbic acid, 10 mM β-glycerolphosphate and 10−8
M dexamethasone); ii) MM with CGFe (concentration 50%); iii) MM
with TNF-α (10 ng/ml); or iv) MM with CGFe+TNF-α (10 ng/ml). The
medium was replaced every 3 days.
Alizarin Red staining was used to detect and
quantify the formation of mineralized nodules after 21 days. This
assay was performed according to a previously described protocol,
with minor modifications (9,23).
Specifically, the hPDLCs were fixed with 95% ethanol for 15 min at
room temperature, washed twice with dH2O and stained
with 0.1% Alizarin Red S solution (pH 4.1) for 20 min at room
temperature. hPDLCs were observed under an inverted phase-contrast
microscope (IX73; Olympus Corporation, Tokyo, Japan; magnification,
×20 or ×40). Next, the hPDLCs were washed three times with
dH2O. To semi-quantify the content of mineralized matrix
nodules generated from the hPDLCs, 100 mM cetyl pyridinium chloride
was added to the 24-well plates to dissolve and release the
calcium-combined Alizarin Red S into solution. OD values were read
at 570 nm, which represented the relative quantity of
mineralization nodules.
Western blotting
Cell lysates were prepared in
radioimmunoprecipitation assay buffer (150 mM NaCl, 0.1% SDS, 1 mM
PMSF, 10 mM Tris-Cl, pH 7.4, 1% sodium deoxycholate, 1% Triton
X-100). The cells were treated with four different media (control,
CGFe, TNF-α, or CGFe+TNF-α) for 14 days. The cell lysates were
incubated for 30 min on ice, then clarified by centrifugation at
6,000 × g for 10 min at 4°C. Protein concentration was determined
with a bicinchoninic acid assay. Lysate samples (20 µl) were
denatured and resolved by 10% SDS-PAGE, transferred onto
polyvinylidene difluoride membranes and run at 300 mA for 2 h.
Membranes were blocked in 5% non-fat milk at room temperature for 1
h, then incubated with primary antibodies against RUNX2 (1:1,000;
cat. no. 12556; Cell Signaling Technology, Inc., Danvers, MA, USA)
and Osterix (1:1,000; cat. no. ab229258; Abcam) and GAPDH overnight
at 4°C. Membranes were subsequently washed three times in
Tris-buffered saline with 0.1% Tween 20 (TBST) and incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:500; cat.
no. 10285–1-AP; ProteinTech Group, Inc., Chicago, IL, USA) for 1 h
at room temperature. Following three washes with TBST, the protein
bands were visualized by using an Enhanced Chemiluminescence kit
(GE Healthcare Life Sciences) and exposed to X-ray film. GAPDH was
used as internal reference and the ImageJ (version 1.48; National
Institutes of Health, Bethesda, MD, USA) was used for
densitometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
hPDLCs (2 ml; 1×105/well) were seeded
into 6-well plates in standard medium until 60–70% confluence was
reached. The cells were treated with four different media (control,
CGFe, TNF-α, or CGFe+TNF-α). The control group was incubated with
osteogenic induction medium [α-MEM, l50 µg/ml ascorbic acid and 10
mM β-sodium glycerophosphate (Sigma-Aldrich; Merck KGaA)]. The
experimental groups were treated with osteogenic induction medium
plus CGFe, osteogenic induction medium plus TNF-α (10 ng/ml), or
osteogenic induction medium plus CGFe+TNF-α (10 ng/ml). RNA was
extracted and reverse transcribed into cDNA using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) after 4, 7 and 14 days. cDNA synthesis was performed with 1
µg of total RNA, random hexamer primers, 5X First-Strand Buffer,
and dNTP Mix (10 mM each) using SuperScript™ II RT (cat. no.
18064014; Invitrogen; Thermo Fisher Scientific, Inc.). The
temperature protocol for RT was: Denaturing RNA at 65°C for 5 min,
then incubation at 25 °C for 10 min and 42°C for 60 min, followed
by 70°C for 15 min.
A 10 µl volume reaction system was adopted for qPCR
determination of the expression of the osteogenic genes OCN,
RUNX2 and OSX using the SYBR® Premix Ex Taq™
II kit (Takara Bio, Inc., Otsu, Japan). qPCR primers were designed
to span an intron so that only RNA-specific amplification was
possible. PCR was performed with the following thermocycling
conditions: 95°C for 3 min, followed by 40 cycles of 95°C for 3 sec
and 60°C for 60 sec. Each sample was tested in triplicate, and fold
differences in gene expression were calculated using the
2−∆∆Cq method (24)
with normalization to β-actin. The primer sequences are presented
in Table I.
 | Table I.Primers used for quantitative
polymerase chain reaction analysis. |
Table I.
Primers used for quantitative
polymerase chain reaction analysis.
|
| Primers |
|
|---|
|
|
|
|
|---|
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | PCR products
(bp) |
|---|
| β-actin |
AGAAAATCTGGCACCACACC |
GGGGTGTTGAAGGTCTAAA | 139 |
| Osteocalcin |
GGCGCTACCTGTATCAATGG |
TCAGCCAACTCGTCACAGTC | 106 |
| RUNX2 |
CACCATGTCAGCAAAACTTCTT |
TCACGTCGCTCATTTTGC | 96 |
| Osterix |
TGCTTGAGGAGGAAGTTCAC |
AGGTCACTGCCCACAGAGTA | 148 |
Statistical analysis
hPDLC proliferation and ALP activity assays were
analyzed by one-way analysis of variance (ANOVA) followed by
Tukey's post-hoc test. Western blotting and Alizarin Red staining
assay data were analyzed using two-way ANOVA, followed by
Bonferroni's post-hoc comparisons test for independent samples.
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used to perform
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference. All data were expressed as
the mean ± standard deviation, and experiments were performed in
triplicate.
Results
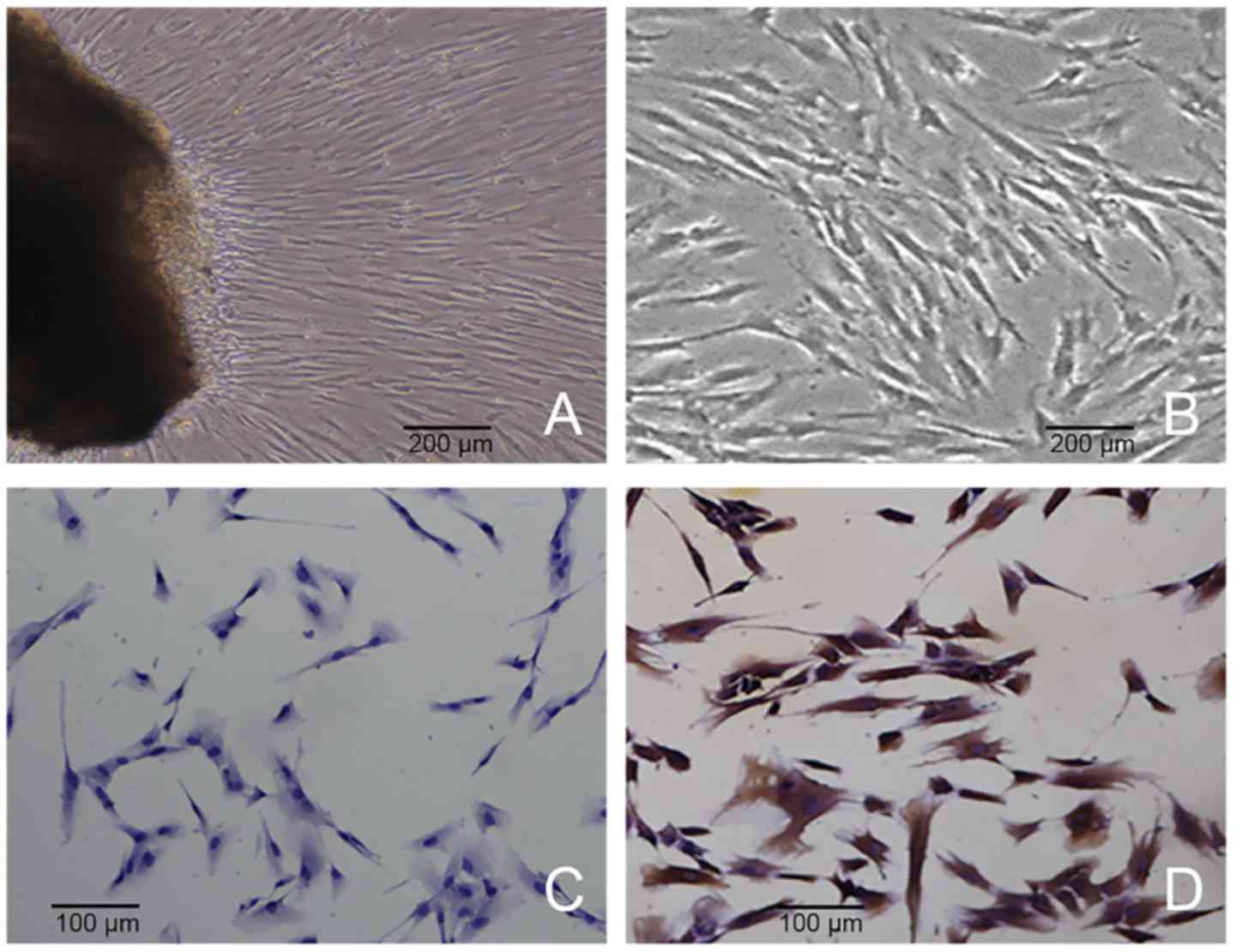
Characterization of hPDLCs
hPDLCs grew out from the tissue explant after 7 and
10 days of culture (Fig. 1A).
Spindle shapes were observed, and a number of cells were
distributed in a circinate pattern with rapid proliferation
(Fig. 1B). The CGFe obtained was a
yellowish clear fluid, and each 50 ml blood sample produced 4 ml of
CGFe. The cells were vimentin positive (Fig. 1C) and keratin negative (Fig. 1D) according to immunochemistry
staining, indicating that these primary cells were of mesenchymal
origin.
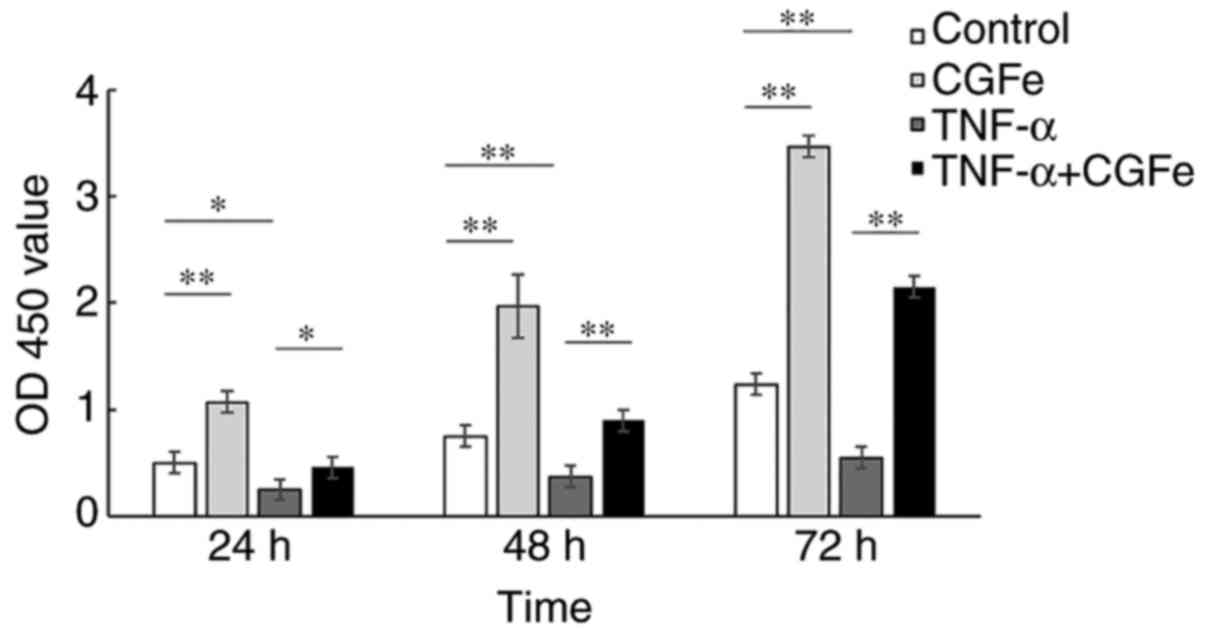
CGFe increases hPDLC
proliferation
The proliferation of hPDLCs was measured with CCK-8
assays (Fig. 2). After 24, 48 and
72 h, CGFe significantly enhanced the proliferation of hPDLCs
compared with the control group (P<0.01), while TNF-α
markedly inhibited the proliferation of hPDLCs (for TNF-α vs.
control, P<0.05 at 24 and 48 h; P<0.01 at 72
h). In addition, the proliferation rate of the CGFe group was
significantly higher than that of the CGFe+TNF-α group, and the
proliferation of the CGFe group exceeded the TNF-α group
substantially (for TNF-α vs. CGFe+TNF-α, P<0.05 at 24 h;
P<0.01 at 48 and 72 h).
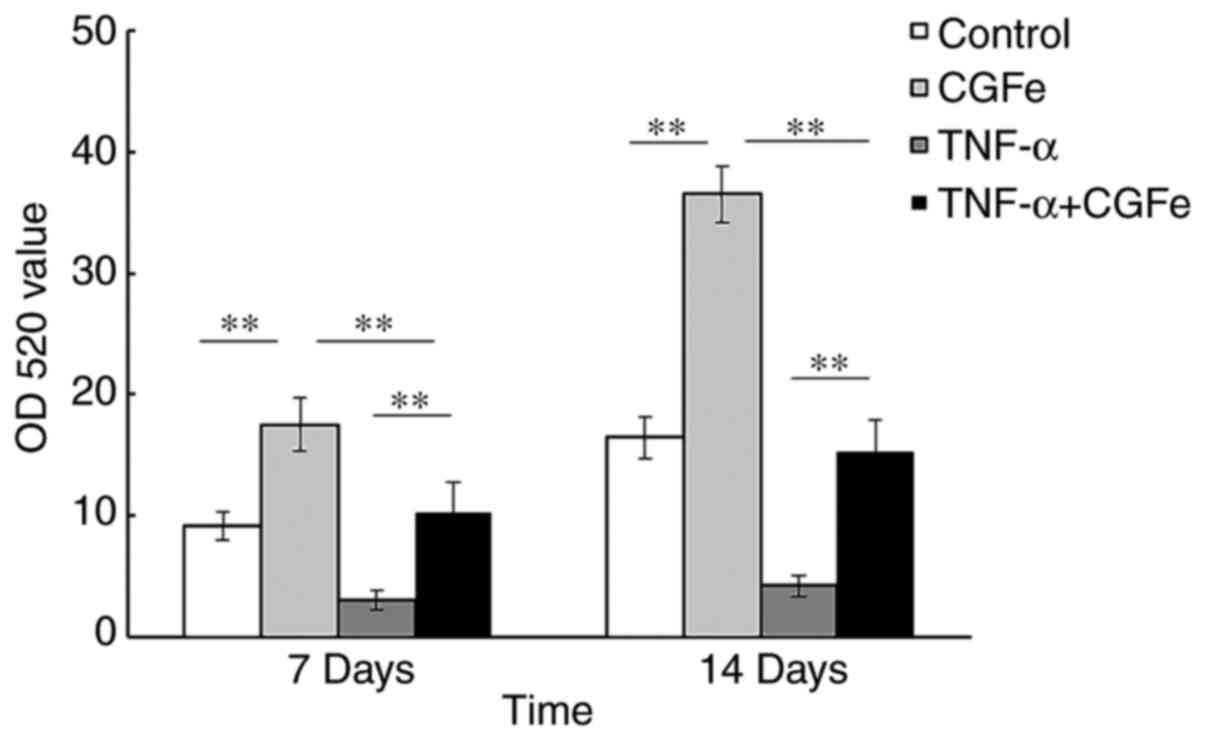
CGFe increases ALP activity
After 7 and 14 days of culture, the hPDLCs cultured
in the CGFe group showed the highest levels of ALP activity
compared with the other experimental groups (Fig. 3). Treatment with TNF-α
significantly inhibited the ALP activity of hPDLCs, as shown by
comparison of the TNF-α treatment group with the CGFe and
TNF-α+CGFe groups, and the difference between these groups was
significant (P<0.01) at both time-points. As expected,
the ALP activity in all groups progressively increased over
time.
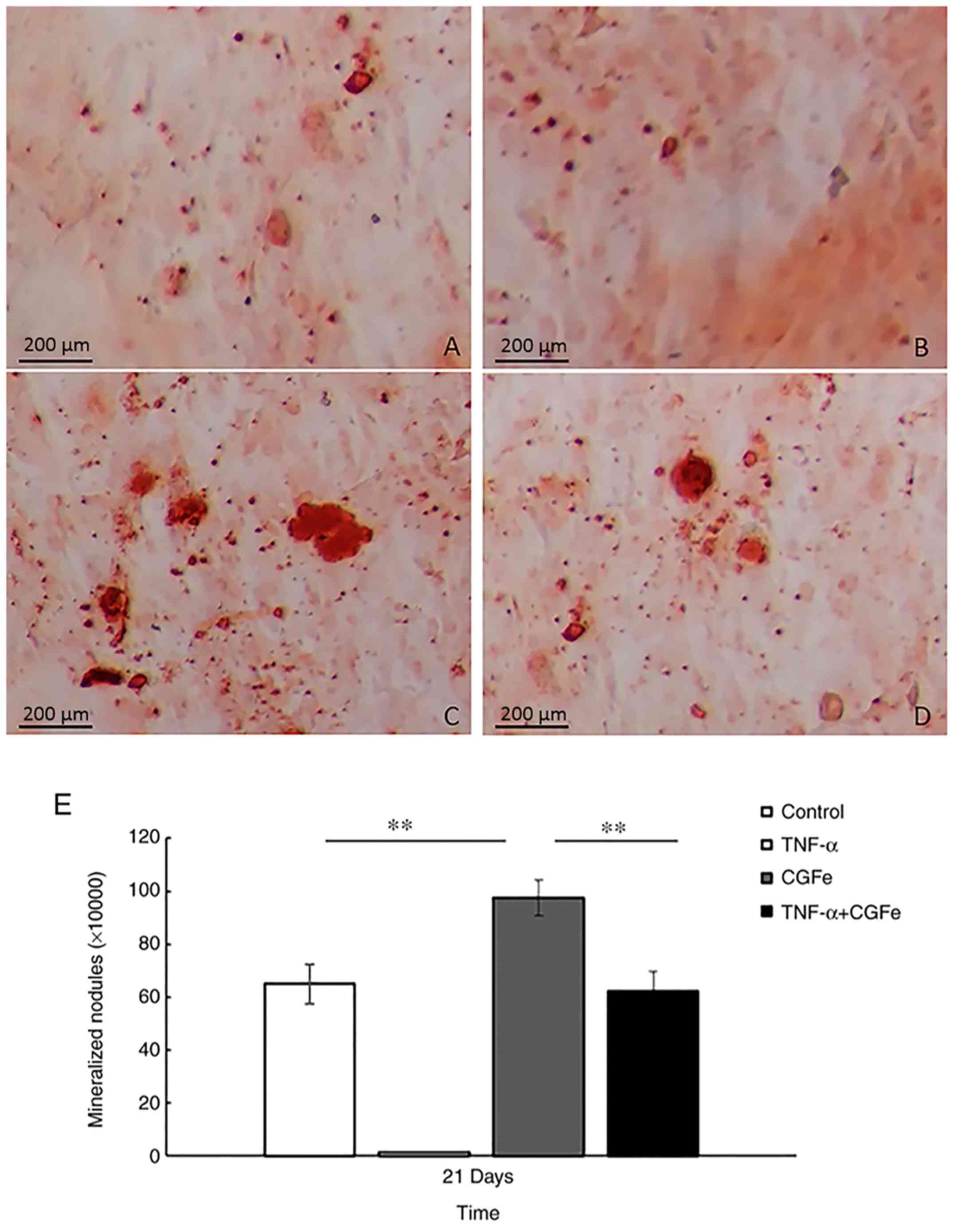
Alizarin Red S staining and
semi-quantification of mineralized matrix nodules
In order to detect the formation of mineralized
matrix nodules, Alizarin Red S staining was performed (Fig. 4A-D). After 21 days of osteogenic
induction, mineralized nodules were observed with an inverted
phase-contrast microscope, and the number of nodules was notably
higher in the CGFe group (Fig.
4C), compared with the control (Fig. 4A) or CGFe+TNF-α (Fig. 4D) groups. The TNF-α group (Fig. 4B) formed the fewest mineralized
nodules.
The mineralized matrix nodules were quantified after
21 days of induction (Fig. 4E).
The absorbance values at 570 nm revealed that extracellular calcium
deposition in the CGFe group was significantly higher than the
control or CGFe+TNF-α groups (P<0.01).
CGFe increases osteogenic-associated
gene and protein expression
The gene expression of the RUNX2, OSX and
OCN was determined after 4, 7 and 14 days of osteogenic
induction (Fig. 5). It was
observed that on days 4, 7 and 14, the expression of RUNX2
and OSX in the TNF-α (10 ng/ml) groups was decreased
compared with the control group (P<0.01; excluding
OSX expression on day 7, P<0.05), and the
expression of these two genes in the CGFe+TNF-α group showed no
significant increase compared with the control group. By contrast,
on days 4, 7, and 14, expression of the RUNX2 and OSX
genes in the CGFe group was significantly increased, compared with
the control group (P<0.01; Fig. 5A and B). The expression of the
downstream OCN gene was not significantly different between
groups on day 4. By day 7, the CGFe group began to surpass the
control group (P<0.01). After 14 days of culture,
OCN expression in the TNF-α group was lower than that of the
control group (P<0.01; Fig.
5C).
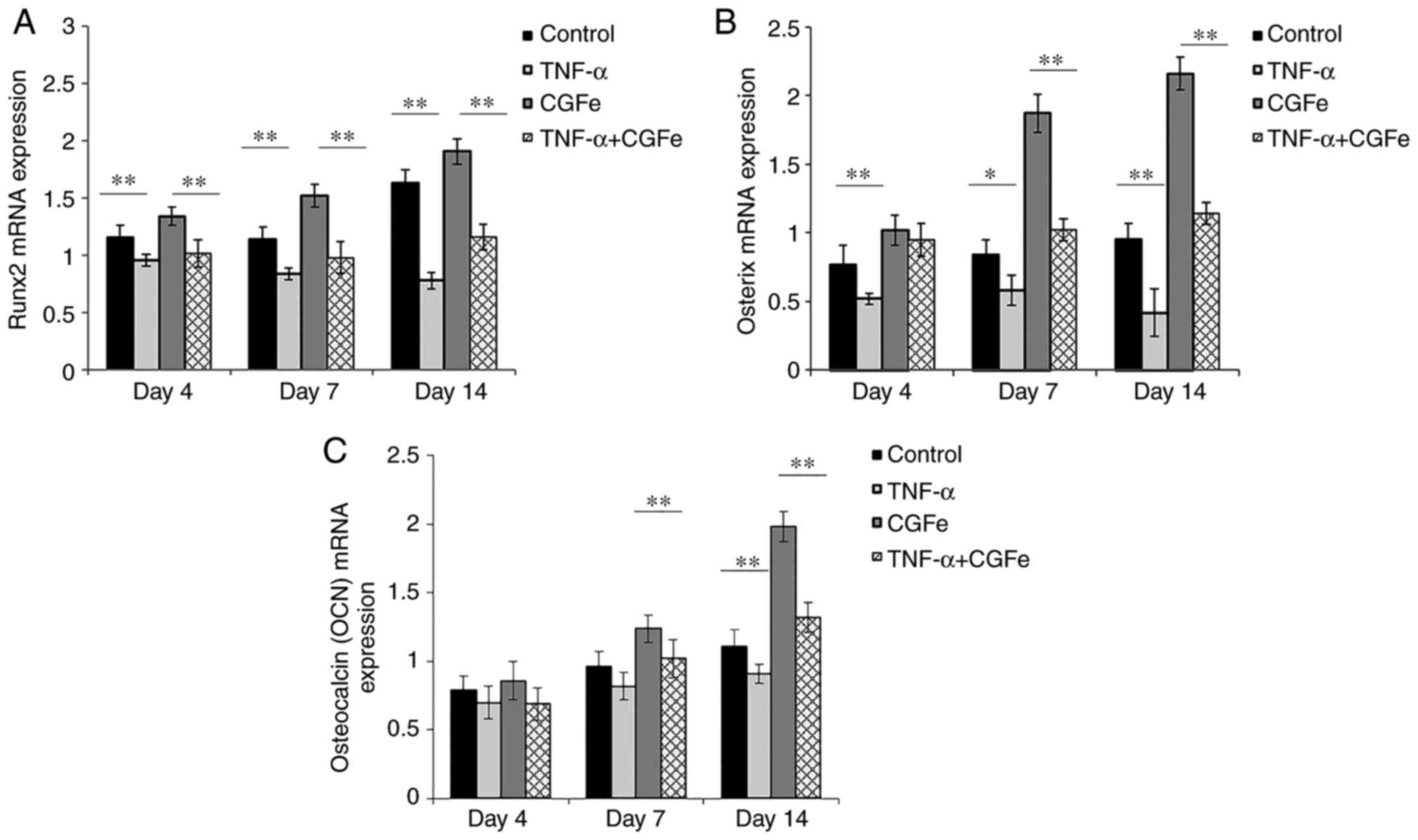 | Figure 5.CGFe increases the expression of
osteogenic-associated genes. Following osteogenic induction for 7
or 14 days, the expression of (A) RUNX2, (B) OSX and
(C) OCN was determined by reverse transcription-quantitative
polymerase chain reaction. The expression of RUNX2 and
OSX in the TNF-α groups was lower than that of the control
group. On days 4, 7 and 14, the expression of RUNX2 and
OSX in the CGFe groups was higher than the control group.
OCN gene expression was not significantly different between
the experimental groups on day 4. By day 7, OCN expression
in the CGFe group began to surpass the control group. After 14 days
of culture, OCN expression in the TNF-α was lower than that
of the control group. **P<0.01. CGFe, concentrated growth factor
exudate; TNF-α, tumor necrosis factor-α; RUNX2, runt-related
transcription factor 2; OCN, osteocalcin; OSX,
Osterix. |
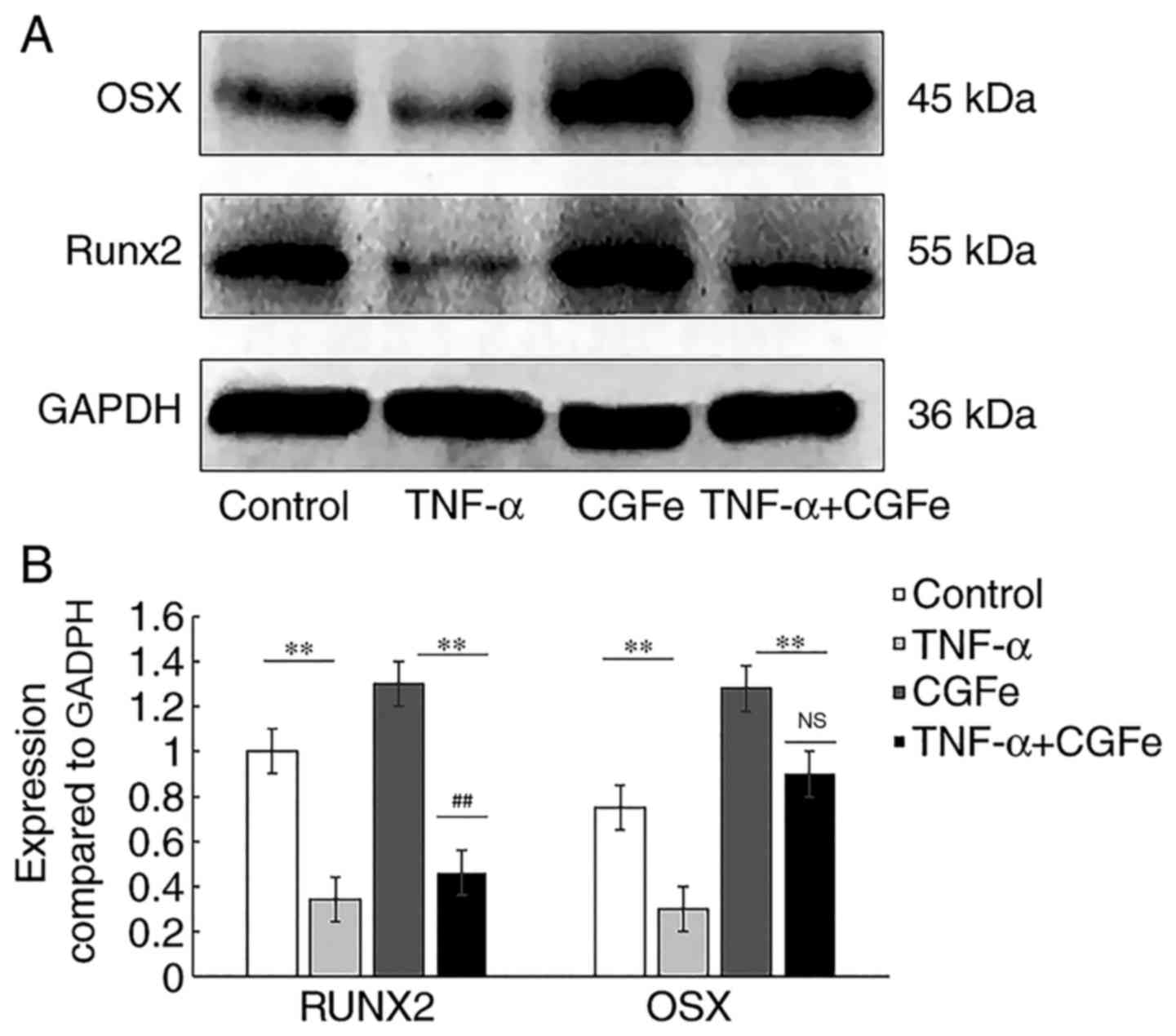
Western blotting was performed to examine the
effects of CGFe on hPDLC differentiation. hPDLCs were cultured in
each medium for 14 days. As shown in Fig. 6A, compared with the control group,
the protein expression of RUNX2 was upregulated in the CGFe group,
and downregulated in the CGFe+TNF-α and TNF-α groups
(P<0.01; Fig. 6B). A
similar trend was observed for OSX expression. Although the protein
level of OSX was upregulated in the CGFe+TNF-α group, there was no
significant difference compared with the control group
(P>0.05; Fig. 6B).
Discussion
CGFe can be obtained from the venous blood of
patients and thus is convenient and economical to prepare. CGFe
contains high concentrations of growth factors and provokes no
immune reaction (8–10,22,25).
According to previous studies (4–8), the
CGFe obtained from different patients in the present study
contained different inventories of components, yet the major
components were the same. These included epidermal growth factor,
platelet-derived growth factor, transforming growth factor (TGF-β),
vascular endothelium growth factor, insulin-like growth factor,
bone morphogenetic protein (BMP), and fibroblast growth factor
(4–8). In the last decade, CGFe has been
widely used in the reconstruction of bone tissue in the surgical
field (8,9). These growth factors have functions in
accelerating the revascularization of injured tissues and inducing
the migration, proliferation, and differentiation of fibroblasts
and osteoblasts (8–10,25).
TNF-α is a common and crucial inflammatory factor in
the development of periodontal inflammation and alveolar bone
resorption (20). It has been
established that TNF-α significantly inhibits the proliferation of
hPDLCs and mesenchymal stem cells (5,26,27).
In addition, activation of the nuclear factor (NF)-κB signaling
pathway inhibits the expression of RUNX2, which is a
downstream transcription factor induced by the exogenous
BMP2-mothers against decapentaplegic homolog (Smad) signaling
pathway controlling osteoblast differentiation, thus inhibiting the
formation of bone (28).
In the present study, TNF-α (10 ng/ml) was used to
construct an inflammatory microenvironment. It was demonstrated
TNF-α reduced ALP activity, mineralization ability and the
expression of RUNX2 and OSX, compared with the control group. This
result was concordant with the findings of Yang et al
(18), which revealed that the
bone formation efficiency of gingival mesenchymal stem cells and
periodontal ligament stem cells decreased under TNF-α-induced
inflammatory conditions.
Previous studies have shown that RUNX2 and OSX are
two essential transcription factors in the osteogenic pathway
(29,30). In particular, RUNX2 regulates cell
osteogenic differentiation and serves a central role in multiple
osteogenic signaling pathways (29). OSX is an osteogenic-specific
transcription factor with a zinc finger structure. A previous study
revealed that no bone formation occurs in mice lacking the
OSX gene (30). The level
of ALP activity reflects the tendency of cells to transform in the
osteogenic direction midway during the osteoproductive process,
while OCN is a late marker of osteogenesis. OCN is the most
abundant non-procollagen protein in bone tissue and promotes
osteogenic differentiation by combining with minerals (31,32).
Positive Alizarin Red S staining of the mineralized nodules formed
indicates the expression of OCN and osteogenesis at a later stage
(31,32).
In the present study, CGFe was added to cell
cultures to observe the osteogenic efficiency of hPDLCs, by
detecting the expression of RUNX2, OSX and OCN genes,
as well as ALP activity and mineralized nodule formation in each
group of hPDLCs. The formation of mineralized nodules was used to
determine the osteogenic capacity of each group of hPDLCs. It was
observed that even in the presence of inflammatory cytokine
stimulation, hPDLCs cultured with CGFe still exhibited osteogenic
differentiation and mineralization ability. By comparing the assay
and imaging results of the CGFe group with the CGFe+TNF-α group, it
was demonstrated that ALP activity in the CGFe group was stronger
than that of the CGFe+TNF-α group, and the formation of mineralized
nodules was notable in both groups, although the CGFe group was
stained more prominently. In addition, ALP activity in the
CGFe+TNF-α group was significantly higher than the TNF-α group, and
the CGFe+TNF-α group had deep mineralized staining with a large
area and high density, while no mineralized nodule formation was
observed in the TNF-α group.
The western blotting results of the present study
revealed that RUNX2 and OSX expression was higher in the CGFe
group, compared with the CGFe+TNF-α group, indicating that TNF-α
may play an inhibitory role upstream of protein translation. By
contrast, growth factors contained in the CGFe may antagonize the
inhibitory effect of TNF-α on cell proliferation. For example,
TGF-β1 induces the phosphorylation of Smad2/Smad3, which
upregulates RUNX2 transcription (29), thereby impeding TNF-α activation of
the NF-κB signaling pathway, which would result in RUNX2
inhibition.
In conclusion, CGFe not only has an osteogenic
effect on hPDLCs in a normal culture, but also promotes hPDLC
osteogenesis in a TNF-α-induced inflammatory microenvironment. In
the present work, a single inflammatory factor, TNF-α, was used to
mimic the microenvironment of periodontal disease, and this imposed
certain limitations. The enhancing effect of CGFe on osteogenic
differentiation of hPDLCs stimulated by other inflammatory factors
will be examined in follow-up experiments. To study the effects of
CGFe in the treatment of alveolar bone defects, in vivo
should be performed. In addition, the exact mechanism of how CGFe
enhanced the proliferation and osteogenic differentiation of hPDLCs
should be determined.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the People's
Hospital of Longhua (Shenzhen, China) and the Health and Family
Planning Commission of Shenzhen Municipality (grant no.
SZFZ2018035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, HY and BW conceived and designed the study. XL,
YZ, and HL performed cell culture, immunostaining and proliferation
analysis. XL and YZ performed the experimental procedures of
osteogenic differentiation induction, RT-qPCR, and western
blotting. HY, ZZ and ZY provided reagents and interpreted the data.
XL, HY and BW performed data analysis and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Jilin University Health Science Center (Changchun, China).
Written consent was obtained prior to experimentation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alsaadi G, Quirynen M, Komárek A and Van
Steenberghe D: Impact of local and systemic factors on the
incidence of oral implant failures, up to abutment connection. J
Clin Periodontol. 34:610–617. 2010. View Article : Google Scholar
|
|
2
|
Del Fabbro M, Boggian C and Taschieri S:
Immediate implant placement into fresh extraction sites with
chronic periapical pathologic features combined with plasma rich in
growth factors: Preliminary results of single-cohort study. J Oral
Maxillofac Surg. 67:2476–2484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiao J, Duan J, Zhang Y, Chu Y and Sun C:
The effect of concentrated growth factors in the treatment of
periodontal intrabony defects. Future Sci OA. 2:FS1362016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jang SJ, Kim JD and Cha SS: Platelet-rich
plasma (PRP) injections as an effective treatment for early
osteoarthritis. Eur J Orthop Surg Traumatol. 23:573–580. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He L, Lin Y, Hu X, Zhang Y and Wu H: A
comparative study of platelet-rich fibrin (PRF) and platelet-rich
plasma (PRP) on the effect of proliferation and differentiation of
rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 108:707–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suba Z, Takács D, Gyulai-Gaál S and Kovács
K: Facilitation of beta-tricalcium phosphate-induced alveolar bone
regeneration by platelet rich plasma in beagle dogs: A histologic
and histomorphometric study. Int J Oral Maxillofac Implants.
19:832–838. 2004.PubMed/NCBI
|
|
7
|
Fennis JP, Stoelinga PJ and Jansen JA:
Mandibular reconstruction: A histologic and histomorphomrtric study
on the use of autogenous scaffolds, particulate cortico-cancellous
bone grafts and platelet rich plasma in goats. Int J Oral
Maxillofac Surg. 33:48–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim TH, Kim SH, Sándor GK and Kim YD:
Comparison of platelet-rich plasma (PRP), platelet-rich fibrin
(PRF), and concentrated growth factor (CGF) in rabbit-skull defect
healing. Arch Oral Biol. 59:550–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park HC, Kim SG, Oh JS, You JS, Kim JS,
Lim SC, Jeong MA, Kim JS, Jung C, Kwon YS and Ji H: Early bone
formation at a femur defect using CGF and PRF grafts in adult dogs:
A comparative study. Implant Dent. 25:387–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodella LF, Favero G, Boninsegna R,
Buffoli B, Labanca M, Scarì G, Sacco L, Batani T and Rezzani R:
Growth factors, CD34 positive cells, and fibrin network analysis in
concentrated growth factors fraction. Microsc Res Tech. 74:772–777.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XJ, Yang HX, Zhang ZJ, Yan ZH, Lv HL,
Zhang Y and Wu B: Platelet-rich fibrin exudate promotes the
proliferation and osteogenic differentiation of human periodontal
ligament cells in vitro. Mol Med Rep. 18:4477–4485. 2018.PubMed/NCBI
|
|
12
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second generation platelet concentrate. Part I: Technological
concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e37–e44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tavassoli-Hojjati S, Sattari M, Ghasemi T,
Ahmadi R and Mashayekhi A: Effect of platelet-rich plasma
concentrations on the proliferation of periodontal cells: An in
vitro study. Eur J Dent. 10:469–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part III: Leucocyte
activation: A new feature for platelet concentrates? Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 101:e51–e55. 2006. View Article : Google Scholar
|
|
15
|
Li B and Wang Y: Contour changes in human
alveolar bone following tooth extraction of the maxillary central
incisor. J Zhejiang Univ Sci B. 15:1064–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lacey DC, Simmons PJ, Graves SE and
Hamilton JA: Proinflammatory cytokines inhibit osteogenic
differentiation from stem cells: Implications for bone repair
during inflammation. Osteoarthritis Cartilage. 17:735–742. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu B and Wang Z: Effect of concentrated
growth factors on beagle periodontal ligament stem cells in vitro.
Mol Med Rep. 9:235–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou
J, Jin Y and Chen FM: Comparison of mesenchymal stem cells derived
from gingival tissue and periodontal ligament in different
incubation conditions. Biomaterials. 34:7033–7047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Górska R, Gregorek H, Kowalski J,
Laskus-Perendyk A, Syczewska M and Madaliński K: Relationship
between clinical parameters and cytokine profiles in inflamed
gingival tissue and serum samples from patients with chronic
periodontitis. J Clin Periodontal. 30:1046–1052. 2003. View Article : Google Scholar
|
|
20
|
Li W, Yu B, Li M, Sun D, Hu Y, Zhao M, Cui
CB and Hou S: NEMO-binding domain peptide promotes osteoblast
differentiation impaired by tumor necrosis factor alpha. Biochem
Biophys Res Commun. 391:1228–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sacco L: Lecture: International Academy of
Implant Prosthesis and Osteoconnection. Lecture. 12:42006.
|
|
22
|
Sohn DS, Heo JU, Kwak DH, Kim DE, Kim JM,
Moon JW, Lee JH and Park IS: Bone regeneration in the maxillary
sinus using an autologous fibrin-rich block with concentrated
growth factors alone. Implant Dent. 20:389–395. 2011.PubMed/NCBI
|
|
23
|
Yamaguchi A: Application of BMP to bone
repair. Clin Calcium. 17:263–369. 2007.(In Japanese). PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Honda H, Tamai N, Naka N, Yoshikawa H and
Myoui A: Bone tissue engineering with bone marrow-derived stromal
cells integrated with concentrated growth factor in Rattus
norvegicus calvaria defect model. J Artif Organs. 16:305–315. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choukroun J, Diss A, Simonpieri A, Girard
MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J and Dohan DM:
Platelet-rich fibrin (PRF): A second-generation platelet
concentrate. Part V: Histologic evaluations of PRF effects on bone
allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 101:299–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang YH, Jeon SH, Park JY, Chung JH,
Choung YH, Choung HW, Kim ES and Choung PH: Platelet-rich fibrin is
a Bioscaffold and reservoir of growth factors for tissue
regeneration. Tissue Eng Part A. 17:349–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clipet F, Tricot S, Alno N, Massot M,
Solhi H, Cathelineau G, Perez F, De Mello G and Pellen-Mussi P: In
vitro effects of Choukroun's platelet-rich fibrin conditioned
medium on 3 different cell lines implicated in dental implantology.
Implant Dent. 21:51–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Franceschi RT and Xiao G: Regulation of
the osteoblast-specific transcription factor, Runx2: Responsiveness
to multiple signal transduction pathways. J Cell Biochem.
88:446–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piek E, Ju WJ, Heeyer J, Escalante-Alcalde
D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP
and Roberts AB: Functional characterization of transforming growth
factor beta signaling in Smad2-and Smad3-deficient fibroblasts. J
Biol Chem. 276:19945–19953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Glowacki J, Rey C, Glimcher MJ, Cox KA and
Lian J: A role for osteocalcin in osteoclast differentiation. J
Cell Biochem. 45:292–302. 1991. View Article : Google Scholar : PubMed/NCBI
|